Abstract
Mucus secretion from individual tracheal glands in adult ferrets was studied with time-lapse optical imaging of mucus droplets under an oil layer. Density of functional glands (determined by responses to 1 μM carbachol) was 1.5 ± 0.3 per mm2 (n = 6). Secretion rates (in pl·min−1·gland−1) were as follows: 4.1 ± 0.7 basal (unstimulated; n = 27, 669 glands), 338 ± 70 to 10 μM forskolin (n = 8, 90 glands), 234 ± 13 to 1 μM VIP (n = 6, 57 glands), 183 ± 92 to 10 μM isoproterenol (n = 3, 33 glands), 978 ± 145 to 1 μM carbachol (n = 11, 131 glands), and 1,348 ± 325 to 10 μM phenylephrine (n = 7, 74 glands). The potency (EC50, in μM) and efficacy (Vmax, in pl·min−1·gland−1) were 7.6 (EC50) and 338 ± 16 (Vmax) to forskolin, 1.0 (EC50) and 479 ± 19 (Vmax) to VIP, 0.6 (EC50) and 1,817 ± 268 (Vmax) to carbachol, and 3.7 (EC50) and 1,801 ± 95 (Vmax) to phenylephrine. Although carbachol and phenylephrine were equally effective secretagogues, only carbachol caused contractions of the trachealis muscle. Synergy was demonstrated between 300 nM isoproterenol and 100 nM carbachol, which, when combined, produced a secretion rate almost fourfold greater than predicted from their additive effect. The dependence of fluid secretion on Cl− and HCO3− varied depending on the mode of stimulation. Secretion stimulated by VIP or forskolin was reduced by ∼60% by blocking either anion, while carbachol-stimulated secretion was blocked 68% by bumetanide and only 32% by HEPES replacement of HCO3−. These results provide parametric data for comparison with fluid secretion from glands in ferrets lacking CFTR.
Keywords: cystic fibrosis transmembrane conductance regulator, airway, adrenergic, cholinergic, muscle contraction
innate defenses of the airways eliminate pathogens by physically removing them (mucus clearance) or suppressing or killing them with antimicrobial compounds and leukocytes present in the mucus (1, 11, 28, 33). In the upper airways, most mucus originates from submucosal glands, which are distributed along the tracheobronchial airways and are under complex neural control. Stimulation of glands leads to expansion of airway surface liquid volume, which accelerates the rate of mucociliary transport (33).
Chronic bacterial lung infections are the major cause of death in patients with cystic fibrosis (CF). Defective submucosal gland secretion has been hypothesized to play an important role in the pathogenesis of CF lung disease, because the glands are an important site of CFTR expression in human airways (18, 48) and because CF patients have deficient gland fluid secretion to all agonists tested (9, 30, 46, 51, 53), alone or in combination. Comparative functional data from control and CF human airway glands are difficult to acquire, because such data rely on tissues obtained at transplant or via invasive biopsies. Thus there is great interest in studying airway submucosal glands in animal models of CF. CF mice are the most readily available models, but their airway submucosal glands are restricted to the anterior trachea and rely only minimally on CFTR (23); perhaps partly for these reasons, CF mice do not display CF-like airway disease.
Recently, CF pigs (44) and CF ferrets (52) have been produced, and each is predicted to offer an improved model of human CF airway disease. We and others carried out extensive studies of pig glands to provide a basis for comparison with the CF pig model. However, although ferrets have been the focus of many studies of airway gland secretion (2, 13, 20, 21, 31, 41, 43, 47), those studies mainly relied on labeling of secreted compounds or morphometric analyses; no single-gland studies of fluid secretion have been carried out in ferrets. Ferrets are appealing for studies of the possible involvement of airway glands in CF airway disease because ferrets have abundant tracheal glands, the glands express CFTR, and airway morphology, with respect to the presence of surface airway goblet cells and submucosal glands, is similar to that of the human (48).
We have developed an optical method to study fluid secretion from many individual submucosal glands in parallel with minute-by-minute resolution (30). In this study, we apply that method to ferret airway glands, with the primary goal of establishing a good basis for eventual comparison with CF ferrets. We demonstrate that ferret airway glands have many features in common with human glands, including a reliance on Cl− and HCO3− for secretion, a similar proportionality in the magnitude of responses to forskolin and carbachol, and synergy between low levels of agonists that work via these pathways. One marked difference is a large secretory response to α-adrenergic stimulation with phenylephrine, similar to that seen in cats, but not observed in humans, sheep, or pigs.
MATERIALS AND METHODS
Tissues.
Ferret (Mustela putorius furo, n = 45), pig (Yorkshire, n = 4), and sheep (Suffolk-Rambouillet, n = 5) tracheas were obtained ∼1 h postmortem (pentobarbital sodium injection) following experiments carried out for other purposes. No animals were euthanized specifically for these experiments; nevertheless, the experiments were approved by the Stanford University Institutional Animal Care and Use Committee. The mean age of the ferrets was 141.1 ± 23.7 days (n = 43); the youngest ferret was 59 days old and had abundant airway glands along its whole trachea. Pigs and sheep were adults. Human tracheas (n = 5) were obtained from surgical trim from lung transplantation donors, under procedures approved by Stanford's Institutional Review Board.
All tissues were transported to the laboratory in cold Physiosol solution (Abbott Laboratories) and transferred to ice-cold Krebs-Ringer bicarbonate buffer, bubbled with 95% O2-5% CO2, in which they were maintained until use, usually within 24 h. The Krebs-Ringer bicarbonate buffer composition was 115 mM NaCl, 2.4 mM K2HPO4, 0.4 mM KH2PO4, 25 mM NaHCO3, 1.2 mM MgCl2, 1.2 mM CaCl2, 10 mM glucose, and 1.0 μM indomethacin to minimize tissue exposure to endogenously released prostaglandins during tissue preparation and mounting. Osmolarity was adjusted to 290 ± 5 mosM with a vapor pressure osmometer (model 5500, Wescor), and pH was verified to be 7.4 (Orion 420A pH meter) after the solution was bubbled with 95% O2-5% CO2. To prepare the ferret tracheal tissue for optical recording of mucus secretion from individual glands, the dissection was modified from our previous methods (30). The trachea was cut dorsally along its muscular portion, and a ∼3- to 4-cm2 piece was used. The tracheal mucosa with underlying glands was not dissected from the cartilage, to which it was more adherent than in other species we have studied. We tried several times to work with mucosa dissected from the cartilage, but gland secretion was sparse. Instead, tissue external to the cartilage was removed, and the cartilage was hatched along its outer surface with a surgical blade under microscopic control to facilitate drug access to the underlying glands. From this point, the procedure was standard: the scored trachea was pinned mucosal-side-up with the serosa in the bath in a 35-mm-diameter plastic petri dish filled with Sylgard (Dow Corning). The luminal surface was cleaned gently with cotton swabs and dried with a stream of 95% O2-5% CO2 gas; the surface was coated with 50–80 μl of water-saturated mineral oil. The tissue was warmed to 37°C at a rate of <2°C/min with a temperature-controlled system (TC-202A, Harvard Apparatus) and continuously exposed to warmed, humidified 95% O2-5% CO2 gas. For bicarbonate-free experiments, 25 mM HCO3− in the Krebs solution was replaced with 1 mM HEPES + 24 mM NaCl that had been pregassed with 100% O2. The pH for the HEPES solution was 7.4 after it was gassed with 100% O2. Procedures for other species were identical, except the mucosa was removed from cartilage.
Optical measurement and calculation of mucus secretion.
Bubbles of mucus within the oil layer were visualized by oblique illumination, and digital images were captured automatically at 5-min intervals using krinnicam software and a Nikon digital camera in macro mode. Stored images were analyzed using NIH ImageJ software (http://rsb.info.nih.gov/ij/). Mucus volumes were determined as previously described (30) from the size of the spherical bubbles using the following formula: V = 4/3πr3, where r is radius. Bubbles that were approximately spherical with clear circular margins were selected for analysis. Bubbles from adjacent duct openings sometimes merged; in such cases, measurement was stopped. If merging occurred more often with faster-secreting glands, the exclusion of these glands could lead to an underestimate of average secretion rates during the later periods when their data were excluded. However, only 25 of 389 (5.7%) glands measured for secretion rate merged during our observation period, and no significant difference in secretion rates was noticed when their data were entirely excluded.
Stimulation and inhibition protocols.
Different agonists caused secretion to reach mean maximal values with markedly different latencies, but once attained, the mean maximal rates were stable for as long as we followed them. For a given agonist, there was great variation in the time to reach maximal steady rates of the individual glands. Therefore, to obtain unbiased comparisons of secretory rates produced by each agonist, we extrapolated backward from the last rates obtained (30–60 min after agonist addition) using a linear fit of the data until we reached an obvious shift to a region of slower secretion (see Fig. 2A). For comparisons among agonists, we used the mean maximal rate as just defined, and we also recorded the average latency from agonist addition to the earliest point where the average mean maximal rate began. As noted in many studies, onset times of responses to agonists that raise intracellular cAMP concentration ([cAMP]i) were slower than those of responses to agents that raise intracellular Ca2+ concentration ([Ca2+]i). Additional delays common to all agonists resulted from the presence of cartilage in these preparations, but these were minor (∼2-fold compared with off-cartilage times measured in previous studies with other species).
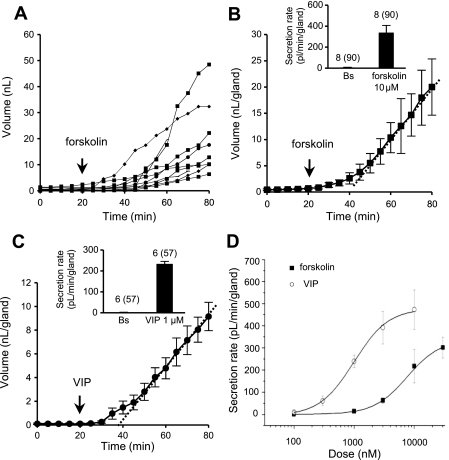
Fig. 2.
Volume vs. time responses to forskolin and VIP. A: plot of cumulative volume of secretion for each of 10 glands as a function of time and presence of 10 μM forskolin from minute 20. B: plot of mean cumulative volume of mucus from glands in A. Dotted line is best linear fit to volumes from 35 to 80 min; slope of this line is taken as the mean secretion rate for each experiment. In this example, secretion rate was 470 pl·min−1·gland−1 and latency to reach this rate was 30 min. Inset: average secretion rate in 90 glands in 8 ferrets treated with 10 μM forskolin. Bs, basal secretion. C: plot of mean cumulative volume of mucus from 8 glands treated with 1 μM VIP. Dotted line is best linear fit to volumes from 35 to 80 min; slope of this line is taken as mean secretion rate. In this experiment, rate was 151 pl·min−1·gland−1 and latency to reach this rate was 25–30 min. Inset: average secretion rate in 57 glands in 6 ferrets treated with 1 μM VIP. Values (points or bars) are means ± SE. D: dose-response relations for gland mucus secretion to forskolin vs. VIP. Vmax values (in pl·min−1·gland−1) are 338 ± 16 for forskolin and 479 ± 19 for VIP. EC50 values (in nM) are 8,691 ± 1,671 for forskolin and 993 ± 61 for VIP. (For additional information see supplemental Fig. S1.)
We used bumetanide (100 μM) to block the Na+-K+-2Cl− cotransporter and, thereby, eliminate Cl−-mediated fluid secretion. To block HCO3−-mediated fluid secretion, HCO3− in the Krebs solution was replaced with HEPES, and the solution was gassed with O2 to block both anions; bumetanide was added to the HEPES-buffered solution. Bumetanide, HEPES, or bumetanide + HEPES was added to the bath at time 0, and we observed the effects on 20 min of basal secretion followed by 30 min with the chosen agonists. For the inhibition experiments, we used 1 μM VIP + 10 μM forskolin to raise [cAMP]i, because there is evidence that this combination was more effective than either VIP or forskolin alone (and higher levels of VIP were precluded because of its expense). To raise [Ca2+]i, we used 1 μM carbachol as a acetylcholinesterase-resistant substitute for ACh. At 1 μM carbachol, the response is submaximal, but there are reasons to think that 1 μM is a more physiologically relevant concentration, and higher concentrations caused merging of the mucus bubbles. Control tissues were treated identically without the inhibitors. At least three of the four conditions (control, bumetanide, HEPES, and bumetanide + HEPES) were run on tissues from the same trachea in most experiments.
Chemicals.
All compounds were purchased from Sigma and made fresh or maintained at −20°C. Stock solutions of carbachol, phenylephrine, isoproterenol, and VIP were dissolved in distilled water; forskolin was dissolved in DMSO; bumetanide was dissolved in an alkaline solution. The highest DMSO concentration was 0.01%; 0.5% DMSO as vehicle alone had no effect on gland secretion. For all drugs, stock aliquots of <15 μl were kept frozen until the day of use, when they were diluted 1:1,000 with freshly made bath solution or appropriate buffer.
Statistics.
Values are means ± SE unless otherwise indicated. Unless otherwise stated, n refers to animals. Student's t-test for paired data was used to compare the means of different treatment groups. One-way ANOVA with Bonferroni's post hoc method was used for comparisons of three or more data groups having parametric distributions. The Kruskal-Wallis method was used for nonparametric data among three or more groups. Parametric distribution of data was checked with the Kolmogorov-Smirnov test. Differences were considered to be significant when P < 0.05. Dose-response curves were generated with Origin 6 software (OriginLab) using a sigmoidal fit function.
RESULTS
Overview of ferret tracheal submucosal glands.
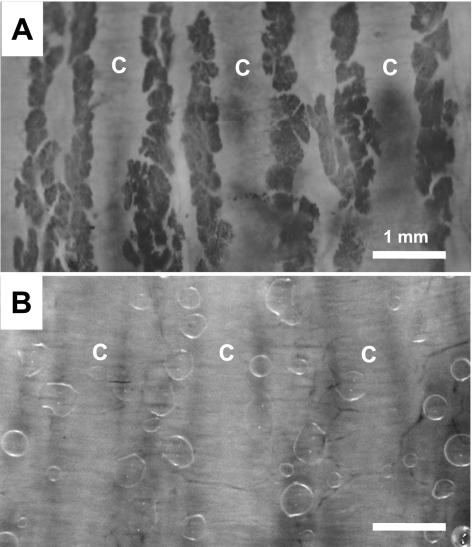
To identify the distribution and abundance of ferret airway glands, tracheas were stained with neutral red dye (see materials and methods), and the unfixed tracheas were photographed as whole mounts with transillumination. In agreement with prior studies of other mammalian species (7), submucosal glands were concentrated between tracheal cartilaginous rings and were distributed along the full length of the trachea (Fig. 1A). Removal of the cartilage was more difficult in ferrets than in larger animals and often led to damage (see materials and methods). Therefore, we assessed the ability of agonists to stimulate secretions in preparations with intact cartilaginous rings, as well as our ability to image such secretions. Images of gland secretions in the cartilage-intact preparation (Fig. 1B) were somewhat less clear than those of larger glands in the off-cartilage preparations from larger species (humans, pigs, and sheep), but images were clear enough to quantify secretion with an accuracy of 97.9 ± 0.03% determined by independent measurements by two raters. In six ferrets, we measured the density of secreting glands by counting the number of mucus bubbles produced by 1 μM carbachol, being careful to avoid undercounting caused by merging of bubbles. For the ventral, cartilage-containing portion of the trachea, gland density was 1.5 ± 0.3 per mm2 (142 glands, 6 ferrets); it was 1.8 ± 0.4 per mm2 when calculated only for gland openings located in the area of intercartilaginous rings (81 glands, 6 ferrets). We did not estimate the gland density in the posterior, membranous portion of the trachea.
Fig. 1.
Ferret submucosal glands and secreted mucus bubbles under oil. A: tracheal submucosal glands of an adult ferret stained with neutral red and viewed from the mucosal surface. Abundant glands are shown between 5 tracheal cartilaginous rings, 3 of which are marked (C). B: representative image of mucus bubbles secreted into the oil layer by individual tracheal glands after 5 min of 1 μM carbachol applied to the bath. Preparation in B is different from that in A, but scale is the same. Both images are converted from color, with contrast enhanced.
Basal secretion from unstimulated glands.
Approximately one-fifth of the glands that eventually responded to agonists secreted at a low level during the 20-min period prior to any deliberate stimulation (basal secretion). The average basal secretion rate for the basally active glands was 4 ± 1 pl·min−1·gland−1 (669 glands, 27 ferrets). Most of the basally secreting glands responded to agonists (95.0% to forskolin, 93.0% to VIP, 97.8% to carbachol, and 97.3% to phenylephrine). The small percentage (<3%) of basally secreting glands that did not respond to any of the agonists might have been injured during the dissection, which could have provoked some secretion but then created a leak pathway that would shunt secretion into the bath, instead of onto the airway surface.
Forskolin- or VIP-stimulated steady rates of gland secretion.
VIP or forskolin elevates [cAMP]i and stimulates CFTR-dependent submucosal gland secretion in humans (8, 26). The response to 10 μM forskolin is shown in Fig. 2, A and B. Figure 2B is a representative plot of the mean cumulative volume increase from 10 ferret tracheal glands (Fig. 2A). In these on-cartilage preparations, the response to forskolin took 20 ± 3 min (n = 8) to reach a maximal rate, in contrast with latencies of 10–15 min in off-cartilage preparations of humans and pigs (26, 29). However, similar to the responses in these species, once they reached the maximal rate, they showed a nearly linear increase in mucus volume, with no peaks or trends in the secretion rates. The average secretion rate for 90 glands in 8 ferrets was 338 ± 70 pl·min−1·gland−1 (Fig. 2B, inset). This value was obtained from experiments in which the mean duration measured after forskolin addition was 46.3 ± 5.7 min and, therefore, might be a slight underestimate, because secretion for three of the ferrets was followed for only 30 min, at which time the secretion rate might not have reached its maximum. The mean time to reach maximal secretion was 20 ± 3 min (n = 8).
A similar response was produced by 1 μM VIP (Fig. 2C). The average secretion rate was 234 ± 12 pl·min−1·gland−1 (57 glands, 6 ferrets; Fig. 2C, inset) on the basis of 30–60 min of secretion measurements. Maximal VIP secretion was reached at 18 ± 2 min (n = 6).
The potencies and efficacies of forskolin and VIP were determined by sequential addition of increasing concentrations of forskolin or VIP. In these experiments, each concentration was present for 30 min before the next increase. At lower agonist concentrations, secretion may not have reached steady state by the time of the next addition. Therefore, a rate reported for a lower concentration may underestimate the secretion rate at that concentration.
For the other concentrations, the secretion rates had stabilized by the end of the 30-min period and, thus, provide an accurate estimate. VIP was more potent and slightly more efficacious than forskolin (Fig. 2D). The EC50 of VIP was 1.0 μM and Vmax was 479 ± 19 pl·min−1·gland−1 (31–54 glands, 5 ferrets) vs. 7.6 μM and 338 ± 16 pl·min−1·gland−1, respectively, for forskolin (32 glands, 3 ferrets). The mean secretion rate response to VIP varied 19.1-fold among individual glands in the same preparation, while the average response across ferrets (10–12 glands per ferret) varied only 1.3-fold (see dose-response curves for forskolin and VIP, with 95% confidence intervals, in supplemental Figs. S1 and S2 in the online version of this article).
Carbachol or phenylephrine stimulated robust, sustained gland secretion.
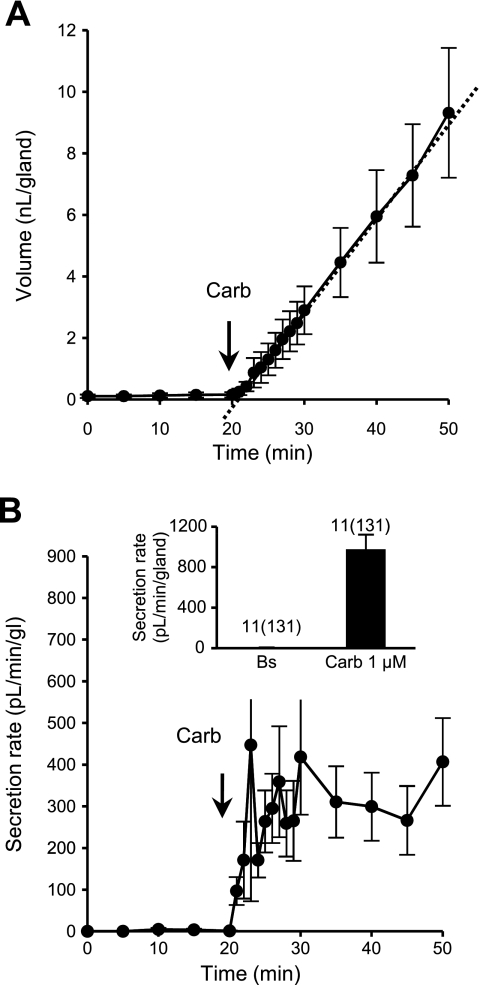
ACh is the major natural agonist of submucosal gland secretion, acting on muscarinic receptors to elevate [Ca2+]i (35, 36). In ferrets, 1 μM carbachol stimulated a high rate of secretion (Fig. 3A). The mean maximal secretion rate was 978 ± 145 pl·min−1·gland−1 (131 glands, 11 ferrets; Fig. 3B, inset). Secretion rates plotted at 1-min intervals (Fig. 3B) showed variable, transient peaks, with a mean peak rate of 1,613 ± 658 pl·min−1·gland−1 and a mean latency to peak of 4 min 53 s (53 glands, 5 ferrets). These transient peaks occurred at staggered times in different glands (see supplemental Fig. S3), producing a smoother, more gradual rise in averaged secretion to a sustained rate of secretion to a maximum that continued for as long as we followed it. This pattern differs somewhat from that seen in other species, which showed an earlier, sharper peak that was two to three times greater than the sustained response (29, 30). The different kinetics can probably be attributed to the use of 1 μM, instead of 10 μM, carbachol; the intact cartilage may also play a role.
Fig. 3.
Responses to carbachol (Carb). A: representative experiment showing mean cumulative volume of mucus secreted from 9 glands to 1 μM carbachol from minute 20. B: corresponding secretion rates for glands shown in A. Inset: average secretion rate measured from the start of maximal secretion at 2 min (n = 11, 131 glands).
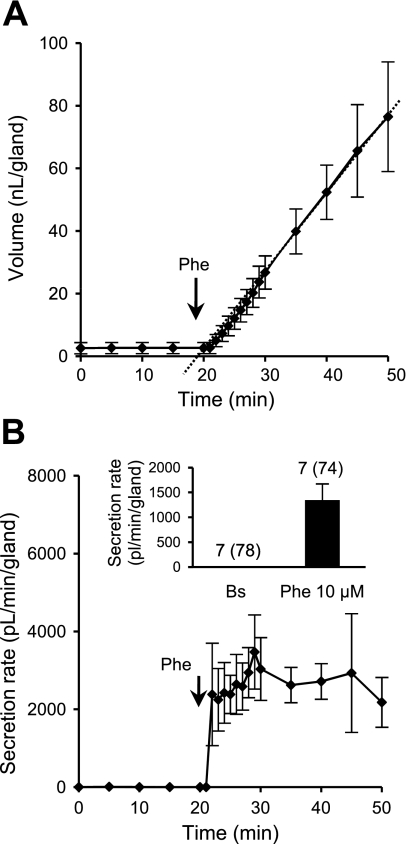
Phenylephrine is an α-adrenergic, [Ca2+]i-elevating agonist that strongly stimulates tracheal submucosal gland secretion in cats but has minimal effects on human, sheep, or pig submucosal glands (30). In ferrets, phenylephrine (10 μM) produced robust, sustained responses (Fig. 4A), with an initial near-peak response followed by a high sustained secretion rate (Fig. 4B). The mean maximal secretion rate was 1,348 ± 325 pl·min−1·gland−1 (74 glands, 7 ferrets; Fig. 4B, inset). Thus, ferret glands resemble cat glands with respect to their responsiveness to phenylephrine, with a response magnitude similar to that produced by carbachol.
Fig. 4.
Responses to the α1-adrenergic agonist phenylephrine (Phe). A: cumulative volume of secreted mucus from 12 glands from a representative experiment. Phenylephrine (10 μM) was present from minute 20, and during the first 10 min after phenylephrine, response was measured at 1-min intervals. B: plot of corresponding secretion rates for glands stimulated by phenylephrine. Inset: average secretion rate measured from minute 2 (n = 7, 74 glands). Values (points or bars) are means ± SE.
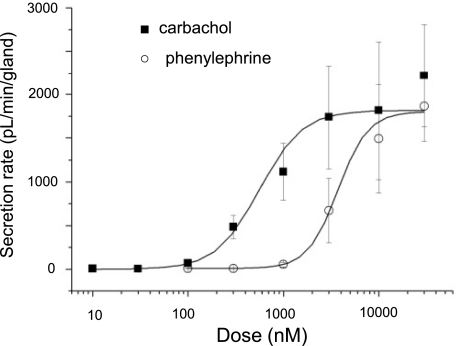
Dose-response relations for carbachol and phenylephrine are compared in Fig. 5. Carbachol was more potent than phenylephrine but equally as efficacious, with an EC50 of 0.6 ± 0.1 μM and a Vmax of 1,817 ± 268 pl·min−1·gland−1 (30–53 glands, 3–4 ferrets) vs. 3.7 ± 0.4 μM and 1,801 ± 95 pl·min−1·gland−1, respectively, for phenylephrine (34 glands, 3 ferrets).
Fig. 5.
Dose-response relations for gland mucus secretion to carbachol and phenylephrine. Vmax values were (in pl·min−1·gland−1) 1,817 ± 268 for carbachol and 1,801 ± 95 for phenylephrine; EC50 values (in nM) were 559 ± 144 for carbachol and 3,731 ± 388 for phenylephrine. (For additional information see supplemental Fig. S2.)
Phenylephrine and carbachol differed markedly in their effects on the trachealis muscle.
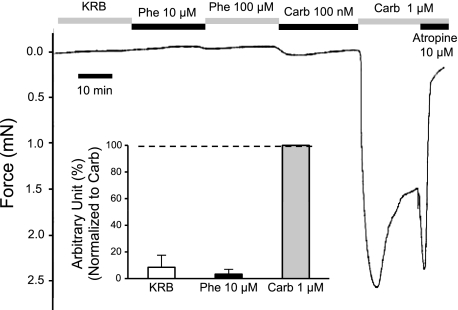
Airway diameter is reduced and airway resistance is increased when the trachealis muscle contracts. Carbachol contracts the trachealis muscle and also induces strong secretion of mucus from the glands; these dual effects act in concert during the lung defense reflex. Because phenylephrine is almost as efficacious as carbachol for stimulating fluid secretion from the tracheal submucosal glands, we expected that it would have a similar effect on the trachealis muscle. However, in our hands, phenylephrine did not stimulate contractions of the trachealis muscle at ≤100 μM, whereas carbachol caused clear contractions at concentrations as low as 100 nM and strong contractions at 1 μM. Agonist effects on the trachealis muscle were studied with a strain gauge (Fig. 6) and by optical measurements. For the tension measurements, the mean tension produced by 1 μM carbachol was 1.3 ± 0.7 mN (n = 4). This value depended on idiosyncrasies of each muscle arrangement, because we did not rigorously isolate an equal number of muscle fibers in each experiment. Therefore, for comparison with phenylephrine, in each experiment we set each response to 1 μM carbachol equal to 100 arbitrary units and expressed the response to 10 μM phenylephrine as a percentage of that value. With this method, 10 μM phenylephrine produced only 3.3 ± 3.7% of the contractile force of 1 μM carbachol (n = 4). The optical measures were not quantified but gave the same qualitative answer: the effects of phenylephrine were almost undetectable compared with the powerful contractions produced by carbachol (see supplemental Fig. S4). These results show that high levels of gland secretion can occur in the ferret without airway narrowing; whether this occurs naturally remains to be determined.
Fig. 6.
Representative tension response of trachealis muscle to stimulation with phenylephrine or carbachol; carbachol response was blocked by atropine. Trachea was opened along the ventral midline to preserve trachealis muscle, and one end of the muscle was secured while the other was attached by 26-gauge wire to a previously calibrated strain gauge (series 400A force transducer system, Cambridge Technology). The wire was stretched just enough to remove all slack, and drugs of the indicated concentrations were added with full bath replacement. Inset: average responses from 4 ferrets. Response to 10 μM phenylephrine did not differ significantly from zero (paired t-test, P = 0.35). KRB, Krebs-Ringer bicarbonate buffer.
Combining low-dose isoproterenol and carbachol produced synergistic increases in fluid secretion from ferret tracheal submucosal glands.
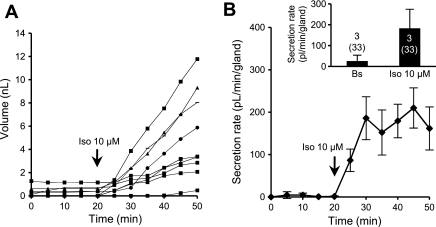
In prior experiments with human submucosal glands, synergistic secretion was observed when low concentrations of agonists that elevated [cAMP]i were combined with low concentrations of agonists that elevated [Ca2+]i (8, 9). We tested for synergy in ferret glands by combining low levels of carbachol with isoproterenol, a β-adrenergic receptor agonist previously shown to increase [cAMP]i in canine, ferret, and feline tracheal submucosal glands (34); in feline glands, the increase occurred in the absence of an increase in [Ca2+]i (25). We first tested isoproterenol alone at high concentration. In 33 glands from 3 ferrets, 10 μM isoproterenol stimulated a steady increase in secretion that reached 183 ± 92 pl·min−1·gland−1 (Fig. 7), with a latency of 10.0 ± 3.5 min to reach maximal secretion. The isoproterenol secretion rate is only 54% of the Vmax for forskolin and 38% of the Vmax for VIP. A similar weak activation of tracheal gland secretion by isoproterenol was reported previously for sheep (30).
Fig. 7.
Responses to isoproterenol (Iso). A: representative experiment showing cumulative volume secreted by 9 glands as a function of time and stimulation with 10 μM isoproterenol, a β2-adrenergic agonist. B: mean secretion rates for 9 glands as a function of time. Inset: mean basal and stimulated secretion rates for 33 glands in 3 ferrets.
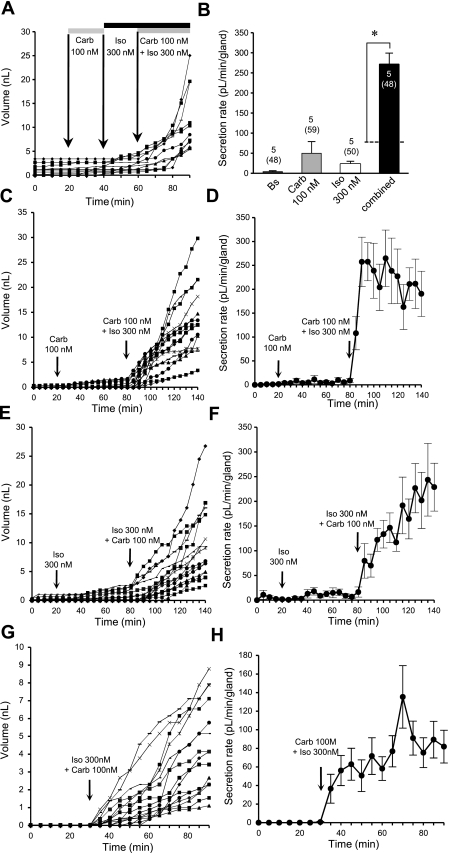
To determine whether synergy exists between cAMP and Ca2+ agonists, we administered low levels of carbachol and isoproterenol alone and in combination (Fig. 8, A and B). The secretion rate responses were 50 ± 29 pl·min−1·gland−1 to 100 nM carbachol alone (59 glands, 5 ferrets) and 23 ± 7 pl·min−1·gland−1 to 300 nM isoproterenol alone (50 glands, 5 ferrets). Isoproterenol + carbachol produced 271 ± 28 pl·min−1·gland−1, which is four times the rate predicted for a purely additive effect (48 glands, 5 ferrets, P < 0.001, paired t-test). To test for the possibility that the rise seen after addition of the second agonist might be superimposed on a slowly developing response to the first agonist, we extended the exposure times to 60 min and added the agonists in reverse order (Fig. 8, C–F). We carried out these experiments using tissues from a single ferret trachea that had a low basal secretion rate and a minimal response to carbachol alone. We saw synergy in both conditions (Fig. 8, C–F). Interestingly, in these experiments with a single animal, the synergistic response patterns differed, showing a faster rise when the combination followed long exposure to carbachol. As an additional control, we applied both agonists together without prior exposure to either agonist alone (Fig. 8, G and H). We again saw a synergistic response when measured against the responses to agonists alone from the same animal in Fig. 8, C–F.
Fig. 8.
Synergy between intracellular concentrations of cAMP ([cAMP]i) and Ca2+ ([Ca2+]i) agonists for mucus secretion. A: representative experiment showing cumulative volume of mucus secreted from 11 individual glands after sequential exposure to low-dose (100 nM) carbachol, low-dose (300 nM) isoproterenol, and carbachol + isoproterenol. B: summary data demonstrating synergistic stimulation of mucus secretion. Response to combined agonists was significantly (4-fold) greater than predicted additive effects of either agonist alone (dotted line). *P < 0.001 (paired t-test). Note large error in response to 100 nM carbachol. C–H: experiments with prolonged exposure to agonists; these 3 experiments used tissues from the same ferret trachea. C: cumulative volume of secretion for each of 15 glands after 60 min of exposure to 100 nM carbachol and then 100 nM carbachol + 300 nM isoproterenol. D: corresponding averaged secretion rates. E: cumulative volume of secretion for each of the 15 glands after 60 min of exposure to 300 nM isoproterenol and then 300 nM isoproterenol + 100 nM carbachol. F: corresponding averaged secretion rates. G and H: simultaneous application of agonists showing cumulative responses of individual glands (G) and average rates (H). Glands were exposed to combined agonists for only 60 min, and average secretion rate was still increasing at the end of the experiment.
Ferret tracheal gland secretion is driven by Cl− and HCO3−, but their proportions differed depending on the method of stimulation.
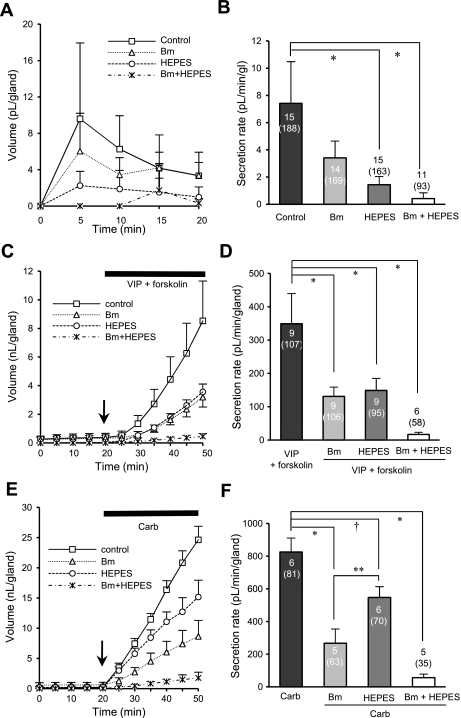
The fluid component of mucus is produced by anion-driven secretion, with Cl− and HCO3− being the major ions involved (24, 27). To assess the contribution of Cl−, 100 μM bumetanide was applied to block the basolateral Na+-K+-2Cl− (NKCC1) cotransporter, while the contribution of HCO3− was assessed by replacing HCO3− with HEPES and gassing with 100% O2. The contribution of each anion differed according to the method of stimulation.
The basal secretion rate was reduced 48.9 ± 13.2% by bumetanide (n = 14, 169 glands), 64.8 ± 23.7% by HEPES (n = 15, 163 glands), and 93.6 ± 6.5% by bumetanide + HEPES (n = 11, 93 glands), but only HEPES or bumetanide + HEPES differed significantly from control [P < 0.001 and P < 0.0001 respectively, Kruskal-Wallis method (Scheffé's and Dunn's post hoc test for nonparametric data); Fig. 9, A and B]. To test the inhibition of [cAMP]i-mediated secretion, we used VIP (1 μM) + forskolin (10 μM) as the agonist. The secretion response to this combination was reduced equally by bumetanide or HEPES (Fig. 9, C and D). Bumetanide decreased secretion by 57.1 ± 6.0% (106 glands, 9 ferrets, P < 0.0001), HEPES replacement reduced secretion by 59.4 ± 4.3% (93 glands, 9 ferrets, P < 0.0001), and HEPES + bumetanide reduced secretion by 95.0 ± 1.1% (58 glands, 6 ferrets, P < 0.0001). Thus, for secretion mediated by increased [cAMP]i, the magnitude of inhibition produced by blocking Cl− or HCO3− did not differ significantly (P > 0.98).
Fig. 9.
Anion component of ferret gland fluid secretion is agonist dependent. A, C, and E: each curve indicates accumulated volume of mucus secretion to KRB buffer (A), 1 μM VIP + 10 μM forskolin (C), or 1 μM carbachol (E) applied at minute 20 with or without other agents. B, D, and F: summary data. Each bar shows secretion rate (mean ± SE) with agonist alone or with agonist + bumetanide (Bm), HEPES, or bumetanide + HEPES. Number of ferrets and glands tested are shown (within or above bars). Differences among values were tested for significance using 1-way ANOVA with Bonferroni's post hoc test (D and F) or the Kruskal-Wallis method with Scheffé's and Dunn's post hoc test (B): *P < 0.0001, †P < 0.005, **P < 0.05.
By contrast, secretion stimulated by 1 μM carbachol (increased [Ca2+]i) was more sensitive to blocking Cl− than to blocking HCO3−. Bumetanide decreased carbachol-stimulated secretion by 68.3 ± 7.8% (63 glands, 5 ferrets, P < 0.0001), but HEPES replacement reduced secretion by only 31.5 ± 8.3% (70 glands, 6 ferrets, P < 0.005). The difference between HEPES- and bumetanide-produced inhibition was also significant (P < 0.05). The combined treatments eliminated 93.2 ± 2.2% of secretion (35 glands, 5 ferrets, P < 0.0001; Fig. 9, E and F). Thus, for secretion mediated by increased [Ca2+]i, the magnitude of inhibition produced by blocking Cl− was significantly greater than that produced by blocking HCO3− (P < 0.05).
Contributions of CFTR to fluid secretion are similar in ferret and human submucosal glands.
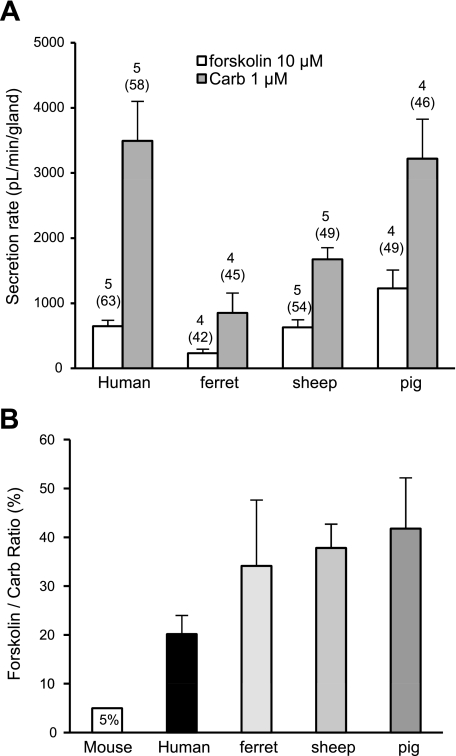
With regard to CF, one of the more important questions about the utility of ferret glands as a model of human glands is whether they depend on CFTR-mediated fluid secretion to a similar extent. In humans and mice, forskolin-stimulated fluid secretion from glands is entirely dependent on CFTR (23, 26). In mice, CFTR-dependent, forskolin-stimulated fluid secretion from airway glands was ∼5% of carbachol-stimulated secretion (23). To directly and contemporaneously compare the ratio of forskolin to carbachol secretion rates in ferret and human glands, we obtained the secretion rates to 10 μM forskolin and 1 μM carbachol and expressed the ratio as follows: (rate produced by 10 μM forskolin ÷ rate produced by 1 μM carbachol) × 100.
Because we used 1 μM carbachol as a comparison in these studies, we also performed contemporaneous studies of gland secretion in sheep and pigs stimulated with 1 μM carbachol and 10 μM forskolin. The results are shown in Fig. 10 and Table 2. The mean secretion rates (Fig. 10A) scale with the size of the glands. The main finding is that the forskolin-to-carbachol secretion ratios for ferrets, pigs, sheep, and humans did not differ significantly from one another, and all were at least fourfold greater than the ratio for mice.
Fig. 10.
Responses to forskolin vs. carbachol in 4 species. A: average secretion rate produced by 10 μM forskolin vs. 1 μM carbachol. Numbers of subjects (or animals) and glands (in parentheses) are shown above each bar. B: forskolin-to-carbachol ratio (mean ± SE) for data shown in A. Ratios did not differ significantly among the 4 species when tested by 1-way ANOVA (P = 0.1278). Leftmost bar represents ratio for mouse based on data reported in Ref. 23.
Table 2.
Summary of responses to forskolin and carbachol in four species
| Forskolin |
Carbachol |
||||||
|---|---|---|---|---|---|---|---|
|
n |
n |
||||||
| Rate, pl•min−1•gland−1 | Subjects | Glands | Rate, pl•min−1•gland−1 | Subjects | Glands | Ratio, % | |
| Humans | 647.8 ± 91.2 | 5 | 63 | 3,494.5 ± 606.4 | 5 | 58 | 20.2 ± 3.8 |
| Ferrets | 231.7 ± 63.3 | 4 | 42 | 851.2 ± 305.5 | 4 | 45 | 34.1 ± 13.5 |
| Sheep | 630.7 ± 117.2 | 5 | 54 | 1,673.7 ± 179.5 | 5 | 49 | 37.8 ± 4.9 |
| Pigs | 1,229.6 ± 281.1 | 4 | 49 | 3,220.2 ± 606.9 | 4 | 46 | 41.8 ± 10.4 |
Values are means ± SE, shown as airway tracheal submucosal gland fluid secretion rates to 10 μM forskolin and 1 μM carbachol. Ratio shows percentage of the rates to carbachol, based on the mean of ratios obtained for each subject or animal. Differences did not reach significance when tested by 1-way ANOVA (P = 0.1278).
DISCUSSION
The twin purposes of these studies were 1) to establish normative data from wild-type (WT) adult ferrets to help guide studies of gland secretion from CFTR−/− ferrets and 2) to allow comparison between ferret and human gland secretion. CFTR−/− ferrets are predicted to be useful models of human CF airway disease, in part because of the hypothesis that airway glands contribute to protecting the airways from infections in ferrets and humans. In ferrets, the most direct evidence for a role of glands in innate defense comes from experiments comparing tracheal xenografts with and without glands. Secretions from the gland-containing xenografts produce much more lysozyme and kill bacteria much more effectively than xenografts without glands (11, 56). In humans, the affirmation for a gland contribution to innate defense is based on four interrelated forms of evidence: 1) human glands produce abundant and highly diversified antimicrobials, 2) human glands express CFTR, 3) gland secretion is severely disrupted in CF, and 4) CF airways are notoriously prone to infections, even though the adaptive immune system is intact or, perhaps, enhanced in CF patients, indicating that local innate defenses of the airways are the source of the defect.
Main features of optically measured fluid secretion from ferret airway glands: agonists.
In the present study, we quantified mucus secretion from individual glands in the ferret trachea using optical methods previously used in mice, pigs, sheep, and humans, allowing direct comparisons with those species. Secretion rates to agonists depend on gland size (N. S. Joo, unpublished observations), which varies widely within a given preparation, while total gland volume appears to scale with the diameter of the trachea (7). For these reasons, comparisons of absolute rates of secretion are less informative than rates normalized to some standard. We chose carbachol-stimulated secretion rates as the basis for normalization, because carbachol is the most potent and efficacious agonist for gland secretion among the species we have examined. As shown in Table 1, the agonists we tested rank in potency as follows: carbachol > VIP > phenylephrine > forskolin. In terms of efficacy, they rank as follows: carbachol ≈ phenylephrine > VIP ≈ forskolin. The main difference between ferrets and humans with regard to these four agonists is the strong response of ferret glands to phenylephrine, in contrast with the near absence of a response in human glands (30). Carbachol and phenylephrine elevate [Ca2+]i and can be expected to stimulate anion-mediated fluid secretion, at least in part via Ca2+-activated Cl− channels (CACC), whereas forskolin and VIP elevate [cAMP]i. In human glands, they appear to mediate fluid secretion entirely via CFTR-dependent mechanisms (26); thus this pathway is of special interest.
Table 1.
Parameters of mucus secretion from individual submucosal glands from ferrets
|
n |
|||||||
|---|---|---|---|---|---|---|---|
| Mean Stable Secretion Rate, pl•min−1•gland−1 | EC50, μM | Latency to Start of Stable Secretion, min | Secretion Rate at Vmax, pl•min−1•gland−1 | Carbachol Vmax Ratio | Ferrets | Glands | |
| Basal | 4.1 ± 0.7 | NA | NA | NA | 0.002* | 27 | 669 |
| VIP (1 μM) | 234 ± 12 | 1.0 | 18 | 479 ± 19 | 0.264 | 6 | 57 |
| Forskolin (10 μM) | 338 ± 70 | 8.7 | 20 | 338 ± 16 | 0.186 | 8 | 90 |
| Carbachol (1 μM) | 978 ± 145 | 0.6 | 2 | 1,817 ± 268 | 1.000 | 11 | 131 |
| Phenylephrine (10 μM) | 1,348 ± 325 | 3.7 | 2 | 1,801 ± 94.7 | 0.991 | 7 | 74 |
| Isoproterenol (10 μM) | 183 ± 92 | ND | 10 | ND | 0.100* | 3 | 33 |
Values are means ± SE. Mean stable secretion rates to the indicated concentrations of agonists are based on as many measurements as possible during the period when secretion had reached a stable value. Vmax ratio is given relative to 10 μM carbachol; Vmax for basal or 10 μM isoproterenol was not determined, so, in those cases, the mean value was used to compute the ratio relative to carbachol-stimulated secretion (indicated with *). NA, not available; ND, not determined.
VIP as an agonist for airway glands.
VIP is released from intrinsic airway neurons and signals exclusively via [cAMP]i. VIP has been localized to the airway glands of humans (19, 49, 54) and ferrets (15, 38) and has been shown to be an agonist for fluid secretion from airway submucosal glands in human, mouse, and pig (23, 26, 29). VIP (2 μM) induced degranulation (measured morphometrically) of serous cells in ferrets (20), and numerous studies in ferrets established that VIP stimulates release of 35S-labeled macromolecules that represent the mucin component of gland mucus secretion (20, 37, 41). VIP (Vmax, 1 μM) stimulated release of 35S-labeled macromolecules from tracheal explants of ferrets with no change of electrical properties measured in the Ussing chamber (41). This was interpreted to mean that VIP stimulated release of macromolecules without stimulating anion transport. However, we know that VIP stimulates anion-mediated fluid transport via CFTR, and a more likely explanation for the observations of Peatfield et al. (41) is that electrical changes across the epithelia lining the glands is poorly reflected in Ussing chamber measurements (J. H. Widdicombe, personal communication).
In our experiments, Vmax was ∼29% greater for VIP than forskolin, although neither drug was tested at high enough concentrations or in a large enough sample to ensure that this difference is real. It will be of interest to eventually clarify this point, because in Calu-3 cells, a serous cell model, VIP produces an increase in apical expression of CFTR by reducing endocytosis, an effect that did not occur with forskolin and appears to depend on a PKC pathway (6). In contrast with its stimulatory effect on gland secretion, VIP inhibits tension in the trachealis muscle (57). It will be important to determine whether normal, neural activation of glands involves a significant VIP component, because that would produce higher volumes of gland secretion for any given level of trachealis tone.
Sympathetic agonist effects on gland secretion and trachealis muscle.
We observed that phenylephrine stimulates ferret gland secretion but not trachealis muscle contractions. Phenylephrine acts on α1-adrenergic receptors and is a partial surrogate for actions of the sympathetic nervous system. In many species, including humans, α1-adrenergic agonists produce only small effects on the lungs that are thought to mainly consist of increases in resistance of the pulmonary vasculature, but their effects even on blood vessels are apparently modest compared with the nitrergic system (5, 22). However, cat submucosal glands receive adrenergic innervation (40) and respond strongly to phenylephrine (30, 42, 55). Ferret submucosal glands also receive strong α-adrenergic neural input, because electrical stimulation of tracheal explants produced gland fluid secretion that was only partially blocked (27–32%) by atropine or the α-adrenergic antagonist phentolamine but was blocked almost completely (92%) by both inhibitors (3). In morphometric studies of ferret airway glands, phenylephrine and methacholine stimulated the release of serous cell granule contents, but only methacholine stimulated significant increases in vacuole formation, which was taken as an indicator of ion and fluid transport (2). However, the present results confirm a powerful effect of phenylephrine on airway gland fluid secretion in ferrets, which, together with cats, are the two species of the six we have tested that respond in this way. However, in a one-sentence comment, phenylephrine-induced gland fluid secretion has also been reported for dog airways (12).
Although phenylephrine was similar to carbachol as a secretagogue, we did not observe a contractile effect on the trachealis muscle. By contrast, carbachol is a powerful secretagogue and a powerful bronchoconstrictor (Fig. 6). Our finding that phenylephrine did not contract the trachealis muscle is interesting in light of prior reports that phenylephrine increases tracheal intraluminal pressure and decreases luminal volume in the cannulated ferret trachea (32, 57). One explanation for the different results might be provided by the finding that, in dog trachealis muscle, phenylephrine alone produced very small contractile responses, but these were augmented manyfold by prior exposure to histamine or serotonin (4), substances that might have been present in the cannulated ferret preparations, but not in our preparation. We do not understand why the α-adrenergic, sympathomimetic agonist phenylephrine is such a powerful gland secretagogue in cats, ferrets, and dogs relative to its trivial effects in pigs, sheep, and humans, nor do we understand the implications of being able to produce copious gland secretion without airway narrowing.
In contrast with the strong α1-adrenergic response, isoproterenol, a β-adrenergic agonist that elevates [cAMP]i, was the weakest agonist we tested for fluid secretion, consistent with previous findings in ferrets for the β2-agonist terbutaline (3) and with prior results with sheep (30).
Role of CFTR in ferret and human glands.
Additional animal models of CF are needed, because mouse models do not adequately mimic CF airway disease. Many factors contribute to the different expression of CF in humans and mice, but probably the most significant is the relative extent to which different organ systems rely on CFTR or CACC for anion-mediated fluid secretion (10). One way to estimate the relative roles of these two anion pathways in glands is to compare fluid secretion rates to forskolin, which is known to be entirely CFTR-dependent in mice and humans, with secretion rates to cholinergic agonists, which are dependent on CFTR and CACC. Previous work in mice showed that maximal responses to forskolin stimulation are only a few percentage of maximal responses to carbachol (23). As shown in Fig. 10 and Table 2, the forskolin response in humans is ∼20% of the carbachol response, while responses in ferrets, sheep, and pigs exceed that value, with ferrets being closest to the human value. We recognize that these ratios are unlikely to only reflect the relative role of CFTR. Nevertheless, to the extent that this ratio serves as an indicator of CFTR's contribution to airway gland function, and if airway glands are important components of airway innate defenses, then these data suggest that CF ferrets, pigs, and sheep might develop human-like airway disease, perhaps even more severe disease. However, the role of airway glands in human CF disease has not been established, and the forskolin-to-carbachol ratio may not accurately reflect the CFTR/CACC contribution for many reasons, including the observation that normal activation of the glands almost certainly involves multiple transmitters acting in concert (8, 9, 58).
Synergy and inhibition among secretagogues in ferret airway glands.
Airway glands are richly innervated by neurons that release at least four transmitters, ACh, nitric oxide, substance P, and VIP (14–17, 39, 59, 60), and it has been demonstrated that human airway glands show marked, CFTR-dependent synergy between agents that increase [cAMP]i and [Ca2+]i (8, 9). Therefore, it seemed important to determine whether synergy is also a property of ferret airway glands. Only one set of synergy experiments was done using nanomolar concentrations of carbachol and isoproterenol. These produced small responses when used alone but showed marked (∼4-fold) synergy when combined (Fig. 8). No further experiments were done in this initial survey, but when CFTR−/− ferrets are available, it will be important to test whether, as in humans, synergy is CFTR-dependent. If it is, it will be important to determine the mechanism of synergy and its role in the normal operation of airway glands. Synergy between VIP and methacholine was previously reported in cats (50); however, VIP inhibited neural, cholinergic-mediated 35S-labeled macromolecular secretion (produced by electrical stimulation) from the ferret trachea (37). This result is of considerable interest, because the effect was near maximal at 1 nM and the EC50 was ∼10 pM, whereas our threshold stimulation for fluid secretion was ∼2–300 nM VIP. Liu et al. (37) also found that 1–10 μM VIP stimulated release of 35S-labeled macromolecular secretion and concluded that VIP has two effects: excitatory on the gland itself and inhibitory on cholinergic terminals. This result highlights the pressing need for a better understanding of the neural control of airway glands; direct application of agonists can take us only so far and may actually produce misleading results.
Anion dependence of cholinergic and noncholinergic secretion.
Gland fluid secretion is dependent on Cl− and HCO3−, but the relative contributions of these two anions may vary across species and conditions. We assessed their contributions by blocking Cl− secretion with bumetanide and HCO3− secretion with HEPES replacement + O2 gassing. In prior experiments with pigs, each of these maneuvers reduced forskolin- and carbachol-stimulated secretion to similar extents, and, in agreement with this finding, the pH of forskolin- or carbachol-stimulated mucus did not differ significantly (29). In ferrets, Cl− or HCO3− block also caused similar decreases in forskolin-stimulated secretion rates, but carbachol-stimulated secretion was decreased significantly more by Cl− block than by HCO3− replacement. It will be interesting to determine whether the pH of ferret mucus also shows agonist dependence and, if so, whether that affects its properties.
Will CF ferret airways be good models for airway disease in humans with CF?
It was a disappointment that CF mice did not manifest a human-like CF airway disease, but the comparison of mouse and human airways revealed many structural and physiological differences that could explain the different outcomes observed when CFTR function is lost. The production of CF models from pigs (45) and ferrets (52) will eventually provide us with an eight-cell matrix (4 WT and CF species) for comparative studies, and this greatly increases the opportunity to define the critical roles that CFTR plays in the innate defense of the airways. Airway submucosal glands are just one component of the airway defense, but there are reasons to think that defective gland secretion plays a role in CF airway disease, and so it is reasonable to propose that we should learn as much as we can about gland function in WT and CF humans, mice, pigs, and ferrets. Focusing just on airway submucosal glands, we found that in humans and mice the component of fluid secretion produced by [cAMP]i-elevating agents was CFTR-dependent and, hence, missing in CF glands, while the fluid component produced by [Ca2+]i-elevating agents was mainly CACC-dependent and, hence, at least partially spared in CF glands.
One way to assess the importance of the CFTR-dependent component is to stimulate secretion with forskolin to elevate [cAMP]i or with carbachol to elevate [Ca2+]i. In humans and mice, the forskolin component of fluid secretion is entirely CFTR-dependent. As shown in Fig. 10B, the forskolin-to-carbachol ratio for gland secretion is as follows: pigs ≥ sheep ≥ ferrets ≥ humans >> mice. The key point of this comparison is that ferrets, sheep, and pigs are similar to humans and are, if anything, more dependent on CFTR for gland fluid secretion, unlike mice, which depend far less on CFTR. It will be interesting to see the extent to which this comparison predicts airway disease in the four CF species; we predict that CF pigs and ferrets will develop airway disease that is at least as severe as human CF airway disease.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5 R01 DK-051817, an award from Cystic Fibrosis Foundation Therapeutics (WINE07XX0), and the Cystic Fibrosis Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tran V. Kim, Jonathan Chen, Dr. Rosemary Broome, and Armando Navarro for expert technical support, including the provision of ferret tracheas. We also thank Mauri E. Krouse and Jin V. Wu for extensive discussions about all aspects of this research and Mauri E. Krouse for expert assistance with data analysis and interpretation.
REFERENCES
- 1.Basbaum CB, Jany B, Finkbeiner WE. The serous cell. Annu Rev Physiol 52: 97–113, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Basbaum CB, Ueki I, Brezina L, Nadel JA. Tracheal submucosal gland serous cells stimulated in vitro with adrenergic and cholinergic agonists. A morphometric study. Cell Tissue Res 220: 481–498, 1981 [DOI] [PubMed] [Google Scholar]
- 3.Borson DB, Chin RA, Davis B, Nadel JA. Adrenergic and cholinergic nerves mediate fluid secretion from tracheal glands of ferrets. J Appl Physiol 49: 1027–1031, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Brown JK, Shields R, Jones C, Gold WM. Augmentation of α-adrenergic contractions in the trachealis muscle of living dogs. J Appl Physiol 54: 1558–1566, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Carvalho P, Thompson WH, Charan NB. Comparative effects of α-receptor stimulation and nitrergic inhibition on bronchovascular tone. J Appl Physiol 88: 1685–1689, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chappe F, Loewen ME, Hanrahan JW, Chappe V. Vasoactive intestinal peptide increases cystic fibrosis transmembrane conductance regulator levels in the apical membrane of Calu-3 cells through a protein kinase C-dependent mechanism. J Pharmacol Exp Ther 327: 226–238, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat 197: 361–372, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JY, Joo NS, Krouse ME, Wu JV, Robbins RC, Ianowski JP, Hanrahan JW, Wine JJ. Synergistic airway gland mucus secretion in response to vasoactive intestinal peptide and carbachol is lost in cystic fibrosis. J Clin Invest 117: 3118–3127, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JY, Khansaheb M, Joo NS, Krouse ME, Robbins RC, Weill D, Wine JJ. Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process. J Clin Invest 119: 1189–2000, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr−/− mice. Proc Natl Acad Sci USA 91: 479–483, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dajani R, Zhang Y, Taft PJ, Travis SM, Starner TD, Olsen A, Zabner J, Welsh MJ, Engelhardt JF. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol 32: 548–552, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis B, Nadel JA. New methods used to investigate the control of mucus secretion and ion transport in airways. Environ Health Perspect 35: 121–130, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis B, Tseng HC. Neural regulation of lysozyme secretion from tracheal submucosal glands of ferrets in vivo. J Appl Physiol 71: 939–944, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Dey RD, Altemus JB, Michalkiewicz M. Distribution of vasoactive intestinal peptide- and substance P-containing nerves originating from neurons of airway ganglia in cat bronchi. J Comp Neurol 304: 330–340, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Dey RD, Altemus JB, Rodd A, Mayer B, Said SI, Coburn RF. Neurochemical characterization of intrinsic neurons in ferret tracheal plexus. Am J Respir Cell Mol Biol 14: 207–216, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Dey RD, Hoffpauir J, Said SI. Co-localization of vasoactive intestinal peptide- and substance P-containing nerves in cat bronchi. Neuroscience 24: 275–281, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Dey RD, Mayer B, Said SI. Colocalization of vasoactive intestinal peptide and nitric oxide synthase in neurons of the ferret trachea. Neuroscience 54: 839–843, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2: 240–248, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Fischer A, Canning BJ, Kummer W. Correlation of vasoactive intestinal peptide and nitric oxide synthase with choline acetyltransferase in the airway innervation. Ann NY Acad Sci 805: 717–722, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Gashi AA, Borson DB, Finkbeiner WE, Nadel JA, Basbaum CB. Neuropeptides degranulate serous cells of ferret tracheal glands. Am J Physiol Cell Physiol 251: C223–C229, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Gashi AA, Nadel JA, Basbaum CB. Tracheal gland mucous cells stimulated in vitro with adrenergic and cholinergic drugs. Tissue Cell 21: 59–67, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Grandordy BM, Paiva de Carvalho J, Regnard J, Florentin D, de Lauture D, Marsac J, Lockhart A. The effect of intravenous phenylephrine on airway calibre in asthma. Eur Respir J 8: 624–631, 1995 [PubMed] [Google Scholar]
- 23.Ianowski JP, Choi JY, Wine JJ, Hanrahan JW. Mucus secretion by single tracheal submucosal glands from normal and cystic fibrosis transmembrane conductance regulator CFTR knock-out mice. J Physiol 580: 301–314, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inglis SK, Corboz MR, Ballard ST. Effect of anion secretion inhibitors on mucin content of airway submucosal gland ducts. Am J Physiol Lung Cell Mol Physiol 274: L762–L766, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Ishihara H, Shimura S, Sato M, Masuda T, Ishide N, Miura M, Sasaki T, Sasaki H, Takishima T. Intracellular calcium concentration of acinar cells in feline tracheal submucosal glands. Am J Physiol Lung Cell Mol Physiol 259: L345–L350, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem 277: 50710–50715, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Joo NS, Krouse ME, Wu JV, Saenz Y, Jayaraman S, Verkman AS, Wine JJ. HCO3− transport in relation to mucus secretion from submucosal glands. JOP 2: 280–284, 2001 [PubMed] [Google Scholar]
- 28.Joo NS, Lee DJ, Winges KM, Rustagi A, Wine JJ. Regulation of antiprotease and antimicrobial protein secretion by airway submucosal gland serous cells. J Biol Chem 279: 38854–38860, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Joo NS, Saenz Y, Krouse ME, Wine JJ. Mucus secretion from single submucosal glands of pig. Stimulation by carbachol and vasoactive intestinal peptide. J Biol Chem 277: 28167–28175, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Joo NS, Wu JV, Krouse ME, Saenz Y, Wine JJ. Optical method for quantifying rates of mucus secretion from single submucosal glands. Am J Physiol Lung Cell Mol Physiol 281: L458–L468, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kishioka C, Okamoto K, Kim J, Rubin BK. Regulation of secretion from mucous and serous cells in the excised ferret trachea. Respir Physiol 126: 163–171, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Kitano S, Wells UM, Webber SE, Widdicombe JG. The effects of intraluminal and extraluminal drug application on secretion and smooth muscle tone in the ferret liquid-filled trachea in vitro. Pulm Pharmacol 5: 167–174, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109: 571–577, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarus SC, Basbaum CB, Gold WM. Localization of cAMP in dog and cat trachea: effects of β-adrenergic agonists. Am J Physiol Cell Physiol 247: C327–C334, 1984 [DOI] [PubMed] [Google Scholar]
- 35.Lee RJ, Foskett JK. Mechanisms of Ca2+-stimulated fluid secretion by porcine bronchial submucosal gland serous acinar cells. Am J Physiol Lung Cell Mol Physiol 298: L210–L231, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Lee RJ, Limberis MP, Hennessy MF, Wilson JM, Foskett JK. Optical imaging of Ca2+-evoked fluid secretion by murine nasal submucosal gland serous acinar cells. J Physiol 582: 1099–1124, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu YC, Khawaja AM, Rogers DF. Effect of vasoactive intestinal peptide (VIP)-related peptides on cholinergic neurogenic and direct mucus secretion in ferret trachea in vitro. Br J Pharmacol 128: 1353–1359, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YC, Patel HJ, Khawaja AM, Belvisi MG, Rogers DF. Neuroregulation by vasoactive intestinal peptide (VIP) of mucus secretion in ferret trachea: activation of BKCa channels and inhibition of neurotransmitter release. Br J Pharmacol 126: 147–158, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maggi CA, Giachetti A, Dey RD, Said SI. Neuropeptides as regulators of airway function: vasoactive intestinal peptide and the tachykinins. Physiol Rev 75: 277–322, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Murlas C, Nadel JA, Basbaum CB. A morphometric analysis of the autonomic innervation of cat tracheal glands. J Auton Nerv Syst 2: 23–37, 1980 [DOI] [PubMed] [Google Scholar]
- 41.Peatfield AC, Barnes PJ, Bratcher C, Nadel JA, Davis B. Vasoactive intestinal peptide stimulates tracheal submucosal gland secretion in ferret. Am Rev Respir Dis 128: 89–93, 1983 [DOI] [PubMed] [Google Scholar]
- 42.Quinton PM. Composition and control of secretions from tracheal bronchial submucosal glands. Nature 279: 551–552, 1979 [DOI] [PubMed] [Google Scholar]
- 43.Ramnarine SI, Haddad EB, Khawaja AM, Mak JC, Rogers DF. On muscarinic control of neurogenic mucus secretion in ferret trachea. J Physiol 494: 577–586, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB, Jr, McLennan G, Meyerholz DK, Namati E, Ostedgaard LS, Prather RS, Sabater JR, Stoltz DA, Zabner J, Welsh MJ. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 295: L240–L263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321: 1837–1841, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, Nielson DW, Verkman AS. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J 19: 431–433, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Schuster A, Ueki I, Nadel JA. Neutrophil elastase stimulates tracheal submucosal gland secretion that is inhibited by ICI 200355. Am J Physiol Lung Cell Mol Physiol 262: L86–L91, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Sehgal A, Presente A, Engelhardt JF. Developmental expression patterns of CFTR in ferret tracheal surface airway and submucosal gland epithelia. Am J Respir Cell Mol Biol 15: 122–131, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Sharma RK, Addis BJ, Jeffery PK. The distribution and density of airway vasoactive intestinal polypeptide (VIP) binding sites in cystic fibrosis and asthma. Pulm Pharmacol 8: 91–96, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Shimura S, Sasaki T, Ikeda K, Sasaki H, Takishima T. VIP augments cholinergic-induced glycoconjugate secretion in tracheal submucosal glands. J Appl Physiol 65: 2537–2544, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol 290: C741–C749, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Sun X, Yan Z, Yi Y, Li Z, Lei D, Rogers CS, Chen J, Zhang Y, Welsh MJ, Leno GH, Engelhardt JF. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest 118: 1578–1583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thiagarajah JR, Song Y, Haggie PM, Verkman AS. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J 18: 875–877, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Uddman R, Alumets J, Densert O, Hakanson R, Sundler F. Occurrence and distribution of VIP nerves in the nasal mucosa and tracheobronchial wall. Acta Otolaryngol (Stockh) 86: 443–448, 1978 [DOI] [PubMed] [Google Scholar]
- 55.Ueki I, German VF, Nadel JA. Micropipette measurement of airway submucosal gland secretion. Autonomic effects. Am Rev Respir Dis 121: 351–357, 1980 [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Zhang Y, Amberson A, Engelhardt JF. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am J Respir Cell Mol Biol 24: 195–202, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Webber SE, Widdicombe JG. The effect of vasoactive intestinal peptide on smooth muscle tone and mucus secretion from the ferret trachea. Br J Pharmacol 91: 139–148, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wine JJ. Parasympathetic control of airway submucosal glands: central reflexes and the airway intrinsic nervous system. Auton Neurosci 133: 35–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu ZX, Satterfield BE, Dey RD. Substance P released from intrinsic airway neurons contributes to ozone-enhanced airway hyperresponsiveness in ferret trachea. J Appl Physiol 95: 742–750, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Zhu W, Dey RD. Projections and pathways of VIP- and nNOS-containing airway neurons in ferret trachea. Am J Respir Cell Mol Biol 24: 38–43, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.