Abstract
It has been shown that compounds containing the p-N,N,-dialkylaminobenzylidene cyanoacetate motif can serve as fluorescent non-mechanical viscosity sensors. These compounds, referred to as molecular rotors, belong to a class of fluorescent probes that are known to form twisted intramolecular charge-transfer complexes in the excited state. In this study we present the synthesis and spectroscopic characterization of these compounds as viscosity sensors. The effects of the molecular structure and electronic density of these rotors to the emission wavelength, fluorescence intensity and viscosity sensitivity are discussed.
1. Introduction
Variations in fluid viscosity are associated with a variety of functions and diseases both at the cellular1 and organismal level.2 For instance, cell membrane viscosity depends on the chemical composition of the bilayer and must have optimum values for the proper function of various membrane bound enzymes and receptors.3 Consequently, changes of membrane viscosity affect cellular signaling pathways and are associated with a variety of disorders, such as cardiovascular disease,4 cell malignancy,5 and Alzheimer’s disease.6 In a similar fashion, changes in the viscosity of blood, plasma or lymphatic fluids have been linked to diabetes,7 hypertension,8 infarction9 and aging.10
The importance of membrane viscosity in cellular biology and physiology led to the development of several methods for quantitative measurements. Among them are included mechanical methods, where the lipids and proteins are tested in a cone-and-plate viscometer or a capillary viscometer.11 However, these methods are limited by the large sample size and are not effective in measuring real-time changes.12 Alternatively, fluorescence-based methods benefit from the rapid response time and good spatial resolution of the fluorescent probes.13 Techniques based on fluorescence anisotropy14 (FA) and fluorescence recovery after photobleaching15 (FRAP) are commonly used in biological measurements but suffer from the need of specialized instruments, high energy light and limited spatial resolution.16 An alternative method for measuring viscosity is based on the use of a special group of environment-sensitive fluorescent probes.17 These probes, referred to as molecular rotors, are known to form twisted intramolecular charge transfer (TICT) complexes in the excited state producing a fluorescence quantum yield that is dependent on the surrounding environment.18 The chemical structure of the molecular rotors contains an electron donor unit in conjugation with an electron acceptor unit (D-π-A motif). Following photoexcitation, this motif has the unique ability to relax either via fluorescence emission or via an internal non-radiative molecular rotation. This internal rotation occurs around the σ-bonds that connect the electronically rich π-system with the donor and acceptor groups and can be modulated by the microenvironment of the probe.19 For instance, if such rotation is hindered due to the high viscosity of their microenvironment, the relaxation occurs via an increased fluorescence emission. In contrast, in solvents of low viscosity the relaxation proceeds mainly via a non-radiative pathway. Overall, this property results in a fluorescence emission whose quantum yield is proportional to the viscosity of the environment.
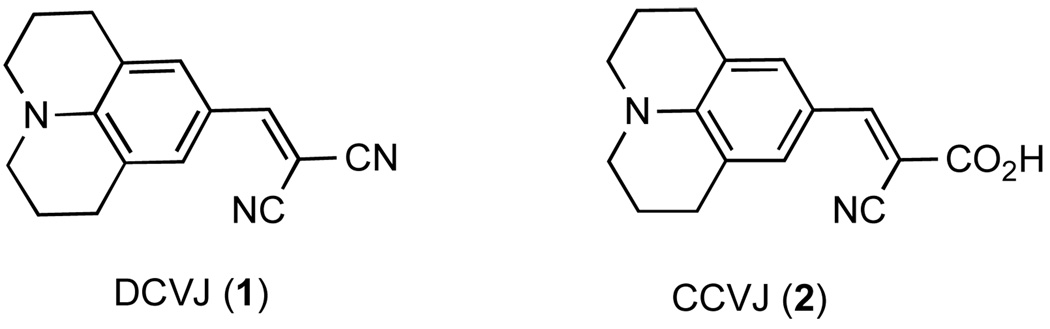
Figure 1 shows the chemical structures of two commonly used molecular rotors based on the julolidine scaffold: 9-(dicyanovinyl)-julolidine (DCVJ)20 and 9-(2-carboxy-2-cyano)vinyl-julolidine (CCVJ).21 TICT formation takes place by photoinduced electron transfer from the julolidine nitrogen to one of the nitrile groups with subsequent intramolecular rotation around the julolidine–vinyl bond. Over the past years we have been exploring the applicability of molecular rotors, containing the N,N-dialkylaniline motif,22 as sensors for various biological and engineering applications.23 The subject of this investigation was to evaluate whether and how changes in the chemical structure of the molecular rotors can change the emission wavelength, fluorescence intensity and sensitivity of the viscosity measurements.
Figure 1.
Representative structures of molecular rotors based on the julolidine scaffold.
2. Results
Synthesis of molecular rotors
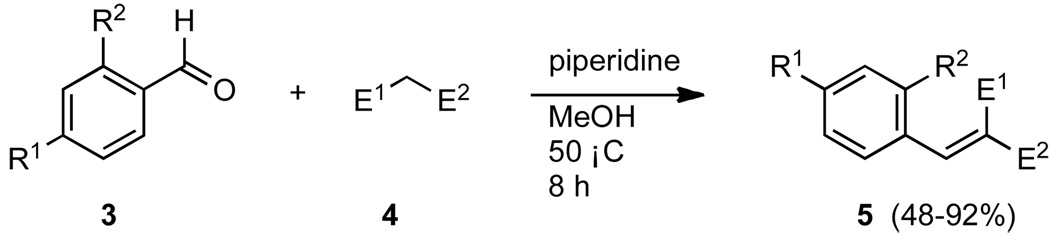
The synthesis of all molecular rotors was accomplished in one step by Knoevenagel condensation of 1 equivalent of the appropriate aldehyde with 1.1 equivalents of malonic acid derivative 4. This reaction was catalyzed by piperidine (10%) and was completed within 8 hours.24 The synthesis of rotors based on the phenyl scaffold is illustrated in Scheme 1. In most cases, the desired product 5 crystallized out of methanol (Table 1).
Scheme 1.
Reagents and conditions: 1.0 equiv 3, 1.1 equiv 4, 0.1 equiv piperidine, MeOH, 50 °C, 8 h.
Table 1.
Structures and yields of rotors 5a-5m
| Cmp # | R1 | R2 | E1 | E2 | Yield (%) |
|---|---|---|---|---|---|
| 5a | OMe | H | CN | CO2Me | 84 |
| 5b | NH2 | H | CN | CO2Me | 87 |
| 5c | NH2 | H | CN | CN | 65 |
| 5d | NMe2 | H | CN | CO2Me | 89 |
| 5e | NMe2 | H | CN | CN | 91 |
| 5f | NMe2 | H | CN | SO2Ph | 92 |
| 5g | NMe2 | H | CO2Me | CO2Me | 17 |
| 5h | NMe2 | OMe | CN | SO2Ph | 81 |
| 5i | NMe2 | OMe | CN | CO2Me | 89 |
| 5j | NMe2 | Me | CN | CO2Me | 86 |
| 5k | NEt2 | H | CN | CO2Me | 67 |
| 5l | NMe2 | H | SO2Ph | SO2Ph | 7 |
| 5m | NBu2 | H | CN | CO2Me | 72 |
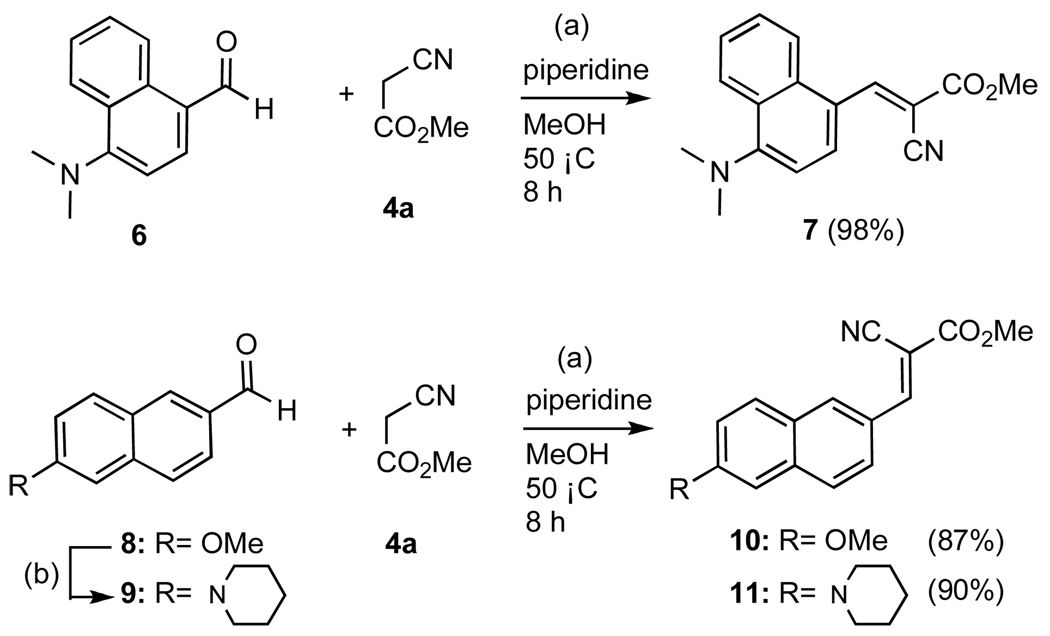
The synthesis of molecular rotors based on the naphthalene scaffold is illustrated in Scheme 2. The p-substituted naphthaldehyde 9 was prepared by treatment of commercially available methoxy naphthaldehyde 8 with 8 equivalents of lithiated piperidine.25 Condensation of 6, 8 and 9 with methyl 2-cyanoacetate (4a) afforded rotors 7, 10 and 11 respectively in excellent yields.
Scheme 2.
Reagents and conditions: (a) 1.0 equiv aldehyde, 1.1 equiv α-cyanomethylester, 0.1 equiv piperidine, MeOH, 50 °C, 8h; (b) 8.0 equiv piperidine, benzene/HMPA: 1/1, 0 °C, 8.0 equiv nBuLi, 0 °C, 15 min; then 1.0 equiv aldehyde, 25 °C, 12 h, 35%.
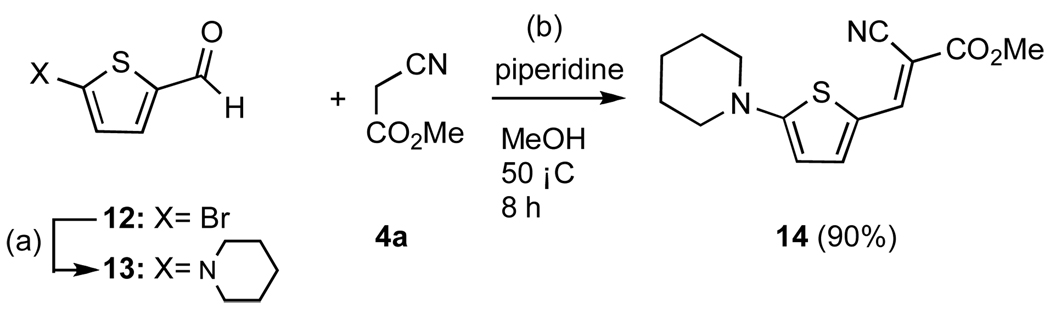
The synthesis of molecular rotor 14, in which the donor and acceptor groups are conjugated through a thiophene scaffold, is shown in Scheme 3. Commercially available aldehyde 12 was converted to the piperidine derivative 13 in 72% yield.26 Knoevenagel condensation of 13 with 4a produced 14 in 90% yield.
Scheme 3.
Reagents and conditions: (a) 1.0 equiv aldehyde, 1.0 equiv piperidine, 0.1 equiv pTsOH, toluene, 120 °C, 24 h, 72%; (b) 1.0 equiv 13, 1.1 equiv 4a, 0.1 equiv piperidine, MeOH, 50 °C, 8h, 90%.
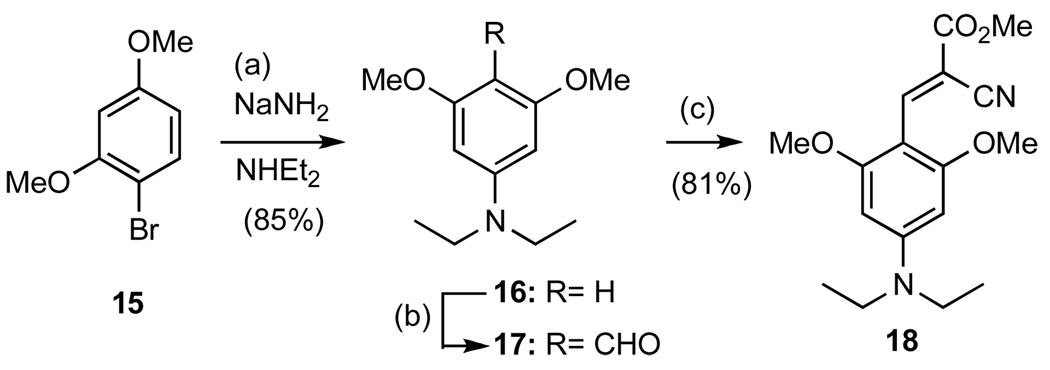
The synthesis of molecular rotor 18, containing two methoxy groups ortho to the acceptor unit, is shown in Scheme 4. Treatment of 1-bromo-2,4-dimethoxybenzene (15) with sodium amide in the presence of diethylamine produced, via the formation of an aryne intermediate, aniline 16 in 85% yield.27 Formylation of 16 under Vilsmeier conditions, followed by condensation of the resulting aldehyde 17 with 4a produced compound 18 in 53% combined yield.28
Scheme 4.
Reagents and conditions: (a) 1 equiv 15, 10 equiv NaNH2, 75 equiv NHEt2, 25 °C, 24h, 85% (b) 1.0 equiv 16, 2.0 equiv DMF, 1.1 equiv POCl3, 2M NaOH, 8h, 65%; (c) 1.0 equiv aldehyde, 1.1 equiv cyanomethylester, 0.1 equiv piperidine, MeOH, reflux, 50 °C, 8h, 81%
Spectroscopic characterization of molecular rotors
In principle a good molecular rotor should have the following properties: (a) a large Stokes shift that will provide good separation between the excitation and emission light; (b) a tunable emission wavelength that can be modified as a function of the chemical structure and can be optimized for a specific application and; (c) high brightness, which translates to a high overall emission quantum yield; and (d) high sensitivity which translates to bigger changes in emission quantum yield as a function of changes in viscosity.
As indicated above, two excited-state deactivation pathways exist for molecular rotors, the nonradiative intramolecular rotation and the fluorescent emission. The viscosity of the solvent influences the rate of the nonradiative intramolecular rotation that subsequently influences the fluorescence quantum yield.29 Förster and Hoffmann found a nonlinear relationship between fluorescent quantum yield ΦF and viscosity η as described in equation 1:30
| (equation 1) |
where x is a dye-dependent constant and C is an empirical proportionality constant. Furthermore, fluorescence emission intensity (IF) and quantum yield (ΦF) are proportional. With steady-state fluorescence, equation 1 can be reformulated as a power law equation 2:
| (equation 2) |
where the new constant αis 10C multiplied with predominantly instrument factors and dye concentration. The constants α and x can be determined experimentally by measuring the peak emission intensity in solvents of different viscosities and fitting a regression line into the logarithms of the data points. Notably, α is a comparative metric for the dye's overall brightness and the slope x is a measure for the change of the intensity with viscosity, that is, the dye's viscosity sensitivity. A possible interpretation of the regression's y-intercept α is the logarithm of the emission intensity at a viscosity of 1 mPa•sec. We therefore use the scaled y-intercept α, given in 1000 photon counts per second, as a relative metric of brightness. Finally, the Stokes shift was calculated as the difference between emission and excitation wavelength. Table 2 summarizes these properties for the synthesized rotors.
Table 2.
Fluorescent properties and viscosity profile of molecular rotors
| Cmp # | Excitation λ (nm) |
Emission λ (nm) |
Stokes shift |
Viscosity Sensitivity x |
R2 | Brightness y-intercept α |
|---|---|---|---|---|---|---|
| 1 | 470 | 503 | 33 | 0.542 | > 0.98 | 70 |
| 2 | 431 | 489 | 58 | 0.543 | > 0.99 | 147 |
| 5a[a] | 321 | 404 | 83 | 0.359 | > 0.99 | 7 |
| 5b[a] | 423 | 460 | 37 | 0.553 | > 0.99 | 50 |
| 5c[a] | 430 | 460 | 30 | 0.425 | > 0.99 | 24 |
| 5d[b] | 440 | 484 | 44 | 0.535 | > 0.99 | 85 |
| 5e | 447 | 485 | 38 | 0.538 | > 0.98 | 61 |
| 5f | 436 | 486 | 50 | 0.531 | > 0.99 | 146 |
| 5g | 393 | 485 | 92 | 0.519 | > 0.99 | 17 |
| 5h | 448 | 480 | 32 | 0.477 | >0.99 | 295 |
| 5i[a] | 450 | 480 | 30 | 0.528 | >0.99 | 99 |
| 5j | 448 | 491 | 43 | 0.614 | >0.99 | 43 |
| 5k | 443 | 486 | 43 | 0.554 | >0.99 | 127 |
| 5l | 419 | 470 | 51 | 0.440 | >0.99 | 22 |
| 5m | 443 | 488 | 45 | 0.512 | >0.99 | 200 |
| 7[a] | 466 | 532 | 66 | 0.521 | >0.99 | 18 |
| 10 | 373 | 476 | 103 | 0.403 | >0.98 | 81 |
| 11 | 447 | 598 | 151 | 0.223 | >0.99 | 822 |
| 14 | 470 | 492 | 22 | 0.517 | >0.99 | 112 |
| 18 | 433 | 461 | 28 | 0.562 | >0.99 | 11 |
average of N= 2,
average of N= 4
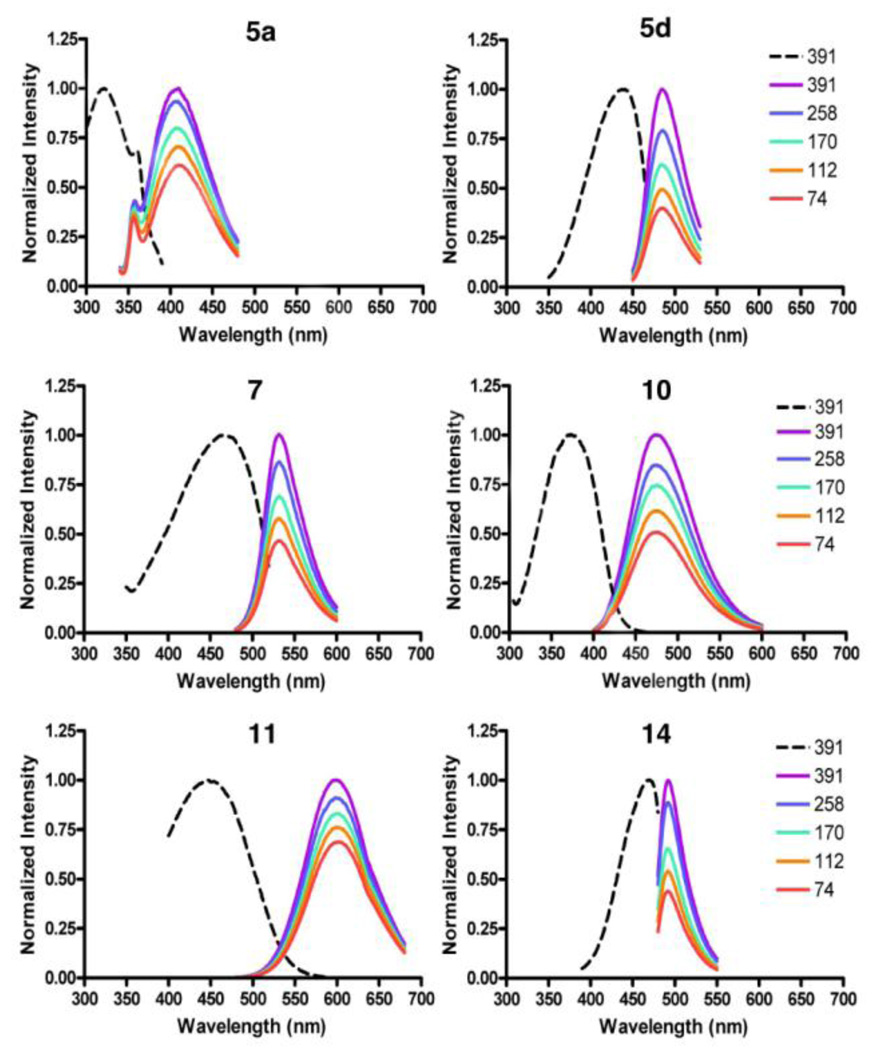
Excitation and emission spectra of selected molecular rotors are shown in Figure 2. To allow better comparison, the spectra were normalized so that the emission from the highest viscosity is unity. The dashed line represents an excitation acquisition for each derivative while solid lines are the emission spectra in different mixtures of ethylene glycol and glycerol of varying viscosity (given in mPa•sec). A high glycerol content increases the solvent viscosity leading to an increase in the quantum yield of the fluorescence emission.
Figure 2.
Normalized fluorescence excitation and emission spectra for compounds 5a, 5d, 7, 10, 11 and 14.
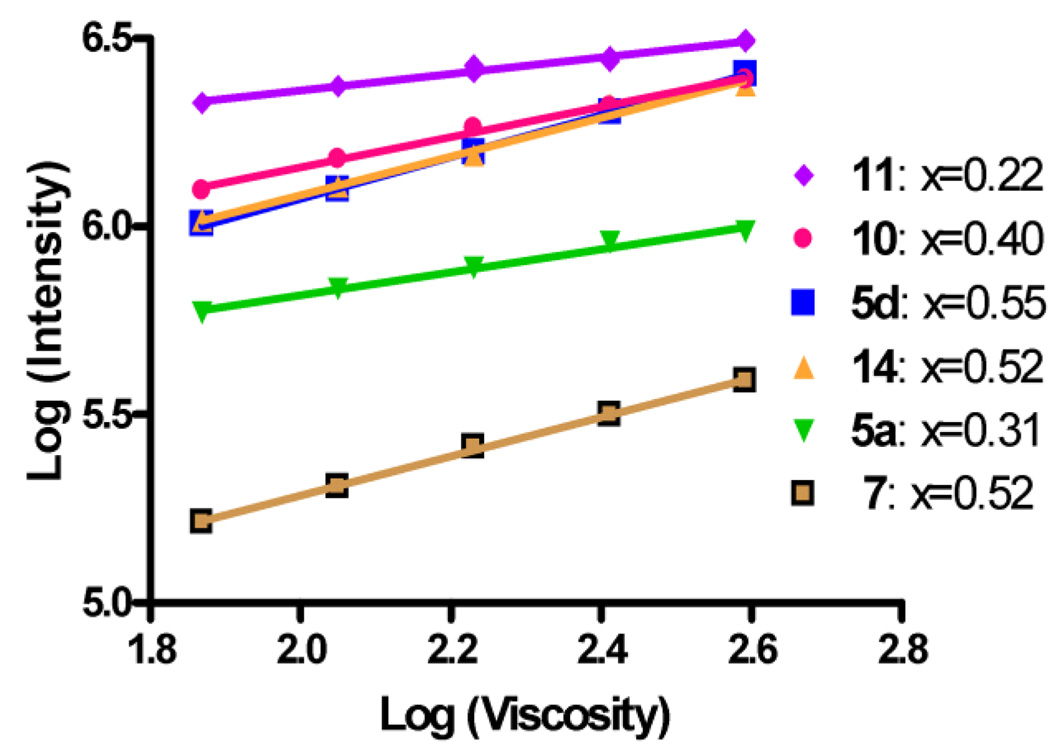
As shown in equation 2, there is a power law relationship between viscosity and emission quantum yield. Figure 3 represents peak emission intensities taken from Figure 2 and drawn over viscosity in a double-logarithmic scale. The slope and the y-intercept provide the desired metrics for sensitivity and brightness.
Figure 3.
Emission intensities of rotors 5a, 5d, 7, 10, 11 and 14 in mixtures of ethylene glycol/glycerol (emission maxima in Table 2) drawn over the viscosity of the mixtures in a double-logarithmic scale.
3. Discussion
In terms of chemistry, all rotors are characterized by a small molecular weight (less than 500), a facile one- to two-steps synthesis from commercially available materials and a good solubility in the solvents of interest. With the exception of the low yielding condensation reaction for the formation of 5g and 5l, all yields were high. Their chemical structure is defined by the presence of an electron donor group (oxygen or nitrogen) that is in conjugation with an electron acceptor group (nitrile, methyl ester of phenyl sulfone). Replacing the amino group with a weaker electron donor, such as the methoxy group,31 induces a blue shift to both the excitation and emission maxima in accordance to previous published results.32 This effect is manifested in both the phenyl (Table 2, compounds 5a, 5b, 5d) and naphthyl systems (Table 2, compounds 10, 11). Moreover, the extent of conjugation between the donor and the acceptor group influences significantly the emission wavelength and increases the Stokes shift. This effect is evident when we compare rotor 5d (emission max 460, Stoke shift= 44) with 11 (emission max= 598, Stoke shift= 151). On the other hand, the alkyl side chains of the nitrogen have no significant effect on the excitation and emission wavelengths (Table 2, compounds 5d, 5k, 5m). Similarly, minimal changes are observed between the different acceptor groups (Table 2, compounds 5d, 5e, 5f) and between differentially substituted phenyl rings (Table 2, compounds 5d, 5i, 5j).
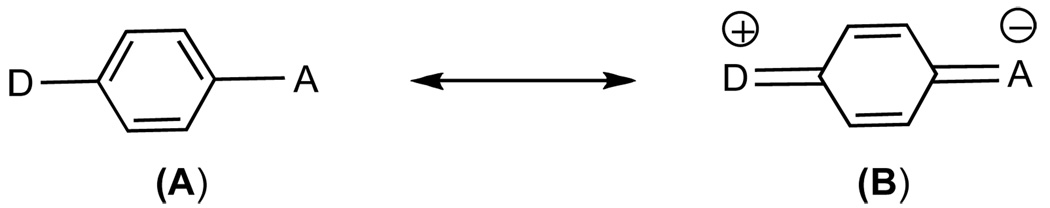
Although the size of the alkyl side chain of the nitrogen does not influence the viscosity sensitivity (all slope numbers are between 0.51 and 0.55) they do affect the brightness of the dye as shown by the y intercept data (Table 2: 5b= 50, 5d= 85, 5k= 127 and 5m= 200). Replacement of the nitrile group by a methyl ester or phenyl sulfonyl motif resulted in increase of the emission quantum yield (Table 2, compounds 5c, 5e, 5f). These results can be explained by considering the effect of these changes to the overall dipole moment of the probes. It is known that, upon excitation, the TICT probes assume a planar and dipolar quinoid structure (B), the stability of which is affected by the dipole moment and charge distribution (Figure 4). Thus, changes in the molecular structure that stabilize the excited state lead to an increase of the fluorescence intensity. It should be noted that a similar increase of fluorescence has been observed with a series of dialkylaminobenzonitriles and has been explained based on the dipole moment.33
Figure 4.
Mesomeric structures of a TICT probe. Upon photoexcitation, the ground-state structure (A) is converted to a dipolar quinoid structure (B).
An increase in the brightness is also observed when the phenyl group of 5d or 5f is functionalized with an additional methoxy substituent (rotors 5i or 5h respectively). Similarly, the more electronically rich thiophenyl group of rotor 14 is brighter than 5d.34 These results suggest that increasing the electron density between the donor and acceptor units leads to an increase of fluorescence. This electronic effect can, however, be compromised by steric effects.35 The steric congestion due to substitution at the periphery of the phenyl ring can destabilize the planar structure (B) of the excited state. Along these lines, compound 18 is among the least bright dyes despite the presence of the two electronically donating methoxy groups. Similar effects have been recorded in a series of methoxy-substituted stilbenes.35,36
It is also interesting to compare the naphthyl compounds 7 and 11. The presence of the donor and acceptor groups along the long axis of naphthalene of rotor 11 increases significantly the Stokes shift and intensity, as compared to 7. This can be explained by considering that substituents along the long axis of naphthalene decrease significantly the lowest singlet excited state.37 This results to an increase of the energy gap between that excited state and the TICT states rendering the compound more bright but less sensitive to environmental changes. On the other hand, substituents along the short axis of naphthalene, such as in rotor 7, decrease the second lowest excited state and thus increase the coupling of this state with the TICT states.37
Theoretical studies by Förster and Hoffmann30 have predicted that the maximum viscosity sensitivity of a molecular rotor is 0.66 assuming that the deexcitation of this molecule takes place predominantly via intramolecular rotation. In solvents with the viscosity range used in our study, this prerequisite is met. In most cases the viscosity sensitivity of the molecular rotors was found to be between 0.4 and 0.6. It is interesting to observe that most chemical modifications that increase the fluorescence intensity result in a decrease of the viscosity sensitivity. In fact, compound 18, one of the least bright dyes of this study, exhibits one of the highest sensitivity values. On the other hand, naphthalene-based rotor 11 is the brightest dye but has the smallest value of viscosity sensitivity. This suggests that a dye that exhibits low intensity at a low viscosity can increase its viscosity-related intensity faster, and thus have a higher sensitivity, as compared to a bright dye.
4. Conclusions
In this study we examined the effects of the chemical functionalities of molecular rotors to the fluorescence profile, brightness and viscosity sensitivity. The excitation and emission maxima for these compounds can be tuned by the extent of conjugation between the electron donor and electron acceptor groups. In addition, the brightness of these dyes can be tuned by modifying the electron density between the electron donor and electron acceptor groups and by the presence of alkyl side chains on the donor group. Moreover, chemical changes that increase the brightness of a molecular rotor, lead to a decrease of its viscosity sensitivity. The understanding of the effect of the chemical structure to the fluorescence profile should help the rational design of molecular rotors that are optimized for a specific application.
5. Experimental Section
General notes
6-methoxy-2-naphthaldehyde 8 was purchased from Alfa Aesar. The rest of the reagents were obtained (Aldrich, Acros) at highest commercial quality and used without further purification except where noted. Air- and moisture-sensitive liquids and solutions were transferred via syringe or stainless steel cannula. Organic solutions were concentrated by rotary evaporation below 45 °C at approximately 20 mmHg. All non-aqueous reactions were carried out under anhydrous conditions. Yields refer to chromatographically and spectroscopically (1H NMR, 13C NMR) homogeneous materials, unless otherwise stated. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm E. Merck silica gel plates (60F-254) and visualized under UV light and/or developed by dipping in solutions of 10% ethanolic phosphomolybdic acid (PMA) or p-anisaldehyde and applying heat. E. Merck silica gel (60, particle size 0.040-0.063 mm) was used for flash chromatography. Preparative thin-layer chromatography separations were carried out on 0.25 or 0.50 mm E. Merck silica gel plates (60F-254). NMR spectra were recorded on Varian Mercury 400 and/or Unity 500 MHz instruments and calibrated using the residual non-deuterated solvent as an internal reference. The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, b = broad. High resolution mass spectra (HRMS) were recorded on a VG 7070 HS mass spectrometer under electron spray ionization (ESI) or electron impact (EI) conditions. Fluorescence spectroscopy data were recorded on a Jobin-Yvon Fluoromax-3 instrument.
General procedure for the preparation of molecular rotors
To a round bottom flask containing a solution of aldehyde (5.0 mmol) and methyl 2-cyanoacetate (5.5 mmol) in 20 ml of methanol was added 0.50 mmol of piperidine and the mixture was heated at 50 °C. The formation of the product was monitored by TLC and was completed within 8 hours. Note that in certain cases the product precipitates out of the reaction mixture. The crude mixture was concentrated under reduced pressure and the product was collected by a vacuum filtration. Alternatively, the product was purified via flash chromatography (10–30% ethyl acetate in hexane).
(E)-methyl 2-cyano-3-(4-methoxyphenyl)acrylate (5a)
48% yield; yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.19 (s, 1H), 8.01 (d, 2H, J= 8.9 Hz), 7.00 (d, 2H, J= 8.9 Hz), 3.92 (s, 3H), 3.90 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 163.8, 163.5, 154.6, 133.6, 124.1, 116.1, 114.7, 98.6, 55.5, 53.1; HRMS calc for C12H11NO3 (M+Na)+ 240.0631 found 240.0633.
(E)-methyl 3-(4-aminophenyl)-2-cyanoacrylate (5b)
87% yield; yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.09 (s, 1H), 7.88 (d, 2H, J= 8.7 Hz), 6.69 (d, 2H, J= 8.7 Hz), 4.36 (s, 2H), 3.89 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 164.2, 154.8, 152.8, 134.1, 120.3, 117.1, 114.0, 93.7, 52.6; HRMS calc for C11H10N2O2 (M+H)+ 203.0815 found 203.0822.
2-(4-aminobenzylidene) malononitrile (5c)
65% yield; dark yellow solid; 1H NMR (400 MHz, DMSO) δ 7.97 (s, 1H), 7.72 (d, 2H, J= 8.8 Hz), 7.02 (s, 2H), 6.66 (d, 2H, J= 8.8 Hz); 13C NMR (100 MHz, DMSO) δ 159.7, 156.7, 135.1, 119.6, 117.1, 116.3, 114.3, 68.5; HRMS calc for C10H7N3 (M)+ 169.0634 found 169.0636.
(E)-methyl 2-cyano-3-(4-(dimethylamino)phenyl) acrylate (5d)
89% yield; yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.06 (s, 1H), 7.92 (d, 2H, J= 9.0Hz), 6.68 (d, 2H, J= 9.1 Hz), 3.87 (s, 3H), 3.10 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 165.0, 155.0, 153.9, 134.3, 119.5, 117.8, 111.7, 93.6, 53.0, 40.2; HRMS calc for C13H14N2O2 (M+H)+ 231.1128 found 231.1126.
2-(4-dimethylaminobenzylidene)malononitrile (5e)
91% yield; orange solid; 1H NMR (400 MHz, CDCl3) δ 7.78 (d, 2H, J= 8.8 Hz), 7.41 (s, 1H), 6.68 (d, 2H, J= 8.9 Hz), 3.14 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 158.0, 154.1, 133.7, 119.1, 116.0, 114.9, 111.5, 71.3, 40.0; HRMS calc for C12H11N3 (M+H)+ 198.1026 found 198.1024.
(E)-3-(4-(dimethylamino)phenyl)-2 (phenylsulfonyl) acrylonitrile (5f)
92% yield; orange solid; 1H NMR (400 MHz, CDCl3) δ 7.99 (s, 1H), 7.97 (s, 2H), 7.81 (d, 2H, J= 9.1 Hz), 7.62 (m, 1H), 7.55 (m, 2H), 6.65 (d, 2H, J= 9.1 Hz), 3.10 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 154.0, 151.1, 139.6, 134.0, 133.7, 129.3, 127.9, 117.6, 115.0, 111.5, 104.6, 40.0; HRMS calc for C17H16N2O2S (M+H)+ 313.1005 found 313.1004.
Dimethyl 2-(4-(dimethylamino) benzylidene) malonate (5g)
17% yield; yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.67 (s, 1H), 7.33 (d, 2H, J= 8.9 Hz), 6.63 (d, 2H, J= 9.0 Hz), 3.88 (s, 3H), 3.81 (s, 3H), 3.03 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 168.4, 165.3, 151.9, 143.5, 131.8, 119.8, 118.9, 111.5, 52.4, 52.2, 39.9; HRMS calc for C14H17O4N (M)+ 263.1155 found 263.1152.
(E)-3-(4-(dimethylamino)-2-methoxyphenyl)-2-(phenylsulfonyl) acrylonitrile (5h)
81% yield; yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.51 (s, 1H), 8.12 (d, 1H, J= 9.2 Hz), 7.97 (d, 2H, J= 7.5 Hz), 7.60 (m, 1H), 7.53 (m, 2H), 6.26 (dd, 1H, J= 2.1 Hz, J= 9.2 Hz), 5.98 (d, 1H J= 2.1 Hz), 3.89 (s, 3H), 3.11 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 162.1, 156.2, 144.8, 140.2, 133.4, 130.7, 129.2, 127.8, 115.8, 107.9, 105.3, 102.4, 92.6, 55.4, 40.2; HRMS calc for C18H18N2O3S (M+H)+ 343.1111 found 343.1110.
(E)-methyl 2-cyano-3-(4-(dimethylamino)-2-methoxyphenyl)acrylate (5i)
89% yield; orange solid; 1H NMR (400 MHz, CDCl3) δ 8.62 (s, 1H), 8.36 (d, 1H, J= 9.2 Hz), 6.31 (dd, 1H, J= 2.4 Hz, J= 9.2 Hz), 6.00 (d, 1H, J= 2.4 Hz), 3.87 (s, 3H), 3.85 (s, 3H), 3.09 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 165.5, 162.2, 155.9, 148.4, 131.2, 118.6, 109.6, 105.4, 93.1, 91.8, 55.6, 52.8, 40.4; HRMS calc for C14H16N2O3 (M+H)+ 261.1234 found 261.1235.
(E)-methyl 2-cyano-3-(4-(dimethylamino)-2-methylphenyl) acrylate (5j)
86% yield; yellow solid; 1H NMR (300 MHz, CDCl3) δ 8.44 (s, 1H), 8.41 (s, 1H), 6.57 (dd, 1H, J= 2.5 Hz, J= 9.1 Hz), 6.47 (d, 1H, J= 2.3 Hz), 3.87 (s, 3H), 3.07 (s, 6H), 2.43 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 165.3, 153.8, 151.5, 144.0, 131.3, 118.4, 118.2, 113.1, 110.1, 93.5, 53.0, 44.4, 40.2, 20.8; HRMS calc for C14H16N2O2 (M+H)+ 245.1285 found 245.1289.
(E)-methyl 2-cyano-3-(4-diethylamino-phenyl) acrylate (5k)
67% yield; brown solid; 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 1H), 7.88 (d, 2H, J= 9.1 Hz), 6.64 (d, 2H, J= 9.2 Hz), 3.84 (s, 3H), 3.42 (q, 4H, J= 7.1 Hz), 1.19 (t, 6H, J= 7.1 Hz); 13C NMR (100 MHz, CDCl3) δ 164.8, 154.4, 151.6, 134.4, 118.6, 117.7, 111.0, 92.4, 52.6, 44.7, 12.4; HRMS calc for C15H18N2O2 (M+H)+ 259.1441 found 259.1430.
4-(2,2-bis(phenylsulfonyl)vinyl)-N,N-dimethylaniline (5l)
2% yield; yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.45 (s, 1H), 8.02 (t, 4H, J= 7.5 Hz), 7.90 (d, 2H, J= 9.0 Hz), 7.43-7.59 (m, 6H), 6.61 (d, 2H, J= 9.0 Hz), 3.09 (s, 6H); 13C NMR (400 MHz, CDCl3) δ 153.6, 151.5, 141.9, 141.7, 137.5, 133.3, 133.0, 128.9, 128.8, 128.7, 128.1, 127.2, 117.0, 112.2, 40.0; HRMS calc for C22H21NO4S2 (M+H)+ 428.0985 found 428.0984.
(E)-methyl 2-cyano-3-(4-(dibutylamino)phenyl) acrylate (5m)
72% yield; orange solid; 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 7.91 (d, 2H, J= 9.1 Hz), 6.64 (d, 2H, J= 9.2 Hz), 3.88 (s, 3H), 3.36 (m, 4H), 1.60 (m, 4H), 1.37 (m, 4H), 0.97 (t, 6H, J= 7.3 Hz); 13C NMR (100 MHz, CDCl3) δ 164.9, 154.4, 152.0, 134.3, 118.6, 117.8, 111.2, 92.4, 52.6, 50.8, 29.2, 20.1, 13.8; HRMS calc for C19H26N2O2 (M+H)+ 315.2067 found 315.2056.
(E)-methyl 2-cyano-3-(4-(dimethylamino) naphthalene-1-yl) acrylate (7)
98% yield; yellow solid; 1H NMR (400 MHz, CDCl3) δ 9.07 (s, 1H), 8.51 (d, 1H, J= 8.3 Hz), 8.20 (d, 1H, J= 8.4 Hz), 8.11 (d, 1H, J= 8.4 Hz), 7.61 (m, 1H), 7.55 (m, 1H,), 7.06 (d, 1H, J= 8.3 Hz), 3.96 (s, 3H), 3.05 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 164.1, 156.7, 151.9, 134.2, 130.2, 128.0, 127.6, 126.0, 125.5, 123.3, 121.2, 117.0, 112.7, 100.0, 53.4, 44.8; HRMS calc for C17H16N2O2 (M+H)+ 281.1285 found 281.1287.
(E)-methyl 2-cyano-3-(6-methoxynaphthalen-2-yl)acrylate (10)
87% yield; yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.37 (s, 1H), 8.32 (d, 1H, J= 2.4 Hz), 8.18 (dd, 1H, J= 1.8 Hz, J= 8.7 Hz), 7.85-7.79 (m, 2H), 7.21 (dd, 1H, J= 2.5 Hz, J= 8.9 Hz), 7.16 (s, 1H), 3.97 (s, 3H), 3.95 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 163.5, 160.4, 155.3, 137.3, 134.3, 131.1, 128.1, 127.8, 126.8, 126.0, 120.2, 116.1, 105.9, 100.3, 55.5, 55.3; HRMS calc for C16H13NO3 (M+Na)+ 290.0788 found 290.0791.
(E)-methyl 2-cyano-3-(6-piperidin-1-yl naphthalen-2-yl) acrylate (11)
90% yield; orange solid; 1H NMR (300 MHz, CDCl3) δ 8.29 (s, 1H), 8.19 (s, 1H), 8.09 (dd, 1H, J= 1.8 Hz, J= 8.8 Hz), 7.74 (d, 1H, J= 9.2 Hz), 7.64 (d, 1H, J= 8.8 Hz), 7.28 (dd, 1H, J= 2.6 Hz, J= 9.3 Hz), 7.03 (d, 1H, J= 2.2 Hz), 3.93 (s, 3H), 3.39 (t, 4H, J= 5.1 Hz), 1.73-1.66 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 163.9, 155.5, 151.9, 137.7, 134.7, 130.6, 127.2, 126.4, 126.0, 125.6, 119.3, 116.6, 108.3, 98.3, 53.1, 49.3, 25.5, 24.3; HRMS calc for C20H20N2O2 (M+H)+ 321.1598 found 321.1601.
(E)-methyl 2-cyano-3-(5-(piperidin-1-yl)thiophen-2-yl)acrylate (14)
90% yield; orange solid; 1H NMR (400 MHz, CDCl3) δ 7.95 (s, 1H), 7.38 (bs, 1H), 6.07 (d, 1H, J= 4.6 Hz), 3.76 (s, 3H), 3.39 (bs, 4H), 1.64 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 169.2, 165.4, 146.1, 144.1, 119.4, 118.4, 104.8, 61.5, 51.1, 24.8, 23.3; HRMS calc for C14H16N2O2S (M+H)+ 277.1005 found 277.1006.
(E)-methyl 2-cyano-3-(4-(diethylamino)-2,6-dimethoxyphenyl)acrylate (18)
81% yield; yellow solid; 1H NMR (300 MHz, CDCl3) δ 8.47 (s, 1H) 5.72 (s, 2H) 3.86 (s, 6H) 3 .84 (s, 3H) 3.41 (q, 4H, J= 7.1 Hz) 1.22 (t, 6H, J= 7.1 Hz); 13C NMR (100 MHz, CDCl3) δ 166.5, 162.6, 153.9, 146.3, 118.0, 101.1, 95.4, 87.0, 55.0, 52.8, 45.2, 12.9; HRMS calc for C17H22N2O4(M+H)+ 319.1652 found 319.1657.
6-(piperidin-1-yl)-2-naphthaldehyde (9)
To a 50 ml round bottom flask containing benzene (3 mL), HMPA (3 mL) and piperidine (1.65 ml, 16.7 mmol) was added via syringe, at 0 °C, n-BuLi (1.6 M in hexane, 10.4 mL, 16.7 mmol). After stirring for 15 min, the reaction mixture was treated with a solution of 6-methoxy-2-naphthaldehyde (390 mg, 2.09 mmol) in benzene: HMPA 1:1 (2 ml). The reaction mixture was warmed to room temperature, left stirring for 12 hours and then it was poured into cold 5% aqueous NaCl (30 ml). The mixture was extracted with diethyl ether (3 × 20 mL), dried over MgSO4 and concentrated. The product was purified via flash chromatography (20% EtOAc in hexanes) to give compound 9. 9: 35% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 10.02 (s, 1H), 8.14 (s, 1H), 7.88-7.73 (m, 2H), 7.67 (d, 1H, J= 8.6 Hz), 7.32 (dd, 1H, J= 2.5 Hz, J= 9.1 Hz), 7.08 (d, 1H, J= 2.4 Hz), 3.42-3.32 (m, 4H), 1.85-1.57 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 192.2, 152.2, 138.8, 134.7, 131.6, 130.7, 127.5, 126.5, 123.6, 119.7, 109.0, 49.8, 25.8, 24.6; HRMS calc for C16H17NO (M+H)+ 240.1383 found 240.1387.
5-(piperidin-1-yl)thiophene-2-carbaldehyde (13)
A 200 ml round bottom flask containing 5-bromothiophene-2-carbaldehyde (7.62 g, 400 mmol), piperidine (3.40 g, 400 mmol), toluene (30 mL) and p-toluene sulfonic acid (0.69 g, 40 mmol) was refluxed for 24 hours. The crude mixture was concentrated and the residue was subjected to flash chromatography using dichloromethane/ethyl ether/hexane: 1/1/8 to give aldehyde 13. This compound was further crystallized from dichloromethane/hexane. 13: 72% yield, dark blue solid; 1H NMR (400 MHz, CDCl3) δ 9.51 (s, 1H), 7.47 (d, 1H, J= 4.4 Hz), 6.06 (d, 1H, J= 4.4 Hz), 3.35 (t, 4H, J= 4.7 Hz, J= 11.0 Hz), 1.73-1.65 (m, 6H); 13C NMR (100 MHz, CDCl3) 180.4, 168.6, 140.6, 126.6, 104.2, 51.1, 25.1, 23.7; HRMS calc for C10H13NOS (M+H)+ 196.0791 found 196.0792.
4-(diethylamino)-2,6-dimethoxybenzaldehyde (17)
To a 10 mL round bottom flask containing N,N-diethyl-3,5-dimethoxyaniline27 (99.7 mg, 0.48 mmol) and DMF (150 µL, 0.96 mmol) in DCM (2 mL) was added dropwise POCl3 (82.2 mg, 0.05 mL, 0.54 mmol) and the reaction was stirred at 25 °C for 5 h. Sodium hydroxide (2 M, 15 drops) was then added until the solution became neutral causing the color to change to dark blue and the mixture was stirred at 0 °C for 3 h. The mixture was extracted with ether (2 × 10 mL) and the organic extracts were dried over MgSO4 and concentrated. The product was purified by flash column chromatography (50% EtOAc in hexanes). 17: 65% yield, white crystals; 1H NMR (400 MHz, CDCl3) 10.17 (s, 1H), 5.68 (s, 2H), 3.82 (s, 6H), 3.38 (q, 4H, J= 7.1 Hz), 1.19 (t, 6H, J= 7.1 Hz); 13C NMR (75 MHz, CDCl3) 186.6, 164.6, 153.9, 105.1, 86.8, 55.9, 55.8, 45.0, 12.9; HRMS calc for C13H19NO3 (M)+ 237.1359 found 237.1356.
General procedure for the determination of spectral properties
Each viscosity sample was mixed according to column A in Table 3 shown below. The glycerol (Gly) was heated to ensure more exact measuring during pipetting. The pre-stained ethylene glycol (EG) for each sample contained 100 µM of dye resulting in a final concentration of 10 µM for each sample. All samples were placed on rotating mixer for 1 hour before pouring into cuvettes for scanning. Preliminary fluorescent scanning was done on each dye dissolved in 391.4 mPa•sec viscosity solvent to determine optimal excitation and peak emission and slit settings for each molecular rotor derivative. All fluorescent scanning was done with the temperature-controlled turret set at room temperature (22 ± 0.8 C); each sample was inserted in the turret and allowed to equilibrate for 10 minutes before testing. For each solvent the fluorescent emission in a 11 nm range was averaged and the logarithm of the average peak intensity was plotted against the logarithm of the viscosity. The slope was obtained for each molecular rotor derivative by linear regression (Graphpad Prism 4.01, San Diego, CA). The exponent x of each viscosity gradient was used to evaluate viscosity sensitivity, with a higher value of the exponent x indicating higher sensitivity. R2 values indicate the linear regression of the log-transformed data (intensity over viscosity). When two or more similar experiments were performed the lowest value of R2 is given (Table 2).
| A | B | C |
|---|---|---|
| Pre-stained EG / EG / Gly volumes (ml) |
Viscosity (mPa •sec) |
Log Viscosity |
| 0.5 / 0.5 / 4.0 | 391.4 | 2.593 |
| 0.5 / 1.0 / 3.5 | 258.1 | 2.412 |
| 0.5 / 1.5 / 3.0 | 170.2 | 2.231 |
| 0.5 / 2.0 / 2.5 | 112.2 | 2.050 |
| 0.5 / 2.5 / 2.0 | 74.0 | 1.869 |
Supplementary Material
Acknowledgments
Financial support by the NIH (1R21RR025358) and the NSF (CMMI-0652476) is gratefully acknowledged. We thank the National Science Foundation for instrumentation grants CHE-9709183 and CHE-0741968. We also thank Drs. A. Mrse and Dr. Y. Su for NMR spectroscopic and mass spectrometric assistance respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information
1H and 13C NMR spectra of compounds 5a-5m, 7–11, 13, 14 and 16–18.
References
- 1. For selected reviews and monographs see: Luby-Phelps K. Intern. Rev. Cytol. 2000;192:189–221. doi: 10.1016/s0074-7696(08)60527-6. Rohrbach DH, Timpl R, editors. Molecular and cellular aspects of basement membranes. San Diego: Academic Press; 1993. Yeagle PL, editor. The membranes of cells. San Diego: Academic Press; 1993. Ohnishi ST, Ohnishi T, editors. Membrane abnormalities in sickle cell disease and in other red blood cell disorders. Boca Raton, Fla.: CRC Press; 1994.
- 2. For representative reviews see: Reinhart WH. Biorheology. 2001;38:203–212. Moriarty PM, Gibson CA. Cardiovascular Rev. & Rep. 2003;24:321–325. Uchimura I, Numano F. Diabetes Frontier. 1997;8:33–37. Simon A, Gariepy J, Chironi G, Megnien J-L, Levenson J. J. Hypertension. 2002;20:159–169. doi: 10.1097/00004872-200202000-00001.
- 3.Oppenheimer N, Diamant H. Biophys. J. 2009;96:3041–3049. doi: 10.1016/j.bpj.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Owen DM, Williamson D, Rentero C, Gaus K. Traffic. 2009;10:962–971. doi: 10.1111/j.1600-0854.2009.00908.x. [DOI] [PubMed] [Google Scholar]; Frick M, Schmidt K, Nichols BJ. Curr. Biol. 2007;17:462–467. doi: 10.1016/j.cub.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 4.Luneva OG, Brazhe NA, Maksimova NV, Rodnenkov OV, Parsina EY, Bryzgalova NY, Maksimov GV, Rubin AB, Orlov SN, Chazov EI. Pathophysiol. 2007;14:41–46. doi: 10.1016/j.pathophys.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin JS, Drake KR, Remment CL, Kenworthy AK. Biophys. J. 2005;89:1398–1410. doi: 10.1529/biophysj.104.055640. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dibner MD, Ireland KA, Koerner LA, Dexter DL. Cancer Res. 1985;45:4998–5003. [PubMed] [Google Scholar]
- 6.Aleardi AM, Benard G, Augereau O, Malgat M, Talbot JC, Mazat JP, Letellier T, Dachary-Prigent J, Solaini GC, Rossignol R. J. Bioenerg. & Biomembr. 2005;37:207–225. doi: 10.1007/s10863-005-6631-3. [DOI] [PubMed] [Google Scholar]; Hou X, Richardson SJ, Aguilar M-I, Small DH. Biochemistry. 2005;44:11618–11627. doi: 10.1021/bi050700m. [DOI] [PubMed] [Google Scholar]
- 7.Ahn JH, Kim TY, Kim Y-J, Han MW, Yoon TH, Chung JW. Diabet. Med. 2006;23:1339–1343. doi: 10.1111/j.1464-5491.2006.01993.x. [DOI] [PubMed] [Google Scholar]; Salazar Vazquez BY, Salazar Vasquez MA, Venzor VC, Negrete AC, Cabrales P, Diaz JS, Intaglietta M. Clin. Hemorheol. Microcircul. 2008;38:67–74. [PubMed] [Google Scholar]
- 8.Kearney-Schwartz A, Virion JM, Stoltz J-F, Drouin P, Zannad F. Fund. & Clin. Pharmacol. 2007;21:387–396. doi: 10.1111/j.1472-8206.2007.00496.x. [DOI] [PubMed] [Google Scholar]
- 9.Velcheva I, Antonova N, Dimitrova V, Dimitrov N, Ivanov I. Hemorheol. & Microcircul. 2006;35:155–157. [PubMed] [Google Scholar]
- 10.Bosman GJCGM, Bartholomeus IGP, de Grip WJ. Gerontology. 1991;37:95–112. doi: 10.1159/000213253. [DOI] [PubMed] [Google Scholar]
- 11.International Committee for Standardization in Haematology. J. Clin. Pathol. 1984;37:1147–1152. doi: 10.1136/jcp.37.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Boss AH, Kensey KR, Rosenson RS. Clin. Chim. Acta. 2003;332:79–82. doi: 10.1016/s0009-8981(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 13.Lakowicz JR. “Principles of Fluorescence Spectroscopy”. New York: KLUWER ACADEMIC/PLENUM; 1999. [Google Scholar]
- 14.Shinitzky M, Barenholz Y. Biochim. Biophys. Acta. 1978;515:367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- 15.(a) Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. Biophys. J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Soumpasis DM. Biophys. J. 1983;41:95–97. doi: 10.1016/S0006-3495(83)84410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Swaminathan R, Bicknese S, Periasamy N, Verkman AS. Biophys. J. 1996;71:1140–1151. doi: 10.1016/S0006-3495(96)79316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blonk JCG, Don A, van Aalst H, Birmingham JJ. J. Microsc. 1993;169:363–374. [Google Scholar]
- 17.(a) Haidekker MA, Theodorakis EA. Org. & Biomol. Chem. 2007;5:1669–1678. doi: 10.1039/b618415d. [DOI] [PubMed] [Google Scholar]; (b) Demchenko P, Mely Y, Duportail G, Klymchenko AS. Biophys. J. 2009;96:3461–3470. doi: 10.1016/j.bpj.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabowski ZR, Rotkiewicz K, Rettig W. Chem. Rev. 2003;103:3899–4031. doi: 10.1021/cr940745l. [DOI] [PubMed] [Google Scholar]
- 19.(a) Loutfy RO. Pure Appl. Chem. 1986;58:1239–1248. [Google Scholar]; (b) Levitt JA, Kuimova MK, Yahioglu G, Chung P-H, Suhling K, Phillips D. J. Phys. Chem. C. 2009;113:11634–11642. [Google Scholar]
- 20.Haidekker MA, L'Heureux N, Frangos JA. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1401–H1406. doi: 10.1152/ajpheart.2000.278.4.H1401. [DOI] [PubMed] [Google Scholar]
- 21.Haidekker MA, Tsai AG, Brady T, Stevens HY, Frangos JA, Theodorakis EA, Intaglietta M. Amer. J. Physiol. Heart Circ. Physiol. 2002;282:H1609–H1614. doi: 10.1152/ajpheart.00712.2001. [DOI] [PubMed] [Google Scholar]
- 22.(a) Loutfy RO, Law KY. J. Phys. Chem. 1980;84:2803–2808. [Google Scholar]; (b) Haidekker MA, Brady TP, Lichlyter D, Theodorakis EA. Bioorg. Chem. 2005;33:415–425. doi: 10.1016/j.bioorg.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 23.(a) Haidekker MA, Akers W, Lichlyter D, Brady TP, Theodorakis EA. Sensor Lett. 2005;3:42–48. [Google Scholar]; (b) Haidekker MA, Brady TP, Lichlyter D, Theodorakis EA. J. Am. Chem. Soc. 2006;128:398–399. doi: 10.1021/ja056370a. [DOI] [PubMed] [Google Scholar]; (c) Fischer D, Theodorakis EA, Haidekker MA. Nature Protocols. 2007;2:227–236. doi: 10.1038/nprot.2006.455. [DOI] [PubMed] [Google Scholar]; (d) Nipper ME, Majd S, Mayer M, Lee J, Theodorakis EA, Haidekker MA. Biochim. Biophys. Acta Biomembr. 2008;1778:1148–1153. doi: 10.1016/j.bbamem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Chen X-X, Zhao Z, Liu Y, Lu P, Wang Y-G. Chem. Lett. 2008;37:570–571. [Google Scholar]
- 25.Guo H-M, Tanaka F. J. Org. Chem. 2009;74:2417–2424. doi: 10.1021/jo900013w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wuerthner F, Yao S, Schilling J, Wortmann R, Redi-Abshiro M, Mecher E, Gallego-Gomez F, Meerholz K. J. Am. Chem. Soc. 2001;123:2810–2824. doi: 10.1021/ja002321g. [DOI] [PubMed] [Google Scholar]
- 27.Razzuk A, Biehl ER. J. Org. Chem. 1987;52:2619–2622. [Google Scholar]
- 28.Haidekker MA, Ling T, Anglo M, Stevens HY, Frangos JA, Theodorakis EA. Chemistry & Biology. 2001;8:123–131. doi: 10.1016/s1074-5521(00)90061-9. [DOI] [PubMed] [Google Scholar]
- 29.Law KY. Chem. Phys. Lett. 1980;75:545–549. [Google Scholar]
- 30.Förster Th, Hoffmann G. Z. Phys. Chem. 1971;75:63–76. [Google Scholar]
- 31.DiCesare N, Lakowicz JR. J. Phys. Chem. A. 2001;105:6834–6840. doi: 10.1021/jp010076x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.(a) Minakata S, Moriwaki S, Inada H, Komatsu M, Kajii H, Ohmori Y, Tsumura M, Namura K. Chem. Lett. 2007;36:1014–1015. [Google Scholar]; (b) Duan L, Xu Y, Qian X, Zhang Y, Liu Y. Tetrahedron Lett. 2009;50:22–25. [Google Scholar]
- 33.(a) Schuddeboom W, Jonker SA, Warman JM, Leinhos U, Kuehnle W, Zachariasse KA. J. Phys. Chem. 1992;96:10809–10819. [Google Scholar]; (b) Il’chev YV, Kuehnle W, Zachariasse KA. J. Phys. Chem. 1998;102:5670–5680. [Google Scholar]
- 34.Yang X, Jiang X, Zhao C, Chen R, Qin P, Sun L. Tetrahedron Lett. 2006;47:4961–4964. [Google Scholar]
- 35.Shinohara Y, Arai T. Bull. Chem. Soc. Jpn. 2008;81:1500–1504. [Google Scholar]
- 36.(a) Roberts JC, Pincock JA. J. Org. Chem. 2006;71:1480–1492. doi: 10.1021/jo052123d. [DOI] [PubMed] [Google Scholar]; (b) Hayakawa J, Ikegami M, Mizutani T, Wahadoszamen Md, Monotake A, Nishimura Y, Arai T. J. Phys. Chem. A. 2006;110:12566–12571. doi: 10.1021/jp065075p. [DOI] [PubMed] [Google Scholar]
- 37.Zachariasse KA, Grobys M, von der Haar Th, Hebecker A, Il’ichev YV, Jiang Y–B, Morawski O, Kuehnle W. J. Photochem & Photobiol. A: Chem. 1996;102:59–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.