Abstract
Studies centered on understanding how molecular structure affects biological function have historically focused on proteins. Circular dichroism (CD) is commonly used to analyze protein secondary structure, yet its application to other molecules is far less explored. In fact, little is known about how glycan conformation might affect function, likely because of a lack of tools for measuring dynamic structural changes of carbohydrates. In the present study, we developed a method based on CD to monitor conformational changes in the zwitterionic T-cell-activating glycoantigen polysaccharide A1 (PSA). We found that PSA helical structure produces a CD spectrum that is strikingly similar to proteins rich in α-helical content and is equally sensitive to nonpolar solvents. Like conventional T-cell-dependent proteins, PSA requires processing before major histocompatibility complex class II (MHCII) binding. CD spectra of PSA fragments of varying sizes indicated that fragments smaller than three repeating units lack helical content and are incapable of MHCII binding. Likewise, neutralization of charged groups in the repeating unit resulted in major conformational changes as measured by CD, which correlated with a lack of MHCII presentation. These data represent two significant findings: CD can be used to measure conformational changes in carbohydrates and the functional epitope from PSA is dependent on a specific conformation that is stabilized by adjacent repeating units and a zwitterionic charge motif. As a result, this work demonstrates that CD is a valuable tool for use in functional glycomics efforts that seek to align chemical and conformational structure with biological activity.
Keywords: circular dichroism, conformation, glycoantigen, MHC class II, polysaccharide
Introduction
The predominant emphasis in carbohydrate research is on elucidating the primary sequence, repeating unit composition, and glycosidic linkages of complex glycans, although investigation of conformation and carbohydrate dynamics in solution is much less explored. The inherent flexibility of carbohydrate linkages and the resulting dynamic and variable secondary and higher order structures lends considerable difficulty to these types of analyses.
Monitoring the solution structure of biological molecules via optical properties is traditionally accomplished using a number of methods, including Raman and infrared spectroscopies and circular dichroism (CD) polarimetry. In CD, chiral molecules show a difference in their absorption of left-and right-circularly polarized light as they interact with neighboring groups and their solution, and this difference can be expressed as molar ellipticity, θ. CD is most commonly used to study the concentration, based on Beer’s law, of protein secondary structure, as α-helices and β-sheets produce characteristic far ultraviolet (UV) spectra between 190 and 250 nm, and changes in protein conformation upon ligand binding or thermodynamic unfolding are readily apparent. Because most monosaccharides absorb light that is <200 nm, vacuum UV CD at wavelengths between 160 and 200 nm can also be used to study the stereochemistry of sac-charide monomers and the arrangement of linkages between saccharides (Johnson 1987). In fact, changes in ellipticity can be used to differentiate between the complex equilibrium individual asymmetric monosaccharides freely adopt in solution, including the pyranose, furanose, α and β anomer, and the acyclic and septanose forms (Nelson and Johnson 1976). Using these methodologies provides the ability to monitor shifts in chair conformation, optical rotation, alternations in dipole moments, and variations in linkage in response to the solution environment of individual saccharides (Pysh 1976).
The secondary and higher order structures of polysacchar-ides have been studied very little, because of the flexibility of the glycosidic bonds and the difficulty of attributing fixed structures to the molecules. Most investigations of polysaccharide structure have utilized 13C- and/or 1H–Nuclear Overhauser enhancement spectroscopy (NOESY) nuclear magnetic resonance (NMR) (Koerner et al. 1988), and although some linear polysaccharides such as the capsule of group B meningococci display a random coil structure with extreme heterogeneity in polysaccharide length and flexibility (Henderson et al. 2003), others such as carrageenan form rigid helices stabilized by extensive electrostatic interactions (Piculell et al. 1997). Analysis of carbohydrate conformation has also been performed with limited success using X-ray diffraction, particularly on the nonzwitterionic type III Streptococcus pneumonia capsular polysaccharide (Marchessault et al. 1980). It was found that the standard colorimetric and NMR techniques were not able to provide structural information of the polysaccharide Vi antigen from Salmonella typhi, because of its unusual chemical composition (Stone and Szu 1988; Szu et al. 1991).
The average solution structures of several polysaccharides have been elucidated with CD, most notably for hyaluronic acid and the hyaluronate anion in ethanol and dimethylsufoxide, and at various pH values. The results revealed that the structural rearrangement of HA strands reflects cooperative association (Staskus and Johnson 1988) and provided information on the ways in which the polysaccharides interact with their solution environment (Park and Chakrabarti 1978). Similar studies utilizing heparin (Park and Chakrabarti 1977; Mukherjee et al. 1978; Stone 1985) and chondroitin (Cowman et al. 1981) have been performed.
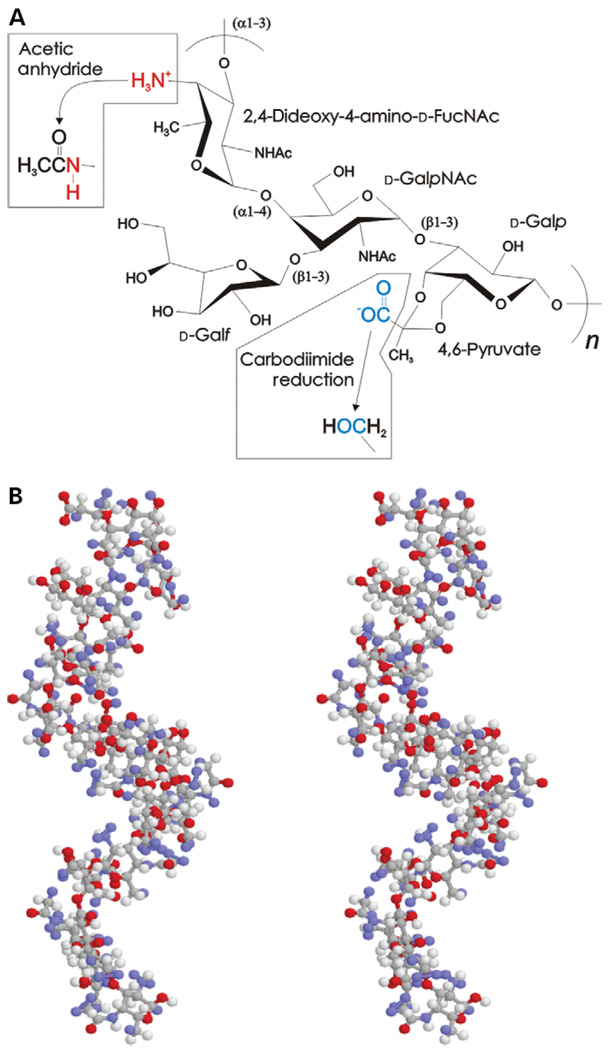
The polysaccharide PS A1 (PSA) is the predominant capsular component from the NCTC9343 strain of Bacteroides fragilis (Krinos et al. 2001) and has been shown to have unusual immunogenic activity (Tzianabos et al. 1993; Cobb et al. 2004; Mazmanian et al. 2005). The primary structure of the glycoantigen PSA consists of a zwitterionic tetrasaccharide repeating unit containing 2,4-dideoxy-4-amino-d-N-acetyl-fucose, d-N-acetylgalactosamine, d-galactopyranose, and d-galactofuranose, with 4,6-pyruvate attached to the galactopyranose, as determined by butanolysis, peracetylation, gas chromatography–mass spectroscopy, and NMR (Figure 1A) (Baumann et al. 1992). The structure of PSA has also previously been investigated using total correlated spectroscopy and NOESY NMR, where it was found that the average solution structure of PSA is characterized by a stable extended helical conformation with two repeating units per turn and a pitch of 20 Å (Figure 1B). The zwitterionic motif is formed with alternating anionic carboxylate lying in repeated grooves every 15 Å and cationic-free amines exposed on the outer surface of the carbohydrate (Wang et al. 2000).
Fig. 1.
PSA structure. (A) Chemical composition of one tetrasaccharide repeating unit of PSA, illustrating the chemical modifications to eliminate the negatively charged free amine (red) and the negatively charged carboxylate (blue) with acetic anhydride and carbodiimide reduction, respectively. (B) Stereo ball-and-stick average solution structure of PSA using coordinates determined by the NMR spectroscopy, showing the right-handed helical conformation (Wang et al. 2000).
It has been known for a number of years that B. fragilis is able to activate T cells and that the specific component inducing activation is PSA (Tzianabos et al. 1993, 2000). The T cells activated via PSA are able to stimulate the generation of antibodies protective against bacteremia (Kasper and Onderdonk 1982) while provoking abscess formation at the sites of infection (Kasper et al. 1989). We recently showed that PSA-mediated T-cell activation is dependent on processing via oxidative cleavage of the polysaccharide into small antigenic fragments inside the endo-lysosomal vesicles and major histocompatibility complex class II (MHCII) loading in an acid pH-dependent process (Cobb et al. 2004). Animals deficient in their ability to produce nitric oxide were unable to form abscesses upon PSA challenge, but if the antigen was “pre-processed” by in vitro ozonolysis prior to challenge, the animals regained their ability to activate T cells and form abscesses. After processing and MHCII loading, the PSA–MHCII complex moves to the cell surface where it is recognized by αβ T-cell receptors to form the classical immune synapse to activate T cells. Because this pathway was previously known only for protein antigens, the discovery of MHCII-dependent T-cell-activating glycoantigens represents a major shift in the known paradigm of antigen processing and presentation.
The immunogenic activity of PSA is dependent on the presence of the zwitterionic motif and molecular mass. Elimination of either charge group in PSA results in a lack of in vivo T-cell activation (Tzianabos et al. 1993). This feature is common to other T-cell-activating glycoantigens, such as the polysaccharides from S. pneumonia type I (Choi et al. 2002; Dominguez et al. 2006) and Staphylococcus aureus (Choi et al. 2002; O’Riordan and Lee 2004). In addition, oxidative processing of PSA from its native size of >100 kDa to fragments < 15 kDa is critical for MHCII presentation (Cobb et al. 2004), whereas other studies suggest that fragments smaller than approximately 5 kDa have diminished T-cell stimulatory activity (Kalka-Moll et al. 2000). These reports suggest that the epitope derived from PSA is more complex than a single repeating unit and therefore may involve a conformational component. As a result, PSA is an excellent model carbohydrate to use in the development of new tools to study conformational structures and their role in biological activity of important polysaccharides, such as carbohydrates from Francisella tularensis (Tarnvik 1989; Hartley et al. 2006), Cryptococcus neoformans (Yauch et al. 2005; Mansour et al. 2006), and S. typhi Vi (Raffatellu et al. 2005, 2006). The development of these tools should prove to be very important in the creation and expansion of modern glycomics initiatives.
In the present report, we sought to understand the impact of the zwitterionic motif and varied molecular mass on the conformation of PSA by developing a method that employs far UV CD analysis as a measure of structural integrity. We were able to observe the helical conformation of full-length PSA using CD that was sensitive to the inclusion of a nonpolar solvent (i.e., acetonitrile), as commonly seen with protein secondary structures (Rosenblatt et al. 1980; Carbone et al. 1987). The helical structure is also present in ozone-processed polysaccharide at sizes greater than two repeating units, yet the CD spectra of lower molecular-weight (MW) PSA samples show a lack of helical content. The removal of either or both of the alternating charges that constitute the zwitterionic charge motif also obliterates the helices and the ability to bind MHCII. Our results show that the presence or absence of helical structure is directly related to the ability to bind MHCII molecules. This is the first report of CD being utilized to study the solution secondary structure of an antigenic linear polysaccharide, which could prove to be an important tool both in the study of carbohydrate structure and function relations and in glycomics-based approaches for identifying additional carbohydrates that may have an interesting immunological activity.
Results
Aqueous CD measurements
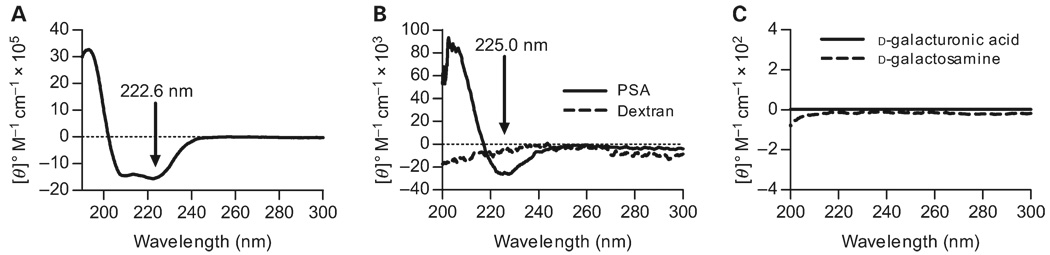
On the basis of the average solution structures solved by 2D NMR, the zwitterionic carbohydrate antigen PSA forms an extended right-handed helix (Figure 1B) (Wang et al. 2000). To test whether the helicticity of PSA can be monitored by CD, we compared scans of myoglobin, a protein made of nearly all α-helices, a nonhelical polysaccharide (80 000 kDa dextran), and PSA. Myoglobin scans agreed well with the expected profile, including the local minimum at 222.6 nm and the local maximum at or near 200 nm (Figure 2A). The CD profile for PSA was strikingly similar to the myoglobin scan in that a local minimum was seen at 225.0 nm and a local maximum at 205 nm (Figure 2B). The signal strength was approximately 30 times less for PSA compared with protein but was easily and reproducibly measured. In addition, the dual minima seen in classic α-helical scans (Figure 2A) are not seen in the data for helical polysaccharide. In contrast, the nonhelical dextran failed to show a significant optical activity (Figure 2B).
Fig. 2.
CD spectra of proteins and carbohydrates. The dotted line in each panel represents the zero ellipticity point. (A) CD spectrum of myoglobin at 10 µM in PBS, showing the primarily α-helical structure. The characteristic minima are indicated with an arrow at 222.6 nm and a maximum at approximately 195 nm. (B) CD spectra of PSA (solid line) and dextran (dashed line) at 50 µM in PBS. The nonstructured dextran does not show significant ellipticity, whereas PSA has both a minimum similar to that found in myoglobin at 225.0 nm (arrow) and a maximum at approximately 205 nm, suggesting comparable conformations. The total molar ellipticity for PSA is approximately 30-fold less than myoglobin. (C) Monosaccharides similar to those found in the PSA repeating unit fail to yield CD signals at 2.5 mM each in PBS.
To confirm that the ellipticity seen with PSA was due to conformational characteristics rather than the monosaccharide constituents, spectra were collected of two monosaccharides in phosphate-buffered saline (PBS), which are similar to those found in the PSA repeating unit that provide the negative and positive charges in PSA. For both d-galacturonic acid and d-galactosamine, no significant CD signal was observed (Figure 2C).
The effect of nonpolar solvent on CD spectra
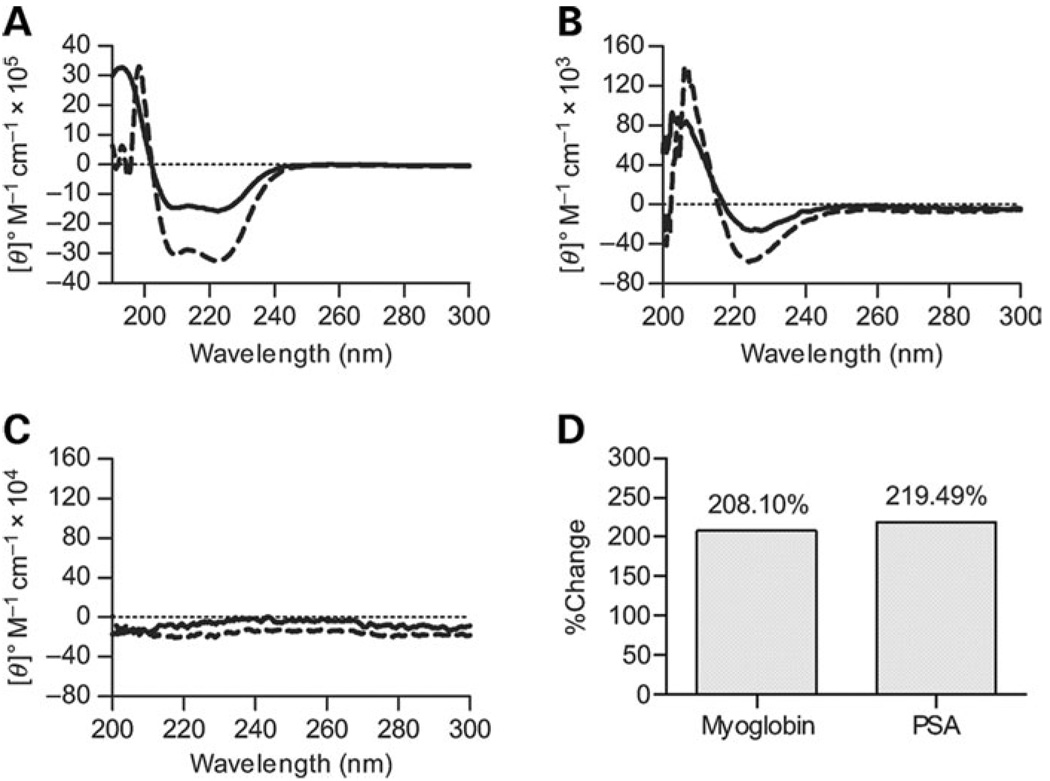
It is common to use nonpolar organic solvents such as aceto-nitrile to increase the ellipticity measured by CD. This effect is due in part to the strengthening of hydrogen bonding between key contacts that stabilize protein secondary structure by forcing those groups away from the nonpolar solvent. As would be expected, myoglobin showed an increased molar ellipticity in 20% acetonitrile (Figure 3A). It was therefore reasonable to hypothesize that if the CD spectrum for PSA is due to the helical conformation of PSA, acetonitrile should enhance ellipticity. We found that 20% acetonitrile increased the molar ellipticity seen with PSA in solution (Figure 3B), whereas no change in the essentially flat spectrum of dextran was seen (Figure 3C). Indeed, the percent change when comparing the minimum at or near 225 nm for both myoglobin and PSA was nearly identical (208.1% versus 219.5%, respectively, Figure 3D), demonstrating indistinguishable acetonitrile effects on both molecules.
Fig. 3.
Nonpolar solvent effects. In all panels, CD spectra collected in PBS are represented as solid lines, those collected in PBS with 20% acetonitrile as dashed lines, and the zero point as a dotted line. (A) CD spectra of 10-µM myoglobin in PBS with and without acetonitrile, showing an increase in negative molar ellipticity at 223 and 210 nm and a narrowing of the maximum peak at 200 nm characteristic of helical conformations. (B) CD spectra of 50 µM PSA in PBS with and without acetonitrile, showing a similar increase in negative molar ellipticity at 225 nm as seen with myoglobin. The maximum peak also narrows and shifts slightly to a higher wavelength, suggesting that the spectra shape is due to helical conformation. (C) CD spectra of dextran under the same two conditions, with no signal in either solvent system. (D) Quantification of the percent change at 223 nm for both myoglobin and PSA, illustrating the similarity of the acetonitrile effect on both CD spectra.
The thermal stability of PSA
Dynamic folding studies as a function of temperature are often accomplished by CD because the loss of tertiary structure (measured by near UV CD) to form a molten globule conformation that retains secondary structure (measured by far UV CD) is a common intermediate step in the folding or unfolding pathway of many proteins and can be easily distinguished by CD. Likewise, the final loss of secondary structure is easily monitored.
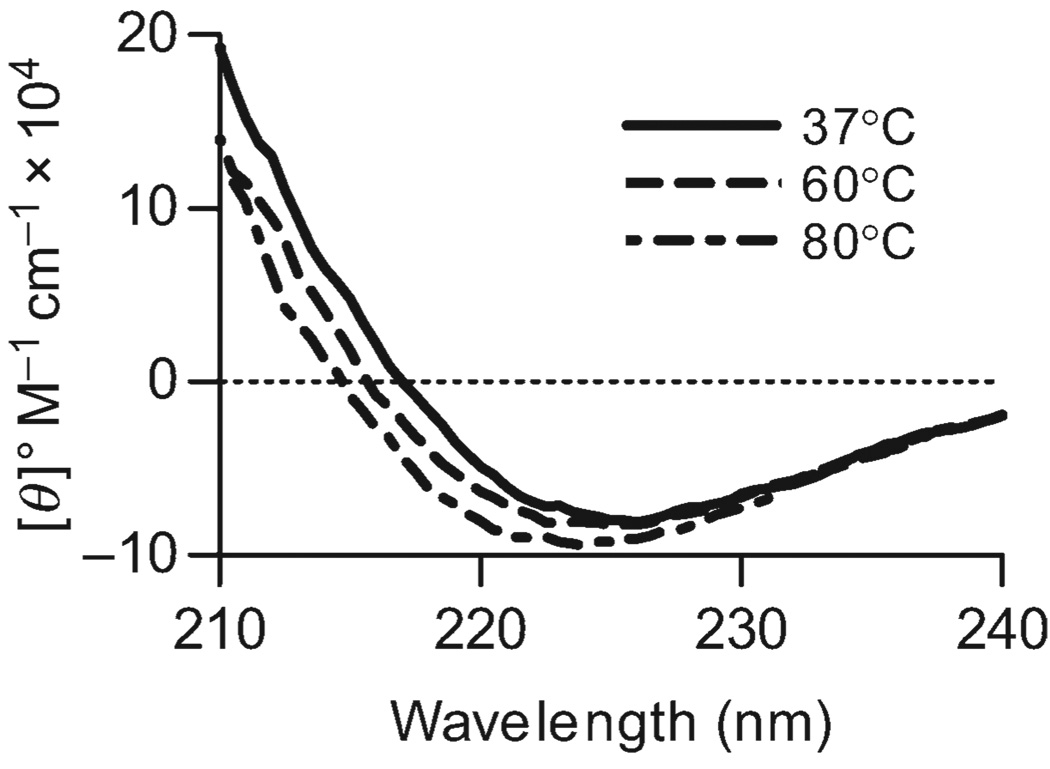
With CD as a new tool for measuring the structural integrity of PSA, we first sought to understand the thermal stability of this conformation. The carbohydrate solution was incubated at a range of temperatures between 37 and 80 °C for approximately 10 min before collecting the CD spectra. We found that even at high temperatures, no significant difference in the average structure can be seen (Figure 4).
Fig. 4.
Thermal stability of PSA. A 50-µM solution of PSA in PBS was incubated at varying temperatures and CD spectra collected. At a range of temperatures between 37 and 80 °C (not all shown), no significant changes in the spectra were seen, suggesting that the conformation of PSA is remarkably stable.
The structure and function relation for PSA: MW
We recently demonstrated for the first time that PSA activates T cells in a manner that requires processing to low MW fragments for binding to and presentation by MHCII molecules. The mechanism of cellular processing of carbohydrate antigens is mediated by the production of nitric oxide, likely through a direct chemical oxidation that can be mimicked in solution with ozone (Cobb et al. 2004) and other strong oxidant-mediated cleavage at glycosidic bonds (Kokki and Uprichard 1991). Because MHCII binding is dependent on oxidative cleavage of PSA, we oxidized PSA with ozone for varying lengths of time and then correlated size with structure as measured by CD and function as measured by in vitro MHCII binding.
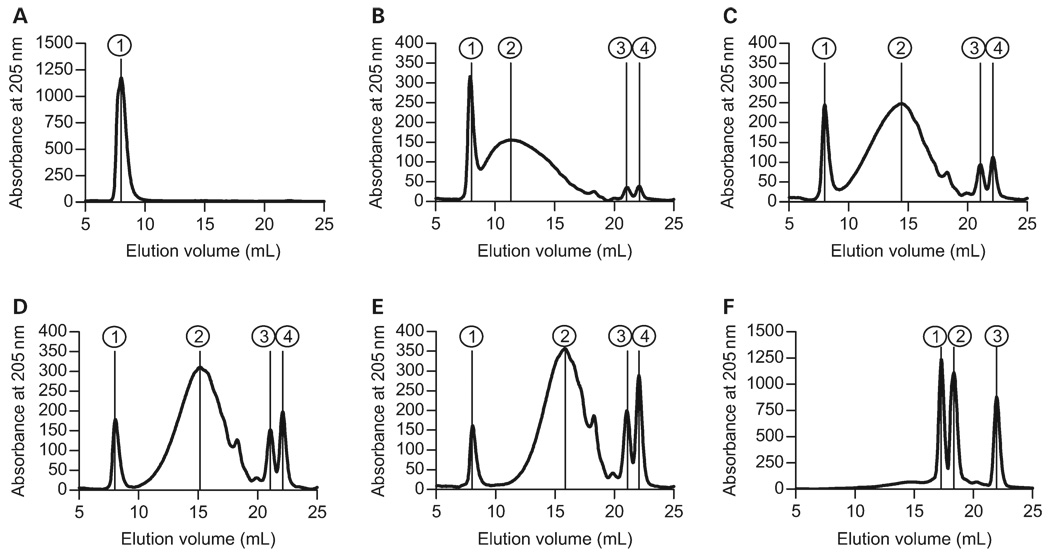
First, ozone was bubbled through a concentrated solution of purified PSA in PBS at a constant flow rate of 100 mg/h. At 0, 15, 30, 45, 60, and 90 min, aliquots were removed for analysis. Using a dextran-standardized Superdex75 (GE Healthcare Biosciences, Piscataway, NJ) size exclusion column on an high-pressure liquid chromatography (HPLC) system, we found that untreated “native” PSA elutes at the void volume of the column, indicating a size >50 kDa (Figure 5A and Table I). By 15 min of ozone treatment, the high MW PSA had been cleaved into a number of fragments including the intact high MW peak (Figure 5B, Peak 1, >50 kDa) and two low MW peaks that likely represent monosaccharides (Figure 5B, Peaks 3 and 4, <1 kDa). We also observed that the major fraction of PSA spanned nearly the entire column elution profile, with a peak at approximately 17.3 kDa (Figure 5B, Peak 2). Likewise, as the length of ozone treatment increased, the high MW peak continued to become a smaller proportion of the sample, whereas the low MW monosaccharides became a larger proportion of the total sample (Figure 5C–E and Table I). The main peak in between these sizes continued to move to lower MWs, arriving at about 1.0 kDa after 90 min of treatment (Figure 5F, Peak 1), which corresponds to a single repeating unit. A summary of the average MW of each peak is presented in Table I and an overlay of these elution profiles is shown in Figure 6A for direct comparison.
Fig. 5.
MW of ozone-treated PSA samples (100 µg per injection), as measured by size exclusion chromatography on a Superdex75 column fitted to an HPLC system. The column was standardized with dextrans of known MW (1, 5, 12, 50, and 150 kDa). All data represent the absorbance of 205 nm and peaks are numbered. Peak and MW details are listed in Table I: (A) untreated PSA; (B) PSA ozone cleaved for 15 min; (C) PSA ozone cleaved for 30 min; (D) PSA ozone cleaved for 45 min; (E) PSA ozone cleaved for 60 min; (F) PSA ozone cleaved for 90 min. Increased exposure to ozone results in the cleavage of PSA into progressively smaller fragments. Native PSA is >50 kDa (A, peak 1), but by 90 min, the largest PSA fragment (F, peak 1) is approximately 1 kDa.
Table I.
Summary of PSA fragment sizes from ozone cleavage time course
| Sample (min) | Peak no. | Vr, mL | MW (kDa) |
|---|---|---|---|
| No ozone | 1 | 8.008 | >50 |
| 15 | 1 | 8.008 | >50 |
| 2 | 11.323 | 17.3 | |
| 3 | 21.044 | <1 | |
| 4 | 22.124 | ≪1 | |
| 30 | 1 | 8.008 | >50 |
| 2 | 14.452 | 4.0 | |
| 3 | 21.081 | <1 | |
| 4 | 22.124 | ≪1 | |
| 45 | 1 | 8.045 | >50 |
| 2 | 15.159 | 2.9 | |
| 3 | 21.081 | <1 | |
| 4 | 22.124 | ≪1 | |
| 60 | 1 | 8.082 | >50 |
| 2 | 15.867 | 2.1 | |
| 3 | 21.119 | <1 | |
| 4 | 22.087 | ≪1 | |
| 90 | 1 | 17.282 | 1.06 |
| 2 | 18.362 | <1 | |
| 3 | 21.975 | ≪1 |
Fig. 6.
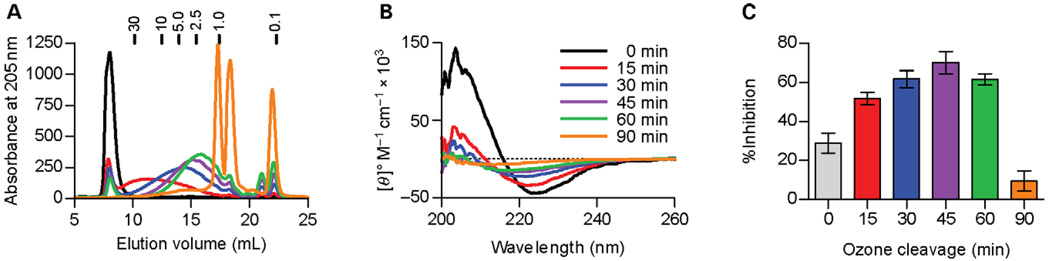
Effects of size on structure and function. (A) Overlaid Superdex75 elution patterns of ozone-cleaved PSA samples with MW markers listed for reference. (B) CD spectra of ozone-cleaved PSA samples, showing a progressive loss of helical character as more ozone-mediated breaks are introduced. The full-length PSA (0 min) shows the greatest helicticity, whereas the sample with fragments 1 kDa and smaller (90 min) shows no helical content. (C) Binding of PSA fragments to MHCII protein as measured by competition with a conventional peptide antigen, expressed as percentage of peptide binding inhibition. No significant difference (P > 0.05) in MHCII binding is seen between the full-length (0 min) and < 1 kDa (90 min) PSA; however, samples treated with ozone for 15, 30, 45, or 60 min all bound to a much higher extent (P < 0.001). These data demonstrate that the presence of PSA fragments eluting from the Superdex75 column between 10 and 15 mL (approximately 30–3 kDa), which retain helical content, is required for MHCII binding. Statistical comparisons of these data are shown in Table II.
Next, we collected the CD spectra of each ozone time point aliquot to determine the impact of size on helical content. We found that the minimum present at 225 nm shifts very slightly to shorter wavelengths and to lower intensity as the size of the fragment pool is decreased, indicating a progressive loss of helicticity in the average PSA conformation. Indeed, by the time that most of the fragments are one repeating unit or less (90 min of ozone, Figure 6A), the spectrum appears similar to the flat spectrum of unstructured dextran (Figure 3C).
To correlate these structural changes with function, we performed binding competition assays with human leukocyte antigen DR1 (HLA-DR1). In these assays, DR1 was coated onto the wells of a high protein binding enzyme linked immunosorbent assay (ELISA) plate, and then biotiny-lated myelin basic protein peptide (bMBPp) was added with and without the ozone-treated PSA samples. Competition was used to enable binding measurements without the complicating factor of differential biotinylation of the various sizes of PSA, which would skew any direct analysis. Equilibrium was allowed to establish at 37 °C for 24 h and then the amount of bound bMBPp was determined with europium-conjugated streptavidin and time-resolved fluorometry (TRF). Data were normalized to 100% binding in the absence of PSA and expressed as percent inhibition of bMBPp binding. Intact (untreated) and very small PSA (90-min ozone) did not bind well (approximately 25% bMBPp inhibition), showing indistinguishable levels of binding inhibition (P > 0.05, Table II). In contrast, samples of PSA having sizes ranging from 3 to 20 kDa (15-, 30-, 45-, and 60-min samples) showed as much as 70% peptide binding competition (Figure 6C). No significant difference in binding was seen for these intermediate PSA samples (Table II). These results correlate well with the previously published observations showing a requirement for processing (Cobb et al. 2004), however the loss of helical content as measured by CD (Figure 6B) correlates directly with a near complete loss of PSA binding to DR1 (Figure 6C), collectively suggesting a direct relation between structural conformation and antigenic function.
Table II.
Summary of statistical comparisons for ozone-cleaved PSA samples binding to MHCII
| Comparison (min) | P value | Significantly different |
|---|---|---|
| 0 versus 15 | <0.01 | Yes |
| 0 versus 30 | <0.001 | Yes |
| 0 versus 45 | <0.001 | Yes |
| 0 versus 60 | <0.001 | Yes |
| 0 versus 90 | >0.05 | No |
| 15 versus 30 | >0.05 | No |
| 15 versus 45 | >0.05 | No |
| 15 versus 60 | >0.05 | No |
| 15 versus 90 | <0.001 | Yes |
| 30 versus 45 | >0.05 | No |
| 30 versus 60 | >0.05 | No |
| 30 versus 90 | <0.001 | Yes |
| 45 versus 60 | >0.05 | No |
| 45 versus 90 | <0.001 | Yes |
| 60 versus 90 | <0.001 | Yes |
One-way ANOVA was performed using the Tukey–Kramer multiple comparisons test.
The structure and function relation for PSA: zwitterionic charges
The defining characteristic of all known T-cell-activating carbohydrates is the presence of a zwitterionic charge motif within each repeating unit. Previous reports demonstrate that chemical modification of either the positively charged free amines or the negatively charged carboxylate groups results in a lack of T-cell stimulatory activity (Tzianabos et al. 1993). To determine whether the charges present in PSA are required for MHCII-mediated T-cell activation due to simple electrostatic interactions with MHCII protein or whether these groups play a role in maintenance of the helical structure and therefore immunological function of PSA, the effect of charge modification on structure and function was explored.
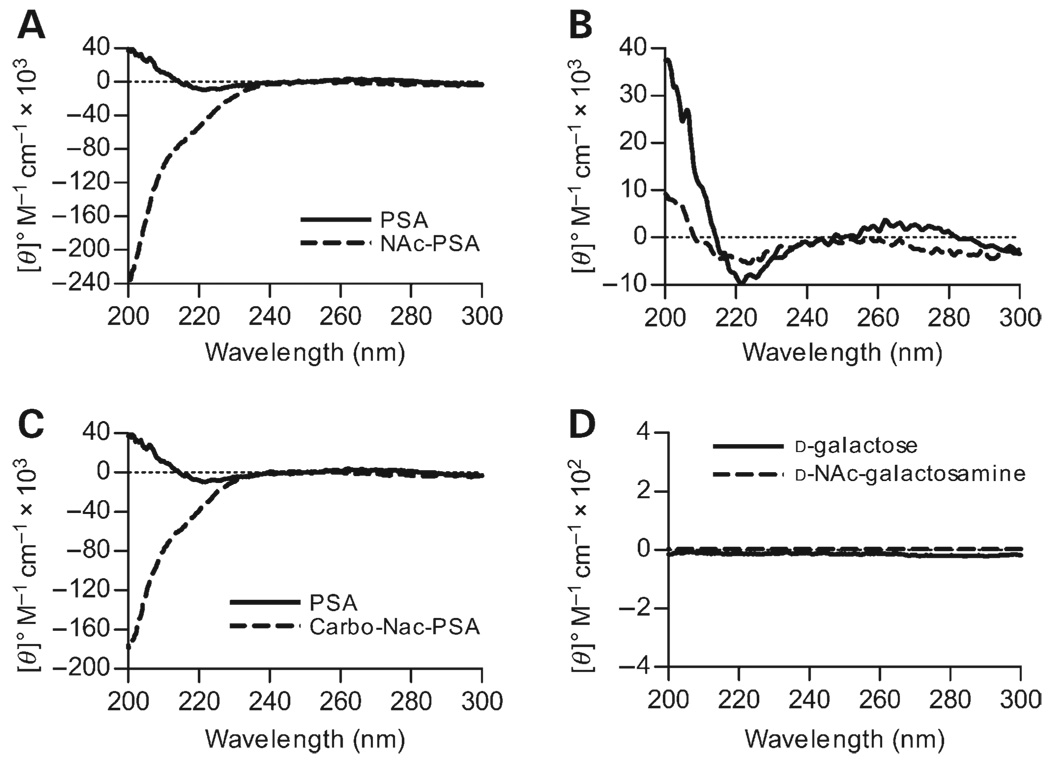
Free amines on low MW PSA (pre-treated for 45 min with ozone and biotinylated) were neutralized by N-acetylation using acetic anhydride, producing a negatively charged poly-saccharide. This N-acetylated PSA (NAc-PSA) sample was first analyzed by CD. We found that when compared with zwitterionic low MW and biotinylated PSA, the CD spectra changed radically (Figure 7A). In contrast to a local minimum at 225 nm and a maximum at approximately 205 nm in the zwitterionic form, we observed only a minimum <200 nm with a >100-fold increase in ellipticity, demonstrating a major shift in the average structure, likely to a random coil conformation, upon removal of the alternating charge motif.
Fig. 7.
Structural effects of chemical neutralization of charged groups in PSA. All spectra were collected with 80 µM PSA in PBS, with the dotted line representing the zero point. (A) CD spectra of native PSA and N-acetylated PSA without positive charges. The maximum at 205 nm is completely lost and the shape and amplitude of the spectrum are highly altered, most resembling a random coil conformation. (B) CD spectra of native PSA compared with Carbo-PSA that has the negative charges neutralized. The ellipticity at 223 nm decreases 2-fold, whereas the maximum at 205 nm shifts to a lower wavelength and is decreased by 4-fold, suggesting a significant loss of helical character. (C) CD spectra of neutralized PSA with both N-acetylation and carbodiimide reduction compared with native PSA. Similar to the structural change in NAc-PSA (A), neutral PSA shows a remarkable resemblance to a random coil conformation compared with the helical native PSA. (D) No difference in the signals of monosaccharides (2.5 mM in PBS) similarly neutralized was seen.
Carbodiimide reduction was used to neutralize the carboxylate groups, producing a positively charged PSA (Carbo-PSA). The changes in the CD spectrum are more subtle for this modification, yet the minimum at 225 nm had approximately 50% of the ellipticity found in zwitterionic PSA, whereas the maximum at 200 nm was reduced 4-fold compared with native PSA (Figure 7B). The spectrum of Carbo-PSA suggests that the helical content of PSA has been reduced by 2–4-fold. In combination, these two modifications, which produce a totally neutral PSA (Carbo-NAc-PSA, Figure 7C), appeared similar to the random coil seen with NAc-PSA (Figure 7A).
To confirm that N-acetylation and carbodiimide reduction reactions alone do not correspond to any notable changes in the CD spectra independent of conformation, we analyzed the CD spectra of N-acetylated galactosamine (GalNAc) and galactose (Gal). GalNAc is the product of acetic anhydride modification of galactosamine, whereas Gal is the product of carbodiimide reduction of galacturonic acid. We found that like the “unmodified” monosaccharides (Figure 2C), neither of these “charge-modified” monosaccharides produced measurable signals by CD (Figure 7D); therefore, alterations in the CD spectra generated in PSA upon removal of these charges are intrinsic to the conformation of the molecule rather than chemical changes in the monosaccharides.
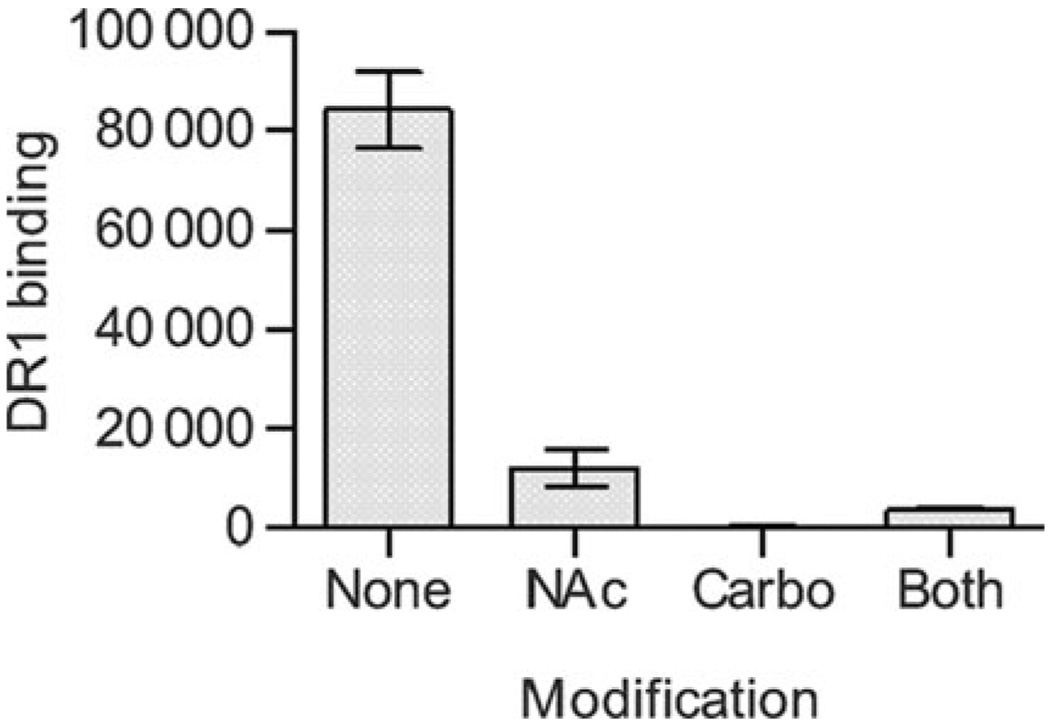
If the helical structure of PSA is required for MHCII presentation, then we would expect that these modified antigens would show reduced binding to MHCII compared with the zwitterionic form. In direct binding assays with immobilized HLA-DR1, we found that only the zwitterionic form of PSA significantly binds to MHCII. The chemical charge modifications result in both significant structural rearrangements (Figure 7A–C) and at least an 80% reduction in the ability to associate with MHCII (Figure 8), once again linking conformation and antigenic function.
Fig. 8.
Binding of charge-modified PSA samples. All PSA samples were of same MW (averaged approximately 3 kDa) and biotinylated. The MHCII protein HLA-DR1 was coated onto ELISA plates and binding of biotin-PSA samples were detected by streptavidin binding and time-resolved fluorescence. Native PSA without modification (“none”) showed significant binding, but all other charge-modified PSA samples were MHCII-binding-deficient.
Discussion
Peptides that are presented by MHC proteins do not require a specific conformation for binding or recognition. Crystal structures of the MHC–peptide complexes show that these epitopes depend on their amino acid sequence to fit into the MHC binding cleft in a linear, unfolded fashion (Smith et al. 1998). In fact, conventional antigenic proteins cannot bind MHC without first being processed to small peptides. For T-cell-activating glycoantigens such as PSA, it has been shown that processing to a low MW species is required for MHC-mediated presentation and in vivo T-cell activation (Cobb et al. 2004). Likewise, it has been demonstrated that the T-cell stimulatory activity of glycoantigens requires the presence of a zwitterionic motif (Tzianabos et al. 1993). As a result, it has been assumed that these charged groups were solely responsible for the activity of these antigens because this is the only common characteristic of all the known T-cell-dependent glycoantigens. In addition, these groups could form unique electrostatic interactions with immune system molecules during complex formation. However, the solution structures of two such glycoantigens (PSA and the capsule from S. pneumoniae) have nearly identical average conformations in solution (Choi et al. 2002), which leads to the possibility that antigenic activity might be the result of a particular 3D structure rather than simply the chemistry of the primary repeating unit structure. It is conceivable that the unusual zwitterionic chemistry of the repeating units found in T-cell-activating glycoantigens results in particular conformations that are required for MHC presentation.
Very little is known about the relation between glycoantigen structure and function, likely stemming from the fact that few analytical tools exist for easily monitoring dynamic con-formational changes of flexible carbohydrates. For this reason, the present report represents two major findings. First, we demonstrate for the first time that far UV CD is capable of measuring changes in the glycoantigen conformation. Second, we show that changes in the glycoantigen structure correlate with defects in their ability to be presented by MHCII proteins. More specifically, we found that the zwitterionic motif found in all known T-cell-activating glycoanti-gens stabilizes the PSA conformation required for activity.
In order to establish CD as a tool for measuring conformational changes in glycoantigens, we first compared the far UV spectra of native PSA, dextran, and myoglobin. Like many flexible polysaccharides, dextran is not known to form any particular structure and this is reflected in the lack of signal within its CD spectra, yet both the α-helical protein myoglobin and PSA, which forms right-handed helices, have strikingly similar CD spectra and nearly overlapping minima (222.6 versus 225.0 nm).
Confirmation that the spectra collected with PSA represents the conformation came in two forms. First, monosaccharides similar to those found in PSA failed to produce signals on CD. Second, the inclusion of a nonpolar organic solvent (i.e., acetonitrile), which is often used to enhance the observed ellipticity from proteins with α-helical content (Rosenblatt et al. 1980; Carbone et al. 1987) through strengthening intramolecular hydrogen bonds and sequestration from water molecules (e.g., Figure 3A), increased the PSA ellipticity amplitude without significantly altering the wavelength of the minimum seen in the spectrum (224.2 nm with acetonitrile versus 225.0 nm without). The percent change in ellipticity was the same for myoglobin and PSA when compared at 223 nm, and the maximum peak for both myoglobin and PSA became more narrow and shifted to a slightly higher wavelength with acetonitrile. Because monosaccharides do not show ellipticity >190 nm and solvation impacted the signal from two predominantly helical molecules to the same degree, we conclude that the CD spectrum for PSA is the result of all conformations in solution, which are predominantly helical in nature.
With this observation, the use of CD in a number of applications relating to carbohydrate structure and stability becomes possible. For example, near and far UV CD are commonly used in thermal or chemical denaturation curves of proteins because of the ability to readily monitor tertiary and secondary structures (reviewed in Kelly et al. 2005). Such studies have provided important insights into protein dynamics, stability, and folding and has helped lead to the common use of temperature as a means to determine whether particular biological activities in a crude extract is due to protein or other molecules, such as lipids or carbohydrates. This is based on the observation that typical proteins denature at high temperatures and the assumption that carbohydrates fail to form functional conformations that might be sensitive to the environment. In a test of this assumption, we measured the temperature stability of the PSA structure up to 80 °C. To our surprise, the PSA helical content was found to be remarkably stable even at high temperatures, suggesting that PSA helicticity is significantly more stable than the average protein. To date, it remains unclear whether this stability translates to other folded carbohydrates, but the discovery that far UV CD can be used to monitor these qualities is exciting in terms of impact on efforts of the Consortium for Functional Glycomics.
Another use of CD with respect to understanding carbohydrates is centered on traditional structure and function relations. Historically, this topic has not been explored thoroughly because of a lack of conformational information on complex carbohydrates. This is also true for T-cell-activating glycoantigens such as PSA, even though a structure is already known (Wang et al. 2000). We therefore applied CD to better understand the two key aspects of the T-cell-activating glycoantigen biology: size and charge character.
First, to assess the impact of molecular size on the PSA structure and function, ozone was used for varying lengths of time to generate cleavage products similar to those found inside antigen-presenting cells, following oxidative processing. We found that native PSA with a high MW binds only modestly to MHCII molecules, yet after only 15 min of ozone treatment, the fragments were able to bind quite well while maintaining significant helicticity. The ability to bind MHCII peaks at 45 min of ozone treatment, which correlates to a dominant fragment size of about 3 kDa or three repeating units. This fragment shows less ellipticity than the unprocessed PSA, but the minimum and maximum recorded remain in the same wavelength position on the CD spectrum. However, once the fragment size of PSA is reduced to approximately 1 kDa (one repeating unit), the CD spectrum becomes essentially flat like the spectrum of nonstructured dextran and binding functionality is lost.
On the basis of these data, we hypothesize that the high MW PSA binds less efficiently not because of a lack of helicticity, but potentially because of masking the binding domain in a higher order structure that is not readily apparent by CD measurements. It is also possible that native PSA does not bind because of unfavorable thermodynamics. However, given that the helical structure of PSA requires two repeating units per turn (Wang et al. 2000) and this helical conformation changes significantly <2 kDa as measured by CD, it is reasonable to conclude that the PSA with the most efficient MHCII binding, which contains at least three repeating units, is preferred because it has the minimum number of repeating units needed to form a stable binding-competent structure and therefore is thermodynamically favored.
Second, the zwitterionic charge motif is the defining characteristic of all known T-cell-dependent glycoantigens. As such, we sought to determine the relation between this charge motif and the structure of PSA by using CD to measure structural differences following simple chemical modifications to eliminate the positive and negative groups from 3-kDa PSA samples. We found that neutralization of the positively charged free amines resulted in a strikingly large conformational change that appears similar to the CD spectra of random coils and was accompanied with near ablation of the binding activity. Interestingly, neutralization of the negatively charged carboxy-late group also resulted in a conformational change and ablation of binding, but the structural change appeared to be simply a reduction in helical content. In combination, these two neutralization reactions generated a totally neutral PSA molecule characterized by an overall conformation more similar to the negatively charged random coil PSA molecule and a lack of MHC binding capability, congruent with the inability of charge-modified PSA to activate T cells or provoke abscesses in vivo (Tzianabos et al. 1993).
The positive charges on the PSA helix are localized on the outer edge of the helical turns, facing away from the helical groove (Wang et al. 2000). The addition of an acetyl group on the free amine, therefore, would not be expected to result in a major change by virtue of adding a bulky group. In the previous reports comparing deaminated PSA, where the amine was simply removed, with NAc-PSA, indistinguishable deleterious effects on the activity of PSA was observed (Tzianabos et al. 1993). With respect to the carbodiimide reduction, the chemical reaction results in a smaller but still hydrophilic group. Again on the basis of the NMR structure, there is no obvious reason that the change in group size alone would cause major conformational instability within the groove. As a result, we conclude that the changes in conformation reported in the present study are the result of the loss of charges and therefore that the zwitterionic charge motif is essential for the maintenance of a helical conformation of PSA. Collectively, our findings strongly support a model in which the PSA structure and function are closely tied in that PSA must maintain significant helical content in order to enable MHCII binding and presentation.
This report represents a significant step forward in the study of complex carbohydrates by showing far UV CD can be used to analyze the structure of some carbohydrates and structure can dictate function in polysaccharides in much the same way as proteins. In the modern age of functional glycomics, new tools can prove to be invaluable to gaining a complete understanding of carbohydrates in biology. To illustrate this point, we used this approach to show that the T-cell-activating glycoantigen PSA is the first known processed MHCII-dependent antigen that requires a particular conformation for presentation.
Materials and methods
Carbohydrates and proteins
All monosaccharides, dextran, and myoglobin were purchased from Sigma-Aldrich (St Louis, MO). PSA was purified as previously described (Tzianabos et al. 1992) from a mutant B. fragilis strain expressing only PSA within the capsule (Krinos et al. 2001). Biotin-MBP peptide (residues 85–99) was custom synthesized by New England Peptide (Gardner, MA). Purified ectodomain HLA-DR1 was provided by Dr Stephen DeWall. DR1 was expressed and purified as previously described (Stratikos et al. 2002).
CD measurements
All CD spectra were collected on a Jasco J-810 Spectropolarimeter (Jasco Inc., Easton, MD) fitted with a temperature controlled cuvette holder. Except as indicated, samples were analyzed in PBS (pH 7.2; Invitrogen, Carlsbad, CA) and a quartz cuvette (path length 0.2 cm) at room temperature. Data are reported as average molar ellipticity of four accumulated scans ([θ]° M−1 cm−1) at wavelengths between 190 and 300 nm. Protein samples were collected at 10 µM and carbohydrate samples at 2.5 mg/mL (50 µM at native MW). Acetonitrile effects were measured at 20% acetonitrile in PBS.
HPLC size analyses
All carbohydrate size measurements were performed on a Superdex75 size exclusion column fitted to an Akta® Purifier10 HPLC system (GE Healthcare Biosciences, Piscataway, NJ). The column was standardized with 1-, 5-, 12-, 50-, and 150-kDa dextrans monitored by refractive index (RI) using a ProStar 355 RI Flow Cell (Varian Inc., Walnut Creek, CA). Elution volumes were then used to calculate a standard curve for size calculations using Prism v.4.03 (GraphPad Software, San Diego, CA). Experimental PSA sample elution was monitored by absorbance at 205 nm and RI. The elution profiles of ozone-cleaved PSA mixtures were integrated and an average MW determined on the basis of the percentage of PSA in a given peak.
Oxidation and chemical modifications
Positive free amine groups were neutralized by N-acetylation (Figure 1A) with 20-fold molar excess of acetic anhydride in 100-mM sodium bicarbonate, as previously described (Tzianabos et al. 1993). Negative carboxylate groups were modified to alcohols by carbodiimide reduction. Briefly, n-cyclohexyl-N′-[2,4-morpholinyl-ethyl] carbodiimide-methyl-tolulenesulfonate was added at 10-fold excess to polysaccharide by weight and maintained at pH 4.75 for 2 h at room temperature. The resulting intermediate was reduced with 10fold molar excess of sodium borohydride to yield the neutral hydroxyl (Figure 1A). Oxidation by ozone was accomplished using an ozone generator (AquaZone, Red Sea Ltd, Houston, TX) at a constant flow rate (100 mg/h) for the indicated lengths of time in PBS, as previously described (Wang et al. 1998, 1999]. mod-PSA for binding assays was created by first cleaving PSA for 45 min in ozone, performing the charge modifications as described above, then conjugating the poly-saccharide fragments with hydrazide-biotin as previously reported (Cobb et al. 2004).
Binding analyses
Purified DR1 protein (100 ng) was coated in ELISA wells (Immulon 4HBX, 384 wells, Thermo Electron Corp., Waltham, MA). Negative DR1 controls were performed with blocked wells only. Ozone-cleaved PSA samples were analyzed for DR1 binding by peptide competition in which bMBPp (200 nM) and 2.5 µg of PSA were added at the same time in 50 mM citrate-buffered saline at pH 5.0. The percentage of binding compared with bMBPp assays without PSA was calculated and plotted as a function of ozone treatment length. Assays were performed at 37° C for 24 h and bound bMBPp was detected using europium-conjugated streptavidin (Eu-SA) and TRF on a Victor3 V Multilabel Counter (Perkin-Elmer, Oak Brook, IL). Wells were blocked with 5% fetal bovine serum for 2 h. For mod-PSA assays, direct binding of biotin-PSA was detected under identical conditions as above, only without peptide present and with 300-ng DR1.
Acknowledgments
We would like to thank Dr Mike Zagorski for use of his CD polarimeter and many helpful discussions on CD measurements. We also thank Dr Daved Fremont for technical suggestions during the completion of this project. This work was supported by grants to B.A. Cobb through the National Institutes of Health, National Institute of Allergy and Infectious Disease (AI 062707) and Case Western Reserve University School of Medicine.
Abbreviations
- bMBPp
biotinylated myelin basic protein peptide
- Carbo-NAc-PSA
neutralized PSA
- Carbo-PSA
carbodiimide-reduced PSA
- CD
circular dichroism
- ELISA
enzyme linked immunosorbent assay
- FucNAc
N-acetylfucosamine
- Gal
galactose
- GalNAc
N-acetylated galactosamine
- HLA-DR1
human leukocyte antigen DR1
- HPLC
high-pressure liquid chromatography
- MHCII
major histocompatibility complex class II
- MW
molecular weight
- NAc-PSA
N-acetylated PSA
- NMR
nuclear magnetic resonance
- NOESY
Nuclear overhauser enhancement spectroscopy
- PBS
phosphate-buffered saline
- PSA
polysaccharide A1
- RI
refractive index
- TRF
time-resolved fluorometry
- UV
ultraviolet
Footnotes
Conflict of interest statement
None declared.
References
- Baumann H, Tzianabos AO, Brisson JR, Kasper DL, Jennings HJ. Structural elucidation of two capsular polysaccharides from one strain of Bacteroides fragilis using high-resolution NMR spectroscopy. Biochemistry. 1992;31:4081–4089. doi: 10.1021/bi00131a026. [DOI] [PubMed] [Google Scholar]
- Carbone FR, Fox BS, Schwartz RH, Paterson Y. The use of hydro-phobic, alpha-helix-defined peptides in delineating the T cell determinant for pigeon cytochrome C. J Immunol. 1987;138:1838–1844. [PubMed] [Google Scholar]
- Choi YH, Roehrl MH, Kasper DL, Wang JY. A unique structural pattern shared by T-cell-activating and abscess-regulating zwitterionic polysaccharides. Biochemistry. 2002;41:15144–15151. doi: 10.1021/bi020491v. [DOI] [PubMed] [Google Scholar]
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman MK, Balazs EA, Bergmann CW, Meyer K. Preparation and circular dichroism analysis of sodium hyaluronate oligosaccharides and chondroitin. Biochemistry. 1981;20:1379–1385. doi: 10.1021/bi00508a053. [DOI] [PubMed] [Google Scholar]
- Dominguez J, Andreo F, Blanco S, Ruiz-Manzano J, Prat C, Latorre I, Gali N, Rivelo R, Matas L, Ausina V. Rapid detection of pneumococcal antigen in serum samples for diagnosing Pneumococcal pneumonia. J Infect. 2006;53:21–24. doi: 10.1016/j.jinf.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Hartley G, Taylor R, Prior J, Newstead S, Hitchen PG, Morris HR, Dell A, Titball RW. Grey variants of the live vaccine strain of Francisella tularensis lack lipopolysaccharide O-antigen, show reduced ability to survive in macrophages and do not induce protective immunity in mice. Vaccine. 2006;24:989–996. doi: 10.1016/j.vaccine.2005.08.075. [DOI] [PubMed] [Google Scholar]
- Henderson TJ, Venable RM, Egan W. Conformational flexibility of the group B meningococcal polysaccharide in solution. J Am Chem Soc. 2003;125:2930–2939. doi: 10.1021/ja0210087. [DOI] [PubMed] [Google Scholar]
- Johnson WC., Jr The circular dichroism of carbohydrates. Adv Carbohydr Chem Biochem. 1987;45:73–124. [PubMed] [Google Scholar]
- Kalka-Moll WM, Tzianabos AO, Wang Y, Carey VJ, Finberg RW, Onderdonk AB, Kasper DL. Effect of molecular size on the ability of zwitterionic polysaccharides to stimulate cellular immunity. J Immunol. 2000;164:719–724. doi: 10.4049/jimmunol.164.2.719. [DOI] [PubMed] [Google Scholar]
- Kasper DL, Finberg RF, Crabb J, Onderdonk AB. Immune mechanisms in the prevention of intra-abdominal abscess formation. Scand J Infect Dis Suppl. 1989;62:29–34. [PubMed] [Google Scholar]
- Kasper DL, Onderdonk AB. Infection with Bacteroides fragilis: pathogenesis and immunoprophylaxis in an animal model. Scand J Infect Dis Suppl. 1982;31:28–33. [PubMed] [Google Scholar]
- Kelly SM, Jess TJ, Price NC. How to study proteins by circular dichroism. Biochim Biophys Acta. 2005;1751:119–139. doi: 10.1016/j.bbapap.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Koerner TA, Yu RK, Scarsdale JN, Demou PC, Prestegard JH. Analysis of complex carbohydrate primary and secondary structure via two-dimensional proton nuclear magnetic resonance spectroscopy. Adv Exp Med Biol. 1988;228:759–784. doi: 10.1007/978-1-4613-1663-3_32. [DOI] [PubMed] [Google Scholar]
- Kokki S, Uprichard JM. Ozone degradation of cellulose model compounds. J Fac Agric Kyushu Univ. 1991;36:45–53. [Google Scholar]
- Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 2001;414:555–558. doi: 10.1038/35107092. [DOI] [PubMed] [Google Scholar]
- Mansour MK, Latz E, Levitz SM. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J Immunol. 2006;176:3053–3061. doi: 10.4049/jimmunol.176.5.3053. [DOI] [PubMed] [Google Scholar]
- Marchessault RH, Imada K, Bluhm T, Sundararajan PR. Conformation of crystalline type III pneumococcal polysaccharide. Carbohydr Res. 1980;83:287–302. doi: 10.1016/s0008-6215(00)84541-3. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immuno-modulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mukherjee DC, Park JW, Chakrabarti B. Optical properties of Cu(II) complexes with heparin and related glycosaminoglycans. Arch Biochem Biophys. 1978;191:393–399. doi: 10.1016/0003-9861(78)90103-0. [DOI] [PubMed] [Google Scholar]
- Nelson RG, Johnson WC., Jr Optical properties of sugars. 3. Circular dichroism of aldo- and ketopyranose anomers. J Am Chem Soc. 1976;98:4290–4295. doi: 10.1021/ja00430a047. [DOI] [PubMed] [Google Scholar]
- O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Chakrabarti B. Acid–base and optical properties of heparin. Biochem Biophys Res Commun. 1977;78:604–608. doi: 10.1016/0006-291x(77)90222-4. [DOI] [PubMed] [Google Scholar]
- Park JW, Chakrabarti B. Conformational transition of hyaluronic acid: carboxylic group participation and thermal effect. Biochim Biophys Acta. 1978;541:263–269. doi: 10.1016/0304-4165(78)90399-9. [DOI] [PubMed] [Google Scholar]
- Piculell L, Borgstrom J, Chronakis IS, Quist PO, Viebke C. Organisation and association of kappa-carrageenan helices under different salt conditions. Int J Biol Macromol. 1997;21:141–153. doi: 10.1016/s0141-8130(97)00054-8. [DOI] [PubMed] [Google Scholar]
- Pysh ES. Optical activity in the vacuum ultraviolet. Ann Rev Biophys Bioeng. 1976;5:63–75. doi: 10.1146/annurev.bb.05.060176.000431. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Chessa D, Wilson RP, Dusold R, Rubino S, Baumler AJ. The Vi capsular antigen of Salmonella enterica serotype typhi reduces toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect Immun. 2005;73:3367–3374. doi: 10.1128/IAI.73.6.3367-3374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Chessa D, Wilson RP, Tukel C, Akcelik M, Baumler AJ. Capsule-mediated immune evasion: a new hypothesis explaining aspects of typhoid fever pathogenesis. Infect Immun. 2006;74:19–27. doi: 10.1128/IAI.74.1.19-27.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt M, Beaudette NV, Fasman GD. Conformational studies of the synthetic precursor-specific region of preproparathyroid hormone. Proc Natl Acad Sci U S A. 1980;77:3983–3987. doi: 10.1073/pnas.77.7.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998;188:1511–1520. doi: 10.1084/jem.188.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskus PW, Johnson WC., Jr Double-stranded structure for hyaluronic acid in ethanol-aqueous solution as revealed by circular dichroism of oligomers. Biochemistry. 1988;27:1528–1534. doi: 10.1021/bi00405a020. [DOI] [PubMed] [Google Scholar]
- Stone AL. Far-ultraviolet circular dichroism and uronic acid components of anticoagulant deca-, dodeca-, tetradeca-, and octadecasaccharide heparin fractions. Arch Biochem Biophys. 1985;236:342–353. doi: 10.1016/0003-9861(85)90635-6. [DOI] [PubMed] [Google Scholar]
- Stone AL, Szu SC. Application of optical properties of the Vi capsular polysaccharide for quantitation of the Vi antigen in vaccines for typhoid fever. J Clin Microbiol. 1988;26:719–725. doi: 10.1128/jcm.26.4.719-725.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratikos E, Mosyak L, Zaller DM, Wiley DC. Identification of the lateral interaction surfaces of human histocompatibility leukocyte antigen (HLA)-DM with HLA-DR1 by formation of tethered complexes that present enhanced HLA-DM catalysis. J Exp Med. 2002;196:173–183. doi: 10.1084/jem.20020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu SC, Li XR, Stone AL, Robbins JB. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect Immun. 1991;59:4555–4561. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989;11:440–451. [PubMed] [Google Scholar]
- Tzianabos AO, Finberg RW, Wang Y, Chan M, Onderdonk AB, Jennings HJ, Kasper DL. T cells activated by zwitterionic molecules prevent abscesses induced by pathogenic bacteria. J Biol Chem. 2000;275:6733–6740. doi: 10.1074/jbc.275.10.6733. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Pantosti A, Baumann H, Brisson JR, Jennings HJ, Kasper DL. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 1992;267:18230–18235. [PubMed] [Google Scholar]
- Wang Y, Hollingsworth RI, Kasper DL. Ozonolysis for selectively depolymerizing polysaccharides containing beta-d-aldosidic linkages. Proc Natl Acad Sci U S A. 1998;95:6584–6589. doi: 10.1073/pnas.95.12.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hollingsworth RI, Kasper DL. Ozonolytic depolymeriza-tion of polysaccharides in aqueous solution. Carbohydr Res. 1999;319:141–147. doi: 10.1016/s0008-6215(99)00113-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kalka-Moll WM, Roehrl MH, Kasper DL. Structural basis of the abscess-modulating polysaccharide A2 from Bacteroides fragilis. Proc Natl Acad Sci U S A. 2000;97:13478–13483. doi: 10.1073/pnas.97.25.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch LE, Mansour MK, Levitz SM. Receptor-mediated clearance of Cryptococcus neoformans capsular polysaccharide in vivo. Infect Immun. 2005;73:8429–8432. doi: 10.1128/IAI.73.12.8429-8432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]