Abstract
Viral infections have been associated with the rejection of transplanted allografts in humans and mice, and the induction of tolerance to allogeneic tissues in mice is abrogated by an ongoing viral infection and inhibited in virus-immune mice. One proposed mechanism for this “heterologous immunity” is the induction of alloreactive T cell responses that cross-react with virus-derived antigens. These cross-reactive CD8 T cells are generated during acute viral infection and survive into memory, but their ability to partake in the immune response to allografts in vivo is not known. We show here that cross-reactive, virus-specific memory CD8 T cells from mice infected with LCMV proliferated in response to allografts. CD8 T cells specific to several LCMV epitopes proliferated in response to alloantigens, with the magnitude and hierarchy of epitope-specific responses varying with the private specificities of the host memory T cell repertoire, as shown by adoptive transfer studies. Lastly, we show that purified LCMV-specific CD8 T cells rejected skin allografts in SCID mice. These findings therefore implicate a potential role for heterologous immunity in virus-induced allograft rejection.
INTRODUCTION
Acute and chronic rejection of transplanted foreign tissues has been associated with viral infections, in particular by members of the herpesvirus group in humans (1–4). Virus-induced rejection of transplanted tissues is also evident in mice, even occurring under conditions of tolerization to alloantigens by a costimulation blockade that substantially reduces the number of alloreactive T cells (4–6). The association of viral infections with allograft rejection might be predicted, due to the fact that viral infections often stimulate the outgrowth of alloreactive T cells that cross-react with virus-derived antigens in the absence of alloantigens (7–12). For example, acute LCMV infection of C57BL/6 (B6) (H2b) mice activates CD8 T cells that cross-react between syngeneic (H2b) cells presenting viral peptides and uninfected allogeneic cells expressing H2d-MHC alloantigens (13–15). Cross-reactivity between virus-specific T cells and alloantigens has also been shown in CD8 T cell lines derived from mice infected with LCMV, influenza virus, Sendai virus, vesicular stomatitis virus (VSV), and herpes simplex virus (HSV) and from humans infected with EBV, CMV, and HSV (16–20). However, it has never been clarified whether virus-specific T cells that cross-react with alloantigens proliferate in response to a transplanted allograft in vivo. In fact, some evidence suggests that virus-specific T cells have low affinity to the cross-reactive alloantigens (6, 14). In order to determine the importance of cross-reactive or “heterologous” immunity in the rejection of foreign tissue grafts it is necessary to evaluate the recruitment of cross-reactive virus-specific CD8 T cells into the immune response to allografts.
The LCMV-induced alloreactive CD8 T cell population enters the memory pool after resolution of infection, indicating that the alloreactive T cell repertoire in virus-immune mice can be comprised of cross-reactive memory as well as naïve T cells (13, 14, 21). Memory-phenotype alloreactive T cells are detectable in humans who have never been exposed to alloantigens, and this may be accounted for by their exposure instead to cross-reactive pathogens (22, 23). Although the presence of memory alloreactive T cells that cross-react with viral antigens is well documented, it again has never been clarified whether they are of sufficient functionality to respond to allografts. The possibility that cross-reactive memory T cells may respond to allografts in vivo is suggested by experiments, which showed that EBV- and CMV-specific memory CD8 T cells from humans proliferate after in vitro culture with allogeneic cells (17, 24).
Here we examined the impact of virus infection on the alloreactive T cell repertoire and the ability of virus-specific memory CD8 T cells to respond against alloantigens in vitro and in vivo. Using our newly developed assay to differentiate naïve from effector/memory alloreactive T cells (25), we show that the alloreactive T cell repertoire in an LCMV-immune mouse is comprised of both memory and naïve phenotype CD8 T cells. Our findings also demonstrate that LCMV-specific CD8 T cells readily respond to in vitro stimulation with allogeneic cell lines and in vivo to skin allografts. The extent of T cell proliferation and the peptide-specific populations that responded to the alloantigens varied between individual mice, and adoptive transfer experiments showed that this diversity reflected the unique private specificities of virus-specific T cell repertoires in individual mice. Finally, our data show that virus-specific CD8 T cells mediate the rejection of skin allografts. These results indicate that virus-specific memory T cells will respond to alloantigens and that their presence may influence the host response against transplanted foreign tissues.
MATERIALS AND METHODS
Mice
Male C57BL/6J (B6, H2b), BALB/cJ (H2d), CBA/J (H2k) and B6.CB17-Prkdcscid/SzJ (B6/SCID) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 4 to 5 weeks of age. B6/SJL mice (B6.SJL-Ptprca/BoyAiTac, CD45.1, stock No: 004007) were purchased from Taconic Farms, Inc. (Hudson, NY). All experiments were done in compliance with institutional guidelines as approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Viruses and Cell Lines
Stocks of LCMV, strain Armstrong, were prepared in baby hamster kidney cells (BHK21) as previously described (26). For the generation of virus-specific T cell responses, mice were inoculated ip with 5 × 104 PFU of LCMV and were considered immune 6 weeks or longer after infection. The P815 cell line (H2d), a DBA/2 mouse-derived, methylcholanthrene-induced mastocytoma, was maintained in MEM (Gibco) previously described (13).
Preparation of LPS-treated splenocytes
LPS-treated splenocytes were prepared as described previously (25). Briefly, splenocytes from the indicated mouse strains (2 × 106 cells/ml in supplemented RPMI) were treated with LPS (15 μg/ml) for 3 days in vitro. Following incubation, the cultures were washed 3 times with supplemented RPMI, g-irradiated (20 Gy), and frozen at −70°C until used.
Intracellular Cytokine Staining
Cytokine-producing CD8 T cells were detected using the Cytofix/Cytoperm Kit Plus™ (with GolgiPlug™, BD Biosciences, San Diego, CA), as described previously (27). Splenocytes (2 × 106 cells) were incubated with 1 μM synthetic peptide, with the indicated LPS-treated splenocyte populations (1 × 106 stimulator cells per sample) or with 250 ng/ml of anti-mouse CD3e mAb (145-2C11, BD Biosciences) in the presence of 10 U/ml of human recombinant IL-2 (BD Biosciences) and 1 ml/ml GolgiPlug™ for 5 h at 37°C. Following the incubation, splenocytes were stained with mAb specific for CD8 (53-6.7), CD11a (M17/4) and CD44 (IM7), fixed and permeabilized and stained with mAb specific for IFN-γ or TNF (XMG1.2 and MP6-XT22, respectively, BD Biosciences).
In vitro splenocyte cultures
Splenocytes from LCMV-immune or naïve B6 mice were labeled with 2 μM carboxyfluorescein diacetate succinimidyl ester (CFSE, Sigma, St. Louis, MO), washed, and cultured with mitomycin-C treated P815 (H2d) in 12 well tissue culture plates (1 × 107 splenocytes and 1 × 106 allogeneic cells), as previously described (28). After 6 days the cells were recovered and evaluated for proliferation by dilution of CFSE and antigen specificity by intracellular cytokine assay. In some instances splenocytes from individual mice were used to set up duplicate cultures, which were kept separate during in vitro culture and analysis
Skin Transplantation
BALB/c or B6 skin (1 to 2 cm in diameter) was transplanted onto the dorsal flanks of B6 mice or B6/SCID mice (29). Graft rejection was defined as the first day that the entire graft was rejected. The generation of alloreactive T cell responses was examined by intracellular cytokine assay (25).
Adoptive transfer of LCMV-immune splenocytes
Splenocytes from LCMV-immune B6 mice (CD45.2) were labeled with CFSE (2 μM), washed and adoptively transferred (3 × 107 cells) intravenously into naïve B6/SJL mice (CD45.1) mice. One day after transfer, recipient mice received either syngeneic or allogeneic skin grafts. T cell responses were examined at the indicated time points, and donor CD8 T cells were identified by staining with a mAb specific for the congenic marker CD45.2 (104, BD Biosciences). The donor cells were evaluated for proliferation by dilution of CFSE and for antigen specificity by intracellular cytokine assay.
Purification and adoptive transfer of virus-specific CD8 T cells
Splenocytes from B6.SJL (CD45.1) mice infected 8 days previously with LCMV were stained with NP396-MHC tetramers and with mAb specific for CD8 as described previously (13). Purified populations of double positive (tetramer and CD8) cells were then sorted using a MoFlo XDP Digital Cell sorter (Beckman Coulter). The sorted populations were then incubated for 1 hr at 37°C and then 1–2 × 106 cells were transferred into the indicated recipient mice. Transferred LCMV-specific CD8 T cells were monitored by staining with NP396-tetramer, and mAb against CD8 and CD45.1 (A20, BD Biosciences).
RESULTS
The alloreactive T cell repertoire in LCMV-immune mice contains both naïve and memory cells
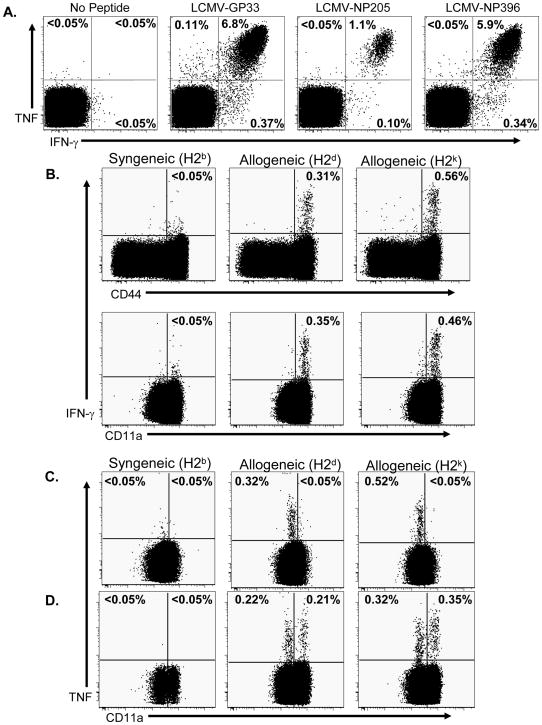
We compared the alloreactive T cell repertoire between naïve and LCMV-immune B6 mice, we used TNF-production to distinguish naïve alloreactive T cells (TNF+/IFN–γ−/CD11alo) from memory/effector cells (TNF+/IFN–γ+/CD11ahi), as we have previously described (30). Splenocytes from LCMV-immune and naïve B6 mice (H2b) were stimulated in vitro with either LCMV-derived peptides or with allogeneic splenocytes (BALB/c, H2d or CBA/J, H2k), and 5 hours later cytokine production was evaluated by intracellular cytokine assay. Following stimulation with LCMV-derived peptides, CD8 T cells from LCMV-immune mice produced both IFN–γ and TNF (Figure 1A). In contrast CD8 T cells from naïve mice did not (Supplemental Figure 1A), as has been previously described (31). Following stimulation with alloantigens, a detectable population of memory phenotype (CD44hi and CD11ahi) CD8 T cells produced IFN–γ (Figure 1B) over background levels stimulated by syngeneic cells, confirming that memory alloreactive CD8 T cells are generated by infection with LCMV (13). IFN-γ producing, alloreactive CD8 T cells were not detected in splenocytes from naïve B6 mice (Supplemental Figure 1B) (25). We next examined the production of TNF by the CD8 T cells from naïve B6 mice following stimulation with either syngeneic or allogeneic splenocytes. Figure 1C shows that TNF was produced exclusively within the CD11alo population of these naïve mice. In contrast, stimulation of LCMV-immune splenocytes with alloantigens resulted in TNF production by both naïve (CD11alo) and memory (CD11ahi) CD8 T cells (Figure 1D). Of note is that the frequencies of alloreactive T cells detected by TNF production were only slightly elevated after virus infection, though approximately 50% were now of a memory phenotype. In addition the memory phenotype alloreactive CD8 T cells from LCMV-immune mice were poly-functional, with approximately 50% of the IFN-γ producing cells, also secreting TNF (Supplemental Figure 1C). Together these results confirm that following a viral infection the alloreactive T cell population is altered and contains both memory and naïve CD8 T cells.
Figure 1. Naive and memory phenotype alloreactive CD8 T cells are detectable in LCMV-immune mice.
Splenocytes from LCMV-immune B6 mice (A, B and D) or naïve B6 mice (C) were stimulated with peptides or with splenocytes (allogeneic, irradiated LPS-treated cells) that were either syngeneic (H2b) or allogeneic (BALB/c, H2d or CBA, H2k), as described in Materials and Methods. After culture, splenocytes were stained for cell surface markers and for intracellular IFN–γ and TNF. For analysis, samples were gated on CD8+ cells, and the values shown represent the percentage of CD8 T cells staining positive for either cytokine. The data are representative of 4 experiments.
Alloantigens stimulate the in vitro proliferation of LCMV-specific memory CD8 T cells
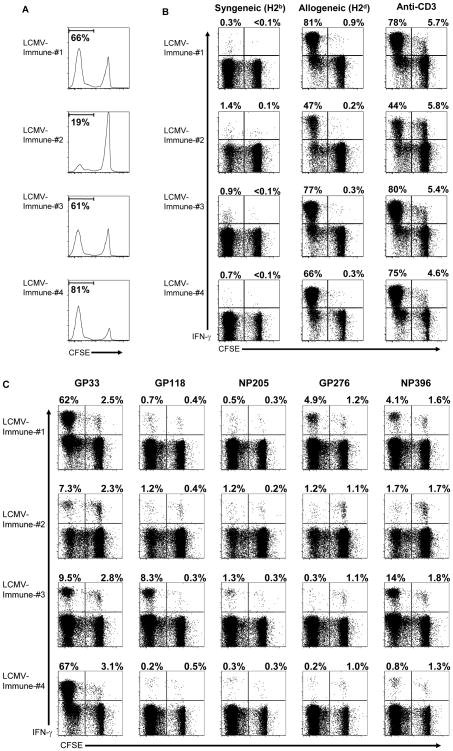
To address whether memory alloreactive T cells that are cross-reactive with viruses participate in the immune response to alloantigens, CFSE-labeled splenocytes from LCMV-immune mice were cultured in vitro with an allogeneic (H2d) cell line (P815) for 6 days. Following the culture period, CD8 T cells were examined for division by CFSE dilution (Figure 2A). In vitro culture with P815 cells stimulated CD8 T cells from LCMV-immune mice to undergo multiple rounds of division, with 19 to 81% of the T cells being CFSElo. To determine the antigen-specificity of the CFSElo populations, cells recovered from the cultures were re-stimulated in vitro with either the indicated cell lines (Figure 2B), anti-CD3 (Figure 2B) or with LCMV-specific peptides (Figure 2C) and then evaluated for IFN-γ production. A high proportion of the CFSElo CD8 T cells produced IFN–γ following re-stimulation with P815 cells, indicating that many of the divided T cells were specific for H2d-antigens (Figure 2B).
Figure 2. H2d-antigens stimulate LCMV-specific memory CD8 T cells to proliferate in vitro.
CFSE-labeled splenocytes from LCMV-immune B6 mice were cultured in vitro for 6 days with P815 cells (H2d) as described in Materials and Methods. (A) After the in vitro culture, CD8 T cells were evaluated for division by dilution of CFSE, and the values shown represent the percentage of CD8 T cells that are CFSElo. (B) Cultured cells were incubated with either syngeneic (H2b) or allogeneic (H2d) splenocytes or with a mAb specific for CD3 for 5 hr and then evaluated for the production of IFN–γ̃ (C) Alternatively, cultured cells were incubated with the indicated peptides for 5 hr and then evaluated for the production of IFN–γ. For the intracellular cytokine assays, samples were gated on CD8+ cells, and the values shown represent the percentage of either CFSElo or CFSEhi CD8 T cells staining positive for IFN–γ. The data are representative of 5 experiments.
As shown in Figure 2C, LCMV-specific CD8 T cells (identified by the production of IFN-γ after stimulation with LCMV-derived peptides) underwent multiple rounds of division following exposure to alloantigens, but distinct epitope-specific populations were observed among individual mice. GP33-specific CD8 T cells proliferated in all mice examined, representing a substantial proportion (62% and 67%) of the divided CD8 T cells in 2 of the mice (1 and 4, Figure 2C). The high proportion of the GP33-specific CD8 T cells in the CFSElo population indicates that many of the virus-specific cells must have specificity for the alloantigen. For other LCMV-derived peptides distinct patterns were observed, with strong proliferation of NP396-specific CD8 T cells in mice 1 and 3, of GP276-specific CD8 T cells in mouse 1, and of GP118-specific CD8 T cells in mouse 3. GP33-specific CD8 T cells comprised the dominant response in 3 out of the 4 mice, with the NP396-specific response dominating in one mouse. It is also important to note that in each case where epitope-specific T cells were stimulated to divide, only a proportion of the total population responded, as there were some remaining CFSEhi LCMV-specific CD8 T cells not responding to the alloantigens. This would be expected for an antigen-specific response as opposed to a non-specific stimulation. Moreover, while CD8 T cells from naïve mice were stimulated to divided in response to stimulation with allogeneic cells, none of the CFSElo cells were LCMV-specific (Supplemental Figure 2).
Skin allografts stimulate the in vivo proliferation of LCMV-specific memory CD8 T cells
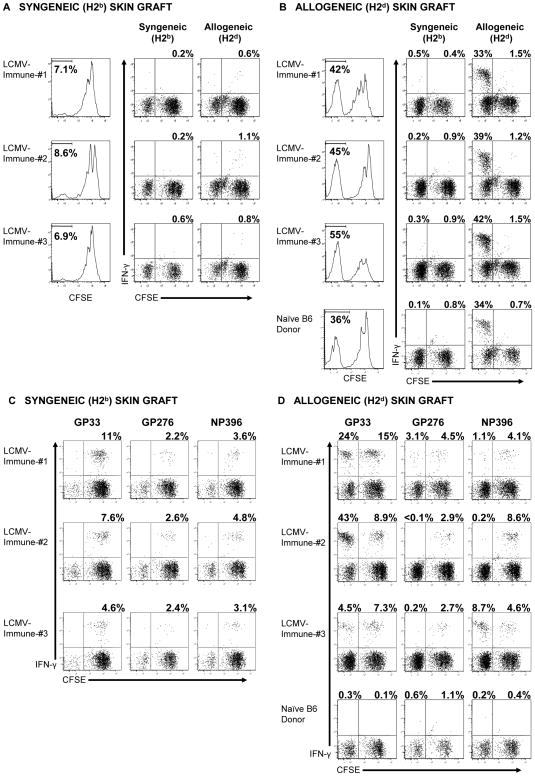
To test whether skin allografts would stimulate LCMV-specific memory CD8 T cells to proliferate in vivo, splenocytes from individual LCMV-immune mice (CD45.2) were labeled with CFSE and transferred into separate congenic mice (CD45.1). One day after transfer recipient mice were engrafted with either syngeneic (H2b) or allogeneic (H2d) skin. Allogeneic skin grafts were rejected by all recipients within 12 days, while syngeneic grafts remained intact. Thirteen days after engraftment donor CD8 T cells from the spleen were examined for proliferation by CFSE dilution and for antigen specificity by intracellular cytokine assay (Figure 3). Only a small percentage of donor CD8 T cells were stimulated to proliferate in mice engrafted with syngeneic skin (Figure 3A). In contrast, a large proportion of donor CD8 T cells (42 to 55% being CFSElo) were stimulated to divide in mice receiving skin allografts (H2d) (Figure 3B). CFSElo donor CD8 T cells produced IFN–γ following re-stimulation in vitro with H2d-splenocytes (Figure 3B), indicating that many of the divided T cells were specific for H2d-antigens. Donor CD8 T cells derived from naïve B6 mice also proliferated in response to an H2d-skin allograft, and these CFSElo cells also produced IFN–γ following re-stimulation in vitro with H2d-splenocytes (Figure 3B).
Figure 3. H2d-skin allografts stimulate LCMV-specific memory CD8 T cells to proliferate in vivo.
CFSE-labeled splenocytes (3 × 107) from LCMV-immune B6 mice were adoptively transferred into naïve congenic recipient mice, which were engrafted with either syngeneic (B6, H2b) skin (A and C) or BALB/c (H2d) skin (B and D) 1 day later, as described in the Materials and Methods. (A and B) Thirteen days after engraftment, donor CD8 T cells (CD45.2) from the spleens of recipient mice were evaluated for division by dilution of CFSE, and the values represent the percentage of CD8 T cells that are CFSElo. Recovered splenocytes were incubated with either syngeneic (H2b) or allogeneic (H2d) splenocytes for 5 hr and then evaluated for the production of IFN–γ. (C and D) Alternatively, recovered splenocytes were incubated with the indicated peptides for 5 hr and then evaluated for the production of IFN–γ. For analysis, samples were gated on CD8+ cells, and the values shown represent the percentage of either CFSElo or CFSEhi CD8 T cells staining positive for IFN–γ. The data are representative of 4 experiments.
To determine if the immune donor cells proliferating in the presence of skin allografts were specific to LCMV, donor CD8 T cells were examined for their ability to recognize LCMV-derived peptides by intracellular cytokine assay. As shown in Figure 3C, LCMV-specific CD8 T cells were detectable after transfer into mice that received syngeneic skin grafts, but these donor CD8 T cells were still CFSEhi, indicating that only a low level, if any, of homeostatic division had occurred. In contrast, some LCMV-specific CD8 T cells divided multiple times in mice receiving skin allografts, with distinct epitope-specific populations responding in individual mice (Figure 3D). As observed with the in vitro culture experiments (Figure 2), GP33-specific CD8 T cells underwent division in a majority of mice examined (14 out of 18 mice in 4 experiments) and represented a large proportion of the divided cells in two mice shown in Figure 3D (24 and 43% of the CFSElo cells). For other LCMV-derived peptides distinct patterns were observed, with strong proliferation of NP396-specific CD8 T cells in mouse 3. Although some donor CD8 T cells from naïve B6 mice divided in response to skin allografts, these cells did not recognize LCMV-specific peptides and were probably part of a normal alloreactive T cell response from naïve cells (Figure 3D). Together these results demonstrated that skin allografts will stimulate LCMV-specific memory CD8 T cells to proliferate in vivo.
Alloantigen-stimulated proliferation of cross-reactive LCMV-specific memory CD8 T cells is determined by private specificity
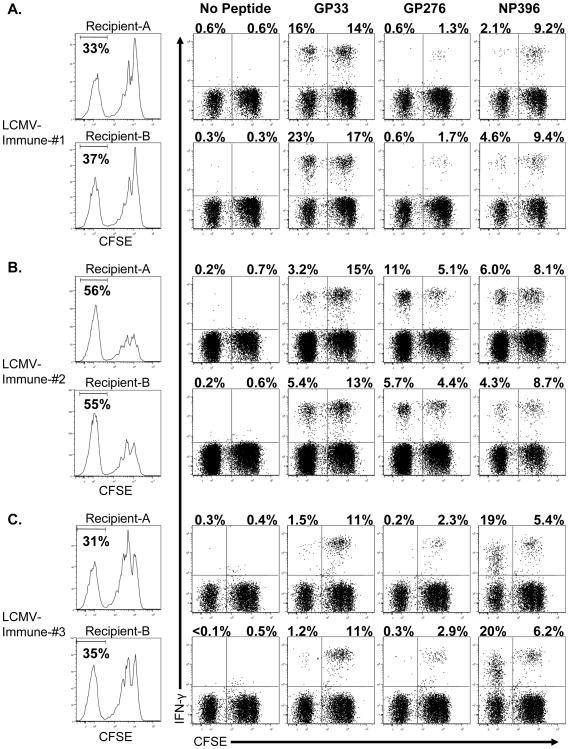
The different patterns of epitope-specific proliferation shown in Figures 2 and 3 may indicate either a stochastic process or a reflection of the private specificity of the TCR-repertoire for epitope-specific T cells within individual mice. Previous work has shown that virus-specific CD8 T cells from individual mice use different TCR repertoires (32), and that this private specificity of virus-specific T cells in individuals dictated the patterns of cross-reactivity (33, 34). To determine if private specificity accounts for the diversity in the proliferation of virus-specific CD8 T cells to skin allografts in vivo, CFSE-labeled splenocytes from individual mice were divided and adoptively transferred into separate congenic mice, which received allografts 1 day later. Thirteen days after engraftment, the donor CD8 T cells were evaluated for proliferation and for antigen-specificity (Figure 4). The donor CD8 T cells derived from individual mice displayed similar levels of division by the CD8 T cells and similar patterns of epitope-specific cross-reactivity. In both recipient mice that received splenocytes from mouse 1 (Figure 4A), GP33-specific CD8 T cells comprised the dominant proportion of the divided cells, with a small proportion of NP396-specific CD8 T cells and no detectable GP276-specific CD8 T cells in the divided population. In both recipient mice that received splenocytes from mouse 2 (Figure 4B), GP33-, GP276- and NP396-specific CD8 T cells were all detectable in the divided population. In both recipient mice that received splenocytes from mouse 3 (Figure 4C), NP396-specific CD8 T cells comprised the dominant proportion of the divided cells, with a small proportion of GP33-specific CD8 T cells and no detectable GP276-specific CD8 T cells in the divided population. We observed similar patterns of private specificty with replicate cultures from individual LCMV-immune mice (Supplemental Figure 3). These results indicate that the diversity of epitope-specific CD8 T cells stimulated to divide in vivo is a reflection of the private specificity of an individual.
Figure 4. LCMV-immune CD8 T cells from a single immune mouse exhibit similar responses to H2d-skin allografts in separate recipients.
CFSE-labeled splenocytes (3 × 107) from individual LCMV-immune B6 mice (A, B and C) were adoptively transferred into separate congenic recipient mice, which were engrafted with BALB/c (H2d) skin 1 day later, as described in the Materials and Methods. Thirteen days after engraftment, donor CD8 T cells (CD45.2) from the spleens of recipient mice were incubated with the indicated peptides for 5 hr and then evaluated for the production of IFN–γ. For analysis, samples were gated on CD8+ cells, and the values shown represent the percentage of either CFSElo or CFSEhi CD8 T cells staining positive for IFN–γ. The data are representative of 3 experiments.
Virus-specific CD8 T cells mediate rejection of skin allografts
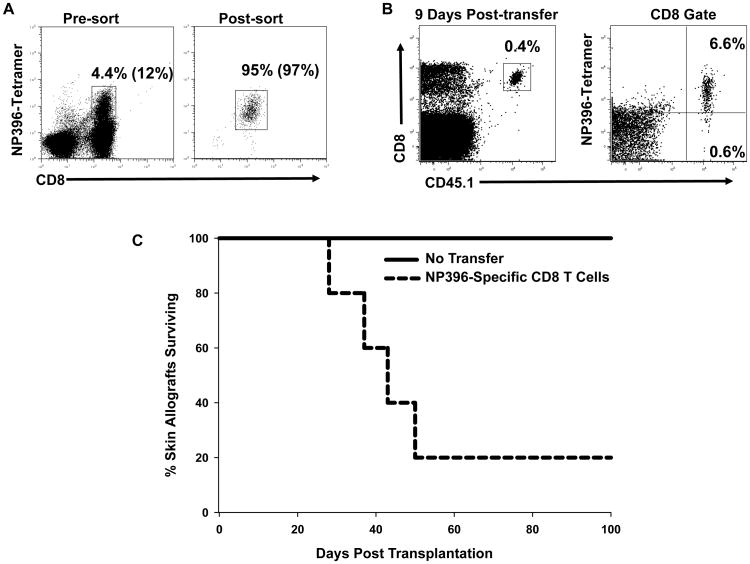
Even though virus-specific CD8 T cells cross-react with alloantigens (8, 10, 13–15, 24), the ability of virus-specific CD8 T cells to directly mediate rejection of allogeneic tissues in vivo has not been shown. To formally demonstrate this, CD8 T cells specific for the LCMV-epitope NP396 were purified from mice acutely infected with LCMV and adoptively transferred into B6/SCID mice engrafted with BALB/c skin (H2d). This epitope was chosen because we had previously demonstrated that approximately 12% of NP396-specific CD8 T cells from B6 mice acutely infected with LCMV are cross-reactive with H2d-antigens (13) and because this is the largest epitope-specific response in B6 mice (35). NP396-specific CD8 T cells were purified by sorting NP396-tetramer positive CD8 T cells from acutely infected B6/SJL (CD45.1) mice (8 day pi). We used mice that were acutely infected with LCMV because this allowed us to recover the necessary number of NP396-specific CD8 T cells for the adoptive transfer experiment. The frequency of NP396-tetramer positive cells both pre- and post-sort is shown in Figure 5A, with the sorted population being 95% tetramer positive. One or two million CD8 T cells were transferred into 4 recipient B6/SCID mice in 2 separate experiments. NP396-specific cells were detected in the peripheral blood of 5 out of 8 recipient mice 9 days after transfer (Figure 5B). Of these 5 recipients that still retained NP396-specific CD8 T cells, 4 rejected the skin allografts over a 50 day period, while B6/SCID mice receiving no cells (N = 6) or mice in which the transferred cells were not detectable (N = 3) maintained grafts until the end of the experiment at day 100 (Figure 5C, p = 0.013). These data indicate that effector virus-specific CD8 T cells are able to reject skin allografts.
Figure 5. LCMV-specific CD8 T cells mediate rejection of skin allografts.
B6/SJL mice (CD45.1) were infected with LCMV and 8 days later spleens were harvested and pooled. (A) Splenocytes were stained with NP396-MHC tetramers and with mAb to CD8, and double positive cells were purified by sorting. The purity of NP396-tetramer positive cells is shown as the percentage of total cells and as the percentage of CD8 T cells (values in parentheses). (B) Purified NP396-specific CD8 T cells (1–2×106 cells) were then adoptively transferred into B6/SCID mice bearing BALB/c skin grafts. (C) Skin graft survival was monitored over the next 100 days. The data are representative of 2 experiments.
DISCUSSION
Viral infections are a serious complication for transplant recipients and are often associated with rejection of engrafted tissues. Historically, herpes viruses such as CMV, EBV and HSV, were the primary concern for transplant recipients, but other viruses, including influenza virus and polyoma viruses (BK and JC virus) are also problematic (2, 36–39). In this report, we show that LCMV-specific memory CD8 T cells proliferate in response to alloantigens both in vitro and in vivo. This represents, to our knowledge, the first demonstration that virus-specific memory CD8 T cells are recruited into the immune response against tissue allografts in vivo. The alloantigen-induced division of LCMV-specific memory CD8 T cells involved multiple epitope-specific populations, which varied between individual mice. The variation between individuals was attributed to the unique private specificity of the memory T cell pool for each mouse. These results demonstrate that virus-specific CD8 T cells generated by past viral infections will respond against foreign tissue grafts and that the epitope-specificity of this cross-reactive response will be dictated by an individual’s unique TCR repertoire.
Our study makes the observation that virus-reactive CD8 T cells proliferate in response to alloantigens and that the epitope-specificity of the proliferating cells is variable between individual mice, with distinct patterns of epitope-specific populations responding. This diversity in the activation of cross-reactive memory CD8 T cells was also observed in models of sequential viral infections with LCMV and VV and with LCMV and PV (33, 34). Our results reinforce the concept that the virus-specific CD8 T cells from individual mice have a unique epitope-specific TCR-repertoire and that this private specificity will impact directly on the induction of an allo-cross-reactive T cell response (33, 34).
Memory alloreactive T cells are a serious concern for transplant patients, as the pre-transplant frequency of donor-reactive memory T cells correlates with the likelihood of an acute rejection episode (22). Memory alloreactive CD8 T cells respond rapidly to transplanted tissues in mice and produce inflammatory cytokines, which stimulate the allograft to secrete chemokines that enhance the recruitment of innate immune cells, such as polymorphonuclear leukocytes, to the engraftment site (40). The survival of virus-induced cross-reactive T cells into memory may account for the detection of alloreactive memory CD8 T cells in humans never been exposed to alloantigens (13, 22). Previous studies have suggested that cross-reactive virus-specific CD8 T cells are an important consideration for transplantation patients. For example, human CD8 T cells specific for an EBV-epitope (FLRGRAYGL) presented by HLA-B8 recognize HLA molecules B14, B44 and B35 as alloantigens (12, 17), and HLA-B44-positive renal transplants show decreased survival in HLA-B8 positive recipients (7).
Our findings presented in Figure 5 indicate that a population of effector CD8 T cells specific for an individual virus-derived epitope can mediate the rejection of a skin allograft. Although we used mice acutely infected with LCMV for this experiment in order to obtain sufficient numbers of T cells, we predict that virus-specific memory CD8 T cells will also mediate rejection of transplanted tissues and that this population will differ between LCMV-immune hosts due to the variability in the allo-cross-reactive repertoire. In these experiments it was not meaningful to use a control non-tetramer-specific population, because cross-reactive alloreactive T cells are found among T cells with other viral epitope specificities (Figures 2, 3 and 4) and there are more than 20 LCMV epitopes. Further, in these experiments some alloreactive T cells would not be cross-reactive with the virus. What we find convincing though, is that graft rejection only occurred in hosts that retained and had apparently stimulated the proliferation of the NP396-specific T cells. When those T cells were not recovered, the allografts remained intact. While we anticipate that the donor NP396-specific CD8 T cells infiltrate the skin allografts and mediate rejection, it is also possible that the small population of donor CD8 T cells that did not bind NP396 tetramer could be undergoing homeostatic expansion in the B6/SCID host and then contributing to the rejection process. We are currently evaluating this possibility.
The generation of alloreactive memory T cells by infection may represent a long-term barrier for the use of costimulation blockade to induce allograft tolerance, as memory T cells are less reliant on costimulation for activation as compared to naïve cells (13, 21, 41–45). LCMV-specific CD8 T cells were still capable of responding to stimulation with alloantigen after costimulation blockade. Even if cross-reactive, alloreactive memory cells can be identified prior to transplantation, it may be difficult to predict whether they would proliferate in response to the allograft, as division of cross-reactive cells can be quite stringent and is variable between individuals. Virus-specific human CD4 T cells also cross-react with alloantigens, suggesting that both memory CD8 and CD4 T cells generated by past infection may respond to allografts in vivo (46, 47).
Supplementary Material
Acknowledgments
We thank Dr. Liisa K. Selin for helpful discussions.
This work was supported by National Institutes of Health research grants AR-35506, AI46629, AI083911, an institutional Diabetes Endocrinology Research Center (DERC) grant DK32520, an institutional Center for AIDS Research (CFAR) grant AI042845 and a fellowship from the Charles A. King Trust, Bank of America, Co-Trustee (Boston, MA) to M.A.B.
References
- 1.Chen JH, Mao YY, He Q, Wu JY, Lv R. The impact of pretransplant cytomegalovirus infection on acute renal allograft rejection. Transplant Proc. 2005;37(10):4203–4207. doi: 10.1016/j.transproceed.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Gaston JSH, Waer M. Virus-specific MHC-restricted T lymphocytes may initiate allograft rejection. Immunol Today. 1985;6(8):237–239. doi: 10.1016/0167-5699(85)90010-6. [DOI] [PubMed] [Google Scholar]
- 3.Jakel KT, Loning T. Herpes virus infections, acute rejection, and transplant arteriosclerosis in human cardiac allografts. Transplant Proc. 1993;25(2):2029–2030. [PubMed] [Google Scholar]
- 4.Brehm MA, Daniels KA, Ortaldo JR, Welsh RM. Rapid conversion of effector mechanisms from NK to T cells during virus-induced lysis of allogeneic implants in vivo. J Immunol. 2005;174(11):6663–6671. doi: 10.4049/jimmunol.174.11.6663. [DOI] [PubMed] [Google Scholar]
- 5.Welsh RM, Markees TG, Woda BA, Daniels KA, Brehm MA, Mordes JP, et al. Virus-induced abrogation of transplantation tolerance induced by donor- specific transfusion and anti-CD154 antibody. J Virol. 2000;74(5):2210–2218. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams MA, Tan JT, Adams AB, Durham MM, Shirasugi N, Whitmire JK, et al. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J Immunol. 2001;167(9):4987–4995. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 7.Burrows SR, Khanna R, Silins SL, Moss DJ. The influence of antiviral T-cell responses on the alloreactive repertoire. Immunol Today. 1999;20(5):203–207. doi: 10.1016/s0167-5699(98)01429-7. [DOI] [PubMed] [Google Scholar]
- 8.Yang HY, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142(5):1710–1718. [PubMed] [Google Scholar]
- 9.Strang G, Rickinson AB. Multiple HLA class I-dependent cytotoxicities constitute the “non-HLA-restricted” response in infectious mononucleosis. Eur J Immunol. 1987;17(7):1007–1013. doi: 10.1002/eji.1830170717. [DOI] [PubMed] [Google Scholar]
- 10.Tomkinson BE, Maziarz R, Sullivan JL. Characterization of the T cell-mediated cellular cytotoxicity during acute infectious mononucleosis. J Immunol. 1989;143(2):660–670. [PubMed] [Google Scholar]
- 11.Brehm MA, Selin LK, Welsh RM. CD8 T cell responses to viral infections in sequence. Cell Microbiol. 2004;6(5):411–421. doi: 10.1111/j.1462-5822.2004.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows SR, Silins SL, Khanna R, Burrows JM, Rischmueller M, McCluskey J, et al. Cross-reactive memory T cells for Epstein-Barr virus augment the alloresponse to common human leukocyte antigens: degenerate recognition of major histocompatibility complex-bound peptide by T cells and its role in alloreactivity. Eur J Immunol. 1997;27(7):1726–1736. doi: 10.1002/eji.1830270720. [DOI] [PubMed] [Google Scholar]
- 13.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170(8):4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 14.Nahill SR, Welsh RM. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J Exp Med. 1993;177(2):317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Welsh RM. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J Immunol. 1986;136(4):1186–1193. [PubMed] [Google Scholar]
- 16.Braciale TJ, Andrew ME, Braciale VL. Simultaneous expression of H-2-restricted and alloreactive recognition by a cloned line of influenza virus-specific cytotoxic T lymphocytes. J Exp Med. 1981;153(5):1371–1376. doi: 10.1084/jem.153.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179(4):1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finberg R, Burakoff SJ, Cantor H, Benacerraf B. Biological significance of alloreactivity: T cells stimulated by Sendai virus-coated syngeneic cells specifically lyse allogeneic target cells. Proc Natl Acad Sci U S A. 1978;75(10):5145–5149. doi: 10.1073/pnas.75.10.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koelle DM, Chen HB, McClurkan CM, Petersdorf EW. Herpes simplex virus type 2-specific CD8 cytotoxic T lymphocyte cross-reactivity against prevalent HLA class I alleles. Blood. 2002;99(10):3844–3847. doi: 10.1182/blood.v99.10.3844. [DOI] [PubMed] [Google Scholar]
- 20.Sheil JM, Bevan MJ, Lefrancois L. Characterization of dual-reactive H-2Kb-restricted anti-vesicular stomatitus virus and alloreactive cytotoxic T cells. J Immunol. 1987;138(11):3654–3660. [PubMed] [Google Scholar]
- 21.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163(4):2267–2275. [PubMed] [Google Scholar]
- 23.Lombardi G, Sidhu S, Daly M, Batchelor JR, Makgoba W, Lechler RI. Are primary alloresponses truly primary? Int Immunol. 1990;2(1):9–13. doi: 10.1093/intimm/2.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Gamadia LE, Remmerswaal EB, Surachno S, Lardy NM, Wertheim-van Dillen PM, van Lier RA, et al. Cross-reactivity of cytomegalovirus-specific CD8+ T cells to allo-major histocompatibility complex class I molecules. Transplantation. 2004;77(12):1879–1885. doi: 10.1097/01.tp.0000131158.81346.64. [DOI] [PubMed] [Google Scholar]
- 25.Brehm MA, Mangada J, Markees TG, Pearson T, Daniels KA, Thornley TB, et al. Rapid quantification of naive alloreactive T cells by TNF-alpha production and correlation with allograft rejection in mice. Blood. 2007;109(2):819–826. doi: 10.1182/blood-2006-03-008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selin LK, Nahill SR, Welsh RM. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179(6):1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3(7):627–634. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 28.Bonneau RH, Jennings SR. Herpes simplex virus-specific cytolytic T lymphocytes restricted to a normally low responder H-2 allele are protective in vivo. Virology. 1990;174(2):599–604. doi: 10.1016/0042-6822(90)90113-6. [DOI] [PubMed] [Google Scholar]
- 29.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, et al. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64(2):329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 30.Brehm MA, Daniels KA, Welsh RM. Rapid production of TNF-alpha following TCR-engagement of naïve CD8 T cells. J Immunol. 2005;175(8):5043–5049. doi: 10.4049/jimmunol.175.8.5043. [DOI] [PubMed] [Google Scholar]
- 31.Slifka MK, Whitton JL. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J Immunol. 2000;164(1):208–216. doi: 10.4049/jimmunol.164.1.208. [DOI] [PubMed] [Google Scholar]
- 32.Lin MY, Welsh RM. Stability and diversity of T cell receptor repertoire usage during lymphocytic choriomeningitis virus infection of mice. J Exp Med. 1998;188(11):1993–2005. doi: 10.1084/jem.188.11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornberg M, Chen AT, Wilkinson LA, Brehm MA, Kim SK, Calcagno C, et al. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest. 2006;116(5):1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SK, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J Exp Med. 2005;201(4):523–533. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 36.Binggeli S, Egli A, Schaub S, Binet I, Mayr M, Steiger J, et al. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7(5):1131–1139. doi: 10.1111/j.1600-6143.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 37.Briggs JD, Timbury MC, Paton AM, Bell PR. Viral infection and renal transplant rejection. Br Med J. 1972;4(5839):520–522. doi: 10.1136/bmj.4.5839.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duncan MD, Wilkes DS. Transplant-related immunosuppression: a review of immunosuppression and pulmonary infections. Proc Am Thorac Soc. 2005;2(5):449–455. doi: 10.1513/pats.200507-073JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss PA, Cobbold M, Craddock C. The cellular immunotherapy of viral infection. Transfus Med. 2003;13(6):405–415. doi: 10.1111/j.1365-3148.2003.00468.x. [DOI] [PubMed] [Google Scholar]
- 40.El-Sawy T, Miura M, Fairchild R. Early T cell response to allografts occurring prior to alloantigen priming up-regulates innate-mediated inflammation and graft necrosis. Am J Pathol. 2004;165(1):147–157. doi: 10.1016/s0002-9440(10)63283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bingaman AW, Farber DL. Memory T cells in transplantation: generation, function, and potential role in rejection. Am J Transplant. 2004;4(6):846–852. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 42.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164(1):265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 43.Valujskikh A, Lakkis FG. In remembrance of things past: memory T cells and transplant rejection. Immunol Rev. 2003;196:65–74. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8(+) T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169(8):4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 45.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169(7):3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 46.Elkington R, Shoukry NH, Walker S, Crough T, Fazou C, Kaur A, et al. Cross-reactive recognition of human and primate cytomegalovirus sequences by human CD4 cytotoxic T lymphocytes specific for glycoprotein B and H. Eur J Immunol. 2004;34(11):3216–3226. doi: 10.1002/eji.200425203. [DOI] [PubMed] [Google Scholar]
- 47.Landais E, Morice A, Long HM, Haigh TA, Charreau B, Bonneville M, et al. EBV-specific CD4+ T cell clones exhibit vigorous allogeneic responses. J Immunol. 2006;177(3):1427–1433. doi: 10.4049/jimmunol.177.3.1427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.