Abstract
Aminoacyl-tRNA synthetases hydrolyze aminoacyl adenylates and aminoacyl-tRNAs formed from near-cognate amino acids, thereby increasing translational fidelity. The contributions of pre- and post-transfer editing pathways to the fidelity of Escherichia coli threonyl-tRNA synthetase (ThrRS) were investigated by rapid kinetics. In the pre-steady state, asymmetric activation of cognate threonine and noncognate serine was observed in the active sites of dimeric ThrRS, with similar rates of activation. In the absence of tRNA, seryl-adenylate was hydrolyzed 29-fold faster by the ThrRS catalytic domain than threonyl-adenylate. The rate of seryl transfer to cognate tRNA was only 2-fold slower than threonine. Experiments comparing the rate of ATP consumption to the rate of aminoacyl-tRNAAA formation demonstrated that pre-transfer hydrolysis contributes to proofreading only when the rate of transfer is slowed significantly. Thus, the relative contributions of pre- and post-transfer editing in ThrRS are subject to modulation by the rate of aminoacyl transfer.
Keywords: Amino Acid, Aminoacyl tRNA Synthesis, Aminoacyl tRNA Synthetase, RNA-Protein Interaction, Transfer RNA (tRNA), Amino Acid Editing, Fidelity, Threonine
Introduction
The accurate transfer of genetic information in living systems requires multiple mechanisms to enhance and preserve the fidelity of gene expression, particularly in protein synthesis. The 1–3 errors per 10,000 amino acids incorporated during protein synthesis largely originate from errors in decoding (1) but can arise from previous steps, including the aminoacylation reaction catalyzed by aminoacyl-tRNA synthetases (ARSs)2 (2). In the first of two half-reactions, ARSs condense amino acid and ATP to form a noncovalently enzyme-bound adenylate, followed by a transfer reaction that produces aminoacyl-tRNA and AMP as products. This highly accurate reaction (on the order of 1 error in 105) reflects the ability of ARSs to carefully discriminate among chemically similar standard and nonstandard amino acids (3). Mutations in ARSs or their cognate tRNAs can nonetheless create errors associated with significant molecular pathologies, particularly in neural tissues (4).
Amino acids that differ by a single methyl group pose a particular discrimination challenge for ARSs (3, 5, 6). An additional methyl group on an amino acid typically provides no more than ∼1 kcal/mol of incremental binding energy, imposing an upper limit of discrimination on the order of 1 in 5 (7). To account for the enhanced discrimination seen in living systems, a “double sieve” model was proposed (8). Amino acids larger than cognate are excluded by the “coarse sieve” of the aminoacylation site, whereas smaller or isosteric amino acids are cleaved from the end of the tRNA by the “fine sieve” of the editing site. In principle, noncognate amino acids can be edited both by increased hydrolysis of the adenylate (pre-transfer editing) or by hydrolysis of the mis-acylated tRNA (post-transfer editing). Either or both reactions can formally occur in a dedicated editing site structurally distinct from the standard synthetic site. Because of differences between systems, and the absence of a comprehensive kinetic description of any single editing ARS, the relative contributions of pre- and post-transfer editing mechanisms to the overall fidelity of ARSs are unknown.
Editing functions are widely distributed among members of the two structurally distinct classes of ARSs. The class I isoleucyl-, valyl-, and leucyl- (LeuRS) tRNA synthetases possess a discrete CP-1 domain inserted into the Rossmann fold that serves as the active site for deacylation of mis-acylated tRNAs (5). The link between post-transfer editing mechanisms and the CP-1 domain has been established in many class I systems (9–11). Assessment of the role of pre-transfer editing has proved to be more elusive, because of the potential existence of both tRNA-dependent and tRNA-independent mechanisms and competing proposals about the site of the activity (12–15). Methionyl-tRNA synthetase (MetRS), which edits homocysteine through the formation of a cyclic lactone, represents one of the clearest examples of pre-transfer editing (16).
Among class II ARSs, editing functions have similarly been demonstrated for phenylalanyl- (PheRS) (17–19), alanyl- (AlaRS) (20, 21), threonyl- (ThrRS) (22), and prolyl-tRNA synthetases (ProRS) (23). Unlike class I enzymes, which share a conserved CP-1 editing domain, class II ARSs lack a common editing domain. Nevertheless, there is considerable evidence for the importance of post-transfer editing in numerous systems and localization of that activity to specialized editing domains (3). With regard to pre-transfer editing, tRNA-independent ATPase activities have been documented for the alanyl (21) and prolyl systems (24, 25). However, the role of these mechanisms in fidelity preservation is not well understood, because they have not been studied by detailed kinetic analysis.
ThrRS is a representative class II α2 homodimeric ARS with a dedicated N-terminal domain implicated in post-transfer editing, a central catalytic domain, and C-terminal anticodon binding domain (26). A catalytic Zn2+ ion present in the active site directly coordinates the α-amino group and β-hydroxyl of both threonine and serine, providing discrimination against isosteric valine (27). Serine incorporation is prevented by multiple mechanisms, including its low affinity for the ThrRS active site, and the deacylation of Ser-tRNAThr in the editing domain active site (22, 27, 28). Structural and biochemical evidence implicates His-73 as a likely candidate for general base-catalyzed hydrolysis of Ser-tRNAThr (28, 29). Based on prior structural studies and the failure to observe serine-stimulated hydrolysis of ATP, it has been suggested that post-transfer editing is sufficient to account for the observed fidelity of ThrRS (28, 29). This conclusion has yet to be supported by a kinetic analysis in which the activities of ThrRS on threonine and serine substrates are compared in detail.
The essentially kinetic nature of the editing process underscores the importance of determining the rates of specific individual rate constants in the overall pathway, particularly for the branch points associated with pre- and post-transfer mechanisms. For a number of class II ARSs that have been examined, the amino acid activation reaction is asymmetric (30–32), but the asymmetry can be modulated by the cognate tRNA in some cases (32, 33). Here, the editing of serine by Escherichia coli ThrRS was investigated by application of both pre- and steady state kinetic approaches. The ThrRS catalytic domain was found to discriminate against serine, principally by enhancing the rate of adenylate turnover. The importance of this mechanism is shown to be dependent on the rate of aminoacyl transfer.
EXPERIMENTAL PROCEDURES
Preparation of Enzymes and tRNA
ThrRS and CCA adding enzymes were expressed and purified as described previously (34), employing nickel-nitrilotriacetic acid affinity chromatography. Active fractions were identified by SDS-PAGE, pooled, concentrated by low speed centrifugation through Centricon concentrators, and then dialyzed against buffer A (10 mm Tris, pH 8.0, 100 mm KCl, 10 mm MgCl2, 3 mm β-mercaptoethanol). The final preparations were stored in 50% glycerol at −20 °C. Active site concentrations were determined before use in experiments. The purification of ΔN1N2 ThrRS included 10% glycerol throughout the procedure. The fully modified version of tRNAThr was prepared from an Escherichia coli overexpression strain as described previously (34).
ATP Hydrolysis Experiments
These assays were performed essentially as described previously (35). The reactions were carried out at 37 °C in buffer A with 1 mm dithiothreitol, 0.02 unit/ml pyrophosphatase, 3 mm [γ-32P]ATP, 2 μm enzyme (wild type ThrRS or ΔN1N2 ThrRS) in the presence or absence of 3′-deoxy A76 tRNA. Reactions with cognate amino acid contained threonine at 5 mm, whereas noncognate reactions contained 500 mm l-serine. Ten-μl reaction aliquots were quenched in 7% perchloric acid prior to adsorption on activated charcoal. The mixture was vortexed and spun for 3 min at 12,000 rpm, and the amount of [γ-32P]PPi in 100 μl of supernatant was determined by scintillation counting. A background rate of ATP hydrolysis in the absence of enzyme was subtracted from all determinations. Data were analyzed as described below under “Data Analysis.”
Transient Kinetic Experiments
Pre-steady state adenylation reaction rates were determined by use of an RQF-3 rapid chemical quench instrument (Kin-Tek, Austin, TX). All reactions were carried out at 37 °C at pH 8 in buffer A. The reaction conditions and experimental work up by thin layer chromatography were identical to those described for HisRS (32, 33). As a technical constraint, the [α-32P]ATP concentration was fixed at 100 μm to obtain a desired signal-to-noise ratio (24, 25, 32, 33). After exposing the TLC plates to a Bio-Rad K-screen, the fractional conversion of ATP to AMP was calculated using Bio-Rad Quantity One software. The resulting plots were fit to a complex equation featuring a bi-exponential term followed by a linear phase, as described under “Data Analysis.” Single turnover aminoacyl transfer experiments employing the post-transfer editing deficient H73A ThrRS were performed as described previously (34). Briefly, the pre-formed ThrRS-[14C]seryl-adenylate was rapidly mixed with tRNA and then quenched by addition of 3 m NaOAc. Data from time points collected over the interval of 10 ms to 1 s were used to construct first order progress curves, as described under “Data Analysis.”
Solvent Adenylate Hydrolysis
The adenylate was synthesized in situ in ∼5-min reactions that contained 2 μm ThrRS, 100 μm [α-32P]ATP, 5 mm threonine, or 500 mm serine in buffer A. Quench solution (0.5 m EDTA, pH 8.0, 0.2% SDS) was added to terminate the reaction and denature the enzyme. One-μl reaction aliquots were chromatographed on TLC plates and detected by a Bio-Rad phosphorimager. The resulting data were analyzed by fitting to an exponential decay equation as described under “Data Analysis.”
ATP Consumption versus Aminoacyl-tRNA Formation (Energy Efficiency) Experiments
Aminoacylation reactions with labeled amino acid and ATP were performed in parallel under multiple turnover conditions, employing identical concentration of substrates and reaction conditions at 37 °C and pH 8. To monitor the consumption of ATP and formation of aminoacyl-tRNA, reactions contained 100 μm ATP, 1 mm amino acid, and 15 μm tRNAThr. In the component of the experiment that followed ATP consumption, 100 μm [α-32P]ATP was employed as substrate, although the other component to follow production of aminoacyl-tRNA employed 1 mm 14C-labeled amino acid as the labeled substrate. Product formation was monitored by trichloroacetic acid precipitation in the case of 14C-labeled amino acid and TLC in the case [α-32P]ATP. Reactions involving wild type ThrRS and threonine contained 0.1 μm ThrRS wild type or 2 μm H73A ThrRS when serine was the substrate. Reactions involving H73A,H309A ThrRS and threonine employed an enzyme concentration of 0.5 μm, although those featuring serine used 5 μm of the double mutant enzyme. The resulting plots were analyzed by linear regression (33).
Data Analysis
The TLC experiments measuring the formation of [α-32P]AMP and [α-32P]AA-AMP were fit to burst Equation 1,
where k1 is the rate of burst phase, and k2 is the steady state rate constant for the formation of AA-AMP, and A represents the amplitude of burst phase. Alternatively, Equation 2 was used with double exponential terms followed by a linear phase,
where k1 and k2 are the rate constants for the formation of AMP (or Thr-AMP) in active site 1 and active site 2, respectively; A1 and A2 represent the respective amplitudes, and k3 represents the steady state rate of adenylate turnover. The rate of solvent adenylate hydrolysis was fit to the following exponential decay Equation 3,
where A represents the amplitude; k represents the rate of decay, and C represents the y offset. The single turnover aminoacyl transfer reaction monitoring the formation of [14C]Ser-tRNA was fit to the single exponential Equation 4,
where ktrans is the rate of transfer; A is the amplitude, and C is the y offset. The data were plotted using Kaleidagraph.
The efficiency of editing can be calculated according to Fersht (36). In a simple treatment where there is a branched pathway at an enzyme-intermediate with separate branches for productive synthesis and editing, the fraction of enzyme-adenylate that proceeds to aminoacyl-tRNA product (Fp) is given by Equation 5,
 |
where ks is the rate of conversion of enzyme adenylate to aminoacyl-tRNA (essentially the rate of aminoacyl transfer), and kd is the rate of hydrolytic destruction of the adenylate. The number of ATP molecules consumed per mol of aminoacyl-tRNA formed is equivalent to 1/Fp. For example, if the rates of formation of aminoacyl-tRNA and rates of destruction of enzyme adenylate complex are equal, than 50% of the adenylate complex is hydrolyzed prior to transfer, and 2 mol of ATP would be consumed per mol of aminoacyl-tRNA formed.
RESULTS
Pre-transfer Editing Occurs in the Presence of Serine in ThrRS
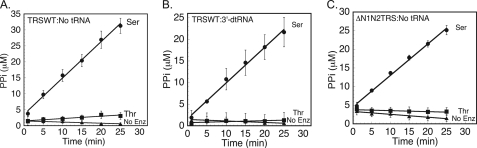
Structural studies of ThrRS in complex with seryl- and threonyl-adenylate analogs (27) showed that the ThrRS active site lacks structural features to discriminate against serine (supplemental Fig. 1). One indication of pre-transfer editing is the enhanced consumption of ATP in the presence of a noncognate amino acid, relative to the cognate (12). To test whether ThrRS exhibits pre-transfer editing, ATP hydrolysis assays were performed in the presence of cognate threonine and noncognate serine. Threonine and serine elicit rates of ATP hydrolysis of 0.08 and 1.1 min−1, respectively, representing a 14-fold stimulation by serine (Fig. 1A). ATP hydrolysis in the absence of enzyme or amino acid was negligible. The inclusion of a nonaminoacylatable version of tRNAThr (3′-deoxy A76) did not affect ATP hydrolysis rates (Fig. 1B). Some models of pre-transfer editing have proposed that the adenylate is translocated to the editing active site prior to hydrolysis (37, 38). This possibility was tested in ATP hydrolysis experiments using the N-terminal deleted mutant version of ThrRS (ΔN1N2 ThrRS), which lacks post-transfer editing (22). Steady state rates of ATP hydrolysis for both serine and threonine with ΔN1N2 ThrRS were comparable with the rates obtained using the full-length enzyme (Fig. 1C). In summary, ThrRS is an efficient serine-dependent ATPase, and this function is independent of the canonic editing domain.
FIGURE 1.
Serine-stimulated ATPase activity in ThrRS occurs in the absence of the editing domain and tRNA. A–C depict steady state ATP hydrolysis assays at 37 °C and pH 8 with wild type ThrRS (TRSWT) and no tRNA (A), wild type ThrRS (TRSWT) and 3′d-A76 tRNAThr (B), or ΔN1N2 ThrRS with no tRNA (C). ATP turnover was detected by the formation of [γ-32P]PPi in reactions containing ThrRS or ΔN1N2 ThrRS (2 μm), [γ-32P]ATP (3 mm), and threonine (5 mm) (filled squares), or serine (500 mm) (filled circles) or no enzyme (No Enz)/amino acid (filled triangles). Error bars represent mean ± S.D. from three independent experiments.
Asymmetric Catalysis of Amino Acid Activation Occurs in the Presence of Threonine and Serine
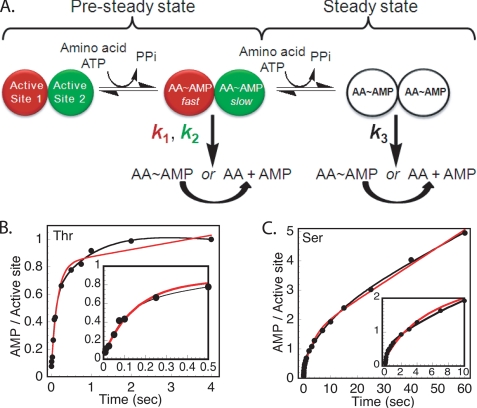
Most class II ARSs are dimers or multiples of dimers, the implications of which for editing function have not been examined. Recently, differential rates of amino acid activation were observed for the dimeric class II HisRS in the absence of tRNA, with the rate in active site 1 (k1) exceeding that of the rate in active site 2 (k2) by a factor of 25 (32, 33). In subsequent turnovers, a steady state is observed characterized by the rate constant k3 (Fig. 2A). This may represent slow reiterative cycles of adenylate formation and hydrolysis (32, 33). To probe for this mechanism in ThrRS, the adenylation reaction was performed under pre-steady state multiple turnover conditions in the presence of excess amino acid and [α-32P]ATP, following the production of [α-32P]AMP and [α-32P]AA-AMP by TLC (supplemental Fig. 2, A and B). As seen previously with HisRS, the resulting progress curves are best described by two exponentials (k1 and k2), followed by a linear steady state phase (k3) (Fig. 2, B and C).
FIGURE 2.
Amino acid activation by ThrRS is asymmetric in the absence of tRNA. A, diagrammatic representation of the basic mechanism of asymmetric catalysis. The two active sites of ThrRS are depicted as circles with k1 and k2 representing the rate of adenylate synthesis in the two active sites. The rate constant k3 represents the steady state rate of adenylate turnover. The formation of [α-32P]AMP was monitored on TLCs (supplemental Fig. 2, A and B), with the data plotted as pre-steady state progress curves in the presence of threonine (B) and serine (C). The experiments were performed using 10 μm ThrRS active sites, 100 μm [α-32P]ATP, and 5 mm threonine or 150 mm serine. Representative data from one of three independent experiments with comparable results are shown. The progress curves were fit either to a burst equation with a single exponential and linear phase (red line) or double exponential with a linear phase (black line). The insets expand the time scale to show the earliest portions of the reactions.
At saturating concentrations of the cognate substrate threonine, the rate of formation of AMP in active site 1 (k1 = 17.7 s−1) was 8-fold higher than that measured in active site 2 (k2 = 2.1 s−1). The amplitude of AMP formation in both active sites was nearly equal (0.5 in active site 1 versus 0.4 in active site 2). The steady state rate of AMP formation (k3) was negligible, at 0.001 s−1 (Table 1). In contrast to HisRS, however, there was an appreciable steady state rate of accumulation of threonyl-adenylate. In comparison with AMP formation, the progress curve for threonyl- (and seryl-adenylate, see below) formation could be readily fit to either a single or double exponential equation, with amplitudes that were sub-stoichiometric with respect to the concentration of active sites (supplemental Fig. 3, A and B). The accumulation of adenylate at sub-stoichiometric concentrations over time suggests that there is partitioning of newly formed molecules into a population that remains stably bound and a portion that undergoes hydrolysis on the enzyme. This remains to be investigated further.
TABLE 1.
Rate constants for adenylate synthesis by ThrRS and HisRS in the absence of tRNA
ThrRS-mediated adenylation reactions were performed in a rapid quench flow instrument at pH 8 and 37 °C. The reaction products were resolved by TLC and quantified as described under “Experimental Procedures.” Values represent the means of more than three independent experiments with standard error. ND indicates not determined.
| ARS/Amino acid | Rate of formation of [32P]AMP measured by rapid chemical quench |
Refs. | ||
|---|---|---|---|---|
| k1 (s−1) active site 1 | k2 (s−1) active site 2 | k3 (s−1) steady state | ||
| ThrRS/threonine | 17.7 ± 4.2 | 2.1 ± 0.33 | 0.001 ± 0.0002 | This work |
| ThrRS/serine | 3.8 ± 1.2 | 0.15 ± 0.02 | 0.029 ± 0.003 | This work |
| HisRS/histidine | 18.1 ± 4.7 | 0.68 ± 0.16 | ND | 32 |
With serine as the amino acid substrate, the rates of formation of AMP during the burst phase were ∼25-fold faster in site 1 than site 2 (Table 1). Unlike threonine, where the amplitudes were essentially equal, the amplitude of the second exponential for serine was higher, likely as a consequence of editing. The other significant difference between cognate serine and cognate threonine was the k3 parameter, a linear rate that probably reflects reiterative synthesis and destruction of the seryl-adenylate. Significantly, the steady state rate of AMP formation in the presence of serine (0.029 s−1) was 29-fold higher than in the presence of threonine (0.001 s−1) (Table 1). This k3 parameter is the singular rate constant that is significantly greater in the case of serine relative to threonine. The rate of Ser-AMP formation was also analyzed in a manner similar to that of threonine (supplemental Fig. 3B). The ratio of Ser-AMP formed to total amount of active sites was also less than 1, arguing that Ser-AMP is not actively released from active sites.
The AMP formed under these conditions reflects the sum of AMP arising from adenylate hydrolysis on the enzyme and that arising from adenylate that dissociates and then becomes hydrolyzed in solution. To resolve the rates of these two separate events, the rate of solvent-induced adenylate hydrolysis was determined. In solution, threonyl- and seryl-adenylate were found to decay with virtually identical rates (0.0007 and 0.0008 s−1) (supplemental Fig. 4, A and B). These rates were ∼40-fold slower than k3 in the presence of serine (Table 1). Hydrolysis of seryl-adenylate in the ThrRS active site is therefore likely to be the predominant pathway, as was observed previously for ProRS (25).
Amino Acid Discrimination Does Not Occur at the Aminoacyl Transfer Step
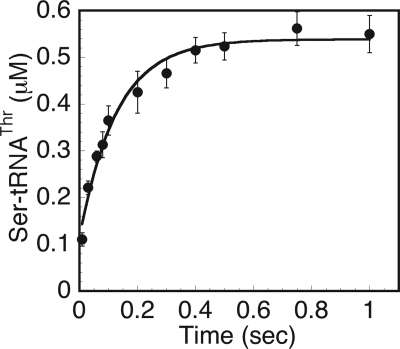
When functional cognate tRNA is present, an adenylate formed from a noncognate amino acid can either undergo hydrolysis or undergo transfer to the 3′ end of the tRNA. If the rate of transfer is substantially greater than hydrolysis, then little pre-transfer editing can occur; if it is similar or less, then pre-transfer editing can contribute to fidelity (36). The rate of aminoacyl transfer employing a noncognate seryl-adenylate was measured in a single turnover experiment using rapid chemical quench, as described under “Experimental Procedures.” To prevent the hydrolysis of Ser-tRNAThr, the experiment was performed using H73A ThrRS, which deacylates Ser-tRNAThr 7000-fold slower than wild type ThrRS (29). A value of 7.8 s−1 for ktrans was obtained from single turnover experiments (Fig. 3), which is only 2-fold slower than the rate of wild type ThrRS aminoacyl transfer with threonine (34). This value was 2- and 52-fold faster than the rates of AMP formation (i.e. k1 and k2) in the burst phase of seryl-adenylate formation but 270-fold greater than k3, the apparent steady state rate of adenylate formation and hydrolysis (Table 1).
FIGURE 3.
Aminoacyl transfer of serine is fast relative to adenylate turnover. The [14C]seryl-adenylate-H73A ThrRS (8–10 μm) was prepared as described under “Experimental Procedures” and then rapidly mixed with tRNA (4–6 μm) under rapid quench conditions over the interval of 10 ms–1 s. Then the resulting curve was fit to a single exponential equation to derive the rate of aminoacyl transfer (ktrans). The error bars at each time point represent the mean ± S.E. from three independent experiments.
Energy Efficiency of Aminoacyl-tRNA Formation by ThrRS
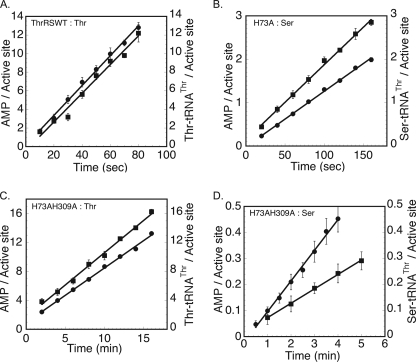
In considering different editing mechanisms, one possibility is that the rate of adenylate hydrolysis on-enzyme is accelerated by the cognate tRNA. This would be expected to produce excess consumption of ATP relative to each mole of aminoacyl-tRNA formed, under conditions where post-transfer editing has been blocked (25, 39). To address this possibility for ThrRS, the ratio of ATP consumption to aminoacyl-tRNA product was determined in multiple turnover experiments, where these two parameters were followed in parallel, as described under “Experimental Procedures.” To eliminate any possibility of post-transfer editing, ratios were also determined for H73A ThrRS and H73A,H309A ThrRS, both of which contain an H73A mutation (28, 29). In the presence of threonine, the steady state rates of AMP formation (0.16 ± 0.01 s−1) and Thr-tRNAThr (0.15 ± 0.01 s−1) formation by wild type ThrRS were equal, indicating that each mole of cognate adenylate is quantitatively converted to aminoacylated-tRNA product (Fig. 4A and summarized in Table 2). With H73A ThrRS as enzyme and noncognate serine as substrate, the rate of formation of AMP (0.013 ± 0.0002 s−1) was also equivalent to that of the rate of formation of Ser-tRNAThr (0.015 ± 0.0003 s−1) (Fig. 4B and summarized in Table 2). The nearly 1 to 1 ratio for moles of [ATP] consumed per mol of Ser-tRNAThr formed indicates that the efficiency of Ser-tRNAThr synthesis by H73A ThrRS is close to 100%. Neither the threonyl- nor the seryl-adenylate was detected by TLC in the presence of functional tRNAThr. This is also consistent with the rapid rate of aminoacyl transfer, relative to the adenylate turnover rates measured in the absence of tRNA. Thus, in the branched path between adenylate hydrolysis and aminoacyl transfer, nearly all of the seryl-adenylate is transferred to tRNAThr.
FIGURE 4.
Determination of energy efficiency in the presence of serine and threonine. The formation of [α-32P]AMP (filled circles) and formation of [14C]aminoacyl-tRNA (filled squares) at pH 8.0 and 37 °C were followed in parallel using 100 μm [α-32P]ATP, 1 mm 14C-labeled amino acid substrate, and 15 μm tRNAThr. A, 0.1 μm wild type ThrRS and threonine as substrate; B, 2 μm H73A ThrRS and serine as substrate; C, 0.5 μm H73A,H309A ThrRS and threonine as substrate; D, 5 μm H73A,H309A ThrRS and serine as substrate. The error bars associated with the amount of reaction product formed at each time point represent the mean ± S.E. from three independent experiments.
TABLE 2.
Estimation of energy efficiencies in the presence of threonine and serine using wild type and mutant ThrRSs
Aminoacylation reactions were carried out at pH 8.0 and 37 °C. To determine the consumption of ATP and AA-tRNA, [α-32P]ATP and 14C-labeled amino acids were used in the parallel reactions, respectively. ThrRS wild type enzyme was used when threonine was the substrate and H73A as the post-transfer editing deficient mutants with serine. When the effect of aminoacyl transfer was investigated, the H73A/H309A double mutant was used. The progress curves were plotted and analyzed by linear regression. Values represent the mean ± S.E. from three experiments.
| Aminoacylation enzyme/AA substrate | Product monitored | Product formation rate | Ratio of ATP consumption to AA-tRNA formation |
|---|---|---|---|
| s−1 | |||
| Wild type ThrRS/Thr | [α-32P]AMP | 0.16 ± 0.01 | 1 |
| [14C]Thr-tRNAThr | 0.15 ± 0.01 | ||
| H73A ThrRS/Ser | [α-32P]AMP | 0.013 ± 0.0002 | 0.9 |
| [14C] Ser-tRNAThr | 0.015 ± 0.0003 | ||
| H73A/H309A ThrRS/Thr | [α-32P]AMP | 0.01 ± 0.0005 | 1 |
| [14C]Thr-tRNAThr | 0.01 ± 0.001 | ||
| H73A/H309A ThrRS/Ser | [α-32P]AMP | 0.002 ± 0.0002 | 2 |
| [14C]Ser-tRNAThr | 0.001 ± 0.0001 |
Accordingly, pre-transfer editing would be predicted to be important only when transfer is significantly slowed. This hypothesis was addressed in measurements using the H73A,H309A ThrRS double mutant. The first mutation eliminates post-transfer editing, although the second reduces the rate of aminoacyl transfer 242-fold with only a minimal effect on amino acid activation (34). With threonine as substrate, the rates of AMP formation and Thr-tRNAThr formation were essentially identical (0.01 ± 0.001 s−1) for H73A,H309A ThrRS (Fig. 4C, summarized in Table 2). However, with serine as substrate, the rate of AMP formation (0.002 ± 0.0002 s−1) was 2-fold higher than the rate of Ser-tRNAThr formation (0.001 ± 0.0001 s−1) (Fig. 4D and summarized in Table 2). Thus, the observation of a 2-fold higher rate of ATP consumption for H73A,H309A ThrRS relative to the rate of Ser-tRNAThr synthesis demonstrates that, when aminoacyl transfer is compromised, pre-transfer editing can contribute to fidelity.
DISCUSSION
Biochemical reactions involving editing depend on kinetic partitioning and possess branch points in which an intermediate either undergoes hydrolysis (either directly on the enzyme or after release into solution) or is transformed in the next step (12, 36, 40). During aminoacylation, the aminoacyl-adenylate and the aminoacyl-tRNA both represent intermediates subject to kinetic partitioning. Measuring the rates of elementary steps in the overall aminoacylation reaction sequence catalyzed by ThrRS by rapid chemical quench allows those steps that dictate the specificity of amino acid to be readily identified and one to thereby assess the contribution of pre- and post-transfer editing to overall fidelity. By using this approach, we found that ThrRS stimulates hydrolysis of the seryl-adenylate, a form of pre-transfer editing. Unexpectedly, the rates of adenylate formation per se and aminoacyl transfer were very nearly identical for serine and threonine. As discussed below, this has important consequences for the relative contribution of pre- and post-transfer editing mechanisms to overall fidelity.
Enzyme-catalyzed Turnover of Noncognate Adenylates Is Broadly Distributed among the Class II ARSs
Prior to this study, it had been concluded that ThrRS largely prevents mis-acylation of tRNAThr by a post-transfer activity localized to the conserved N-terminal domain in prokaryotes and eukaryotes. The failure of past work (22, 27) to detect the ATPase activity reported here may reflect the use of sub-optimal conditions and assays, including too low amino acid and enzyme concentrations and the use of charcoal to capture product. The data reported here indicate that, under conditions where amino acid binding is saturating, ThrRS activates threonine and serine with similar rates, i.e. there is rather modest (less than 10-fold) discrimination against serine at the level of adenylate synthesis chemistry. This is consistent with the results of pyrophosphate exchange experiments, where the kcat values for threonine (36 s−1) and serine (26 s−1) activation were determined to be similar (27). As discussed in further detail below, the steady state rate of seryl-adenylate turnover is significantly faster than that of the threonyl-adenylate when tRNA is absent (Table 1). Rather than a facilitated diffusion of the adenylate from the synthetase active site to the editing site proposed previously (38, 41), this activity was shown to localize to the aminoacylation active site and not to the editing site (Fig. 1, A and C). The residues in the aminoacylation active site responsible for hydrolysis have yet to be identified. At a minimum, selection against serine by ThrRS involves at least three discrete mechanisms, including discrimination at the level of initial binding (27), enhanced hydrolysis of the seryl-adenylate (this work), and de-acylation of Ser-tRNAThr (22, 28). Similar mechanisms may be operative in the related class II ARSs AlaRS, ProRS, and seryl-tRNA synthetase (21, 25, 42). By contrast, pre-transfer editing in class I LeuRS has been shown to be stimulated by the presence of tRNALeu (14).
The structural basis for discrimination by ThrRS between threonine and serine is not immediately evident (27). Although contacts between the β-hydroxyl groups of threonine and serine and the active site Zn2+ ion are conserved, interactions between Thr-482, Ala-513, and the side chain of threonine are not (supplemental Fig. 1). The low affinity of the ThrRS active site for serine (27) has precluded detection of the induced fit conformational changes that accompany threonine binding and adenylate formation (43). One possibility is that a suboptimal arrangement of active site groups associated with serine binding allows entrance of adventitious solvent molecules, enhancing hydrolysis. The stimulation of adenylate turnover by serine in the ThrRS active site is reminiscent of the ATPase behavior of ProRS (24, 25), HisRS (33), and seryl-tRNA synthetase (33, 42). In each of these class II ARSs, reiterative adenylate synthesis and breakdown appears to be occurring in the canonic class II active site. Evolution of the class II ARS active site appears to reflect a balance between efficient synthesis of the cognate adenylate and the facilitated hydrolysis of near-cognates.
Aminoacyl Transfer Does Not Contribute to the Fidelity of Amino Acid Selection in ThrRS
A new finding emerging from this work is that threonine and serine undergo aminoacyl transfer at similar rates (Fig. 3). This suggests that the aminoacyl transfer step, like the chemical step associated with adenylate formation, confers very little amino acid specificity in the ThrRS system. Although this was proposed earlier in the mis-acylation reaction with alanine by ProRS (25), the results reported here provide a specific quantitative comparison of the aminoacyl transfer of cognate and near-cognate amino acids. In glutaminyl-tRNA synthetase, the rate kchem, which reflects both activation and aminoacyl transfer, is at least 165-fold slower for glutamate relative to cognate glutamine (44). In this nonediting ARS, there is considerable specificity mediated at the level of chemistry, which is reminiscent of the DNA polymerases (3). The mis-positioning of incoming nucleotides by DNA polymerases may impose unfavorable geometric or steric constraints on the reaction, providing a significantly highly energetic barrier to incorporation (45). For an amino acid that is smaller than cognate, no such barriers are predicted to exist. Instead, there are increased entropic penalties for a smaller amino acid, but these are “pre-paid” in the binding and activation steps, and this accounts for the limited impact on the rate of aminoacyl transfer.
Comparing Rates of Pre-transfer Editing in the Presence and Absence of tRNA and the Importance of Asymmetry
In HisRS, which does not possess amino acid editing function, amino acid activation in the first site of the dimer occurs at a rate 25-fold faster than that of the second site, and the second mole of adenylate is readily removed by chromatography (32, 33). In the presence of an aminoacylatable cognate tRNA, however, the asymmetry disappears, and both sites catalyze activation at a rate no faster than the overall rate of aminoacylation (33, 46). Here, we found that ThrRS behaves in a qualitatively similar fashion in the tRNA-independent activation reaction with respect to both the cognate threonine and near-cognate serine substrates. For threonine, AMP formation is 8.4-fold faster in one subunit than the other, although for serine, one site is 25-fold faster than the second (Table 1). We take the bi-exponential nature of the AMP product formation curves, and the nearly equivalent amplitudes of the two exponentials in the fitting equation, as presumptive evidence of a functional asymmetry in the ThrRS tRNA-independent dimer. Other possible explanations for the double exponential behavior include the presence of two or more different forms of ThrRS, and/or two different rates of amino acid activation in each active site. Several observations argue against these alternative explanations. Were different chemical forms of ThrRS to be present, we would observe altered and complex steady state kinetics, multiple rates of aminoacyl transfer, and multiple species during purification and/or crystallization. None of these was observed. Moreover, asymmetry in AMP formation by ThrRS can also be unmasked in the presence of tRNA in mutant enzymes (such as H309A ThrRS) where transfer is significantly slowed (34). This is reminiscent of the burst of adenylate formation corresponding to one subunit observed in a version of HisRS where one of the two dimers has been rendered inactive by mutation (32). Hence, the preponderance of highly similar data between the two systems, and the marked absence of significant differences, argues strongly that ThrRS activates adenylate formation asymmetrically in the absence of tRNA, but it converts to symmetrical rates (Fig. 4) when tRNA is present.
One area of difference between the two systems is the apparent accumulation of threonyl- and seryl-adenylate by ThrRS. The accumulation of both adenylate and AMP under conditions where the tRNA is absent strongly suggests that, for the first turnover, a significant fraction of the adenylate formed is rapidly hydrolyzed. Here, the difference between the behavior of threonine and serine is noteworthy. With threonine, there is no pre-transfer editing, so dissociation of products, rebinding of substrates, and new rounds of adenylate synthesis must be suppressed. Based on the observation that AMP was observed as an adventitiously bound ligand in the ThrRS-tRNAThr crystal structure when none was added during purification or crystallization, pre-transfer editing of cognate amino acid appears likely to be blocked at the level of AMP release (26).
With a near-cognate substrate like serine that is subject to editing, two rates (k1 and k2 with k1 > k2) were recorded during the burst phase as well as a slower linear phase (k3) that likely represents the steady rate of seryl-adenylate re-synthesis and destruction. These experiments alone do not uniquely establish the physical process for which k3 is the rate constant; it could represent a limiting rate of substrate re-binding, new adenylate formation, or AMP release. However, it clearly is the one rate that is accelerated for serine relative to threonine, and its magnitude (0.029 s−1) is equal to what was reported as the tRNA-independent rate of pre-transfer editing of alanine by ProRS (25).
To understand how the rate of AMP formation can be altered in the context of overall aminoacylation, it is useful to compare the rates determined in the absence and presence of tRNA. When either threonine or serine is substrate and tRNA is present, there is neither an accumulation of adenylate nor a burst of AMP synthesis, indicating that the rapid hydrolysis of the adenylate in the first turnover seen in the absence of tRNA with both threonine and serine is suppressed by the presence of the tRNA (Fig. 4). As seen with HisRS and ThrRS previously, tRNA therefore acts to coordinate the activities of the two active sites of the dimer so that the rates of adenylate synthesis are normalized (34, 46). Based on experiments following the ratio of ATP consumed relative to aminoacyl-tRNA formed (Fig. 4), only one scenario led to excess ATP consumption (and thus pre-transfer editing). This was the combination of serine as substrate and H309A,H73A ThrRS as enzyme (Fig. 4 and Table 2). As described below, the rate data associated with this particular experimental scenario provide constraints on the likely rate of pre-transfer editing.
With the previous observations as context, the three rates of AMP formation in the presence of serine (k1, k2, and k3) can be compared with the data in Fig. 4 to derive a consistent model for pre-transfer editing. For the fastest of the three rates, k1, the rate of pre-transfer editing would produce a significant consumption of ATP under conditions where aminoacyl transfer is not significantly slowed. This was not observed (Fig. 4B). This fast rate would also predict such a rapid decomposition of the ThrRS-seryl adenylate complex that its isolation by spin chromatography might be precluded, which also did not occur. Accordingly, k1 is unlikely to represent the rate of pre-transfer editing. This leaves a k2 (0.15 s−1) and k3 (0.029 s−1). Relative to the measured rate of aminoacyl transfer of serine, k2 is 52-fold slower, whereas k3 is 268-fold slower. Although there is clearly a numerical difference between k2 and k3, the qualitative conclusions associated with either value with respect to the overall central finding of the study are the same. In both cases, pre-transfer editing would be significantly slower than the rate of aminoacyl transfer, such that pre-transfer editing would only be revealed when aminoacyl transfer is slowed significantly.
As a further point of comparison, the values associated with k2 and k3 can be used to calculate the respective costs of editing under either scenario, and these produce different predictions. Using the approach of Fersht (36) as described under “Experimental Procedures,” one can calculate the efficiencies of aminoacyl-tRNA synthesis using either k2 or k3 as estimates of the rate of pre-transfer editing, and these lead to different ATP consumption values. For the H309A,H73A ThrRS mutant, a value of ktrans (serine) = 0.031 s−1 can be estimated based on the single turnover transfer rate (0.06 s−1) for H309A ThrRS in the presence of threonine (34) corrected by the 50% decrease in rate associated with serine relative to threonine (Fig. 3). Using the values of either k2 or k3, one obtains a value of 0.17 for the Fp (fraction of product formed) for k2 and a value of 0.51 for k3. Converting these values to stoichiometries of ATP consumed, we obtain values of 5.8 for k2 and 1.9 for k3. Experimentally, a value of 2 was obtained (Table 2). Considering that the pre-transfer editing rate is a steady state value and that the stoichiometry of ATP consumption is derived from the steady state slope of product formation, this indirect rate calculation analysis suggests that k3 is likely to be a better estimate of the editing rate than k2.
Role of Aminoacyl Transfer in Editing Pathway Selection by ThrRS
Based on the data shown and the arguments presented above, pre-transfer editing therefore makes a minimal contribution to fidelity in the case of either wild type or H73A ThrRS, but it can become important when transfer is slowed significantly, as in the case of H73A,H309A. Strikingly, recent work comparing the editing properties of class I isoleucyl- and valyl-tRNA synthetases suggests that this conclusion may be general to both classes of ARSs. The rate of aminoacyl transfer catalyzed by the valyl enzyme is at least 150 times faster than the isoleucyl-tRNA synthetase, and this correlates with the observation that valyl-tRNA synthetase exclusively employs post-transfer editing, whereas isoleucyl-tRNA synthetase uses both (47). Considering valyl-tRNA synthetase and ThrRS as representatives of each of the two classes of ARSs, this raises the possibility that the preference for pre- or post-transfer editing mechanism may be driven by similar mechanistic constraints in the two classes.
Conclusions
Here, we have demonstrated that the catalytic domain of ThrRS possesses the inherent ability to reject noncognate serine. In the absence of tRNA, cognate threonine and noncognate serine are activated at asymmetric rates with respect to the dimer. The seryl-adenylate is hydrolyzed at a steady state rate 29-fold higher than threonine. No discrimination against serine is exhibited in either the rate of amino activation per se or aminoacyl transfer. The rate of the transfer step is sufficiently fast that, in comparison with the rate of adenylate turnover, pre-transfer editing is expected to contribute little to overall fidelity, except in the case of H73A,H309A ThrRS. The minimal kinetic scheme for ThrRS aminoacylation presented here underscores the roles that amino acid activation and aminoacyl transfer play in determining amino acid specificity. Accordingly, the application of pre-steady state kinetics to other editing ARSs may similarly help to establish which, of the different possible editing pathways, is the preferred pathway.
Supplementary Material
Acknowledgment
We thank Dr. John Perona, University of California, Santa Barbara, for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM54899 from NIGMS.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- ARS
- aminoacyl-tRNA Synthetase
- ThrRS
- threonyl-tRNA synthetase
- HisRS
- histidyl-tRNA synthetase
- ProRS
- Prolyl-tRNA synthetase.
REFERENCES
- 1.Zaher H. S., Green R. (2009) Nature 457, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling J., Reynolds N., Ibba M. (2009) Annu. Rev. Microbiol. 63, 61–78 [DOI] [PubMed] [Google Scholar]
- 3.Francklyn C. S. (2008) Biochemistry 47, 11695–11703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J. W., Beebe K., Nangle L. A., Jang J., Longo-Guess C. M., Cook S. A., Davisson M. T., Sundberg J. P., Schimmel P., Ackerman S. L. (2006) Nature 443, 50–55 [DOI] [PubMed] [Google Scholar]
- 5.Ibba M., Soll D. (2000) Annu. Rev. Biochem. 69, 617–650 [DOI] [PubMed] [Google Scholar]
- 6.Ling J., Yadavalli S. S., Ibba M. (2007) RNA 13, 1881–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauling L. (1957) in Festchrift for Prof. Dr. Arthur Stoll (Birkhauser A. ed) pp. 597–602, Birkhauser, Basel, Switzerland [Google Scholar]
- 8.Fersht A. R. (1999) Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding, pp. 377–400, W. H. Freeman & Co., New York [Google Scholar]
- 9.Schmidt E., Schimmel P. (1994) Science 264, 265–267 [DOI] [PubMed] [Google Scholar]
- 10.Lin L., Hale S. P., Schimmel P. (1996) Nature 384, 33–34 [DOI] [PubMed] [Google Scholar]
- 11.Mursinna R. S., Lincecum T. L., Jr., Martinis S. A. (2001) Biochemistry 40, 5376–5381 [DOI] [PubMed] [Google Scholar]
- 12.Fersht A. R. (1977) Biochemistry 16, 1025–1030 [DOI] [PubMed] [Google Scholar]
- 13.Boniecki M. T., Vu M. T., Betha A. K., Martinis S. A. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 19223–19228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan M., Zhu B., Zhou X. L., He R., Chen X., Eriani G., Wang E. D. (2010) J. Biol. Chem. 285, 3235–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lincecum T. L., Jr., Tukalo M., Yaremchuk A., Mursinna R. S., Williams A. M., Sproat B. S., Van Den Eynde W., Link A., Van Calenbergh S., Grøtli M., Martinis S. A., Cusack S. (2003) Mol. Cell 11, 951–963 [DOI] [PubMed] [Google Scholar]
- 16.Jakubowski H., Fersht A. R. (1981) Nucleic Acids Res. 9, 3105–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarus M., Mertes M. (1973) J. Biol. Chem. 248, 6744–6749 [PubMed] [Google Scholar]
- 18.Lin S. X., Baltzinger M., Remy P. (1983) Biochemistry 22, 681–689 [DOI] [PubMed] [Google Scholar]
- 19.Roy H., Ling J., Irnov M., Ibba M. (2004) EMBO J. 23, 4639–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beebe K., Ribas De Pouplana L., Schimmel P. (2003) EMBO J. 22, 668–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsui W. C., Fersht A. R. (1981) Nucleic Acids Res. 9, 4627–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dock-Bregeon A., Sankaranarayanan R., Romby P., Caillet J., Springer M., Rees B., Francklyn C. S., Ehresmann C., Moras D. (2000) Cell 103, 877–884 [DOI] [PubMed] [Google Scholar]
- 23.Beuning P. J., Musier-Forsyth K. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8916–8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hati S., Ziervogel B., Sternjohn J., Wong F. C., Nagan M. C., Rosen A. E., Siliciano P. G., Chihade J. W., Musier-Forsyth K. (2006) J. Biol. Chem. 281, 27862–27872 [DOI] [PubMed] [Google Scholar]
- 25.Splan K. E., Ignatov M. E., Musier-Forsyth K. (2008) J. Biol. Chem. 283, 7128–7134 [DOI] [PubMed] [Google Scholar]
- 26.Sankaranarayanan R., Dock-Bregeon A. C., Romby P., Caillet J., Springer M., Rees B., Ehresmann C., Ehresmann B., Moras D. (1999) Cell 97, 371–381 [DOI] [PubMed] [Google Scholar]
- 27.Sankaranarayanan R., Dock-Bregeon A. C., Rees B., Bovee M., Caillet J., Romby P., Francklyn C. S., Moras D. (2000) Nat. Struct. Biol. 7, 461–465 [DOI] [PubMed] [Google Scholar]
- 28.Dock-Bregeon A. C., Rees B., Torres-Larios A., Bey G., Caillet J., Moras D. (2004) Mol. Cell 16, 375–386 [DOI] [PubMed] [Google Scholar]
- 29.Waas W. F., Schimmel P. (2007) Biochemistry 46, 12062–12070 [DOI] [PubMed] [Google Scholar]
- 30.Ambrogelly A., Kamtekar S., Stathopoulos C., Kennedy D., Söll D. (2005) FEBS Lett. 579, 6017–6022 [DOI] [PubMed] [Google Scholar]
- 31.Hauenstein S. I., Hou Y. M., Perona J. J. (2008) J. Biol. Chem. 283, 21997–22006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guth E., Farris M., Bovee M., Francklyn C. S. (2009) J. Biol. Chem. 284, 20753–20762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guth E. C., Francklyn C. S. (2007) Mol. Cell 25, 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minajigi A., Francklyn C. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17748–17753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Splan K. E., Musier-Forsyth K., Boniecki M. T., Martinis S. A. (2008) Methods 44, 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fersht A. R. (1981) Proc. R. Soc. Lond. B Biol. Sci. 212, 351–379 [DOI] [PubMed] [Google Scholar]
- 37.Silvian L. F., Wang J., Steitz T. A. (1999) Science 285, 1074–1077 [PubMed] [Google Scholar]
- 38.Nomanbhoy T. K., Hendrickson T. L., Schimmel P. (1999) Mol. Cell 4, 519–528 [DOI] [PubMed] [Google Scholar]
- 39.Yamane T., Hopfield J. J. (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 2246–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopfield J. J. (1974) Proc. Natl. Acad. Sci. U.S.A. 71, 4135–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomanbhoy T. K., Schimmel P. R. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5119–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gruic-Sovulj I., Rokov-Plavec J., Weygand-Durasevic I. (2007) FEBS Lett. 581, 5110–5114 [DOI] [PubMed] [Google Scholar]
- 43.Bovee M. L., Pierce M. A., Francklyn C. S. (2003) Biochemistry 42, 15102–15113 [DOI] [PubMed] [Google Scholar]
- 44.Uter N. T., Gruic-Sovulj I., Perona J. J. (2005) J. Biol. Chem. 280, 23966–23977 [DOI] [PubMed] [Google Scholar]
- 45.Tsai Y. C., Johnson K. A. (2006) Biochemistry 45, 9675–9687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guth E., Connolly S. H., Bovee M., Francklyn C. S. (2005) Biochemistry 44, 3785–3794 [DOI] [PubMed] [Google Scholar]
- 47.Dulic M., Cvetesic N., Perona J. J., Gruic-Sovulj I. (2010) J. Biol. Chem. 285, 23799–23809 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.