Abstract
Lymphatic metastasis is the main prognostic factor for survival of patients with breast cancer and other epithelial malignancies. Mounting clinical and experimental data suggest that migration of tumor cells into the lymph nodes is greatly facilitated by lymphangiogenesis, a process that generates new lymphatic vessels from pre-existing lymphatics with the aid of circulating lymphatic endothelial progenitor cells. The key protein that induces lymphangiogenesis is vascular endothelial growth factor receptor-3 (VEGFR-3), which is activated by vascular endothelial growth factor-C and -D (VEGF-C and VEGF-D). These lymphangiogenic factors are commonly expressed in malignant, tumor-infiltrating and stromal cells, creating a favorable environment for generation of new lymphatic vessels. Clinical evidence demonstrates that increased lymphatic vessel density in and around tumors is associated with lymphatic metastasis and reduced patient survival. Recent evidence shows that breast cancers induce remodeling of the local lymphatic vessels and the regional lymphatic network in the sentinel and distal lymph nodes. These changes include an increase in number and diameter of tumor-draining lymphatic vessels. Consequently, lymph flow away from the tumor is increased, which significantly increases tumor cell metastasis to draining lymph nodes and may contribute to systemic spread. Collectively, recent advances in the biology of tumor-induced lymphangiogenesis suggest that chemical inhibitors of this process may be an attractive target for inhibiting tumor metastasis and cancer-related death. Nevertheless, this is a relatively new field of study and much remains to be established before the concept of tumor-induced lymphangiogenesis is accepted as a viable anti-metastatic target. This review summarizes the current concepts related to breast cancer lymphangiogenesis and lymphatic metastasis while highlighting controversies and unanswered questions.
Keywords: lymphangiogenesis, VEGF-C•VEGFR-3 axis, breast cancer, lymph nodes, lymphatic metastasis
1. Introduction
Metastasis is the leading cause of mortality in patients diagnosed with breast cancer [1;2] and other solid tumors [3;4]. Frequently, the initial sites of metastasis are the regional lymph nodes [5;6]. Mounting clinical and experimental data suggest that migration of tumor cells into the lymph nodes is greatly facilitated by lymphangiogenesis, a process that generates new lymphatic vessels from pre-existing lymphatics [7;8] or lymphatic endothelial progenitors [9]. This process is dynamic during embryogenesis but is relatively rare in adulthood. The main protein that regulates lymphangiogenesis is vascular endothelial growth factor receptor-3 (VEGFR-3) [10], a tyrosine kinase receptor expressed primarily on lymphatic endothelial cells (LEC) [11]. The VEGFR-3 pathway is activated by binding vascular endothelial growth factor-C (VEGF-C) [10;12] or a related protein, VEGF-D [13–15]. These lymphangiogenic factors are commonly expressed in malignant [16–18], tumor infiltrating [19;20] and stromal cells [21], creating a favorable environment for generation of new lymphatic vessels [12;14;22]. Studies of clinical breast cancers [23;24] and experimental breast tumor models [12;25;26] have provided substantial evidence of increased densities of both intratumoral and peritumoral lymphatic vessels, and their associations with metastasis as well as reduced survival. Recently, enhanced lymph node lymphangiogenesis and lymph flow in tumor draining lymphatic vessels have also been reported to contribute to metastatic spread. Agents that neutralize VEGF-C and VEGF-D, or block VEGFR-3 signaling, reportedly suppress development of new lymphatic vessels, lymphatic hyperplasia, and tumor metastasis in experimental cancer models [27;28]. Importantly, lower VEGFR-3 expression correlates with fewer positive lymph nodes and longer patient survival [22].
Collectively, these data implicate lymphangiogenesis in promoting metastasis to lymph nodes that are likely reservoirs for further dissemination to distant organs, suggesting that targeting tumor-induced lymphangiogenesis would prevent or reduce cancer-related death. Nevertheless, this is a relatively new field of study and much remains to be established before this concept is accepted. The following review summarizes the established and recently emerged concepts related to breast cancer lymphangiogenesis and lymphatic metastasis while highlighting current controversies and unanswered questions.
2. Incidence and clinical significance of lymph node (LN) metastasis in breast cancer
2.1. Prevalence of lymphatic vessel invasion (LVI) and metastasis through lymphatic channels in breast cancer
Numerous reports suggest that lymphatic vessels facilitate metastasis by providing a portal for tumor cell dissemination [29;30]. Compared with blood vessels, a lymphatic vessel pathway offers many advantages for invasion and transport of pre-metastatic cells, such as: 1) discontinuous basement membrane and loose cell-cell junctions; 2) a much lower flow rate that increases survival by minimizing shear stress; and 3) a 1000-fold higher lymph concentration of hyaluronic acid, a molecule with potent cell-protecting and pro-survival properties [31]. The lymphatic system is naturally equipped to transport cells throughout the body while ensuring their survival and activity. Epithelial tumors, including breast [1;7], melanoma [32], prostate [33], and head and neck [34] cancers, take advantage of the cell-transport capabilities of the lymphatic system and preferentially disseminate through the lymphatic vessels rather than through the hematogenous route.
The preferential spreading through lymphatic vessels might stem from the high frequency of lymphatic vessel invasion (LVI) in breast cancer as compared with blood vessel invasion (BVI) (Table 1 and Table 2). Vascular invasion encompassing both types of vessels has long been recognized as a poor prognostic indicator [35–37]. However, early studies did not distinguish between lymphatic and blood vessels due to the absence of specific lymphatic markers. The emergence of specific lymphatic markers, such as LYVE-1 [38], Prox1 [39], and podoplanin/D2-40 [40;41] has enabled clear distinction between lymphatic and blood vessel invasion by tumor cells [42–44]. Using these markers in conjunction with double immunostaining yielded extensive evidence for preferential invasion of the lymphatic, rather than blood vessels by breast carcinoma cells [42;44]. This is illustrated by several independent studies involving a substantial number of patients (Table 2). One of the largest studies conducted to date (N=1,408) showed the presence of LVI in 34.2% of all cases while BVI was detected in only 4.2% [2]. A separate study of 177 invasive breast carcinomas [36] detected lymphatic invasion in 96.4% of all specimens, whereas BVI was detected in only 3.5% [36]. A similar tendency of breast tumor cells to invade lymphatics has been shown in additional studies collectively involving more than 3,000 patients that demonstrated a 2–3-fold higher frequency of LVI in comparison with BVI (Table 2).
Table 1.
Association of lymphatic vessel invasion (LVI) with lymphatic metastasis in breast cancer
| # of Patients |
Method of Analysis |
Comments | P valuea | Reference |
|---|---|---|---|---|
| 4,351 | H&Eb | Sentinel LNb metastasis was strongly associated with peritumoral vascular invasion (pVI) |
P<0.0001 | [37] |
| 2,606 | H&E | LN metastasis significantly correlated with perivascular invasion encompassing both LVIb or BVIb |
P<0.0001 | [110] |
| 850 | H&E | LVI detected in 51% of patients was the most significant predictor for axillary LN metastases |
P<0.001 | [51] |
| 400 | IHCb | Patients with LVI were more likely to have sLNb metastases (51.3% of LVI-positive patients had positive sLN) |
P<0.001 | [46] |
| 374 | IHC | LVI was significantly associated with a higher risk for developing lymph node metastasis |
P=0.004 | [50] |
| 254 | H&E and IHC |
In a multivariate analysis, LVI was the strongest predictor for ALNMb with an odds ratio of 3.489 |
P=0.0003 | [41] |
| 206 | H&E | LVI was significantly associated with non-sentinel LN involvement (41% of LVI-positive patients had also positive LN versus 26% of LVI-negative patients) |
P=0.021 | [230] |
| 194 | H&E and IHC |
Strong association was found between pLVI and non-sentinel LN metastasis (65% versus 37%) |
P=0.001 | [231] |
| 177 | IHC | LVI was strongly associated with LN metastasis, distant metastasis, decreased disease-free interval and overall survival |
P<0.001 | [36] |
| 165 | H&E | LVI in the primary tumor was the only factor significantly associated with metastases in the non-sentinel LN |
P<0.01 | [45] |
| 123 | IHC | LVI but not BVI significantly correlated with lymphatic metastasis |
P=0.002 | [42] |
| 118 | H&E | LVI was a strong predictor of axillary LN metastasis regardless of tumor size |
P<0.0001 | [232] |
| 113 | IHC | LVI correlated with LN metastasis, LVDb and VEGF-Cb | P< 0.0001 | [70] |
| 98 | IHC | LVI correlated with VEGF-C, which was expressed in ~40% of breast cancers but not in adjacent normal mammary glands |
P=0.0004 | [67] |
| 95 | H&E and IHC |
Only peritumoral LVI, but not BVI, was associated with LN metastases. LVI exceeded BVI in both the number of invaded vessels and the size of the emboli. (P=0.004) |
P=0.002 | [44] |
P value indicates association of intratumoral or peritumoral lymphatic vessel invasion with LN metastasis.
Abbreviations: H&E, hematoxylin & eosin; LN, lymph node; LVI, lymphatic vascular invasion; BVI, blood vascular invasion; IHC, immunohistochemistry; sLN, sentinel lymph node; pLVI, peritumoral lymphatic vascular invasion; DFS, disease-free survival; OS, overall survival; ALNM, axillary lymph node metastasis; LEC, lymphatic endothelial cells; LVD, lymphatic vessel density; VEGF-C, vascular endothelial growth factor C.
Table 2.
Incidence of LVI and BVI in breast cancer and the impact on patient survival
| # of Study |
# of Patients |
% Invasiona |
Comments | P valuec | Reference | |
|---|---|---|---|---|---|---|
| LVIb | BVIb | |||||
| 1 | 1,408 | 34.2 | 4.2 | LVI and BVI were both indicators of poor survival |
P=0.001 | [2] |
| 1 | 1,408 | 34.2 | 4.2 | Multivariate analysis showed that LVI is a strong prognostic indicator of significantly increased risk of death in both LNb positive and negative patients whereas BVI has no prognostic value |
P=0.001 | [2] |
| 2 | 1,258 | 27.6 | NDd | LVI was a highly significant independent predictor of disease outcome |
P<0.0001 | [49] |
| 3 | 374 | 28.0 | ND | LVI presented as an independent prognostic parameter for DFSb as well as OSb |
P=0.001 | [50] |
| 4 | 303 | 27.0 | ND | Multivariate analysis revealed that LVI was the only significant predictor of distal recurrence. LVI was significantly associated with shorter overall survival |
P=0.009 | {3497} |
| 5 | 177 | 96.4 | 3.5 | LVI was strongly associated with LN metastasis, distant metastasis, decreased DFS and OS |
P<0.001 | [36] |
| 6 | 123 | 28.5 | 15.4 | LVI but not BVI positively correlated with tumor cell Ki67 score and other markers associated with poor prognosis |
Not assessede |
[42] |
| 7 | 95 | 69.5 | 37.9 | When LVI and BVI were both present, 80% of the invaded vessels were of lymphatic origin |
Not assessed |
[44] |
% Invasion is presented as a fraction of cases in which tumor cells were immunohistochemically in the lumen of lymphatic or blood vessels.
Abbreviations: LN, lymph node; LVI, lymphatic vascular invasion; BVI, blood vascular invasion; DFS, disease-free survival; OS, overall survival.
P values for association of LVI disease-free survival (DFS) and overall survival (OS).
ND, not determined.
Association of LVI or BVI with patient survival was not assessed in this study.
LVI has also been shown to significantly correlate with sentinel [45–47], non-sentinel [45] and axillary LN metastasis [48] as well as with disease outcome[2;49]. Sentinel LN metastasis was detected in 51% of LVI-positive as compared with 30% of LVI-negative patients [46]. A separate study showed that peritumoral LVI (pLVI) is significantly associated with LN metastasis (p=0.004), being present nearly three-fold more frequently in node-positive than in node-negative patients (65% vs. 23%) [50]. This was also supported by an independent study of 1,258 patients that identified pLVI as a highly significant predictor of disease outcome [49]. A strong association between pLVI and axillary metastasis was shown in a large study (N=850) in which positive LN were detected in 51% of the LVI-positive group compared with 19% of the LVI-negative patients [51].
Some studies identified both LVI and BVI as poor prognostic markers [2], but the majority of studies identified only LVI as an indicator of short survival {1105, 3497, 3160, 329}(Table 2). This does not indicate that BVI and hematogenous metastasis do not play a significant role in mortality from breast cancer. Clearly, the main cause of death from cancer is not metastasis to lymph nodes per se, but distant metastasis that interferes with the function of vital organs such as lung, bone, brain, etc. Nevertheless, strong association of LVI with poor outcome in breast cancer suggests that local LVI eventually leads to BVI that might occur in lymph nodes, or contributes independently of local BVI to distant metastasis through the lymphatic vessel trafficking. Since in breast cancer LVI occurs more frequently than BVI (Table 2), the evidence for LVI has more prognostic power for the disease outcome than that of BVI. Collectively, these studies underscore the prevalence of LVI over BVI in breast cancer. These data also suggest that tumor-associated both pre-existing and newly-generated lymphatic vessels are the main means of transportation for metastatic cells to loco-regional nodes.
2.2. Metastatic incidence to sentinel, axillary, and distant lymph nodes
In patients with clinically staged I and II breast tumors, lymph node status is one of the most important prognostic factors for survival independent of tumor size, histological grade and other clinicopathological parameters [53;54]. Until recently, complete axillary lymph node dissections were conducted routinely and were found to be positive in 30% of patients [55]. However, axillary lymph node dissection is associated with long-term morbidity and poor quality of life, manifesting in reduced shoulder mobility, sensory disturbance, and lymphedema [55]. A much less intrusive procedure, the sentinel lymph node (sLN) biopsy was first reported in 1993 to accurately predict spread of tumor cells to regional nodes [56]. The sentinel lymph node is the first node receiving lymphatic drainage from the tumor that might contain metastatic cells [56]. Patients with positive sLN undergo a complete axillary lymph node dissection, while sLN-negative patients can be spared the procedure. Overall, the sLN is positive in 20–35% of patients with early-stage breast cancer [55–59]. Metastasis to the sentinel lymph node can be defined as macrometastases (>2 mm), micrometastases (<2 mm), or as isolated tumor cells identified by immunostaining using epithelial cell markers [59]. Cytokeratin-based identification of tumor cells improves staging [60] and may prevent local recurrence by removing positive nodes undetectable by routine H&E analysis.
Dissemination of tumor cells from the sentinel to distal non-sentinel nodes, and then to axillary lymph nodes, occurs in 30–35% of patients with positive sLN [61;62]. Presence of macrometastases in the sLN is associated with a significantly higher risk for axillary node metastasis (49% vs. 11%) [61]. Tumor cells can also metastasize to intramammary lymph nodes, particularly in those having extensive axillary involvement and presence of LVI at the primary site [63]. As summarized in Table 3, metastasis to sLN [49], non-sentinel, intramammary [63] and axillary nodes [53;64;65] is strongly associated with poor disease-free survival (DFS) and shorter overall survival (OS). The size of the metastatic lesion in the lymph node [53;64;66] and the number of positive nodes [54;66] exacerbate the prognosis. Combined, these studies indicate that metastatic breast tumor cells preferentially use lymphatic channels to exit the primary site and that the sentinel LN metastasis is associated with spread to distal nodes and non-lymphoid organs, as manifested by significantly reduced DFS and OS rates.
Table 3.
Association of lymphatic metastasis with distant metastasis and survival
| Correlation with survival | |||||
|---|---|---|---|---|---|
| # of Patients |
Time of observation |
Comments | Disease free (DFS) a |
Overall (OS) a |
Reference |
| N/A b | N/A | Distant metastasis did not occur in the absence of lymph node metastasis in an experimental model |
N/A | N/A | [121] |
| 62,557 | 10 years | Ten-year DFS and OS were significantly lower in LN positive patients compared to those with pN0 (82.3% versus 91.9% and OS 68.1% versus 75.7%) |
P<0.001 | P<0.001 | [65] |
| 6,959 | >10 years | Multivariate analysis adjusted for patient-, histopathologic-, and loco-regional therapeutic variables showed that LN positive patients had a significantly higher risk of death relative to negative cases |
Not assessed |
P<0.01 | [53] |
| 1,258 | 12 years | Both lymph node status and the presence of LVI were highly significant independent predictors of outcome |
Not assessed |
P<0.0001 | [49] |
| 1,126 | 9 years | Axillary metastasis developed in 18% of patients and significantly correlated with survival |
P = 0.02 | P = 0.01 | [118] |
| 939 | >5 years | Nodal involvement and % positive nodes were the major factors for both OS and DFS with % positive nodes the most significant prognostic factor for survival |
P<0.001 | P<0.001 | [233] |
| 813 | 5 years | Node-negative patients survived 42 months after relapse compared with 20 months for patients with 1–3 nodes and 13 months for those having > 4 positive nodes |
P<0.0001 | P<0.0001 | [66] |
| 813 | >75 years | 62% of 813 patients had positive sLN. There was a significant difference in DFS and OS between patients who had 0–4 non-sentinel LN+ and those who had >5 non-sentinel LN+ suggesting that once the tumor passes sLN, it is much likely to spread systemically |
P = 0.001 | P = 0.003 | [58] |
| 453 | 6–65 months |
A 10 year survival rate for patients with less than 14 nodes removed was 79% compared with 89% for patients with more than 14 nodes removed |
P<0.0001 | P=0.005 | [117] |
| 157 c* | 9 years | Omission of axillary dissection occurred in 157 cases and correlated with reductions in OS, DFS, and breast cancer-specific survival in all patients |
P<0.001 | P<0.001 | [118] |
| 152 | 75 months | 73% of node-negative patients were free of distant metastasis 5 years after diagnosis versus 48% of node- positive patients |
P=0.01 | P=0.03 | [234] |
| 130 | 5 years | Patients with intramammary metastases had poorer 5- year rates of DFS, disease-specific (66% vs. 90%; P = 0.001), and OS |
P = 0.001 | P = 0.004 | [63] |
| 122 | >5 years | The size of the largest LN was associated with worse outcome in both univariate and multivariate analysis |
P < .0001 | P < .0001 | [64] |
| 118 | 10 years | 91% of node-negative patients were free of distant metastasis versus 77% of node-positive patients |
P = 0.001 | Not assessed |
[232] |
P values for disease-free survival (DFS) and overall survival (OS).
N/A, not applicable.
157 patients from this study [118] who did not undergo axillary dissection were analyzed separately for survival. The effect of axillary dissection omission on survival is reported.
3. Clinical evidence for association of lymphangiogenesis and LN metastasis in breast cancer
While clinical significance of lymphatic metastasis in breast cancer is well recognized, the means of tumor cell transportation to regional LN and relevance to distant metastasis is a matter of considerable debate. Central to this debate are the following questions: (1) Do clinical breast tumors induce lymphangiogenesis either intratumorally or in the peritumoral tissue? (2) How do tumor cells access lymphatic vessels? (3) Are tumor-induced new lymphatic vessels capable of transporting tumor cells? (4) Can lymphatic metastasis occur in the absence of lymphangiogenesis? (5) Do metastatic cells in LN contribute to distant spread? The answers to these questions are crucial to understanding whether tumor-induced lymphangiogenesis is a viable target for inhibition of distant metastasis that is, undoubtedly, the major cause of cancer-related mortality. The next chapter summarizes the current clinical evidence that provides insights to these questions.
3.1. Do clinical breast tumors induce lymphangiogenesis?
To answer this question, multiple studies analyzed the three main parameters defining tumor lymphangiogenesis: (A) expression of lymphangiogenic factors, VEGF-C and VEGF-D; (B) the presence of dividing lymphatic endothelial cells identified by proliferative markers, Ki-67 or PCNA; and (C) increase in lymphatic vessel density (LVD) and invasion (LVI) at the tumor periphery or in the tumor proper.
3.1.1. Expression of the main lymphangiogenic mediators, VEGF-C and VEGF-D
A number of studies correlated the expression of VEGF-C and VEGF-D with induction of lymphangiogenesis, lymphatic metastasis and disease outcome (Table 4). Elevated VEGF-C expression has been reported in 30–40% of breast cancers and has been shown to associate with high incidence of LVI, lymph node metastasis and a lower DFS [67–69]. Another study found positive VEGF-C breast tumor cells in 39% of specimens (n=98) with no expression in adjacent normal mammary glands [67]. Additional studies demonstrated VEGF-C expression in 48% of 113 [70] and 87% of 123 [71] breast tumors, respectively, and a significant correlation between tumor VEGF-C positivity, LVD, LN metastasis, DFS and OS [70;71]. In contrast, some studies detected VEGF-C in the majority of the examined tumors, but found no correlation with LVI or LN metastasis [72;73]. These discrepancies might suggest a role for other factors besides VEGF-C and VEGF-D in induction of lymphangiogenesis, or may relate to heterogeneity of analyzed patient populations.
Table 4.
Correlation of VEGF-C and VEGF-D with lymphatic metastasis
| # of Patients |
Method of Analysis |
Comments | P valuea | Reference |
|---|---|---|---|---|
| 177 | IHCb | VEGF-Cb was highly expressed in 37% of specimens. VEGF-C expression was associated with a higher LVDb (P=0.014), lymph node and distant metastasis (P<0.001 and P=0.008, respectively) and a shorter OSb (P= 0.028) |
P<0.001 | [68] |
| 123 | IHC | VEGF-C was detected in 87.3% of specimens. High VEGF-C was associated with poor DFSb and OS (P=0.0165 & P=0.0175) |
P=0.0131 | [71] |
| 121 | IHC | VEGF-D correlated with the presence of intralymphatic tumor cells |
[80] | |
| 113 | qRT-PCRb | VEGF-C mRNA level also correlated with LVD (P=0.0409) |
P=0.0074 | [70] |
| 105 | IHC | VEGF-Db expression was identified in 86 cases (81.9% of all cases) and highly correlated with LN metastasis |
0.0238 | [74] |
| 98 | IHC | No correlations were found between VEGF-C or VEGF-D expression and tumor size, grade, estrogen receptor status, axillary lymph node metastases and other parameters |
NS | [235] |
| 80 | IHC | VEGF-C was also significantly associated with higher peritumoral LVD, lymphatic invasion and number of positive nodes in patients with micropapillary breast carcinoma |
P=0.003 | [236] |
| 70 | IHC | Both VEGF-C and COX-2 correlated with LN metastasis, LVD, LVIb as well as with worse DFS and OS |
P=0.01 | [237] |
| 51 | IHC | There was no significant correlation between VEGF-C and LN status, LVI, tumor size, grade or other parameters |
NS | [73] |
| 33 | RT-PCR | VEGF-C mRNA expression and LVI or lymph node metastasis |
NS | [72] |
| 29 | qRT-PCR & IHC |
Intratumoral VEGF-C and VEGF-D expression by quantitative RT-PCR correlated with D2-40c but not with CD31c microvessel density |
P=0.0414 | [238] |
P value indicates association of VEGF-C or -D expression with LN metastasis.
Abbreviations: IHC, immunohistochemistry; VEGF-C and VEGF-D, vascular endothelial growth factor C or D; OS, overall survival; DFS, disease-free survival; LVD, lymphatic vessel density; qRT-PCR, quantitative reverse-transcriptase polymerase chain reaction; LVI, lymphatic vascular invasion.
D2-40 and CD31 are markers of lymphatic and blood vascular endothelial cells, respectively.
Up-regulation of VEGF-D and correlation with LN metastasis was found in a smaller number of studies, although one study reported immunohistochemical VEGF-D detection in as many as 81% of 105 breast cancer specimens [74]. Yet, other studies found no correlation of VEGF-D with metastasis or survival, or decreased VEGF-D expression in malignant as compared with normal breast tissue [72]. Studies of other tumor types also reported high expression of VEGF-D in normal tissues [75] and reduced detection in malignant tumors [75;76], suggesting that VEGF-C and VEGF-D play distinct physiological roles. It should also be mentioned that both factors are highly expressed in tumor-associated macrophages [19] suggesting that the tumor environment might be pro-lymphangiogenic regardless of VEGF-C/VEGF-D expression by neoplastic cells.
3.1.2 Presence of dividing lymphatic endothelial cells in clinical breast cancer
Detection of dividing lymphatic endothelial cells (LEC) became central to the current controversy on whether lymphangiogenesis occurs in breast cancer and whether lymphatic metastasis occurs through pre-existing or newly-formed lymphatic vessels [77–80]. This controversy exists because neither the mere presence of pro-lymphangiogenic factors nor frequent incidence of LN metastasis constitutes a proof of tumor-induced, actively ongoing formation of new lymphatic vessels. Resolving this question has been sought by double staining using antibodies to specific lymphatic markers (LYVE-1 or D2-40) combined with antibodies to proliferative markers such Ki-67 (MIB-1) or PCNA. This approach represents a significant improvement over previously employed histological evaluation (i.e., by H&E) to identify dividing LEC. However, most studies still use colorimetric stains, which are inferior to immunofluorescence in terms of discerning among individually stained structures. As a result, interpretation of overlapped lymphatic marker/Ki-67 positivity might depend on whether the positive cells are seen to be proliferating LEC, or dividing tumor cells that had invaded lymphatic vessels. Additional challenges in detection of proliferating LEC are: 1) a relatively low rate of vessel formation in well-established tumors; 2) a lower density and heterogeneity of tumor lymphatics compared with tumor blood vessels; and 3) variability in sprouting of new vessels at different points along the parental lymphatic vessel [81], with the latter being undetectable in two-dimensional evaluation. Moreover, the formation of new lymphatic vessels might not require endothelial mitotic division if they originate from circulating progenitors or non-endothelial cells via transdifferentiation [82].
Given these technical and biological limitations, it is not surprising that several studies failed to detect Ki-67 or PCNA markers on LYVE-1 or D2-40-labeled structures [77;78;80] (Table 5). The authors of these studies concluded that active lymphangiogenesis is absent in clinical human breast cancer, and lymphatic metastasis occurs either through undefined non-vascular routes or through invasion of pre-existing lymphatic vessels juxtaposed to the tumor-stroma border. These explanations are entirely possible as nothing prevents tumor cells to invade pre-existing lymphatic vessels if the new lymphatic vessels are not formed in a particular tumor environment. However, evidence from several research groups also supports tumor-induced lymphangiogenesis and shows its clinical relevance to lymphatic metastasis (Table 5). For instance, double Ki-67/podoplanin staining of a large panel (N=177) of invasive breast carcinomas determined that 29% of specimens displayed Ki-67 positive nuclei in 2.2% of intratumoral, peritumoral and peripheral lymphatics [23]. Frequency of positive nuclei was strongly associated with a high lymphatic density (P=0.001), LN metastasis and survival [23]. An independent study detected a similar fraction of proliferating LEC (LECP%) in peritumoral lymphatics and also identified LECP% as an independent prognostic factor for LN metastasis [83]. Studies that compared LECP% in inflammatory and non-inflammatory breast cancers found that the former have both a higher incidence of Ki-67 positive lymphatics (80% versus 50%) and an increased median LECP% [24;84]. Active lymphangiogenesis was also detected in positive sentinel LN [85;86] that displayed a significantly higher median LECP% (P<0.001) than uninvolved LN [86]. Moreover, high frequency of Ki-67-labeled lymphatics in positive sLN was strongly associated (P=0.01) with axillary metastasis [85], supporting the contention that tumor-induced lymphangiogenesis promotes dissemination from both the primary tumor and secondary metastatic sites. Nevertheless, with the exception of very active lymphangiogenesis in inflammatory breast cancer [24;84], a relatively low fraction of dividing lymphatic endothelial cells (2–6%) and some discrepancy between high %LVI and low %LECP in other breast cancer types suggest that both new and existing lymphatic vessels partake in lymphatic metastasis.
Table 5.
Correlation of lymphatic endothelial cell proliferation and lymphatic metastasis
| # of Patients |
Method of Analysis |
Comments | P valuea | Reference |
|---|---|---|---|---|
| 177 | Double IHC, D2-40/Ki-67 |
Proliferating lymphatics were detected in 29% of specimens and were significantly associated with inflammatory infiltrate |
Not assessed | [23] |
| 123 | IHC | Higher incidence of LVI was associated with increased LEC proliferation and correlated to the presence of micrometastases |
P=0.002 | [42] |
| 121 | Double IHC, podoplanin & Ki-67 |
No correlation between intratumoral lymphatic vessel density inside the lymph node metastases and patient survival |
NS | [80] |
| 110 | Double IHC, D2-40/Ki-67 |
Median intra- and perinodal lymphatic endothelial cell proliferation fractions were higher in metastatic LN |
P<0.001 | [86] |
| 75 | Double IHC, LYVE-1/Ki-67 |
None of the breast carcinomas displayed dividing lymphatic endothelial cells, but a fraction of the peritumoral lymphatics contained tumor emboli |
NS | [77] |
| 65 | Double IHC, D2-40/Ki-67 |
LECP%b correlated with a positive non-sentinel LN status | P = 0.01 | [85] |
| 56 | Double IHC, D2-40/Ki-67 |
The degree of lymphatic endothelial cell proliferation was predictive of LN metastasis. Inflammatory breast cancer specimens displayed significantly higher LECP% than non-inflammatory breast tumors (5.74% vs. 1.83%, P=0.005) |
P = 0.01 | [24] |
| 32 | Double IHC, LYVE-1/Ki-67 |
Inflammatory breast cancers contained significantly higher LECP% than non-inflammatory specimens (P = 0.033) |
Not assessed | [84] |
P value indicates significant association of a fraction of lymphatic endothelial cells undergoing division with lymphatic metastasis.
LECP%, lymphatic endothelial cell proliferation fraction or percent of total LEC identified by specific lymphatic endothelial cell markers.
3.1.3 Clinical evidence of increase in lymphatic vessel density (LVD)
In contrast to challenges mentioned earlier in the detection of dividing LEC, enumerating lymphatic vessels seemed initially a straightforward measure of lymphangiogenesis. It was expected that quantitative analysis of intratumoral, peritumoral and non-tumor lymphatic vessel densities (LVD) should settle the question of induction of breast cancer lymphangiogenesis, analogously to the previous establishment of intratumoral blood vessel density (BVD) as a reliable indicator of tumor-induced angiogenesis [87]. Technically, however, LVD quantification is much more challenging than BVD because of the natural heterogeneous distribution of lymphatic vessels. Additional complexity arises from the fact that, in contrast to blood vessels, lymphatic vessels support spread of metastatic cells, but not tumor cell proliferation and expansion of the tumor mass. Therefore, subtle increases in LVD might be missed in tumor sections set aside for immunohistochemical analysis, although they might suffice for tumor dissemination in a patient. As a result, findings and interpretations from the studies that focused on infrequently occurring intratumoral lymphatic vessels [79], or those that compared a heterogeneous LVD pattern to more orderly tumor blood vessel distribution [77], fueled the debate whether lymphangiogenesis exists in breast cancer [77–80].
The main evidence supporting the claim that lymphangiogenesis does not exit in tumors is detection of decreased LVD or absence of intratumoral lymphatic vessels (LV) compared with normal breast tissue [77–80]. The same studies, however, reported a significant increase (P=0.0001) in peritumoral LVD [78;80] with some lymphatic vessels (LV) containing tumor emboli [77]. Similar findings were also reported in an independent study (N=180) in which the intratumoral LV were detected in only 12% of the tumors, whereas the peritumoral LV were present in 94% [88]. The bias toward development of peritumoral lymphatics has also been noted in a study on 177 specimens of invasive breast cancer, in which intra- and peritumoral lymphatic vessels were detected in 41% and 100% of the samples, respectively [23]. However, a study comparing LVD in a highly metastatic inflammatory breast cancer (IBC, N=29) with invasive but non-IBC (N=59) tumors, found intratumoral lymphatic vessels in as many as 80–82% of samples in both tumor groups [24]. As shown in Table 6, there is a wide range of opinions with regard to a prognostic value of intratumoral LVD. However, a consensus seems to exist with regard to increased density of peritumoral lymphatic vessels (Table 6) that might be sufficient for tumor cell transit to LN even in the absence of intratumoral lymphatics.
Table 6.
Association of lymphatic vessel density (LVD) with lymphatic metastasis in breast cancer
| # of Patients |
Method of Analysis |
Comments | P valueb | Reference |
|---|---|---|---|---|
| 180 | IHCa | Higher than median LVD a was significantly associated with unfavorable prognosis |
P=0.033 | [88] |
| 177 | IHC | Tumors with higher total LVD were significantly associated with LN metastasis and shorter OS a |
P<0.001 | [23] |
| 121 | IHC | Intratumoral lymphatic vessels were present in ~10% of all patients and did not correlate with LN metastasis. Peritumoral lymphatic vessel density was not assessed |
NS | [80] |
| 113 | IHC | LVD also strongly correlated with poor DFS a and OS (P=0.0033 & P=0.0391) |
P<0.0001 | [70] |
| 87 | IHC | D2-40-stained intratumoral lymph vessels were present in 80% of non-inflammatory and 82.8% of inflammatory breast cancers specimens |
P=0.001 | [24] |
| 80 | IHC | Higher LVD correlated with the number of positive LN in patients with invasive micropapillary breast carcinoma |
P=0.045 | [236] |
| 75 | IHC | Lymphatic vessel density was unrelated to LN status | NS | [77] |
| 61 | IHC | LVD was higher in breast carcinoma than in benign mammary lesions and significantly correlated with higher expression of VEGF-C and VEGF-D, P<0.01 |
P<0.01 | [69] |
| 61 | IHC | Intratumoral lymphatic vessels were reduced or absent in tumors compared with normal breast tissue. Peritumoral LVD was not assessed |
N/A | [79] |
| 55 | IHC | There was an increase in peritumoral LVD as compared with normal breast) but a decrease in intratumoral LVD (both P = 0.0001) |
P = 0.0001 | [78] |
| 29 | qRT-PCR | LVD also correlated with VEGF-C and VEGF-D expression measured by qRT-PCR (P=0.0291) |
P=0.0558 | [238] |
Abbreviations: IHC, immunohistochemistry; LVD, lymphatic vessel density; OS, overall survival; DFS, disease-free survival; other abbreviations are given under Table 1.
P value indicates association of intratumoral or peritumoral LVD with LN metastasis.
3.2. How do tumor cells access lymphatic vessels?
It has been proposed that tumor cells, in addition to passive invasion of lymphatic vessels, may use physiological attractants SDF-1 (also known as CXCL12) and CCL21 to migrate through the lymphatic channels toward regional lymph nodes. This hypothesis has been supported by several lines of evidence. First, significant number of breast cancers (30% to up to 70%) over-express CXCR4 and/or CCR7, which are the receptors for SDF-1 and CCL21, respectively [89–91]. Up-regulation of the expression of either CXCR4 or CCR7 strongly correlates with lymph node metastasis [89;92]. Second, SDF-1 is highly expressed in tissues that are often sites for breast cancer metastasis, including lymph node, lung, liver, and bone marrow, and the extracts from these tissues stimulates migration of breast carcinoma cells [93]. Third, stimulation of either CXCR4 or CCR7 with respective ligands increases migration and invasiveness of cultured breast cancer cells [94–96]. Fourth, antagonists or neutralizing antibodies against these chemokine receptors significantly reduce both lymphatic and distant metastasis in breast cancer animal models [93]. Collectively, these studies suggest that CXCR4 or CCR7-positive breast tumors may exhibit enhanced lymphatic metastasis due to active chemo-attraction and directional migration toward ligand-overexpressing lymph nodes.
3.3. Are tumor-induced new lymphatic vessels capable of transporting tumor cells?
3.3.1 Evidence for the ability of tumor-induced lymphatic vessels to transfer fluid and metastatic cells
Lymphatic metastasis was reported also to occur in the absence of intratumoral lymphatic vasculature [97;98]. It has also been shown in some experimental systems that intratumoral lymphatic vessels might be present but conduct no fluid whereas the only peritumoral lymphatics are functional [99;100]. This conclusion was further supported by an experimental study of B16-F10 melanoma over-expressing VEGF-C that demonstrated functionality in 40% of peritumoral but not intratumoral lymphatic vessels [101]. The peritumoral lymphatics incorporated a proliferative marker [101], indicating actively ongoing lymphangiogenesis rather than lymphatics existing prior to tumor implantation. This study [101] showed a tracer uptake by peritumoral lymphatics, but did not demonstrate actual lymph flow from the tumor to loco-regional nodes. In contrast, a separate study using a fluorescent dye injected proximally to B16-F10 melanoma tumors implanted into the foot of mice showed a 23-fold increase in lymph flow to the popliteal LN as compared with the nontumor bearing leg [102]. Studies in the T241 fibrosarcoma model over-expressing VEGF-C also showed an increase in volumetric lymph flow by 40%, coincident with a 200-fold increase in tumor cell dissemination and a 4-fold increase in LN metastasis [103]. Moreover, blocking VEGF-C reduced lymphatic hyperplasia, lymph flow, and lymph node metastasis [104].
A recent study of orthotopic MDA-MB-231 breast tumors showed that tumors not only enhanced lymph fluid flow but also transfer of metastatic cells [105]. Tumor cells labeled by red fluorescence were imaged in real-time during invasion of “green” lymphatic vessels visualized by FITC-dextran, then exiting the primary site through the lymphatics, clustering into clumps at the lymphatic vessel junction, entering the subcapsular sinuses of the inguinal lymph node and intruding the node parenchyma [105]. This work was the first to provide unambiguous fluorescent imagery demonstrating the utilization of lymphatic vessels by tumor cells for transit from the primary site to LN destination. It also highlighted specific mechanistic steps occurring during this transfer, and demonstrated the role of high interstitial pressure that might enhance the drainage rate and increase intravasation into the peritumoral lymphatics [105].
3.3.2. Evidence for presence and functionality of lymphatic vessels associated with sentinel lymph nodes
It was initially thought that the main factors driving LN metastasis are access of tumor cells to lymphatic vessels and the flow rate of lymph fluid in the draining vessels. Another significant factor is tumor-dependent induction of lymphangiogenesis and increased lymph flow in the sentinel and other draining lymph nodes [106]. This process, termed Lymph Node Lymphangiogenesis (LNL), has been observed in multiple animal models of tumor and inflammation, and is supported by clinical observations.
LNL was detected in the B16-F10 melanoma model, in which tumors implanted into the footpad of C57BL/J6 mice increased lymphangiogenesis in the tumor-draining popliteal lymph node by 9-fold (P<0.0001) compared with the non-tumor-draining lymph node [102]. Significantly, morphologic changes in LN lymphatics occurred prior to detection of metastasis suggesting that such changes might predict a metastatic risk. A nearly 15-fold increase in lymph flow in the tumor-bearing footpads was detected by fluorescent imaging, which recorded the arrival of quantum dots to the tumor-draining node within 2 minutes, as compared with 30 minutes to the contralateral node [102]. Similar findings were obtained in a model of nasopharyngeal carcinoma, which increased volume of the sentinel lymph nodes by 3–4-fold compared with contralateral lymph nodes [107]. Lymph node enlargement was accompanied by robust lymphangiogenesis manifested by both an increase in number of lymphatic sinuses and diameter of their lumens [107]. LNL was also shown in a mouse skin inflammation model that expresses VEGF-A in keratinocytes under the K14-promoter [108]. Compared with normal non-stimulated lymph nodes, the draining nodes from the chronically inflamed skin had increased weight (4.3-fold), cellularity (3.9-fold) and density of LYVE-1 positive vessels [108]. These studies showed that functionality of tumor-draining lymphatic vessels is increased, rather than decreased, as has been previously suggested [109]. The enhanced drainage that results from both hyperplasia and increased lymphatic vessel density may increase transport of metastatic cells from the tumor periphery to sentinel nodes and beyond.
3.4. Can lymphatic metastasis occur in the absence of lymphangiogenesis?
Although several studies suggested that LEC proliferation occurs in intratumoral and more frequently, in peritumoral vessels, the pre-existing lymphatic vessels may equally contribute to metastatic spread. In other words, lymphatic metastasis may not exclusively depend on generation of new vessels, although, undoubtedly, increase in LVD significantly increases a potential for a tumor cell to invade lymphatic vessel surface. This may account for strong statistical associations between LVI encompassing both pre-existing and new vessels and LN metastasis [37;51;110]. It is possible, however, that in some cases breast tumors fail to induce lymphangiogenesis and lymphatic metastasis occurs only through the pre-existing vessels [77]. Collectively, current reports suggest that new lymphatic vessels may provide an additional track for tumor cell transit, thus bolstering the pro-metastatic activity of the pre-existing lymphatic vessels situated at the tumor border.
3.5. Do metastatic cells in LN contribute to distant spread?
Current controversies include the prognostic value of lymphangiogenic markers, the active status of lymphangiogenesis and the role of new lymphatic vessels in transportation of tumor cells to lymph nodes. However, the most crucial issue to resolve is whether metastatic cells stop their travel in lymph nodes or continue to disseminate throughout the body. This is because lymph node metastases by themselves are not life-threatening and could be removed surgically; it is only the distant metastasis to bone and visceral organs that causes death in patients of breast [44] and other solid tumors.
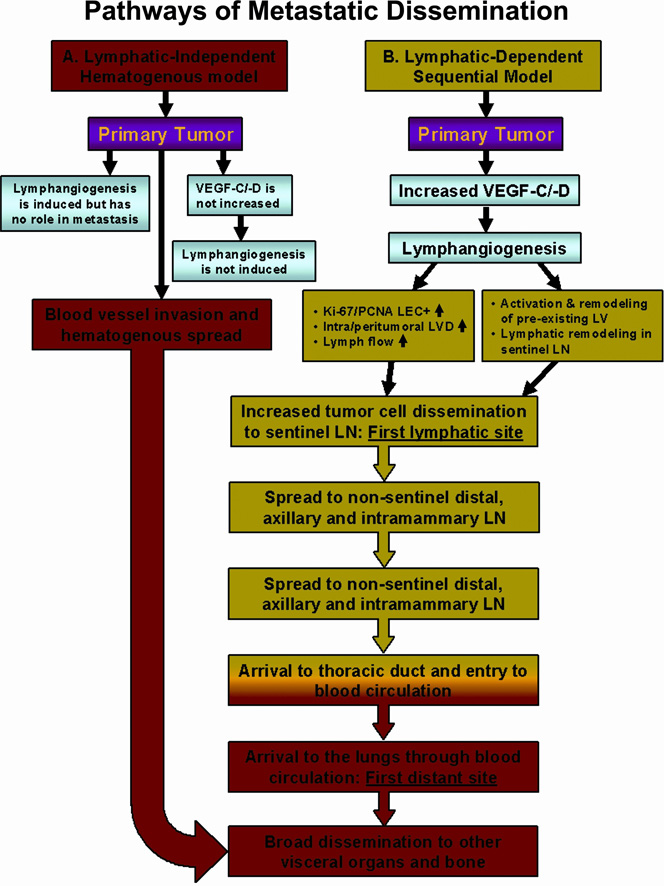
There are two opposing views on this subject. One school of thought is that metastasis to lymph nodes merely reflects tumor aggressiveness, with little to no contribution of nodal metastatic cells to spread to distant organs (Fig. 1, pathway A describing lymphatic-independent hematogenous model of metastasis). This proposal is mainly based on evidence from clinical trials that showed no survival benefits for patients undergoing lymphadenectomy (lymph node removal) [111]. The second argument supporting this notion is based on the concept that tumor dissemination is organ-specific and, therefore, metastatic cells adapted to lymphatic circulation and establishing lesions within the lymphatic system may not be able to develop metastasis elsewhere [112]. Opponents of this view maintain that, although primary tumors may disseminate independently from LN metastases, the latter greatly contribute to the spread by generating metastatic cells that may use either LN blood vessels or efferent lymphatic vessels to colonize distal organs (Fig. 1, pathway B describing lymphatic-dependent sequential model of metastasis and Fig. 2, panels 1–5).
Figure 1. Comparison of lymphatic-independent (hematogenous) and lymphatic-dependent (lymphogenous) pathways of metastatic dissemination.
A. Lymphatic-independent, hematogenous metastatic model. This concept implies that lymphangiogenesis is either not induced or, if induced, new lymphatic vessels do not contribute to tumor dissemination. It is envisioned that the main disseminating pathway is through invasion of intratumoral blood vessels that deliver metastatic cells to distant organs such as lung, liver, bone, and brain. Based on this model, aggressive tumor cells may also invade pre-existing peritumoral lymphatics that transport tumor cells to loco-regional nodes; however, node-derived metastatic cells do not contribute to distant metastasis. B. Lymphatic-dependent, sequential model of dissemination in breast cancer. The second model suggests that breast tumors induce intratumoral or peritumoral lymphangiogenesis as well as remodeling of the lymphatic system in the sentinel and distal lymph nodes. This is manifested by increased number of lymphatic vessels, increased frequency of dividing lymphatic endothelial cells and increased lymph flow between the primary tumor and the sentinel node. These attributes promote tumor cell dissemination through tumor-associated lymphatic vessels to the sLN and spread to intramammary and axillary lymph nodes. Nodal metastatic cells can use either lymphatic or blood vessels for subsequent dissemination. Transport through the lymphatic system ends in the entering the thoracic duct whose contents are subsequently mixed with the venous blood, giving rise to metastatic lesions in the lungs and other visceral organs. Two models are not mutually excluding and in some tumors, hematogenous and lymphogenous metastasis may occur simultaneously.
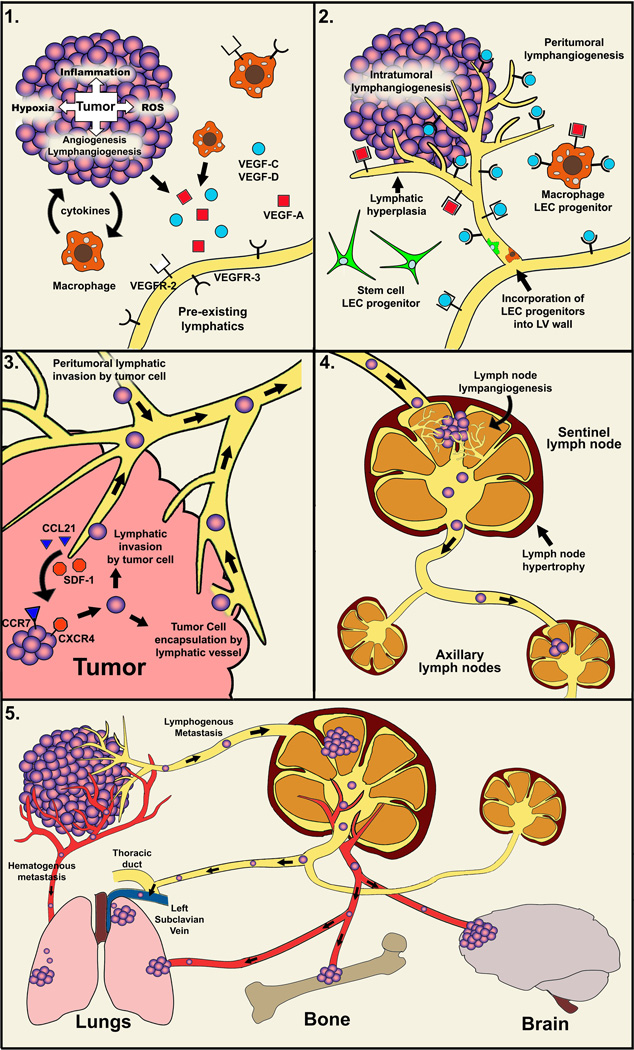
Figure 2. Mechanisms of lymphatic metastasis.
Panel 1: Inflammation, hypoxia and reactive oxygen species (ROS) up-regulate expression of pro-lymphangiogenic factors VEGF-C, VEGF-D and VEGF-A produced by both tumor neoplastic and tumor-associated stromal cells (e.g. macrophages and fibroblasts). Panel 2: New lymphatic vessels are formed mainly in the peritumoral region but in some cases also intratumorally. The formation of new lymphatic vessels involves activation of VEGFR-3 by VEGF-C/-D and VEGFR-2 by VEGF-A as well as mature forms of VEGF-C/-D. Following, LEC proliferate, migrate and assemble into new vessels. The process may include incorporation of tumor-recruited circulating LEC progenitor cells. Panel 3: Tumor cells might actively invade intratumoral or peritumoral lymphatic vessels, or might be passively encapsulated by lymphatic vessels. Both types of interactions promote LVI leading to lymphatic metastasis to the sentinel lymph node. The active metastasis includes chemotactic recruitment of tumor cells expressing CCR7 or CXCR4 receptors by corresponding chemokines (CCL21 and SDF-1) expressed by the local lymphatic system. Panel 4: Unknown tumor-secreted factors that may include, but not limited to VEGF-A and VEGF-C, elicit profound morphological changes in the sentinel LN culminating in lymph node lymphangiogenesis (LNL) and lymphatic hypertrophy. Dramatically increased lymph flow in and out of the sLN promotes both lymphatic metastasis and further dissemination to distant organs. Panel 5: The pathway to distant organs may occur through both blood and lymphatic vasculature. Following dissemination through lymphatic vessels to the sentinel LN, tumor cells may enter the blood circulation through nodal blood vessels, or proceed with the lymphatic flow and enter the venous circulation via the thoracic duct, both pathways leading to distant organ metastasis.
The issue of survival benefits from lymphadenectomy remains controversial with several caveats to be considered. First, the lack of therapeutic value of lymphadenectomy is mainly based on trials conducted in the years 1980–2000 [111], when breast cancer detection was delayed and patients were presented with more advanced disease. Second, node positivity in earlier studies was primarily determined histologically, a method that misses 20–50% of micrometastases and isolated tumor cells compared with cytokeratin immunostaining [113]. Indeed, re-examination of node specimens by anti-cytokeratin staining detected micrometastases in 20% more cases than previously determined by H&E and demonstrated differences in disease-free and overall survival in some of the groups with or without node dissection [60]. Third, recent clinical trials showed significant benefit for patients with small melanoma tumors who underwent lymphadenectomy immediately, thus disputing the previous conclusion [114]. Fourth, experimental assessment of this question in a melanoma model also showed significant survival benefit of sentinel lymphadenectomy for mice bearing small tumors [115]. The same study also found that cells from primary tumors and sLN have equal metastatic potential to colonize distant organs [115], thus arguing against the hypothesis that nodal metastatic cells are biologically restricted to their first destination site. Lastly, much of the recent information on tumor lymphatic biology directly contradicts the opinion that metastatic cells in the lymph nodes “venture no farther” [116]. Several recent lines of evidence support the active role of the lymphatic system in the distant spread of metastatic breast carcinoma cells. Table 3 presents a selective list of large clinical studies that demonstrated highly significant associations between LN metastasis and distant metastasis, and survival. Table 1, Table 2, Table 4 and Table 6 show correlation of poor outcome with additional lymphangiogenic parameters including VEGF-C expression [71], LVD [70;88], LVI [18;36;50] and the consequences of partial [117] or omitted [118] axillary dissection in sLN-positive patients.
Because clinical studies are correlative by nature, one can argue that all of the above indicates tumor aggressiveness rather than promotion of metastasis by the lymphatic system, although a perennial finding of pLVI as an independent prognostic factor for survival hardly fits this explanation. However, it is even more difficult to explain mounting data from experimental studies implicating lymphatic metastases as essential precursors for systemic dissemination. Ectopic expression of a single protein, VEGF-C [12] or VEGF-D [119], factors, which primarily affect lymphatic endothelial cells and not tumor cells [120], invariably increases not only lymph node metastasis but also lung metastasis [7;119]. Moreover, distant metastasis does not develop in mice lacking LN lesions [121], suggesting that nodal metastatic cells are pre-requisite for the formation of lung lesions. Defined molecular agents neutralizing VEGF-C/-D suppress both LN and lung metastasis [122–124], which is unexplainable if lung metastasis occurs independently of the lymphatic dissemination. Additional argument in the favor of a sequential manner of dissemination (Fig. 1, pathway B) is the observation that in many experimental systems LN metastasis precedes metastasis to visceral organs [25;98;120]. The role of the lymphatic system in distant metastasis is also supported by findings that tumor-induced LNL (section 3.2.2) increases lymphatic surface and lymph flow in both afferent and efferent vessels of the sLN [102], suggesting that tumor cell transit is accelerated not only toward the sLN but also toward distal nodes located along the lymphatic trunk. Arrival of metastatic cells to the first node as schematically illustrated in panel 4 of Fig. 2 further promotes remodeling and enlargement of the lymphatic system [121]. The new concept of LNL strongly suggests that both lymphatic and distant metastases are the combined result of tumor-inherent behavior and reactivity of the host immune system, of which the sLN is the first organ, to tumor-derived stimuli. Remodeling and expansion of the tumor associated lymphatic system has been documented not only before metastasis occurred [102;107;121], but even before the primary malignant tumors were fully formed in transgenic mice [98]. This recent information compels the conclusion that lymphangiogenesis plays an active role in distant dissemination, which is critically relevant to development of effective anti-metastatic strategies.
4. Experimental evidence supporting causality between lymphangiogenesis and LN metastasis
4.1 Induction of lymphangiogenesis and LN metastasis by forced VEGF-C/VEGF-D expression
The causality between tumor-induced lymphangiogenesis and lymph node metastasis has been demonstrated in several experimental models targeting lymphangiogenic factors and receptors through forced expression, RNA interference, antagonistic antibodies, soluble receptor traps, and multi-kinase inhibitors. One of the first reports in an orthotopic green fluorescent protein (GFP)-tagged MDA-MB-435 breast cancer model showed that overexpression of VEGF-C increased intratumoral lymphangiogenesis by 4.6-fold, which coincided with a 60% increase in incidence of LN metastasis [7]. Pulmonary metastatic lesions were up to 60% larger in mice with VEGF-C overexpressing tumors compared with control mice [7], suggesting that LN metastasis contributes to dissemination to visceral organs.
Similar findings were demonstrated in studies using the MCF-7 breast carcinoma line. The parental low-expressing VEGF-C line neither induced lymphangiogenesis nor generated spontaneous metastasis upon implantation in the mammary fat pad of immunodeficient mice [14]. In contrast, MCF-7 cells with ectopic VEGF-C expression developed tumors with extensive intra- and peritumoral hyperplastic lymphatic vessels [14]. Significantly, LN metastasis was detected in 70% of mice with VEGF-C overexpressing MCF-7 tumors as compared with 0% in mice implanted with parental MCF-7 line [12;14]. A VEGF-C-dependent increase in lymphangiogenesis and LN metastasis has also been demonstrated in other models of solid tumors including lung [27], prostate [125], melanoma [120;126], gastric carcinoma [127], fibrosarcoma [120] and colorectal cancer [128]. Most of these studies reported a correlation between LN and distant metastasis [27;120;125], favoring the hypothesis that lymph node metastasis is an intermediate step toward systemic dissemination.
Whereas experimentally-induced VEGF-C invariably increased lymphangiogenesis and metastasis, tumor lines with an endogenously high level of VEGF-C have been shown to preferentially undergo spontaneous lymphogenous metastasis as shown in models of breast [25;123;129], prostate [130] and gastric carcinomas [131]. Depletion of VEGF-C by stable shRNA in mouse breast carcinoma C166 and BJMC3879 models drastically reduced intratumoral lymphangiogenesis and inhibited LN and lung metastasis [123;124]. In contrast, suppression of VEGF-C in a PC3 prostate cancer model reduced intratumoral LVD by 3-fold but had no effect on peritumoral lymphatics or the incidence of LN metastasis [130], suggesting that tumor cells can also disseminate through pre-existing vessels whose maintenance does not depend on VEGF-C.
A role for VEGF-D in lymphatic metastasis has been shown in experimental models of lines overexpressing this factor including HEK293 [15], hepatocellular [132] and pancreatic [133] cancer cells. VEGF-D overexpression induced intratumoral or peritumoral lymphangiogenesis and lymphatic hyperplasia, which coincided with a significant increase in LN metastasis. A transgenic RipTag model that forms spontaneous VEGF-D overexpressing pancreatic β-cell tumors showed a significantly increased intratumoral and peritumoral LVD accompanied by a 60–80% increase in LN and lung, but not liver, metastases compared with β-cell tumors that did not express VEGF-D [119]. The high incidence of pulmonary and lack of hepatic metastasis suggested that tumors metastasized first to the lymph nodes and subsequently colonized the lungs but did not spread hematogenously to colonize the liver.
4.2 Role of VEGF-A in promotion of breast tumor-induced lymphangiogenesis
In addition to being a potent angiogenic factor [134;135], VEGF-A has recently emerged as a strong promoter of inflammatory [108;136;137] and tumor lymphangiogenesis [138–140]. This was first demonstrated by induction of new lymphatic vessels in the ear and peritoneal lining of nude mice infected with VEGF-A over-expressing adenovirus [136]. These neo-lymphatics resembled hyperplasic vessels found in lymphatic malformations, suggesting that elevated VEGF-A at malignant and chronically inflamed sites might contribute to pathological lymphangiogenesis. This hypothesis was subsequently supported by studies demonstrating induction of a strong lymphangiogenic response in the rat [141] and mouse [142] models of corneal injury, chronic inflammation [137] and VEGF-A-induced skin tumorigenesis [138]. Additionally, VEGF-A mediated lymphangiogenesis has been shown in T241 fibrosarcoma [140] and MDA-MB-435 breast carcinoma cells [26] that have been engineered to overexpress VEGF-A. In all tumor experimental models studied to date, induction of VEGF-A-dependent intratumoral [12] or peritumoral [140] lymphatic vessels correlated with lymphatic invasion [138], and lymph node and distant metastasis [26;138;140]. We recently demonstrated, using an orthotopic luciferase-tagged MDA-MB-231 tumor model, that neutralizing VEGF-A reduces the density of intratumoral lymphatic vessels by 80% [25] and inhibits LN and lung metastasis by 3.2-fold and 4.5-fold, respectively [25]. Inhibition of VEGF-A signaling by anti-VEGFR-2 antibody also suppressed new lymphatic vessels and tumor spread, although less efficiently than anti-VEGFR-3 antibody treatment [26]. These studies support the causality between tumor induction of lymphangiogenesis and both lymphatic and distant metastasis, thus suggesting that targeting VEGF-A may suppress both hematogenous and lymphatic metastasis.
4.3 Potential role of other factors mediating lymphangiogenesis
Additional factors implicated in lymphangiogenesis include angiopoietins (Ang-1, Ang-2, Ang-3), fibroblast growth factor (FGF)-2, platelet-derived growth factor (PDGF), insulin growth factor-1 and -2 (IGF-1,-2), hepatocyte growth factor (HGF) and growth hormone (GH). Ang-1 and Ang-2, ligands for the tyrosine kinase receptor Tie-2 expressed by endothelial and stromal cells regulate both angiogenesis and lymphangiogenesis. In contrast to antagonistic effects of Ang-1 and Ang-2 on blood vessels, both factors are agonists for Tie-2 activation in cultured LEC [143] and lymphatic endothelium in vivo, as illustrated by Ang-1-dependent rescue of lymphatic defects in Ang-2 knockout mice [144]. Ang-1 and Ang-2 are elevated in plasma of breast cancer patients [145;146] and are indicators of lymphatic metastasis [147–149]. Lymphangiogenic effects of FGF-2 [150], PDGF, IGF-1 and -2 [151], as well as HGF [152], have been shown in a mouse corneal assay whereas the effect of GH was shown in a wound healing model [153]. FGF-2 and HGF-induced lymphangiogenesis correlated with increased VEGF-C expression and was inhibited by anti-VEGFR-3 blocking antibody [150;152]. In contrast, the effects of PDGF isoforms [154] and IGF-1 and IGF-2 factors [151] were not inhibited by soluble VEGFR-3, suggesting an independent mechanism. Although extensive circumstantial data link these factors to both vessel formation and metastasis [155–157], none was examined systematically with regard to breast cancer-induced lymphangiogenesis, lymphatic invasion and lymphatic metastasis. Currently, their roles in these processes are not fully established.
4.4 Role of circulating LEC progenitors in tumor-induced lymphangiogenesis
Circulating stem progenitor endothelial cells have been shown to contribute to growth of blood vasculature during embryonic development, inflammation, tissue remodeling and malignancy [158–161]. In comparison, few studies have focused on LEC progenitors, particularly with regard to their contribution in tumor lymphangiogenesis. The cell types that potentially comprise adult LEC progenitors include CD34-positive hematopoietic stem cells (HSC) [9;162], CD14+ monocytes [163] and CD11b+ macrophages [82]. Contribution of HSC to lymphangiogenesis has been shown by bone marrow (BM) transplantation of GFP-labeled cells co-expressing VEGFR-3 and LYVE-1 that incorporated into 1–3% of lymphatic vessels in the liver, intestine, gastric tract, and the kidney [164]. GFP+ labeled HSC injected into APCMin/+ mice with spontaneous intestinal adenomas have been shown to incorporate into tumor lymphatic vessels [164]. Lymphatic vessel integration of LYVE-1-positive, GFP+/CD34+ cells has also been shown in a tumor T241 fibrosarcoma model and a model of inflammatory lymphangiogenesis induced by FGF-2 in the mouse cornea [162]. A corneal inflammation assay also showed incorporation of CD11b+ macrophages to new lymphatic vessels [82]. A notably elegant study examining lymphangiogenesis in gender-mismatched renal transplant patients undergoing rejection identified donor-derived Prox1+ LEC progenitors incorporated into the lymphatic vessels [163]. This strongly supports an active pro-lymphangiogenic role of circulating progenitors in inflammatory lymphangiogenesis in humans.
In contrast to the aforementioned studies, transplantation of unpurified GFP+ BM cells into mice bearing Lewis lung carcinoma showed extensive BM cell recruitment to the vicinity of tumor lymphatic vessels but did not show incorporation of progenitors into the vessels [165]. The discrepancy with other studies [162;164] could relate to the fact that total BM cell mixture, not purified CD34+ hematopoietic cells, was used for transplantation, which diluted the number of transplanted LEC progenitors of CD34 lineage that comprise a relatively low (1–3%) fraction [164]. This topic is currently a subject of debate, and the extent and specific functions of LEC progenitors in breast tumor lymphangiogenesis are yet to be established.
5. Cellular and molecular mechanisms of lymphangiogenesis
Although tumor lymphangiogenesis might be regulated in a distinct manner from physiologically driven lymphangiogenesis occurring during embryogenesis, or, in adults during wound healing, such differences are not readily apparent. The common pathway involved in embryonic, adult, physiological and pathological lymphangiogenesis is VEGFR-3. This conclusion is reached because antibodies blocking this pathway, through either receptor or ligand binding impairment, suppress the formation of new lymphatic vessels in all examined settings, regardless of the developmental stage or health status. We, therefore, will focus on regulation of the VEGFR-3 pathway as it is the main established contributor to development of new lymphatic vessels, including those generated in or around breast tumors.
5.1 Regulation of expression of VEGF-3 ligands, VEGF-C and VEGF-D
VEGF-C and VEGF-D are secreted glycoproteins that induce proliferation, migration, and survival of LEC through activation of VEGFR-3 [10;166]. VEGF-C/-D undergo proteolytic processing to form 31/29kD immature isoforms that exclusively activate VEGFR-3 and 21kD mature isoforms that have increased affinity to VEGFR-3 and additional capacity to bind VEGFR-2 [167;168]. Both VEGF-C and VEGF-D are processed by the furin convertases, PC5 and PC7 [169;170], and the serine protease, plasmin [171]. VEGF-C [172] but not VEGF-D [173] is required for embryonic development of the lymphatic system [172]. VEGF-C induced lymphatic vessel hyperplasia and lymphangiogenesis in embryonic and postnatal mice, whereas VEGF-D induced these effects only in postnatal mice [174]. Comparative frequency of VEGF-C and VEGF-D expression in normal and malignant tissues suggest that VEGF-C plays a more important role in tumor lymphangiogenesis. Taken together, these studies suggest that although VEGF-C and VEGF-D bind the same receptor, these two ligands might play distinct functions in maintenance of normal lymphatic vessels and inducing new ones.
5.2. Role of VEGFR-3 receptor pathway in LEC activation and induction of lymphangiogenesis
VEGFR-3 is a member of the VEGF-receptor family, a group of structurally related receptor tyrosine kinases comprised of seven immunoglobulin-like domains, a single transmembrane domain, and an interrupted kinase domain [175]. VEGFR-3 exists in two alternatively spliced isoforms, long (VEGFR-3L) and short (VEGFR-3S), differing by 65 amino acids in the C-terminus [176]. VEGFR-3L contains 16 tyrosine residues in the intracellular domain, six of which are located in the cytoplasmic tail [177]. Three of the six most distal phosphorylated tyrosine residues are absent in VEGFR-3S, suggesting the two isoforms have different signaling capabilities [178]. RT-PCR analysis of breast and prostate carcinomas showed that VEGFR-3S isoform is expressed at a higher level than VEGFR-3L and correlates with lymphatic metastasis [179;180]. This suggests that VEGFR-3S is the predominant splice variant in malignant cells; however, it remains unclear whether mRNA for the two isoforms correlates with protein expression.
The VEGFR-3 pathway follows canonical activation of tyrosine kinase receptors, including binding of VEGF-C/-D homodimers followed by receptor dimerization and phosphorylation of tyrosine residues in the kinase domains. The VEGFR-3 pathway has been partially elucidated using tryptic mapping and deletion mutants. Phosphorylation of VEGFR-3 activates Akt, JNK and Erk signaling pathways through interaction with adaptor proteins Shc and Grb2 [166;181]. VEGFR-3 signaling in LEC is also mediated through PKC-dependent activation of the p42/44 MAPK pathway [166]. Deletion of tyrosines Y1230/1231 and Y1337 decreased endothelial cell proliferation, migration and survival in vitro [178] while mutation of Y1337 drastically reduced phosphorylation of Shc protein [178]. Another means to regulate VEGFR-3 is through the formation of heterodimers with VEGFR-2 receptor [182]. This interaction may block phosphorylation of the two most distal tyrosines, thus inhibiting downstream VEGFR-3 signaling [183].
VEGFR-3 can also be regulated through its interactions with integrins that control endothelial cell adhesion to the extracellular matrix (ECM) necessary for cell survival [184]. Both fibronectin, the major ligand for α5β1 integrin, and VEGF-C156S, a specific activator of VEGFR-3, promote VEGFR-3/α5β1 interactions leading to phosphorylation of PI3K and enhanced endothelial cell survival [185]. Likewise, ECM proteins enhance VEGF-D-induced migration of LEC [186]. In a corneal inflammation assay, inhibition of α5 integrin signaling by the inhibitor JSM6424 reduced lymphovascular area by 50% compared with control animals [187]. These studies suggest that α5β1 integrin contributes to VEGFR-3 signaling in inflammatory lymphangiogenesis, although the extent of integrins to tumor-induced lymphangiogenesis remains to be determined.
5.3. Promotion of lymphangiogenesis and LN metastasis by tumor-associated inflammation
Chronic inflammation is a hallmark of breast cancer [103;188] and has been repeatedly linked to increased tumorigenesis [189;190], angiogenesis [191;192], lymphangiogenesis [20;193] and metastatic progression [194–196]. Lymphangiogenesis, driven by chronically inflamed tumor environment, substantially increases the area of lymphatic-tumor interface, thus increasing intravasation of tumor cells and their potential to reach regional lymph nodes. The tumor inflammatory environment is created mainly by infiltrates of immune cells [197;198] that may contain macrophages [18;19;141], dendritic cells [199], neutrophils [193] and mast cells [200]. The presence of infiltrates that secrete an array of pro-angiogenic factors[18;201] is associated with poor prognosis [202;203]. Additional sources of inflammatory mediators are tumor fibroblasts[193] and endothelial cells [204;205], as well as cytokine-overexpressing neoplastic cells. The inflammatory stimuli derived from these cells mediate their effects mainly through activation of the NF-κB pathway [206]. This key intracellular mediator of inflammation plays a paramount role in induction of lymphangiogenesis through several complementary mechanisms. First, NF-κB induces expression of lymphangiogenic factors VEGF-C [207] and VEGF-A [208]. Second, many genes transcribed by NF-κB (e.g., TNF-α, IL-1β, IL-6, IL-8, IL-7 and COX-2) increase lymphangiogenesis indirectly, by up regulating expression of VEGF-C or VEGF-D [21;209;210]. Third, NF-κB regulated enzymes, metalloproteinases, and uPA degrade extracellular matrix and proteolytically cleave VEGF-C and VEGF-D to create mature forms with higher affinity to VEGFR-3 and the new ability to activate VEGFR-2 receptor [171]. Lastly, NF-κB activates transcription of the VEGFR-2 receptor that binds VEGF-A as well as mature versions of VEGF-C/-D factors [196;211]. Our laboratory recently established that NF-κB also directly regulates VEGFR-3 expression, thus enhancing LEC responsiveness to VEGF-C in vivo [212]. Collectively, this evidence suggests that inflammation-induced activation of NF-κB facilitates metastasis not only by enhancing proliferation [213;214], migration [215;216], survival [217;218] and invasion [195;216]of the tumor cells but also through transcription of critical lymphangiogenic genes. The strategies to counteract the traits endowed by tumor-associated inflammation are likely to reduce node metastasis and improve patient survival.
6. Therapeutic opportunities for inhibiting lymphatic metastasis in breast cancer
Given strong associations among tumor-induced lymphangiogenesis, LVI, regional lymphatic spread, distant metastasis and survival (Table 1–Table 6), inhibition of tumor lymphatic vessels appears to be an attractive target. Based on the current understanding of mechanisms governing malignant lymphangiogenesis (Fig. 2, panels 1 and 2), the most logical targets for pharmacological inhibition are the main lymphangiogenic factors and receptors (VEGF-C, VEGF-D, VEGF-A, VEGFR-3 and VEGFR-2), lymphatic invasion ligand•receptor promoting pairs (e.g., SDF-1•CXCR4, CCL21•CCR7) and a variety of pro-lymphangiogenic ancillary proteins (e.g., Ang-2, neuropilin-2, inflammatory mediators). Inhibition of lymphangiogenic factors or receptors has been assessed in several experimental models that demonstrated a significant anti-metastatic efficacy when administrated in animals with recently implanted or small-sized tumors [26;120;219]. However, monotherapies with either anti-VEGFR-3 and anti-VEGFR-2 antibodies were significantly less efficacious in suppressing metastasis in mice with well-established tumors [26], which is an anticipated situation in clinics. This is probably because, at the time of diagnosis, lymphangiogenesis is already induced at both the primary site and the sentinel lymph node, pre-existing lymphatic vessels are activated, tumor lymph drainage is increased and micrometastases might be present at least in the sentinel node. It is, therefore, unlikely that depletion of lymphangiogenic mediators, even at earlier stages of the disease, would significantly affect the rate of lymphatic metastasis, although it is likely to reduce the incidence of subsequent distant metastasis. Likewise, bevacizumab monotherapy that targets the most potent tumor angiogenic factor, VEGF-A, is minimally effective in clinics [220] despite strong performance in experimental models [221;222]. In contrast, combination therapies using bevacizumab and various chemotherapeutic drugs [223] are highly successful in both tumor models [129;224] and clinical studies [220;225;226]. This is mainly because VEGF-A [227;228], like VEGF-C [166;229] and VEGF-D [166;229], is a potent survival factor for endothelial cells that cannot recover from damage inflicted by simultaneously administered cytotoxic therapies. Analogously, a combination of chemo- and anti-lymphangiogenic therapies might be highly effective in eliminating metastatic cells while preventing the re-building of the damaged lymphatic vasculature. Agents that may block lymphangiogenesis include antibodies, fusion proteins, or shRNA, as well as orally-administered inhibitors that target tyrosine kinase receptors required for angiogenesis and lymphangiogenesis (Sunitinib, E7080, cediranib, Vadnetanib, PTK/ZK, and MAZ51) [229]. This approach is particularly appealing given the new concept of LEC division induced at both the tumor vicinity [23] and the draining nodes [85;86], which suggests susceptibility of tumor-associated lymphatic endothelium to commonly used anti-proliferative anti-cancer drugs.
Acknowledgements
The authors acknowledge support of NIH (#1R15CA125682-01) and Illinois William E. McElroy Foundation.
Abbreviations
- ALNM
axillary lymph node metastasis
- BM
bone marrow
- BVI
blood vascular invasion
- DFS
disease-free survival
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- LEC
lymphatic endothelial cells
- LN
lymph node(s)
- LNL
lymph node lymphangiogenesis
- LVD
lymphatic vessel density
- LVI
lymphatic vessel invasion
- MFP
mammary fat pad
- OS
overall survival
- sLN
sentinel lymph node
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- pLVI
peritumoral lymphatic vascular invasion
- VEGF-A-C,-D
vascular endothelial growth factor-A, -C, -D
- VEGFR-2, -3
vascular endothelial growth factor receptor-2, -3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schoppmann SF, Horvat R, Birner P. Lymphatic vessels and lymphangiogenesis in female cancer: mechanisms, clinical impact and possible implications for anti-lymphangiogenic therapies (Review) Oncol.Rep. 2002;9:455–460. [PubMed] [Google Scholar]
- 2.Lauria R, Perrone F, Carlomagno C, De Laurentiis M, Morabito A, Gallo C, Varriale E, Pettinato G, Panico L, Petrella G. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer. 1995;76:1772–1778. doi: 10.1002/1097-0142(19951115)76:10<1772::aid-cncr2820761014>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Kyzas PA, Geleff S, Batistatou A, Agnantis NJ, Stefanou D. Evidence for lymphangiogenesis and its prognostic implications in head and neck squamous cell carcinoma. J.Pathol. 2005;206:170–177. doi: 10.1002/path.1776. [DOI] [PubMed] [Google Scholar]
- 4.Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–1757. doi: 10.1016/S0140-6736(07)60781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chua B, Ung O, Taylor R, Boyages J. Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ.J.Surg. 2001;71:723–728. doi: 10.1046/j.1445-1433.2001.02266.x. [DOI] [PubMed] [Google Scholar]
- 6.Cunnick GH, Jiang WG, Gomez KF, Mansel RE. Lymphangiogenesis and breast cancer metastasis. Histol.Histopathol. 2002;17:863–870. doi: 10.14670/HH-17.863. [DOI] [PubMed] [Google Scholar]
- 7.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat.Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Karpanen T, Alitalo K. Role of lymphangiogenic factors in tumor metastasis. Biochim.Biophys.Acta. 2004;1654:3–12. doi: 10.1016/j.bbcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 10.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc.Natl.Acad.Sci.U.S.A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Harkonen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int.J.Cancer. 2002;98:946–951. doi: 10.1002/ijc.10283. [DOI] [PubMed] [Google Scholar]
- 13.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 14.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 15.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat.Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 16.Tsai PW, Shiah SG, Lin MT, Wu CW, Kuo ML. Up-regulation of vascular endothelial growth factor C in breast cancer cells by heregulin-beta 1. A critical role of p38/nuclear factor-kappa B signaling pathway. J.Biol.Chem. 2003;278:5750–5759. doi: 10.1074/jbc.M204863200. [DOI] [PubMed] [Google Scholar]
- 17.Wulfing P, Kersting C, Buerger H, Mattsson B, Mesters R, Gustmann C, Hinrichs B, Tio J, Bocker W, Kiesel L. Expression patterns of angiogenic and lymphangiogenic factors in ductal breast carcinoma in situ. Br.J.Cancer. 2005;92:1720–1728. doi: 10.1038/sj.bjc.6602567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139:839–846. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am.J.Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouta C, Heroult M. Inflammatory triggers of lymphangiogenesis. Lymphat.Res.Biol. 2003;1:201–218. doi: 10.1089/153968503768330247. [DOI] [PubMed] [Google Scholar]
- 21.Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J.Biol.Chem. 1998;273:8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Flt-4-positive vessel density correlates with vascular endothelial growth factor-d expression, nodal status, and prognosis in breast cancer. Clin.Cancer Res. 2003;9:5313–5317. [PubMed] [Google Scholar]
- 23.Mohammed RA, Ellis IO, Elsheikh S, Paish EC, Martin SG. Lymphatic and angiogenic characteristics in breast cancer: morphometric analysis and prognostic implications. Breast Cancer Res.Treat. 2008:261–273. doi: 10.1007/s10549-008-9936-1. [DOI] [PubMed] [Google Scholar]
- 24.van der Auwera I, Van den Eynden GG, Colpaert CG, Van Laere SJ, van Dam P, Van Marck EA, Dirix LY, Vermeulen PB. Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clin.Cancer Res. 2005;11:7637–7642. doi: 10.1158/1078-0432.CCR-05-1142. [DOI] [PubMed] [Google Scholar]
- 25.Whitehurst B, Flister MJ, Bagaitkar J, Volk L, Bivens CM, Pickett B, Castro-Rivera E, Brekken RA, Gerard RD, Ran S. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int.J.Cancer. 2007;121:2181–2191. doi: 10.1002/ijc.22937. [DOI] [PubMed] [Google Scholar]
- 26.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 27.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J.Natl.Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, Alitalo K, Jooss K. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 29.Bando H, Weich HA, Horiguchi S, Funata N, Ogawa T, Toi M. The association between vascular endothelial growth factor-C, its corresponding receptor, VEGFR-3, and prognosis in primary breast cancer: a study with 193 cases. Oncol.Rep. 2006;15:653–659. [PubMed] [Google Scholar]
- 30.Schoppmann SF. Lymphangiogenesis, inflammation and metastasis. Anticancer Res. 2005;25:4503–4511. [PubMed] [Google Scholar]
- 31.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 32.Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod.Pathol. 2005;18:1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 33.Jennbacken K, Vallbo C, Wang W, Damber JE. Expression of vascular endothelial growth factor C (VEGF-C) and VEGF receptor-3 in human prostate cancer is associated with regional lymph node metastasis. Prostate. 2005:110–116. doi: 10.1002/pros.20276. [DOI] [PubMed] [Google Scholar]
- 34.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. [PubMed] [Google Scholar]
- 35.Dua RS, Gui GP, Isacke CM. Endothelial adhesion molecules in breast cancer invasion into the vascular and lymphatic systems. Eur.J.Surg.Oncol. 2005;31:824–832. doi: 10.1016/j.ejso.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am.J.Surg.Pathol. 2007;31:1825–1833. doi: 10.1097/PAS.0b013e31806841f6. [DOI] [PubMed] [Google Scholar]
- 37.Viale G, Zurrida S, Maiorano E, Mazzarol G, Pruneri G, Paganelli G, Maisonneuve P, Veronesi U. Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer. 2005;103:492–500. doi: 10.1002/cncr.20809. [DOI] [PubMed] [Google Scholar]
- 38.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J.Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilting J, Papoutsi M, Christ B, Nicolaides KH, von Kaisenberg CS, Borges J, Stark GB, Alitalo K, Tomarev SI, Niemeyer C, Rossler J. The transcription factor Prox1 is a marker for lymphatic endothelial cells in normal and diseased human tissues. FASEB J. 2002;16:1271–1273. doi: 10.1096/fj.01-1010fje. [DOI] [PubMed] [Google Scholar]
- 40.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun M, Flucke U, Debald M, Walgenbach-Bruenagel G, Walgenbach KJ, Holler T, Polcher M, Wolfgarten M, Sauerwald A, Keyver-Paik M, Kuhr M, Buttner R, Kuhn W. Detection of lymphovascular invasion in early breast cancer by D2-40 (podoplanin): a clinically useful predictor for axillary lymph node metastases. Breast Cancer Res.Treat. 2008;112:503–511. doi: 10.1007/s10549-007-9875-2. [DOI] [PubMed] [Google Scholar]
- 42.Marinho VF, Metze K, Sanches FS, Rocha GF, Gobbi H. Lymph vascular invasion in invasive mammary carcinomas identified by the endothelial lymphatic marker D2-40 is associated with other indicators of poor prognosis. BMC.Cancer. 2008;8:64. doi: 10.1186/1471-2407-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammed RA, Ellis IO, Lee AH, Martin SG. Vascular invasion in breast cancer; an overview of recent prognostic developments and molecular pathophysiological mechanisms. Histopathology. 2008:1–9. doi: 10.1111/j.1365-2559.2008.03169.x. [DOI] [PubMed] [Google Scholar]
- 44.Van den Eynden GG, van der Auwera I, Van Laere SJ, Colpaert CG, van DP, Dirix LY, Vermeulen PB, Van Marck EA. Distinguishing blood and lymph vessel invasion in breast cancer: a prospective immunohistochemical study. Br.J.Cancer. 2006;94:1643–1649. doi: 10.1038/sj.bjc.6603152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Travagli JP, Atallah D, Mathieu MC, Rochard F, Camatte S, Lumbroso J, Garbay JR, Rouzier R. Sentinel lymphadenectomy without systematic axillary dissection in breast cancer patients: predictors of non-sentinel lymph node metastasis. Eur.J.Surg.Oncol. 2003;29:403–406. doi: 10.1053/ejso.2002.1427. [DOI] [PubMed] [Google Scholar]
- 46.Ozmen V, Karanlik H, Cabioglu N, Igci A, Kecer M, Asoglu O, Tuzlali S, Mudun A. Factors predicting the sentinel and non-sentinel lymph node metastases in breast cancer. Breast Cancer Res.Treat. 2006;95:1–6. doi: 10.1007/s10549-005-9007-9. [DOI] [PubMed] [Google Scholar]
- 47.Beriwal S, Soran A, Kocer B, Wilson JW, Ahrendt GM, Johnson R. Factors that predict the burden of axillary disease in breast cancer patients with a positive sentinel node. Am.J.Clin.Oncol. 2008;31:34–38. doi: 10.1097/COC.0b013e318068419b. [DOI] [PubMed] [Google Scholar]
- 48.Bader AA, Tio J, Petru E, Buhner M, Pfahlberg A, Volkholz H, Tulusan AH. T1 breast cancer: identification of patients at low risk of axillary lymph node metastases. Breast Cancer Res.Treat. 2002;76:11–17. doi: 10.1023/a:1020231300974. [DOI] [PubMed] [Google Scholar]
- 49.Woo CS, Silberman H, Nakamura SK, Ye W, Sposto R, Colburn W, Waisman JR, Silverstein MJ. Lymph node status combined with lymphovascular invasion creates a more powerful tool for predicting outcome in patients with invasive breast cancer. Am.J.Surg. 2002;184:337–340. doi: 10.1016/s0002-9610(02)00950-9. [DOI] [PubMed] [Google Scholar]
- 50.Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, Jakesz R, Horvat R. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann.Surg. 2004;240:306–312. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gajdos C, Tartter PI, Bleiweiss IJ. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Ann.Surg. 1999;230:692–696. doi: 10.1097/00000658-199911000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod.Pathol. 2007;20:183–191. doi: 10.1038/modpathol.3800728. [DOI] [PubMed] [Google Scholar]
- 53.Grabau D, Jensen MB, Rank F, Blichert-Toft M. Axillary lymph node micrometastases in invasive breast cancer: national figures on incidence and overall survival. APMIS. 2007;115:828–837. doi: 10.1111/j.1600-0463.2007.apm_442.x. [DOI] [PubMed] [Google Scholar]
- 54.Sivridis E, Giatromanolaki A, Galazios G, Koukourakis MI. Node-related factors and survival in node-positive breast carcinomas. Breast. 2006;15:382–389. doi: 10.1016/j.breast.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Behm EC, Buckingham JM. Sentinel node biopsy in larger or multifocal breast cancers: to do or not to do. ANZ.J.Surg. 2008;78:151–157. doi: 10.1111/j.1445-2197.2007.04392.x. [DOI] [PubMed] [Google Scholar]
- 56.Nagashima T, Sakakibara M, Nakano S, Tanabe N, Nakamura R, Nakatani Y, Nagai Y, Koda K, Miyazaki M. Sentinel node micrometastasis and distant failure in breast cancer patients. Breast Cancer. 2006;13:186–191. doi: 10.2325/jbcs.13.186. [DOI] [PubMed] [Google Scholar]
- 57.Chagpar AB, Scoggins CR, Martin RC, Sahoo S, Carlson DJ, Laidley AL, El-Eid SE, McGlothin TQ, McMasters KM. Factors determining adequacy of axillary node dissection in breast cancer patients. Breast J. 2007;13:233–237. doi: 10.1111/j.1524-4741.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 58.Cox C, DuPont EL, Furman B, Stowell N, Clark J, Ebert M, Diaz NM, Cantor A. The clinical relevance of positive sentinel nodes only versus positive nonsentinel lymph nodes in breast cancer patients. Am.J.Surg. 2003;186:333–336. doi: 10.1016/s0002-9610(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 59.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De CC, De LF, Gennari R. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N.Engl.J.Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 60.Cote RJ, Peterson HF, Chaiwun B, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, Gusterson B, Neville AM. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet. 1999;354:896–900. doi: 10.1016/s0140-6736(98)11104-2. [DOI] [PubMed] [Google Scholar]
- 61.van Iterson V, Leidenius M, Krogerus L, von Smitten K. Predictive factors for the status of non-sentinel nodes in breast cancer patients with tumor positive sentinel nodes. Breast Cancer Res.Treat. 2003;82:39–45. doi: 10.1023/B:BREA.0000003918.59396.e4. [DOI] [PubMed] [Google Scholar]
- 62.Zavagno G, De Salvo GL, Bozza F, Scalco G, Marconato R, Valletta S, Racano C, Burelli P, Nitti D, Lise M. Number of metastatic sentinel nodes as predictor of axillary involvement in patients with breast cancer. Breast Cancer Res.Treat. 2004;86:171–179. doi: 10.1023/B:BREA.0000032985.28558.6d. [DOI] [PubMed] [Google Scholar]
- 63.Shen J, Hunt KK, Mirza NQ, Krishnamurthy S, Singletary SE, Kuerer HM, Meric-Bernstam F, Feig B, Ross MI, Ames FC, Babiera GV. Intramammary lymph node metastases are an independent predictor of poor outcome in patients with breast carcinoma. Cancer. 2004;101:1330–1337. doi: 10.1002/cncr.20515. [DOI] [PubMed] [Google Scholar]
- 64.Klauber-DeMore N, Ollila DW, Moore DT, Livasy C, Calvo BF, Kim HJ, Dees EC, Sartor CI, Sawyer LR, Graham M, Carey LA. Size of residual lymph node metastasis after neoadjuvant chemotherapy in locally advanced breast cancer patients is prognostic. Ann.Surg.Oncol. 2006;13:685–691. doi: 10.1245/ASO.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Truong PT, Vinh-Hung V, Cserni G, Woodward WA, Tai P, Vlastos G. The number of positive nodes and the ratio of positive to excised nodes are significant predictors of survival in women with micrometastatic node-positive breast cancer. Eur.J.Cancer. 2008;44:1670–1677. doi: 10.1016/j.ejca.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Rack B, Janni W, Gerber B, Strobl B, Schindlbeck C, Klanner E, Rammel G, Sommer H, Dimpfl T, Friese K. Patients with recurrent breast cancer: does the primary axillary lymph node status predict more aggressive tumor progression? Breast Cancer Res.Treat. 2003;82:83–92. doi: 10.1023/B:BREA.0000003955.73738.9e. [DOI] [PubMed] [Google Scholar]
- 67.Kinoshita J, Kitamura K, Kabashima A, Saeki H, Tanaka S, Sugimachi K. Clinical significance of vascular endothelial growth factor-C (VEGF-C) in breast cancer. Breast Cancer Res.Treat. 2001;66:159–164. doi: 10.1023/a:1010692132669. [DOI] [PubMed] [Google Scholar]
- 68.Mohammed RA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br.J.Cancer. 2007;96:1092–1100. doi: 10.1038/sj.bjc.6603678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu Y, Qi X, Guo S. Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: a retrospective study of 61 cases. Clin.Exp.Metastasis. 2008;25:717–725. doi: 10.1007/s10585-008-9180-4. [DOI] [PubMed] [Google Scholar]
- 70.Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res.Treat. 2005;91:125–132. doi: 10.1007/s10549-004-5783-x. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Tsukiyama A, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Clinicopathological significance of vascular endothelial growth factor-C in breast carcinoma with long-term follow-up. Mod.Pathol. 2003;16:309–314. doi: 10.1097/01.MP.0000062858.98295.9F. [DOI] [PubMed] [Google Scholar]
- 72.Koyama Y, Kaneko K, Akazawa K, Kanbayashi C, Kanda T, Hatakeyama K. Vascular endothelial growth factor-C and vascular endothelial growth factor-D messenger RNA expression in breast cancer: association with lymph node metastasis. Clin.Breast Cancer. 2003;4:354–360. doi: 10.3816/cbc.2003.n.041. [DOI] [PubMed] [Google Scholar]
- 73.Hoar FJ, Chaudhri S, Wadley MS, Stonelake PS. Co-expression of vascular endothelial growth factor C (VEGF-C) and c-erbB2 in human breast carcinoma. Eur.J.Cancer. 2003;39:1698–1703. doi: 10.1016/s0959-8049(03)00382-4. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long-term follow-up. Clin.Cancer Res. 2003;9:716–721. [PubMed] [Google Scholar]
- 75.Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, Fox SB. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression 7. J.Pathol. 2003;200:183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- 76.George ML, Tutton MG, Janssen F, Arnaout A, Abulafi AM, Eccles SA, Swift RI. VEGF-A, VEGF-C, and VEGF-D in colorectal cancer progression 1. Neoplasia. 2001;3:420–427. doi: 10.1038/sj.neo.7900186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams CS, Leek RD, Robson AM, Banerji S, Prevo R, Harris AL, Jackson DG. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer 6. J.Pathol. 2003;200:195–206. doi: 10.1002/path.1343. [DOI] [PubMed] [Google Scholar]
- 78.Agarwal B, Saxena R, Morimiya A, Mehrotra S, Badve S. Lymphangiogenesis does not occur in breast cancer. Am.J.Surg.Pathol. 2005;29:1449–1455. doi: 10.1097/01.pas.0000174269.99459.9d. [DOI] [PubMed] [Google Scholar]
- 79.Vleugel MM, Bos R, van der GP, Greijer AE, Shvarts A, Stel HV, van der WE, van Diest PJ. Lack of lymphangiogenesis during breast carcinogenesis. J.Clin.Pathol. 2004;57:746–751. doi: 10.1136/jcp.2003.014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Schaft DW, Pauwels P, Hulsmans S, Zimmermann M, van de Poll-Franse LV, Griffioen AW. Absence of lymphangiogenesis in ductal breast cancer at the primary tumor site. Cancer Lett. 2007;254:128–136. doi: 10.1016/j.canlet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat.Rev.Mol.Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 82.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J.Clin.Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van den Eynden GG, van der Auwera I, Van Laere SJ, Trinh XB, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Comparison of molecular determinants of angiogenesis and lymphangiogenesis in lymph node metastases and in primary tumours of patients with breast cancer. J.Pathol. 2007;213:56–64. doi: 10.1002/path.2211. [DOI] [PubMed] [Google Scholar]
- 84.van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, Vermeulen PB, Dirix LY. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin.Cancer Res. 2004;10:7965–7971. doi: 10.1158/1078-0432.CCR-04-0063. [DOI] [PubMed] [Google Scholar]
- 85.Van den Eynden GG, Vandenberghe MK, van Dam PJ, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin.Cancer Res. 2007;13:5391–5397. doi: 10.1158/1078-0432.CCR-07-1230. [DOI] [PubMed] [Google Scholar]
- 86.Van den Eynden GG, van der Auwera I, Van Laere SJ, Huygelen V, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Induction of lymphangiogenesis in and around axillary lymph node metastases of patients with breast cancer. Br.J.Cancer. 2006;95:1362–1366. doi: 10.1038/sj.bjc.6603443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weidner N. Tumoural vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J.Pathol. 1998;184:119–122. doi: 10.1002/(SICI)1096-9896(199802)184:2<119::AID-PATH17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 88.Bono P, Wasenius VM, Heikkila P, Lundin J, Jackson DG, Joensuu H. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin.Cancer Res. 2004;10:7144–7149. doi: 10.1158/1078-0432.CCR-03-0826. [DOI] [PubMed] [Google Scholar]
- 89.Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, Sahin A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin.Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 90.Andre F, Cabioglu N, Assi H, Sabourin JC, Delaloge S, Sahin A, Broglio K, Spano JP, Combadiere C, Bucana C, Soria JC, Cristofanilli M. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann.Oncol. 2006;17:945–951. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- 91.Cabioglu N, Gong Y, Islam R, Broglio KR, Sneige N, Sahin A, Gonzalez-Angulo AM, Morandi P, Bucana C, Hortobagyi GN, Cristofanilli M. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann.Oncol. 2007;18:1021–1029. doi: 10.1093/annonc/mdm060. [DOI] [PubMed] [Google Scholar]
- 92.Yasuoka H, Tsujimoto M, Yoshidome K, Nakahara M, Kodama R, Sanke T, Nakamura Y. Cytoplasmic CXCR4 expression in breast cancer: induction by nitric oxide and correlation with lymph node metastasis and poor prognosis. BMC.Cancer. 2008;8:340. doi: 10.1186/1471-2407-8-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 94.Wong SY, Crowley D, Bronson RT, Hynes RO. Analyses of the role of endogenous SPARC in mouse models of prostate and breast cancer. Clin.Exp.Metastasis. 2008;25:109–118. doi: 10.1007/s10585-007-9126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 2005;7:R402–R410. doi: 10.1186/bcr1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, Nie S, Umbreit J, Shim H. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 97.Achen MG, Stacker SA. Tumor lymphangiogenesis and metastatic spread-New players begin to emerge. Int.J.Cancer. 2006:1755–1760. doi: 10.1002/ijc.21899. [DOI] [PubMed] [Google Scholar]
- 98.Ruddell A, Mezquita P, Brandvold KA, Farr A, Iritani BM. B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis. Am.J.Pathol. 2003;163:2233–2245. doi: 10.1016/S0002-9440(10)63581-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 100.Saaristo A, Veikkola T, Enholm B, Hytonen M, Arola J, Pajusola K, Turunen P, Jeltsch M, Karkkainen MJ, Kerjaschki D, Bueler H, Yla-Herttuala S, Alitalo K. Adenoviral VEGF-C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J. 2002;16:1041–1049. doi: 10.1096/fj.01-1042com. [DOI] [PubMed] [Google Scholar]
- 101.Isaka N, Padera TP, Hagendoorn J, Fukumura D, Jain RK. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res. 2004;64:4400–4404. doi: 10.1158/0008-5472.CAN-04-0752. [DOI] [PubMed] [Google Scholar]
- 102.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am.J.Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ben Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003;5:31–36. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Padera TP, Kuo AH, Hoshida T, Liao S, Lobo J, Kozak KR, Fukumura D, Jain RK. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol.Cancer Ther. 2008;7:2272–2279. doi: 10.1158/1535-7163.MCT-08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dadiani M, Kalchenko V, Yosepovich A, Margalit R, Hassid Y, Degani H, Seger D. Real-time imaging of lymphogenic metastasis in orthotopic human breast cancer. Cancer Res. 2006;66:8037–8041. doi: 10.1158/0008-5472.CAN-06-0728. [DOI] [PubMed] [Google Scholar]
- 106.Das S, Skobe M. Lymphatic vessel activation in cancer. Ann.N.Y.Acad.Sci. 2008;1131:235–241. doi: 10.1196/annals.1413.021. [DOI] [PubMed] [Google Scholar]
- 107.Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, Chen J, Resau JH, Teh BT. Preparing the "soil": the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365–10376. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 108.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–3167. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 110.Colleoni M, Rotmensz N, Maisonneuve P, Sonzogni A, Pruneri G, Casadio C, Luini A, Veronesi P, Intra M, Galimberti V, Torrisi R, Andrighetto S, Ghisini R, Goldhirsch A, Viale G. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann.Oncol. 2007;18:1632–1640. doi: 10.1093/annonc/mdm268. [DOI] [PubMed] [Google Scholar]
- 111.Gervasoni JE, Jr, Sbayi S, Cady B. Role of lymphadenectomy in surgical treatment of solid tumors: an update on the clinical data. Ann.Surg.Oncol. 2007;14:2443–2462. doi: 10.1245/s10434-007-9360-5. [DOI] [PubMed] [Google Scholar]
- 112.Cady B. Regional lymph node metastases; a singular manifestation of the process of clinical metastases in cancer: contemporary animal research and clinical reports suggest unifying concepts. Ann.Surg.Oncol. 2007;14:1790–1800. doi: 10.1245/s10434-006-9234-2. [DOI] [PubMed] [Google Scholar]
- 113.Abdul-Rasool S, Kidson SH, Panieri E, Dent D, Pillay K, Hanekom GS. An evaluation of molecular markers for improved detection of breast cancer metastases in sentinel nodes. J.Clin.Pathol. 2006;59:289–297. doi: 10.1136/jcp.2005.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Reintgen DS, Coventry BJ, Glass EC, Wang HJ. Sentinel-node biopsy or nodal observation in melanoma. N.Engl.J.Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 115.Rebhun RB, Lazar AJ, Fidler IJ, Gershenwald JE. Impact of sentinel lymphadenectomy on survival in a murine model of melanoma. Clin.Exp.Metastasis. 2008;25:191–199. doi: 10.1007/s10585-008-9141-y. [DOI] [PubMed] [Google Scholar]
- 116.Weinberg RA. Moving out:Invasion and Metastasis, The biology of cancer, Garland Science. New York: Taylor &Francis Group, LLC; 2007. pp. 631–634. [Google Scholar]
- 117.van der Wal BC, Butzelaar RM, van der MS, Boermeester MA. Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur.J.Surg.Oncol. 2002;28:481–489. doi: 10.1053/ejso.2002.1239. [DOI] [PubMed] [Google Scholar]
- 118.White RE, Vezeridis MP, Konstadoulakis M, Cole BF, Wanebo HJ, Bland KI. Therapeutic options and results for the management of minimally invasive carcinoma of the breast: influence of axillary dissection for treatment of T1a and T1b lesions. J.Am.Coll.Surg. 1996;183:575–582. [PubMed] [Google Scholar]
- 119.Kopfstein L, Veikkola T, Djonov VG, Baeriswyl V, Schomber T, Strittmatter K, Stacker SA, Achen MG, Alitalo K, Christofori G. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am.J.Pathol. 2007;170:1348–1361. doi: 10.2353/ajpath.2007.060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP, Jain RK. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 121.Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–722. [PubMed] [Google Scholar]
- 123.Chen Z, Varney ML, Backora MW, Cowan K, Solheim JC, Talmadge JE, Singh RK. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–9011. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- 124.Shibata MA, Morimoto J, Shibata E, Otsuki Y. Combination therapy with short interfering RNA vectors against VEGF-C and VEGF-A suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther. 2008;15:776–786. doi: 10.1038/cgt.2008.43. [DOI] [PubMed] [Google Scholar]
- 125.Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, Alitalo K, Wu L. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res. 2008;68:7828–7837. doi: 10.1158/0008-5472.CAN-08-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am.J.Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yanai Y, Furuhata T, Kimura Y, Yamaguchi K, Yasoshima T, Mitaka T, Mochizuki Y, Hirata K. Vascular endothelial growth factor C promotes human gastric carcinoma lymph node metastasis in mice. J.Exp.Clin.Cancer Res. 2001;20:419–428. [PubMed] [Google Scholar]
- 128.Kawakami M, Yanai Y, Hata F, Hirata K. Vascular endothelial growth factor C promotes lymph node metastasis in a rectal cancer orthotopic model. Surg.Today. 2005;35:131–138. doi: 10.1007/s00595-004-2896-0. [DOI] [PubMed] [Google Scholar]
- 129.Volk LD, Flister MJ, Bivens CM, Stutzman A, Desai N, Trieu V, Ran S. Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor A therapy. Neoplasia. 2008;10:613–623. doi: 10.1593/neo.08302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–9798. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- 131.Shimizu K, Kubo H, Yamaguchi K, Kawashima K, Ueda Y, Matsuo K, Awane M, Shimahara Y, Takabayashi A, Yamaoka Y, Satoh S. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci. 2004;95:328–333. doi: 10.1111/j.1349-7006.2004.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thelen A, Scholz A, Benckert C, Von Marschall Z, Schroder M, Wiedenmann B, Neuhaus P, Rosewicz S, Jonas S. VEGF-D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int.J.Cancer. 2008;122:2471–2481. doi: 10.1002/ijc.23439. [DOI] [PubMed] [Google Scholar]
- 133.Von MZ, Scholz A, Stacker SA, Achen MG, Jackson DG, Alves F, Schirner M, Haberey M, Thierauch KH, Wiedenmann B, Rosewicz S. Vascular endothelial growth factor-D induces lymphangiogenesis and lymphatic metastasis in models of ductal pancreatic cancer. Int.J.Oncol. 2005;27:669–679. [PubMed] [Google Scholar]
- 134.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J.Clin.Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 135.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69 Suppl 3:11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 136.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J.Exp.Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 138.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J.Exp.Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wislez M, Fujimoto N, Izzo JG, Hanna AE, Cody DD, Langley RR, Tang H, Burdick MD, Sato M, Minna JD, Mao L, Wistuba I, Strieter RM, Kurie JM. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic Kras. Cancer Res. 2006;66:4198–4207. doi: 10.1158/0008-5472.CAN-05-3842. [DOI] [PubMed] [Google Scholar]
- 140.Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, Wu L, Cao Y. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261–9268. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- 141.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J.Clin.Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, Kruse FE, Wiegand SJ, Dana MR, Streilein JW. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol.Vis.Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 143.Nguyen VP, Chen SH, Trinh J, Kim H, Coomber BL, Dumont DJ. Differential response of lymphatic, venous and arterial endothelial cells to angiopoietin-1 and angiopoietin-2. BMC.Cell Biol. 2007;8:10. doi: 10.1186/1471-2121-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev.Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 145.Caine GJ, Stonelake PS, Lip GY, Blann AD. Changes in plasma vascular endothelial growth factor, angiopoietins, and their receptors following surgery for breast cancer. Cancer Lett. 2007;248:131–136. doi: 10.1016/j.canlet.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 146.Caine GJ, Blann AD, Stonelake PS, Ryan P, Lip GY. Plasma angiopoietin-1, angiopoietin-2 and Tie-2 in breast and prostate cancer: a comparison with VEGF and Flt-1. Eur.J.Clin.Invest. 2003;33:883–890. doi: 10.1046/j.1365-2362.2003.01243.x. [DOI] [PubMed] [Google Scholar]
- 147.Sfiligoi C, de Luca A, Cascone I, Sorbello V, Fuso L, Ponzone R, Biglia N, Audero E, Arisio R, Bussolino F, Sismondi P, De Bortoli M. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int.J.Cancer. 2003;103:466–474. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- 148.Rmali KA, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Angiopoietins lack of prognostic significance in ductal mammary carcinoma. Int.Semin.Surg.Oncol. 2007;4:6. doi: 10.1186/1477-7800-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Currie MJ, Gunningham SP, Han C, Scott PA, Robinson BA, Harris AL, Fox SB. Angiopoietin-1 is inversely related to thymidine phosphorylase expression in human breast cancer, indicating a role in vascular remodeling. Clin.Cancer Res. 2001;7:918–927. [PubMed] [Google Scholar]
- 150.Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc.Natl.Acad.Sci.U.S.A. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc.Natl.Acad.Sci.U.S.A. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 153.Banziger-Tobler NE, Halin C, Kajiya K, Detmar M. Growth hormone promotes lymphangiogenesis. Am.J.Pathol. 2008;173:586–597. doi: 10.2353/ajpath.2008.080060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 155.Da MX, Wu Z, Tian HW. Tumor lymphangiogenesis and lymphangiogenic growth factors. Arch.Med.Res. 2008;39:365–372. doi: 10.1016/j.arcmed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 156.Kucab JE, Dunn SE. Role of IGF-1R in mediating breast cancer invasion and metastasis. Breast Dis. 2003;17:41–47. doi: 10.3233/bd-2003-17105. [DOI] [PubMed] [Google Scholar]
- 157.Parr C, Watkins G, Mansel RE, Jiang WG. The hepatocyte growth factor regulatory factors in human breast cancer. Clin.Cancer Res. 2004;10:202–211. doi: 10.1158/1078-0432.ccr-0553-3. [DOI] [PubMed] [Google Scholar]
- 158.De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim.Biophys.Acta. 2006;1766:159–166. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 159.Garmy-Susini B, Varner JA. Circulating endothelial progenitor cells. Br.J.Cancer. 2005;93:855–858. doi: 10.1038/sj.bjc.6602808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Real C, Caiado F, Dias S. Endothelial progenitors in vascular repair and angiogenesis: how many are needed and what to do? Cardiovasc.Hematol.Disord.Drug Targets. 2008;8:185–193. doi: 10.2174/187152908785849071. [DOI] [PubMed] [Google Scholar]
- 161.Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J.Vasc.Surg. 2007;45 Suppl A:A39–A47. doi: 10.1016/j.jvs.2007.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 163.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Krober SM, Greinix H, Rosenmaier A, Karlhofer F, Wick N, Mazal PR. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat.Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 164.Jiang S, Bailey AS, Goldman DC, Swain JR, Wong MH, Streeter PR, Fleming WH. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS.ONE. 2008;3:e3812. doi: 10.1371/journal.pone.0003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 166.Makinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc.Natl.Acad.Sci.U.S.A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Siegfried G, Basak A, Cromlish JA, Benjannet S, Marcinkiewicz J, Chretien M, Seidah NG, Khatib AM. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J.Clin.Invest. 2003;111:1723–1732. doi: 10.1172/JCI17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McColl BK, Paavonen K, Karnezis T, Harris NC, Davydova N, Rothacker J, Nice EC, Harder KW, Roufail S, Hibbs ML, Rogers PA, Alitalo K, Stacker SA, Achen MG. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR-2. FASEB J. 2007;21:1088–1098. doi: 10.1096/fj.06-7060com. [DOI] [PubMed] [Google Scholar]
- 171.McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL, Simpson RJ, Alitalo K, Stacker SA, Achen MG. Plasmin Activates the Lymphangiogenic Growth Factors VEGF-C and VEGF-D. J.Exp.Med. 2003;198:863–868. doi: 10.1084/jem.20030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 173.Baldwin ME, Halford MM, Roufail S, Williams RA, Hibbs ML, Grail D, Kubo H, Stacker SA, Achen MG. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol.Cell Biol. 2005;25:2441–2449. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karpanen T, Wirzenius M, Makinen T, Veikkola T, Haisma HJ, Achen MG, Stacker SA, Pytowski B, Yla-Herttuala S, Alitalo K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am.J.Pathol. 2006;169:708–718. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Galland F, Karamysheva A, Pebusque MJ, Borg JP, Rottapel R, Dubreuil P, Rosnet O, Birnbaum D. The FLT4 gene encodes a transmembrane tyrosine kinase related to the vascular endothelial growth factor receptor. Oncogene. 1993;8:1233–1240. [PubMed] [Google Scholar]
- 176.Hughes DC. Alternative splicing of the human VEGFGR-3/FLT4 gene as a consequence of an integrated human endogenous retrovirus. J.Mol.Evol. 2001;53:77–79. doi: 10.1007/s002390010195. [DOI] [PubMed] [Google Scholar]
- 177.Borg JP, deLapeyriere O, Noguchi T, Rottapel R, Dubreuil P, Birnbaum D. Biochemical characterization of two isoforms of FLT4, a VEGF receptor-related tyrosine kinase. Oncogene. 1995;10:973–984. [PubMed] [Google Scholar]
- 178.Fournier E, Rosnet O, Marchetto S, Turck CW, Rottapel R, Pelicci PG, Birnbaum D, Borg JP. Interaction with the phosphotyrosine binding domain/phosphotyrosine interacting domain of SHC is required for the transforming activity of the FLT4/VEGFR3 receptor tyrosine kinase. J.Biol.Chem. 1996;271:12956–12963. doi: 10.1074/jbc.271.22.12956. [DOI] [PubMed] [Google Scholar]
- 179.Gunningham SP, Currie MJ, Han C, Robinson BA, Scott PA, Harris AL, Fox SB. The short form of the alternatively spliced flt-4 but not its ligand vascular endothelial growth factor C is related to lymph node metastasis in human breast cancers. Clin.Cancer Res. 2000;6:4278–4286. [PubMed] [Google Scholar]
- 180.Wang JF, Zhang X, Groopman JE. Activation of vascular endothelial growth factor receptor-3 and its downstream signaling promote cell survival under oxidative stress. J.Biol.Chem. 2004;279:27088–27097. doi: 10.1074/jbc.M314015200. [DOI] [PubMed] [Google Scholar]
- 181.Salameh A, Galvagni F, Bardelli M, Bussolino F, Oliviero S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. 2005;106:3423–3431. doi: 10.1182/blood-2005-04-1388. [DOI] [PubMed] [Google Scholar]
- 182.Alam A, Herault JP, Barron P, Favier B, Fons P, Delesque-Touchard N, Senegas I, Laboudie P, Bonnin J, Cassan C, Savi P, Ruggeri B, Carmeliet P, Bono F, Herbert JM. Heterodimerization with vascular endothelial growth factor receptor-2 (VEGFR-2) is necessary for VEGFR-3 activity. Biochem.Biophys.Res.Commun. 2004;324:909–915. doi: 10.1016/j.bbrc.2004.08.237. [DOI] [PubMed] [Google Scholar]
- 183.Dixelius J, Makinen T, Wirzenius M, Karkkainen MJ, Wernstedt C, Alitalo K, Claesson-Welsh L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J.Biol.Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- 184.Ji RC. Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat.Res.Biol. 2006;4:83–100. doi: 10.1089/lrb.2006.4.83. [DOI] [PubMed] [Google Scholar]
- 185.Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J.Cell Physiol. 2005;202:205–214. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
- 186.Wang JF, Zhang XF, Groopman JE. Stimulation of beta 1 integrin induces tyrosine phosphorylation of vascular endothelial growth factor receptor-3 and modulates cell migration. J.Biol.Chem. 2001;276:41950–41957. doi: 10.1074/jbc.M101370200. [DOI] [PubMed] [Google Scholar]
- 187.Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer D, Stragies R, Zahn G, Cursiefen C. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am.J.Pathol. 2007;171:361–372. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yu JL, Rak JW. Host microenvironment in breast cancer development: inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res. 2003;5:83–88. doi: 10.1186/bcr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF-kappaB amalgamates the perilous partnership. Curr.Cancer Drug Targets. 2005;5:325–344. doi: 10.2174/1568009054629645. [DOI] [PubMed] [Google Scholar]
- 190.Ditsworth D, Zong WX. NF-kappaB: key mediator of inflammation-associated cancer. Cancer Biol.Ther. 2004;3:1214–1216. doi: 10.4161/cbt.3.12.1391. [DOI] [PubMed] [Google Scholar]
- 191.Albini A, Tosetti F, Benelli R, Noonan DM. Tumor inflammatory angiogenesis and its chemoprevention. Cancer Res. 2005;65:10637–10641. doi: 10.1158/0008-5472.CAN-05-3473. [DOI] [PubMed] [Google Scholar]
- 192.Matsumoto G, Namekawa J, Muta M, Nakamura T, Bando H, Tohyama K, Toi M, Umezawa K. Targeting of nuclear factor kappaB pathways by dehydroxymethylepoxyquinomicin, a novel inhibitor of breast carcinomas: antitumor and antiangiogenic potential in vivo. Clin.Cancer Res. 2005;11:1287–1293. [PubMed] [Google Scholar]
- 193.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J.Clin.Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bharti AC, Aggarwal BB. Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Ann.N.Y.Acad.Sci. 2002;973:392–395. doi: 10.1111/j.1749-6632.2002.tb04671.x. [DOI] [PubMed] [Google Scholar]
- 195.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 196.Zhang C, Chakravarty D, Sakabe I, Mewani RR, Boudreau HE, Kumar D, Ahmad I, Kasid UN. Role of SCC-S2 in experimental metastasis and modulation of VEGFR-2, MMP-1, and MMP-9 expression. Mol.Ther. 2006;13:947–955. doi: 10.1016/j.ymthe.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 197.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 198.Colombo MP, Mantovani A. Targeting myelomonocytic cells to revert inflammation-dependent cancer promotion. Cancer Res. 2005;65:9113–9116. doi: 10.1158/0008-5472.CAN-05-2714. [DOI] [PubMed] [Google Scholar]
- 199.Hamrah P, Chen L, Zhang Q, Dana MR. Novel expression of vascular endothelial growth factor receptor (VEGFR)-3 and VEGF-C on corneal dendritic cells. Am.J.Pathol. 2003;163:57–68. doi: 10.1016/S0002-9440(10)63630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, Bevilacqua G, Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J.Clin.Pathol. 2004;57:630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 202.Grimshaw MJ, Wilson JL, Balkwill FR. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. Eur.J.Immunol. 2002;32:2393–2400. doi: 10.1002/1521-4141(200209)32:9<2393::AID-IMMU2393>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 203.Lin EY, Pollard JW. Macrophages: modulators of breast cancer progression. Novartis.Found.Symp. 2004;256:158–168. [PubMed] [Google Scholar]
- 204.Kriehuber E, Breiteneder-Geleff S, Groeger M, Soleiman A, Schoppmann SF, Stingl G, Kerjaschki D, Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J.Exp.Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Whitehurst B, Eversgerd C, Flister M, Bivens CM, Pickett B, Zawieja DC, Ran S. Molecular profile and proliferative responses of rat lymphatic endothelial cells in culture. Lymphat.Res.Biol. 2006;4:119–142. doi: 10.1089/lrb.2006.4.119. [DOI] [PubMed] [Google Scholar]
- 206.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu.Rev.Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 207.Hsieh CY, Chen CA, Chou CH, Lai KP, Jeng YM, Kuo ML, Wei LH. Overexpression of Her-2/NEU in epithelial ovarian carcinoma induces vascular endothelial growth factor C by activating NF-kappa B: implications for malignant ascites formation and tumor lymphangiogenesis. J.Biomed.Sci. 2004;11:249–259. doi: 10.1007/BF02256568. [DOI] [PubMed] [Google Scholar]
- 208.Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res.Treat. 2002;73:237–243. doi: 10.1023/a:1015872531675. [DOI] [PubMed] [Google Scholar]
- 209.Trompezinski S, Berthier-Vergnes O, Denis A, Schmitt D, Viac J. Comparative expression of vascular endothelial growth factor family members, VEGF-B, -C and -D, by normal human keratinocytes and fibroblasts. Exp.Dermatol. 2004;13:98–105. doi: 10.1111/j.0906-6705.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- 210.Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br.J.Cancer. 2006;94:1154–1163. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S, Klagsbrun M, Ferrara N, Bussolino F. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J.Biol.Chem. 1998;273:22128–22135. doi: 10.1074/jbc.273.34.22128. [DOI] [PubMed] [Google Scholar]
- 212.Flister MJ, Hall K, Miele LA, Ran S. NF-κB and Prox1 directly regulate expression of vascular endothelial growth factor receptor (VEGFR)-3. Proceedings of 99th American Association for Cancer Research Annual Meeting; San Diego, CA. 2008. [Google Scholar]
- 213.Chuang SE, Cheng AL, Lin JK, Kuo ML. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem.Toxicol. 2000;38:991–995. doi: 10.1016/s0278-6915(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 214.Haffner MC, Berlato C, Doppler W. Exploiting our knowledge of NF-kappaB signaling for the treatment of mammary cancer. J.Mammary.Gland.Biol.Neoplasia. 2006;11:63–73. doi: 10.1007/s10911-006-9013-5. [DOI] [PubMed] [Google Scholar]
- 215.Montesano R, Soulie P, Eble JA, Carrozzino F. Tumour necrosis factor alpha confers an invasive, transformed phenotype on mammary epithelial cells. J.Cell Sci. 2005;118:3487–3500. doi: 10.1242/jcs.02467. [DOI] [PubMed] [Google Scholar]
- 216.Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer TW, Somcio RJ, Wu Y, Hooper A, Hicklin DJ, Ellis LM. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–2653. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 217.Das R, Philip S, Mahabeleshwar GH, Bulbule A, Kundu GC. Osteopontin: it's role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression. IUBMB.Life. 2005;57:441–447. doi: 10.1080/15216540500159424. [DOI] [PubMed] [Google Scholar]
- 218.Hamada K, Oike Y, Takakura N, Ito Y, Jussila L, Dumont DJ, Alitalo K, Suda T. VEGF-C signaling pathways through VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis. Blood. 2000;96:3793–3800. [PubMed] [Google Scholar]
- 219.Izumi Y, di Tomaso E, Hooper A, Huang P, Huber J, Hicklin DJ, Fukumura D, Jain RK, Suit HD. Responses to antiangiogenesis treatment of spontaneous autochthonous tumors and their isografts. Cancer Res. 2003;63:747–751. [PubMed] [Google Scholar]
- 220.Bando H. Vascular endothelial growth factor and bevacizumab in breast cancer. Breast Cancer. 2007;14:163–173. doi: 10.2325/jbcs.968. [DOI] [PubMed] [Google Scholar]
- 221.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem.Biophys.Res.Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 222.Zhang W, Ran S, Sambade M, Huang X, Thorpe PE. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis. 2002;5:35–44. doi: 10.1023/a:1021540120521. [DOI] [PubMed] [Google Scholar]
- 223.Link JS, Waisman JR, Nguyen B, Jacobs CI. Bevacizumab and albumin-bound paclitaxel treatment in metastatic breast cancer. Clin.Breast Cancer. 2007;7:779–783. doi: 10.3816/CBC.2007.n.039. [DOI] [PubMed] [Google Scholar]
- 224.Hu L, Hofmann J, Holash J, Yancopoulos GD, Sood AK, Jaffe RB. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin.Cancer Res. 2005;11:6966–6971. doi: 10.1158/1078-0432.CCR-05-0910. [DOI] [PubMed] [Google Scholar]
- 225.Hurwitz H. Integrating the anti-VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin.Colorectal Cancer. 2004;4(Suppl 2):S62–S68. doi: 10.3816/ccc.2004.s.010. [DOI] [PubMed] [Google Scholar]
- 226.Lyseng-Williamson KA, Robinson DM. Spotlight on bevacizumab in advanced colorectal cancer, breast cancer, and non-small cell lung cancer. BioDrugs. 2006;20:193–195. doi: 10.2165/00063030-200620030-00007. [DOI] [PubMed] [Google Scholar]
- 227.Tran J, Rak J, Sheehan C, Saibil SD, LaCasse E, Korneluk RG, Kerbel RS. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem.Biophys.Res.Commun. 1999;264:781–788. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- 228.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J.Biol.Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 229.Wissmann C, Detmar M. Pathways targeting tumor lymphangiogenesis. Clin.Cancer Res. 2006;12:6865–6868. doi: 10.1158/1078-0432.CCR-06-1800. [DOI] [PubMed] [Google Scholar]
- 230.Weiser MR, Montgomery LL, Tan LK, Susnik B, Leung DY, Borgen PI, Cody HS., III Lymphovascular invasion enhances the prediction of non-sentinel node metastases in breast cancer patients with positive sentinel nodes. Ann.Surg.Oncol. 2001;8:145–149. doi: 10.1007/s10434-001-0145-y. [DOI] [PubMed] [Google Scholar]
- 231.Turner RR, Chu KU, Qi K, Botnick LE, Hansen NM, Glass EC, Giuliano AE. Pathologic features associated with nonsentinel lymph node metastases in patients with metastatic breast carcinoma in a sentinel lymph node. Cancer. 2000;89:574–581. doi: 10.1002/1097-0142(20000801)89:3<574::aid-cncr12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 232.Abner AL, Collins L, Peiro G, Recht A, Come S, Shulman LN, Silver B, Nixon A, Harris JR, Schnitt SJ, Connolly JL. Correlation of tumor size and axillary lymph node involvement with prognosis in patients with T1 breast carcinoma. Cancer. 1998;83:2502–2508. [PubMed] [Google Scholar]
- 233.Atahan IA, Yildiz F, Ozyigit G, Sari S, Gurkaynak M, Selek U, Hayran M. Percent positive axillary lymph node metastasis predicts survival in patients with non-metastatic breast cancer. Acta Oncol. 2008;47:232–238. doi: 10.1080/02841860701678761. [DOI] [PubMed] [Google Scholar]
- 234.Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, Vielh P, Bourstyn E. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J.Clin.Oncol. 2002;20:1304–1310. doi: 10.1200/JCO.2002.20.5.1304. [DOI] [PubMed] [Google Scholar]
- 235.Gisterek I, Matkowski R, Kozlak J, Dus D, Lacko A, Szelachowska J, Kornafel J. Evaluation of prognostic value of VEGF-C and VEGF-D in breast cancer--10 years follow-up analysis. Anticancer Res. 2007;27:2797–2802. [PubMed] [Google Scholar]
- 236.Li YS, Kaneko M, Amatya VJ, Takeshima Y, Arihiro K, Inai K. Expression of vascular endothelial growth factor-C and its receptor in invasive micropapillary carcinoma of the breast. Pathol.Int. 2006;56:256–261. doi: 10.1111/j.1440-1827.2006.01961.x. [DOI] [PubMed] [Google Scholar]
- 237.Zhang XH, Huang DP, Guo GL, Chen GR, Zhang HX, Wan L, Chen SY. Coexpression of VEGF-C and COX-2 and its association with lymphangiogenesis in human breast cancer. BMC.Cancer. 2008;8:4. doi: 10.1186/1471-2407-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Choi WW, Lewis MM, Lawson D, Yin-Goen Q, Birdsong GG, Cotsonis GA, Cohen C, Young AN. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod.Pathol. 2005;18:143–152. doi: 10.1038/modpathol.3800253. [DOI] [PubMed] [Google Scholar]