Abstract
The later steps of carotenoid biosynthesis involve the formation of cyclic carotenoids. The reaction is catalyzed by lycopene β-cyclase (LCY-B), which converts lycopene into β-carotene, and by capsanthin-capsorubin synthase (CCS), which is mainly dedicated to the synthesis of κ-cyclic carotenoids (capsanthin and capsorubin) but also has LCY-B activity. Although the peptide sequences of plant LCY-Bs and CCS contain a putative dinucleotide-binding motif, it is believed that these two carotenoid cyclases proceed via protic activation and stabilization of resulting carbocation intermediates. Using pepper (Capsicum annuum) CCS as a prototypic carotenoid cyclase, we show that the monomeric protein contains one noncovalently bound flavin adenine dinucleotide (FAD) that is essential for enzyme activity only in the presence of NADPH, which functions as the FAD reductant. The reaction proceeds without transfer of hydrogen from the dinucleotide cofactors to β-carotene or capsanthin. Using site-directed mutagenesis, amino acids potentially involved in the protic activation were identified. Substitutions of alanine, lysine, and arginine for glutamate-295 in the conserved 293-FLEET-297 motif of pepper CCS or LCY-B abolish the formation of β-carotene and κ-cyclic carotenoids. We also found that mutations of the equivalent glutamate-196 located in the 194-LIEDT-198 domain of structurally divergent bacterial LCY-B abolish the formation of β-carotene. The data herein reveal plant carotenoid cyclases to be novel enzymes that combine characteristics of non-metal-assisted terpene cyclases with those attributes typically found in flavoenzymes that catalyze reactions, with no net redox, such as type 2 isopentenyl diphosphate isomerase. Thus, FAD in its reduced form could be implicated in the stabilization of the carbocation intermediate.
Later steps of carotenoid biosynthesis involve the formation of diverse cyclic carotenoids. For example, β-carotene, the vitamin A precursor, is synthesized de novo by photosynthetic organisms, limited nonphototrophic bacteria and fungi, and also by aphids (Moran and Jarvik, 2010) according to a multistep pathway that ends with the cyclization of lycopene by lycopene β-cyclase (LCY-B). Similarly, in pepper (Capsicum annuum) chromoplasts, antheraxanthin and violaxanthin are converted into the κ-cyclic carotenoids capsanthin and capsorubin, respectively, by capsanthin-capsorubin synthase (CCS). In both cases, the proposed mechanism involves a concerted protic attack and stabilization of a transient carbocation without any net redox change (Camara, 1980; Bouvier et al., 1994; Britton, 1998). Several cDNAs for LCY-B have been cloned from bacteria (Misawa et al., 1990; Cunningham et al., 1994; Armstrong, 1997; Cunningham and Gantt, 2001), fungi (Verdoes et al., 1999; Velayos et al., 2000; Arrach et al., 2001), and plants (Hugueney et al., 1995; Ronen et al., 2000) using functional complementation. Information available from primary structures suggest that the cyclization of lycopene is catalyzed by holomeric proteins in photosynthetic organisms (Cunningham et al., 1994; Maresca et al., 2007), by holomeric (Misawa et al., 1990) or heteromeric (Krubasik and Sandmann, 2000; Viveiros et al., 2000) proteins in nonphotosynthetic bacteria, and by holomeric, bifunctional proteins in fungi that combine the activities of phytoene synthase and lycopene cyclase (Verdoes et al., 1999; Velayos et al., 2000; Arrach et al., 2001). This structural diversity of LCY-Bs coupled to a lack of significant amino acid sequence identity between the lycopene cyclases from bacteria, fungi, and plants hinder our understanding of the catalytic mechanism of LCY-Bs and CCS. In addition, the N terminus of plant LCY-B and CCS contains an amino sequence motif characteristic of a polypeptide predicted to adopt a Rossmann fold (Rossmann et al., 1974) and suggests the binding of an as yet unknown dinucleotide prosthetic ligand. It has been shown using recombinant bacterial enzyme that the cyclization of lycopene into β-carotene strictly requires NADPH but proceeds without any net redox change (Schnurr et al., 1996; Hornero-Mendez and Britton, 2002). Under the same conditions, FAD alone could not sustain bacterial LCY-B activity (Schnurr et al., 1996). Much less is known about the dinucleotide requirements of plant carotenoid cyclases, which are highly conserved within plants but are extremely divergent in nonplant organisms. Previously, a crucial acidic domain for lycopene cyclase activity was identified using an affinity-labeling strategy followed by site-directed mutagenesis (Bouvier et al., 1997) in the absence of any crystal structures. This so-called 293-FLEET-297 motif of LCY-B and CCS contained two tandem Glu-295-Glu-296 residues that were essential for LCY-B- and κ-cyclase activities (Bouvier et al., 1997). However, it still remains unclear how the protic mechanism is compatible with the requirement of dinucleotide cofactors.
To further explore the mechanism of plant carotenoid cyclases, we first choose pepper CCS as a prototypic enzyme because it displays a strong identity (52%) to pepper LCY-B, and we have shown previously that CCS could also catalyze the cyclization of lycopene into β-carotene (up to 25% of activity compared with LCY-B; Hugueney et al., 1995). Herein, we have shown that monomeric CCS purified to homogeneity from plant chromoplasts or recombinant CCS purified from Escherichia coli-transformed cells are typical flavoproteins containing one noncovalently bound FAD. We also observed that CCS-bound FAD is required for enzyme activity in the presence of NADPH, which functions as a reductant of FAD. During this process, no hydrogen is transferred to β-carotene or κ-cyclic carotenoids. In addition to this cofactor requirement, we also show from extensive site-directed mutagenesis using pepper CCS and LCY-B and Erwinia herbicola LCY-B (Mialoundama, 2009) that Glu-295 of pepper CCS and LCY-B plays a key role in the formation of β-carotene and κ-cyclic carotenoids, and we demonstrate that a similar role is played in structurally divergent bacterial LCY-Bs by Glu-196. These characteristics suggest that plant CCS and LCY-Bs are mechanistically similar to non-metal-assisted terpene cyclases, such as squalene:hopene cyclase and oxidosqualene cyclase, and additionally represent a new subfamily of flavoproteins like isopentenyl diphosphate isomerase type II, which catalyze carotenoid cyclization without any net redox modification of the substrate.
RESULTS
Identification of CCS as a Typical Flavoprotein
Amino acid sequence alignment indicates that plant-type LCY-Bs and CCS contain dinucleotide-binding motifs characteristic of an N-terminal Rossmann fold of FAD-dependent oxidoreductases (Rossmann et al., 1974; Cunningham et al., 1994; Hugueney et al., 1995). To analyze the nature of the potential dinucleotide prosthetic group, we noted during the initial purification steps that it was more tightly bound to CCS prepared from pepper chromoplasts than that of recombinant CCS produced in E. coli. Based on this evidence, we first purified CCS from pepper chromoplasts using a previously described procedure (Bouvier et al., 1994). When homogenous fractions (Fig. 1A) were subjected to mild electrophoretic conditions, the band corresponding to homogenous CCS protein could be observed without further treatment due to its yellow color, as observed previously (Bouvier et al., 1994; Fig. 1B). Further analysis of chromoplast-derived CCS showed UV-visible absorption (458 nm) and fluorescence (520 nm) spectra characteristic of flavoproteins (Fig. 1C).
Figure 1.
SDS-PAGE analysis and spectral analysis of ChrCCS. A, Chromoplast membrane proteins (MB) and purified ChrCCS fractions (lanes 1–8) eluted after the Superose 6 column were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. Molecular mass markers (PageRuler Prestained Protein Ladder; Fermentas) are shown on the left. B, Unstained gel showing the yellow ChrCCS band corresponding to fractions 5 to 7 from the Superose 6 column shown in A. The SDS concentration in the solubilization buffer was reduced by half, and the separation was carried out at 6°C. Molecular mass markers are shown on the left. C, Visible absorption (peak 1) of purified ChrCCS (5.5 μm) in 10 mm phosphate buffer (pH 7) containing 15% glycerol and 0.5% Tween 80. Peak 2 shows the fluorescence spectrum of purified ChrCCS (2 μm) in the same buffer. Excitation was set at 458 nm.
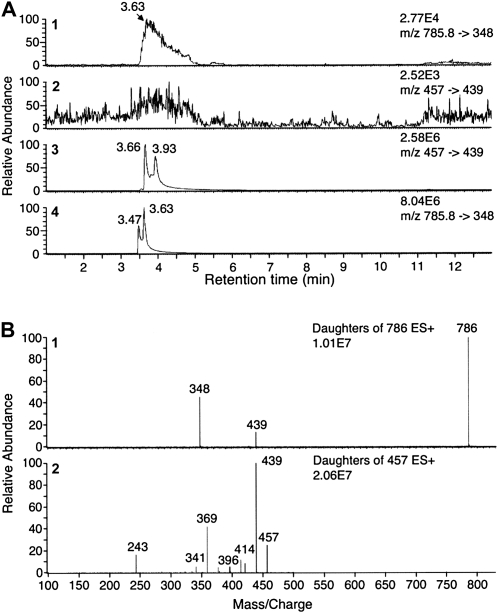
To further characterize the binding and the structure of the flavin prosthetic group, the yellow gel bands (Fig. 1B) were subjected to in-gel digestion using trypsin. The resulting peptide mixture was then extracted and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). We could unambiguously identify CCS peptides, but no peptide fragments carrying covalently linked FAD or FMN were detected (data not shown). Subsequently, we extracted the yellow gel band using acidic acetonitrile before chromatographic analysis using FAD and FMN standards. Both cofactors provide the best signal in positive electrospray mode, and the best MS signal was obtained with 25 V (data not shown). The best specific multiple reaction monitoring (MRM) transitions were mass-to-charge ratio (m/z) 786→348 for FAD and 457→439 for FMN (Fig. 2). MRM analysis revealed the presence of FAD in the yellow gel extract (Fig. 2), whereas no FMN was detected (Fig. 2). The broadening of FAD and FMN peaks is probably due to the phosphate group, which is known to be poorly ionized under electrospray conditions. Collectively, these data demonstrate that FAD is noncovalently bound to chromoplast CCS (ChrCCS). The stoichiometry of 0.95 FAD per monomeric CCS could be determined at 458 nm, assuming that free and bound FAD have the same millimolar absorbance coefficient (11.3 mm−1cm−1; Whitby, 1953). Thus, one could postulate that each native CCS monomer (52 kD) contains one noncovalently bound FAD. Very similar data were obtained using purified recombinant pepper CCS (reCCS; Supplemental Fig. S1) and recombinant LCY-B (reLCY-B; data not shown). Binding affinities for FAD were determined for ChrCCs, reCCS, and reLCY-B. The dissociation constant values of 0.4 ± 0.02, 0.6 ± 0.03, and 0.9 ± 0.02 μm were obtained for ChrCCS, reCCS, and reLCY-B. These data collectively demonstrate that CCS and plant LCY-B are FAD-binding flavoproteins.
Figure 2.
Analysis of prosthetic flavin bound to purified ChrCCS by UPLC-MS/MS. A, From top to bottom, UPLC-MS/MS chromatograms showing the identification of FAD and FMN using MRM with positive electrospray ionization. Flavin extract obtained from homogenous ChrCCS separated by SDS-PAGE as described in Figure 1B was used for the identification of FAD (1) using the transition m/z 785.8→348 (tR = 3.47 and tR = 3.63). The absence of FMN (2) in the gel extract was based on the FMN transition m/z 457→439 obtained for FMN standard (3) with the transition m/z 457→439 (tR = 3.66 and tR = 3.93) compared with FAD standard with the transition m/z 785.8→348 (tR = 3.47 and tR = 3.63). B, UPLC-MS/MS spectra representing the fragmentation pattern determined for FAD and FMN standards using daughter scans to validate the above transitions: FAD, transition m/z 785.8→348; FMN, transition m/z 457→439.
Implication of FAD and NADPH during CCS Catalysis
To determine if FAD is implicated in the cyclization of lycopene into β-carotene or the formation of κ-cyclic carotenoids, we removed noncovalently bound FAD from ChrCCS and reCCS using hydrophobic interaction chromatography and determined the activity of the resulting apoenzymes under diverse reconstitution conditions. Apo-ChrCCS and apo-reCCS revealed no enzymatic activity toward carotenoid cyclization in the absence of added cofactors (Fig. 3A). Next, we analyzed the role of FAD following the reconstitution of apo-ChrCCS and apo-reCCS with FAD (Fig. 3A). The resulting holoenzyme catalyzed the cyclization of lycopene into β-carotene and the synthesis of κ-cyclic carotenoids only in the presence of FAD and NADPH (Fig. 3A). The NADPH requirement was also observed for purified ChrCCS and reCCS that contained noncovalently bound FAD in their native form. Similar data were obtained for reLCY-B (Fig. 3B). The Km values for NADPH were 0.15 ± 0.07, 0.25 ± 0.03, and 0.22 ± 0.02 mm for ChrCCS, reCCS, and reLCY-B, respectively. Subsequently, we tested whether the requirement of FAD and NADPH is coupled to hydrogen transfer to the cyclic carotenoids. To this end, we determined the CCS activity in the presence of FAD and (4R)-[4-3H]NADPH prepared as described previously (McCracken et al., 2004). Under these conditions, we did not observe tritium incorporation in the purified β-carotene or in capsanthin (data not shown). Thus, no hydrogen from NADPH is transferred to β-carotene and capsanthin, in agreement with previous data (Hornero-Mendez and Britton, 2002). Finally, we noted that upon incubation of the holoenzyme with NADPH, a strong modification of the absorption spectrum of holo-CCS was observed under anaerobic conditions (Fig. 3C). This change is reminiscent of the direct reduction of CCS-bound FAD, as noted for isopentenyl diphosphate isomerase type II, where noncovalently bound FMN could be reduced directly by NADH or NADPH (Hemmi et al., 2004; Rothman et al., 2007).
Figure 3.
Effects of FAD and NADPH on the activity of ChrCCS, reCCS, and reLCY-B using different reconstitution conditions. The different holoenzymes were deflavinylated as described in “Materials and Methods” before incubation in a reaction mixture containing 50 μg of protein and appropriate cofactors as indicated. A, For CCS, the lycopene cyclase (conversion of lycopene to β-carotene) and the κ-cyclase (conversion of antheraxanthin into capsanthin) activities were determined. Values were normalized with respect to the holoenzymes or apoenzymes incubated with the substrate in the presence of FAD and NADPH. The 100% values refer to ChrCCS (β-carotene, 81 ± 7.2 nmol mg−1 h−1; capsanthin, 232 ± 15.6 nmol mg−1 h−1); apo-ChrCCS (β-carotene, 67 ± 5.2 nmol mg−1 h−1; capsanthin, 178 ± 11.6 nmol mg−1 h−1); reCCS (β-carotene, 72 ± 6.3 nmol mg−1 h−1; capsanthin, 156 ± 10.4 nmol mg−1 h−1); apo-reCCS (β-carotene, 40 ± 5.7 nmol mg−1 h−1; capsanthin, 125 ± 15.7 nmol mg−1 h−1); reLCY-B (β-carotene, 115 ± 12.3 nmol mg−1 h−1); and apo-reLCY-B (β-carotene, 77 ± 6.7 nmol mg−1 h−1). B, ReLCY-B and apo-reLCY-B were used to determine the conversion of lycopene into β-carotene. C, UV-visible absorption of purified ChrCCS incubated with 5 mm NADPH under argon atmosphere. Absorption of oxidized (peak 1) ChrCCS (5.5 μm) in 10 mm phosphate buffer (pH 7) containing 15% glycerol, 0.5% Tween 80, and 5 mm NADPH (peak 2) is shown.
Implication of the Acidic FLEET Motif in CCS and LCY-B Activity
Based on the indirect implication of FAD and NADPH in the cyclization of carotenoid, we decided to explore further the role of amino acids potentially involved in the protic activation during carotenoid cyclization. A homology-based, structural alignment reveals that lycopene cyclases identified to date cannot be placed into a single homogenous group. The 293-FLEET-297 motif in LCY-Bs and in CCS (Supplemental Fig. S2) was previously shown to be crucial for cyclization of lycopene to β-carotene (Bouvier et al., 1997). Because neither crystal structure nor homology modeling is yet possible for LCY-B and CCS, we further analyzed the contribution of this motif in reCCS and reLCY-B (Mialoundama, 2009) by substituting Ala for targeted residues and by substituting Lys and Arg for selected acidic amino acids to convert them to positive charges. We also substituted Ala for conserved Asp-127, Asp-259, Glu-128, Glu-332, and His-360 residues, which are conserved between highly divergent plant and bacterial carotenoid cyclases (Supplemental Fig. S2). This strategy was used because Ala substitutions generate low distortions in protein structure (Fersht, 1987), while Lys and Arg substitutions eliminate the protic activation potential of acidic amino acid residues. Before mutant proteins were expressed in E. coli, these substitutions were verified by DNA sequencing. Recombinant LCY-B and CCS were then purified by His-tag affinity chromatography as described in “Materials and Methods.” They migrated as a 66-kD band and were immunoreactive to antibodies directed against LCY-B or CCS (Fig. 4). The intensity of the protein signals as shown revealed that mutations of LCY-B and CCS have no effect on the expression or stability of proteins. Through the use of a FAD-agarose affinity column, we observed that the mutant and the wild-type reCCS and reLCY-B proteins had practically the same affinity for FAD (data not shown). The effects of different mutations on the cyclization of lycopene into β-carotene were compared with wild-type enzymes, and the kinetic properties were determined from Lineweaver-Burk plots (Lineweaver and Burk, 1934).
Figure 4.
Expression of plant-type LCY-Bs. Soluble lysate from cells harboring the control vector and lines expressing wild or mutated plant reLCY-B or reCCS was used for affinity purification as described in “Materials and Methods.” Affinity-purified wild and mutant reLCY-B or reCCS were probed with anti-LCY-B or anti-CCS. The arrowheads indicate the signal at approximately 66 kD.
Substituting Ala, Lys, or Arg for Glu-295 completely abolishes reLCY-B (Table I) and reCCS (Table II) activity. Substituting Ala for Glu-296 reduces the lycopene cyclase activities of reLCY-B (Table I) and reCCS (Table II) to residual levels. Similarly, substituting Ala for Asp-127, Glu-128, Asp-259, and Glu-332 drastically reduced the activity up to 90%. In the case of reLCY-B H360A (Table I) or reCCS H360A (Table II), the activity was abolished but was partially recovered in reLCY-B H360K/R and reCCS H360K/R. Compared with the wild-type cyclases, the apparent Vmax values of the different mutant cyclases were reduced while the Km values were not altered (Tables I and II). Thus, the reduction of the catalytic activity was probably not due to a modified binding of lycopene. This indicates that the Asp-127, Glu-128, Asp-259, Glu-332, and His-360 residues of reLCY-B and reCCS have an important catalytic role but are not likely to directly initiate cyclization.
Table I. Kinetic parameters of wild-type and mutant reLCY-B.
The values shown are means ± se for three determinations. ND, Not determined.
| Enzyme | Vmax | Km | Relative Activity |
| nmol mg−1 h−1 | μm | % | |
| Wild type | 130.0 ± 9 | 4.5 ± 0.03 | 100 |
| D127A | 75.0 ± 3 | 4.2 ± 0.04 | 57.6 ± 2 |
| E128A | 6.5 ± 0.3 | 4.0 ± 0.04 | 5.0 ± 0.2 |
| D259A | 32.5 ± 2.5 | 4.2 ± 0.02 | 25.0 ± 2 |
| E295A | 0 | – | 0 |
| E295K | 0 | – | 0 |
| E295R | 0 | – | 0 |
| E296A | 1.3 ± 0.04 | ND | 1.0 ± 0.04 |
| E296K | 0.3 ± 0.02 | ND | 0.2 ± 0.01 |
| E296R | 0.3 ± 0.02 | ND | 0.2 ± 0.01 |
| E332A | 6.5 ± 0.2 | 3.9 ± 0.03 | 5.0 ± 0.09 |
| H360A | 0 | – | 0 |
| H360K | 19.5 ± 1.5 | 5.0 ± 0.05 | 15.0 ± 0.8 |
| H360R | 26.0 ± 2 | 4.8 ± 0.07 | 20.0 ± 2 |
Table II. Kinetic parameters of wild-type and mutant reCCS.
The values shown are means ± se for three determinations. ND, Not determined.
| Enzyme | Vmax | Km | Relative Activity |
| nmol mg−1 h−1 | μm | % | |
| Wild type | 82 ± 6 | 3.7 ± 0.05 | 100 |
| D127A | 62 ± 3 | 4.2 ± 0.03 | 75.6 ± 4 |
| E128A | 3.6 ± 0.04 | 4.1 ± 0.04 | 4.4 ± 0.03 |
| D259A | 25.0 ± 6 | 3.8 ± 6 | 30.5 ± 2 |
| E295A | 0 | – | – |
| E295K | 0 | – | – |
| E295R | 0 | – | – |
| E296A | 1.2 ± 0.01 | ND | 1.5 ± 0.03 |
| E296K | 0.1 ± 0.01 | ND | 0.1 ± 0.01 |
| E296R | 0.1 ± 0.01 | ND | 0.1 ± 0.01 |
| E332A | 8.3 ± 0.4 | 3.5 ± 0.04 | 10.1 ± 0.5 |
| H360A | 0 | – | – |
| H360K | 25.0 ± 2 | 3.6 ± 0.05 | 30.5 ± 2 |
| H360R | 22.0 ± 2 | 3.8 ± 0.03 | 26.8 ± 1 |
Examination of protein sequence data revealed that plant LCY-B or CCS Glu-295 is structurally equivalent to bacterial Glu-196 of Erwinia LCY-B (Fig. 5). This led us to envisage that this invariant residue may have an identical function. This idea was explored by site-directed mutations substituting Glu-196 of Erwinia LCY-B to Ala, Lys, and Arg. To test these mutant strains, an in vitro complementation procedure was employed using E. coli that synthesized lycopene. This offered an indirect and rapid means of screening for lycopene cyclase activity by measuring β-carotene accumulation following transformation of E. coli with plasmids that contained wild-type or mutated lycopene cyclase. Under these conditions, plasmids containing lycopene cyclase allowed up to 80% conversion of endogenous lycopene into β-carotene. However, none of the plasmids containing the Erwinia Glu-196Ala, Glu-196Lys, or Glu-196Arg mutants of lycopene cyclases was able to direct β-carotene synthesis (Fig. 5). Thus, an active Glu residue was needed to initiate lycopene cyclization by pepper CCS, plant-type LCY-Bs, and bacterial LCY-B.
Figure 5.
Functional characterization of bacterial wild-type and mutant LCY-Bs. Following the identification of Glu-196 as an active-site residue of Erwinia lycopene cyclase, a plasmid-based assay was used to test wild-type and mutant Erwinia LCY-B. E. coli JM101 harboring the plasmid pACYC-EBI (chloramphenicol), which produces lycopene, was used. After transformation of E. coli JM101-pACYC-EBI (chloramphenicol) with plasmids encoding wild-type or mutant Erwinia LCY-Bs and induction with 0.5 mm isopropyl-β-thiogalactopyranoside, total carotenoids were extracted from the bacterial pellets and analyzed by HPLC as described in “Materials and Methods.” Data shown represent means ± sd for two independent experiments.
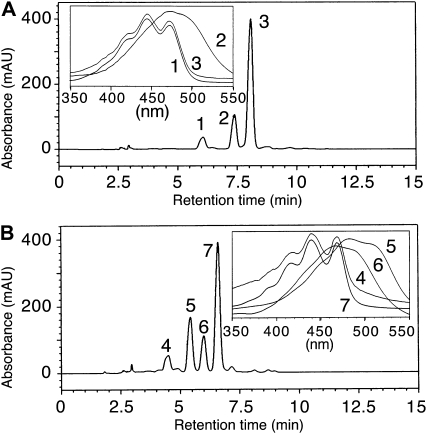
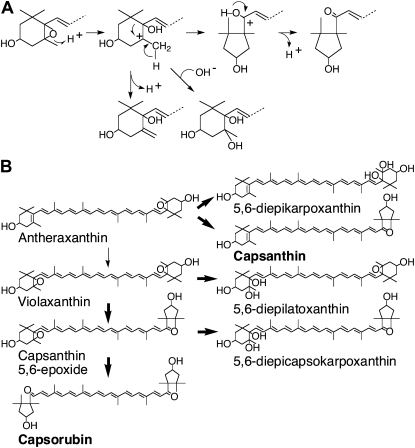
To further analyze the catalytic mechanism of plant carotenoid cyclase, we incubated wild-type and mutant reCCS with different 5,6-epoxy-xanthophylls and further analyzed the reaction products using ultra-performance liquid chromatography (UPLC)-MS/MS. When wild-type reCCS was incubated with antheraxanthin, we noted that, in addition to capsanthin, the normal product, a new xanthophyll (peak 1), was formed (Fig. 6A). The polarity, absorption maxima in the eluting solvent, and the total ion chromatogram of the new xanthophylls (Supplemental Fig. S3) showed it to be 5,6-diepikarpoxanthin (peak 1; Deli et al., 1998). In a similar vein, we incubated wild-type reCCS with violaxanthin. HPLC and MS analyses of reaction products showed that when violaxanthin was used as a substrate, capsanthin-5,6-epoxide (peak 6) and capsorubin (peak 5) were formed as expected and a new xanthophyll (peak 4) appeared as well (Fig. 6B). The relative elution of peak 4 on HPLC and its spectral characteristics in the eluting solvent are consistent with peak 4 being 5,6-diepilatoxanthin (Deli et al., 1998), but we could not further analyze its structure. The formation of these new xanthophylls was abolished when reCCSAla-295, reCCSLys-295, and reCCSArg-295 mutants were used for the enzymatic tests (data not shown). As these new xanthophylls might represent trapped intermediates during the formation of κ-cyclic carotenoids, they were also tested as possible substrates of reCCS. The results revealed that they were not converted into κ-cyclic xanthophylls (data not shown). Thus, these new xanthophylls are not transient intermediates en route to κ-cyclic carotenoid synthesis. They most probably represent dead-end products of the trapped carbocation produced during the formation of capsanthin and capsorubin (Fig. 7).
Figure 6.
HPLC analysis of κ-cyclic carotenoids synthesized by reCCS from xanthophyll epoxides. A, Antheraxanthin conversion into κ-cyclic and 3,5,6-trihydroxy xanthophyll by reCCS. ReCCS was incubated with antheraxanthin as described in “Materials and Methods,” and products were analyzed by HPLC and online diode array detection. Peaks refer to 5,6-diepikarpoxanthin (peak 1), capsanthin (peak 2), and antheraxanthin (peak 3). B, Violaxanthin conversion into κ-cyclic and 3,5,6-trihydroxy xanthophyll by reCCS. ReCCS was incubated with violaxanthin, and products were analyzed as described in A. Peaks refer to putative 5,6-diepilatoxanthin (peak 4), capsorubin (peak 5), capsanthin-5,6-epoxide (peak 6), and violaxanthin (peak 7).
Figure 7.
Molecular diversity of carotenoids generated by CCS. A, Flexible stabilization of incipient carbocation generated by CCS. B, Possible pathways of carotenoids produced by CCS during the formation of κ-cyclic carotenoids.
DISCUSSION
FAD and NADPH Dependence of Plant Carotenoid Cyclases
Amino acid sequence alignment of plant-type LCY-Bs suggests that the N-terminal region could adopt a Rossmann-fold characteristic of flavoproteins (Dym and Eisenberg, 2001). The nature of the putative prosthetic flavin group had remained elusive. Previously, a strict requirement of exogenous NADPH, and nonessentially, of exogenous FAD, have been reported for the enzymatic cyclization of lycopene into β-carotene using Erwinia uredovora LCY-B (Schnurr et al., 1996). However, although E. uredovora LCY-B functions in the presence of NADPH, no hydrogen is transferred from NADPH to β-carotene (Hornero-Mendez and Britton, 2002). This reinforced the accepted protic cyclization mechanism of carotenoids triggered by unknown amino acid residues but leaves unexplained the indirect role played by NADPH. Using CCS as a carotenoid cyclase prototype has allowed us to identify nonbound FAD as the prosthetic group implicated in the synthesis of β-carotene and κ-cyclic carotenoids. Our results also reveal that the cyclization reaction takes place only in the presence of FAD and NADPH (Fig. 3, A and B). Data further revealed that when NADPH is incubated with CCS holoenzyme, its absorption spectrum is drastically modified (Fig. 3C). The characteristic absorption at approximately 450 nm was lost (Fig. 3C). A similar change was observed when dithionite was used instead of NADPH (data not shown). The spectral change suggests a direct reduction of FAD by NADPH (Fig. 3C). In E. uredovora, FAD reduced by Ti(III)-citrate in the absence of NADPH could sustain the conversion of lycopene into β-carotene (Yu et al., 2010; P. Beyer, personal communication). Very similar observations have been described for isopentenyl diphosphate isomerase type II (Hemmi et al., 2004; Rothman et al., 2007), for which the reaction requires the reduction of FMN-bound enzyme by NADPH. In this context, it is interesting that the characteristics of CCS are reminiscent of those described for isopentenyl diphosphate isomerase type II, which is a flavoenzyme, and requires FMN and NADPH for activity but functions without net redox modifications of the substrate. Because flavoproteins usually catalyze redox reactions, the question arises of how CCS-bound FAD functions. With respect to data obtained from isopentenyl diphosphate isomerase type II, which has been crystallized, two likely explanations could be offered (Rothman et al., 2008; Unno et al., 2009). In one case, CCS-bound FAD, when reduced by NADPH, could act as an acid-base catalyst (Unno et al., 2009). Alternatively, reduced CCS-bound FAD could be involved in the stabilization of the transient carbocation formed during the reaction (Rothman et al., 2008). The latter conclusion is supported by the recent finding that E. uredovora LCY-B could function with reduced 1- and 5-deazaflavin analogs that probably have limited potential to trigger the protic activation of lycopene (Yu et al., 2010).
Importance of Invariant Acidic Residues in Plant Carotenoid Cyclases
LCY-Bs cloned from bacteria, plants, and fungi do not contain consensus motifs. We have previously used a site-directed mutant approach to identify several amino acid residues critical for differential inhibition of plant LCY-B and CCS (Bouvier et al., 1997). In this study, we made use of an Ala scanning mutagenesis strategy combined with the introduction of positive or negative residues to replace several conserved residues between plant cyclases LCY-B, CCS, and bacterial LCY-B. Stably expressed recombinant proteins were purified and used for enzyme assays. The mutant proteins allowed us to define Glu-295 as a critical residue for the cyclization of lycopene into β-carotene and also for the formation of κ-cyclic carotenoids in plants. Glu-295A/K/R failed to synthesize β-carotene or κ-cyclic carotenoids. In addition, the mutation of Glu-196 of E. herbicola (Fig. 5) or E. uredovora (Yu et al., 2010; P. Beyer, personal communication), equivalent to Glu-295 of plant-type LCY-Bs, abolished the cyclization of lycopene into β-carotene. Therefore, the presence of a Glu residue at position 295 in plant enzymes or at position 196 in bacteria is essential for the catalytic activity of carotenoid cyclases. Cloning of fungal LCY-B from wild-type and mutant strains of Phycomyces blakesleeanus (Arrach et al., 2001) supports our conclusion. Fungal LCY-Bs have no homology with plant cyclases; however, massive amounts of lycopene accumulated in the Phycomyces Car23 mutant, which has a Glu-77-to-Lys-77 substitution. Glu-77 represents the acidic residue in the PxE(E/D) motif found in LCY-Bs of different organisms, including Mycobacterium aurum, Sulfolobus solfataricus, and fungi. Thus, although bacterial, fungal, and plant-type LCY-Bs are highly divergent, one could suggest that they have evolved to convert lycopene into β-carotene via an acid-catalyzed cyclization mechanism. Indeed, the formation of 5,6-diepikarpoxanthin and of putative 5,6-diepilatoxanthin catalyzed by CCS (Fig. 6) could be rationalized by considering that following the protic attack, the incipient carotenoid carbocation generated is flexibly stabilized to yield different structures (Fig. 7). This has been hypothesized previously (Deli et al., 1998), because these new xanthophylls are present in trace amounts in red pepper fruits and absent in yellow varieties, which are known to be devoid of CCS (Bouvier et al., 1994).
Protic activations of carbon-carbon double bonds are uncommon in nature. Typical examples are Δ8-Δ7 cholesterol isomerase (Wilton et al., 1969), isopentenyl diphosphate isomerase (Street et al., 1990), and squalene cyclase (Wendt et al., 2000). While Cys-67 has been identified as the protic activator in isopentenyl diphosphate isomerase (Street et al., 1990), Asp residues are the proton initiators in squalene cyclase. For squalene cyclase, protonation of the terminal double bond of squalene is triggered by Asp-376 of the D376DTAVV motif and assisted by His-451 (Wendt et al., 2000). It has been suggested that the tandem aspartic residues in the DDTAVV motif of squalene cyclase offer higher acidity, which is required for protonating the terminal double bond of squalene compared with the terminal oxirane of oxidosqualene, the phytosterol precursor, which is more easily protonated (Wendt et al., 2000). It is possible that the tandem Glu residues in the FLEET motif of LCY-Bs and CCS could play the same role. Interestingly, the similarity between the catalytic mechanism of squalene cyclase and LCY-B or CCS is supported by the fact that aza-squalene, a potent transient-state inhibitor of squalene cyclase (Reinert et al., 2004), is also a strong inhibitor of pepper CCS and LCY-B (Camara et al., 1985; Bouvier et al., 1997). Due to these characteristics, and because flavin prosthetic groups are not involved in squalene cyclase catalysis, one may suggest that CCS- or plant LCY-B-bound FAD, in its reduced form, is probably involved in the stabilization of the incipient carotenoid carbocation (Yu et al., 2010) and could function as previously envisioned “negative point charges” (Bouvier et al., 1997). Given previous biochemical data (Schnurr et al., 1996; Hornero-Mendez and Britton, 2002) indicating the requirement of NADPH for LCY-B activity, we show that FAD is likely reduced by NADPH. Further studies are required to fully evaluate this hypothesis.
In conclusion, the data presented herein reveal that plant-type carotenoid cyclases have evolved to integrate two characteristics. First, they belong to FAD flavoproteins that catalyze reactions without net redox changes (Bornemann, 2002). In the terpene series, this feature is shared with isopentenyl diphosphate isomerase II. Second, they belong to the class of non-metal-assisted cyclases such as squalene and oxidosqualene cyclases that operate via a protic attack. With respect to the latter mechanism, we show that Glu-295 of plant LCY-B and CCS or the equivalent Glu-196 from bacterial LCY-B plays a key role. Further work based on the CCS crystal is required to delineate the catalytic mechanism.
MATERIALS AND METHODS
Materials
All-trans-lycopene extracted from red tomato (Solanum lycopersicum) fruits and all-trans-xanthophylls extracted from red pepper (Capsicum annuum) fruits and Viola tricolor flowers (Deli et al., 1998) were purified (Davies, 1976) and used as substrates. Erwinia herbicola (renamed Pantoea agglomerans) DNA was used as the source for the amplification of bacterial LCY-B by PCR. CCS and LCY-B antibodies were prepared as described previously (Bouvier et al., 1994). The FAD-agarose affinity column and all chemicals were obtained from Sigma-Aldrich and GE Healthcare unless otherwise stated.
Site-Directed Mutagenesis
The different point mutations within the peptide sequences of pepper cyclase, pepper CCS, and E. herbicola lycopene cyclase were available from previous work (Mialoundama, 2009) using QuickChange site-directed mutagenesis (Stratagene) as described previously (Bouvier et al., 1997). The introduced mutations were verified by DNA sequencing using an automatic DNA sequencer. For Capsicum LCY-B (accession no. CAA60119), the following mutagenic oligonucleotides were used: D127A, 5′-GGTGTTTGGGTGGCTGAATTTGAGGCT-3′; E128A, 5′-GTTTGGGTGGATGCATTTGAGGCTATG-3′; D259A, 5′-ATGGTTTTCATGGCTTGGCGCGACTCT-3′; E295A, 5′-AGGATATTTCTTGCAGAAACCTCACTT-3′; E295K, 5′-AGGATATTTCTTAAAGAAACCTCACTT-3′; E295R, 5′-AGGATATTTCTTCGAGAAACCTCACTT-3′; E296A, 5′-ATATTTCTTGAAGCAACCTCACTTGTT-3′; E296K, 5′-ATATTTCTTGAAAAAACCTCACTTGTT-3′; E296R, 5′-ATATTTCTTGAACGAACCTCACTTGTT-3′; E332A, 5′-ATTGAAGAGGATGCACATTGTGTAATA-3′; H360A, 5′-GCCGGTATGGTTGCTCCATCCACCGGTT-3′; H360K, 5′-GCCGGTATGGTTAAACCATCCACCGGTT-3′; H360R, 5′-GCCGGTATGGTTCGTCCATCCACCGGTT-3′. For Capsicum CCS (accession no. CAA53759), the following mutagenic oligonucleotides were used: D127A, 5′-GGTGTTTGGGTTGCTGAGTTTGAAAAG-3′; E128A, 5′-GTTTGGGTTGATGCGTTTGAAAAGTTG-3′; D259A, 5′-ATGATGCTTATGGCTTGGAGGGATTCT-3′; E295A, 5′-TTGGTATTCTTGGCAGAGACTTCTTTA-3′; E295K, 5′-TTGGTATTCTTGAAAGAGACTTCTTTA-3′; E295R, 5′-TTGGTATTCTTGCGAGAGACTTCTTTA-3′; E296A, 5′-GTATTCTTGGAAGCGACTTCTTTAGTG-3′; E296K, 5′-GTATTCTTGGAAAAGACTTCTTTAGTG-3′; E296R, 5′-GTATTCTTGGAACGGACTTCTTTAGTG-3′; E332A, 5′-GTCCTTGAGGAAGCGAAGTGTGTGATC-3′; H360A, 5′-TCAGGGATAGTTGCTCCATCGTCTGGG-3′; H360K, 5′-TCAGGGATAGTTAAACCATCGTCTGGG-3′; H360R, 5′-TCAGGGATAGTTCGTCCATCGTCTGGG-3′. For Erwinia LCY-B (accession no. Q01331), the following mutagenic oligonucleotides were used: E196A, 5′-ACGCTGCTGATCGCGGATACGCGCTAC-3′; E196K, 5′-ACGCTGCTGATCAAGGATACGCGCTAC-3′; E196R, 5′-ACGCTGCTGATCAGGGATACGCGCTAC-3′. Mutated positions are underlined.
Expression and Characterization of Recombinant Enzymes
Recombinant LCY-B and CCS (reLCY-B and reCCS) devoid of transit peptide sequences were expressed in Escherichia coli (TOPO10) using pBAD/TOPOThiofusion vector (Invitrogen) before purification by metal affinity chromatography on TALON resin (Clontech; Bouvier et al., 1997). The mature part of pepper LCY-B (amino acid residues 54–498 of the precursor protein) and the mature part of CCS (amino acid residues 41–498 of the precursor protein) were amplified by PCR using the forward primer 5′-GCTCTTTTGGAGCTTGTACCTGAGAC-3′ and the reverse primer 5′-TTCTTTATCCTGTAACAAATTGTTGAT-3′ for LCY-B. For CCS, the forward primer 5′-TTCCATTATAGAAACAAAAGCAGTAC-3′ and the reverse primer 5′-AAGGCTCTCTATTGCTAGATTGCCCAG-3′ were used. The protein composition of each fraction was analyzed by SDS-PAGE and western blotting using the enhanced chemiluminescence western blotting detection kit (GE Healthcare) and anti-LCY-B or anti-CCS antibodies (Bouvier et al., 1994, 1997) followed by densitometry scanning using UN-SCAN-IT gel software (Silk Scientific). Protein concentrations were determined according to Smith et al. (1985).
Purification of ChrCCS and Analysis of Flavin Prosthetic Groups
ChrCCS was purified essentially as described previously from pepper chromoplasts (Bouvier et al., 1994) using sequentially Q-Sepharose, Affigel 501, Mono-P, and Sephacryl S-200 HR column chromatography and a last step including size-exclusion chromatography on a Superose-6 column using a Waters 650 FPLC system (Bouvier et al., 1994). Because the homogenous protein preparation displayed a yellow color that was preserved under mild SDS-PAGE (lowering by half the SDS concentration in the solubilization buffer and separation at 6°C), we first adopted an in-gel digestion procedure followed by MS analysis using an automated protein digestion system (MassPREP Station; Waters) as described previously (Heintz et al., 2009) to determine whether the prosthetic group was covalently bound. The gel was subsequently extracted using acetonitrile acidified with 1% formic acid. The resulting yellow supernatant was clarified by centrifugation, dried under argon, and solubilized in 200 μL of water:acetonitrile (90:10, v/v) before UPLC analysis on an Acquity UPLC system (Waters), using authentic FAD and FMN standards. The column was eluted with water containing 0.1% formic acid (solvent A) and acetonitrile containing 0.1% formic acid (solvent B). The flow rate was 0.40 mL min−1. Chromatographic conditions were as follows: linear gradient from 95% A to 5% B (2 min); 95% A to 100% B (6 min); 100% B (2 min); 100% B to 95% A (1 min); 95% A to 100% A (2 min). UV-visible spectra were recorded from 200 to 500 nm. The electrospray ionization source was used in positive and negative mode with capillary voltage of 3.4 kV; radiofrequency lens at 0 V, resolution (LM1, HM1, LM2, HM2) 15, ion energy 1 and 2: 0.5. Source and desolvation temperatures were 135°C and 400°C. MRM with positive electrospray ionization was used for qualitative analysis. Cone tensions and collision energy were optimized at 25 and 20 V for each compound. For FAD, the MRM transition was m/z 785.8→348, and for FMN, the MRM transition was m/z 457→439. The prosthetic group of reCCS or reLCY-B was extracted and analyzed using the same procedure. Purified CCS in phosphate buffer (pH 7) containing 10% glycerol and 0.5% Tween 80 was used for UV-visible absorption spectrophotometry using a NanoDrop 2000 (Thermo Scientific) or a Shimadzu UV2401 spectrophotometer, and fluorescence emission spectra were recorded with a Shimadzu RF5302 fluorescence spectrophotometer at room temperature following excitation at 458 nm. The flavin content was determined as described previously (Whitby, 1953).
Deflavinylation and Reconstitution of Carotenoid Cyclases
To prepare the apoenzyme of carotenoid cyclases, we modified a previously described procedure (Van Berkel et al., 1988). Purified carotenoid cyclases were adsorbed onto a PhenylSepharose column (1 × 5 cm) equilibrated with 50 mm K2HPO4 buffer (pH 7) containing 1 mm EDTA, 2 m NaCl, and 1 m KBr. FAD was released using the same buffer adjusted to pH 5. The apoproteins were subsequently eluted with 50 mm Tris-maleate buffer (pH 6.8) containing 15% glycerol and 40% dimethyl sulfoxide (DMSO). DMSO was eliminated by dialysis against the same buffer devoid of DMSO, and the fluorescence of the apoenzymes was recorded at 520 nm following excitation at 458 nm to assess the elimination of free FAD. For the reconstitution and determination of the binding affinity of FAD, carotenoid cyclase apoenzymes in 0.5 mL of Tris-maleate, pH 6.8, containing 15% glycerol were incubated with 5% molar excess of FAD for 12 h at 4°C. Excess FAD was removed using a Sephadex G-25 column (PD-10; GE Healthcare). The binding affinities were determined using GraphPad Prism 4.0 software.
Carotenoid Cyclase Assay
Unless otherwise stated, assays were performed in a reaction mixture (final volume of 250 μL) containing 50 mm Tris-maleate buffer, pH 6.8, 1 mm dithiothreitol, 2 mm NADPH, 10 μm FAD, 1 mg of Tween 80, 50 nmol of carotenoid substrates, and a definite amount of enzyme. The reaction mixture was flushed with argon and incubated at 25°C for 30 min to 1 h. The reaction products were isolated following the addition of 500 μL of chloroform/methanol (2:1, v/v) before HPLC analysis of the lipid phase (Camara, 1985; Bouvier et al., 2000). Alternatively, a plasmid-based complementation procedure was used to determine the conversion of lycopene into β-carotene using E. coli, which produces lycopene (Bouvier et al., 1997). After transformation with plasmids encoding wild-type and mutant LCY-Bs and CCSs and induction with 0.5 mm isopropyl-β-thiogalactopyranoside, total carotenoids were extracted from the bacterial pellets and subjected to HPLC analysis (Camara, 1985). General methods, including carotenoid analysis and identification, were as described previously (Davies, 1976). The HPLC system consisted of SpectraSYSTEM (ThermoFinnigan) comprising an SCM1000 solvent degasser, a P1-1000XR gradient pump, an AS3000 autosampler, and a UV6000LP diode array detector. Data acquisition and processing were done using ChromQuest version 3 software (ThermoFinnigan). The reaction products were collected, evaporated to dryness, and further characterized by UPLC-MS/MS using the MS mode. The analysis was performed on a Waters Quattro Premier XE equipped with an atmospheric pressure chemical ionization (APCI) source and coupled to an Acquity UPLC system (Waters) with diode array detector. UV spectra were recorded from 200 to 500 nm. Chromatographic separation was achieved using an Acquity UPLC BEH C18 column (100 × 2.1 mm, 1.7 μm; Waters) coupled to an Acquity UPLC BEH C18 precolumn (2.1 × 5 mm, 1.7 μm; Waters). The mobile phases were 75% methanol in water (solvent A) and 100% isopropanol (solvent B). The following separation sequence was adopted: 100% A (5 min); 100% A to 50% B (3 min); 50% A + 50% B (4 min); 50% A to 100% B (5 min); 100% B (10 min); 100% B to 50% A (2 min); 50% B + 50% A (5 min); 50% B to 100% A (6 min). The parameters for MS detection and APCI were as follows: nebulizer gas flow was set to approximately 50 L h−1, and the desolvation gas flow was 150 L h −1. The APCI probe temperature was set to 500°C, and the source temperature was 120°C. The capillary voltage was set at 1.5 kV, and cone voltage after optimization for each carotenoid was set to 25 V. The ionization mode was positive. Low-mass and high-mass resolution were 15 for both mass analyzers, ion energies 1 and 2 were 1 V, entrance and exit potentials were 50 V, and detector (multiplier) gain was 650 V. Mass spectra of carotenoids were acquired with a scan range of m/z 350 to 750 atomic mass units. The combination of the chromatography retention time (tR) and the parent mass was used to selectively monitor the different carotenoids as follows: antheraxanthin ([M+H+] = 586, tR = 8.47), violaxanthin ([M+H+] = 602, tR = 8.13), capsanthin ([M+H+] = 586, tR = 8.32), capsanthin-5,6-epoxide ([M+H+] = 602, tR = 7.96), capsorubin ([M+H+] = 584, tR = 7.81), and 5.6-diepikarpoxanthin ([M+H+] = 602, tR = 7.59). Data acquisition and analysis were performed with MassLynx software (version 4.1) running under Windows XP professional on a Pentium personal computer. The kinetic parameters were obtained by nonlinear regression analysis using the GraphPad Prism 4.0 program (GraphPad Software).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers CAA60119 for Capsicum LCY-B and CAA53759 for Capsicum CCS.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Spectral analysis of purified reCCS.
Supplemental Figure S2. Multiple alignment of deduced amino acid sequences of selected LCYs and pepper CCS.
Supplemental Figure S3. Total ion chromatogram analysis of carotenoid products isolated from the incubation of reCCS with xanthophyll epoxides.
Supplementary Material
Acknowledgments
We thank P. Hammann and M. Alioua for DNA sequencing.
References
- Armstrong GA. (1997) Genetics of eubacterial carotenoid biosynthesis: a colorful tale. Annu Rev Microbiol 51: 629–659 [DOI] [PubMed] [Google Scholar]
- Arrach N, Fernandez-Martin R, Cerdà-Olmedo E, Avalos J. (2001) A single gene for lycopene cyclase, phytoene synthase and regulation of carotene biosynthesis in Phycomyces. Proc Natl Acad Sci USA 98: 1687–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann S. (2002) Flavoenzymes that catalyse reactions with no net redox change. Nat Prod Rep 19: 761–772 [DOI] [PubMed] [Google Scholar]
- Bouvier F, d'Harlingue A, Backhaus RA, Kumagai MH, Camara B. (2000) Identification of neoxanthin synthase as a carotenoid cyclase paralog. Eur J Biochem 267: 6346–6352 [DOI] [PubMed] [Google Scholar]
- Bouvier F, d'Harlingue A, Camara B. (1997) Molecular analysis of carotenoid cyclase inhibition. Arch Biochem Biophys 346: 53–64 [DOI] [PubMed] [Google Scholar]
- Bouvier F, Hugueney P, d'Harlingue A, Kuntz M, Camara B. (1994) Xanthophyll biosynthesis in chromoplast: isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenoid. Plant J 6: 45–54 [DOI] [PubMed] [Google Scholar]
- Britton G. (1998) Overview of carotenoid biosynthesis. Britton G, Liaaen-Jensen S, Pfander H, , Carotenoids, Vol 3 Birkhäuser Verlag, Basel, pp 13–147 [Google Scholar]
- Camara B. (1980) Biosynthesis of keto-carotenoids in Capsicum annuum fruits. FEBS Lett 118: 315–318 [Google Scholar]
- Camara B. (1985) Carotene synthesis in Capsicum chromoplasts. Methods Enzymol 110: 244–253 [Google Scholar]
- Camara B, Dogbo O, d'Harlingue A, Bardat F. (1985) Inhibition of lycopene cyclization by Capsicum chromoplast membranes by 2-aza-2,3-dihydrosqualene. Phytochemistry 24: 2751–2752 [Google Scholar]
- Cunningham FX, Jr, Gantt E. (2001) One ring or two? Determination of ring number in carotenoids by lycopene epsilon-cyclases. Proc Natl Acad Sci USA 98: 2905–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham FX, Jr, Sun Z, Chamovitz D, Hirschberg J, Gantt E. (1994) Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6: 1107–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BH. (1976) Carotenoids. Goodwin TW, , Chemistry and Biochemistry of Plant Pigments, Vol 2 Academic Press, London, pp 38–165 [Google Scholar]
- Deli J, Molnar P, Zoltan M, Toth G, Steck A, Pfander H. (1998) Isolation of carotenoids with 3,5,6,-trihydroxy-5,6-dihydro-β-end groups from red paprika (Capsicum annuum). Helv Chim Acta 81: 1233–1241 [Google Scholar]
- Dym O, Eisenberg D. (2001) Sequence-structure analysis of FAD-containing proteins. Protein Sci 10: 1712–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht AR. (1987) Kinetic aspects of purposely modified proteins. Oxender DL, Fox CF, , Protein Engineering. AR Liss, New York, pp 221–224 [Google Scholar]
- Heintz D, Gallien S, Wischgoll S, Ullmann AK, Schaeffer C, Kretzschmar AK, van Dorsselaer A, Boll M. (2009) Differential membrane proteome analysis reveals novel proteins involved in the degradation of aromatic compounds in Geobacter metallireducens. Mol Cell Proteomics 8: 2159–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Ikeda Y, Yamashita S, Nakayama T, Nishino T. (2004) Catalytic mechanism of type 2 isopentenyl diphosphate:dimethylallyl diphosphate isomerase: verification of a redox role of the flavin cofactor in a reaction with no net redox change. Biochem Biophys Res Commun 322: 905–910 [DOI] [PubMed] [Google Scholar]
- Hornero-Mendez D, Britton G. (2002) Involvement of NADPH in the cyclization reaction of carotenoid biosynthesis. FEBS Lett 515: 133–136 [DOI] [PubMed] [Google Scholar]
- Hugueney P, Badillo A, Chen HC, Klein A, Hirschberg J, Camara B, Kuntz M. (1995) Metabolism of cyclic carotenoids: a model for the alteration of this biosynthetic pathway in Capsicum annuum chromoplasts. Plant J 8: 417–424 [DOI] [PubMed] [Google Scholar]
- Krubasik P, Sandmann G. (2000) A carotenogenic gene cluster from Brevibacterium linens with novel lycopene cyclase genes involved in the synthesis of aromatic carotenoids. Mol Gen Genet 263: 423–432 [DOI] [PubMed] [Google Scholar]
- Lineweaver H, Burk D. (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56: 658–666 [Google Scholar]
- Maresca JA, Graham JE, Wu M, Eisen JA, Bryant DA. (2007) Identification of a fourth family of lycopene cyclases in photosynthetic bacteria. Proc Natl Acad Sci USA 104: 11784–11789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JA, Wang L, Kohen A. (2004) Synthesis of R and S tritiated reduced beta-nicotinamide adenine dinucleotide 2′ phosphate. Anal Biochem 324: 131–136 [DOI] [PubMed] [Google Scholar]
- Mialoundama AS. (2009) Régulation moléculaire du métabolisme secondaire chez les plantes au cours du développement et sous l'effet du stress. PhD thesis Université de Strasbourg, Strasbourg, France [Google Scholar]
- Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa K, Nakamura K, Harashima K. (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172: 6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, Jarvik T. (2010) Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328: 624–627 [DOI] [PubMed] [Google Scholar]
- Reinert DJ, Balliano G, Schulz GE. (2004) Conversion of squalene to the pentacarbocyclic hopene. Chem Biol 11: 121–126 [DOI] [PubMed] [Google Scholar]
- Ronen G, Carmel-Goren L, Zamir D, Hirschberg J. (2000) An alternative pathway to β-carotene formation in plant chromoplasts discovered by map-based cloning of Beta and old-gold color mutations in tomato. Proc Natl Acad Sci USA 97: 11102–11107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann MG, Moras D, Olsen KW. (1974) Chemical and biological evolution of nucleotide-binding protein. Nature 250: 194–199 [DOI] [PubMed] [Google Scholar]
- Rothman SC, Helm TR, Poulter CD. (2007) Kinetic and spectroscopic characterization of type II isopentenyl diphosphate isomerase from Thermus thermophilus: evidence for formation of substrate-induced flavin species. Biochemistry 46: 5437–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SC, Johnston JB, Lee S, Walker JR, Poulter CD. (2008) Type II isopentenyl diphosphate isomerase: irreversible inactivation by covalent modification of flavin. J Am Chem Soc 130: 4906–4913 [DOI] [PubMed] [Google Scholar]
- Schnurr G, Misawa N, Sandmann G. (1996) Expression, purification and properties of lycopene cyclase from Erwinia uredovora. Biochem J 315: 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85 [DOI] [PubMed] [Google Scholar]
- Street IP, Christensen DJ, Poulter CD. (1990) Hydrogen exchange during the enzyme-catalyzed isomerization of isopentenyl diphosphate and dimethylallyl diphosphate. J Am Chem Soc 112: 8577–8578 [Google Scholar]
- Unno H, Yamashita S, Ikeda Y, Sekiguchi SY, Yoshida N, Yoshimura T, Kusunoki M, Nakayama T, Nishino T, Hemmi H. (2009) New role of flavin as a general acid-base catalyst with no redox function in type 2 isopentenyl-diphosphate isomerase. J Biol Chem 284: 9160–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel WJ, Van den Berg WA, Muller F. (1988) Large-scale preparation and reconstitution of apo-flavoproteins with special reference to butyryl-CoA dehydrogenase from Megasphaera elsdenii: hydrophobic-interaction chromatography. Eur J Biochem 178: 197–207 [DOI] [PubMed] [Google Scholar]
- Velayos A, Eslava AP, Iturriaga EA. (2000) A bifunctional enzyme with lycopene cyclase and phytoene synthase activities is encoded by the carRP gene of Mucor circinelloides. Eur J Biochem 267: 5509–5519 [DOI] [PubMed] [Google Scholar]
- Verdoes JC, Krubasik KP, Sandmann G, van Ooyen AJ. (1999) Isolation and functional characterisation of a novel type of carotenoid biosynthetic gene from Xanthophyllomyces dendrorhous. Mol Gen Genet 262: 453–461 [DOI] [PubMed] [Google Scholar]
- Viveiros M, Krubasik P, Sandmann G, Houssaini-Iraqui M. (2000) Structural and functional analysis of the gene cluster encoding carotenoid biosynthesis in Mycobacterium aurum A+. FEMS Microbiol Lett 187: 95–101 [DOI] [PubMed] [Google Scholar]
- Wendt KU, Schulz GE, Corey EJ, Liu DR. (2000) Enzyme mechanisms for polycyclic triterpene formation. Angew Chem Int Ed 39: 2813–2833 [PubMed] [Google Scholar]
- Whitby LG. (1953) A new method for preparing flavin-adenine dinucleotide. Biochem J 54: 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton DC, Rahimtula AD, Akhtar M. (1969) The reversibility of the Δ8-cholesterol-Δ7-cholesterol isomerase reaction in cholesterol biosynthesis. Biochem J 114: 71–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Schaub P, Ghisla S, Al-Babili S, Liszkay-Krieger A, Beyer P. (2010) The lycopene cyclase CrtY from Pantoea ananatis (ex Erwinia uredovora) catalyzes an FADred-dependent non-redox reaction. J Biol Chem 285: 12109–12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.