Introduction
Sepsis or serious infection within the first four weeks of life kills greater than 1 million newborns globally every year1. The attack rate for neonatal sepsis is variable (from <1% to >35% of live births) based on gestational age and time of onset (early[<72 hours after birth] or late[>72 hours after birth])2–5. Neonates with sepsis may present in or progress to septic shock, exemplified initially by cardiovascular dysfunction requiring fluid resuscitation or inotropic support6. If the progression of infection cannot be stopped, end organ damage and death become much more likely. While the true incidence is not known, a recent retrospective cohort study of 3800 neonates admitted to the NICU over a 6 year period reported septic shock in 1.3% with an associated mortality peaking at 71% for extremely low birth weight (ELBW) neonates <1000g7. There are few published data regarding the pathophysiology of septic shock in neonates. Previous clinical investigations into neonatal sepsis and shock have largely focused on diagnostic markers. Descriptions of septic shock are predominantly case reports on very small numbers, mixed populations with severe respiratory distress syndrome (RDS) and sepsis, or pediatric studies that included neonates that were not evaluated as a separate group8–24.
Definitions of the sepsis continuum
In 2005, definitions for pediatric infection, systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, septic shock, and organ dysfunction were suggested that included term neonates (0– 7 days), newborns (1 week – 1 month) and infants (1 month – 1 year) [Box 1A/B]25. Working definitions for the sepsis continuum specific for preterm neonates are needed to provide a uniform basis for clinicians and researchers to study and diagnose severe sepsis in this particularly vulnerable population. We have proposed modifications to the consensus definitions to incorporate preterm infants that are also presented in Box 1A/B.
Box 1.
| Box 1A: Definition of systemic inflammatory response syndrome (SIRS), infection, sepsis, severe sepsis, and septic shock. | |
|---|---|
| Consensus definitions | Suggested modifications for premature infants |
|
SIRS The presence of at least two of the following four criteria, one of which must be abnormal temperature or leukocyte count: |
SIRS The presence of at least two of the following four criteria, one of which must be abnormal temperature or leukocyte count: |
| • Core* temperature of >38.5°C or <36°C. | • Core temperature of >38.0°C1 or <36°C. |
| • Tachycardia, defined as a mean heart rate >2 SD above normal for age in the absence of external stimulus, chronic drugs, or painful stimuli; or otherwise unexplained persistent elevation over a 0.5- to 4-hr time period OR for children <1yr old: bradycardia, defined as a mean heart rate <10th percentile for age in the absence of external vagal stimulus, β blocker drugs, or congenital heart disease; or otherwise unexplained persistent depression over a 0.5-hr time period. |
• Tachycardia, defined as a mean heart rate >2 SD above normal for age in the absence of external stimulus, chronic drugs, or painful stimuli; or otherwise unexplained persistent elevation over a 0.5- to 4-hr time period OR bradycardia, defined as a mean heart rate <10th percentile for age in the absence of β-blocker drugs or congenital heart disease2; or otherwise unexplained persistent bradycardia3 |
| • Mean respiratory rate >2 SD above normal for age or mechanical ventilation for an acute process not related to underlying neuromuscular disease or the receipt of general anesthesia. |
• Mean respiratory rate >2 SD above normal for age or mechanical ventilation for an acute process not related to underlying neuromuscular disease or the receipt of general anesthesia. |
| • Leukocyte count elevated or depressed for age (not secondary to chemotherapy-induced leukopenia) or >10% immature neutrophils. |
• Leukocyte count elevated or depressed for age or >20% immature to total neutrophil ratio4 or C-reactive protein > 10mg/dL. |
|
Infection A suspected or proven (by positive culture, tissue stain, or polymerase chain reaction test) infection caused by any pathogen OR a clinical syndrome associated with a high probability of infection. Evidence of infection includes positive findings on clinical exam, imaging, or laboratory tests (e.g., white blood cells in a normally sterile body fluid, perforated viscus, chest radiograph consistent with pneumonia, petechial or purpuric rash, or purpura fulminans) |
No change suggested |
|
Sepsis SIRS in the presence of or as a result of suspected or proven infection. |
No change suggested |
|
Severe sepsis Sepsis plus one of the following: cardiovascular organ dysfunction OR acute respiratory distress syndrome OR two or more other organ dysfunctions. |
No change suggested |
|
Septic shock Sepsis and cardiovascular organ dysfunction. |
No change suggested |
|
Box 1B: Definitions of organ dysfunction. | |
|---|---|
| Consensus definitions of organ dysfunction25 | Suggested modifications for premature infants |
| Cardiovascular dysfunction | Cardiovascular dysfunction |
| Despite administration of isotonic intravenous fluid bolus >40 mL/kg in 1 hr |
Despite administration of isotonic intravenous fluid bolus >40 mL/kg in 1 hr (>10ml/kg in infants less than 32 weeks)1 |
| • Decrease in BP (hypotension) <5th percentile for age or systolic BP >2 SD below normal for age |
• Decrease in BP (hypotension) <5th percentile for age or systolic BP >2 SD below normal for age or MAP < 30mm Hg with poor capillary refill time (>4 seconds)2 |
| OR | OR |
| • Need for vasoactive drug to maintain BP in normal range (dopamine >5 mcg/kg/min or dobutamine, epinephrine, or norepinephrine at any dose) |
• Need for vasoactive drug to maintain BP in normal range (dopamine >5 mcg/kg/min or dobutamine, or epinephrine at any dose)3 |
| OR | OR |
| • Two of the following: | • Two of the following: |
| -Unexplained metabolic acidosis: base deficit >5.0 mEq/L | -Unexplained metabolic acidosis: base deficit >5.0 mEq/L |
| -Increased arterial lactate >2 times upper limit of normal | -Increased arterial lactate >2 times upper limit of normal |
| -Oliguria: urine output <0.5 mL/kg/hr | -Oliguria: urine output <0.5 mL/kg/hr |
| -Prolonged capillary refill: >5 secs | -Prolonged capillary refill: >4 sec4 |
| -Core to peripheral temperature gap >3°C | -Simultaneous measurement of core and peripheral temperature not common in premature neonates |
| Pulmonarya | Pulmonary |
| • PaO2/FIO2 <300 in absence of cyanotic heart disease or preexisting lung disease |
• Excessive oxygen should be limited to avoid complications including retinopathy of prematurity |
| OR | |
| • PaCO2 >65 torr or 20 mm Hg over baseline PaCO2 | • PaCO2 >65 torr or 20 mm Hg over baseline PaCO2 |
| OR | OR |
| • Proven needb for >50% FIO2 to maintain saturation >92% | • Proven need for >50% FIO2 to maintain saturation >92% (88% for <32 weeks) |
| OR | OR |
| • Need for non-elective invasive or noninvasive mechanical ventilationc |
• Need for non-elective invasive or noninvasive mechanical ventilation |
| Neurologic | Neurologic |
| • Glasgow Coma Score >11 | • Acute change in mental status5 |
| OR | |
| • Acute change in mental status with a decrease in Glasgow Coma Score >3 points from abnormal baseline |
|
| Hematologic | Hematologic |
| • Platelet count <80,000/mm3 or a decline of 50% in platelet count from highest value recorded over the past 3 days (for chronic hematology/oncology patients) |
• Platelet count <80,000/mm3 or a decline of 50% in platelet count from highest value recorded over the past 3 days6 |
| OR | OR |
| • International normalized ratio >2 | • International normalized ratio >2 |
| Renal | Renal |
| • Serum creatinine >2 times upper limit of normal for age or 2-fold increase in baseline creatinine |
• Serum creatinine >2 times upper limit of normal for age or 2-fold increase in baseline creatinine |
| Hepatic | Hepatic |
| • Total bilirubin >4 mg/dL (not applicable for newborn) | • Alanine transaminase 2 times upper limit of normal for age7 or 50% increase over patient’s baseline8 |
| OR | |
| • ALT 2 times upper limit of normal for age | |
core temperature must be measured by rectal, bladder, oral, or central catheter probe
Neonatal fever is considered greater than 38°C;
External vagal stimulus use is very uncommon in preterm infants;
Infrequent self-resolving bradycardic episodes can be common in premature neonates in the absence of sepsis;
more commonly accepted ratio is greater than 20% immature to total ratio and chemotherapy-induced leukopenia is uncommon in premature infants.
From Goldstein et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005 Jan;6(1):2–8, with permission.
BP, blood pressure; ALT, alanine transaminase.
acute respiratory distress syndrome must include a PaO2/FIO2 ratio ≤200 mm Hg, bilateral infiltrates, acute onset, and no evidence of left heart failure. Acute lung injury is defined identically except the PaO2/FIO2 ratio must be ≥300 mm Hg;
proven need assumes oxygen requirement was tested by decreasing flow with subsequent increase in flow if required;
in postoperative patients, this requirement can be met if the patient has developed an acute inflammatory or infectious process in the lungs that prevents him or her from being extubated.
Rapid large volume expansion can be associated with intraventricular hemorrhage;
30mm Hg suggested as minimum MAP;
Norepinephrine not commonly used in premature neonates;
Greater than 4 seconds may reflect a low systemic blood flow264;
Glasgow Coma Score not applicable to term or preterm neonates;
Neonates not frequently chronic hematology-oncology patients;
Indirect hyperbilirubinemia is common in newborn.
Transaminases are commonly elevated in preterm neonates on long-term intravenous hyperalimentation
From Goldstein et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005 Jan;6(1):2–8, with permission.
Why have definitions of sepsis and septic shock not been established for preterm neonates? These patients present diagnostic challenges that are clouded by immaturity of organ systems and transitional physiology. For example, normal blood pressure values for gestational and postnatal age have not been established, particularly in the very low birth weight neonate (VLBW, <1500g), largely because blood pressure alone cannot identify abnormal cardiac output, organ perfusion, and oxygen delivery26. In the absence of normative values, it is nearly impossible to establish parameters that are associated with poor outcome. Perhaps the most obvious limitation is the differences in monitoring capabilities between the preterm neonate and older, physically larger patients. For example, pulmonary artery catheterization may be used in children or adults to monitor the course of septic shock, but this is not feasible in small neonates.. For these reasons, the hemodynamic response to septic shock and optimum clinical interventions in preterm neonates are not well understood.
Risk factors for development of neonatal septic shock
Risk factors for a neonate developing sepsis have been well-described Though risk factors for septic shock will overlap with those for sepsis, specific antenatal and postnatal risks for the development of neonatal septic shock have not been described in depth.
Maternal factors contributing to the risk of neonatal sepsis are shown in Box 2 and include prematurity, low birth weight, rectovaginal colonization with group B streptococcus (GBS), prolonged rupture of membranes, maternal intrapartum fever, and chorioamnionitis 2, 3, 27–33.
Box 2.
Risk factors for the development of neonatal sepsis and septic shock
| Maternal factors | |
|
|
| Delivery room | |
|
|
| Neonatal | |
|
|
Factors in the postnatal period associated with an increased risk of sepsis or septic shock include male gender, birth weight <1000 grams, hypogammaglobulinemia, intravenous alimentation, central venous catheters, use of steroids or drugs that decrease gastric acid acidity, and prolonged duration of mechanical ventilation. The development of severe necrotizing enterocolitis (NEC) is also associated with severe sepsis, shock, multi-organ system failure and death34, 35. Genetic evaluations in children and adults have identified a number of polymorphisms in cytokines and their receptors as well as other host defense proteins that may either increase or decrease risk for sepsis or poor outcome from sepsis 36–41. However, gene polymorphism studies in neonates have not yielded consistent results due to relatively small sample sizes and a general lack of formal prospective validation studies42–56.
Microbiology of sepsis and septic shock in neonates
A number of pathogens have been associated with sepsis in the neonatal period. The predominant agents are bacterial, but viruses including herpes simplex and enteroviruses have been associated fulminant neonatal sepsis with high mortality57–59. In one study, gram-negative infection accounted for 38% of cases of septic shock and 62.5% of sepsis mortality7. These results are similar to those from a previous study that showed Gram-negative infection was associated with 69% of cases of fulminant septic shock (death within 48 hours) 60. Gram-positive etiologies of sepsis are dominated by GBS and coagulase-negative staphylococcus (CoNS)3, 61. While lethality and shock from GBS have been well described, mortality associated with CoNS is extremely low3, 4 and septic shock is rare60. Fungi (primarily Candida albicans) may also lead to fulminant neonatal sepsis and predominantly affect ELBW infants3, 62, 63. It is important to note that studies of neonatal sepsis are confounded by the limitations of sensitivity of the current diagnostic “gold standard” blood culture. Sample volume constraints in newborns may undermine the identification of organisms causing shock, particularly in preterm infants64. For this reason, many studies combine the entities “culture-proven sepsis” and “clinical sepsis” (cultures negative but strong clinical suspicion leading to long-term antibiotic treatment). Improved techniques such as molecular diagnostics, discussed in Chapter xx, may help to delineate which patients with “clinical sepsis” truly have sepsis versus other causes of clinical deterioration.
Pathophysiology of sepsis and shock:Molecular and cellular events
Molecular signaling: PRRs, PAMPs, and DAMPs
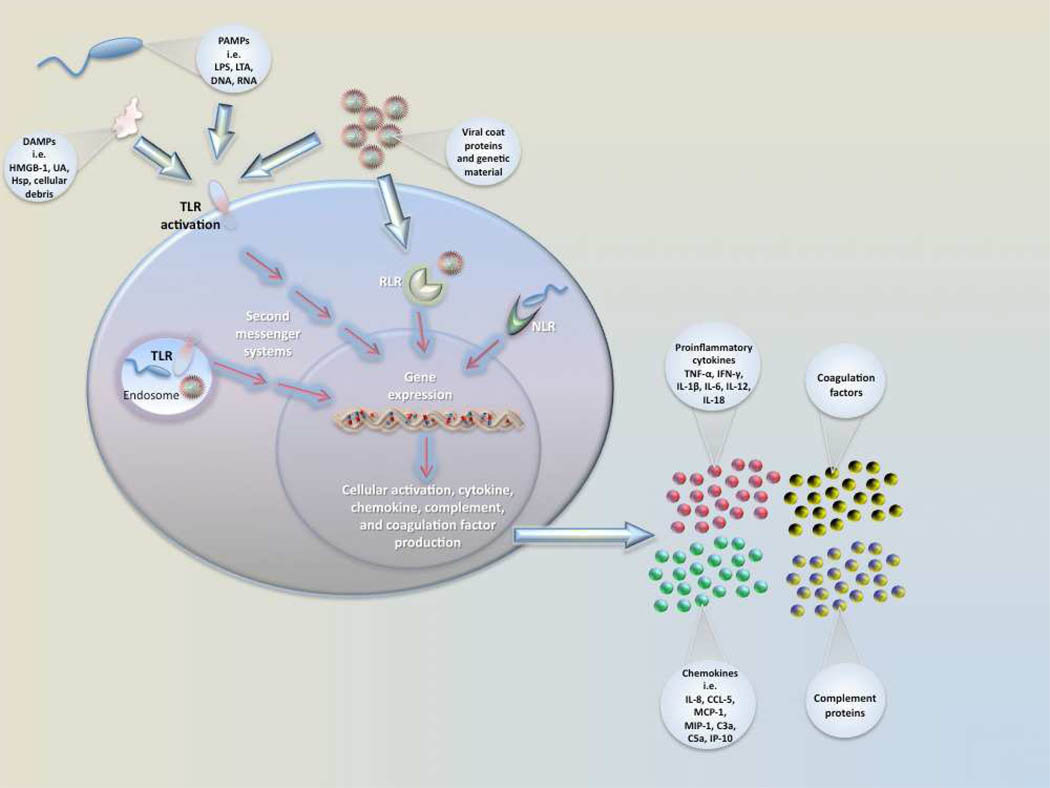
Pathogen recognition by local immune sentinel cells is the first step towards the development of an immune response once local barrier function has been compromised [Figure 1]. Recognition is initiated via the activation of pattern recognition receptors (PRRs)65 including Toll-like receptors (TLRs). There are 10 known TLRs in humans, and each receptor has a specific molecular activation trigger66, 67. TLRs, present on and within multiple cell types, recognize extracellular and intracellular pathogens by their signature microbial products known as pathogen associated molecular patterns (PAMPs). Lipopolysaccharide (LPS, endotoxin) on gram negative bacteria is the prototypic PAMP and a key mediator of systemic inflammation, septic shock, and multi-organ failure and death68. LPS signals primarily through TLR4 in conjunction with the cell surface adaptor proteins CD14 and MD265. Gram positive bacterial PAMPS such as lipoteichoic acid signal primarily through TLR2, while viral PAMPS such as double-stranded RNA signal through TLR3. Microorganisms often stimulate more than one TLR simultaneously allowing for initiation of a pathogen-specific host response67, 69. Ligandreceptor binding results in downstream production of cytokines and chemokines as well as activation of other antimicrobial effector mechanisms66.
Figure 1. Activation of sentinel immune cells.
Sentinel cells (e.g.. monocyte, macrophage) sense pathogens via PAMPs or DAMPs binding to PRRs. Pathogen recognition receptors (PRRs) include TLRs (Toll-like receptor), RLRs (Rig-1-like receptors), and NLRs (NOD-like receptors). Pathogen associated molecular patterns (PAMPs) include LPS (lipopolysaccharide), LTA (lipotechoic acid), DNA, and RNA. Damage/Danger associated molecular patterns (DAMPs) can also be sensed through TLRs and include uric acid (UA), heat shock proteins (Hsp), and HMGB-1. Signaling occurs through a series of second messengers and results in transcription and translation of cytokines and chemokines that amplify the immune response.
Intracellular non-TLR PRRs include NOD-like receptors (NLRs) and RIG-like receptors (RLRs). Nucleotide-binding oligomerization domain (a NLR) detects peptidoglycan of gram positive bacteria in the cytosol, and retinoic-acid-inducible protein I (RIG-I) detects viral double-stranded RNA and induce type I interferon production67. Once engaged by pathogens, these PRRs initiate an immune response including the production of proinflammatory cytokines via mitogen activated protein kinase (MAPK) and the transcription factor nuclear factor κB (NF-κB). To date, RLR and NLR function have not been examined in neonates with sepsis.
Since TLRs play an essential role in recognition and response to pathogens, alterations in their expression, structure, signaling pathways, and function can have consequences to host defense. Polymorphisms or mutations in TLRs are associated with increased risk for infection in adults70–73 and in children74–76 but are less well characterized in neonates. Upregulation of TLR2 and TLR4 mRNA in leukocytes of neonates occurs during Gram-positive and Gram-negative infection, respectively, across gestational ages77. Dysregulation or overexpression of TLR4 is involved in the development of necrotizing enterocolitis in experimental animal models78, demonstrating the importance of TLRs in the initial immune response to pathogens and their role in neonatal sepsis and septic shock. Mutations have been identified in NLRs that are involved in the pathogenesis of Neonatal-Onset Multisystem Inflammatory Disease (cryopyrin)79. Investigation for mutations in specific domains of NLRs has been performed to identify causes of abnormal inflammatory signaling leading to NEC, but no associations have been identified80. RLR mutations have been identified but are of unknown clinical significance81. The role that intracellular PRR play is of particular interest with respect to defense against Listeria monocytogenes, a pathogen particularly virulent in neonates, which can be recognized by NLRs82.
Mutations or decreased expression of co-stimulatory molecules necessary for TLR activation are also associated with an increased risk for infection. For example, the lipopolysaccharide (LPS, endotoxin) co-receptor CD14 and LPS binding protein (LBP, which binds intravascular LPS and facilitates its attachment to CD14) are both increased during neonatal sepsis83–85. Genetic variations in these proteins have been associated with increased risk for sepsis in adults47, 49, 50. Gene polymorphisms in myeloid differentiation-2 (MD-2), a small protein involved in LPS signaling through TLR4, increase the risk for organ dysfunction and sepsis in adults86 but the significance in neonates is unknown. Polymorphisms in select cytokines (IL-6 and IL-10) or their receptors (IL-4ra53), and constituents of their signaling pathways, may be associated with increased risk of infection42, 43, 46, 51, though there is not complete agreement on these findings44, 52, 54. Polymorphisms in post-TLR activation intracellular signaling molecules including myeloid differentiation factor 88(MyD88)87, IL-1-receptor-associated kinase 4(IRAK-4)88, and NF-κB essential modulator (NEMO)89 are associated with invasive bacterial infection in older populations. These genetic factors predisposing to sepsis are likely just the tip of the iceberg as evaluation of intracellular second messenger inflammatory signaling systems is a relatively new and active area of research.
In addition to being activated by PAMPs, TLRs can be activated by DAMPs (damage or danger associated molecular patterns). such as intracellular proteins or mediators released by dying or damaged cells [Figure 1]. High mobility group box-1 (HMGB-1), an important DAMP, is involved in the progression of sepsis to septic shock68, 90. HMGB-1 is produced by macrophages or endothelial cells stimulated with LPS or TNF-α and signals through TLR2, TLR4, and receptor for advanced glycation end products (RAGE) 91. Important actions of HMGB-1 include cytokine production, activation of coagulation, and neutrophil recruitment90, 92. HMGB-1 mediates disruption of epithelial junctions within the gut via the induction of reactive nitrogen intermediates (RNI) leading to increased bacterial translocation93. The role of HMGB-1 and RAGE signaling in septic shock in human neonates has not been well studied, but has been linked to the pathophysiology of NEC in a preclinical model94.
Other DAMPs including heat shock proteins (Hsps) and uric acid may also contribute to the pathophysiology of septic shock. Hsps activate proinflammatory signaling through TLRs, regulate neutrophil function, are immune adjuvants, and are elevated in septic adults and children95. Elevated Hsp60 and Hsp70 measured within 24 hours of PICU admission was associated with pediatric septic shock and there was a strong trend towards a significant association with death96, 97. Hsp production in septic neonates has not been evaluated. Uric acid can increase cytokine production, PMN recruitment, and dendritic cell stimulation98 and may also serve as an antioxidant99. Uric acid is reduced in the serum of septic neonates as compared to control neonates100. The importance of DAMPs in neonatal sepsis and shock has yet to be determined.
Cytokines, Chemokines, and Adhesion molecules
Following PRR stimulation, production of cytokines and chemokines results in amplification of the innate response directed at the invading organisms [Figure 1]. Elevations of pro-inflammatory cytokines during sepsis and septic shock have been identified including interleukin (IL)-1β, IL-6, IL-8, IL-12, IL-18, interferon gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α)101. Compared to septic adults, septic neonates produce less IL-1β, TNF-α, IFN-γ, and IL-12102–107. The decreased cytokine production is due in part to decreased production of important intracellular mediators of TLR signaling including Myeloid Differentiation Factor 88 (MyD88), Interferon Regulatory Factor 5 (IRF5), and p38 which exhibit gestational age-specific diminution108. In a recent comprehensive study (>140 analytes) of serum from neonates evaluated for late-onset sepsis, IL-18 emerged as a predictive biomarker to differentiate infected from non-infected neonates 109, similar to data from adults with sepsis110. IL-18 reduces PMN apoptosis111, potentiates IFN-g production112, and induces production of TNF-α, IL-1β, and IL-8113. IL-18 primes PMNs for degranulation with production of reactive oxygen intermediates (ROI) on subsequent stimulation114. Dysregulation of many of these functions linked to IL-18 are seen in sepsis and septic shock.
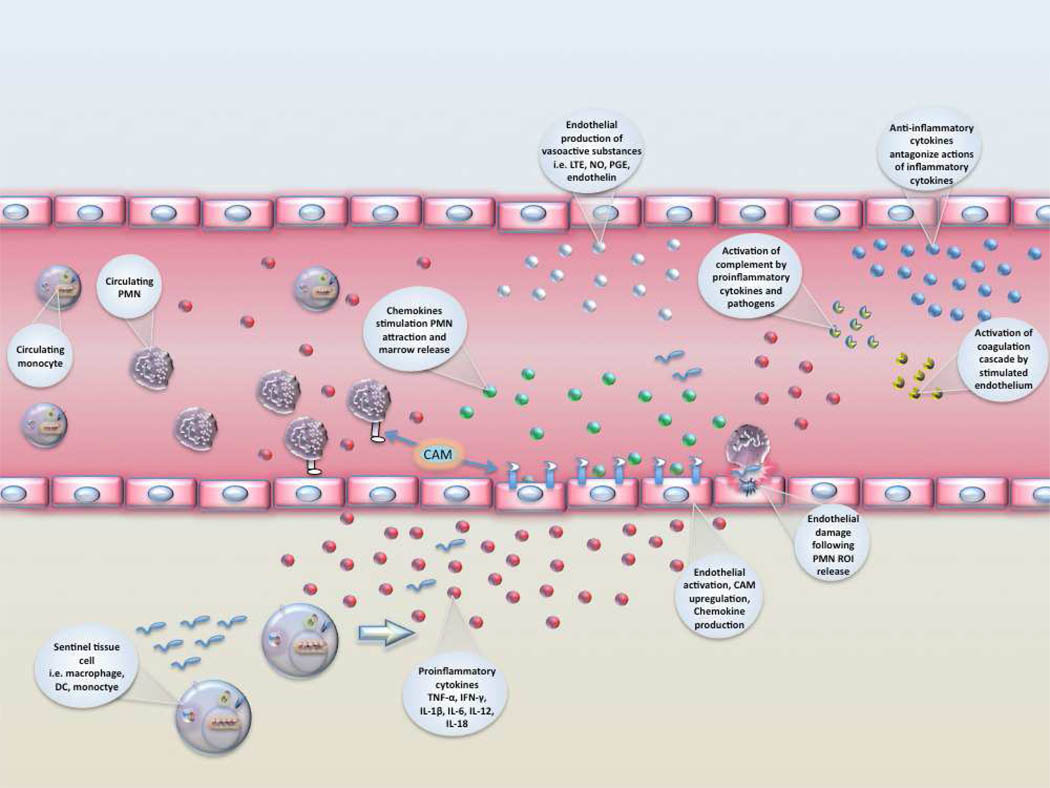
Pro-inflammatory cytokine production leads to activation of endothelial cells including increased expression of cell adhesion molecules (CAMs) that facilitate leukocyte recruitment and diapedesis [Figure 2]. Upregulation of CAMs [soluble ICAM, VCAM, L, P, and E-selectins, and CD11b/CD18] during sepsis facilitates rolling and extravascular migration of leukocytes115–118. Decreased neonatal PMN and monocyte L-selectin and MAC-1 expression impairs accumulation at sites of inflammation119, 120. Chemokine gradients produced by endothelial cells and local macrophages are necessary in addition to CAM interactions for effective and specific leukocyte attraction and accumulation. Without adequate leukocyte recruitment, there is increased risk for propagation from a local to a systemic infection. Although poor cellular chemotaxis in the neonate has been observed, it is not likely a result of reduced serum concentrations of chemokines.121. Suboptimal chemotaxis may be related to other mechanisms such as poor complement receptor upregulation following stimulation122, deficiencies in another downstream signaling process123, or inhibition by bacterial products124.
Figure 2. Cellular recruitment and endothelial activation following pathogen detection.
Pathogen-stimulated tissue/blood monocytes, dendritic cells (DC), and macrophages release proinflammatory cytokines that activate the surrounding endothelium. Endothelial activation results in upregulation of cell adhesion molecules (CAM), production of chemokines and vasoactive substances, activation of complement, and development of a procoagulant state. Recruitment of PMNs occurs along the chemokine gradient surrounding the area of inflammation. Anti-inflammatory cytokines counter the actions of proinflammatory cytokines to prevent excessive cellular activation and recruitment that can result in tissue damage and systemic inflammation. Endothelium can be damaged when PMNs release reactive oxygen intermediates (ROI). LTE-leukotriene, NO-nitric oxide, PMN-neutrophil.
A wide variety of chemokines are increased during sepsis including IP-10, CCL5 (RANTES), MCP-1, MIP-1, and IL-8125. Other chemo-attractive molecules are also increased in sepsis including complement proteins C3a and C5a, host defense proteins or peptides such as cathelicidins and defensins, and components of invading bacteria themselves101, 109. The role of chemoattractive substances in the pathogenesis of severe sepsis is highlighted by recent studies showing IL-8 can be used a stratifying factor for survival.in children126 and C5a is implicated in sepsis-associated organ dysfunction in adults68. Studies of chemokines in neonates with sepsis have shown that IP-10 is a sensitive early marker of infection 125, and decreased levels of CCL5 help predict development of DIC127.
Anti-inflammatory Response
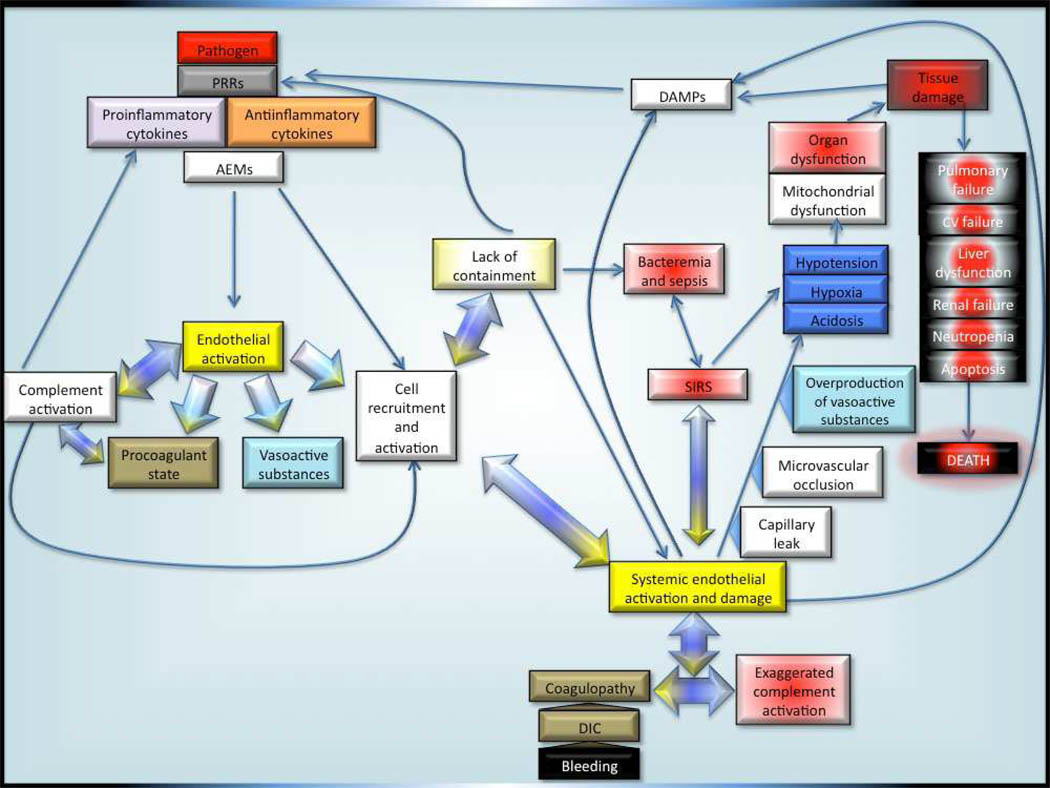
If inflammatory homeostasis is not restored, the consequences can include a systemic inflammatory response syndrome (SIRS) associated with multi-organ failure and death [Figure 3]. The careful interplay between anti and pro-inflammatory stimuli serves to govern the immune response to allow local pathogen containment but prevent systemic activation leading to excessive inflammatory damage though SIRS128. To this end, near simultaneous increases in anti-inflammatory cytokine production occurs during infection, with TGF-β, IL-4, IL-10, IL-11, and IL-13 countering the actions of pro-inflammatory cytokines 101, 129, 130 [Figure 2]. These mediators blunt the activation of phagocytic cells, block fever, modify coagulation factor expression, and decrease production of ROI/RNI, NO, and other vasoactive mediators131–135. In addition to the anti-inflammatory cytokines, specific soluble cytokines and receptor antagonists produced during sepsis modulate pro-inflammatory mediator action, including TNFR2 (which regulates the concentration of TNF-α), sIL-6R, sIL2, and IL-1ra. Elevations of these inhibitors have been documented in neonatal sepsis with resolution following effective treatment130, 136, 137. The role of these regulatory cytokine inhibitors in the immune response to neonatal sepsis and septic shock has been incompletely characterized. Soluble RAGE (sRAGE) competes with cell-bound RAGE for the binding of HMGB-1 and other RAGE ligands138, reduces the intensity of the inflammatory response, and is elevated in adults during sepsis139. In addition, administration of exogenous sRAGE improved survival and reduced inflammation in infected adult rodents140.
Figure 3. Pathophysiology of neonatal sepsis and septic shock.
PRR-Pattern recognition receptors; AEM-antimicrobial effector mechanisms; DAMP-Danger/damage-associated molecular patterns; SIRS-Systemic inflammatory response syndrome; DIC-disseminated intravascular coagulation; CV-cardiovascular.
Role of complement in host defense and sepsis pathophysiology
Complement is an extraordinarily important component of early innate immunity that facilitates killing of bacteria through opsonization and direct microbicidal activity. Complement components also possess chemotactic or anaphylactic activity that increases leukocyte aggregation and local vascular permeability at the site of invasion. In addition, complement components reciprocally activate a number of other important processes such as coagulation, proinflammatory cytokine production, and leukocyte activation [Figure 3]68. Dysregulation of complement activation may participate in the untoward effects seen in neonates with severe sepsis or septic shock. Neonates, particularly the very premature, exhibit decreased basal levels of complement proteins and function for both the alternative and classic pathways141, 142. Additionally, complement-mediated opsonization is poor in premature neonates and limited in term neonates143, 144.
Complement-mediated activation of leukocytes during sepsis occurs via upregulated cell surface receptors (CR1 [CD35], CR3 [Mac-1, CD11b/CD18])145, 146. For example, stimulation of CR1 and C5aR, the receptors for C3b and C5a respectively, facilitate opsonization (CR1-C3b), redistribution of blood flow, increased inflammation, platelet aggregation, and release of ROI (C5a–C5aR)147, 148. Additionally, activation of the multifunctional CR3 facilitates leukocyte adhesion, phagocytosis, migration and activation, as well as recognition of a broad range of microbial products149. Upregulation of CR3 on neutrophils following stimulation is blunted in neonates compared to adults and is believed to play a significant role in diminished chemotaxis and transmigration122. Similar to the effects of TLR stimulation, C5a–mediated local leukocyte activation also results in increased cytokine production with subsequent upregulation of adhesion molecules on vascular endothelium allowing for increased cell recruitment to the site of infection150. Deficiencies in C5aR found in term neonates as compared to adults may limit the ability to respond to C5a and therefore increase the likelihood of infection151. The expression of C5aR on neutrophils of preterm infants has not been quantified.
Complement regulatory proteins modify the effects of complement and prevent potential damage due to over-activation. In particular, CD59 blocks formation of C9 polymerization and target lysis, CD55 destabilizes CR1 and C3 and C5 convertases, and CD35 (CR1) accelerates the deactivation of C3b152. The role of these regulators in the neonatal response to sepsis and septic shock is presently unknown. Dysregulation of complement activation can lead to a vicious activation cycle that results in excessive cellular stimulation, cytokine production, endothelial cell activation, and local tissue damage. Dysregulation likely contributes to the development of SIRS and shock [Figure 3]153.
Data in adults link elevated C5a levels with multiple facets of sepsis-associated pathology such as the development of disseminated intravascular coagulation (DIC) via increased tissue factor expression, cardiomyopathy, increased pro-inflammatory cytokine levels and the development of SIRS, adrenal insufficiency, and neutrophil dysfunction68. Whether or not C5a or other complement proteins play a role in the development of these phenomena in septic neonates remains to be determined.
Other host defense proteins, acute phase reactants, and opsonins
In addition to the initial inflammatory response and complement activation following pathogen recognition, presence of microbes result in increases in other innate proteins that possess valuable immune function154. These components serve to reduce bacterial load and include collectins (e.g. surfactant proteins A and D), lactoferrin, cathelicidins, bacteriocidal permeability increasing protein (BPI), and phospholipase A2155. Acute phase reactant proteins such as CRP (opsonin), haptoglobin and lactoferrin (reduce available iron/antimicrobial peptidelactoferricin), serum amyloid A (cellular recruitment), procalcitonin (unknown function), and others increase during sepsis and provide useful ancillary immune functions101. Neutrophils from term neonates are deficient in BPI, potentially contributing to the increased risk for infection156. Polymorphisms in BPI increase the risk for Gram-negative sepsis in children157, although the impact of these polymorphisms in neonates is unknown. Sepsis results in an increase in other serum components with opsoninic function including fibronectin and natural antibodies (predominantly IgM) produced by circulating B1 lymphocytes158–160. Despite these increases, neonatal plasma has significantly impaired opsonizing activity compared to adults that increases the likelihood of progression to systemic infection161.
Role of dysregulated coagulation in severe sepsis
Development of a pro-coagulant state in the microvasculature surrounding a focal site of infection is a natural host defense mechanism, trapping invading pathogens and preventing further dissemination [Figure 2]. However, like the inflammatory response, if the pro-coagulant response to infection escalates unchecked, it can lead to disseminated intravascular coagulation (DIC) resulting in severe tissue and organ damage. [Figure 3] (DIC)162. Neonates with early elevated ratios of serum inflammatory to anti-inflammatory cytokines during sepsis have an increased risk of developing DIC127. This finding is consistent with the elevated serum levels of IL-659 and high frequency of DIC seen with disseminated HSV infection163.
Initiation of coagulation cascades during infection may begin with activated neutrophils, monocytes, or endothelium, which express increased tissue factor apoprotein164, 165.Activation of tissue factor leads to increased clotting proteins including thrombin-antithrombin complex (TAT), plasminogen activator inhibitor (PAI), and plasmin-α2-antiplasmin complex166. There is also a shift towards inactivation of protein S and depletion of anticoagulant proteins including antithrombin III (ATIII) and protein C167, 168. A small study demonstrated low protein C levels in preterm neonates with sepsis predicted death169. In DIC, platelets are consumed in microthrombi creating a state of thrombocytopenia; a very common finding in infected neonates170. The longest duration and lowest initial and nadir platelet levels have been noted during neonatal Gram-negative and fungal infections171, and this thrombocytopenia may or may not be associated with DIC. Decreased platelet function in preterm neonates with sepsis further increases the risk for bleeding172. In ELBW infants, platelets are hyporeactive for the first few days after birth, complicating the ability of the immune system to contain a microbiological threat and increasing the risk for hemorrhage173.
Role of the neutrophil in septic shock
The most important means of early innate cellular defense against bacterial invasion in neonates is the neutrophil or polymorphonuclear leukocyte (PMN). Neonatal PMNs exhibit quantitative and qualitative deficits as compared to adult cells174, 175. A complete discussion of these deficits is presented within chapter XXXX in this issue. Three aspects of PMN function with particular relevance to neonatal severe sepsis and septic shock deserve brief mention: neutropenia, decreased deformability, and delayed apoptosis.
Rapid depletion of neonatal marrow PMN reserves during infection176 can lead to neutropenia with consequent impaired antimicrobial defenses and significantly increased risk for death177. Neutropenia is particularly common in Gram-negative sepsis in neonates178. Release of immature neutrophil forms (bands) which have even greater dysfunction than mature neonatal neutrophils179 can further predispose to adverse outcomes. PMN respiratory burst activity is also suppressed during sepsis and may contribute to poor microbicidal activity180–182.
PMNs of neonates have reduced deformability compared to PMNs of adults, which, combined with the low blood pressure/flow state associated with septic shock, increases the risk of microvascular occlusion174, 183. Irreversible aggregation of newborn PMNs in the vascular space leads to decreased diapedesis, rapid depletion of bone marrow reserves, vascular crowding183, and increased likelihood of compromised tissue perfusion 184 leading to organ dysfunction.
Neutrophils, while essential for combating pathogens, can also cause significant tissue damage and thus play a role in progression from sepsis to multi-organ system dysfunction. Reactive oxygen and nitrogen intermediates and proteolytic enzymes produced by PMNs can be released extracellularly, via activation of membrane associated-NADPH oxidase. Extracellular release of these reactive intermediates and enzymes can lead to destruction of non-phagocytized bacteria but also can cause local tissue destruction185. Increased levels of neutrophil elastase as well as the neutrophil activators urokinase plasminogen activator, and urokinase plasminogen activator receptor have been described in infected neonates109. Compared to adult PMNs, neonatal PMNs exhibit delayed apoptosis186, 187 as well as sustained capacity for activation (CD11b upregulation) and cytotoxic function (ROI production) that contributes to tissue damage188. Neutrophil-mediated damage may include endothelial and lung injury (including surfactant inactivation189) [Figure 2] in addition to other organ dysfunction [Figure 3].
Other innate cellular contributions to sepsis
Many other cells besides neutrophils are involved in the development of an immune response to infection, but the role that these cells play in the development of neonatal septic shock is incompletely characterized. Monocytes, macrophages, and dendritic cells amplify cellular recruitment through production of inflammatory mediators, phagocytosis and killing of pathogens, and antigen presentation to cells of the adaptive immune system. Important substances produced by stimulated monocytes that may contribute to septic shock include complement components, cytokines (both pro and anti-inflammatory), coagulation factors, and extracellular matrix proteins [Figure 1]190. The role of NK cells in neonatal bacterial sepsis is incompletely defined. Despite activation191, NK cytotoxicity is deficient in both sepsis and recurrent infections192, 193. Circulating NK cells are decreased with neonatal shock194. Further studies are necessary to more clearly define the role of NK cells in neonatal sepsis and shock.
Mast cells play a role in the response to pathogen invasion via production of histamines (which promote vasodilation and upregulation of P-selectin) and cytokines (TNF-α, IL-1α/β), and by promoting neutrophil recruitment, direct bacterial phagocytosis, and antigen presentation195. The production of histamine by mast cells likely contributes to the vasodilation associated with septic shock. Like eosinophils and PMNs, mast cells of adults are also capable of bacterial killing via generation of extracellular traps, like the neutrophil NETS described previously 196. This means of immune protection has not been investigated in neonates. Mast cells may also alter adaptive immune function by patterning the TH2 immunosuppressive phenotype seen in the neonate and therefore contribute to the increased risk of infection. Immature dendritic cells exposed to histamine and LPS during maturation exhibit altered T-cell polarizing activity with predominance of TH2 phenotype via increased production of IL-10 and decreased production of IL-12197. Furthermore, compared to mast cells of adults198 stimulated mast cells from neonates secrete significantly more histamine which may contribute to vasodilation and the development of shock199.
Role of the endothelium and vasoactive mediators in septic shock
Vascular endothelium has not historically been considered part of the innate cellular defenses, but recent studies have shown the importance of these sentinel cells in the early recognition and containment of microbial invasion. The endothelium can be a two-edged sword, however, as excessive activation can lead to vascular dilation and leak which are a driving forces behind the severe consequences of septic shock [Figure 3] 124,200.
Expression of TLRs allows endothelium to become activated in the presence of microbial components, leading to production of cytokines, chemokines, and adhesion molecules which attract circulating leukocytes and facilitate adherence124. Vasoactive substances released from activated leukocytes, platelets, and endothelial cells are shown in Figure 2 and include platelet-activating factor (PAF), thromboxane (TBX), leukotrienes (LTE), nitric oxide (NO), histamine, bradykinin, and prostaglandins (PGE)201, 202. Activated PMNs produce phospholipase A2 (PLA2), which is elevated in the serum of neonates with sepsis203 and leads to generation of vasoactive substances including PGE and LTE. Thromboxane produced by activated platelets and endothelin produced by activated endothelium 204 are potent vasoconstrictors that participate in the development of PPHN205–208. Systemic overproduction of cytokines and vasoactive substances is associated with circulatory alterations and organ failure seen in severe sepsis and septic shock [Figure 3]25, 209–212. For example, the balance of NO and endothelin-1 (ET-1) may be disrupted with endothelial damage, favoring the constrictive effects of ET-1 leading to ischemia and injury. This may explain in part why NO inhibitors increased mortality in adults with septic shock213.
Activated or damaged endothelium establishes a prothrombotic environment that can result in local microvascular occlusion165 or progress to DIC214. Endothelial cell apoptosis, detachment from the lamina, and alterations in vascular tone combine to promote capillary leak of proteins and fluid leading to hypovolemia and shock215. The role of endothelial activation during sepsis and septic shock in neonates, particularly in the premature infant, has not been thoroughly investigated. Adhesion molecules E and P selectin, expressed and secreted by activated endothelium, are increased in the serum of septic neonates109 and likely reflect significant endothelial activation. Toxins from GBS have been shown to damage pulmonary endothelium216 and likely participate in pulmonary complications associated with GBS pneumonia such as ARDS and pulmonary hypertension (PPHN)217. Using transgenic mice, it was recently shown that pulmonary endothelial cells sense bloodborne bacteria and their products124 while alveolar macrophages patrol the airspaces for pathogens218. These data help to explain in part the occurrence of ARDS and PPHN associated with severe sepsis in the absence of a primary pulmonary infectious focus.
Pathophysiology of septic shock: Cardiovascular and other organ effects
Cardiovascular effects
The hemodynamic response to sepsis has been less well characterized in premature and term neonates compared with children and adults, and the hemodynamic abnormalities are significantly more variable219. Factors contributing to developmental differences in hemodynamic responses include altered structure and function of cardiomyocytes, limited ability to increase stroke volume and contractility, and contributions of the transition from fetal to neonatal circulation220. A patent ductus arteriosus (PDA) and the presence of PPHN are significant modifying factors for the management of hypotension and hypoxia. In preterm infants with a PDA, aggressive volume administration to treat low blood pressure may lead to fluid overload, pulmonary edema, or heart failure. In the term infant with severe PPHN, on the other hand, aggressive volume and vasoactive medication administration to maintain a normal blood pressure may be beneficial by reducing right to left shunting and improving oxygenation. Although cardiomyopathy and heart failure may occasionally complicate sepsis in neonates, underlying coronary artery disease or other chronic cardiac conditions often present in septic adults do not complicate septic shock in the neonate.
In adults, septic shock is most commonly characterized by reduced systemic vascular resistance and elevated cardiac index221. In children, a nonhyperdynamic state with reduced cardiac output and increased systemic vascular resistance is most common219, 222–224. The hemodynamic presentation in neonates is much more variable219 and complicated by an unclear association between a “normal” blood pressure and adequate systemic blood flow225, 226. Abnormal peripheral vasoregulation with or with out myocardial dysfunction are the primary mechanisms for the hypotension accompanying septic shock in the neonate227. Neonates with sepsis may present with tachycardia, poor perfusion and “normal” blood pressure (high SVR) or with hypotension and either adequate perfusion (warm shock, vasodilation) or inadequate perfusion (cold shock, vasoconstriction). These distinctions may be important for directing appropriate therapy to restore tissue perfusion, as discussed later.
Multi-organ dysfunction syndrome
Septic shock that leads to multi-organ failure or MODS carries a dismal prognosis. Poor cardiac output and microcirculatory failure, sometimes combined with formation of microthrombi and DIC, can lead to compromised perfusion to the kidney228, 229, liver230, gut231, and CNS232 Figure 3]59, 210, 233, 234 Recent studies suggest that the mechanism of organ failure in sepsis may relate to decreased oxygen utilization associated with mitochondrial dysfunction rather than or in addition to poor oxygen delivery to tissues235, 236. Many other organ systems can be compromised in the setting of septic shock. Pulmonary complications include acute respiratory distress syndrome237, secondary surfactant deficiency238, pulmonary edema, pneumonia23, and PPHN, 220, 237. Endocrine abnormalities may include adrenal insufficiency associated with refractory hypotension239 and altered thyroid function240. Lymphocyte loss secondary to thymic involution and splenocyte apoptosis may also be present and may lead to a state of immune compromise following the acute phase of sepsis241–246. The importance of this finding has been shown in infected adults247–249, but the impact in neonates in whom adaptive immune function is immature is unknown. In a transgenic mouse model, neonatal animals lacking an adaptive immune system showed no difference in survival with polymicrobial sepsis compared to wild-type controls. This is in stark contrast to findings in adult mice250. Hematologic findings during severe sepsis may include thrombocytopenia170, neutropenia177, and coagulation abnormalities including disseminated intravascular coagulation162. Finally, sepsis can lead to metabolic and nutritional consequences. Increased energy expenditure and oxygen consumption251 and decreased mitochondrial oxidative function precipitated by hypoxia and the presence of damaging free radicals may lead to impaired growth and energy failure252, 253. The importance of providing optimum nutritional support in septic adults and children is increasingly recognized and should be considered in septic neonates as well.
Treatment of sepsis and septic shock
Initial resuscitation
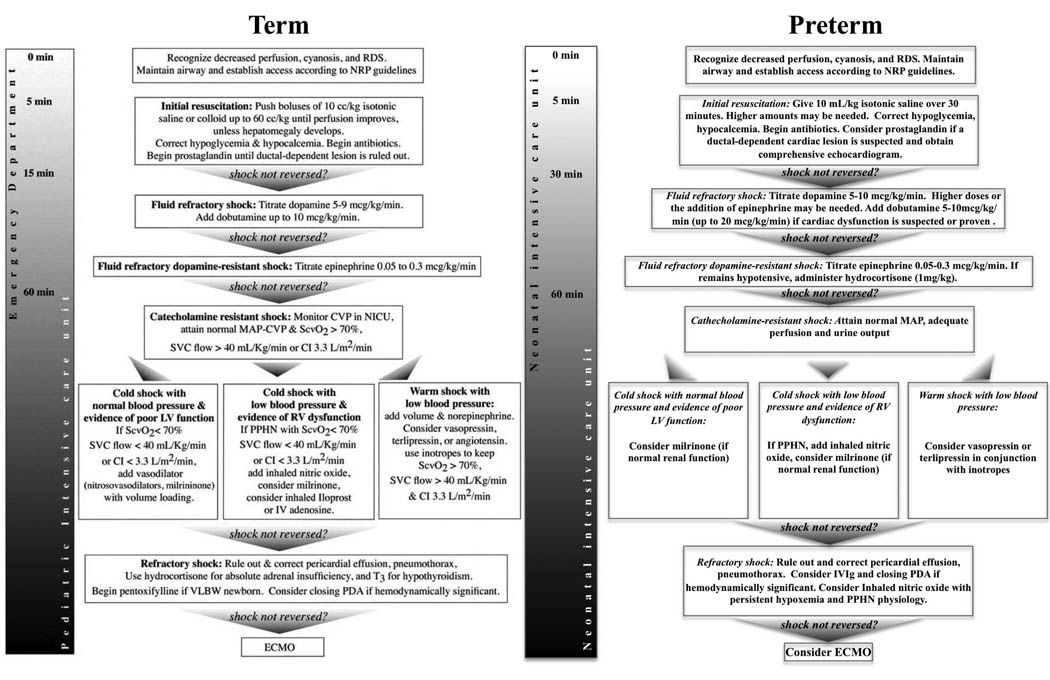
Treatment guidelines for the management of severe sepsis and septic shock have been established for adults254 children and term neonates255, but no such consensus guidelines exist for preterm neonates. We have attempted to incorporate the special circumstances related to premature physiology into the framework of treatment guidelines for term infants [Figure 4]. Development, testing, and acceptance of consensus guidelines for classification and management of preterm neonates with sepsis and septic shock are urgently needed in order to more systematically assess, diagnose, and treat these conditions.
Figure 4. ACCCM consensus guidelines for treatment of shock in term infants and suggested modifications for preterm infants.
RDS-respiratory distress syndrome, NRP-Neonatal Resuscitation Program, CVP-central venous pressure, MAP-mean arterial pressure, ScvO2-central venous oxygen saturation, SVC-superior vena cava, CI-cardiac index, VLBWvery low birth weight, PDA-patent ductus arteriosus, PPHN-persistent pulmonary hypertension of the newborn.
As with all emergencies in neonatology, management of septic shock begins with airway, breathing, and circulation. Septic neonates often present with apnea or severe respiratory distress and may require intubation3, 4. Following establishment of a secure airway and maintenance of lung volume for adequate gas exchange, administration of antibiotics and continuing assessment for cardiovascular dysfunction is critical. Shortly after birth, an umbilical vein catheter can be used for resuscitation but beyond this time, other peripheral or central venous access is essential for volume resuscitation, antibiotic administration, and pressor therapy. Timely therapy, including rapid restoration of adequate tissue perfusion, has been shown to improve outcomes in adults and children with sepsis and should be the goal in neonates as well.
Therapeutic Endpoints
In absence of widely available or well-tested methods for quantifying hemodynamic compromise in septic shock in neonates, clinicians generally rely on vital signs and physical examination for decisions about therapy. Although mean arterial pressure (MAP) may not reflect systemic blood flow, monitoring blood pressure and other measures such as capillary refill time and urine output provide indirect information on the adequacy of organ blood flow. Suggestions for cardiovascular therapeutic endpoints in term neonates include a capillary refill time of < 2 seconds, normal pulses without differential between peripheral and central pulses, warm extremities, urine output greater than 1ml/kg/hr, low serum lactate, and mixed venous saturation of >70%256. Therapeutic endpoints in premature neonates have not been established but the goals for term infants seem reasonable. ELBW infants present the greatest challenge for determination of therapeutic endpoints in septic shock. Assessment of mean arterial pressure, urine output and capillary refill may not be particularly useful determinates of systemic blood flow in ELBW infants, particularly in the first 72 hours of life257. In addition, the contribution of fetal hemoglobin may complicate accurate determination of central venous oxygen saturation (ScvO2) in neonates. ScvO2 obtained using hemoglobin A calibration is 4–7% higher compared to ScvO2 that accounts for fetal hemoglobin258 implying that perhaps the goal ScvO2 should be different in neonates than in older patients for optimum tissue oxygen delivery.
In the future, monitoring techniques such as functional echocardiography (FE) and near-infrared spectroscopy (NIRS) may provide physiologic data to optimize management of septic shock. FE provides a bedside means to assess cardiac output, peripheral vascular resistance, and organ blood flow in response to volume, colloid, and vasoactive medications259, 260. FE can also be used to assess superior vena cava (SVC) flow, which has been suggested as a surrogate marker for cerebral blood flow261 and should be maintained ≥40ml/kg/min262. Prolonged decreases in SVC flow are associated with impaired neurodevelopmental outcome in very preterm neonates263. In the absence of FE to monitor SVC flow, A capillary refill time of >4 seconds combined with a serum lactate concentration of >4 mmol/L had a specificity of 97% for identifying VLBW infants with a low SVC flow state on the first day of life264. NIRS can be used to monitor end organ perfusion non-invasively265 and is used often in neonates with congenital heart disease266. A combination of FE and NIRS, in conjunction with traditional measures (MAP, SpO2, capillary refill, urine output) as well as intermittent laboratory evaluations of tissue perfusion such as pH, mixed venous saturation, lactate, and base deficit would be ideal for monitoring severity of septic shock and response to therapy.
Management of hypotension and cardiovascular support
An algorithm for time-sensitive, goal-directed stepwise management of hemodynamic support for the term newborn with septic shock has been established and should be followed255. Preterm neonates require specific caveats to this algorithm due to their unique physiology and risk for complications [Figure 4].
In contrast to term neonates, the definition of hypotension and shock in preterm neonates is less clear, particularly in the immediate newborn period26. Blood pressure may be a poor indicator of systemic blood flow in preterm neonates225, yet objective measures of adequacy of tissue perfusion and oxygenation delivery are lacking. Another confounding variable in the management of neonatal shock is that inotrope use (dopamine, dobutamine) in hypotensive preterm neonates has not been shown to significantly improve short or long-term outcomes227, 267, 268. These considerations notwithstanding, and in absence of evidence of harm, some neonatologists advocate treating hypotension in preterm neonates to achieve a mean arterial pressure (MAP) of greater than or equal to 30mm Hg. This goal MAP is based in part on a small study showing improved cerebral blood flow autoregulation above this threshold269. However, a gestational age-based cutoff for “normal” blood pressure (goal MAP > GA) is used at many tertiary centers, especially in the first 3 days after birth. Clearly, more studies are required to determine whether targeting a specific blood pressure improves outcomes in preterm infants.
Once a decision is made to treat hypotension with or without shock in a neonate, the recommended initial step is a fluid bolus (crystalloid). Though there is less data in neonates to support this intervention, it remains the accepted clinical practice to treat and monitor closely for signs of intravascular volume depletion227. In term infants or older preterm infants, aggressive volume expansion (20–40 ml/kg) should be considered. In contrast to outcomes with early aggressive fluid resuscitation in older populations270, there is insufficient evidence to support early volume expansion in very preterm neonates271 and there is a significant risk of intracranial hemorrhage associated with rapid volume expansion in the first few days after birth272. In hypotensive preterm neonates, it is recommended that a single bolus of saline (10–20ml/kg over 30–60 minutes) be given and if further intervention is necessary to begin vasoactive medications268. In cases of obvious acute volume loss in preterm infants, more volume may be needed.
Dopamine is generally the first line vasoactive drug, with a starting dose of 5–10µg/kg/minute227 and dose escalation as needed. For neonates with shock, which is unresolved with volume resuscitation and dopamine, several possibilities exist for additional therapy, including glucocorticoids (see below), other catecholamines, and inotropes/vasodilators. Epinephrine or norepinephrine infusions for refractory shock in neonates have been studied to a very limited extent. Neonates with vasodilatory shock may have a positive response to the alpha-adrenergic vasoconstrictive effect of these agents. A recent report in term neonates showed the addition of noradrenaline to existing therapy (after fluid loading and dopamine or dobutamine infusion) resulted in increased blood pressure and decreased tissue lactate18. In another study, low-dose epinephrine was found as effective as low/moderate-dose dopamine for increasing blood pressure, cerebral blood volume, and cerebral oxygen delivery in VLBW infants273. Patients with depressed myocardial function may benefit from infusion of dobutamine for both inotropy and vasodilation. In a study of 42 preterm neonates with low systemic blood flow (as determined by low superior vena cava flow262) in the first 24h after birth, dobutamine treatment improved and maintained systemic blood flow better than dopamine274, 275. As a caution, dobutamine, particularly in high doses, can increase myocardial oxygen demand due to β1 adrenergic stimulation. Dobutamine also has chronotropic actions and severe tachycardia may lead to decreased cardiac output that may be corrected by decreasing the dose. Milrinone, a phosphodiesterase inhibitor and inodilator, has not been studied in neonatal septic shock but has been used in pediatric patients with septic shock276 277. In a study of patients aged 9 months-15 years with volume-resuscitated catecholamine-resistant nonhyperdynamic septic shock milrinone increased cardiac index, stroke volume, and oxygen delivery and decreased systemic vascular resistance without increasing heart rate or blood pressure276. Another alternative agent for treating septic shock is the vasoconstrictor arginine-vasopressin (AVP) or its longer half-life analogue terlipressin278. In a report of six ELBW infants, AVP improved MAP and urine output in patients with septic shock but not in those with non-septic shock279.
Hydrocortisone treatment in neonatal septic shock
Induced by proinflammatory cytokines, endogenous cortisol attenuates the intensity of the systemic inflammatory response associated with severe sepsis and septic shock280. Studies in adults have shown that high-dose glucocorticoid therapy does not impact sepsis mortality while low-dose therapy may be beneficial254. In one randomized clinical trial, low-dose cortisol treatment in conjunction with standard of care measures was associated with a reduction in mortality in adults with septic shock and adrenal insufficiency281. In another study in adults, cortisol treatment sped the reversal of septic shock but had no effect on mortality282.
Cortisol production in the neonate is significantly increased early in septic shock283. However, very preterm neonates can have relative adrenal insufficiency that may contribute to hemodynamic instability and hypotension. In many clinical practices, hydrocortisone is the third-line agent in treatment of neonatal shock after volume resuscitation and dopamine227, 268, 284. In addition to its cytokine-suppressing effects, hydrocortisone has been shown to increase the sensitivity of the cardiovascular system to endogenous or exogenous catecholamines, resulting in improvements in myocardial contractility, stroke volume, effective circulating blood volume, systemic vascular resistance, and urine output. Hydrocortisone has not been evaluated in prospective randomized clinical trials for the treatment of septic shock in the neonate, but it has been shown to increase blood pressure, decrease heart rate and decrease vasoactive medication requirements in preterm and term neonates284, 285. If hydrocortisone treatment is considered, obtaining a pre-treatment serum cortisol level is prudent in order to differentiate contributing causes of hypotension. The reader is referred to a recent review on the diagnosis and treatment of adrenal insufficiency in the premature neonate286.
Pulmonary support
Increased inspired oxygen may be necessary in the setting of neonatal septic shock to maximize tissue oxygen delivery. Decreased pulmonary function (RDS) and/or respiratory failure (apnea) in conjunction with increased tissue demand (increased respiratory and metabolic activity associated with acidosis) contribute to tissue hypoxia. Mechanical ventilation can improve gas exchange through maintenance of lung volume and decreased work of breathing. Administration of exogenous surfactant to neonates with severe pneumonia has been shown to improve oxygenation and gas exchange and reduce the need for ECMO 238.In extremely sick neonates, consideration should be given to maintaining a normal or near-normal pH and oxygen saturations in the 90’s rather than allowing permissive hypercapnia and lower saturations which is standard practice in healthy preterm neonates. Normalizing pH and arterial oxygen content may improve cardiac contractility and improve tissue oxygen content, thus decreasing the risk of multiorgan dysfunction and the risk of pulmonary hypertension. Infants with sepsis and PPHN may require inhaled nitric oxide (iNO) in addition to optimized ventilation strategies such as high frequency oscillatory ventilation287. If oxygenation or tissue perfusion remain severely compromised despite optimal medical management, extracorporeal membrane oxygenation (ECMO) should be considered in neonates >2 kg without contraindications such as presence of or high risk for acute hemorrhage 288, 289.
Other supportive care of neonates with septic shock
Avoidance of hypothermia and hypoglycemia is important in neonates with septic shock. With the exception of patients with acute perinatal hypoxic ischemic encephalopathy290, normothermia should be maintained on a radiant warmer. Use of a 10% glucose solution delivering 4–6 mg/kg/min of glucose combined with frequent monitoring to ensure normoglycemia is recommended. Correction of a significant coagulopathy and anemia (hemoglobin ≤10 g/dL) through the transfusion of fresh frozen plasma or packed red blood cells may also serve to improve blood pressure291 and oxygen delivery. The importance of providing adequate protein and calories to the infant with sepsis and septic shock cannot be overstated. Increased energy demands promote catabolism if adequate nutrition is not provided. Premature neonates have decreased muscles mass and energy reserves as well as higher baseline nutritional requirements as compared to term neonates292. Elevation of serum triglycerides during sepsis293 and increased serum oxygen-derived free radicals associated with infusions of lipid have prompted some clinicians to withhold or decrease intralipid infusions. A recent study showed concurrent administration of intralipids in neonates with infection is not associated with hypertriglyceridemia in the absence of liver dysfunction or fetal growth restriction294. It is suggested that intralipid infusions during sepsis or septic shock in neonates be accompanied by careful monitoring of serum triglycerides to avoid hypertriglyceridemia. Maintenance of a carbohydrate to lipid ratio of ~3:1 increases fat utilization and decreases production of oxygen-derived free radicals to levels seen with fat exclusion295. Protein intakes of 2–3g/kg/day are generally not associated with azotemia, hyperammonemia, or metabolic acidosis296 in the setting of sepsis, but monitoring of blood urea nitrogen is recommended. Monitoring liver and renal function is important for assessing the effectiveness of therapies to improve tissue perfusion and for making decisions about dosing medications that require modification for elimination.
Alternative immunologic and pharmacotherapies for neonatal sepsis/shock
There have been many attempts directed at improving outcomes of sepsis and septic shock in neonates via immunomodulation. A complete review of adjunct immunologic therapies in neonatal sepsis is provided in Chapter xxxx.
Outcomes with sepsis and septic shock
The outcome of septic shock in the neonate is dismal. One study reported death or severe sequelae in 52% of infants, with only 28% of infants < 1000grams alive and free of disability at 18 months of age7. Variables predictive of mortality include cardiac dysfunction manifested as refractory shock, acute renal failure, neutropenia, increased prothrombin time, excessive bleeding, metabolic acidosis, and hypothermia231, 297.
Neurodevelopmental outcomes following neonatal sepsis, without stratification for shock, have been studied in some detail and demonstrate significant risk for impairment, particularly in the most premature neonates298. VLBW infants with sepsis, compared with those without, have been reported to have significantly increased mortality (21% vs. 9%), longer hospital stay (98 vs. 58 days) and a higher risk of chronic lung disease31. ELBW infants are at especially high risk for sepsis-associated adverse neurodevelopmental outcomes, including deafness, cerebral palsy, lower mental and psychomotor development scores, and vision impairment299, 300. In a study of preterm infants, white matter abnormality on MRI at term corrected age predicted neurodevelopmental impairment in those with sepsis compared to those without301. Surgical NEC, which is often accompanied by SIRS or shock, has been associated with significant growth delay and adverse neurodevelopmental outcomes at 18–22 months302. A study of ELBW infants with systemic Candidiasis found that 73% died or developed a neurodevelopmental impairment63 including retinopathy303. These data show that the toll of neonatal sepsis and septic shock reaches far beyond the acute complications of organ dysfunction and mortality.
Future considerations
The field of neonatal sepsis is wide open for translational and clinical research. Definitions for the sepsis continuum and treatment algorithms specific for preterm infants should be developed to improve the quality of clinical trials and facilitate metaanalyses of prophylactic and therapeutic interventions. Systems biology and genomic and proteomic studies have yielded important data on septic shock in older populations304–312 and the utilization of these modern techniques in the study of neonatal inflammation and response to pathogen challenge has begun 109, 138, 313. With further research, real-time sampling using only microliters of blood will allow rapid identification of highest-risk patients, pathogen-specific responses, and sepsis-staging biomarkers.314. Immaturities of immune function and physiology in the neonate necessitate developmental stage-specific evaluations of sepsis pathophysiology and treatment. Exploration of adjuvant treatments including LPS binding proteins (rBPI315, sCD14 or anti-CD14316), anti-inflammatory therapies (pentoxifylline317, nicotinic stimulation318, statins319), synthetic host defense peptides (rhSP-D320, lactoferrin321, 322), combination therapies323 (i.e. IVIg and colony stimulating factor), and innate immune priming using TLR agonists250 may yield improved outcomes. Advances in these areas are urgently needed and are likely to substantially improve long-term outcomes.
Summary
Neonatal septic shock is a devastating condition associated with high morbidity and mortality, Definitions for the sepsis continuum and treatment algorithms specific for premature neonates are needed to improve studies of septic shock and assess benefit from clinical interventions. Unique features of the immature immune system and pathophysiologic responses to sepsis, particularly those of extremely preterm infants, necessitate that clinical trials consider them as a separate group. Keen clinical suspicion and knowledge of risk factors will help to identify those neonates at greatest risk for development of septic shock. Genomic and proteomic approaches, particularly those that utilize very small sample volumes, will increase our understanding of the pathophysiology and direct the development of novel agents for prevention and treatment of severe sepsis and shock in the neonate. Although at present antimicrobial therapy and supportive care remain the foundation of treatment, in the future immunomodulatory agents are likely to improve outcomes for this vulnerable population.
Acknowledgement
The authors thank Associate Professor C. Michael Cotten, MD, MHS for his review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005 Mar 5–11;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002 Jul 25;347(4):240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002 Aug;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005 Jul;24(7):635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 5.Haque KN, Khan MA, Kerry S, Stephenson J, Woods G. Pattern of culture-proven neonatal sepsis in a district general hospital in the United Kingdom. Infect Control Hosp Epidemiol. 2004 Sep;25(9):759–764. doi: 10.1086/502473. [DOI] [PubMed] [Google Scholar]
- 6.Haque KN. Defining common infections in children and neonates. J Hosp Infect. 2007 Jun;65 Suppl 2:110–114. doi: 10.1016/S0195-6701(07)60026-7. [DOI] [PubMed] [Google Scholar]
- 7.Kermorvant-Duchemin E, Laborie S, Rabilloud M, Lapillonne A, Claris O. Outcome and prognostic factors in neonates with septic shock. Pediatr Crit Care Med. 2008 Mar;9(2):186–191. doi: 10.1097/PCC.0b013e31816689a8. [DOI] [PubMed] [Google Scholar]
- 8.Furman WL, Menke JA, Barson WJ, Miller RR. Continuous naloxone infusion in two neonates with septic shock. J Pediatr. 1984 Oct;105(4):649–651. doi: 10.1016/s0022-3476(84)80441-2. [DOI] [PubMed] [Google Scholar]
- 9.Togari H, Mikawa M, Iwanaga T, et al. Endotoxin clearance by exchange blood transfusion in septic shock neonates. Acta Paediatr Scand. 1983 Jan;72(1):87–91. doi: 10.1111/j.1651-2227.1983.tb09669.x. [DOI] [PubMed] [Google Scholar]
- 10.Fenton LJ, Strunk RC. Complement activation and group B streptococcal infection in the newborn: similarities to endotoxin shock. Pediatrics. 1977 Dec;60(6):901–907. [PubMed] [Google Scholar]
- 11.Tollner U, Pohlandt F. Septicemia in the newborn due to gram-negative bacilli. Risk factors, clinical symptoms, and hematologic changes. Eur J Pediatr. 1976 Nov 3;123(4):243–254. doi: 10.1007/BF00444646. [DOI] [PubMed] [Google Scholar]
- 12.Frommhold D, Birle A, Linderkamp O, Zilow E, Poschl J. Drotrecogin alpha (activated) in neonatal septic shock. Scand J Infect Dis. 2005;37(4):306–308. doi: 10.1080/00365540510031412. [DOI] [PubMed] [Google Scholar]
- 13.Miyairi I, Berlingieri D, Protic J, Belko J. Neonatal invasive group A streptococcal disease: case report and review of the literature. Pediatr Infect Dis J. 2004 Feb;23(2):161–165. doi: 10.1097/01.inf.0000109887.40636.07. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed K, Sein PP, Shahnawaz M, Hoosen AA. Pasteurella gallinarum neonatal meningitis. Clin Microbiol Infect. 2002 Jan;8(1):55–57. doi: 10.1046/j.1469-0691.2002.00354.x. [DOI] [PubMed] [Google Scholar]
- 15.Roll C, Schmid EN, Menken U, Hanssler L. Fatal Salmonella enteritidis sepsis acquired prenatally in a premature infant. Obstet Gynecol. 1996 Oct;88(4 Pt 2):692–693. doi: 10.1016/0029-7844(96)00076-2. [DOI] [PubMed] [Google Scholar]
- 16.Wolfler A, Silvani P, Musicco M, Antonelli M, Salvo I. Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian Pediatric Intensive Care Units: a prospective national survey. Intensive Care Med. 2008 Sep;34(9):1690–1697. doi: 10.1007/s00134-008-1148-y. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Nunez A, Lopez-Herce J, Gil-Anton J, Hernandez A, Rey C. Rescue treatment with terlipressin in children with refractory septic shock: a clinical study. Crit Care. 2006 Feb;10(1):R20. doi: 10.1186/cc3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tourneux P, Rakza T, Abazine A, Krim G, Storme L. Noradrenaline for management of septic shock refractory to fluid loading and dopamine or dobutamine in full-term newborn infants. Acta Paediatr. 2008 Feb;97(2):177–180. doi: 10.1111/j.1651-2227.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 19.Filippi L, Poggi C, Serafini L, Fiorini P. Terlipressin as rescue treatment of refractory shock in a neonate. Acta Paediatr. 2008 Apr;97(4):500–502. doi: 10.1111/j.1651-2227.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 20.Meyer S, Loffler G, Polcher T, Gottschling S, Gortner L. Vasopressin in catecholamine-resistant septic and cardiogenic shock in very-low-birthweight infants. Acta Paediatr. 2006 Oct;95(10):1309–1312. doi: 10.1080/08035250500538973. [DOI] [PubMed] [Google Scholar]
- 21.McAdams RM, Garza-Cox S, Yoder BA. Early-onset neonatal pneumococcal sepsis syndrome. Pediatr Crit Care Med. 2005 Sep;6(5):595–597. doi: 10.1097/01.pcc.0000163677.58249.77. [DOI] [PubMed] [Google Scholar]
- 22.Matok I, Leibovitch L, Vardi A, et al. Terlipressin as rescue therapy for intractable hypotension during neonatal septic shock. Pediatr Crit Care Med. 2004 Mar;5(2):116–118. doi: 10.1097/01.pcc.0000112521.93714.b8. [DOI] [PubMed] [Google Scholar]
- 23.Aikio O, Vuopala K, Pokela ML, Hallman M. Diminished inducible nitric oxide synthase expression in fulminant early-onset neonatal pneumonia. Pediatrics. 2000 May;105(5):1013–1019. doi: 10.1542/peds.105.5.1013. [DOI] [PubMed] [Google Scholar]
- 24.Duke TD, Butt W, South M. Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med. 1997 Jun;23(6):684–692. doi: 10.1007/s001340050394. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005 Jan;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 26.Cayabyab R, McLean CW, Seri I. Definition of hypotension and assessment of hemodynamics in the preterm neonate. J Perinatol. 2009 May;29 Suppl 2:S58–S62. doi: 10.1038/jp.2009.29. [DOI] [PubMed] [Google Scholar]
- 27.Shah GS, Budhathoki S, Das BK, Mandal RN. Risk factors in early neonatal sepsis. Kathmandu Univ Med J (KUMJ) 2006 Apr-Jun;4(2):187–191. [PubMed] [Google Scholar]
- 28.Salem SY, Sheiner E, Zmora E, Vardi H, Shoham-Vardi I, Mazor M. Risk factors for early neonatal sepsis. Arch Gynecol Obstet. 2006 Jul;274(4):198–202. doi: 10.1007/s00404-006-0135-1. [DOI] [PubMed] [Google Scholar]
- 29.Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol. 1996 Feb;87(2):188–194. doi: 10.1016/0029-7844(95)00402-5. [DOI] [PubMed] [Google Scholar]
- 30.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999 Jun;103(6):e77. doi: 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 31.Fanaroff AA, Korones SB, Wright LL, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J. 1998 Jul;17(7):593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Schuchat A, Zywicki SS, Dinsmoor MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics. 2000 Jan;105(1 Pt 1):21–26. doi: 10.1542/peds.105.1.21. [DOI] [PubMed] [Google Scholar]
- 33.Escobar GJ, Li DK, Armstrong MA, et al. Neonatal sepsis workups in infants >/=2000 grams at birth: A population-based study. Pediatrics. 2000 Aug;106(2 Pt 1):256–263. doi: 10.1542/peds.106.2.256. [DOI] [PubMed] [Google Scholar]
- 34.Sharma R, Tepas JJ, 3rd, Hudak ML, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007 Mar;42(3):454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 35.Sonntag J, Wagner MH, Waldschmidt J, Wit J, Obladen M. Multisystem organ failure and capillary leak syndrome in severe necrotizing enterocolitis of very low birth weight infants. J Pediatr Surg. 1998 Mar;33(3):481–484. doi: 10.1016/s0022-3468(98)90092-6. [DOI] [PubMed] [Google Scholar]
- 36.Dahmer MK, Randolph A, Vitali S, Quasney MW. Genetic polymorphisms in sepsis. Pediatr Crit Care Med. 2005 May;6(3 Suppl):S61–S73. doi: 10.1097/01.PCC.0000161970.44470.C7. [DOI] [PubMed] [Google Scholar]
- 37.Cogulu O, Onay H, Uzunkaya D, et al. Role of angiotensin-converting enzyme gene polymorphisms in children with sepsis and septic shock. Pediatr Int. 2008 Aug;50(4):477–480. doi: 10.1111/j.1442-200X.2008.02583.x. [DOI] [PubMed] [Google Scholar]
- 38.Lyons EJ, Amos W, Berkley JA, et al. Homozygosity and risk of childhood death due to invasive bacterial disease. BMC Med Genet. 2009;10:55. doi: 10.1186/1471-2350-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liangos O, Jaber BL. Multiple organ dysfunction syndrome in children with sepsis: role of genetic factors. Semin Nephrol. 2008 Sep;28(5):499–509. doi: 10.1016/j.semnephrol.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Kumpf O, Schumann RR. Genetic influence on bloodstream infections and sepsis. Int J Antimicrob Agents. 2008 Nov;32 Suppl 1:S44–S50. doi: 10.1016/j.ijantimicag.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Sutherland AM, Walley KR. Bench-to-bedside review: Association of genetic variation with sepsis. Crit Care. 2009;13(2):210. doi: 10.1186/cc7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahrens P, Kattner E, Kohler B, et al. Mutations of genes involved in the innate immune system as predictors of sepsis in very low birth weight infants. Pediatr Res. 2004 Apr;55(4):652–656. doi: 10.1203/01.PDR.0000112100.61253.85. [DOI] [PubMed] [Google Scholar]
- 43.Baier RJ, Loggins J, Yanamandra K. IL-10, IL-6 and CD14 polymorphisms and sepsis outcome in ventilated very low birth weight infants. BMC Med. 2006;4:10. doi: 10.1186/1741-7015-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chauhan M, McGuire W. Interleukin-6 (−174C) polymorphism and the risk of sepsis in very low birth weight infants: meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008 Nov;93(6):F427–F429. doi: 10.1136/adc.2007.134205. [DOI] [PubMed] [Google Scholar]
- 45.Dzwonek AB, Neth OW, Thiebaut R, et al. The role of mannose-binding lectin in susceptibility to infection in preterm neonates. Pediatr Res. 2008 Jun;63(6):680–685. doi: 10.1203/PDR.0b013e31816fdbff. [DOI] [PubMed] [Google Scholar]
- 46.Gopel W, Hartel C, Ahrens P, et al. Interleukin-6-174-genotype, sepsis and cerebral injury in very low birth weight infants. Genes Immun. 2006 Jan;7(1):65–68. doi: 10.1038/sj.gene.6364264. [DOI] [PubMed] [Google Scholar]
- 47.Hartel C, Rupp J, Hoegemann A, et al. 159C>T CD14 genotype--functional effects on innate immune responses in term neonates. Hum Immunol. 2008 Jun;69(6):338–343. doi: 10.1016/j.humimm.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Hartel C, Schultz C, Herting E, Gopel W. Genetic association studies in VLBW infants exemplifying susceptibility to sepsis--recent findings and implications for future research. Acta Paediatr. 2007 Feb;96(2):158–165. doi: 10.1111/j.1651-2227.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 49.Hubacek JA, Stuber F, Frohlich D, et al. Gene variants of the bactericidal/permeability increasing protein and lipopolysaccharide binding protein in sepsis patients: gender-specific genetic predisposition to sepsis. Crit Care Med. 2001 Mar;29(3):557–561. doi: 10.1097/00003246-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Mollen KP, Gribar SC, Anand RJ, et al. Increased expression and internalization of the endotoxin coreceptor CD14 in enterocytes occur as an early event in the development of experimental necrotizing enterocolitis. J Pediatr Surg. 2008 Jun;43(6):1175–1181. doi: 10.1016/j.jpedsurg.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiman M, Kujari H, Ekholm E, Lapinleimu H, Lehtonen L, Haataja L. Interleukin-6 polymorphism is associated with chorioamnionitis and neonatal infections in preterm infants. J Pediatr. 2008 Jul;153(1):19–24. doi: 10.1016/j.jpeds.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Schueller AC, Heep A, Kattner E, et al. Prevalence of two tumor necrosis factor gene polymorphisms in premature infants with early onset sepsis. Biol Neonate. 2006;90(4):229–232. doi: 10.1159/000093605. [DOI] [PubMed] [Google Scholar]
- 53.Treszl A, Heninger E, Kalman A, Schuler A, Tulassay T, Vasarhelyi B. Lower prevalence of IL-4 receptor alpha-chain gene G variant in very-low-birth-weight infants with necrotizing enterocolitis. J Pediatr Surg. 2003 Sep;38(9):1374–1378. doi: 10.1016/s0022-3468(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 54.Treszl A, Kocsis I, Szathmari M, et al. Genetic variants of TNF-[FC12]a, IL-1beta, IL-4 receptor [FC12]a-chain, IL-6 and IL-10 genes are not risk factors for sepsis in low-birth-weight infants. Biol Neonate. 2003;83(4):241–245. doi: 10.1159/000069484. [DOI] [PubMed] [Google Scholar]
- 55.Treszl A, Kocsis I, Szathmari M, Schuler A, Tulassay T, Vasarhelyi B. Genetic variants of the tumour necrosis factor-alpha promoter gene do not influence the development of necrotizing enterocolitis. Acta Paediatr. 2001 Oct;90(10):1182–1185. doi: 10.1080/080352501317061611. [DOI] [PubMed] [Google Scholar]
- 56.Treszl A, Tulassay T, Vasarhelyi B. Genetic basis for necrotizing enterocolitis--risk factors and their relations to genetic polymorphisms. Front Biosci. 2006;11:570–580. doi: 10.2741/1819. [DOI] [PubMed] [Google Scholar]
- 57.Verboon-Maciolek MA, Krediet TG, Gerards LJ, Fleer A, van Loon TM. Clinical and epidemiologic characteristics of viral infections in a neonatal intensive care unit during a 12-year period. Pediatr Infect Dis J. 2005 Oct;24(10):901–904. doi: 10.1097/01.inf.0000180471.03702.7f. [DOI] [PubMed] [Google Scholar]
- 58.Verboon-Maciolek MA, Krediet TG, Gerards LJ, de Vries LS, Groenendaal F, van Loon AM. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008 Mar;27(3):241–245. doi: 10.1097/INF.0b013e31815c1b07. [DOI] [PubMed] [Google Scholar]
- 59.Kawada J, Kimura H, Ito Y, et al. Evaluation of systemic inflammatory responses in neonates with herpes simplex virus infection. J Infect Dis. 2004 Aug 1;190(3):494–498. doi: 10.1086/422325. [DOI] [PubMed] [Google Scholar]
- 60.Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988–1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics. 2000 Dec;106(6):1387–1390. doi: 10.1542/peds.106.6.1387. [DOI] [PubMed] [Google Scholar]
- 61.Hyde TB, Hilger TM, Reingold A, Farley MM, O'Brien KL, Schuchat A. Trends in incidence and antimicrobial resistance of early-onset sepsis: population-based surveillance in San Francisco and Atlanta. Pediatrics. 2002 Oct;110(4):690–695. doi: 10.1542/peds.110.4.690. [DOI] [PubMed] [Google Scholar]
- 62.Benjamin DK, DeLong E, Cotten CM, Garges HP, Steinbach WJ, Clark RH. Mortality following blood culture in premature infants: increased with Gram-negative bacteremia and candidemia, but not Gram-positive bacteremia. J Perinatol. 2004 Mar;24(3):175–180. doi: 10.1038/sj.jp.7211068. [DOI] [PubMed] [Google Scholar]
- 63.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006 Jan;117(1):84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 64.Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996 Aug;129(2):275–278. doi: 10.1016/s0022-3476(96)70254-8. [DOI] [PubMed] [Google Scholar]
- 65.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009 Apr;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumagai Y, Takeuchi O, Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008 Apr;14(2):86–92. doi: 10.1007/s10156-008-0596-1. [DOI] [PubMed] [Google Scholar]
- 67.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007 Mar;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 68.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008 Oct;8(10):776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007 Oct;68(10):813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Bochud PY, Chien JW, Marr KA, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med. 2008 Oct 23;359(17):1766–1777. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wurfel MM, Gordon AC, Holden TD, et al. Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med. 2008 Oct 1;178(7):710–720. doi: 10.1164/rccm.200803-462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002 Nov 15;186(10):1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 73.Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000 Nov;68(11):6398–6401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang SY, Jouanguy E, Ugolini S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007 Sep 14;317(5844):1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 75.Mockenhaupt FP, Cramer JP, Hamann L, et al. Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. J Commun Dis. 2006 Mar;38(3):230–245. [PubMed] [Google Scholar]
- 76.Faber J, Meyer CU, Gemmer C, et al. Human toll-like receptor 4 mutations are associated with susceptibility to invasive meningococcal disease in infancy. Pediatr Infect Dis J. 2006 Jan;25(1):80–81. doi: 10.1097/01.inf.0000195595.22547.fe. [DOI] [PubMed] [Google Scholar]
- 77.Zhang JP, Chen C, Yang Y. [Changes and clinical significance of Toll-like receptor 2 and 4 expression in neonatal infections] Zhonghua Er Ke Za Zhi. 2007 Feb;45(2):130–133. [PubMed] [Google Scholar]
- 78.Leaphart CL, Cavallo J, Gribar SC, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007 Oct 1;179(7):4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 79.Goldbach-Mansky R, Dailey NJ, Canna SW, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006 Aug 10;355(6):581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szebeni B, Szekeres R, Rusai K, et al. Genetic polymorphisms of CD14, toll-like receptor 4, and caspase-recruitment domain 15 are not associated with necrotizing enterocolitis in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2006 Jan;42(1):27–31. doi: 10.1097/01.mpg.0000192246.47959.b2. [DOI] [PubMed] [Google Scholar]
- 81.Shigemoto T, Kageyama M, Hirai R, Zheng J, Yoneyama M, Fujita T. Identification of loss of function mutations in human genes encoding RIG-I and mda5: Implications for resistance to type I diabetes. J Biol Chem. 2009 Mar 26; doi: 10.1074/jbc.M809449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Warren SE, Mao DP, Rodriguez AE, Miao EA, Aderem A. Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol. 2008 Jun 1;180(11):7558–7564. doi: 10.4049/jimmunol.180.11.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Behrendt D, Dembinski J, Heep A, Bartmann P. Lipopolysaccharide binding protein in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004 Nov;89(6):F551–F554. doi: 10.1136/adc.2003.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berner R, Furll B, Stelter F, Drose J, Muller HP, Schutt C. Elevated levels of lipopolysaccharide-binding protein and soluble CD14 in plasma in neonatal early-onset sepsis. Clin Diagn Lab Immunol. 2002 Mar;9(2):440–445. doi: 10.1128/CDLI.9.2.440-445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blanco A, Solis G, Arranz E, Coto GD, Ramos A, Telleria J. Serum levels of CD14 in neonatal sepsis by Gram-positive and Gram-negative bacteria. Acta Paediatr. 1996 Jun;85(6):728–732. doi: 10.1111/j.1651-2227.1996.tb14135.x. [DOI] [PubMed] [Google Scholar]
- 86.Gu W, Shan YA, Zhou J, et al. Functional significance of gene polymorphisms in the promoter of myeloid differentiation-2. Ann Surg. 2007 Jul;246(1):151–158. doi: 10.1097/01.sla.0000262788.67171.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.von Bernuth H, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008 Aug 1;321(5889):691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Picard C, Puel A, Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003 Mar 28;299(5615):2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 89.Ku CL, Picard C, Erdos M, et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J Med Genet. 2007 Jan;44(1):16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005 Apr;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 91.Mullins GE, Sunden-Cullberg J, Johansson AS, et al. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scand J Immunol. 2004 Dec;60(6):566–573. doi: 10.1111/j.0300-9475.2004.01518.x. [DOI] [PubMed] [Google Scholar]
- 92.van Zoelen MA, Yang H, Florquin S, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009 Mar;31(3):280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002 Sep;123(3):790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 94.Zamora R, Grishin A, Wong C, et al. High-mobility group box 1 protein is an inflammatory mediator in necrotizing enterocolitis: protective effect of the macrophage deactivator semapimod. Am J Physiol Gastrointest Liver Physiol. 2005 Oct;289(4):G643–G652. doi: 10.1152/ajpgi.00067.2005. [DOI] [PubMed] [Google Scholar]
- 95.Pack CD, Kumaraguru U, Suvas S, Rouse BT. Heat-shock protein 70 acts as an effective adjuvant in neonatal mice and confers protection against challenge with herpes simplex virus. Vaccine. 2005 May 20;23(27):3526–3534. doi: 10.1016/j.vaccine.2005.01.152. [DOI] [PubMed] [Google Scholar]
- 96.Wheeler DS, Lahni P, Odoms K, et al. Extracellular heat shock protein 60 (Hsp60) levels in children with septic shock. Inflamm Res. 2007 May;56(5):216–219. doi: 10.1007/s00011-007-6108-4. [DOI] [PubMed] [Google Scholar]
- 97.Wheeler DS, Fisher LE, Jr, Catravas JD, Jacobs BR, Carcillo JA, Wong HR. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med. 2005 May;6(3):308–311. doi: 10.1097/01.PCC.0000161075.97355.2E. [DOI] [PubMed] [Google Scholar]
- 98.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008 Apr;8(4):279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Batra S, Kumar R, Seema, Kapoor AK, Ray G. Alterations in antioxidant status during neonatal sepsis. Ann Trop Paediatr. 2000 Mar;20(1):27–33. doi: 10.1080/02724930092039. [DOI] [PubMed] [Google Scholar]
- 100.Kapoor K, Basu S, Das BK, Bhatia BD. Lipid peroxidation and antioxidants in neonatal septicemia. J Trop Pediatr. 2006 Oct;52(5):372–375. doi: 10.1093/tropej/fml013. [DOI] [PubMed] [Google Scholar]
- 101.Ng PC. Diagnostic markers of infection in neonates. Arch Dis Child Fetal Neonatal Ed. 2004 May;89(3):F229–F235. doi: 10.1136/adc.2002.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ng PC, Li K, Wong RP, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Child Fetal Neonatal Ed. 2003 May;88(3):F209–F213. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004 Oct 1;173(7):4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 104.Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hodge G, Hodge S, Haslam R, et al. Rapid simultaneous measurement of multiple cytokines using 100 microl sample volumes--association with neonatal sepsis. Clin Exp Immunol. 2004 Aug;137(2):402–407. doi: 10.1111/j.1365-2249.2004.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heper Y, Akalin EH, Mistik R, et al. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur J Clin Microbiol Infect Dis. 2006 Aug;25(8):481–491. doi: 10.1007/s10096-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 107.Atici A, Satar M, Cetiner S, Yaman A. Serum tumor necrosis factor-alpha in neonatal sepsis. Am J Perinatol. 1997 Aug;14(7):401–404. doi: 10.1055/s-2007-994168. [DOI] [PubMed] [Google Scholar]
- 108.Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007 Jan 15;195(2):296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 109.Kingsmore SF, Kennedy N, Halliday HL, et al. Identification of diagnostic biomarkers for infection in premature neonates. Mol Cell Proteomics. 2008 Oct;7(10):1863–1875. doi: 10.1074/mcp.M800175-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grobmyer SR, Lin E, Lowry SF, et al. Elevation of IL-18 in human sepsis. J Clin Immunol. 2000 May;20(3):212–215. doi: 10.1023/a:1006641630904. [DOI] [PubMed] [Google Scholar]
- 111.Hirata J, Kotani J, Aoyama M, et al. A role for IL-18 in human neutrophil apoptosis. Shock. 2008 Dec;30(6):628–633. doi: 10.1097/SHK.0b013e31817c0c69. [DOI] [PubMed] [Google Scholar]
- 112.Cusumano V, Midiri A, Cusumano VV, et al. Interleukin-18 is an essential element in host resistance to experimental group B streptococcal disease in neonates. Infect Immun. 2004 Jan;72(1):295–300. doi: 10.1128/IAI.72.1.295-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998 Feb 1;101(3):711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elbim C, Guichard C, Dang PM, et al. Interleukin-18 primes the oxidative burst of neutrophils in response to formyl-peptides: role of cytochrome b558 translocation and N-formyl peptide receptor endocytosis. Clin Diagn Lab Immunol. 2005 Mar;12(3):436–446. doi: 10.1128/CDLI.12.3.436-446.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dollner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J Clin Epidemiol. 2001 Dec;54(12):1251–1257. doi: 10.1016/s0895-4356(01)00400-0. [DOI] [PubMed] [Google Scholar]
- 116.Turunen R, Andersson S, Nupponen I, Kautiainen H, Siitonen S, Repo H. Increased CD11b-density on circulating phagocytes as an early sign of late-onset sepsis in extremely low-birth-weight infants. Pediatr Res. 2005 Feb;57(2):270–275. doi: 10.1203/01.PDR.0000148717.59861.2C. [DOI] [PubMed] [Google Scholar]
- 117.Figueras-Aloy J, Gomez-Lopez L, Rodriguez-Miguelez JM, et al. Serum soluble ICAM-1, VCAM-1, L-selectin, and P-selectin levels as markers of infection and their relation to clinical severity in neonatal sepsis. Am J Perinatol. 2007 Jun;24(6):331–338. doi: 10.1055/s-2007-981851. [DOI] [PubMed] [Google Scholar]
- 118.Kourtis AP, Lee FK, Stoll BJ. Soluble L-selectin, a marker of immune activation, in neonatal infection. Clin Immunol. 2003 Nov;109(2):224–228. doi: 10.1016/s1521-6616(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 119.McEvoy LT, Zakem-Cloud H, Tosi MF. Total cell content of CR3 (CD11b/CD18) and LFA-1 (CD11a/CD18) in neonatal neutrophils: relationship to gestational age. Blood. 1996 May 1;87(9):3929–3933. [PubMed] [Google Scholar]
- 120.Buhrer C, Graulich J, Stibenz D, Dudenhausen JW, Obladen M. L-selectin is down-regulated in umbilical cord blood granulocytes and monocytes of newborn infants with acute bacterial infection. Pediatr Res. 1994 Dec;36(6):799–804. doi: 10.1203/00006450-199412000-00020. [DOI] [PubMed] [Google Scholar]
- 121.Sullivan SE, Staba SL, Gersting JA, et al. Circulating concentrations of chemokines in cord blood, neonates, and adults. Pediatr Res. 2002 May;51(5):653–657. doi: 10.1203/00006450-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 122.Anderson DC, Rothlein R, Marlin SD, Krater SS, Smith CW. Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Blood. 1990 Dec 15;76(12):2613–2621. [PubMed] [Google Scholar]
- 123.Meade VM, Barese CN, Kim C, et al. Rac2 concentrations in umbilical cord neutrophils. Biol Neonate. 2006;90(3):156–159. doi: 10.1159/000092451. [DOI] [PubMed] [Google Scholar]
- 124.Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol. 2009 May;9(5):364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 125.Ng PC, Li K, Chui KM, et al. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr Res. 2007 Jan;61(1):93–98. doi: 10.1203/01.pdr.0000250207.95723.96. [DOI] [PubMed] [Google Scholar]
- 126.Wong HR, Cvijanovich N, Wheeler DS, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med. 2008 Aug 1;178(3):276–282. doi: 10.1164/rccm.200801-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ng PC, Li K, Leung TF, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006 Jun;52(6):1181–1189. doi: 10.1373/clinchem.2005.062075. [DOI] [PubMed] [Google Scholar]
- 128.Sriskandan S, Altmann DM. The immunology of sepsis. J Pathol. 2008 Jan;214(2):211–223. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- 129.Hartel C, Osthues I, Rupp J, et al. Characterisation of the host inflammatory response to Staphylococcus epidermidis in neonatal whole blood. Arch Dis Child Fetal Neonatal Ed. 2008 Mar;93(2):F140–F145. doi: 10.1136/adc.2007.124685. [DOI] [PubMed] [Google Scholar]
- 130.Sikora JP, Chlebna-Sokol D, Krzyzanska-Oberbek A. Proinflammatory cytokines (IL-6, IL-8), cytokine inhibitors (IL-6sR, sTNFRII) and anti-inflammatory cytokines (IL-10, IL-13) in the pathogenesis of sepsis in newborns and infants. Arch Immunol Ther Exp (Warsz) 2001;49(5):399–404. [PubMed] [Google Scholar]
- 131.Opal SM, Esmon CT. Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 2003 Feb;7(1):23–38. doi: 10.1186/cc1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995 Apr 21;270(16):9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 133.Brubaker JO, Montaner LJ. Role of interleukin-13 in innate and adaptive immunity. Cell Mol Biol (Noisy-le-grand) 2001 Jun;47(4):637–651. [PubMed] [Google Scholar]
- 134.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996 Oct 15;157(8):3627–3634. [PubMed] [Google Scholar]
- 135.Koj A. Termination of acute-phase response: role of some cytokines and anti-inflammatory drugs. Gen Pharmacol. 1998 Jul;31(1):9–18. doi: 10.1016/s0306-3623(97)00435-7. [DOI] [PubMed] [Google Scholar]
- 136.Spear ML, Stefano JL, Fawcett P, Proujansky R. Soluble interleukin-2 receptor as a predictor of neonatal sepsis. J Pediatr. 1995 Jun;126(6):982–985. doi: 10.1016/s0022-3476(95)70228-8. [DOI] [PubMed] [Google Scholar]
- 137.Dollner H, Vatten L, Linnebo I, Zanussi GF, Laerdal A, Austgulen R. Inflammatory mediators in umbilical plasma from neonates who develop early-onset sepsis. Biol Neonate. 2001 Jul;80(1):41–47. doi: 10.1159/000047118. [DOI] [PubMed] [Google Scholar]
- 138.Buhimschi CS, Bhandari V, Han YW, et al. Using proteomics in perinatal and neonatal sepsis: hopes and challenges for the future. Curr Opin Infect Dis. 2009 Jun;22(3):235–243. doi: 10.1097/QCO.0b013e32832a5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bopp C, Hofer S, Weitz J, et al. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res. 2008 Jun 1;147(1):79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 140.Liliensiek B, Weigand MA, Bierhaus A, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004 Jun;113(11):1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wolach B, Dolfin T, Regev R, Gilboa S, Schlesinger M. The development of the complement system after 28 weeks' gestation. Acta Paediatr. 1997 May;86(5):523–527. doi: 10.1111/j.1651-2227.1997.tb08924.x. [DOI] [PubMed] [Google Scholar]
- 142.Notarangelo LD, Chirico G, Chiara A, et al. Activity of classical and alternative pathways of complement in preterm and small for gestational age infants. Pediatr Res. 1984 Mar;18(3):281–285. doi: 10.1203/00006450-198403000-00014. [DOI] [PubMed] [Google Scholar]
- 143.Drossou V, Kanakoudi F, Diamanti E, et al. Concentrations of main serum opsonins in early infancy. Arch Dis Child Fetal Neonatal Ed. 1995 May;72(3):F172–F175. doi: 10.1136/fn.72.3.f172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller ME, Stiehm ER. Phagocytic, opsonic and immunoglobulin studies in newborns. Calif Med. 1973 Aug;119(2):43–63. [PMC free article] [PubMed] [Google Scholar]
- 145.Nupponen I, Andersson S, Jarvenpaa AL, Kautiainen H, Repo H. Neutrophil CD11b expression and circulating interleukin-8 as diagnostic markers for early-onset neonatal sepsis. Pediatrics. 2001 Jul;108(1):E12. doi: 10.1542/peds.108.1.e12. [DOI] [PubMed] [Google Scholar]
- 146.Berger M, O'Shea J, Cross AS, et al. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984 Nov;74(5):1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Snyderman R, Goetzl EJ. Molecular and cellular mechanisms of leukocyte chemotaxis. Science. 1981 Aug 21;213(4510):830–837. doi: 10.1126/science.6266014. [DOI] [PubMed] [Google Scholar]
- 148.Vogt W. Anaphylatoxins: possible roles in disease. Complement. 1986;3(3):177–188. doi: 10.1159/000467894. [DOI] [PubMed] [Google Scholar]
- 149.Ehlers MR. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000 Mar;2(3):289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 150.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007 Sep;171(3):715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nybo M, Sorensen O, Leslie R, Wang P. Reduced expression of C5a receptors on neutrophils from cord blood. Arch Dis Child Fetal Neonatal Ed. 1998 Mar;78(2):F129–F132. doi: 10.1136/fn.78.2.f129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lothian C, Dahlgren C, Lagercrantz H, Lundahl J. Different expression and mobilisation of the complement regulatory proteins CD35, CD55 and CD59 in neonatal and adult neutrophils. Biol Neonate. 1997;72(1):15–21. doi: 10.1159/000244461. [DOI] [PubMed] [Google Scholar]
- 153.Castellheim A, Lindenskov PH, Pharo A, Aamodt G, Saugstad OD, Mollnes TE. Meconium aspiration syndrome induces complement-associated systemic inflammatory response in newborn piglets. Scand J Immunol. 2005 Mar;61(3):217–225. doi: 10.1111/j.1365-3083.2005.01532.x. [DOI] [PubMed] [Google Scholar]
- 154.Wong HR, Doughty LA, Wedel N, et al. Plasma bactericidal/permeability-increasing protein concentrations in critically ill children with the sepsis syndrome. Pediatr Infect Dis J. 1995 Dec;14(12):1087–1091. doi: 10.1097/00006454-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 155.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007 May;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 156.Levy O, Martin S, Eichenwald E, et al. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics. 1999 Dec;104(6):1327–1333. doi: 10.1542/peds.104.6.1327. [DOI] [PubMed] [Google Scholar]
- 157.Michalek J, Svetlikova P, Fedora M, et al. Bactericidal permeability increasing protein gene variants in children with sepsis. Intensive Care Med. 2007 Dec;33(12):2158–2164. doi: 10.1007/s00134-007-0860-3. [DOI] [PubMed] [Google Scholar]
- 158.Dyke MP, Forsyth KD. Decreased plasma fibronectin concentrations in preterm infants with septicaemia. Arch Dis Child. 1993 May;68(5 Spec No):557–560. doi: 10.1136/adc.68.5_spec_no.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Romeo MG, Tina LG, Sciacca A, et al. [Decreased plasma fibronectin (pFN) level in preterm infants with infections] Pediatr Med Chir. 1995 Nov-Dec;17(6):563–566. [PubMed] [Google Scholar]
- 160.Kalayci AG, Adam B, Yilmazer F, Uysal S, Gurses N. The value of immunoglobulin and complement levels in the early diagnosis of neonatal sepsis. Acta Paediatr. 1997 Sep;86(9):999–1002. doi: 10.1111/j.1651-2227.1997.tb15187.x. [DOI] [PubMed] [Google Scholar]
- 161.Madden NP, Levinsky RJ, Bayston R, Harvey B, Turner MW, Spitz L. Surgery, sepsis, and nonspecific immune function in neonates. J Pediatr Surg. 1989 Jun;24(6):562–566. doi: 10.1016/s0022-3468(89)80506-8. [DOI] [PubMed] [Google Scholar]
- 162.Hathaway WE, Mull MM, Pechet GS. Disseminated intravascular coagulation in the newborn. Pediatrics. 1969 Feb;43(2):233–240. [PubMed] [Google Scholar]
- 163.Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001 Aug;108(2):223–229. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 164.Rivers RP, Cattermole HE, Wright I. The expression of surface tissue factor apoprotein by blood monocytes in the course of infections in early infancy. Pediatr Res. 1992 Jun;31(6):567–573. doi: 10.1203/00006450-199206000-00006. [DOI] [PubMed] [Google Scholar]
- 165.Markiewski MM, Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007 Apr;28(4):184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 166.Aronis S, Platokouki H, Photopoulos S, Adamtziki E, Xanthou M. Indications of coagulation and/or fibrinolytic system activation in healthy and sick very-low-birth-weight neonates. Biol Neonate. 1998 Nov;74(5):337–344. doi: 10.1159/000014051. [DOI] [PubMed] [Google Scholar]
- 167.Lauterbach R, Pawlik D, Radziszewska R, Wozniak J, Rytlewski K. Plasma antithrombin III and protein C levels in early recognition of late-onset sepsis in newborns. Eur J Pediatr. 2006 Sep;165(9):585–589. doi: 10.1007/s00431-006-0139-7. [DOI] [PubMed] [Google Scholar]
- 168.Roman J, Velasco F, Fernandez F, et al. Coagulation, fibrinolytic and kallikrein systems in neonates with uncomplicated sepsis and septic shock. Haemostasis. 1993 May-Jun;23(3):142–148. doi: 10.1159/000216867. [DOI] [PubMed] [Google Scholar]
- 169.Venkataseshan S, Dutta S, Ahluwalia J, Narang A. Low plasma protein C values predict mortality in low birth weight neonates with septicemia. Pediatr Infect Dis J. 2007 Aug;26(8):684–688. doi: 10.1097/INF.0b013e3180f616f0. [DOI] [PubMed] [Google Scholar]
- 170.Sola MC, Del Vecchio A, Rimsza LM. Evaluation and treatment of thrombocytopenia in the neonatal intensive care unit. Clin Perinatol. 2000 Sep;27(3):655–679. doi: 10.1016/s0095-5108(05)70044-0. [DOI] [PubMed] [Google Scholar]
- 171.Guida JD, Kunig AM, Leef KH, McKenzie SE, Paul DA. Platelet count and sepsis in very low birth weight neonates: is there an organism-specific response? Pediatrics. 2003 Jun;111(6 Pt 1):1411–1415. doi: 10.1542/peds.111.6.1411. [DOI] [PubMed] [Google Scholar]
- 172.Finkelstein Y, Shenkman B, Sirota L, et al. Whole blood platelet deposition on extracellular matrix under flow conditions in preterm neonatal sepsis. Eur J Pediatr. 2002 May;161(5):270–274. doi: 10.1007/s00431-002-0938-4. [DOI] [PubMed] [Google Scholar]
- 173.Bednarek FJ, Bean S, Barnard MR, Frelinger AL, Michelson AD. The platelet hyporeactivity of extremely low birth weight neonates is age-dependent. Thromb Res. 2009 May;124(1):42–45. doi: 10.1016/j.thromres.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 174.Urlichs FSC. Neutrophil function in preterm and term infants. NeoReviews. 2004;5:e417–e430. [Google Scholar]
- 175.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006 Feb-Mar;118(2–3):137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 176.Christensen RD, Rothstein G. Exhaustion of mature marrow neutrophils in neonates with sepsis. J Pediatr. 1980 Feb;96(2):316–318. doi: 10.1016/s0022-3476(80)80837-7. [DOI] [PubMed] [Google Scholar]
- 177.Engle WA, McGuire WA, Schreiner RL, Yu PL. Neutrophil storage pool depletion in neonates with sepsis and neutropenia. J Pediatr. 1988 Oct;113(4):747–749. doi: 10.1016/s0022-3476(88)80394-9. [DOI] [PubMed] [Google Scholar]
- 178.Sarkar S, Bhagat I, Hieber S, Donn SM. Can neutrophil responses in very low birth weight infants predict the organisms responsible for late-onset bacterial or fungal sepsis? J Perinatol. 2006 Aug;26(8):501–505. doi: 10.1038/sj.jp.7211554. [DOI] [PubMed] [Google Scholar]
- 179.Christensen RD, Bradley PP, Rothstein G. The leukocyte left shift in clinical and experimental neonatal sepsis. J Pediatr. 1981 Jan;98(1):101–105. doi: 10.1016/s0022-3476(81)80553-7. [DOI] [PubMed] [Google Scholar]
- 180.Drossou V, Kanakoudi F, Tzimouli V, et al. Impact of prematurity, stress and sepsis on the neutrophil respiratory burst activity of neonates. Biol Neonate. 1997;72(4):201–209. doi: 10.1159/000244485. [DOI] [PubMed] [Google Scholar]
- 181.Shigeoka AO, Santos JI, Hill HR. Functional analysis of neutrophil granulocytes from healthy, infected, and stressed neonates. J Pediatr. 1979 Sep;95(3):454–460. doi: 10.1016/s0022-3476(79)80535-1. [DOI] [PubMed] [Google Scholar]
- 182.Wright WC, Jr, Ank BJ, Herbert J, Stiehm ER. Decreased bactericidal activity of leukocytes of stressed newborn infants. Pediatrics. 1975 Oct;56(4):579–584. [PubMed] [Google Scholar]
- 183.Linderkamp O, Ruef P, Brenner B, Gulbins E, Lang F. Passive deformability of mature, immature, and active neutrophils in healthy and septicemic neonates. Pediatr Res. 1998 Dec;44(6):946–950. doi: 10.1203/00006450-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 184.Mease AD, Burgess DP, Thomas PJ. Irreversible neutrophil aggregation. A mechanism of decreased newborn neutrophil chemotactic response. Am J Pathol. 1981 Jul;104(1):98–102. [PMC free article] [PubMed] [Google Scholar]
- 185.Ohman L, Tullus K, Katouli M, Burman LG, Stendahl O. Correlation between susceptibility of infants to infections and interaction with neutrophils of Escherichia coli strains causing neonatal and infantile septicemia. J Infect Dis. 1995 Jan;171(1):128–133. doi: 10.1093/infdis/171.1.128. [DOI] [PubMed] [Google Scholar]
- 186.Allgaier B, Shi M, Luo D, Koenig JM. Spontaneous and Fas-mediated apoptosis are diminished in umbilical cord blood neutrophils compared with adult neutrophils. J Leukoc Biol. 1998 Sep;64(3):331–336. doi: 10.1002/jlb.64.3.331. [DOI] [PubMed] [Google Scholar]
- 187.Hanna N, Vasquez P, Pham P, et al. Mechanisms underlying reduced apoptosis in neonatal neutrophils. Pediatr Res. 2005 Jan;57(1):56–62. doi: 10.1203/01.PDR.0000147568.14392.F0. [DOI] [PubMed] [Google Scholar]
- 188.Koenig JM, Stegner JJ, Schmeck AC, Saxonhouse MA, Kenigsberg LE. Neonatal neutrophils with prolonged survival exhibit enhanced inflammatory and cytotoxic responsiveness. Pediatr Res. 2005 Mar;57(3):424–429. doi: 10.1203/01.PDR.0000153945.49022.96. [DOI] [PubMed] [Google Scholar]
- 189.Nupponen I, Pesonen E, Andersson S, et al. Neutrophil activation in preterm infants who have respiratory distress syndrome. Pediatrics. 2002 Jul;110(1 Pt 1):36–41. doi: 10.1542/peds.110.1.36. [DOI] [PubMed] [Google Scholar]
- 190.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hodge G, Hodge S, Han P, Haslam R. Multiple leucocyte activation markers to detect neonatal infection. Clin Exp Immunol. 2004 Jan;135(1):125–129. doi: 10.1111/j.1365-2249.2004.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Georgeson GD, Szony BJ, Streitman K, Kovacs A, Kovacs L, Laszlo A. Natural killer cell cytotoxicity is deficient in newborns with sepsis and recurrent infections. Eur J Pediatr. 2001 Aug;160(8):478–482. doi: 10.1007/s004310100773. [DOI] [PubMed] [Google Scholar]
- 193.el-Sameea ER, Metwally SS, Mashhour E, et al. Evaluation of natural killer cells as diagnostic markers of early onset neonatal sepsis: comparison with C-reactive protein and interleukin-8. Egypt J Immunol. 2004;11(1):91–102. [PubMed] [Google Scholar]
- 194.Mazur B, Godula-Stuglik U, Domarecki A, Stojewska M. [The influence of severe infections on the natural killer cells in neonates with vary gestational age] Ginekol Pol. 2000 Jun;71(6):542–549. [PubMed] [Google Scholar]
- 195.Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004 Jul;114(1):21–27. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 196.von Kockritz-Blickwede M, Goldmann O, Thulin P, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008 Mar 15;111(6):3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 197.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001 Dec;108(12):1865–1873. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Damsgaard TE, Nielsen BW, Henriques U, Hansen B, Herlin T, Schiotz PO. Histamine releasing cells of the newborn. Mast cells from the umbilical cord matrix and basophils from cord blood. Pediatr Allergy Immunol. 1996 May;7(2):83–90. doi: 10.1111/j.1399-3038.1996.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 199.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007 Oct 29;204(11):2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003 Oct 15;60(1):49–57. doi: 10.1016/s0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- 201.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003 May;2(5):391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 202.Marom D, Yuhas Y, Sirota L, Livni G, Ashkenazi S. Nitric oxide levels in preterm and term infants and in premature infants with bacteremia. Biol Neonate. 2004;86(3):160–164. doi: 10.1159/000079375. [DOI] [PubMed] [Google Scholar]
- 203.Schrama AJ, de Beaufort AJ, Poorthuis BJ, Berger HM, Walther FJ. Secretory phospholipase A(2) in newborn infants with sepsis. J Perinatol. 2008 Apr;28(4):291–296. doi: 10.1038/sj.jp.7211929. [DOI] [PubMed] [Google Scholar]
- 204.Siauw C, Kobsar A, Dornieden C, et al. Group B streptococcus isolates from septic patients and healthy carriers differentially activate platelet signaling cascades. Thromb Haemost. 2006 May;95(5):836–849. doi: 10.1160/th05-08-0534. [DOI] [PubMed] [Google Scholar]
- 205.Figueras-Aloy J, Gomez-Lopez L, Rodriguez-Miguelez JM, et al. Plasma endothelin-1 and clinical manifestations of neonatal sepsis. J Perinat Med. 2004;32(6):522–526. doi: 10.1515/JPM.2004.126. [DOI] [PubMed] [Google Scholar]
- 206.Kuhl PG, Cotton RB, Schweer H, Seyberth HW. Endogenous formation of prostanoids in neonates with persistent pulmonary hypertension. Arch Dis Child. 1989 Jul;64(7 Spec No):949–952. doi: 10.1136/adc.64.7_spec_no.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rosenberg AA, Kennaugh J, Koppenhafer SL, Loomis M, Chatfield BA, Abman SH. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr. 1993 Jul;123(1):109–114. doi: 10.1016/s0022-3476(05)81552-5. [DOI] [PubMed] [Google Scholar]
- 208.Rossi P, Persson B, Boels PJ, Arner A, Weitzberg E, Oldner A. Endotoxemic pulmonary hypertension is largely mediated by endothelin-induced venous constriction. Intensive Care Med. 2008 May;34(5):873–880. doi: 10.1007/s00134-007-0980-9. [DOI] [PubMed] [Google Scholar]
- 209.Bulger EM, Maier RV. Lipid mediators in the pathophysiology of critical illness. Crit Care Med. 2000 Apr;28(4 Suppl):N27–N36. doi: 10.1097/00003246-200004001-00004. [DOI] [PubMed] [Google Scholar]
- 210.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007 Sep;50(3):652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 211.Shi Y, Li HQ, Shen CK, et al. Plasma nitric oxide levels in newborn infants with sepsis. J Pediatr. 1993 Sep;123(3):435–438. doi: 10.1016/s0022-3476(05)81753-6. [DOI] [PubMed] [Google Scholar]
- 212.Kultursay N, Kantar M, Akisu M, Huseyinov A, Coker I. Platelet-activating factor concentrations in healthy and septic neonates. Eur J Pediatr. 1999 Sep;158(9):740–741. doi: 10.1007/s004310051191. [DOI] [PubMed] [Google Scholar]
- 213.Lopez A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004 Jan;32(1):21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 214.Nimah M, Brilli RJ. Coagulation dysfunction in sepsis and multiple organ system failure. Crit Care Clin. 2003 Jul;19(3):441–458. doi: 10.1016/s0749-0704(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 215.Cribbs SK, Martin GS, Rojas M. Monitoring of endothelial dysfunction in critically ill patients: the role of endothelial progenitor cells. Curr Opin Crit Care. 2008 Jun;14(3):354–360. doi: 10.1097/MCC.0b013e3282fc216d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gibson RL, Nizet V, Rubens CE. Group B streptococcal beta-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr Res. 1999 May;45(5 Pt 1):626–634. doi: 10.1203/00006450-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 217.Dakshinamurti S. Pathophysiologic mechanisms of persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 2005 Jun;39(6):492–503. doi: 10.1002/ppul.20201. [DOI] [PubMed] [Google Scholar]
- 218.Arai H, Matsuda T, Goto R, Takada G. Increased numbers of macrophages in tracheal aspirates in premature infants with funisitis. Pediatr Int. 2008 Apr;50(2):184–188. doi: 10.1111/j.1442-200X.2008.02558.x. [DOI] [PubMed] [Google Scholar]
- 219.McKiernan CA, Lieberman SA. Circulatory shock in children: an overview. Pediatr Rev. 2005 Dec;26(12):451–460. doi: 10.1542/pir.26-12-451. [DOI] [PubMed] [Google Scholar]
- 220.Luce WA, Hoffman TM, Bauer JA. Bench-to-bedside review: Developmental influences on the mechanisms, treatment and outcomes of cardiovascular dysfunction in neonatal versus adult sepsis. Crit Care. 2007;11(5):228. doi: 10.1186/cc6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maeder M, Fehr T, Rickli H, Ammann P. Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006 May;129(5):1349–1366. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 222.Tabbutt S. Heart failure in pediatric septic shock: utilizing inotropic support. Crit Care Med. 2001 Oct;29(10 Suppl):S231–S236. doi: 10.1097/00003246-200110001-00005. [DOI] [PubMed] [Google Scholar]
- 223.Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998 Aug;102(2):e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 224.Carcillo JA, Fields AI. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002 Jun;30(6):1365–1378. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 225.Evans N. Which inotrope for which baby? Arch Dis Child Fetal Neonatal Ed. 2006 May;91(3):F213–F220. doi: 10.1136/adc.2005.071829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kluckow M, Evans N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J Pediatr. 1996 Oct;129(4):506–512. doi: 10.1016/s0022-3476(96)70114-2. [DOI] [PubMed] [Google Scholar]
- 227.Seri I, Noori S. Diagnosis and treatment of neonatal hypotension outside the transitional period. Early Hum Dev. 2005 May;81(5):405–411. doi: 10.1016/j.earlhumdev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 228.Cochat P, Bourgeois J, Gilly J, Cottin X, Larbre F, Bethenod M. [Anatomical study of the kidneys of newborn infants dying after a septic state] Pediatrie. 1986 Jan-Feb;41(1):7–15. [PubMed] [Google Scholar]
- 229.Csaicsich D, Russo-Schlaff N, Messerschmidt A, Weninger M, Pollak A, Aufricht C. Renal failure, comorbidity and mortality in preterm infants. Wien Klin Wochenschr. 2008;120(5–6):153–157. doi: 10.1007/s00508-008-0941-5. [DOI] [PubMed] [Google Scholar]
- 230.Spapen H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat Rec (Hoboken) 2008 Jun;291(6):714–720. doi: 10.1002/ar.20646. [DOI] [PubMed] [Google Scholar]
- 231.Bhutta ZA, Yusuf K. Neonatal sepsis in Karachi: factors determining outcome and mortality. J Trop Pediatr. 1997 Apr;43(2):65–70. doi: 10.1093/tropej/43.2.65. [DOI] [PubMed] [Google Scholar]
- 232.Faix RG, Donn SM. Association of septic shock caused by early-onset group B streptococcal sepsis and periventricular leukomalacia in the preterm infant. Pediatrics. 1985 Sep;76(3):415–419. [PubMed] [Google Scholar]
- 233.Harris MC, D'Angio CT, Gallagher PR, Kaufman D, Evans J, Kilpatrick L. Cytokine elaboration in critically ill infants with bacterial sepsis, necrotizing entercolitis, or sepsis syndrome: correlation with clinical parameters of inflammation and mortality. J Pediatr. 2005 Oct;147(4):462–468. doi: 10.1016/j.jpeds.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 234.Di Naro E, Cromi A, Ghezzi F, et al. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol. 2006 Jan;194(1):153–159. doi: 10.1016/j.ajog.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 235.Cinel I, Dellinger RP. Advances in pathogenesis and management of sepsis. Curr Opin Infect Dis. 2007 Aug;20(4):345–352. doi: 10.1097/QCO.0b013e32818be70a. [DOI] [PubMed] [Google Scholar]
- 236.Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009 Jan;37(1):291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 237.Pfenninger J, Tschaeppeler H, Wagner BP, Weber J, Zimmerman A. The paradox of adult respiratory distress syndrome in neonates. Pediatr Pulmonol. 1991;10(1):18–24. doi: 10.1002/ppul.1950100105. [DOI] [PubMed] [Google Scholar]
- 238.Engle WA. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008 Feb;121(2):419–432. doi: 10.1542/peds.2007-3283. [DOI] [PubMed] [Google Scholar]
- 239.Ng PC, Lam CW, Fok TF, et al. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch Dis Child Fetal Neonatal Ed. 2001 Mar;84(2):F122–F124. doi: 10.1136/fn.84.2.F122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Das BK, Agarwal P, Agarwal JK, Mishra OP. Serum cortisol and thyroid hormone levels in neonates with sepsis. Indian J Pediatr. 2002 Aug;69(8):663–665. doi: 10.1007/BF02722699. [DOI] [PubMed] [Google Scholar]
- 241.Glavina-Durdov M, Springer O, Capkun V, Saratlija-Novakovic Z, Rozic D, Barle M. The grade of acute thymus involution in neonates correlates with the duration of acute illness and with the percentage of lymphocytes in peripheral blood smear. Pathological study. Biol Neonate. 2003;83(4):229–234. doi: 10.1159/000069481. [DOI] [PubMed] [Google Scholar]
- 242.Itoh K, Aihara H, Takada S, et al. Clinicopathological differences between early-onset and late-onset sepsis and pneumonia in very low birth weight infants. Pediatr Pathol. 1990;10(5):757–768. doi: 10.3109/15513819009064710. [DOI] [PubMed] [Google Scholar]
- 243.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J Immunol. 1989 Nov 15;143(10):3200–3206. [PubMed] [Google Scholar]
- 244.Toti P, De Felice C, Occhini R, et al. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol. 2004 Nov;122(5):765–771. doi: 10.1309/RV6E-9BMC-9954-A2WU. [DOI] [PubMed] [Google Scholar]
- 245.Toti P, De Felice C, Stumpo M, et al. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol. 2000 Sep;31(9):1121–1128. doi: 10.1053/hupa.2000.16676. [DOI] [PubMed] [Google Scholar]
- 246.van Baarlen J, Schuurman HJ, Huber J. Acute thymus involution in infancy and childhood: a reliable marker for duration of acute illness. Hum Pathol. 1988 Oct;19(10):1155–1160. doi: 10.1016/s0046-8177(88)80146-1. [DOI] [PubMed] [Google Scholar]
- 247.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis--a potential treatment of sepsis? Clin Infect Dis. 2005 Nov 15;41 Suppl 7:S465–S469. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 248.Kalman L, Lindegren ML, Kobrynski L, et al. Mutations in genes required for T-cell development: IL7R, CD45, IL2RG, JAK3, RAG1, RAG2, ARTEMIS, and ADA and severe combined immunodeficiency: HuGE review. Genet Med. 2004 Jan-Feb;6(1):16–26. doi: 10.1097/01.GIM.0000105752.80592.A3. [DOI] [PubMed] [Google Scholar]
- 249.Le Tulzo Y, Pangault C, Gacouin A, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002 Dec;18(6):487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 250.Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008 Sep 1;112(5):1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bauer J, Hentschel R, Linderkamp O. Effect of sepsis syndrome on neonatal oxygen consumption and energy expenditure. Pediatrics. 2002 Dec;110(6):e69. doi: 10.1542/peds.110.6.e69. [DOI] [PubMed] [Google Scholar]
- 252.Romeo C, Eaton S, Spitz L, Pierro A. Nitric oxide inhibits neonatal hepatocyte oxidative metabolism. J Pediatr Surg. 2000 Jan;35(1):44–48. doi: 10.1016/s0022-3468(00)80011-1. [DOI] [PubMed] [Google Scholar]
- 253.Eaton S. Impaired energy metabolism during neonatal sepsis: the effects of glutamine. Proc Nutr Soc. 2003 Aug;62(3):745–751. doi: 10.1079/PNS2003284. [DOI] [PubMed] [Google Scholar]
- 254.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008 Jan;34(1):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009 Feb;37(2):666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Parker MM, Hazelzet JA, Carcillo JA. Pediatric considerations. Crit Care Med. 2004 Nov;32(11 Suppl):S591–S594. doi: 10.1097/01.ccm.0000145904.97821.0d. [DOI] [PubMed] [Google Scholar]
- 257.Dempsey EM, Al Hazzani F, Barrington KJ. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch Dis Child Fetal Neonatal Ed. 2009 Jul;94(4):F241–F244. doi: 10.1136/adc.2007.124263. [DOI] [PubMed] [Google Scholar]
- 258.Shiao SY. Effects of fetal hemoglobin on accurate measurements of oxygen saturation in neonates. J Perinat Neonatal Nurs. 2005 Oct-Dec;19(4):348–361. doi: 10.1097/00005237-200510000-00010. [DOI] [PubMed] [Google Scholar]
- 259.Kluckow M, Seri I, Evans N. Echocardiography and the neonatologist. Pediatr Cardiol. 2008 Nov;29(6):1043–1047. doi: 10.1007/s00246-008-9275-3. [DOI] [PubMed] [Google Scholar]
- 260.Kluckow M, Seri I, Evans N. Functional echocardiography: an emerging clinical tool for the neonatologist. J Pediatr. 2007 Feb;150(2):125–130. doi: 10.1016/j.jpeds.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 261.Evans N, Kluckow M, Simmons M, Osborn D. Which to measure, systemic or organ blood flow? Middle cerebral artery and superior vena cava flow in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2002 Nov;87(3):F181–F184. doi: 10.1136/fn.87.3.F181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kluckow M, Evans N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed. 2000 May;82(3):F182–F187. doi: 10.1136/fn.82.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hunt RW, Evans N, Rieger I, Kluckow M. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr. 2004 Nov;145(5):588–592. doi: 10.1016/j.jpeds.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 264.Miletin J, Pichova K, Dempsey EM. Bedside detection of low systemic flow in the very low birth weight infant on day 1 of life. Eur J Pediatr. 2009 Jul;168(7):809–813. doi: 10.1007/s00431-008-0840-9. [DOI] [PubMed] [Google Scholar]
- 265.Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. 2001 Aug;27(8):1401–1407. doi: 10.1007/s001340100994. [DOI] [PubMed] [Google Scholar]
- 266.Johnson BA, Hoffman GM, Tweddell JS, et al. Near-infrared spectroscopy in neonates before palliation of hypoplastic left heart syndrome. Ann Thorac Surg. 2009 Feb;87(2):571–577. doi: 10.1016/j.athoracsur.2008.10.043. discussion 577–579. [DOI] [PubMed] [Google Scholar]
- 267.Osborn DA, Evans N, Kluckow M, Bowen JR, Rieger I. Low superior vena cava flow and effect of inotropes on neurodevelopment to 3 years in preterm infants. Pediatrics. 2007 Aug;120(2):372–380. doi: 10.1542/peds.2006-3398. [DOI] [PubMed] [Google Scholar]
- 268.Seri I. Circulatory support of the sick preterm infant. Semin Neonatol. 2001 Feb;6(1):85–95. doi: 10.1053/siny.2000.0034. [DOI] [PubMed] [Google Scholar]
- 269.Munro MJ, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004 Dec;114(6):1591–1596. doi: 10.1542/peds.2004-1073. [DOI] [PubMed] [Google Scholar]
- 270.Han YY, Carcillo JA, Dragotta MA, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003 Oct;112(4):793–799. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 271.Osborn DA, Evans N. Early volume expansion for prevention of morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2004;(2):CD002055. doi: 10.1002/14651858.CD002055.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Goldberg RN, Chung D, Goldman SL, Bancalari E. The association of rapid volume expansion and intraventricular hemorrhage in the preterm infant. J Pediatr. 1980 Jun;96(6):1060–1063. doi: 10.1016/s0022-3476(80)80642-1. [DOI] [PubMed] [Google Scholar]
- 273.Pellicer A, Valverde E, Elorza MD, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics. 2005 Jun;115(6):1501–1512. doi: 10.1542/peds.2004-1396. [DOI] [PubMed] [Google Scholar]
- 274.Osborn D, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr. 2002 Feb;140(2):183–191. doi: 10.1067/mpd.2002.120834. [DOI] [PubMed] [Google Scholar]
- 275.Osborn DA, Paradisis M, Evans N. The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow. Cochrane Database Syst Rev. 2007;(1):CD005090. doi: 10.1002/14651858.CD005090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Barton P, Garcia J, Kouatli A, et al. Hemodynamic effects of i.v. milrinone lactate in pediatric patients with septic shock. A prospective, double-blinded, randomized, placebo-controlled, interventional study. Chest. 1996 May;109(5):1302–1312. doi: 10.1378/chest.109.5.1302. [DOI] [PubMed] [Google Scholar]
- 277.Paradisis M, Evans N, Kluckow M, Osborn D. Randomized trial of milrinone versus placebo for prevention of low systemic blood flow in very preterm infants. J Pediatr. 2009 Feb;154(2):189–195. doi: 10.1016/j.jpeds.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 278.Leone M, Martin C. Role of terlipressin in the treatment of infants and neonates with catecholamine-resistant septic shock. Best Pract Res Clin Anaesthesiol. 2008 Jun;22(2):323–333. doi: 10.1016/j.bpa.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 279.Meyer S, Gottschling S, Baghai A, Wurm D, Gortner L. Arginine-vasopressin in catecholamine-refractory septic versus non-septic shock in extremely low birth weight infants with acute renal injury. Crit Care. 2006;10(3):R71. doi: 10.1186/cc4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004 Aug 28;329(7464):480. doi: 10.1136/bmj.38181.482222.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002 Aug 21;288(7):862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 282.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008 Jan 10;358(2):111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 283.Togari H, Sugiyama S, Ogino T, et al. Interactions of endotoxin with cortisol and acute phase proteins in septic shock neonates. Acta Paediatr Scand. 1986 Jan;75(1):69–74. doi: 10.1111/j.1651-2227.1986.tb10159.x. [DOI] [PubMed] [Google Scholar]
- 284.Seri I, Tan R, Evans J. Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics. 2001 May;107(5):1070–1074. doi: 10.1542/peds.107.5.1070. [DOI] [PubMed] [Google Scholar]
- 285.Noori S, Friedlich P, Wong P, Ebrahimi M, Siassi B, Seri I. Hemodynamic changes after low-dosage hydrocortisone administration in vasopressor-treated preterm and term neonates. Pediatrics. 2006 Oct;118(4):1456–1466. doi: 10.1542/peds.2006-0661. [DOI] [PubMed] [Google Scholar]
- 286.Fernandez EF, Watterberg KL. Relative adrenal insufficiency in the preterm and term infant. J Perinatol. 2009 May;29 Suppl 2:S44–S49. doi: 10.1038/jp.2009.24. [DOI] [PubMed] [Google Scholar]
- 287.Roberts JD, Jr, Fineman JR, Morin FC, 3rd, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997 Feb 27;336(9):605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 288.Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol. 2005 Feb;29(1):8–14. doi: 10.1053/j.semperi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 289.Maclaren G, Butt W. Extracorporeal membrane oxygenation and sepsis. Crit Care Resusc. 2007 Mar;9(1):76–80. [PubMed] [Google Scholar]
- 290.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 291.Emery EF, Greenough A, Gamsu HR. Randomised controlled trial of colloid infusions in hypotensive preterm infants. Arch Dis Child. 1992 Oct;67(10 Spec No):1185–1188. doi: 10.1136/adc.67.10_spec_no.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Neu J, Huang Y. Nutrition of premature and critically ill neonates. Nestle Nutr Workshop Ser Clin Perform Programme. 2003;8:171–181. doi: 10.1159/000072754. discussion 181-175. [DOI] [PubMed] [Google Scholar]
- 293.Park W, Paust H, Schroder H. Lipid infusion in premature infants suffering from sepsis. JPEN J Parenter Enteral Nutr. 1984 May-Jun;8(3):290–292. doi: 10.1177/0148607184008003290. [DOI] [PubMed] [Google Scholar]
- 294.Toce SS, Keenan WJ. Lipid intolerance in newborns is associated with hepatic dysfunction but not infection. Arch Pediatr Adolesc Med. 1995 Nov;149(11):1249–1253. doi: 10.1001/archpedi.1995.02170240067010. [DOI] [PubMed] [Google Scholar]
- 295.Basu R, Muller DP, Eaton S, Merryweather I, Pierro A. Lipid peroxidation can be reduced in infants on total parenteral nutrition by promoting fat utilisation. J Pediatr Surg. 1999 Feb;34(2):255–259. doi: 10.1016/s0022-3468(99)90185-9. [DOI] [PubMed] [Google Scholar]
- 296.Pierro A. Metabolism and nutritional support in the surgical neonate. J Pediatr Surg. 2002 Jun;37(6):811–822. doi: 10.1053/jpsu.2002.32879. [DOI] [PubMed] [Google Scholar]
- 297.Mathur NB, Singh A, Sharma VK, Satyanarayana L. Evaluation of risk factors for fatal neonatal sepsis. Indian Pediatr. 1996 Oct;33(10):817–822. [PubMed] [Google Scholar]
- 298.Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006 Jun;19(3):290–297. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]
- 299.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004 Nov 17;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 300.Bassler D, Stoll BJ, Schmidt B, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009 Jan;123(1):313–318. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008 Aug;153(2):170–175. doi: 10.1016/j.jpeds.2008.02.033. 175 e171. [DOI] [PubMed] [Google Scholar]
- 302.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005 Mar;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 303.Manzoni P, Maestri A, Leonessa M, Mostert M, Farina D, Gomirato G. Fungal and bacterial sepsis and threshold ROP in preterm very low birth weight neonates. J Perinatol. 2006 Jan 1;26(1):23–30. doi: 10.1038/sj.jp.7211420. [DOI] [PubMed] [Google Scholar]
- 304.Wong HR, Cvijanovich N, Lin R, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wong HR, Cvijanovich N, Allen GL, et al. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit Care Med. 2009 May;37(5):1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wong HR, Odoms K, Sakthivel B. Divergence of canonical danger signals: the genome-level expression patterns of human mononuclear cells subjected to heat shock or lipopolysaccharide. BMC Immunol. 2008;9:24. doi: 10.1186/1471-2172-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cvijanovich N, Shanley TP, Lin R, et al. Validating the genomic signature of pediatric septic shock. Physiol Genomics. 2008 Jun 12;34(1):127–134. doi: 10.1152/physiolgenomics.00025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Shanley TP, Cvijanovich N, Lin R, et al. Genome-level longitudinal expression of signaling pathways and gene networks in pediatric septic shock. Mol Med. 2007 Sep-Oct;13(9–10):495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Tang BM, McLean AS, Dawes IW, Huang SJ, Cowley MJ, Lin RC. Gene-expression profiling of gram-positive and gram-negative sepsis in critically ill patients. Crit Care Med. 2008 Apr;36(4):1125–1128. doi: 10.1097/CCM.0b013e3181692c0b. [DOI] [PubMed] [Google Scholar]
- 310.Tang BM, McLean AS, Dawes IW, Huang SJ, Lin RC. The use of gene-expression profiling to identify candidate genes in human sepsis. Am J Respir Crit Care Med. 2007 Oct 1;176(7):676–684. doi: 10.1164/rccm.200612-1819OC. [DOI] [PubMed] [Google Scholar]
- 311.Tang BM, McLean AS, Dawes IW, Huang SJ, Lin RC. Gene-expression profiling of peripheral blood mononuclear cells in sepsis. Crit Care Med. 2009 Mar;37(3):882–888. doi: 10.1097/CCM.0b013e31819b52fd. [DOI] [PubMed] [Google Scholar]
- 312.Karvunidis T, Mares J, Thongboonkerd V, Matejovic M. Recent progress of proteomics in critical illness. Shock. 2009 Jun;31(6):545–552. doi: 10.1097/SHK.0b013e3181986eab. [DOI] [PubMed] [Google Scholar]
- 313.Buhimschi CS, Bhandari V, Hamar BD, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007 Jan;4(1):e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Feezor RJ, Cheng A, Paddock HN, Baker HV, Moldawer LL. Functional genomics and gene expression profiling in sepsis: beyond class prediction. Clin Infect Dis. 2005 Nov 15;41 Suppl 7:S427–S435. doi: 10.1086/431993. [DOI] [PubMed] [Google Scholar]
- 315.Levy O, Sisson RB, Kenyon J, Eichenwald E, Macone AB, Goldmann D. Enhancement of neonatal innate defense: effects of adding an N-terminal recombinant fragment of bactericidal/permeability-increasing protein on growth and tumor necrosis factor-inducing activity of gram-negative bacteria tested in neonatal cord blood ex vivo. Infect Immun. 2000 Sep;68(9):5120–5125. doi: 10.1128/iai.68.9.5120-5125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Reinhart K, Gluck T, Ligtenberg J, et al. CD14 receptor occupancy in severe sepsis: results of a phase I clinical trial with a recombinant chimeric CD14 monoclonal antibody (IC14) Crit Care Med. 2004 May;32(5):1100–1108. doi: 10.1097/01.ccm.0000124870.42312.c4. [DOI] [PubMed] [Google Scholar]
- 317.Lauterbach R, Pawlik D, Kowalczyk D, Ksycinski W, Helwich E, Zembala M. Effect of the immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: a placebo-controlled, double-blind trial. Crit Care Med. 1999 Apr;27(4):807–814. doi: 10.1097/00003246-199904000-00042. [DOI] [PubMed] [Google Scholar]
- 318.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007 Feb;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Pleiner J, Schaller G, Mittermayer F, et al. Simvastatin prevents vascular hyporeactivity during inflammation. Circulation. 2004 Nov 23;110(21):3349–3354. doi: 10.1161/01.CIR.0000147774.90396.ED. [DOI] [PubMed] [Google Scholar]
- 320.Ikegami M, Carter K, Bishop K, et al. Intratracheal recombinant surfactant protein d prevents endotoxin shock in the newborn preterm lamb. Am J Respir Crit Care Med. 2006 Jun 15;173(12):1342–1347. doi: 10.1164/rccm.200509-1485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Mohan P, Abrams SA. Oral lactoferrin for the treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev. 2009;(1):CD007138. doi: 10.1002/14651858.CD007138.pub2. [DOI] [PubMed] [Google Scholar]
- 322.Venkatesh M, Abrams S. Can lactoferrin prevent neonatal sepsis and necrotizing enterocolitis? Expert Rev Anti Infect Ther. 2009 Jun;7(5):515–525. doi: 10.1586/eri.09.25. [DOI] [PubMed] [Google Scholar]
- 323.Suri M, Harrison L, Van de Ven C, Cairo MS. Immunotherapy in the prophylaxis and treatment of neonatal sepsis. Curr Opin Pediatr. 2003 Apr;15(2):155–160. doi: 10.1097/00008480-200304000-00003. [DOI] [PubMed] [Google Scholar]