1. Introduction
In a 1876 report Eugen Baumann described the isolation of a crystalline ‘phenol-forming’ substance from horse urine and showed that the substance was related to m- and p-phenol sulfonic acids. Later the substance was identified as potassium phenyl sulfate and found to be essentially nontoxic because 2.6 g could be administered to rabbits without any adverse consequences. Baumann also noticed that phenol, catechol and indole were excreted extensively as covalently bound sulfuric acid esters when administered to dogs and/or patients. This appears to be the first report on detoxication of phenol and related aromatic molecules and highlights the pioneering work of Eugen Baumann in the chemistry and biochemistry of sulfate esters.1 Sulfate esters are now recognized as modulators of a number of important physiological and pathological processes.
Nature appears to use sulfation of endogenous and exogenous molecules for primarily two purposes including enhanced elimination to avoid potential toxicity and induction of specific cellular or acellular responses. Sulfated molecules may also serve as reservoirs of bioactive principles, which are released upon sulfatase-mediated hydrolysis.
Sulfation of xenobiotics is an important mechanism of removing potentially toxic agents from our body.2 Metabolic sulfation, or more appropriately sulfonation, occurs in the cytosol through the action of one of the sulfotransferases and 3’-phosphoadenosine-5’-phosphosulfate (PAPS), which donates the activated sulfonate group (SO3−) to an acceptor alcohol, phenol or amine group.3 This introduces anionic character in the molecule, thereby enhancing its excretion properties to avoid potential adverse effects.
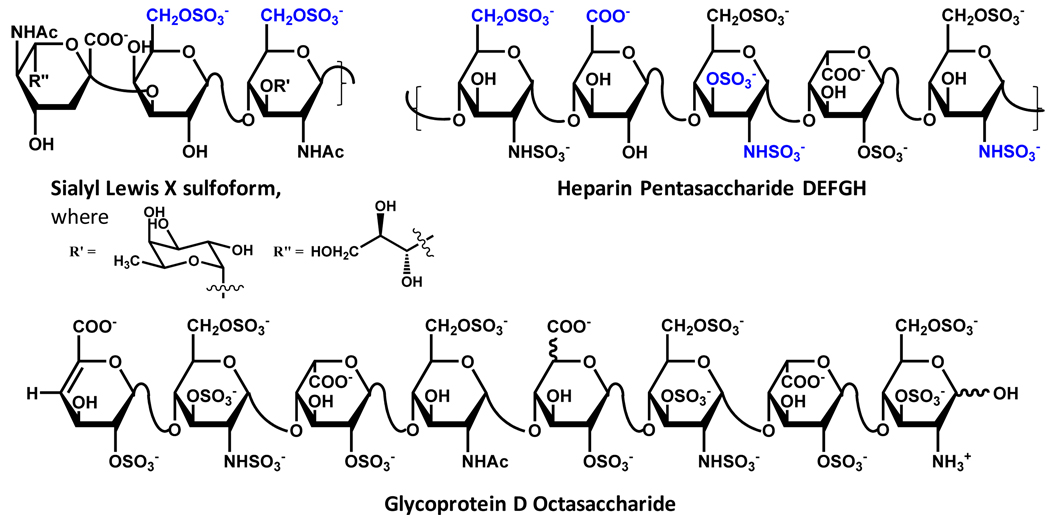
Even more interesting is exploitation of an essentially similar mechanism to induce a specific biological response. This mechanism involves the sulfation of biological molecules, especially carbohydrates, to generate unique sulfated ligands. The enzymes that catalyze these biotransformations are sulfotransferases, i.e., carbohydrate sulfotransferases, which play important roles in cell signaling, adhesion and several other functions.4–6. A key example is sialyl Lewis X for which sulfonation at 6-position of its N-acetyl glucosamine and galactose components enhances the recognition of C-type lectins (L-selectin) (Fig. 1), thus mediating the recruitment of leukocytes to tissue.7 Sulfated tyrosine has been implicated in high affinity binding of P-selectin glycoprotein ligand–1 (PSGL-1) with P-selectin,8–11 which plays a major role in pro-inflammatory response. Likewise, sulfation of tyrosine of the N-terminus of chemokine receptor CCR5 facilitates human immunodeficiency virus-1 (HIV-1) entry into host cells.12 Finally in the class of unique molecules generated by biosynthetic sulfation, the specific sequences present in polymeric heparin/heparan sulfate (H/HS) is nature’s engineering feat.13,14 The sequences engineered, e.g., the antithrombin- and glycoprotein D-binding HS sequences (Fig. 1), possess exquisite specificity for their target proteins primarily because of the three-dimensional constellation of their key sulfate groups (—OSO3−).15–18
Figure 1. Structures of endogeneous sulfated ligands shown to be involved in generating specific physiological or pathological responses.
Sulfate groups highlighted in blue (Sialyl Lewis X and DEFGH) are known to be essential for interaction with target proteins. Such sulfate groups have not been rigorously identified for glycoprotein D octasaccharide.
In addition to the above metabolic and biosynthetic uses, sulfation is an important mechanism for regulating bioactivity of certain molecules. For example, steroid sulfates may not function as hormones, but appear to serve as precursors of active steroids, which are formed by sulfatase-based hydrolysis of the sulfate group. There is growing evidence that intracellular sulfation and desulfation play important roles in regulating the availability of active steroid hormones near the target sites.19
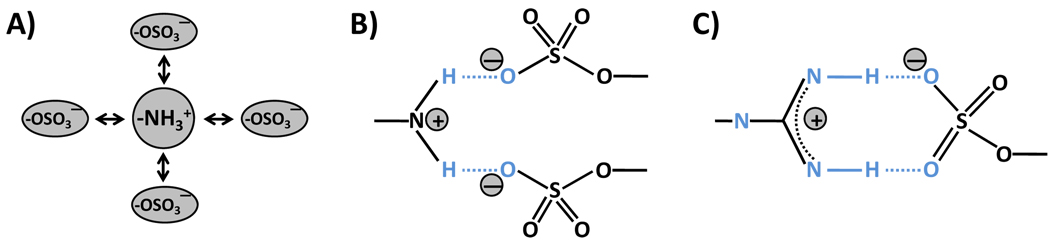
Although many sulfate group – protein interactions are primarily non-specific, i.e., do not require a unique ‘pharmacophore’ for eliciting a biological response, several interactions are known to be driven by specific recognition of one or more sulfate groups. The primary reason for specificity, or lack thereof, is nature of forces dominating the interaction. An electrostatic interaction, as present in a purely anion – cation point pair, is non-directional and hence non-specific (Fig. 2). In contrast, a hydrogen bonding interaction, as may be present between two strongly electronegative or ionic points sandwiching a hydrogen atom, is a directional interaction responsible for generation of specificity. The thrombin – heparin system involves multiple sulfate groups interacting with several arginine and lysine residues. Yet, the system is considered as non-specific because the principal contribution to affinity arises from electrostatic forces.20,21 On the other hand, the heparin pentasaccharide DEFGH – antithrombin system (Fig. 3) is highly specific because non-ionic forces, such as multiple hydrogen bonds, are primary contributors to the free energy of binding.22–24 It is interesting to note that a sulfate group can introduce both specific and non-specific inte ractions, and therein lies the challenge of modulating sulfate – protein interaction.
Figure 2. Interaction of sulfate group(s) with positively charged nitrogen containing groups.
A) The interaction relies solely on the ionic charges on the two groups. This interaction is defined as Coulomb-type interaction and occurs over a longer range in comparison to other atomic interactions. It is isotropic and does not involve any geometrical constraints. B) The interaction between a Lys or an Arg with one or more sulfate groups may sandwich an H atom resulting in the formation of a hydrogen bond. This H-bond may not be linear, yet provides sufficient energy to engineer specificity of recognition. The stoichiometry of interaction here may be 1:1 or 1:2 per nitrogen atom. C) For arginine, a linear H-bond geometry is feasible generating significant bond energy and greater specificity of recognition. The stoichiometry of interaction here is 1:1. Geometry C) is expected to be most stable.
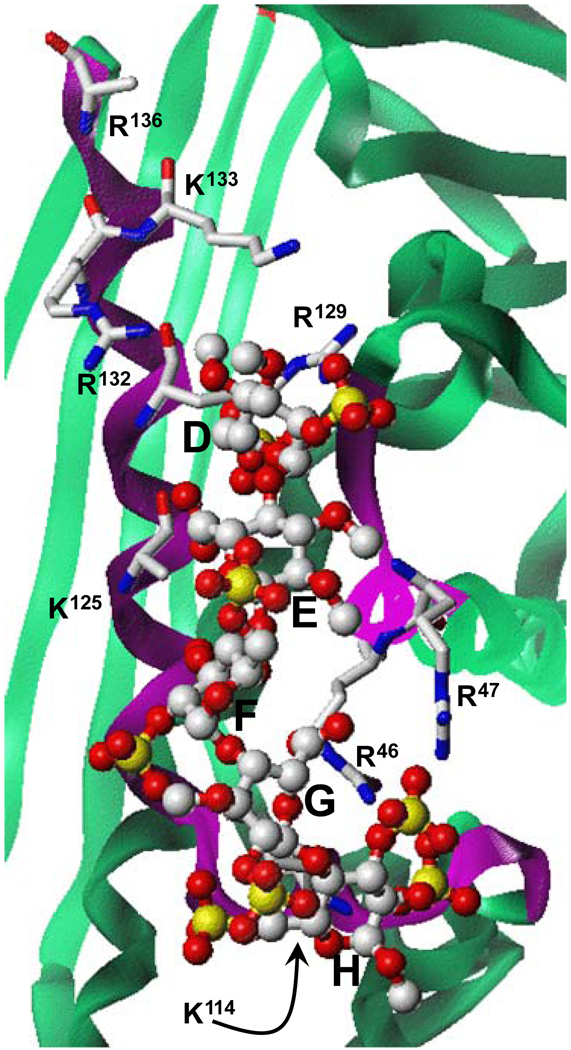
Figure 3. Close-up view of the heparin-binding site in antithrombin.
The structure of co-complex was obtained from PDB (filename ‘1e03’). Green ribbon shows antithrombin and magenta represents the heparin-binding site. Pentasaccharide DEFGH is shown in ball-and-stick representation. Extensive interactions between antithrombin arginine and lysines with multiple sulfate groups of DEFGH engineer the high affinity, high specificity interaction. Majority of the non-ionic binding energy involved in the heparin – antithrombin interaction is thought to arise from the hydrogen bond type interaction with sulfate groups. Figure modified from Desai UR. Med. Res. Rev. 2004; 24:151–181).
The growing importance of natural sulfated ligands, either mono-sulfated or poly-sulfated, conveys similar importance to appropriately designed, non-natural, sulfated scaffolds. For example, Tamura and Nishihara report the stereo-selective synthesis of sulfated glycosyl serine derivatives as mechanistic probes to better understand the mechanism of sorting that occurs in the initial steps of glycosaminoglycan (GAG) biosynthesis.25 Similarly, isosteres of CCR5 tyrosine sulfate have been designed as tools to understand HIV-1 entry.26
Another major application of designed sulfated scaffolds is as agonists/antagonists of biological processes. For example, several large and small aromatic H/HS mimetics including sulfated flavonoids27,28, benzofurans29, isoquinolines30 and sulfated dehydropolymers of the lignin-type31,32 have been designed to modulate the function of coagulation proteins such as antithrombin, thrombin and factor Xa. The design of such highly sulfated, non-natural molecules suggests a strong possibility of discovering novel sulfated non-carbohydrate pharmaceutical agents. Likewise, the carbohydrate literature is replete with numerous reports on the synthesis of non-natural oligosaccharides bearing one or more sulfate groups.16,33 Of these, at least one oligosaccharide, fondaparinux, has reached the clinic to treat deep vein thrombosis.
Although sulfation appears to be a one-step reaction, chemical synthesis of sulfated small molecules is challenging. The introduction of a sulfate group drastically alters the physico-chemical properties of the small molecule. Nearly all sulfated molecules are water soluble, which makes them difficult to isolate in highly pure form. A common problem is the presence of inorganic salts, the proportion of which is usually higher at small synthetic scales34 and which lead to significant inconsistencies. Another challenge is the lability of sulfate groups to acidic conditions and high temperatures.35,36 An additional problem is the lack of maneuverability following introduction of sulfate group. Few functional group transformations can be successfully performed in the presence of sulfate group, which essentially forces the design of the synthetic scheme to include sulfation as the final step. These complications increase geometrically for a poly-sulfated scaffold. Although theoretically, a synthetic approach for a mono-sulfated scaffold should be easy to extend to a poly-sulfated scaffold, yet practically it is a synthetic nightmare because of the generation of significantly higher negative charge density.37 The major challenge is driving the reaction to completion to sulfate all available reactive functional groups (e.g., ROH or PhOH). As the number of alcoholic/phenolic groups increase on a small scaffold, sulfation becomes progressively difficult because of anionic crowding, resulting in numerous partially sulfated side-products.34 Finally, lack of regioselectivity can be expected to become a dominant issue with polyfunctional substrates.
Given the growing importance of sulfated natural as well as non-natural scaffolds, we review the most widely applicable chemical sulfation protocols. Chemical sulfation reaction is an age old reaction and recent developments attempt to make it more ‘user friendly’. Yet, major improvement in technology is necessary to harvest the fruits of significant improvements in understanding the biology of sulfate esters.
2. Chemical Sulfation Approaches
2.1 Sulfation Using Sulfuric Acid
Literature reveals that sulfation used to be carried out with sulfuric acid (H2SO4) in the early part of the 20th century.38 H2SO4 can directly sulfate alkenes and cycloalkenes at moderate temperatures and mild pressures. Mechanistically, a Markownikoff product is obtained. H2SO4 also sulfates saturated monohydric alcohols, although the formation of water in the reaction results in only 65% yield of the sulfate ester from equimolar concentrations of acid and alcohol.39 Addition of excess acid, the removal of water through a Deans-Stark distillation unit and CCl4, adding boron sulfate (a dehydrating chemical) and sulfation in vacuo have been attempted to enhance yields.38
Polyhydric alcohols, e.g., polyethylene glycol, glycerol and polyvinyl alcohol, and polysaccharides, nitrogenous and non-nitrogenous, have been sulfated using H2SO4. Although H2SO4 could sulfate cellulose under mild conditions, e.g. 5 °C in CH2Cl2 or at −10 °C in liquid SO2, it is advisable to convert it into bis-(2-chloroethyl)ether adduct, a less reactive form, with 1,2-dichloroethane as solvent.40 Other approaches in this line of work, such as using SO2 as a moderating solvent, have also been explored.41
A modified form of sulfuric acid exploited for sulfation is sulfamic acid (H2NSO3H), which has been used for synthesis of saturated monohydric alcohol sulfates and carbohydrate sulfates. Since it is less reactive and more expensive than other sulfating agents, it is a reagent to explore when others fail. Sulfamic acid sulfation of long chained primary alcohols gives poor yields and dark-colored products. Nevertheless, phenolic ethylene oxide condensates are preferably sulfated with sulfamic acid.42 Catalysts such as pyridine, urea, thiourea, and acetamide have been used to improve the outcome of sulfation of long chain secondary alcohols and monoand di-esters of glycerol. Cellulose is reported to undergo degradation when heated with sulfamic acid alone, but in presence of urea satisfactory sulfation occurs at 140 °C in 30 min.40 Despite its usefulness for sulfating simple alcohols that are available in abundance, the main problems in H2SO4–based sulfation are the numerous side reactions including dehydration, nonselective sulfation and scaffold degradation.
2.2 Dicyclohexylcarbodiimide-Mediated Sulfation
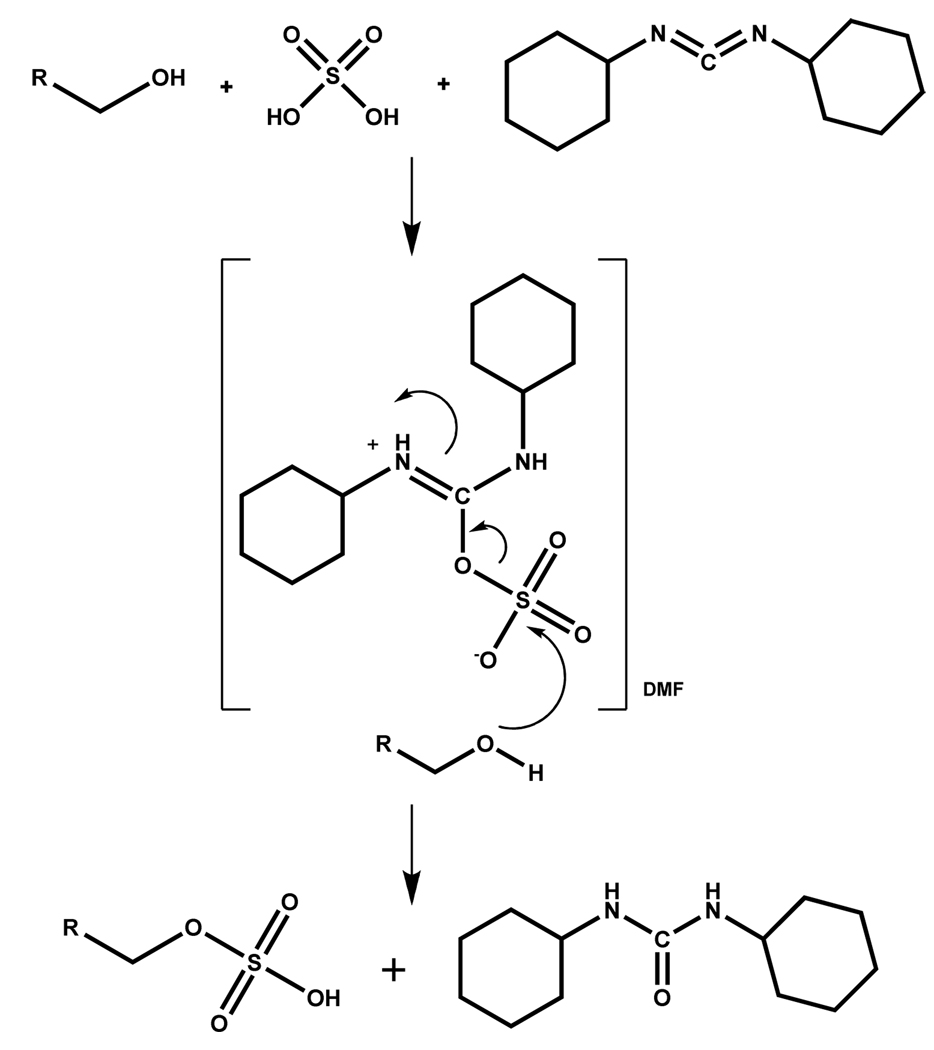
The first sulfation of aliphatic/alicyclic alcohols using dicyclohexylcarbodiimide (DCC) and H2SO4 was reported by Mumma in 1966 (Scheme 1). The alcohol, DCC, and H2SO4 were allowed to react in a fixed ratio of 1:5:1, respectively, in DMF at low temperature (~4 °C) for about 15 minutes. The order of addition of reactants was found to be important with alcohol being added to DCC followed by H2SO4. The proposed mechanism involves the formation of a solvated, protonated DCC-H2SO4 intermediate (Scheme 1), followed by a nucleophilic attack of the alcoholic group onto the sulfur atom producing the monosulfate ester and dicyclohexylurea.
Scheme 1.
The reaction appeared to show concentration-dependent regioselectivity for alcohols. Under dilute conditions only unhindered hydroxyl groups were sulfated, while higher concentrations led to per-sulfated products.43,44 For example, under dilute conditions the phenolic group of estrone or estradiol-17β-acetate could not be sulfated, whereas only one 35S-labeled product was obtained in the case of estradiol and corticosterone suggesting that aliphatic unhindered 1° or 2° alcohols are preferentially sulfated.44 In a similar manner, sulfation of methyl α- and β-d-galactopyranosides and 4-O-β-d-galactopyranosyl-3,6-anhydro-l-galactose dimethylacetal led primarily to the 6-O-sulfated product.45 The steric bulk of DCC-H2SO4 complex is likely to reduce the accessibility of the reagent to the sterically hindered hydroxyl groups.
The versatility of this protocol has been tested through sulfation of other functionalities including phenols, mercaptans, amines, and oximes. These groups can be sulfated in reasonably good yields using an essentially identical protocol with minor modifications. Yet, these functional groups typically require higher concentration of reactants.44
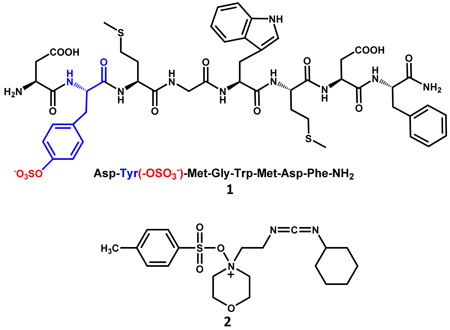
This protocol was also used for O-sulfation of cholecystokinin octapeptide without protection of the amino acid side chains. A maximal yield of 40% could only be achieved with 4-fold excess of H2SO4 and 40-fold excess of DCC highlighting the challenges involved. The purified sulfated octapeptide 1 was active in stimulating amylase secretion from rat pancreatic fragments, and amino acid analysis confirmed the sulfation of tyrosine residue in the peptide.46 Similarly, the tyrosine residue of a synthetic dodecapeptide, designed as a hirudin mimic, was sulfated by the DCC-H2SO4 method. The sulfated hirudin mimic displayed an order of magnitude higher inhibitory activity in traditional clotting assays, the activated partial thromboplastin time (APTT) and thrombin time.47
Although the DCC-mediated sulfation protocol gives good yields of monoalkyl sulfates, the formation of the relatively insoluble dicyclohexylurea complicates the direct isolation of the sulfated product. Rather large volumes of methanol are required for dissolving dicyclohexylurea, especially during the DEAE-cellulose column chromatography.48 To ease the isolation, several carbodiimide derivatives have been investigated. Aromatic derivatives of carbodiimides generally gave poor yields of the final sulfated product in contrast to those with aliphatic groups. The best yields were noted with lcyclohexyl-3-(2-morpholinoethyl)carbodiimide methyl-p-toluene sulfonate 2, which releases a more water soluble carbodiimide derivative. In parallel, reaction conditions were investigated using THF, dioxane, and alcohols as solvents. Although monoalkyl sulfates were the major products with THF and dioxane, many undesirable side products were obtained and reactions were uncontrollable. When alcohols were used as both solvent and reactant, mono- and di- alkyl sulfates were formed.49
2.3 Sulfation Using Sulfur Trioxide–Amine Complexes
The DCC/H2SO4-based is not amenable to sulfation for many sensitive scaffolds considering the strong acidity of sulfuric acid, while direct sulfation of alcoholic or phenolic groups using sulfur trioxide (liquid or gas) is fraught with other multiple problems including polymerization and difficulty of handling SO3.43 Thus, SO3 is preferentially used as adduct with amine, amide, ether, or phosphate containing molecules. These organic complexes are relatively easy to prepare by bubbling SO3 gas into a solution of the preferred organic base or by adding the base to the SO3-organic suspension;50 are solid at room temperature; and are relatively stable at high temperatures. These complexes have been used for sulfating a variety of scaffolds containing alcoholic, phenolic, amine, thiol and other functional groups. Complexes of SO3 with organic bases including pyridine (Py), trimethylamine (NMe3), and triethylamine (NEt3), or amides such as DMF have typically found extensive usage.51–55
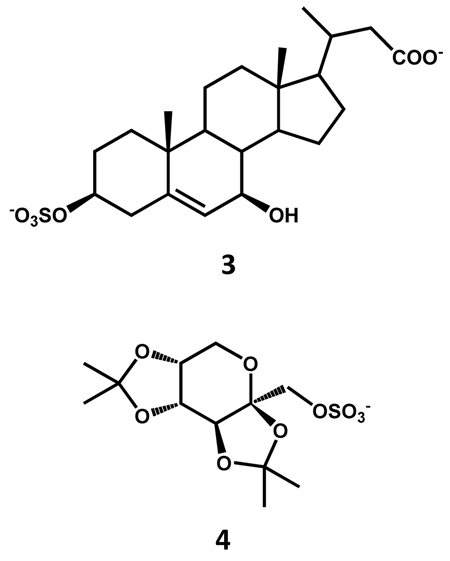
NMe3 and NEt3 complexes with SO3 appear to be well suited for sulfation of alcoholic groups present in carbohydrates, steroids, and aliphatic or alicyclic scaffolds. For instance, Kakiyama et al. report the chemical synthesis of 3β-sulfooxy-7β-hydroxy-24-nor-5-cholenoic acid 3 as an internal standard for mass spectrometric analysis of the abnormal Δ5-bile acids occurring in Niemann-Pick disease.56 The mono-sulfated steroid was synthesized in 76% yield using 5 equivalents of SO3-NMe3 complex per –OH group in dry pyridine at room temperature within 1 hour. Another example is the synthesis of 2-sulfated form of α-l-iduronate glycosides using 1.5 equivalents of SO3-NMe3 per -OH group in dry DMF at 55 °C for 24 h.57 A lengthy series of steroid sulfates have been prepared using SO3-NEt3 complex in which selective sulfation was observed for unhindered hydroxyl groups at room temperature, while heating to 70 – 95 °C led to sulfation of hindered alcoholic groups.58 Polyhydroxysteroids have also been sulfated using 1.5 equivalents of SO3-NEt3 per hydroxyl group at 95 °C.59 Highly sulfated β-d-glucopyranoside derivatives were synthesized in varying yields of 22–86% with SO3-NMe3 complex at 70 °C in dry DMF.60
Scaffolds based on phenolic structures and containing more acidic –OH groups appear to be better sulfated with SO3 complexes with weaker bases such as pyridine and DMF. Additional considerations also play a role including the stability of the resulting quaternary salt, the ease of the product purification, and the simplicity of the sulfating complex preparation. For example, it would be preferable to sulfate tyrosine under neutral or mildly basic conditions. Fujii et al. report SO3-Py complex as more suitable sulfating agent than pyridinium acetyl sulfate for tyrosine sulfation.61 Yet, sulfation with SO3-Py is known to result in considerable coloring and SO3-Py complex is not readily soluble in water or ether leading to difficulties in work-up. Futaki et al. report that sulfation of Boc-tyrosine-OH with SO3-DMF complex (1:5 ratio) at 25 °C gives 20% better yields than SO3-Py complex under similar conditions. DMF is a weaker base than pyridine, which implies that the partial positive charge on the sulfur atom of the SO3-DMF complex would be greater than that in the SO3-Py complex. Thus, it is likely that the nucleophilic attack on SO3-DMF complex would be more favorable resulting in higher yields.62
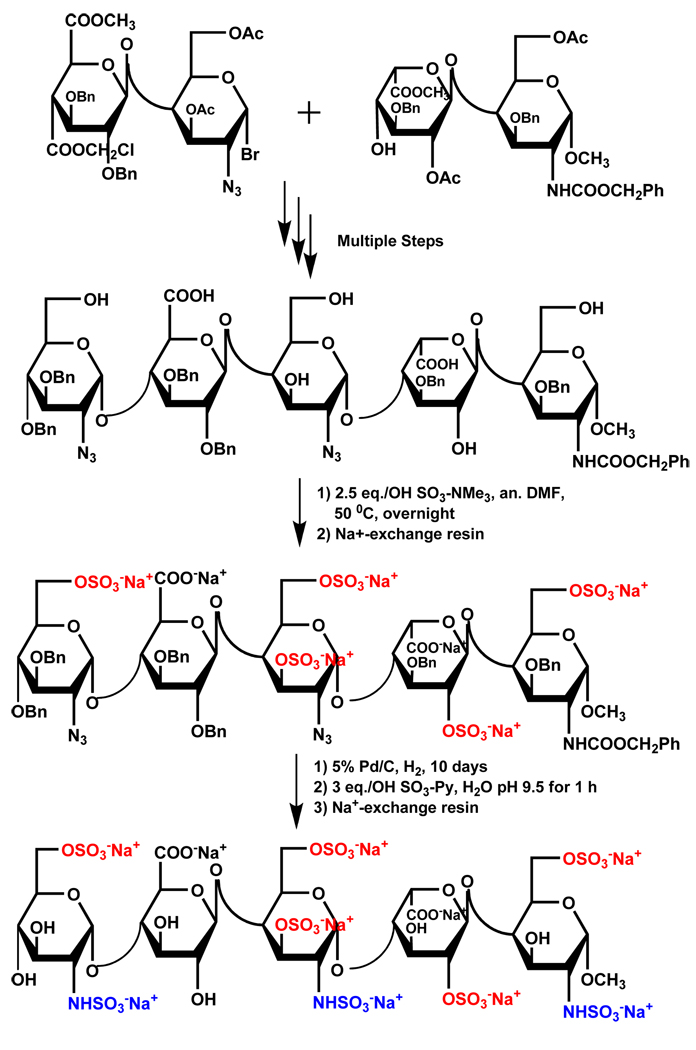
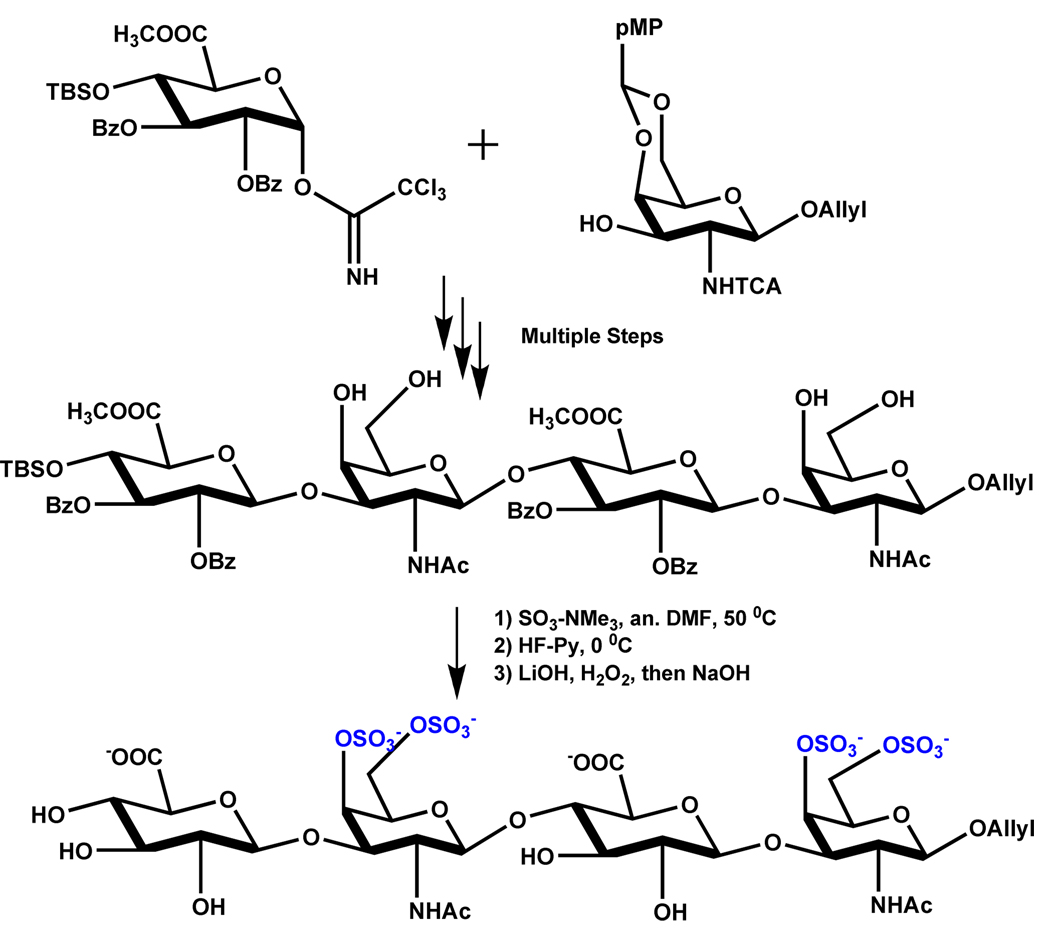
SO3-Py complex has been most often used for sulfation of carbohydrate scaffolds. Popek et al. have prepared 2,3:4,5-di-O-isopropylidene-β-d-fructopyranose-1-sulfate 4 using SO3-Py complex at 55 °C in ~80% yield.63 Another representative example is the synthesis of 2-sulfates of glucose and galactose, which also utilized SO3-Py complex at room temperature for 20 – 36 hours.64 Likewise, synthesis of heparin pentasaccharide and its derivatives have been most often exploited SO3-NMe3 and SO3-Py complexes.16,65,66 In one protocol, O-sulfation was simultaneously accomplished at five positions (D unit: 6-OSO3−, F unit: 3 and 6-OSO3−, G unit: 2-OSO3−, H unit: 6-OSO3−) in 85% yield using 2.5 equivalents per –OH of SO3-NMe3 complex (Scheme 2). Following O-sulfation, triple N-sulfation was achieved with 3 equivalents of SO3-Py complex per –NH2 group in 63% yield (D unit: 2-NSO3−, F unit: 2-NSO3−, H unit: 2-NSO3−). It is interesting to note that the triple N-sulfation was achieved in H2O at pH 9.5 in presence of exposed –OH groups suggesting major regioselectivity in recognition of the amine groups (Scheme 2). A recent report achieves simultaneous sulfation at seven positions (D unit: 6-OSO3−, F unit: 2, 3 and 6-OSO3−, H unit: 2, 3 and 6-OSO3−) to synthesize idraparinux in one step using excess SO3-NEt3 in 93% yield (not shown), which should be considered an achievement from the synthetic perspective.67 Finally, Tully et al. synthesized a tetrasaccharide bearing two sulfate groups at position 4- and -6 of the D-galactosamine unit (Scheme 3), the minimum structural motif in chondroitin sulfate required to promote the neuronal growth, using 10 equivalents of SO3-NMe3 complex per –OH group in DMF at 50 °C for 2 hours.54
Scheme 2.
Scheme 3.
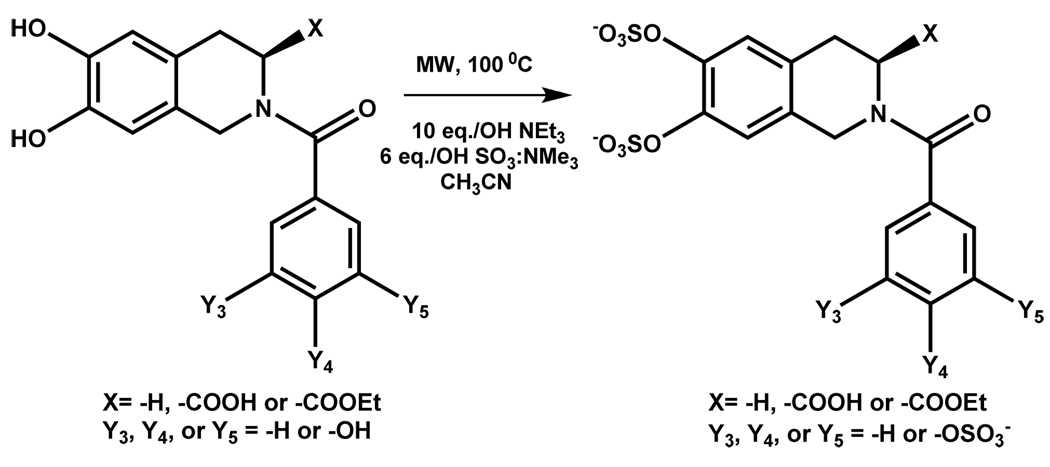
Recently, a microwave-based protocol has been developed to enhance the rate of sulfation of phenolic structures, especially those with multiple phenolic groups.34 Per-sulfation of small polyphenolic scaffold is typically difficult because of anionic overcrowding as well as reduced stability of highly sulfated products to high temperatures. It was reasoned that microwaves are likely to induce significant rate enhancements because the sulfated intermediates may couple to microwaves through ionic conduction. Acetonitrile, instead of the commonly used DMF, was used as the solvent for its microwave-friendliness and ease of evaporation. Further, presence of free base in the reaction mixture was proposed to promote the difficult per-sulfation reaction. Optimal results were achieved by using SO3-amine complex (–NR3 or –Py, where R = Me or Et) at approximately 6 – 10 times molar proportion per phenolic group in the presence of microwaves at 100 °C (Scheme 4). Using these conditions, per-sulfated products could be isolated in ~70–95 % yield.34 Of special note was the synthesis of a crowded 3,4,5-trisulfated structure, which was essentially impossible to isolate through traditional sulfation protocols.
Scheme 4.
Microwave-assisted sulfation approach has opened a new avenue to access polysulfated organic compounds mimicking glycosaminoglycans. This protocol seems to tolerate a range of functional groups such as amides, esters, aldehydes as well as alkenes. Besides, the high yield of the products makes the method convenient for construction of sulfated compounds library. The method can be applied uniformly toward the synthesis of monoto hexa-sulfated compounds, which is important considering that repulsive intra-molecular forces are thought to limit polysulfation resulting in a mixture of partially sulfated products. Both alcoholic and phenolic hydroxyl groups can be sulfated equally well using SO3-Py complex as the sulfating agent. This method appears to provide the per-sulfated product in high purity using a one step aqueous G-10 filtration column. The method is particularly convenient for quantitative isolation of small amounts (<10 mg) of the per-sulfated products and may be possible to scale up. Recently, this protocol has been applied successfully in synthesis of non-saccharide, allosteric antithrombin activators.30 The application of microwave-assisted sulfation to amine, thiols and other functional groups remains unexplored.
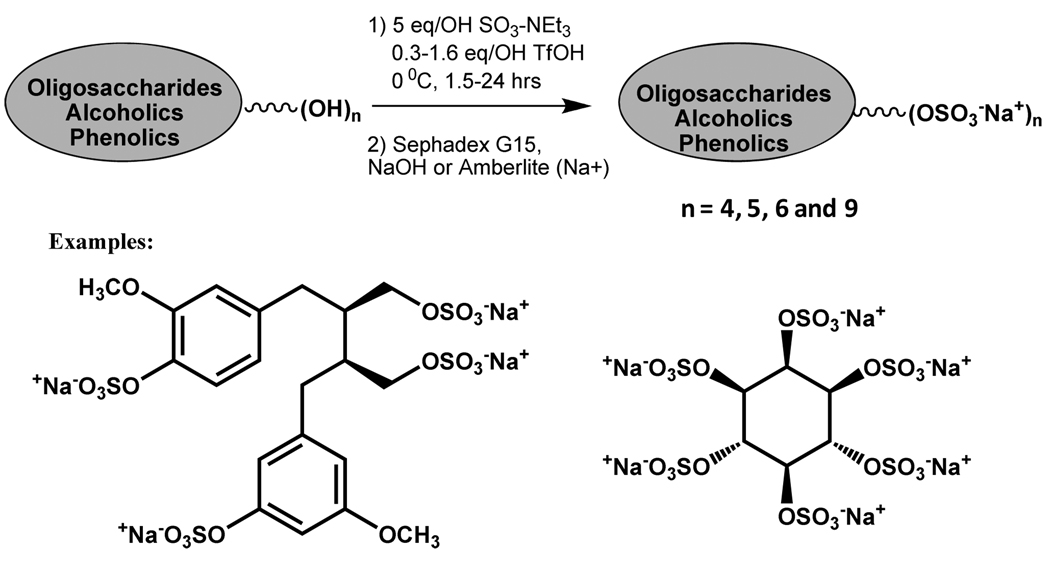
In contrast to the above microwave-assisted sulfation, which can be categorized as high temperature, basecatalyzed reaction, Krylov et al. have recently reported low temperature, acid-catalyzed sulfation reaction.68 The general protocol involves addition of SO3-NEt3 complex to a DMF solution of the poly-alcohol followed by addition of triflic acid (1.6–3.0 eqv./-OH group) at − 20 °C. The suggested mechanism is that triflic acid liberates SO3 from the amine complex in situ and negates the requirement for high temperatures. This approach has been useful for per-O-sulfation of poly-alcoholic scaffolds and has been used to synthesize tetra-sulfated forms of lignans, isolariciresinol and secoisolariciresinol, pentasulfated form of flavonoid dihydroquercetin, hexasulfated form of cyclitol myo-inositol, and nonasulfated form of an oligosaccharide (Scheme 5). The yields of the per-sulfated products were a commendable 53 to 77 % depending on the structure of the reactant.68
Scheme 5.
2.4 Protection/Deprotection Strategies
Despite the success of direct sulfation strategy, syntheses of complex molecules containing multiple sulfate groups may turn out to be challenging. Insolubility of sulfated molecules in organic solvents and limited development of water-based chemical transformations may pose considerable hurdles in the synthesis of complex sulfated molecules. Further, the acid stability of sulfated molecules is also suspect.36 Consequently there is growing interest in developing protection/deprotection strategies in which sulfate group(s) are introduced into the target scaffold in a masked form at an intermediate step. Following appropriate transformations, the unmasking of the sulfate group is achieved through a simple deprotection step. This is an attractive approach because the intermediate containing the masked sulfate can be usually purified using traditional synthetic methods, characterized in detail using standard spectroscopic tools, and is usually stable to a wider range of functional group transformations.
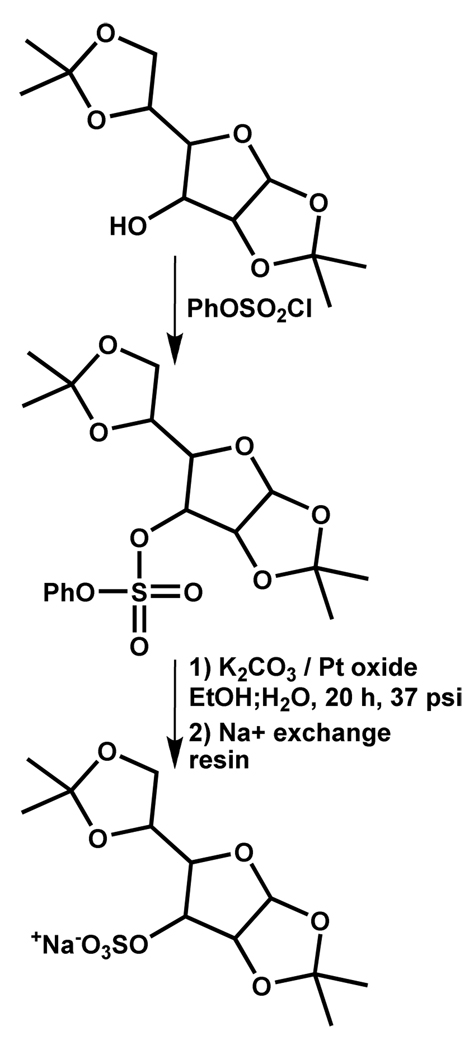
Penney and Perlin describe the strategy of using phenyl chlorosulfate to introduce a masked sulfate in monosaccharides.69 Reaction of phenyl chlorosulfate with 1,2:5,6-di-O-isopropylidene-α-D-glucofuranose (Scheme 6) gave the corresponding sulfated form in 75% yield. The removal of the phenyl group was effected through catalytic hydrogenation on platinum oxide under alkaline conditions. It is important to note that the phenyl group is first perhydrogenated to the cyclohexyl group, which undergoes cycloalkyl fission to release cyclohexanol and the desired sulfated D-glucofuranose.69 The masked sulfate intermediate, phenyl monosaccharyl sulfate diester, can be expected to endure a number of transformations including selective acid-hydrolysis, acetolysis, deacetylation, and fluoride-mediated removal of trialkylsilyl substituents, which may facilitate important structure modifications. However, reports exploiting such an opportunity in the synthesis of sulfated saccharides are limited in the literature.70 A probable reason for this state is the low or variable yield of the deprotection step.
Scheme 6.
To address this deficiency, a new protocol was developed involving 2,2,2-trifluoroethyl protecting group.71 In this strategy, the trifluoroethyl group was introduced by treating sulfate monoesters of carbohydrates with 2,2,2-trifluorodiazoethane, a highly toxic and potentially explosive agent. Following the desired manipulation of carbohydrate structure, the trifluoroethyl protecting group was removed using potassium t-butoxide at high temperature. It is important to note that the demasking conditions in both the above methods are vigorous, which may not be compatible with some sensitive structures.
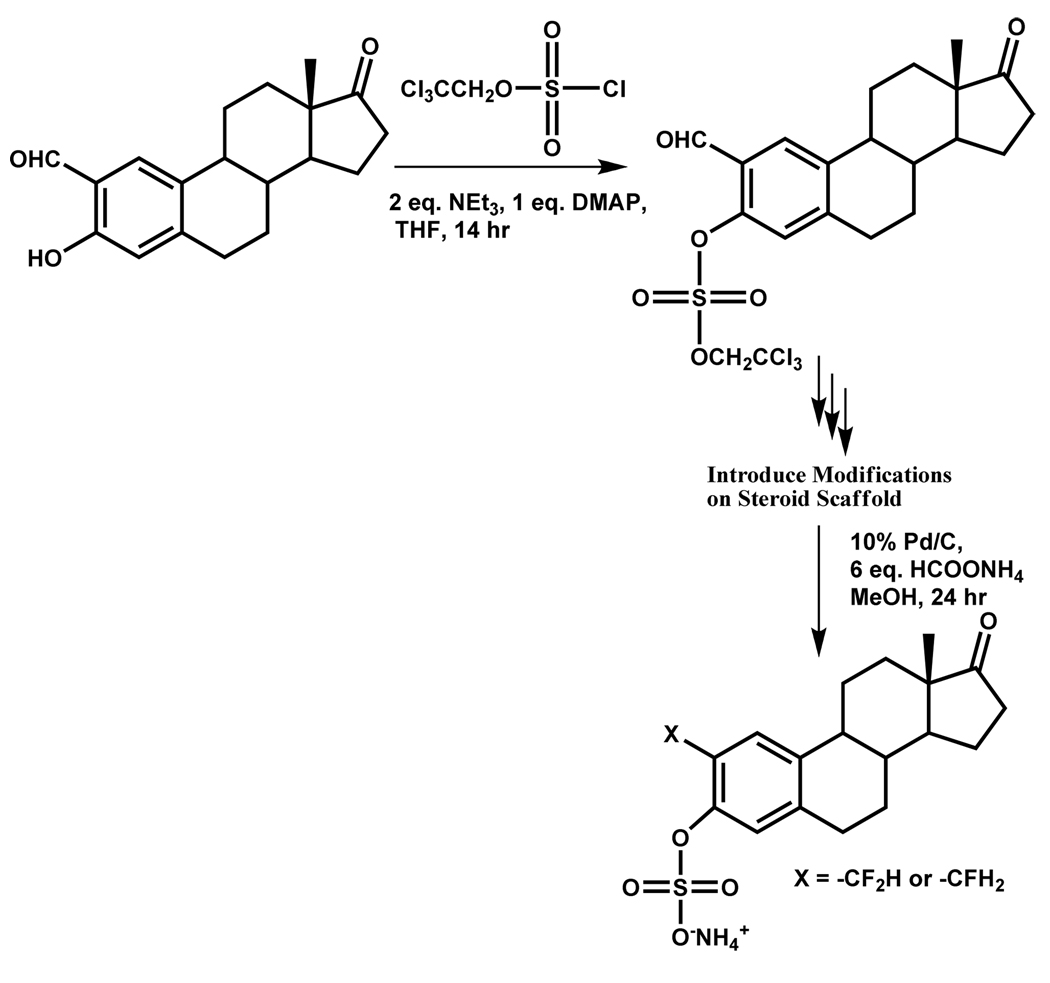
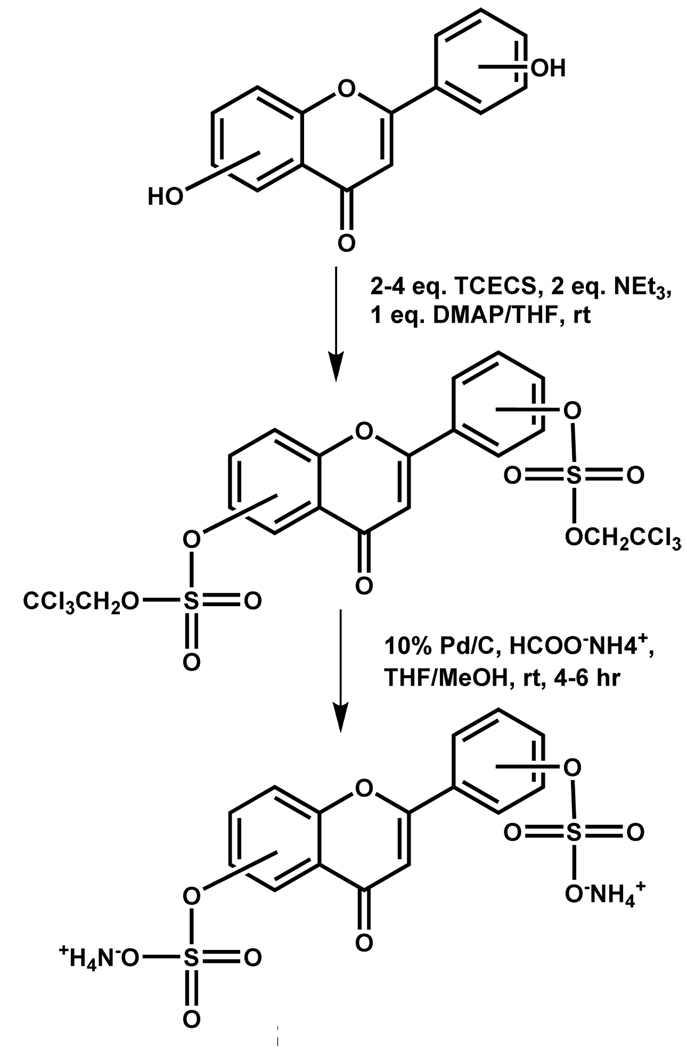
Taylor and coworkers have described the use of a related group, the 2,2,2-trichloroethyl (TCE) group, as the preferred protecting group for synthesizing aryl sulfates. TCE-protected sulfate esters can be prepared in high yields from starting phenols with 2,2,2-trichloroethyl chlorosulfate (TCECS) in the presence of NEt3.72 The resulting TCE aryl disulfate esters could be readily deprotected in excellent yields under neutral conditions with Pd/C and ammonium formate or zinc and ammonium formate. This approach was successfully applied to the synthesis of estrone sulfate derivatives, which were difficult to obtain through other methodologies (Scheme 7). The deblocking conditions were incompatible with functional groups sensitive to reducing agents. The intermediate sulfate diester was stable to strong acid and weak base, but reactive toward nucleophiles or strong organic bases. This strategy was utilized by Gunnarsson et al. to synthesize per-sulfated flavonoids that displayed interesting factor Xa inhibition properties (Scheme 8)73
Scheme 7.
Scheme 8.
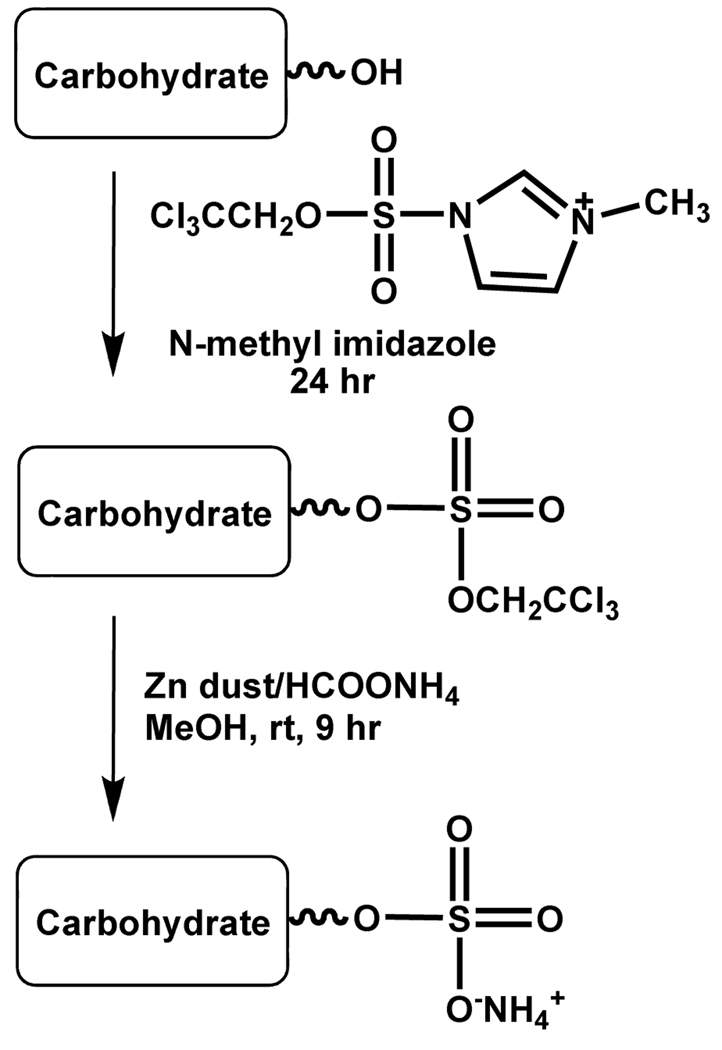
The above TCE-protection protocol appears to fail for certain carbohydrates. TCECS reaction with diisopropylidene-D-glucose gave the corresponding chlorosugar under a variety of different conditions.74 To obviate the chlorosugar product, sulfuryl imidazolium triflate was chosen as a sulfating agent. It was reasoned that replacing the chloride group with another good leaving group that does not possess nucleophilic property would aid TCE-based protection of saccharides. The reaction of TCECS with imidazole gave the corresponding sulfuryl imidazole in 86% yield, which when further treated with methyl triflate resulted in quantitative precipitation of imididazolinium triflate.74,75 Using this agent in 2 – 5-fold excess over the saccharide in the presence of N-methylimidazole, primary and secondary hydroxy groups were sulfated in good to excellent yields at room temperature within 16–48 hours (Scheme 9). Bases such as NEt3, pyridine, 2,6-lutidine, piperidine were found to be considerably less effective.74
Scheme 9.
As mentioned earlier, the TCE-protected saccharides could be deprotected to generate sulfated carbohydrates in good yields by employing zinc- ammonium formate reducing agent in methanol.74 Unmasking of the sulfate groups could also be performed with Pd/C and ammonium formate reducing agent, however, the yields were lower. A specific advantage of this method was that the crude sulfated product was nearly pure with minor inorganic impurities, which could be removed by passing the product through a short column of silica. The TCE-protected sulfates were stable to many of the reaction conditions commonly encountered in carbohydrate chemistry such as ZnCl2/acetic acid/acetic anhydride–mediated debenzylation/acetylation, deacetylation by sodium methoxide, benzylidene ring opening with trifluoromethanesulfonyl or PhBCl2 in the presence of Et3SiH, and formation of trichloroacetimidate derivative using catalytic DBU.74
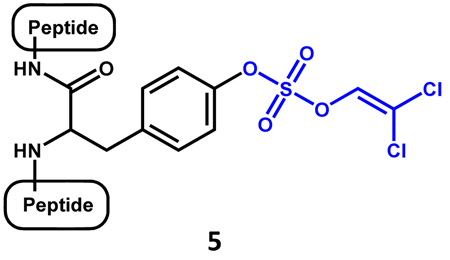
An attempt has been made to integrate the TCE-based sulfation reaction with the solid-phase peptide synthesis (SPPS). Introduction of TCE-protected tyrosine in the SPPS process turned out to be good start.76 The TCE protective group withstands harsh acidic treatment involved in releasing the synthesized peptide from the resin and can also be readily removed using mild reductive conditions. Yet, it is not as stable to organic bases that are commonly used to remove the 9-fluorenylmethoxycarbonyl (Fmoc) protective group during SPPS, such as piperidine, morpholine or DBU. The pitfall inspired Ali et al. to develop a related protecting group, dichlorovinyl (DCV) group (see compound 5), for SPPS. The DCV-protected tyrosine can be easily synthesized, it can withstand a wide variety of reactions particularly trifluoroacetic acid-mediated peptide release and 2-methypiperidine-mediated Fmoc deprotection. Besides, it can be removed using 10 % Pd/C and H2 (gas) or ammonium formate in MeOH at room temperature. This method has been proved to be effective in the synthesis of monosulfated hexapeptide (71% yield), trisulfated octapeptide (46% yield), monosulfated octapeptide (63% yield), disulfated 22-mer (58% yield) and tetrasulfated 20-mer (39% yield).76
Simpson and Widlanski have studied alkyl chlorosulfates, especially isobutyl and neopentyl chlorosulfates, as protected forms of sulfates.77 In this approach, neopentyl-and isobutyl- groups as sulfate protecting groups were investigated. Sulfate diesters containing these protecting groups could be synthesized without much difficulty from the parent phenols or alcohols and neopentyl- or isobutyl- chloro sulfates in high yields (>70%) by nucleophilic displacement of the chloride ion. The neopentylated sulfate diester was found to exhibit high stability to strong acids and bases, thus ruling out efficient protection/ deprotection strategy. On the other hand, the isobutylated sulfate diester was suggested to be stable to strong acids, but susceptible to piperidine. Selective advantages of the two protection groups include the ability to withstand Fmoc and tert-butoxycarbonyl (Boc) amino group protection reaction conditions. The two groups also tolerate hydrogenolysis conditions (Pd/C and H2) for deprotection of benzyl and benzyloxycarbonyl (Cbz) groups of carboxylic acid and amino group, respectively. Likewise, the neopentyl- and isobutylsulfate diesters withstand aqueous acidic conditions suggesting their applicability in complex oligosaccharide synthesis involving isopropylidene protection/deprotection steps. Thus, overall the neopentyl- and isobutyl- sulfate protection groups appears to possess good chemical properties for exploitation in carbohydrate and peptide syntheses.
Deprotection of the neopentyl and isobutyl groups can be effected in a nucleophilic reaction in aprotic polar solvents. Specifically, small nucleophiles such as azide and cyanide in hot DMF (60–70 °C) are effective in quantitative removal of the neopentyl group from the protected aryl sulfate diesters, while deblocking with NaI in hot acetone (55 °C) is effective for isobutyl alkyl sulfate diesters. This condition appears to be problematic for isobutyl aryl sulfate diesters as the iodide can displace the phenoxide through a nucleophilic attack on the sulfur atom. To avoid this problem, sodium thiocyanate in refluxing acetone in the presence of a base (NEt3) was developed.77
2.5 Sulfitylation Oxidation and Release
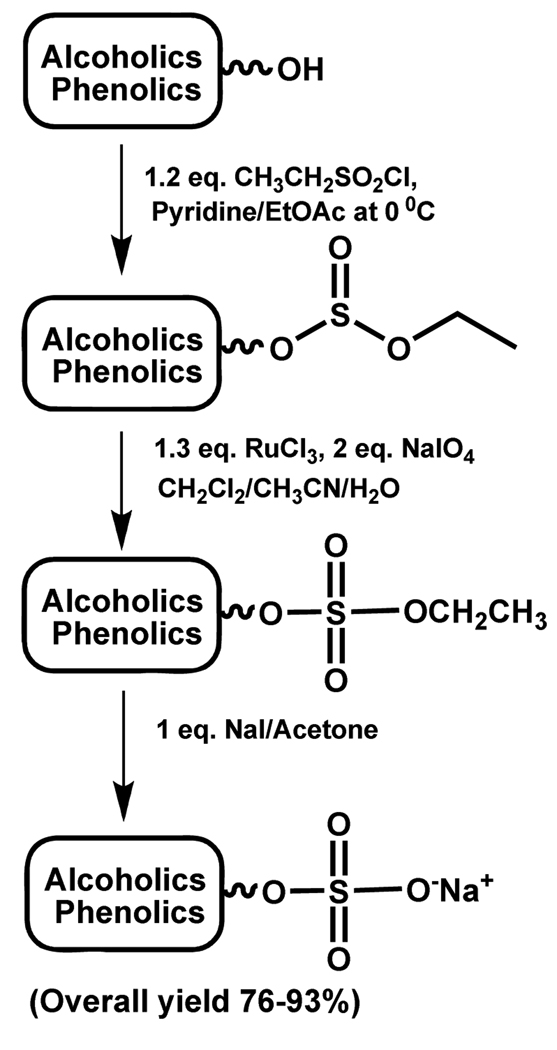
A three-step sulfation strategy was developed by Huibers and co-workers based on the strategy of Gao and Sharpless, who reported the conversion of vicinal diols to cyclic sulfates via cyclic sulfite diesters.78 The protocol proceeds under mild conditions and requires near stoichiometric amounts of the reagents. The first step involves the formation of alkyl ethyl sulfite derivative of the parent alcohol, which is oxidized using sodium periodate and ruthenium III chloride to their sulfate diester forms. The targeted sulfate monoesters were then released in high yields using sodium iodide at ambient temperature (Scheme 10).79
Scheme 10.
This approach, reported as sulfitylation-oxidation protocol, provides several advantages. All three steps are high yielding steps and their products in most cases require minimal purification. Sulfite and sulfate esters of the alcohols tested were found be apolar, stable and readily soluble in organic solvents allowing for traditional chromatographic purification. This strategy appears to be generally applicable as it was found to work well with a number of different aliphatic and secondary alcohols, diols, sugars, and aromatic alcohols. The applicability of this protocol has been demonstrated in the synthesis of methyl-2,3,4,6-tetra-O-sulfonato-β-D-glucopyranoside tetrasodium salt. This tetrasulfated compound was synthesized earlier in 41% yield using SO3-NMe3 complex, while application of the sulfitylation-oxidation and release protocol gave an overall yield of 77%.60,79
Although this protocol appears to be promising for highly sulfatd oligosaccharide synthesis, the potential of ethyl sulfite esters as sulfate precursors requires detailed investigation as acetylation or silylation appear to proceed well, while other transformations, e.g., benzylation and deacetonation, resulted in complete decomposition.79
2.6 Regioselective Sulfation
Majority of sulfation reaction reported to-date revolve around mono-sulfation or per-sulfation of appropriate reactants. However metabolically, sulfation of only selected alcohols or amines among several available would be more relevant and appealing. Although structural properties of substrates, such as steric constraints, do induce some selectivity, specific tools have been developed to engineer regioselectivity in sulfation reactions.
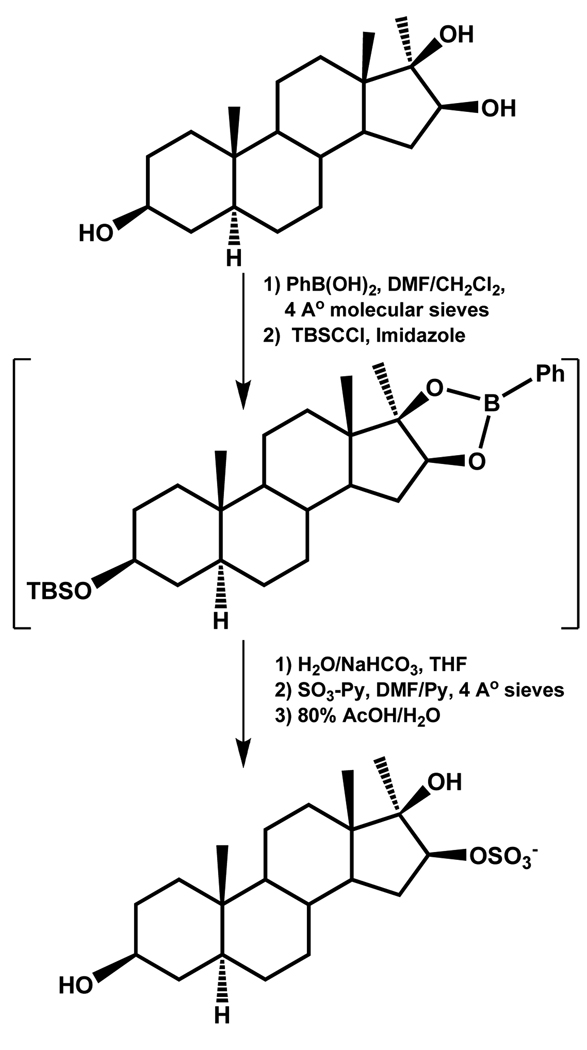
Hungerford et al. report the regioselective sulfation of a steroid scaffold, especially at the 3- and 16-position in the presence of multiple hydroxyl groups using a boronate ester-based approach.80 The C-3 and C-16 sulfated steroids are desirable as standards for metabolites of anabolic steroid, which are routinely abused in the highly competitive world of sports. The regioselective sulfation approach exploited the formation of a 16β,17β-boronate ester to introduce a sulfate at the available hydroxyl group. To prepare a 16β-monosulfated steroid, an orthogonal protecting group, i.e. TBDMS, was introduced at the 3-position followed by oxidative removal of the boronate ester and preferential sulfation of the sterically more accessible 16β-position in the presence of 17β-OH using SO3-Py (Scheme 11). Using these two schemes, the 3-monosulfate and 16β-monosulfate derivatives of 3β,16β,17β-trihydroxy-17α-methyl-5α-androstane were synthesized in an overall yield of 79 and 69 %, respectively.80
Scheme 11.
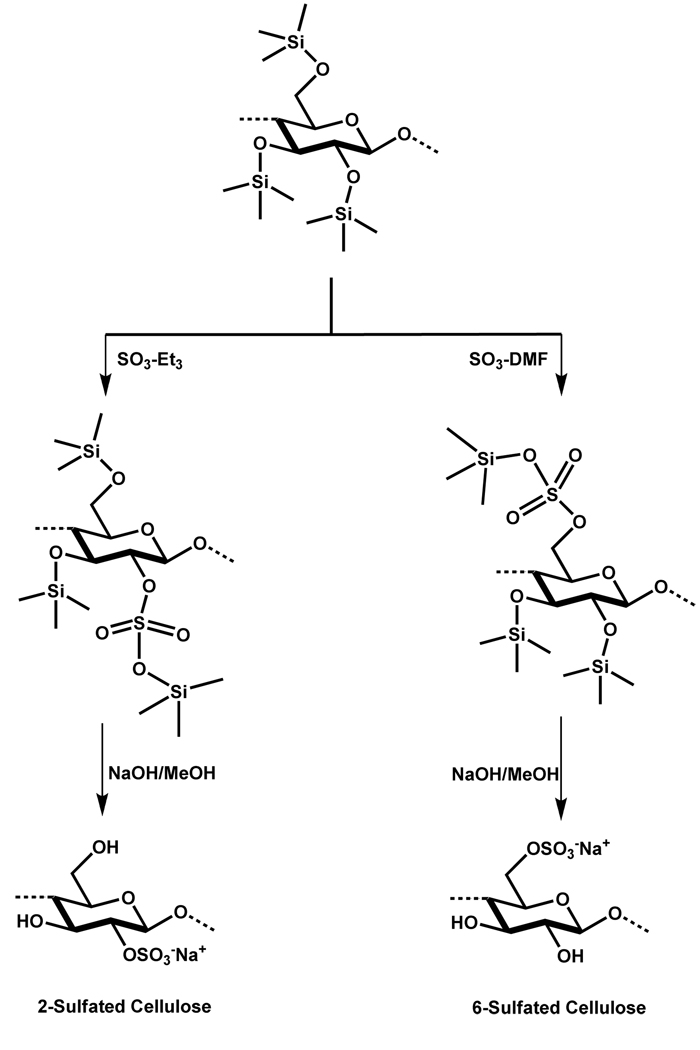
Another reagent that has been explored to regioselectively sulfate polysaccharides is trimethylchlorosilane. This reagent has been shown to be useful in the selective synthesis of either C-6 or C-2 sulfated anhydroglucose unit of cellulose by SO3 insertion into the –O—Si– linkage of trimethylsilyl cellulose.81 The mechanism of this reaction initiates by the nucleophilic attack afforded by one of the oxygens of the SO3 onto the electropositive Si in cellulose resulting in the formation of the corresponding trimethyl silyl sulfate cellulose diester. In order to unmask the sulfate group, basic work up in methanol cleaves the trimethyl sulfate ester by once again exploiting the electrophilicity of the Si atom. Several factors have been reported to explain the observed selectivity. However, the type of the partner of SO3 in sulfating complex and the polarity of –O—Si– have primarily determined the site of sulfation. For instance, SO3 in complex with DMF prefers the least sterically hindered position, which is C-6, while SO3 in complex with NEt3 favors the most polar –O—Si– position, which is C-2 in the polysaccharide (Scheme 12).81
Scheme 12.
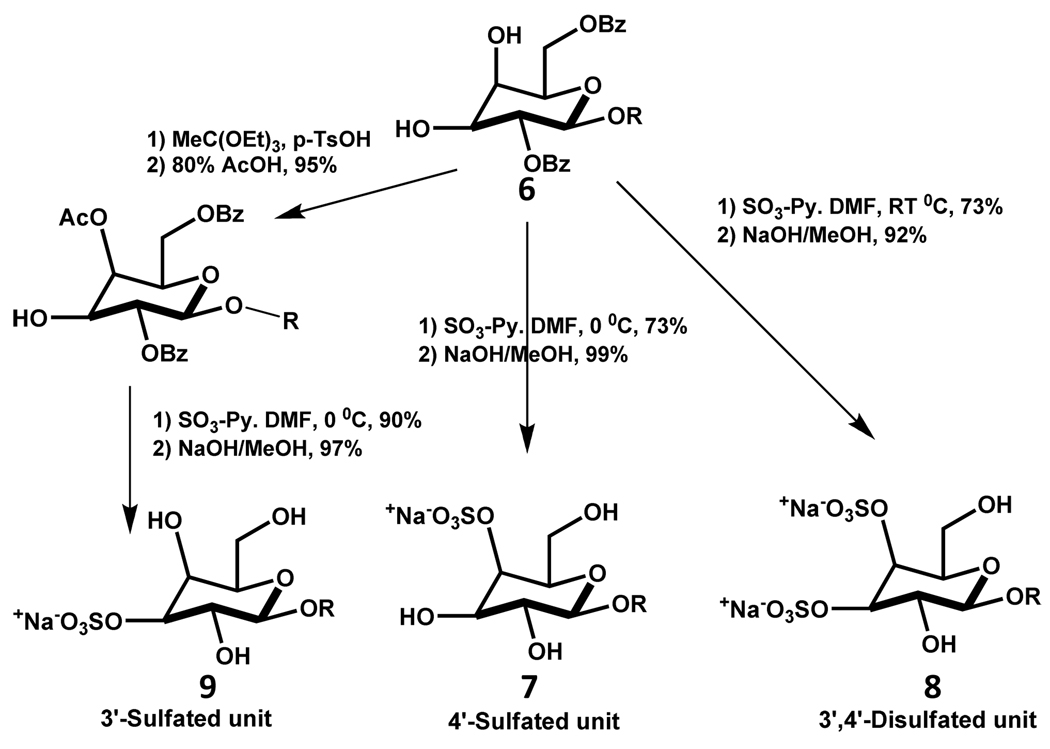
Likewise, regioselective synthesis of 3’-sulfated sialyl Lewisx from the corresponding 3’,4’-diol trisaccharide 6 was found to be temperature dependent (Scheme 13). When diol 6 was sulfated with SO3-Py complex in DMF for one hour at 0°C, followed by sodium methoxide treatment in methanol, the corresponding 4’-sulfated product 7 was obtained in overall yield of about 73%. On the other hand, when 6 sulfated at room temperature, the product was found to be 3’,4’-disulfate 8 in an overall yield of 66%. To regioselectively synthesize the 3’-sulfated derivative 9 an extra protection step was introduced to block the 4’-position, followed by sulfation at 0°C and deblocking of the 4-protecting group (Scheme 13).82
Scheme 13.
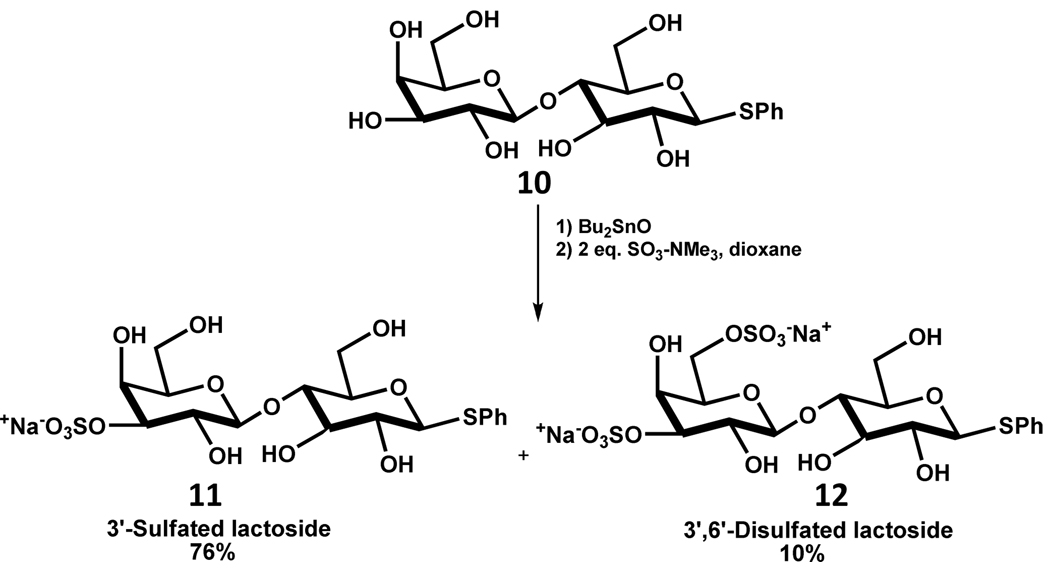
Guilbert et al. report an interesting example of regioselective sulfation in synthesis of sulfated oligosaccharides via the formation of stannyl complexes.83 The preferential formation of stannyl complexes by cis diols was exploited to introduce sulfate groups in sterically less accessible positions. Thiophenyl lactoside 10 was converted to the stannylene acetal with dibutyltin oxide, which was followed by sulfation with SO3-NMe3 complex in dioxane to yield 3’-sulfated lactoside 11 and 3’,6’-disulfated lactoside 12 in 76 and 10% yields, respectively (Scheme 14). In the absence of the stannyl complex, 6 and 6’ mono- and di-sulfated esters were formed indicating the propensity for the normal reaction to primarily sulfate sterically unhindered groups. This selective method was also useful in chemosynthetic synthesis of 3’-sulfo N-acetyl lactosaminide. Interestingly, the regioselectivity changed with maltoside, which preferentially gave the 2’-sulfated derivative. The regioselectivity apparently arises from the increased reactivity of the 2’-hydroxyl group. In addition, the C1’–C2’ cis dioxy configuration next to the α-glycosidic linkage appears to contribute as well because the β-glycosidic linkage in the lactoside seems to favor 3’-sulfation.57,83
Scheme 14.
Dibutyltin oxide has found additional uses in selective 2-O-sulfation of α-l-iduronate glycosides, which are used as substrates in the assay of iduronate-2-sulfatase, an enzyme known to play an important role in Hunter’s syndrome.57 The selective 2-O-sulfation of α-l-iduronate is consistent with the previous reports of selective 2-O-benzoylation and acetylation of monosaccharides and disaccharides, respectively, upon treating with Bu2SnO. Such treatment resulted in the formation of 2’,4’-stannylene acetals, which facilitated regioselective benzoylation and acetylation at the 2’-position by the corresponding chlorides.84,85 As mentioned before, this emphasizes the enhanced reactivity of the 2 position in α-glycosides. Finally, selective 4-O-sulfation of xylopyranoside, xylobioside and xylotrioside using SO3-NMe3 has been reported.86 Several reports have proposed the mechanism of dibutyltin oxide-mediated regioselective reactivity of xylopyranosides towards electrophilic reagents. However, further work is necessary to more accurately arrive at a satisfactory mechanism for the preferential recognition in these substrates for the reaction to be more generally applicable.
2.7 Miscellaneous Approaches
Although S- and P-sulfations are not common in synthetic practice, they can be achieved by essentially the same protocols discussed above for O- and N-sulfations. For instance, thiosulfate esters can be generally prepared by the reaction of thiol with a SO3 complex under anhydrous conditions87 or to lesser extent with H2SO4/DCC.88 Considering the synthesis of sulfatophosphates, aryl sulfatophosphates are relatively simple to prepare and have been obtained by the sulfation of the appropriate aryl phosphoric acid in anhydrous solution with SO3-Py89 or SO3-DMF.90 On the other hand, the preparation of nucleoside sulfatophosphates is more difficult because of the likelihood of sulfating the hydroxyl groups of the sugar moiety. Adenosine-5’-sulfatophosphate has been made by sulfation of its parent with SO3-Py in aqueous medium (5% yield),91 with SO3-NEt3 in anhydrous conditions (60–75% yield)92 and also with H2SO4/DCC (20–25% yield), which gave extensive sulfation of the sugar hydroxyl groups.93
In certain cases, the sensitivity of the scaffold under study and the availability of the reagents urge the invention of new protocol or reagent. For example, some scaffolds have been sulfated using pyridinium sulfate/acetic anhydride.94 Ascorbate-2-sulfate-mediated oxidative sulfation95 and Cu2+-catalyzed sulfation have been reported for synthesis of some sulfated steroids and carbohydrates.96 Finally, most of the above methods introduce a sulfate group on an existing –OH group. In contrast, the Elb’s persulfate oxidation introduces a sulfate group directly onto an aromatic ring by treatment with peroxydisulfate under alkaline conditions. The oxidation of phenols has been extensively studied and most probably involves a nucleophilic attack of p-carbanion resonance form of the phenoxide on the peroxide bond in the peroxydisulfate ion.38
3. Summary and Significance
Highly sulfated GAGs, sulfated steroids and sulfated tyrosine containing peptide sequences play important roles in the large number of physiological and pathological processes. These include morphogenesis, immune response, coagulation, angiogenesis, viral invasion and several others.8–19,97,98 In each of these processes the interaction of a critical protein with an appropriately positioned sulfate group typically drives the signal. A corollary of this observation is that the scaffold positioning the sulfate group(s) is of much less importance. Thus, in principle each of these processes could be regulated by an appropriately designed sulfated antagonist or agonist. This sulfated agonist or antagonist does not have to be GAG- or peptide- based and may advantageously be a small aromatic structure.
The field of sulfated, small, aromatic mimetics of highly sulfated GAGs has put forward some interesting initial results.27–32,73 Yet, the difficulties associated with chemical sulfation highlighted in the current review and the absence of large chemical library of structurally diverse, sulfated aromatic molecules are the major challenges in discovering new drugs targeting the role of sulfated biomolecules.
This review attempts to bring together the current knowledge on chemical sulfation of small scaffolds. As evident from the review, a large number of methods have been developed for this simple reaction, yet none is uniformly applicable with high consistency. Additionally, the primary restriction chemical sulfation places on library construction, especially of structurally diverse – type, is insolubility of products in traditional organic media. This limits functional group transformations following sulfation. Alternatively, it forces construction of scaffolds to include chemical sulfation as the final step. Recent developments that afford manipulation of functional groups in organic media with a masked sulfate functionality (Section C) hold considerable promise, but more robust protecting groups may be necessary to break the cocoon surrounding this important chemical transformation.
Acknowledgements
This work was supported by the grants HL090586 and HL099420 from the National Institutes of Health, grant EIA 0640053N from the American Heart Association National Center, grant 6-46064 from the A. D. Williams Foundation and a grant from the Mizutani Foundation for Glycoscience, Japan.
Abbreviations
- SO3−
Sulfonate
- PAPS
3’-Phosphoadenosine-5’-phosphosulfate
- PSGL-1
P-Selectin glycoprotein ligand-1
- CCR5
Chemokine receptor-5
- HIV-1
Human immunodeficiency virus-1
- H/HS
Heparin/Heparan sulfate
- -OSO3−
Sulfate
- DEFGH
High affinity pentasaccharide sequence of heparin
- H2SO4
Sulfuric acid
- CCl4
Carbon tetrachloride
- CH2Cl2
Dichloromethane
- H2NSO3H
Sulfamic acid
- DCC
N,N’-Dicyclohexylcarbodiimide
- APTT
Activated partial thromboplastin time
- SO2
Sulfur dioxide
- DEAE
Diethylaminoethyl
- THF
Tetrahydrofuran
- SO3
Sulfur trioxide
- Py
Pyridine
- Me3N
Trimethylamine
- Et3N
Triethylamine
- DMF
N,N-Dimethylformamide
- TCE
2,2,2-Trichloroethyl
- TCECS
2,2,2-Trichloroethyl chlorosulfate
- ZnCl2
Zinc chloride
- PhBCl2
Phenyl boron dichloride
- Et3SiH
Triethyl silane
- DBU
1,8-Diazabicyclo[5.4.0]undec-7-ene
- SPPS
Solid-phase peptide synthesis
- Fmoc
9-Fluorenyl-methoxycarbonyl
- DCV
Dichlorovinyl
- Pd/C
Palladium-activated carbon
- H2
Hydrogen gas
- Boc
tert-Butoxy carbonyl
- Cbz
Benzyloxy carbonyl
- NaI
Sodium iodide
- TBDMS
tert-Butyl dimethyl silyl
- GAG’s
Glycosaminoglycans
- eq.’
equivalent
- TfOH
Triflic acid
- EtOH
Ethanol
- Pt. oxide
Platinum oxide
- DMAP
4-Dimethylaminopyridine
- EtOAC
Ethyl acetate
- AcOH
Acetic acid
- PhB(OH)2
Phenyl boronic acid
- p-TsOH
p-Toluenesulfonic acid
- MeC(OEt)3
1,1,1-Trimethoxy ethane
- Bu2SnO
Diutyltin oxide
- rt
Room temperature
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Roy AB. Trends Biochem. Sci. 1976;1:N233–N234. [Google Scholar]
- 2.Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Tallman MN, Brouwer KL. Eur. J. Pharm. Sci. 2006;27:447–486. doi: 10.1016/j.ejps.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Falany CN. FASEB J. 1997;11:206–216. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- 4.Bowman KG, Bertozzi CR. Chem. Biol. 1999;6:R9–R22. doi: 10.1016/S1074-5521(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 5.Grunwell JR, Bertozzi CR. Biochemistry. 2002;41:13117–13126. doi: 10.1021/bi020507h. [DOI] [PubMed] [Google Scholar]
- 6.Kitayama K, Hayashida Y, Nishida K, Akama TO. J. Biol. Chem. 2007;282:30085–30096. doi: 10.1074/jbc.M703695200. [DOI] [PubMed] [Google Scholar]
- 7.Sperandio M. FEBS. J. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- 8.Pouyani T, Seed B. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 9.Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. Cell. 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins PP, Moore KL, McEver RP, Cummings RD. J. Biol. Chem. 1995;270:22677–22680. doi: 10.1074/jbc.270.39.22677. [DOI] [PubMed] [Google Scholar]
- 11.Stone MJ, Chuang S, Hou X, Shoham M, Zhu JZ. N. Biotechnol. 2009;25:299–317. doi: 10.1016/j.nbt.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Choe H, Farzan M. Chapter 7. Methods Enzymol. 2009;461:147–170. doi: 10.1016/S0076-6879(09)05407-X. [DOI] [PubMed] [Google Scholar]
- 13.Lindahl U. Thromb. Haemost. 2007;98:109–115. [PubMed] [Google Scholar]
- 14.Bishop JR, Schuksz M, Esko JD. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 15.Olson ST, Björk I, Bock SC. Trends Cardiovasc. Med. 2002;12:198–205. doi: 10.1016/s1050-1738(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 16.Petitou M, van Boeckel CA. Angew. Chem. Int. Ed. Engl. 2004;43:3118–3133. doi: 10.1002/anie.200300640. [DOI] [PubMed] [Google Scholar]
- 17.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 18.Copeland R, Balasubramaniam A, Tiwari V, Zhang F, Bridges A, Linhardt RJ, Shukla D, Liu J. Biochemistry. 2008;47:774–5783. doi: 10.1021/bi800205t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobkirk R. Trends Endocrinol. Metab. 1993;4:69–74. doi: 10.1016/s1043-2760(05)80018-9. [DOI] [PubMed] [Google Scholar]
- 20.Olson ST, Halvorson HR, Björk I. J. Biol. Chem. 1991;266:6342–6352. [PubMed] [Google Scholar]
- 21.Olson ST, Björk I. J. Biol. Chem. 1991;266:6353–6364. [PubMed] [Google Scholar]
- 22.Olson ST, Björk I, Sheffer R, Craig PA, Shore JD, Choay J. J. Biol. Chem. 1992;267:12528–12538. [PubMed] [Google Scholar]
- 23.Desai UR, Petitou M, Björk I, Olson ST. J. Biol. Chem. 1998;273:7478–7487. doi: 10.1074/jbc.273.13.7478. [DOI] [PubMed] [Google Scholar]
- 24.Desai UR, Petitou M, Björk I, Olson ST. Biochemistry. 1998;37:13033–13041. doi: 10.1021/bi981426h. [DOI] [PubMed] [Google Scholar]
- 25.Tamura J, Nishihara J. Bioorg. Med. Chem. Lett. 1999;9:1911–1914. doi: 10.1016/s0960-894x(99)00300-5. [DOI] [PubMed] [Google Scholar]
- 26.Lam SN, Acharya P, Wyatt R, Kwong PD, Bewley CA. Bioorg. Med. Chem. 2008;16:10113–10120. doi: 10.1016/j.bmc.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunnarsson GT, Desai UR. J. Med. Chem. 2002;45:1233–1243. doi: 10.1021/jm020012q. [DOI] [PubMed] [Google Scholar]
- 28.Gunnarsson GT, Desai UR. Bioorg. Med. Chem. Lett. 2003;13:679–683. doi: 10.1016/s0960-894x(02)01055-7. [DOI] [PubMed] [Google Scholar]
- 29.Verghese J, Liang A, Sidhu PP, Hindle M, Zhou Q, Desai UR. Bioorg. Med. Chem. Lett. 2009;19:4126–4129. doi: 10.1016/j.bmcl.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghuraman A, Liang A, Krishnasamy C, Lauck T, Gunnarsson GT, Desai UR. Eur. J. Med. Chem. 2009;44:2626–2631. doi: 10.1016/j.ejmech.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monien BH, Henry BL, Raghuraman A, Hindle M, Desai UR. Bioorg. Med. Chem. 2006;14:7988–7998. doi: 10.1016/j.bmc.2006.07.066. [DOI] [PubMed] [Google Scholar]
- 32.Henry BL, Connell J, Liang A, Krishnasamy C, Desai UR. J. Biol. Chem. 2009;284:20897–20908. doi: 10.1074/jbc.M109.013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovensky J. Curr. Med. Chem. 2009;16:2338–2344. doi: 10.2174/092986709788453096. [DOI] [PubMed] [Google Scholar]
- 34.Raghuraman A, Riaz M, Hindle M, Desai UR. Tetrahedron Lett. 2007;48:6754–6758. doi: 10.1016/j.tetlet.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jandik KA, Kruep D, Cartier M, Linhardt RJ. J. Pharm. Sci. 1996;85:45–51. doi: 10.1021/js9502736. [DOI] [PubMed] [Google Scholar]
- 36.Liang A, Thakkar JN, Desai UR. J. Pharm. Sci. 2009 doi: 10.1002/jps.21908. (in press) [DOI] [PubMed] [Google Scholar]
- 37.Dantuluri M, Gunnarsson GT, Riaz M, Nguyen H, Desai UR. Anal. Biochem. 2005;336:316–322. doi: 10.1016/j.ab.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert EE. Sulfonation and related reactions. New York: Interscience Publishers; Chapters 1 &6; p. 1965. [Google Scholar]
- 39.Deno NC, Newman MS. J. Am. Chem. Soc. 1950;72:3852–3856. [Google Scholar]
- 40.Gilbert EE. Chem. Rev. 1962;62:549–589. [Google Scholar]
- 41.Whistler RL, Spencer WW. Methods in carbohydrate chemistry. Vol. 3. New York: Academic Press; 1963. pp. 265–267. [Google Scholar]
- 42.Gilbert EE, Veldhuis B. J. Am. Oil Chemist’s Soc. 1960;37:298–300. [Google Scholar]
- 43.Mumma RO. Lipids. 1966;1:221–223. doi: 10.1007/BF02531876. [DOI] [PubMed] [Google Scholar]
- 44.Hoiberg CP, Mumma RO. J. Am. Chem. Soc. 1969;91:4273–4278. [Google Scholar]
- 45.Takano R, Ueda T, Uejima Y, Kamei-Hayashi K, Hara S, Hirase S. Biosci. Biotech. Biochem. 1992;56:1413–1416. doi: 10.1271/bbb.56.1413. [DOI] [PubMed] [Google Scholar]
- 46.Nakahara T, Waki M, Uchimura H, Hirano M, Kim JS, Matsumoto T, Nakamura K, Ishibashi K, Hirano H, Shiraishi A. Anal. Biochem. 1986;154:194–199. doi: 10.1016/0003-2697(86)90514-2. [DOI] [PubMed] [Google Scholar]
- 47.Maraganore JM, Chao B, Joseph ML, Jablonski J, Ramachandran KL. J. Biol. Chem. 1989;264:8692–8698. [PubMed] [Google Scholar]
- 48.Mumma RO, Fujitani K, Hoiberg CP. J. Chem. Eng. Data. 1970;15:358–359. [Google Scholar]
- 49.Mumma RO, Hoiberg CP. J. Chem. Eng. Data. 1971;16:492–494. [Google Scholar]
- 50.Nair V, Bernstein S. Orgn. Prepr. Proc. Int. Briefs. 1987;19:466–467. [Google Scholar]
- 51.Dusza JP, Joseph JP, Bernstein S. Steroids. 1985;45:303–315. doi: 10.1016/0039-128x(85)90079-0. [DOI] [PubMed] [Google Scholar]
- 52.Kitagawa K, Aida C, Fujiwara H, Yagami T, Futaki S, Kogire M, Ida J, Inoue K. J. Org. Chem. 2001;66:1–10. doi: 10.1021/jo000895y. [DOI] [PubMed] [Google Scholar]
- 53.Lee J-C, Lu X-A, Kulkarni SS, Wen Y-S, Hung S-C. J. Am. Chem. Soc. 2004;126:476–477. doi: 10.1021/ja038244h. [DOI] [PubMed] [Google Scholar]
- 54.Tully SE, Mabon R, Gama CI, Tsai SM, Liu X, Hsieh-Wilson LC. J. Am. Chem. Soc. 2004;126:7736–7737. doi: 10.1021/ja0484045. [DOI] [PubMed] [Google Scholar]
- 55.Young T, Kiessling LL. Angew. Chem., Int. Ed. 2002;41:3449–3451. doi: 10.1002/1521-3773(20020916)41:18<3449::AID-ANIE3449>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 56.Kakiyama G, Muto A, Shimada M, Mano N, Goto J, Hofmann AF, Iida T. Steroids. 2009;74:766–772. doi: 10.1016/j.steroids.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Blanchard S, Turecek F, Gelb MH. Carbohydr. Res. 2009;344:1032–1033. doi: 10.1016/j.carres.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dusza JP, Joseph JP, Bernstein S. Steroids. 1968;12:49–61. doi: 10.1016/s0039-128x(68)80079-0. [DOI] [PubMed] [Google Scholar]
- 59.Comin MJ, Maiser MS, Roccatagliata AJ, Pujol CA, Damonte EB. Steroids. 1999;64:335–340. doi: 10.1016/s0039-128x(99)00016-1. [DOI] [PubMed] [Google Scholar]
- 60.Wessel HP, Bartsch S. Carbohydr. Res. 1995;274:1–9. doi: 10.1016/0008-6215(95)00131-c. [DOI] [PubMed] [Google Scholar]
- 61.Fujii N, Futaki S, Funakoshi S, Akaji K, Morimoto H, Doi R, Inoue K, Kogire M, Sumi S, Yun M, Tobe T, Aono M, Matsuda M, Narusawa H, Moriga M, Yajima H. Chem. Phar. Bull. 1988;36:3281–3291. doi: 10.1248/cpb.36.3281. [DOI] [PubMed] [Google Scholar]
- 62.Futaki S, Taike T, Yagami T, Ogawa T, Akita T, Kitagawa K. J. Chem. Soc. Perk. Trans. I. 1990:1739–1744. [Google Scholar]
- 63.Popek T, Lis T. Carbohydr. Res. 2002;337:787–801. doi: 10.1016/s0008-6215(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 64.Peat S, Bowker DM, Turvey JR. Carbohydr. Res. 1968;7:225–231. [Google Scholar]
- 65.Petitou M, Duchaussoy P, Lederman I, Choay J, Jacquinet J-C, Sinaÿ P, Torri G. Carbohydr. Res. 1987;167:67–75. doi: 10.1016/0008-6215(87)80268-9. [DOI] [PubMed] [Google Scholar]
- 66.Sinaÿ P, Jacquinet J-C, Petitou M, Duchaussoy P, Lederman I, Choay J, Torri G. Carbohydr. Res. 1984;132:C5–C9. doi: 10.1016/s0008-6215(00)90633-5. [DOI] [PubMed] [Google Scholar]
- 67.Chen C, Yu B. Bioorg. Med. Chem. Lett. 2009;19:3875–3879. doi: 10.1016/j.bmcl.2009.03.155. [DOI] [PubMed] [Google Scholar]
- 68.Krylov VB, Ustyuzhanina NE, Grachev AA, Nifantiev NE. Tetrahedron Lett. 2008;49:5877–5879. [Google Scholar]
- 69.Penney CL, Perlin AS. Carbohydr. Res. 1981;93:241–246. [Google Scholar]
- 70.Kerns RJ, Linhardt RJ. Synth. Commun. 1996;26:2671–2680. [Google Scholar]
- 71.Proud AD, Prodger JC, Flitsch SL. Tetrahedron Lett. 1997;38:7243–7246. [Google Scholar]
- 72.Liu Y, Lien I-FF, Ruttgaizer S, Dove P, Taylor SD. Org. Lett. 2004;6:209–212. doi: 10.1021/ol036157o. [DOI] [PubMed] [Google Scholar]
- 73.Gunnarsson TG, Riaz M, Adams J, Desai UR. Bioorg. Med. Chem. 2005;13:1783–1789. doi: 10.1016/j.bmc.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 74.Ingram LJ, Taylor SD. Angew. Chem., Int. Ed. 2006;45:3503–3506. doi: 10.1002/anie.200600153. [DOI] [PubMed] [Google Scholar]
- 75.Ingram LJ, Desoky A, Ali AM, Taylor S. J. Org. Chem. 2009;74:6479–6485. doi: 10.1021/jo9014112. [DOI] [PubMed] [Google Scholar]
- 76.Ali AM, Taylor SD. Angew. Chem., Int. Ed. 2009;84:2024–2026. doi: 10.1002/anie.200805642. [DOI] [PubMed] [Google Scholar]
- 77.Simpson LS, Widlanski TS. J. Am. Chem. Soc. 2006;128:1605–1610. doi: 10.1021/ja056086j. [DOI] [PubMed] [Google Scholar]
- 78.Gao Y, Sharpless KB. J. Am. Chem. Soc. 1988;110:7538–7539. [Google Scholar]
- 79.Huibers M, Manuzi I, Rutjes FPJT, van Delft FL. J. Org. Chem. 2006;71:7473–7476. doi: 10.1021/jo060404v. [DOI] [PubMed] [Google Scholar]
- 80.Hungerford NL, McKinney AR, Stenhouse AM, Mcleod MD. Org. Biomol. Chem. 2006;4:3951–3959. doi: 10.1039/b610499a. [DOI] [PubMed] [Google Scholar]
- 81.Richter A, Klemm D. Cellulose. 2003;10:133–138. [Google Scholar]
- 82.Tsukida T, Yoshida M, Kurokawa K, Nakai Y, Achiha T, Kiyoi T, Kondo H. J. Org. Chem. 1997;62:6876–6881. [Google Scholar]
- 83.Guilbert B, Davis NJ, Flitsch SL. Tetrahedron Lett. 1994;35:6563–6566. [Google Scholar]
- 84.Gavard O, Hersant Y, Alais J, Duverger V, Dilhas A, Bascou A, Bonnaffe D. Eur. J. Org. Chem. 2003:3603–3620. [Google Scholar]
- 85.de Paz J-L, Ojeda R, Reichardt N, Lomas-Martin M. Eur. J. Org. Chem. 2003:3308–3324. [Google Scholar]
- 86.Abado-Romero B, Mereiter K, Sixta H, Hofinger A, Kosma P. Carbohydr. Res. 2009;344:21–28. doi: 10.1016/j.carres.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 87.Kice JL, Anderson JM, Pawlowski NE. J. Am. Chem. Soc. 1966;88:5245–5250. [Google Scholar]
- 88.Mumma RO, Fujitani K, Hoiberg CP. J. Chem. Eng. Data. 1970;15:358–359. [Google Scholar]
- 89.Benkovic SJ, Hevey RC. J. Am. Chem. Soc. 1970;92:4971–4977. doi: 10.1021/ja00719a034. [DOI] [PubMed] [Google Scholar]
- 90.Tagaki W, Eiki T, Tanaka I. Bull. Chem. Soc. 1971;44:1139–1141. [Google Scholar]
- 91.Baddiley J, Buchanan JG, Letters R. J. Chem. Soc. 1957:1067–1071. [Google Scholar]
- 92.Cherniak R, Davidson EA. J. Biol. Chem. 1964;239:2986–2990. [PubMed] [Google Scholar]
- 93.Reichard P, Ringertz NR. J. Am. Chem. Soc. 1959;81:878–883. [Google Scholar]
- 94.Levitz M. Steroids. 1963;1:117–120. [Google Scholar]
- 95.Mumma RO. Biochem. Biophys. Acta. 1968;165:571–573. doi: 10.1016/0304-4165(68)90247-x. [DOI] [PubMed] [Google Scholar]
- 96.Nagasawa K, Yoshidome H. J. Org. Chem. 1974;39:1681–1685. [Google Scholar]
- 97.Gandhi NS, Mancera RL. Chem. Biol. Drug. Des. 2008;72:455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 98.Capila I, Linhardt RJ. Angew. Chem. Int. Ed. Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]