Abstract
This paper is concerned with the qualitative analysis of two models (Bonhoeffer et al., Proc. Natl. Acad. Sci. USA 94 (1997), 12106–12111) for different treatment protocols to prevent antibiotic resistance. Detailed qualitative analysis about the local or global stability of the equilibria of both models is carried out in term of the basic reproduction number R0. For the model with a single antibiotic therapy, we show that if R0 < 1, then the disease-free equilibrium is globally asymptotically stable; if R0 > 1, then the disease endemic equilibrium is globally asymptotically stable. For the model with multiple antibiotic therapies, stabilities of various equilibria are analyzed and combining treatment is shown better than cycling treatment. Numerical simulations are performed to show that the dynamical properties depend intimately upon the parameters.
Keywords: Antibiotic resistance, mathematical model, basic reproduction number, equilibrium, stability
1 Introduction
Infections caused by antibiotic-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-resistant enterococci (VRE), are increasing rapidly throughout the world and pose a serious threat to public health (Levy and Marshall, 2004; Grundmann et al., 2006; Spellberg et al., 2008). The transmission dynamics of antibiotic-resistant bacteria in hospitals are complex which involve the patients, health care workers, and their interactions. Antibiotic exposure is crucial to the emergence and spread of these resistant bacteria (D’Agata et al., 2007). Compared to infections caused by antimicrobial-susceptible bacteria, infections with antimicrobial-resistant bacteria cause higher mortality rates, longer hospital stays and greater hospital costs (Daxboeck et al., 2006). It was estimated that in 2005 the deaths in patients with invasive methicillin-resistant S. aureus in the United Sates exceeded the total number of deaths due to HIV/AIDS in the same year (Klevens et al., 2007).
Recently, mathematical models have been extensively used to simulate the spread of the antibiotic-resistant bacteria, to identify various factors responsible for the prevalence of the antibiotic-resistant bacteria, to examine different antibiotic treatments, and to help design effective control programs (Austin and Anderson, 1999; Bonhoeffer et al., 1997; Bonten et al., 2001; Bergstrom et al., 2004; Webb et al., 2005; Boldin et al., 2007; D’Agata et al., 2007). We refer to the survey papers of Grundmann and Hellriegel (2006) and Temime et al. (2008) for more details and references on this topic.
To generate predictions concerning the effects of various patterns of antibiotic treatment at the population level, Bonhoeffer et al. (1997) proposed two mathematical models. In the first model, patients with bacterial infections may be treated with a single antibiotic. The model consists of three ordinary differential equations:
| (1.1) |
where x(t), yw(t), and yr(t) denote the density of uninfected patients, infected by sensitive(wild type) bacteria to the treating antibiotic, and infected by resistant bacteria to the treating antibiotic at time t, respectively. We refer to Fig. 1A in Bonhoeffer et al. (1997) for a chart diagram for the three compartment model. Λ is the recruitment rate of the population, d is the per capita removal rate from the population, b is the transmission rate parameter, c is the death rate of the infected host, which includes natural and disease-associated mortality. rw and rr are the rates of patients infected with wild type and resistant bacteria recover from the infection in the absence of treatment. Patients infected with wild type bacteria are removed from the wild type infected compartment at a rate fh, where f is a scaling parameter (between 0 and 1) reflecting the fraction of patients treated and h is the maximum rate when all patients are treated. A fraction s of treated wt-infected develops resistance during treatment. Bonhoeffer et al. (1997) considered treatment with a single antibiotic and resistance to that antibiotic and analyzed the model to predict the consequences of different usage patterns.
In the second model, two antibiotics A and B are used. The model takes the following form:
| (1.2) |
where the variables are x(t) for the susceptible, yw(t), ya(t), yb(t) and yab(t) for patients infected with wild type(wt), A-resistant(A-res), B-resistant(B-res), and AB-resistant(AB-res) bacteria, respectively (see Fig. 1B in Bonhoeffer et al. (1997) for a chart diagram for the model). Λ is the recruitment rate of the population, d is the per capita removal rate from the population, b is the transmission rate parameter, c is the death rate of the infected host, which includes natural and disease-associated mortality. rw, ra, rb and rab are the recovery rates of wt, A-res, B-res and AB-res infected, respectively; fa, fb and fab reflect the fraction of patients treated with antibiotic A, B, or AB, they fulfill the relation 0 ≤ fa, fb, fab ≤ 1, and fa + fb + fab ≤ 1. h is the maximum rate when all patients are treated. A fraction s or q of treated wt-infected develop resistance with single antibiotic treatment or two antibiotics treatment. Bonhoeffer et al. (1997) analyzed the population-level consequences of different usage patterns of the two antibiotics and made various conclusions based on numerical analysis of their models. In this paper we provide detailed qualitative analysis of the two mathematical models (1.1) and (1.2), including the existence and stability of all possible equilibria, and numerical simulations to support these conclusions.
We would like to make some remarks about the comparisons of models (1.1) and (1.2) with the competition models of resources (see, for example, Amarasekar [1] and Smith and Li [22]) and the multi-strain models in epidemiology (see Bremermann and Thieme [9] and Webb et al. [25]). Firstly models (1.1) and (1.2) are not competition models since the two strains of bacteria, sensitive and resistant, are not competitors. Secondly, patients infected with the sensitive strain can be infected with the resistant strain due to the treatment of antibiotics or the interaction from the contaminated health-care workers, and patients infected with the resistant strain can be cleaned due to treatment. So models (1.1) and (1.2) are different from the multi-strain models in epidemiology (see Bremermann and Thieme [9]) and the two-resistant strains model studied by Webb et al. [25]. Moreover, our results are not about which strain will win, it is about how the resistant strains establish in the patients and how to control that.
The paper is organized as follows. In section 2, we consider the compartment model (1.1) with a single antibiotic therapy and evaluate a threshold, the basic reproduction number R0 (Brauer and Castillo-Chavez, 2000), for two cases: (i) in the absence of treatment fh = 0 and (ii) with treatment fh > 0. The disease-free equilibrium always exists and is globally stable if R0 < 1 and the disease-endemic equilibrium exists and is globally stable if R0 > 1. Section 3 is devoted to discussing the existence and stability of equilibria of the model (1.2) with multiple antibiotic therapies. In order to understand how antibiotic usage patterns may be optimized to preserve or restore antibiotic effectiveness, we consider four different modes of antibiotic therapy, namely, (i) in the absence of treatment fa = fb = fab = 0; (ii) cycling treatment fa = 1, fb = fab = 0 or fb = 1, fa = fab = 0; (iii) 50-50 treatment , fab = 0; and (iv) combination treatment fa = fb = 0, fab = 1. We present stability results for all different cases. In section 4, we present some numerical simulations to illustrate the obtained results and present a brief discussion.
2 The Model with a Single Antibiotic Therapy
In this section, we discuss the existence and stability of equilibria of the compartment model (1.1). In this model, we assume that the fitness cost associated with resistance is manifest by a higher rate of clearance of the infection (recovery) of hosts infected with resistant bacteria relative to those infected with sensitive (rr > rw) and the death rate of infected patients is higher than that of susceptible one, that is c > d (Bonhoeffer et al., 1997).
Because of the biological meaning of the components (x(t), yw (t), yr(t)), we focus on the model in the first octant of R3. We first consider the existence of equilibria of system (1.1). By some calculation, we find that system (1.1) has at most three equilibria:
and
under certain conditions (to be specified later).
We define the basic reproduction number as follows
First we determine the stability of the disease-free equilibrium E0. The Jacobian matrix of system (1.1) at E0 is given by
We can see that E0 is locally stable if R0 < 1.
In the following, we shall study the existence and stability property of other equilibria of model (1.1). We consider two cases.
2.1 In the absence of treatment
fh = 0
We first consider the case of absence of therapy, that is fh = 0. To deduce the threshold for the antibiotic resistance in the patient, we analyze the existence of equilibria and their stability for model (1.1). Now the basic reproduction number is . By examining the linearized form of system (1.1) at the equilibrium, we obtain the following result.
Theorem 2.1
Assume fh = 0, then .
If R0 < 1, then system (1.1) has a disease-free equilibrium E0 = (Λ/d, 0, 0), which is locally asymptotically stable.
-
If R0 > 1, then system (1.1) has two or three equilibria, the disease-free equilibrium E0 = (Λ/d, 0, 0), which is a saddle point and unstable, the nontrivial equilibriumwhich is locally asymptotically stable, and another nontrivial equilibrium
which is unstable if it exists.
2.2 With treatment
fh > 0
We first consider the case rr < rw + fh. In this case
and system (1.1) has at most two equilibria, the disease-free equilibrium E0 and the semitrivial equilibrium if R0 > 1.
The Jacobian matrix of system (1.1) at Er is
It follows that Er is locally asymptotically stable under the assumption of R0 > 1.
From the above discussion, we have the following result.
Theorem 2.2
Suppose rr < rw + fh, then . If R0 < 1, then system (1.1) has a disease-free equilibrium E0 = (Λ/d, 0, 0), which is locally asymptotically stable. If R0 > 1, then E0 is unstable, and the semitrivial equilibrium exists and is locally asymptotically stable.
Next we discuss the case rr > rw + fh. In this case
and system (1.1) may have three equilibria, the disease-free equilibrium E0, the semitrivial equilibrium with the resistant strain Er and a positive equilibrium Ẽ = (x̃, ỹw, ỹr). For convenience, we denote
From the expressions of ỹw and ỹr, we know that Ẽ exists if and only if R0 > 1. The Jacobian matrix of system (1.1) at Ẽ is
Therefore, the corresponding characteristic equation is
| (2.1) |
where
Furthermore, by the relation
we have
Thus, in view of the new expression of a2, it is easy to see that a1, a2, a3 > 0 and a1a2−a3 > 0. By Routh-Hurwitz criteria (see Korn and Korn, 2000, Section 1.6-6(b)), all roots of equation (2.1) have negative real parts. Therefore, when R0 > 1, the positive equilibrium Ẽ is locally stable. Thus, we have the following conclusion.
Theorem 2.3
Assume rr > rw + fh, then . If R0 < 1, then system (1.1) has a disease-free equilibrium E0 = (Λ/d, 0, 0), which is locally asymptotically stable. If R0 > 1, then E0 is unstable and the disease endemic equilibrium
is locally asymptotically stable. Furthermor, if , then the semitrivial equilibrium with the resistant strain
exists and is unstable.
To explore the global stability of the positive equilibrium, we define the new variables
and parameters
Using these changes of variables and parameters, system (1.1) becomes
| (2.2) |
with Ñ(t) = X(t) + Yw(t) + Yr(t). The equation for the total population Ñ is
| (2.3) |
Clearly, Ñ∈ (0, 1] since at the disease-free equilibrium Ñ = X = 1 and the natural expectation is that the spread of the disease in the population will reduce Ñ (that is Ñ < 1). Therefore, we study the stability of the model (2.2) in the region
Consider the subset D* of D given by
From (2.3), it is obvious that in D*. If X + c̃Yw + c̃Yr > 1, then and if X + c̃Yw + c̃Yr < 1, then . It follows that D* is a positively invariant set in D. Thus the ω-limit set of each solution of model (2.2) is contained in D*. Moreover, it is easy to see that Ẽ0 attracts the region D0 = {(X, Yw, Yr) ∈ D : Yw = Yr = 0}.
In the next result, we will show that there cannot be any closed orbit around the equilibrium.
Lemma 2.4
The model (2.2) has no periodic orbits, homoclinic orbits or polygons in D*.
Proof
Let f1, f2, f3 denote the three functions on the right hand sides in system (2.2), respectively. Denote f = (f1, f2, f3)T (T denotes transpose), , (where r = (X, Yw, Yr)T), then
By straightforward calculation, we have in the interior of domain D that
Using the normal vector n= (1, c̃, c̃) on D*, it can be shown that
In view of the assumption of c̃ > 1, we have that curlg · (1, c̃, c̃) is negative on D\∂D. From Corollary 4.2 in Busenberg and van den Driessche (1990), there are no solutions of the stated type in D\∂D. The desired result is obtained.
From Theorems 2.1, 2.2, 2.3 and Lemma 2.4, the following theorem can be obtained.
Theorem 2.5
For system (1.1), the following results hold.
Assume fh < rr − rw, then system (1.1) has at most three equilibria E0, Er, Ẽ with . If, R0 > 1 then the DFE E0 is globally asymptotically stable, if R0 < 1, E0 is unstable, the positive equilibrium Ẽ is globally asymptotically stable, the semitrivial equilibrium Er is unstable if it exists.
Assume fh > rr − rw, then system (1.1) has at most two equilibria E0, Er with . If R0< 1, then the DFE E0 is globally stable, if R0> 1, E0 is unstable, the semitrivial equilibrium Er is globally asymptotically stable.
The above results can be summarized in the following chart (see Table 1. BRN=Basic Reproduction Number).
Table 1.
Stability chart for system (1.1).
| Condition | BRN | Cases | E0 | Er | Ẽ | |
|---|---|---|---|---|---|---|
| fh < rr − rw |
|
R0 < 1 | globally stable | ∄ | ∄ | |
| R0 > 1 | unstable | if exist, unstable | globally stable | |||
| fh > rr − rw |
|
R0 < 1 | globally stable | ∄ | ∄ | |
| R0 > 1 | unstable | globally stable | ∄ |
3 The Model with Multiple Antibiotic Therapies
In this section we consider model (1.2), where we assume that the fitness cost associated with resistance is manifest by a higher rate of clearance of the infection (recovery) of hosts infected with resistant bacteria relative to those infected with sensitive bacteria (rr > rw) and the death rate of infected patients is higher than that of susceptible one, that is c > d (Bonhoeffer et al., 1997).
Because of the components of (x(t), yw (t), ya(t), yb(t), yab(t)) have to be nonnegative, we focus on the model in the first octant of R5. We first consider the existence of equilibria of system (1.2). For any values of parameters, model (1.2) always has a disease-free equilibrium E0 = (Λ/d, 0, 0, 0, 0).
We first determine the stability of the disease-free equilibrium E0. The Jacobian matrix of system (1.2) at E0 is
where
The eigenvalues of J0 are −d, j22, j33, j44, j55. So from the expressions of jii(i = 2, 3, 4, 5), we can see that the steady state E0 is locally asymptotically stable if
and unstable if
By using the next generation operator approach as described by Diekmann, Heesterbeek, and Metz (1999), we obtain the basic reproduction number as follows
Observe that
We have the following result.
Theorem 3.1
For model (1.2), the disease-free equilibrium E0 is locally asymptotically stable if R0 < 1 and unstable if R0 > 1.
In order to analyze the stability of other equilibria of model (1.2), we consider four cases.
3.1 In the absence of treatment
fa = fb = fab = 0.
Theorem 3.2
When fa = fb = fab = 0, for system (1.2), there are at most five possible steady state
The existence and stability of equilibria are described in Table 2.
Table 2.
Existence and Stability Chart for System (1.2) with no Treatment.
| Condition | BRN | Cases | E0 | Ew | Ea | Eb | Eab | |
|---|---|---|---|---|---|---|---|---|
| rw < min{ra, rb, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | stable | * | * | * | |||
| ra < min{rw, rb, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | stable | * | * | |||
| rb < min{rw, ra, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | * | stable | * | |||
| rab < min{rw, ra, rb} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | * | * | stable |
Here * represents that if the equilibrium exists, it is unstable.
Proof
When fa = fb = fab = 0, the Jacobian matrix of system (1.2) at the nontrivial equilibrium Ew is
We can see that the eigenvalues of JEw are rw − ra, rw − rb, rw − rab, and the roots of the polynomial equation
Thus, under the condition rw < min{ra, rb, rab}, if R0 < 1, namely , the equilibria Ew, Ea, Eb, Eab do not exist and the trivial equilibrium E0 is locally stable. If R0 > 1, all eigenvalues of JEw have negative real parts, so Ew is locally stable. Equilibria Ea, Eb, Eab are unstable, since their corresponding Jacobian matrices have positive eigenvalues ra − rw, rb − rw, rab − rw, respectively.
For the other cases, the discussion is similar, we omit it here.
3.2 Cycling treatment
fa = 1, fb = fab = 0 or fb = 1, fa = fab = 0
When fa = 1, fb = fab = 0, system (1.2) becomes
| (3.1) |
It has at most five possible steady state
The basic reproduction number is defined by
Theorem 3.3
When fa = 1, fb = fab = 0, the existence and stability of equilibria are described in Table 3.
Table 3.
Existence and Stability Chart for System (1.2) with Cycling Treatment.
| Condition | BRN | Cases | E0 | Ea | Eab | Ew,a | Eb,ab | |
|---|---|---|---|---|---|---|---|---|
| ra < min{rw + h, rb + h, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | stable | * | * | * | |||
| rab < min{rw + h, ra, rb + h} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | stable | * | * | |||
| rw + h < min{ra, rb + h, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | * | stable | * | |||
| rb + h < min{rw + h, ra, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | * | * | stable |
Here * represents that if the equilibrium exists, it is unstable.
Proof
-
When fa = 1, fb = fab = 0, the Jacobian matrix of system (1.2) at the semitrivial equilibrium Ea isIt follows that ra − rw − h, ra − rb − h, ra − rab are the eigenvalues of JEa, the other two eigenvalues of JEa are the roots of the quadratic polynomial equation
In view of the assumption and above discussion, the existence and stability the equilibrium Ea can be obtained. For the other equilibria, the discussion is similar, we omit it here.
The existence and stability of Eab is similar to that of Ea, we omit it.
-
By the expression of Ew,a, we find that when rw + h < ra and Λ < d(c + rw + b)/b, the semitrivial equilibrium Ew,a exists. For the convenience of discussion, we denoteThen the Jacobian matrix of system (3.2) at the equilibrium Ew,a = ((c+rw +f h)/b, ŷw, ŷa, 0, 0) has the formIt is easy to see that rw − rb and rw + h − rab are the eigenvalues of JEw,a. After some algebra, we can find that the other three eigenvalues of JEw,a are the roots ofwith
From the expressions of a1, a2, a3, it is easy to see that under the assumption rw + h < min{ra, rb + h, rab}, we have a1, a2, a3 > 0 and a1a2 − a3 > 0. Therefore, by Routh-Hurwitz criteria (Korn and Korn, 2000), the local stability of Ew,a is obtained. Equilibria Ea, Eab and Eb,ab are unstable since their corresponding Jacobian matrices have positive eigenvalues ra − rw − h, rab − rw − h, rb − rw respectively.
The existence and stability discussion of Eb,ab is similar to that of Ew,a, we omit it here.
The case that fb = 1, fa = fab = 0 can be analyzed similarly and analogue results can be obtained.
3.3 50-50 treatment , fab = 0
When , fab = 0, system (1.2) becomes
| (3.2) |
It has at most five possible steady state
where
The basic reproduction number is defined by
Theorem 3.4
When fa = fb = 1/2, fab = 0, the existence and stability of equilibria can be summarized in Table 4.
Table 4.
Existence and Stability Chart for System (1.2) with 50-50 Treatment.
| Condition | BRN | Cases | E0 | Eab | Ea,ab | Eb,ab | Ẽ | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | ||
| R0 > 1 | unstable | stable | * | * | * | ||||
|
|
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | ||
| R0 > 1 | unstable | * | stable | * | * | ||||
|
|
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | ||
| R0 > 1 | unstable | * | * | stable | * | ||||
|
|
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | ||
| R0 > 1 | unstable | * | * | * | stable |
Here * represents that if the equilibrium exists, it is unstable.
Proof
We find the steady states of system (3.2) by equating the derivatives on the left-hand sides to zero and solving the resulting algebraic equations. The discussion (acquirement) of the trivial or semitrivial equilibria are easy, we omit it here. Now we consider the existence of the positive equilibrium.
From the corresponding second equilibrium equation of (3.2), we obtain the solution . Substituting into the corresponding third, fourth, fifth equilibrium equations of (3.2), we obtain
| (3.3) |
| (3.4) |
| (3.5) |
Combining equations (3.3), (3.4), (3.5) with the first one of (3.2), after some calculation we obtain that
Thus, when rw + h < min{ra + h/2, rb + h/2, rab} and , system (3.2) has a unique componentwise positive equilibrium Ẽ.
Linearizing system (3.2) about the positive equilibrium (x̃, ỹw, ỹa, ỹb, ỹab) yields the Jacobian matrix
Substituting x̃ = (c + rw + h)/b into JẼ and expanding the determinant of the obtained matrix λI−JẼ by the second row, after some calculation, it can be seen that the eigenvalues are , and the roots of
where
It is noted that in the expression of a3, we have used the relation
From the expressions of a1, a2, a3, it is clear that under the assumption rw + h < min{ra + h/2, rb + h/2, rab}, we have a1, a2, a3 > 0 and a1a2 − a3 > 0. Therefore, by Routh-Hurwitz criteria (Korn and Korn, 2000), the stability of the positive equilibrium Ẽ is established.
3.4 Combination treatment
fa = fb = 0, fab = 1.
When fa = fb = 0, fab = 1, system (1.2) becomes
| (3.6) |
The basic reproduction number is defined as
It has at most five possible steady state
Similar to the discussion of the case fa = 1, fb = fab = 0, we have the following conclusion.
Theorem 3.5
When fa = fb = 0, fab = 1, the existence and stability of equilibria of system (3.6) are described in Table 5.
Table 5.
Existence and Stability Chart for System (1.2) with Combining Treatment.
| Condition | BRN | Cases | E0 | Eab | Ea,ab | Eb,ab | Ew,ab | |
|---|---|---|---|---|---|---|---|---|
| rab < min{rw + h, ra + h, rb + h} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | stable | * | * | * | |||
| ra + h < min{rw + h, rb + h, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | stable | * | * | |||
| rb + h < min{rw + h, ra + h, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | * | stable | * | |||
| rw + h < min{ra + h, rb + h, rab} |
|
R0 < 1 | stable | ∄ | ∄ | ∄ | ∄ | |
| R0 > 1 | unstable | * | * | * | stable |
Here * represents that if the equilibrium exists, it is unstable.
4 Numerical Simulations
In this section, we perform some numerical simulations on the two models to illustrate the results obtained in sections 2 and 3. For the purpose of simulations, we fix most of the parameters in the models and let the other parameters vary.
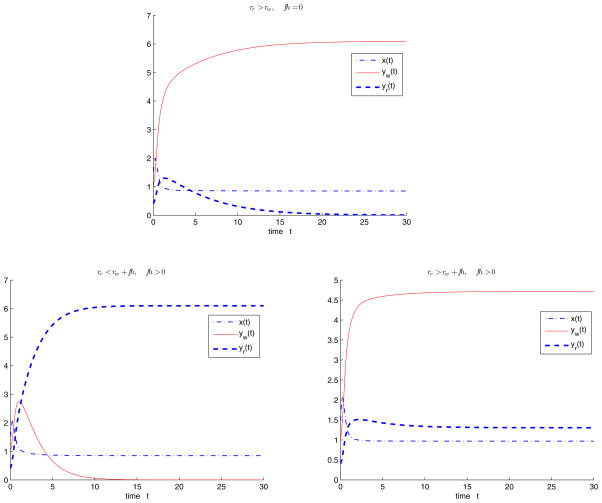
For the model with single antibiotic therapy, we fix the values of Λ, d, b, s, c as Λ = 10, d = 1, b = 2, s = 0.3, c = 1.5 and initial values x(0) = 1.4, yw (0) = 1.0, ya(0) = 0.4, let the parameters rw, rr, f, h vary such that R0 > 1. When rr > rw and f h = 0, the semitrivial equilibrium with the wild type strain Ew is stable (Figure 4.1(i)). When rr < rw + f h and f h > 0, the semitrivial equilibrium with the resistant strain Er is stable (Figure 4.1(ii)). When rr > rw + f h and f h > 0, the endemic equilibrium with both the wild type strain and resistant strain Ẽ is stable (Figure 4.1(iii)).
Figure 4.1.
Solution trajectories of the model with a single antibiotic therapy, the parameters Λ = 10, d = 1, b = 2, s = 0.3, c = 1.5 and initial values x(0) = 1.4, yw (0) = 1, yr (0) = 0.4 are fixed and the parameters rw, rr, f, h vary such that R0 > 1. (i) When rr > rw and f h = 0, the semitrivial equilibrium Ew is stable; (ii) when rr < rw + f h and f h > 0, the semitrivial equilibrium Er is stable; (iii) when rr > rw + f h and f h > 0, the endemic equilibrium Ẽ is stable.
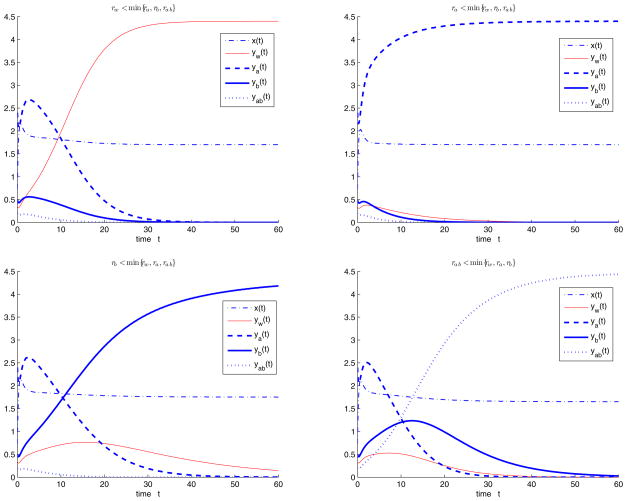
For the model with multiple antibiotic therapies, we first consider the case in the absence of treatment, that is, fa = fb = fab = 0. We choose parameters Λ = 10, d = 2, b = 1, q = 0.1, s = 0.3, c = 1.5, h = 0.2 and initial values x(0) = 0.65, yw (0) = 0.35, ya(0) = 2.4, yb(0) = 1.5, yab(0) = 0.2, and let rw, ra, rb, rab and h vary such that R0 > 1. When rw < min{ra, rb, rab}, the semitrivial equilibrium with the wild type strain Ew is stable (Figure 4.2(i)). When ra < min{rw, rb, rab}, the semitrivial equilibrium with the resistant strain A Ea is stable (Figure 4.2(ii)). When rb < min{rw, ra, rab}, the semitrivial equilibrium with the resistant strain B Eb is stable (Figure 4.2(iii)). When rab < min{rw, ra, rb}, the semitrivial equilibrium with the resistant strains A and B Eab is stable (Figure 4.2(iv)).
Figure 4.2.
System (1.2) with no treatment, i.e. fa = fb = fab = 0, we choose parameters Λ = 10, d = 2, b = 1, q = 0.1, s = 0.3, c = 1.5, h = 0.2, initial values x(0) = 0.65, yw (0) = 0.35, ya(0) = 2.4, yb(0) = 0.5, yab(0) = 0.2, and let rw, ra, rb, rab and h vary such that R0 > 1. (i) When rw < min{ra, rb, rab}, the semitrivial equilibrium Ew is stable; (ii) when ra < min{rw, rb, rab}, the semitrivial equilibrium Ea is stable; (iii) when rb < min{rw, ra, rab}, the semitrivial equilibrium Eb is stable; (iv) when rab < min{rw, ra, rb}, the semitrivial equilibrium Eab is stable.
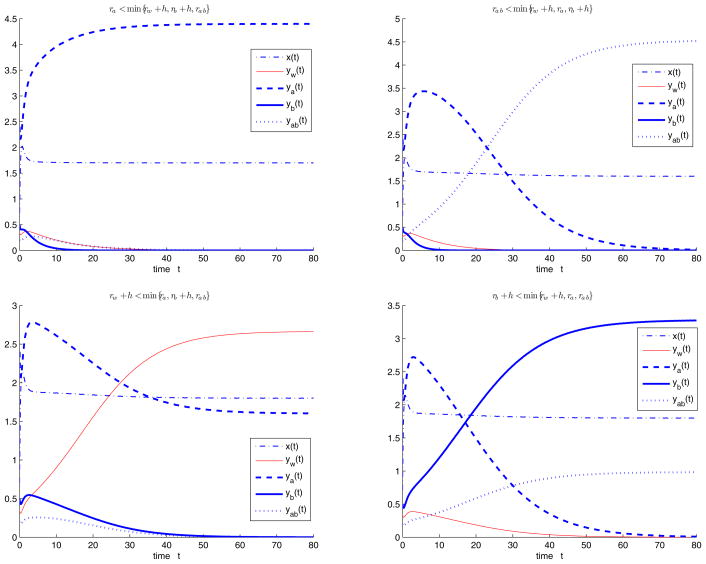
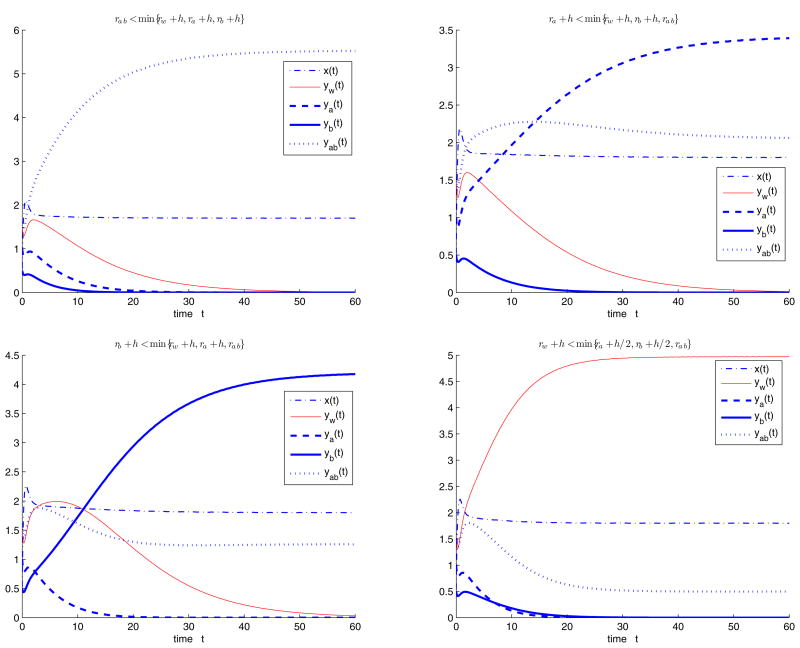
Next we consider the case with cycling treatment, that is, fa = 1, fb = fab = 0. Choose parameters Λ = 10, d = 2, b = 1, q = 0.1, s = 0.3, c = 1.5, h = 0.2 initial values x(0) = 0.65, yw (0) = 0.35, ya(0) = 2.4, yb(0) = 0.5, yab(0) = 0.2, and let rw, ra, rb, rab and h vary such that R0 > 1. When ra < min{rw + h, rb + h, rab}, the semitrivial equilibrium with resistant strain A Ea is stable (Figure 4.3(i)). When rab < min{rw + h, ra, rb + h}, the semitrivial equilibrium with the resistant strains A and B Eab is stable (Figure 4.3(ii)). when rw + h < min{ra, rb + h, rab}, the semitrivial equilibrium with wild type strain and resistant strain A Ew,a is stable (Figure 4.3(iii)). (iv) when rb + h < min{rw + h, ra, rab}, the semitrivial equilibrium with resistant strain B and both strains Eb,ab is stable (Figure 4.3(iv)).
Figure 4.3.
System (1.2) with cycling treatment, i.e., when fa = 1, fb = fab = 0, we choose parameters Λ = 10, d = 2, b = 1, q = 0.1, s = 0.3, c = 1.5, h = 0.2, initial values x(0) = 0.65, yw (0) = 0.35, ya(0) = 2.4, yb(0) = 0.5, yab(0) = 0.2, and let rw, ra, rb, rab and h vary such that R0 > 1. (i) When ra < min{rw + h, rb + h, rab}, the semitrivial equilibrium Ea is stable; (ii) when rab < min{rw + h, ra, rb + h}, the semitrivial equilibrium Eab is stable; (iii) when rw + h < min{ra, rb + h, rab}, the semitrivial equilibrium Ew,a is stable; (iv) when rb + h < min{rw + h, ra, rab}, the semitrivial equilibrium Eb,ab is stable.
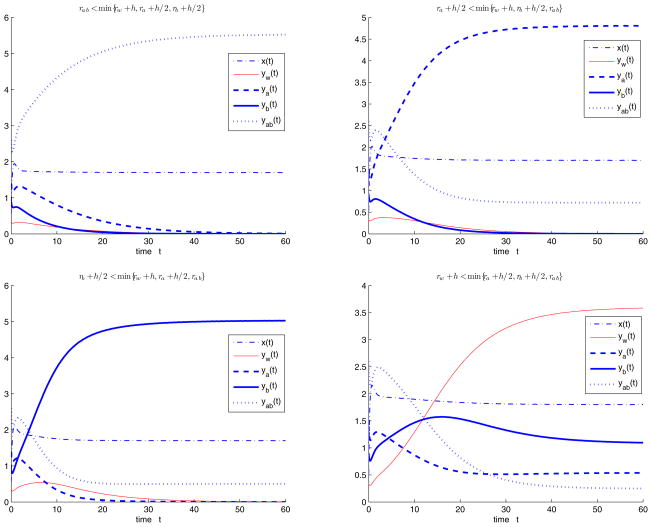
Now we consider the case with 50-50 treatment, that is, , fab = 0, Choose the parameters Λ = 10, d = 1, b = 1, q = 0.1, s = 0.3, c = 1.5, h = 0.2, initial values x(0) = 0.45, yw (0) = 0.35, ya(0) = 1.4, yb(0) = 0.9, yab(0) = 2.6, and let rw, ra, rb, rab and h vary such that R0 > 1. When rab < min{rw + h, ra + h/2, rb + h/2}, the semitrivial equilibrium with both resistant strains A and B Eab is stable (Figure 4.4(i)). When ra +h/2 < min{rw +h, rb +h/2, rab}, the semitrivial equilibrium with resistant strain A and both resistant strains A and B Ea,ab is stable (Figure 4.4(ii)). When rb + h/2 < min{rw + h, ra + h/2, rab }, the semitrivial equilibrium with resistant strain B and both resistant strains A and B Eb,ab is stable (Figure 4.4(iii)). When rw +h < min{ra +h/2, rb +h/2, rab}, the positive equilibrium with all strains Ẽ is stable (Figure 4.4(iv)).
Figure 4.4.
System (1.2) with 50-50 treatment, i.e., when , fab = 0, we choose the same parameters Λ = 10, d = 1, b = 1, q = 0.1, s = 0.3, c = 1.5, h = 0.2, the same initial values x(0) = 0.45, yw (0) = 0.35, ya (0) = 1.4, yb(0) = 0.9, yab(0) = 2.6, and let rw, ra, rb, rab and h vary such that R0 > 1. (i) When rab < min{rw + h, ra + h/2, rb + h/2}, the semitrivial equilibrium Eab is stable;(ii) when ra + h/2 < min{rw + h, rb + h/2, rab }, the semitrivial equilibrium Ea,ab is stable; (iii) when rb + h/2 < min{rw + h, ra + h/2, rab}, the semitrivial equilibrium Eb,ab is stable; (iv) when rw + h < min{ra + h/2, rb + h/2, rab}, the positive equilibrium Ẽ is stable.
Finally we consider the case with combining treatment, that is, fa = fb = 0, fab = 1. Choose parameters Λ = 10, d = 1, b = 1, q = 0.1, s = 0.3, c = 1.5, h = 0.2, initial values x(0) = 0.45, yw (0) = 1.5, ya(0) = 1.0, yb(0) = 0.5, yab(0) = 1.6, and let rw, ra, rb, rab and h vary such that R0 > 1. When rab < min{rw + h, ra + h, rb + h}, the semitrivial equilibrium with both resistant strains A and B Eab is stable (Figure 4.5(i)). When ra + h < min{rw + h, rb + h, rab}, the semitrivial equilibrium with resistant strain A and both resistant strains A and B Ea,ab is stable (Figure 4.5(ii)). When rb + h < min{rw + h, ra + h, rab}, the semitrivial equilibrium with resistant strain B and both resistant strains A and B Eb,ab is stable (Figure 4.5(iii)). When rw + h < min{ra + h, rb + h, rab}, the semitrivial equilibrium with the wild type strain and both resistant strains A and B Ew,ab is stable (Figure 4.5(iv)).
Figure 4.5.
System (1.2) with combining treatment, i.e., when fa = fb = 0, fab = 1, we choose the same parameters Λ = 10, d = 1, b = 1, q = 0.1, s = 0.3, c = 1.5, h = 0.2, the same initial values x(0) = 0.45, yw (0) = 1.5, ya(0) = 1.0, yb(0) = 0.5, yab(0) = 1.6, and let rw, ra, rb, rab and h vary such that R0 > 1. (i) When rab < min{rw + h, ra + h, rb + h}, the semitrivial equilibrium Eab is stable; (ii) when ra + h < min{rw + h, rb + h, rab}, the semitrivial equilibrium Ea,ab is stable; (iii) when rb + h < min{rw + h, ra + h, rab}, the semitrivial equilibrium Eb,ab is stable; (iv) when rw + h < min{ra + h, rb + h, rab}, the semitrivial equilibrium Ew,ab is stable.
5 Discussion
We provided qualitative analysis of models for different treatment protocols to prevent antibiotic resistance. For the model with a single antibiotic therapy, we carried out a global qualitative analysis and studied the existence and stability of the disease-free and endemic equilibria. In terms of the basic reproduction number , our results indicate that when R0 < 1, then the disease-free equilibrium is globally asymptotically stable. If R0 > 1, when the rate of patients infected with wild type bacteria recover from the wild type infected compartment (f h) is less than the difference of the rates of patients infected with resistant bacteria (rr) and wild type (rw) recover from the infection in the absence of treatment, the endemic equilibrium with both strains is globally stable; when f h > rr − rw, the semitrivial equilibrium with the resistant strain is globally stable. Which shows that preventing the initiation or enhancing the discontinuation of unnecessary antibiotic therapy will have a great impact to preserve antibiotic effectiveness (Bonhoeffer et al., 1997).
For the model with multiple antibiotic therapies, stability of various equilibria are analyzed. The model allows quantification of the consequences of different therapy regimens and hospital controls in terms of the complex dynamics of competing bacterial strains (Bonhoeffer et al., 1997). The results show that, in the absence of treatment, when R0 < 1, the disease free equilibrium is stable, when R0 > 1, the semitrivial equilibrium with the strain which has the lowest recovery rate is stable. The results for the cases with 50-50 treatment, cycling treatment and combining treatment demonstrate the essential difficulties in controlling the advance of resistant bacterial infections in hospitals. When more than one antibiotic is employed, as shown by Bonhoeffer et al. (1997), Bergstrom et al. (2004), Reluga (2005), D’Agata et al. (2008), cycling use of different antibiotics is not as good as that with a combination of antibiotics.
An ultimate goal is to validate these models by applying it to a particular hospital to compare the predicted endemic states with the prevalence data. We leave this for future study.
Acknowledgments
We would like to thank the referee and the handling Editor for their helpful comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amarasekar P. Interference competition and species coexistence. Proc R Soc Lond B. 2002;269:2541–2550. doi: 10.1098/rspb.2002.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin DJ, Anderson RM. Studies of antibiotic resistance within the patient, hospitals and the community using simple mathematical models. Phil Trans R Soc Lond B. 1999;354:721–738. doi: 10.1098/rstb.1999.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin DJ, Bonten MJM, Weinstein RA, Slaughter S, Anderson RM. Vancomycin-resistant enterococci in intensive-care hospital settings: Transmission dynamics, persistence, and the impact of infection control programs. Proc Natl Acad Sci USA. 1999;96:6908–6913. doi: 10.1073/pnas.96.12.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergstrom CT, Lo M, Lipsitch M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc Natl Acad Sci USA. 2004;101:13285–13290. doi: 10.1073/pnas.0402298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boldin B, Bonten MJ, Diekmann O. Relative effects of barrier precautions and topical antibiotics on nosocomial bacterial transmission: results of multi-compartment models. Bull Math Biol. 2007;69:2227–2248. doi: 10.1007/s11538-007-9205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonhoeffer S, Lipsitch M, Levin BR. Evaluating treatment protocols to prevent antibiotic resistance. Proc Natl Acad Sci USA. 1997;94:12106–12111. doi: 10.1073/pnas.94.22.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonten MJM, Austin DJ, Lipsitch M. Understanding the spread of antibiotic resistant pathogens in hospitals: mathematical models as tools for control. Clin Infect Dis. 2001;33:1739–1746. doi: 10.1086/323761. [DOI] [PubMed] [Google Scholar]
- 8.Brauer F, Castillo-Chavez C. Mathematical Models in Population Biology and Epidemiology. Springer; New York: 2000. [Google Scholar]
- 9.Bremermann HJ, Thieme HR. A competitive exclusion principle for pathogen virulence. J Math Bio. 1989;27:179–190. doi: 10.1007/BF00276102. [DOI] [PubMed] [Google Scholar]
- 10.Busenberg S, van den Driessche P. Analysis of a disease transmission model in a population with varying size. J Math Biol. 1990;28:257–270. doi: 10.1007/BF00178776. [DOI] [PubMed] [Google Scholar]
- 11.D’Agata EMC, Dupont-Rouzeyrol M, Magal P, Olivier D, Ruan S. The impact of different antibiotic regimens on the emergence of antimicrobial-resistant bacteria. PLoS ONE. 2008 doi: 10.1371/journal.pone.0004036. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Agata EMC, Magal P, Olivier D, Ruan S, Webb GF. Modeling antibiotic resistance in hospitals: The impact of minimizing treatment duration. J Theor Biol. 2007;249:487–499. doi: 10.1016/j.jtbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diekmann O, Heesterbeek JAP, Metz JAJ. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J Math Biol. 1999;28:365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 14.Daxboeck F, et al. Economic burden associated with multi-resistant Gram-negative organisms compared with that for methicillin-resistant Staphylococcus au-reus in a university teaching hospital. J Hosp Infect. 2006;62:214–218. doi: 10.1016/j.jhin.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 16.Grundmann H, Hellriegel B. Mathematical modeling: A tool for hospital infection control. Lancet Infect Dis. 2006;6:39–45. doi: 10.1016/S1473-3099(05)70325-X. [DOI] [PubMed] [Google Scholar]
- 17.Klevens RM, et al. Invasive methicillin-resistant Staphylococcu aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 18.Korn GA, Korn TM. Theorems and Formulas for Reference and Review. Dover Publications; Mineola, NY: 2000. Mathematical Handbook for Scientists and Engineers: Definitions. [Google Scholar]
- 19.Levy SB, Marshall B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 20.Reluga TC. Simple models of antibiotic cycling. Math Med Biol. 2005;22:187–208. doi: 10.1093/imammb/dqi002. [DOI] [PubMed] [Google Scholar]
- 21.Spellberg B, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 22.Smith HL, Li B. Competition for essential resources: a brief review. Fields Institute Communications. 2003;36:213–227. [Google Scholar]
- 23.Temime L, et al. The rising impact of mathematical modeling in epidemiology: Antibiotic resistance research as a case study. Epidemiol Infec. 2008;136:289–298. doi: 10.1017/S0950268807009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb GF, D’Agata EMC, Magal P, Ruan S. A model of antibiotic-resistant bacterial epidemics in hospitals. Proc Natl Acad Sci USA. 2005;102:13343–13348. doi: 10.1073/pnas.0504053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webb GF, Horn MA, D’Agata EMC, Moellering RC, Ruan S. Competition of hospital-acquired and community-acquired methicillin-resistant Staphylococcus aureus strains in hospitals. J Biol Dynamics. 2010;4:115–129. doi: 10.1080/17513750903026411. [DOI] [PMC free article] [PubMed] [Google Scholar]