Abstract
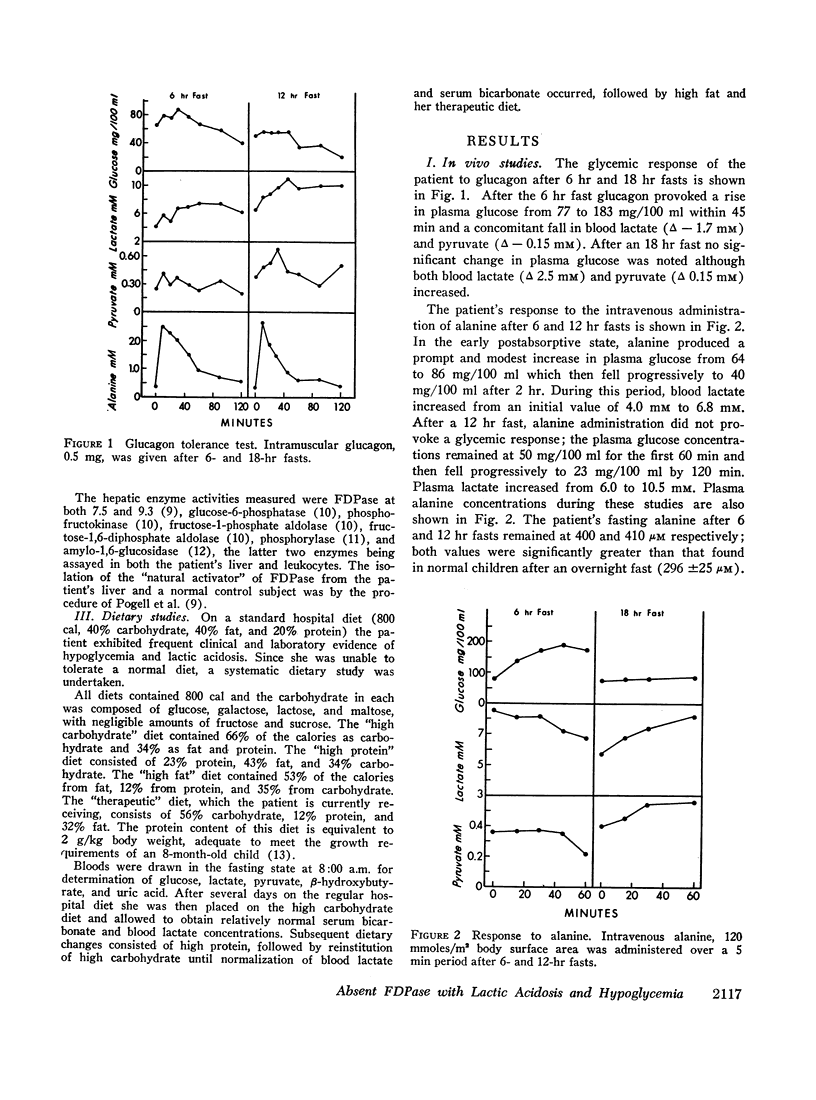
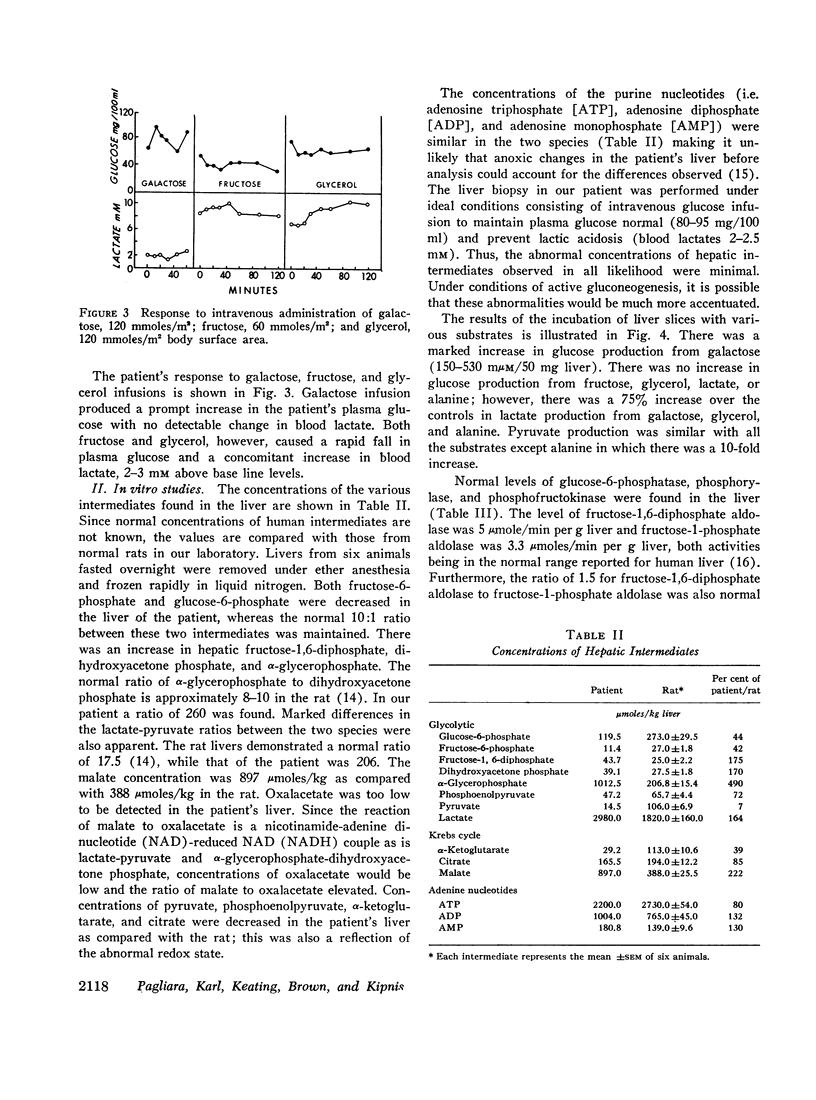
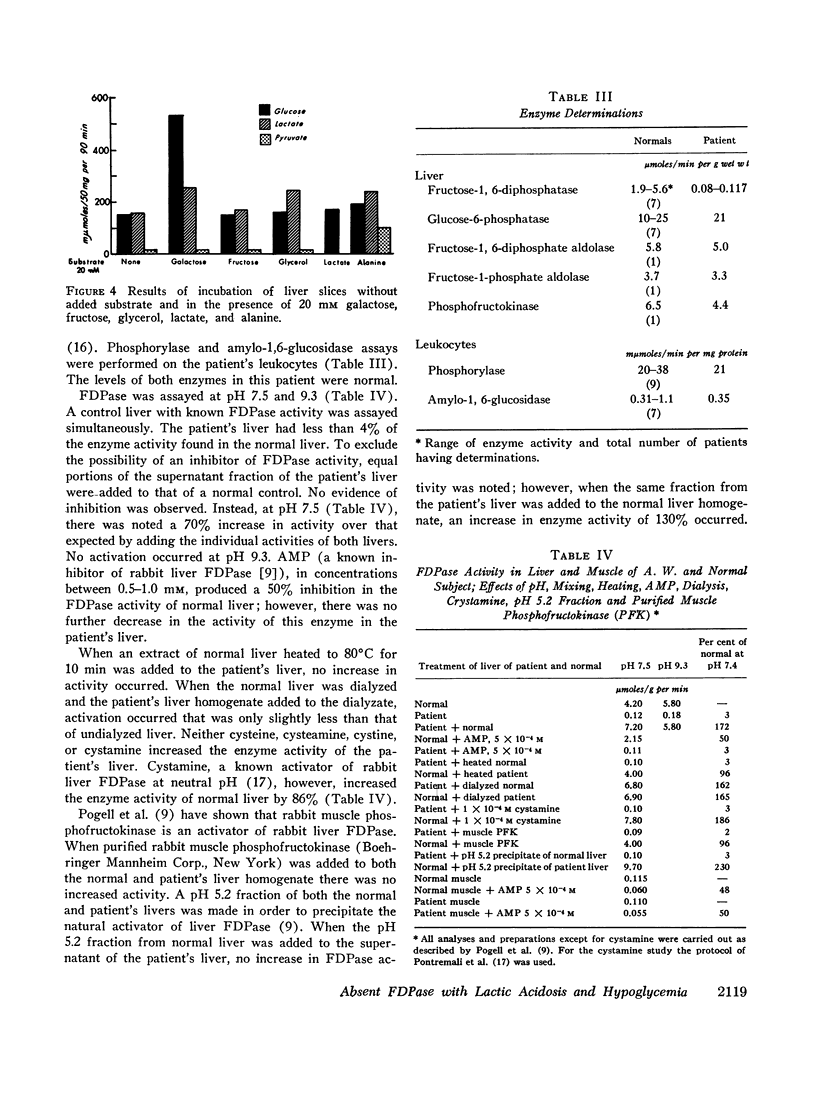
An 8-month-old female, maintained on breast feeding for 6 months, experienced numerous attacks of hyperventilation when weaned to baby food and was admitted with severe lactic acidosis (20 mM) and hypoglycemia. Physical examination was negative except for hepatomegaly. Fasting (18 hr) after stabilization on a high carbohydrate diet resulted in hypoglycemia (plasma glucose 40 mg/100 ml), lactic acidosis (6-10 mM), and a rise in plasma alanine. Glucagon produced a glycemic response after 6 hr, but not after 18 hr fasting. Intravenous galactose increased plasma glucose (Δ 45 mg/100 ml) but intravenous fructose, glycerol, and alanine caused a 40-50% fall in plasma glucose and a significant rise in lactate (Δ 3-4 mM).
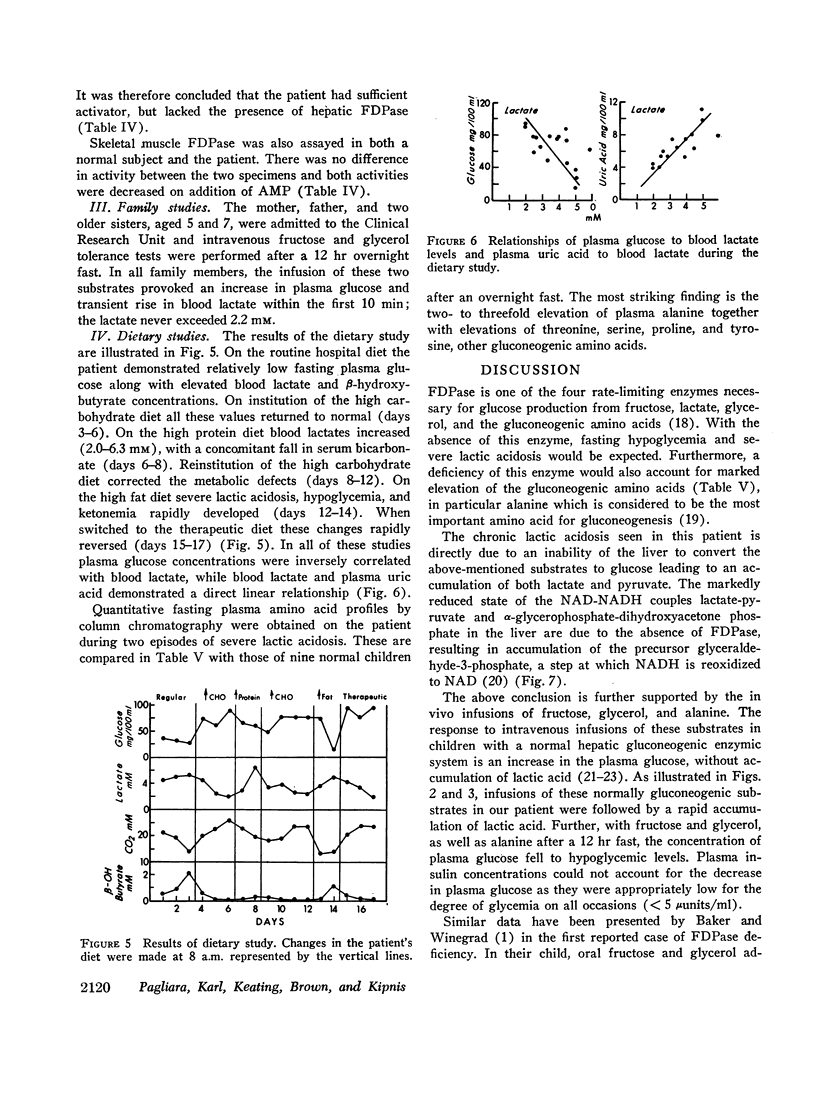
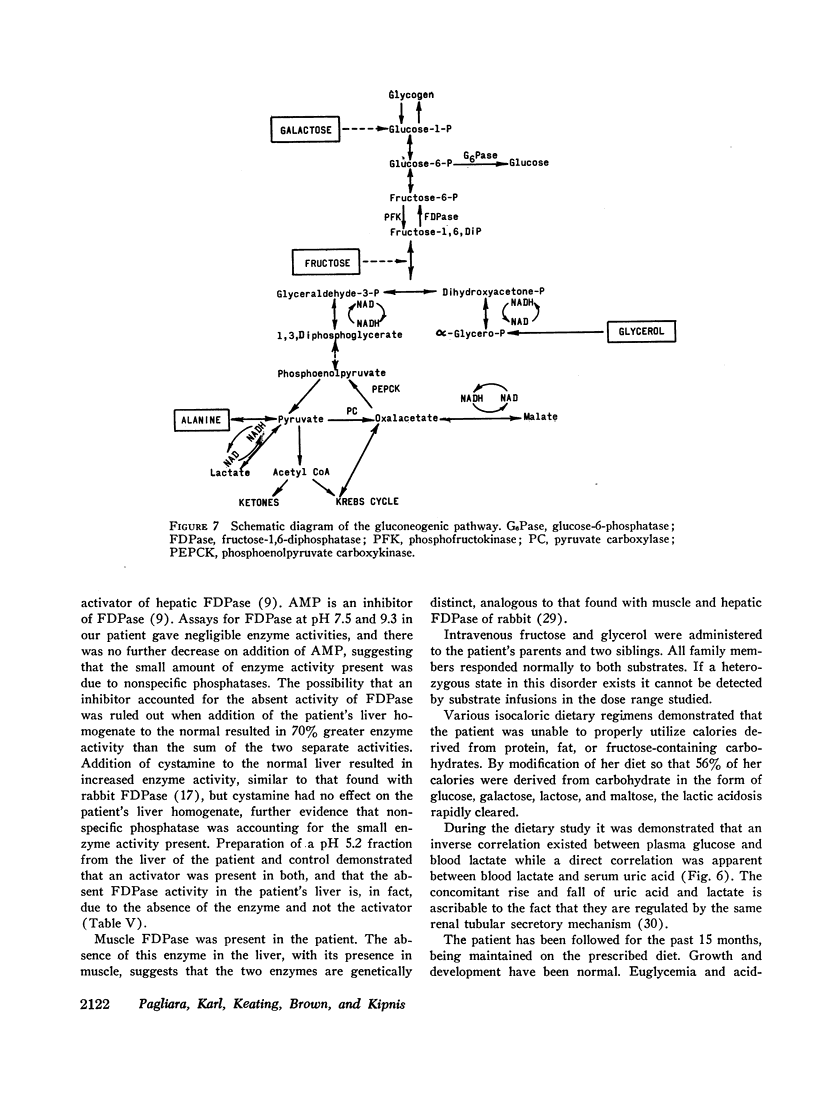
Liver biopsy showed fatty infiltration. Liver slices incubated with galactose, lactate, fructose, alanine, or glycerol converted only galactose to glucose. Hepatic glycolytic intermediates were increased below the level of fructose-1,6-diphosphate and decreased above. Hepatic phosphorylase, glucose-6-phosphatase, amylo-1,6-glucosidase, phosphofructokinase, fructose-1-phosphate aldolase, and fructose-1,6-diphosphate aldolase levels were normal, but no fructose-1,6-diphosphatase (FDPase) activity was detected. Further studies on the liver homogenate of this patient revealed the presence of an acid-precipitable activator of FDPase.
Normal plasma glucose and lactate levels were maintained on an 800 cal diet of 66% carbohydrate (sucrose and fructose excluded). 5% protein, and 20% fat. When carbohydrate was reduced to 35% and protein or fat increased to 23 and 53% respectively, lactic acidosis and hypoglycemia recurred. These studies show that a deficiency of FDPase produced infantile lactic acidosis and hypoglycemia and can be controlled by an appropriate diet.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKEDO H., CHRISTENSEN H. N. Nature of insulin action on amino acid uptake by the isolated diaphragm. J Biol Chem. 1962 Jan;237:118–122. [PubMed] [Google Scholar]
- BURCH H. B., LOWRY O. H., KUHLMAN A. M., SKERJANCE J., DIAMANT E. J., LOWRY S. R., VON DIPPE P. Changes in patterns of enzymes of carbohydrate metabolism in the developing rat liver. J Biol Chem. 1963 Jul;238:2267–2273. [PubMed] [Google Scholar]
- Baker L., Winegrad A. I. Fasting hypoglycaemia and metabolic acidosis associated with deficiency of hepatic fructose-1,6-diphosphatase activity. Lancet. 1970 Jul 4;2(7662):13–16. doi: 10.1016/s0140-6736(70)92474-8. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Enser M., Shapiro S., Horecker B. L. Immunological studies of liver, kidney, and muscle fructose 1,6-diphosphatases. Arch Biochem Biophys. 1969 Jan;129(1):377–383. doi: 10.1016/0003-9861(69)90189-1. [DOI] [PubMed] [Google Scholar]
- FROESCH E. R., WOLF H. P., BAITSCH H., PRADER A., LABHART A. Hereditary fructose intolerance. An inborn defect of hepatic fructose-1-phosphate splitting aldolase. Am J Med. 1963 Feb;34:151–167. doi: 10.1016/0002-9343(63)90050-0. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Passonneau J. V., Lowry O. H. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem. 1966 Sep 10;241(17):3997–4003. [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., REIM M., HUEBENER H. J. The oxidation/reduction state of the extramitochondrial DPN/DPNH system in rat liver and the hormonal control of substrate levels in vivo. Biochem Biophys Res Commun. 1961 Mar 10;4:163–168. doi: 10.1016/0006-291x(61)90263-7. [DOI] [PubMed] [Google Scholar]
- HOWELL R. R., ASHTON D. M., WYNGAARDEN J. B. Glucose-6-phosphatase deficiency glycogen storage disease. Studies on the interrelationships of carbohydrate, lipid, and purine abnormalities. Pediatrics. 1962 Apr;29:553–565. [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Heinz F., Lamprecht W., Kirsch J. Enzymes of fructose metabolism in human liver. J Clin Invest. 1968 Aug;47(8):1826–1832. doi: 10.1172/JCI105872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Gascoyne T., Notton B. M. Generation of extramitochondrial reducing power in gluconeogenesis. Biochem J. 1967 Jan;102(1):275–282. doi: 10.1042/bj1020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDDLE L., SEEGMILLER J. E., LASTER L. The enzymatic spectrophotometric method for determination of uric acid. J Lab Clin Med. 1959 Dec;54:903–913. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of alanine on glucagon secretion. J Clin Invest. 1971 Oct;50(10):2215–2218. doi: 10.1172/JCI106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Kari I. E., De Vivo D. C., Feigin R. D., Kipnis D. M. Hypoalaninemia: a concomitant of ketotic hypoglycemia. J Clin Invest. 1972 Jun;51(6):1440–1449. doi: 10.1172/JCI106940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogell B. M., Tanaka A., Siddons R. C. Natural activators for liver fructose 1,6-diphosphatase and the reversal of adenosine 5'-monophosphate inhibition by muscle phosphofructokinase. J Biol Chem. 1968 Apr 10;243(7):1356–1367. [PubMed] [Google Scholar]
- Pontremoli S., Traniello S., Enser M., Shapiro S., Horecker B. L. Regulation of fructose diphosphatase activity by disulfide exchange. Proc Natl Acad Sci U S A. 1967 Jul;58(1):286–293. doi: 10.1073/pnas.58.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrago E., Young J. W., Lardy H. A. Carbohydrate supply as a regulator of rat liver phosphoenolpyruvate carboxykinase activity. Science. 1967 Dec 22;158(3808):1572–1573. doi: 10.1126/science.158.3808.1572. [DOI] [PubMed] [Google Scholar]
- Senior B., Loridan L. Studies of liver glycogenoses, with particular reference to the metabolism of intravenously administered glycerol. N Engl J Med. 1968 Oct 31;279(18):958–965. doi: 10.1056/NEJM196810312791802. [DOI] [PubMed] [Google Scholar]


