Abstract
T cell-mediated viral clearance is classically attributed to the CD8+ T cell subset, but CD4+ T cells can sometimes assume this role. One such instance was illustrated by the immunization of C57BL/6 mice with HIV-1 envelope, followed by challenge with a recombinant Sendai virus (rSeV-env) carrying a gene for secreted HIV-1 envelope protein. Vaccinated mice that lacked both B cells (μMT) and CD8+ T cells controlled virus, but control was lost when CD4+ T cells were depleted. To explain this activity, we questioned whether CD4+ T cells might utilize perforin for killing of MHC class II-positive targets. We also asked if the process might depend on IFN-γ, which can upregulate MHC expression and enhance T cell recruitment to sites of virus challenge. To address these possibilities, we vaccinated perforin-KO mice with HIV-1 envelope and challenged them with rSeV-env. We found that perforin was not required for (1) CD4+ T cell homing to the site of virus challenge, (2) expression of Th1 and Th2 cytokines (including IFN-γ), or (3) virus clearance. To determine if IFN-γ was required for protection, we repeated experiments in IFN-γ-KO animals. In this case, significant protection was lost, although the CD4+ T cells trafficked readily to the site of infection. In fact, local CD4+ T cell numbers in vaccinated IFN-γ- KO mice exceeded those in wild type animals. In both cases, cells were αß TCR+, NK-1.1–, and CD44+, typifying an activated CD4+ T cell subset. Taken together, our results showed that HIV-1 envelope recombinant virus clearance was dependent on CD4+ T cells and IFN-γ, but occurred in the absence of B cells, CD8+ T cells, or perforin.
Introduction
Antigen-specific CD4+ T cells play important, but varied roles in experimental models of viral immunity. Their presence is generally required for the activation of B cells and production of virus-specific neutralizing antibodies.1–3 CD4+ T cells also assist CD8+ cytotoxic T lymphocyte function.4 Although most researchers agree that CD4+ T cells are “helpers,” there are only a few definitive examples of CD4+ T cell-mediated virus control in the absence of B cell or CD8+ T cell input.5–7
One clear example of CD4+ T cell-mediated virus protection was revealed by our studies of HIV-1 envelope-specific T cells in mice.5,8–10 Because there was (and remains) no gold standard mouse model for HIV-1 infection, envelope-vaccinated mice were challenged with a recombinant virus (Sendai virus, SeV) engineered to encode HIV-1 envelope gp120 protein. The SeV vehicle was specifically designed to carry the gene for secreted HIV-1 envelope protein so that the foreign antigen would not tag viruses or SeV-infected cells for clearance by antibodies. With this system, HIV-1 envelope-specific CD4+ T cells were shown to clear recombinant virus following intranasal challenge in the absence of both B cells and CD8+ T cell partners.5
Recent human and mouse studies have suggested that CD4+ T cells can utilize perforin, a pore-forming polymer often associated with CD8+ T cells, to mediate direct MHC class II-restricted killing of virus-infected targets in vitro and in vivo.11–15 For example, murine influenza virus-specific perforin-positive CD4+ T cells were shown to kill virus-infected targets in vitro.13,14 Additionally, human CMV-specific CD4+ T cells from chronically infected patients were shown to exhibit direct cytolytic activity associated with the intracellular expression of perforin.15 In the CMV system, direct cytolytic activity by CD4+ T cells was associated with interferon (IFN)-γ expression, a cytokine that was also implicated as necessary for CD4+ T cell-mediated clearance of gamma herpes virus in a murine model.6 IFN-γ can upregulate MHC class II glycoprotein expression on target cells to enhance cytotoxicity, and can also increase CD4+ T cell trafficking to a site of virus infection.16–18
To determine the relevance of both perforin and IFN-γ to the HIV-1 envelope-specific CD4+ T cell “protector” function in our system, we vaccinated and challenged both CD8-depleted perforin knock-out (KO) and IFN-γ-KO mice. Our results with perforin-KO mice showed that envelope-specific CD4+ T cells did not utilize perforin for protection in our system. However, when experiments were conducted in IFN-γ-KO mice, significant protection was ablated. This was despite a vigorous influx of activated TCR αß+ CD4+ T cells to the site of virus infection following vaccination and challenge, coincident with a cytokine profile skewed toward Th2 products. Taken together, our results illustrate that the mechanism for rSeV-env virus clearance by CD4+ T cells is dependent on IFN-γ, but can occur in the absence of B cells, CD8+ T cells, or perforin.
Materials and Methods
Mice
Female C57BL/6J (B6, H2b) and KO mice for perforin (Prftm1Sd2) or IFN-γ (Ifngtm1Ts) genes (on a B6 background) were purchased from the Jackson Laboratory (Bar Harbor, ME). Ig-/- μMT mice on a B6 background were bred at St. Jude Children's Research Hospital (SJCRH). Animals were housed under specific pathogen-free conditions in a BL1/BL2 or BL3 containment area at the SJCRH animal facility, as specified by the Association for Assessment and Accreditation for Laboratory Animal Care (AAALAC) guidelines. All studies were conducted under AAALAC guidelines. Mice were approximately 2 months of age at the initiation of the immunization protocols. The μMT mice were confirmed to lack B cells by FACSCalibur analysis with a B220-specific antibody. Data analyses with BD CellQuest Pro (Becton Dickinson, Franklin Lakes, NJ) showed that B cells represented <0.5% of the lymphocyte population in these animals.
Immunogens/immunization
Mice were immunized as described previously8–10 with a recombinant DNA vector expressing HIV-1 envelope from a CCR5-tropic primary isolate, HIV-1UG92005 (UG, GenBank accession no. AF338704). The DNA vaccine was prepared by incorporating envelope sequence (gp140) into a kanamycin-selectable pVVKan vector containing a cytomegalovirus enhancer/promoter, cytomegalovirus intron A, tissue plasminogen activator leader, and bovine growth hormone poly(A) sequence. The plasmid was purified (EndoFree Plasmid Giga kit, Qiagen,Valencia, CA) and reconstituted in phosphate-buffered saline (PBS) before injection into mice. Mice were primed and boosted (with a ≥ 3-week interval) at least once with DNA with a 100 μg dose (administered as 50 μg per gastrocnemius muscle). Prior to challenge experiments, mice were also boosted once by intraperitoneal (ip) injection with a recombinant vaccinia virus (WRWT, bromodeoxyuridine-selected, 107 PFU/mouse) expressing the same UG92005 gp140 envelope protein. Experimental details for individual experiments are described in the figure legends.
Recombinant Sendai virus challenge
The HIV-1UG92005 gp120 envelope gene was cloned between Sendai virus P and M genes and virus was rescued as described previously.5,19–21 Mice were challenged at least 3 weeks after immunization. In μmt mouse experiments, animals were treated by ip injections with the GK1.5 mAb (to remove CD4+ T cells) or the 2.43.1 mAb (to remove CD8+ T cells22,23) on days −5, −3, −1, +1, and +3 relative to rSeV-env challenge. The antibodies were administered as ascites fluid diluted in PBS. Splenocytes were stained and checked to ensure cell depletion using flow cytometry with non-cross-reactive mAbs (BD Biosciences Pharmingen, Franklin Lakes, NJ) to CD4 (RM4–4) and CD8β (53–5.8). Whenever experiments included perforin-KO or IFN-γ-KO mice, all mice [both KO and B6 wild type (wt) mice] were treated by ip injections of the 2.43.1 mAb on days −5, −3, −1, +1, and +3 relative to rSeV-env challenge. All challenges were by intranasal inoculation (see the figure legends for virus dose).
Cytokine measurements
Bronchoalveolar lavage (BAL) was performed on euthanized, virus-infected mice by exposing the trachea, inserting catheters, and washing the lungs each with 1 ml of PBS × 3 (3 ml total). Wash samples were centrifuged to remove cellular material and the supernatants were tested for the presence of four different cytokines using a cytokine bioplex technology (BioRad, Hercules, CA).
Membrane stainin
To characterize cell populations in the respiratory tract airways, the site of virus challenge, cells from the BAL were analyzed by cytofluorimetry. BAL cells were first incubated on a 60 × 15-mm cell culture dish for 1 h at 37°C in a 10% CO2 incubator to remove macrophages. Nonadherent cells were removed by gentle washing. Cells were stained with fluorochrome-conjugated antibody reagents including anti-CD4 (RM4-5), anti-CD3 (145-2C11), anti-TCR αβ (H57-597), anti-TCR γδ (TCR- GL3), anti-NK-1.1(PK136), and anti-B220 (RA36B2, BD Pharmingen, Franklin Lakes, NJ; eBiosciences, San Diego, CA). Data were collected on a BD FACSCalibur and analyzed using FlowJo Software.
Virus titers
The lungs were removed sterilely, washed 4× in PBS, and homogenized in a total volume of 1 ml PBS. The suspensions were centrifuged at 2000 × g for 10 min to clear cellular debris. Virus titers were determined as measured by tissue culture infectious dose-50 (TCID50). TCID50 measurements were performed by plating serial 10× dilutions of lung suspension on LLC-MK2 cells with minimal essential medium containing 0.1% bovine serum albumin in the presence of 5 μg/ml of acetylated trypsin and 50 μg/ml of gentamicin. Cell supernatants were collected after 4–5 days of incubation and mixed 1:1 with chicken red blood cells (0.5%) in PBS for hemagglutination detection. TCID50 values were calculated by the Reed–Muench formula.24
Statistical analyses
Mann–Whitney tests were performed using GraphPad Prism software (GraphPad Software, Inc. San Diego, CA).
Results
Envelope-specific CD4+ T cells protect against an envelope-recombinant virus infection in the absence of CD8+ T cells or B cell activity
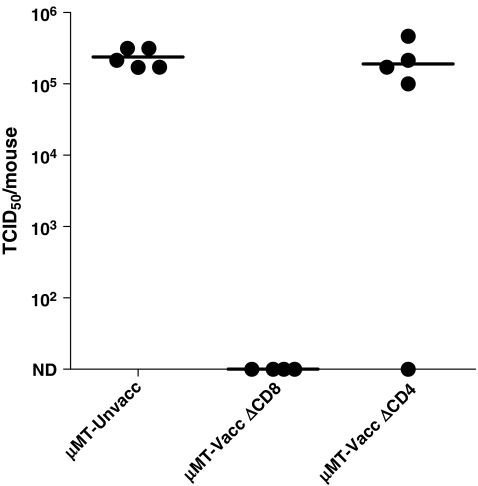
Our previous studies demonstrated that the priming of mice with HIV-1 envelope recombinant antigens elicited a protective response against infection with an envelope-recombinant challenge virus (rSeV-env5). The recombinant challenge virus encompassed a gene for HIV-1 envelope protein (gp120), which lacked the transmembrane region, to avoid the expression of the passenger gene on virus membranes or virus-infected cells and thus avoid antibody-mediated protection. In this system, protection occurred in the absence of both B cell and CD8+ T cell activity. An example of experimental results is shown in Fig. 1. In this experiment, μmt mice (mice lacking B cells, B6 background) were immunized with the HIV-1 envelope vaccine and then challenged with the recombinant Sendai virus expressing the UG92005 gp120 envelope (rSeV-env). On days −5, −3, −1, 1, and 3 relative to challenge, groups of animals were treated with either anti-CD8 or anti-CD4 antibodies. Five days after challenge, animals were sacrificed. As demonstrated in Fig. 1, the μmt mice that were treated with anti-CD8 antibodies before and after challenge exhibited extraordinary control of the rSeV-env. However, when CD4+ T cells were depleted from vaccinated μmt animals, significant protection was lost.5 Results encouraged a further investigation of the mechanism required for HIV-1 envelope-specific CD4+ T cell-dependent virus protection.
FIG. 1.
CD4+ but not CD8+ T cells or B cells are required for protection against HIV-1 envelope-recombinant virus challenge. μMT mice were vaccinated with DNA (D) and vaccinia virus (V) in a prime-boost regimen. DNA was administered intramuscularly at a dose of 100 μg (50 μg per gastrocnemius muscle). Vaccinia virus was administered intraperitoneally at a dose of 107 PFU/mouse. Inoculations were in the order D-D-D-V-D. Two months after the last injection, vaccinated and unvaccinated mice were challenged with rSeV-env. The vaccinated mice were treated with the GK1.5 antibody (to remove CD4+ cells, ΔCD4) or the 2.43 antibody (to remove CD8+ T cells, ΔCD8) on days −5, −3, −1, +1, and +3 relative to challenge with rSeV-env (1 × 105 PFU/animal). On day 5 following rSeV-env challenge, lungs were harvested to measure virus load (TCID50 measurements on LLC-MK2 cells). The Reed–Muench formula was used to calculate the TCID50. Each symbol represents the TCID50 of a different animal.
Vaccinated perforin-KO mice are protected from virus challenge
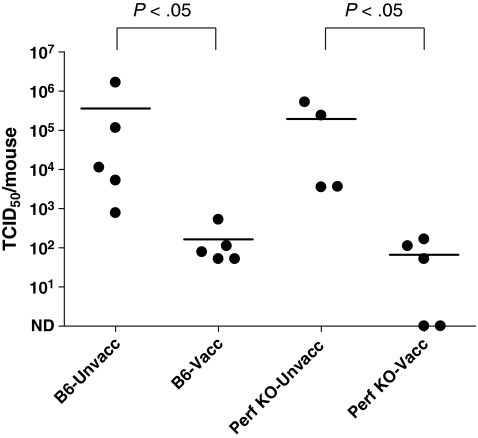
To determine if perforin played a role in protection, either via expression within the CD4+ T cells or via expression by a downstream effector, experiments were conducted in perforin-KO mice. In this set of experiments, groups of perforin-KO mice and wild type (B6) controls were immunized with the HIV-1 envelope vaccine and then challenged with rSeV-env. For all experiments with KO mice, every animal (KO and B6) was treated on days −5, −3, −1, 1, and 3 relative to challenge with the anti-CD8 antibodies to ensure that CD8+ T cells did not contribute to virus control. Five days after challenge, lungs were homogenized in PBS, and the virus in clarified supernatants was titered by TCID50 assays on LLC-MK2 cells. As shown in Fig. 2, the virus titers were substantially lower in vaccinated animals compared to controls for both perforin-KO and B6 wild type animals (p < 0.05, Mann–Whitney test). In repeat experiments, the protection in perforin-KO mice remained significant and trended toward better protection than that observed in the vaccinated B6 counterparts. These results showed that perforin was not required for viral clearance.
FIG. 2.
Vaccinated perforin-KO mice control virus challenge. Perforin-KO and wild type (B6) mice were vaccinated with DNA (D) and vaccinia virus (V) in a prime-boost regimen. Inoculations were in the order D-D-V. One month after the last injection, vaccinated and unvaccinated mice were challenged with rSeV-env (1 × 105 PFU/animal, intranasal administration). Mice were administered ip injections with the anti-CD8 2.43.1 mAb on days −5, −3, −1, +1, and +3 relative to rSeV-env challenge. On day 5 after challenge, groups of vaccinated challenge and groups of vaccinated and unvaccinated mice were sacrificed. Lungs were harvested and the titers of challenge virus in the lungs were determined by a TCID50 measurement on LLC- MK2 cells. The Reed–Muench formula was used to calculate the TCID50. Each symbol represents the TCID50 of a different animal. The levels of protection demonstrated in the vaccinated mice of both perforin-KO and wild type strains were statistically significant (p < 0.05).
CD4+ T cells migrate to the site of virus challenge in vaccinated mice
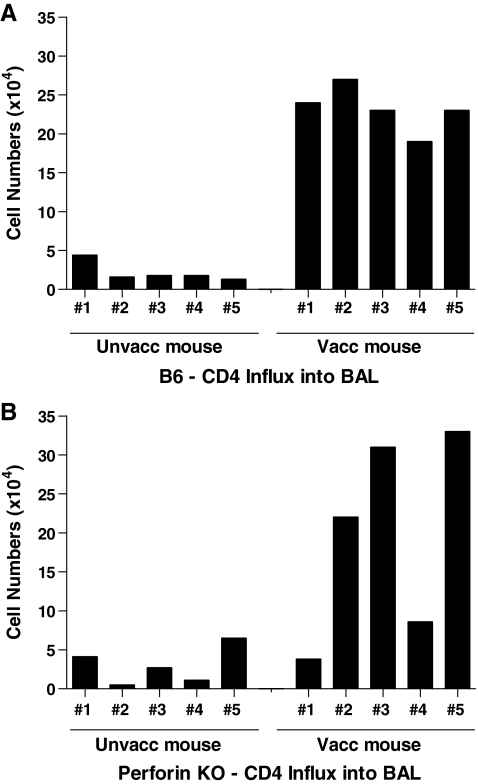
Our previous studies demonstrated an impressive influx of CD4+ T cells into the respiratory tract airways (the site of virus challenge) in vaccinated/challenged mice.5 To determine if the CD4+ T cell influx was similar between B6 and perforin-KO strains, lymphocytes in the BAL of vaccinated and unvaccinated animals were compared. As shown in Fig. 3, CD4+ T cells were detected in large numbers in the lung airways of vaccinated B6 animals on day 5 postchallenge. The cell numbers in most vaccinated perforin-KO animals were also greater than those in unvaccinated controls.
FIG. 3.
CD4+ T cells home to the lung airways upon virus challenge of perforin KO and wild type animals. Perforin-KO and B6 mice were vaccinated with DNA (D) and vaccinia virus (V) in a prime-boost regimen. Inoculations were in the order D-D-V. One month after the last injection, vaccinated and unvaccinated mice were challenged with rSeV-env (1 × 105 PFU/animal). Mice were given ip injections with the anti-CD8 2.43.1 mAb on days −5, −3, −1, +1, and +3 relative to rSeV-env challenge. BAL lymphocytes from individual vaccinated and unvaccinated animals were counted 5 days after challenge. The fraction of CD4+ T cells among BAL lymphocytes was determined by flow cytometry (samples were combined from each test group to ensure sufficient numbers for the CD4+ T cell analysis). Total lymphocyte numbers were multiplied by the CD4+ T cell fraction to determine the approximate CD4+ T cell count in the BAL of each animal.
The lung airways exhibit both Th1 and Th2 cytokines in challenged animals
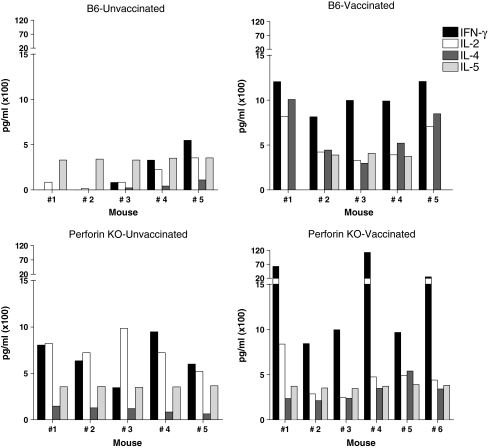
Our previous studies with B6 animals also demonstrated that the lung airways of challenged animals exhibited a mixed Th1/Th2 cytokine profile.5 As shown in Fig. 4, the perforin-KO mice were again similar to the B6 strain in that both Th1 and Th2 cytokines were observed in the BAL.
FIG. 4.
Cytokines detected in the lungs of vaccinated and unvaccinated perforin KO and wild type animals. Perforin-KO and wild type (B6) mice were vaccinated with DNA (D) and vaccinia virus (V) in a prime-boost regimen. Inoculations were in the order D-D-D-V. Two months after the last injection, vaccinated and unvaccinated mice were challenged with rSeV-env. Mice were given ip injections with the anti-CD8 2.43.1 mAb on days −5, −3, −1, +1, and +3 relative to rSeV-env challenge. BAL fluid from vaccinated and unvaccinated mice was examined for IL-2, INF-γ, IL-4, and IL-5 on day 5 after challenge. Results are shown for individual B6 (top) and perforin-KO (bottom) mice.
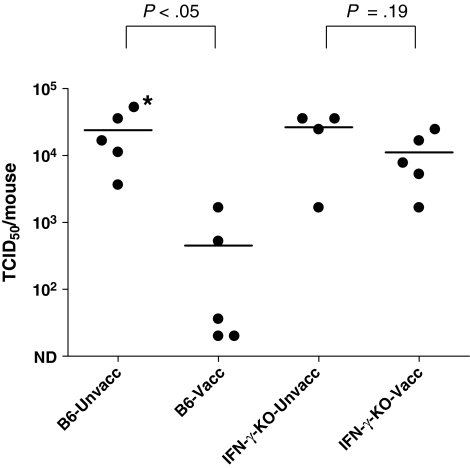
Protection is insignificant in IFN-γ-KO mice
Previous experiments in other systems have suggested that IFN-γ may be necessary for T cell maturation, upregulation of MHC class II on target cells,17 and homing of T cells to the site of virus challenge.18 We therefore questioned whether IFN-γ was required for CD4+ T cell-mediated protection against rSeV-env. To answer this question, we vaccinated and challenged IFN-γ-KO mice, after which lungs were tested for virus load. As before, all KO and B6 wild type animals were treated on days −5, −3, −1, 1, and 3 relative to challenge with the anti-CD8 antibodies. After challenge, lungs were homogenized in PBS, and the virus in clarified supernatants was titered by TCID50 assays on LLC-MK2 cells. As shown in Fig. 5, the challenge virus was significantly reduced in vaccinated B6 animals (p < 0.05, Mann–Whitney test), but not in vaccinated IFN-γ-KO animals (p = 0.19, Mann–Whitney test). The deficiency in CD4+ T cell-associated protection in IFN-γ-KO animals may have occurred at multiple levels, including the initial T cell response to vaccination, the reactivation of T cells upon virus challenge, the homing of cells to the site of infection, and the function of cells or downstream effectors at that site. Repeat experiments showed similar results in that there were some trends toward protection, but statistically significant virus control was not observed in vaccinated IFN-γ-KO animal sets. Results thus demonstrated that IFN-γ was required for significant virus protection against HIV-1 envelope recombinant virus in this system.
FIG. 5.
Vaccinated INF-γ-KO mice fail to control virus challenge. IFN-γ-KO and B6 mice were vaccinated with DNA (D) and vaccinia virus (V) in a prime-boost regimen. Inoculations were in the order D-D-V-D. One month after the last injection, vaccinated and unvaccinated mice were challenged with recombinant Sendai virus (2 × 103 PFU/animal, intranasal administration) and sacrificed on day 5. Mice were given ip injections with the anti-CD8 2.43.1 mAb on days −5, −3, −1, +1, and +3 relative to rSeV-env challenge. Lungs were harvested and the titers of challenge virus in the lungs were determined by a TCID50 measurement on LLC- MK2 cells. The Reed–Muench formula was used to calculate the TCID50. Each symbol represents the TCID50 of a different animal. The asterisk indicates that the titer reached the assay peak and may therefore have been higher than indicated. The level of protection demonstrated in the vaccinated B6 mice was statistically significant (p < 0.05), but protection was not significant in the interferon γ-KO mice (p = 0.19).
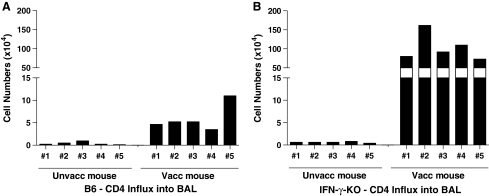
CD4+ T cells infiltrate lung airways in vaccinated IFN-γ-KO mice upon virus challenge
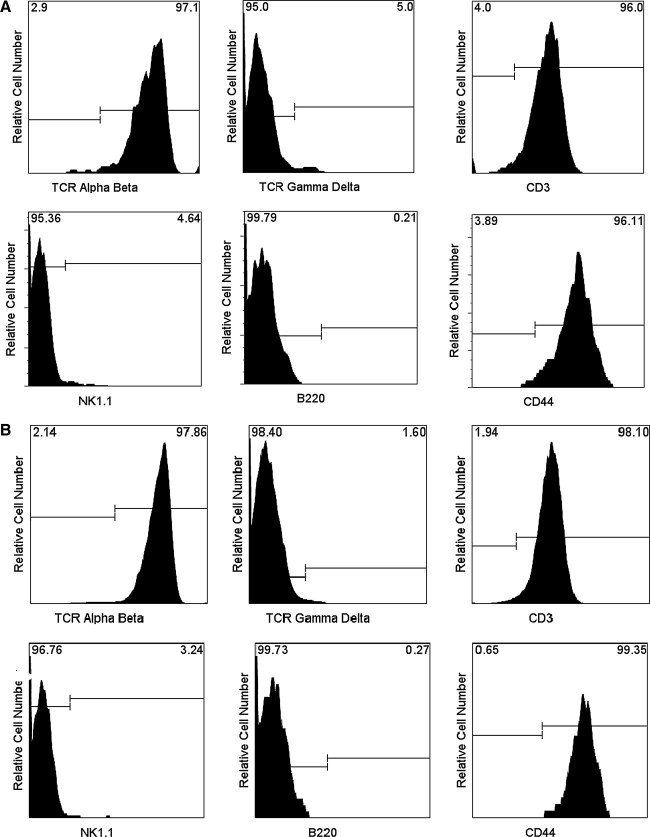
Based on previous suggestions that IFN-γ may be necessary for the homing of vaccinated CD4+ T cells to the site of virus infection, we questioned whether IFN-γ-KO animals had a general defect in the capacity of CD4+ T cells to traffic to the lung. To address this question, we examined lymphocytes in the BAL after rSeV-env challenge. Surprisingly, the numbers of CD4+ lymphocytes in the BAL of vaccinated IFN-γ-KO animals were significantly higher than those in vaccinated B6 controls (Fig. 6). CD4+ T cell phenotypes were similar between the two mouse strains (Fig. 7), in that CD4+ cells in the BAL of IFN-γ-KO and B6 animals were positive for TCR αβ (but not TCR γδ), CD3, the activation antigen CD44, but not NK-1.1 (suggesting that cells were not NK-T cells) or B220. Results demonstrated that there was no general defect in the capacity of CD4+ T cells to home to the site of virus challenge in IFN-γ-KO animals.
FIG. 6.
CD4+ T cells in vaccinated IFN-γ-KO mice home to the lung airways upon virus challenge. IFN-γ-KO and B6 mice were vaccinated with DNA (D) and vaccinia virus (V) in a prime-boost regimen. Inoculations were in the order D-D-V-D. One month after the last injection, vaccinated and unvaccinated mice were challenged with rSeV-env (2 × 103 PFU/animal). Mice were given ip injections with the anti-CD8 2.43.1 mAb on days −5, −3, −1, +1, and +3 relative to rSeV-env challenge. Results show the approximate number of CD4+ T cells in the BAL from individual B6 (A) and IFN-γ- KO (B) mice on day 5 postchallenge, as described in Fig. 3.
FIG. 7.
Activated CD4+ T-lymphocytes in the lung airways of vaccinated, challenged animals. The phenotype of airway-resident CD4+ cells was determined by FACS analyses. The cells were gated on lymphocytes, and then CD4+ cells. (A) Membrane markers are shown among CD4+ lymphocytes in B6 mice. (B) Membrane markers are shown among CD4+ lymphocytes in IFN-γ-KO mice. The majority of CD4+ T cells in the BAL were activated as indicated by membrane CD44 expression. Results were reproducible among animals. For example, among four vaccinated IFN-γ-KO animals tested in one experiment, the means and standard deviations for percentage positive cells within the gated CD4+ population were 97 ± 3 for CD3, 96 ± 6 for CD44, 2 ± 0.7 for NK1.1, and 0.5 ± 0.4 for B220.
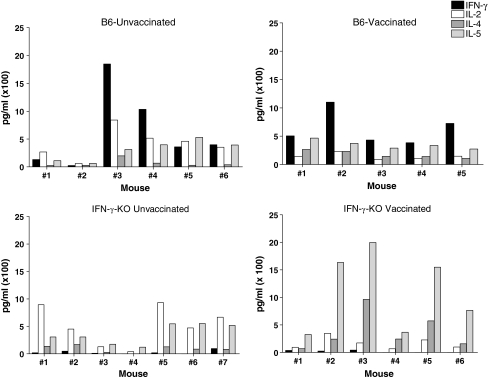
Cytokine production in the lung airways of challenged animals
The IFN-γ-KO mice were also tested for cytokine production in the BAL. Results are shown In Fig. 8. As expected, the IFN-γ-KO mice lacked IFN-γ and therefore showed a skewing of cytokines toward the Th2 profile as compared to the wild type B6 mouse strain.
FIG. 8.
Cytokines detected in the lungs of vaccinated IFN-γ-KO and wild type animals. IFN-γ-KO and B6 mice were vaccinated with DNA (D) and vaccinia virus (V) in a prime-boost regimen. Inoculations were in the order D-D-V-D. One month after the last injection, vaccinated and unvaccinated mice were challenged with rSeV-env. Mice were given ip injections with the anti-CD8 2.43.1 mAb on days −5, −3, −1, +1, and +3 relative to rSeV-env challenge. BAL fluids from vaccinated and unvaccinated mice were examined for IL-2, IFN-γ, IL-4, and IL-5. Results are shown for individual mice.
Discussion
Complex mechanisms of CD4+T cell-associated protection against rSeV-env
The study described in this report provided evidence that unlike the situation for some other viral systems, protection against an HIV-1-envelope recombinant virus challenge (rSeV-env) was independent of perforin, CD8+ T cells, or B cells, but dependent on CD4+ T cells and IFN-γ. These results emphasized that CD4+ T cells have capacities for viral clearance that surpass simple “help” for CD8+ T cell or B cell function. The vaccines did not need to be administered at the mucosal surface or at the site of draining lymph nodes25–28 to be effective. Rather, vaccine administration by intramuscular and intraperitoneal inoculations elicited CD4+ T cells able to home to lung airways and protect.
The lack of perforin dependence in the current study does not negate its importance in other systems. Influenza virus-specific CD4+ T cells have been demonstrated to have the capacity for direct perforin-mediated killing of virus-infected cells in vitro.14 For the study of influenza virus-specific CD4+ T cells, researchers designed model systems in which either B cell (μMT mice) or CD8+ T cell (nude mice) activities were removed.13 In these situations, in vitro-stimulated CD4+ T cells had the capacity to utilize both classical helper and perforin-mediated killer activity to protect against low-dose virus. When the virus load was increased, or when (in a different model) influenza virus-specific CD4+ T cells were tested in mice lacking both B cells and CD8+ T cells, activity was lost.13 It was also shown that CD4+ T cell activity was not dependent on IFN-γ.13,14 The influenza virus system thus differed from our rSeV-env system by mode of function, demonstrating the complexity of the CD4+ T cell-associated antiviral immune response.
A role for IFN-γ in protection against rSeV-env
IFN-γ has been repeatedly recognized as important for CD4+ T cell-mediated virus control6 as it can promote T cell maturation, enhance MHC class II expression on cytotoxic T cell targets, and enhance trafficking of CD4+ T cells to sites of viral infection.16–18 In our system, the dependence of viral clearance on IFN-γ may have occurred at multiple levels including envelope-induced induction of naive CD4+ T cells, cell maturation, cell homing, cell reactivation, and/or responses by down-stream effectors. There was nonetheless no general defect in trafficking of CD4+ T cells to the site of virus challenge in IFN-γ-KO animals as demonstrated by the large numbers of CD4+, CD44+ T cell numbers in lung airways after rSeV-env challenge. In fact, the CD4+ T cell magnitude in the airways of IFN-γ-KO mice was greater than that of vaccinated B6 controls, perhaps because there was uncurbed virus infection and prolonged expression of HIV-1-envelope protein in the lung. The lack of IFN-γ was associated with a relatively low Th1/Th2 cytokine ratio in the lung airways, which may have represented cytokine secretion by both T cells and non-T cells at that site. These experiments thus highlighted IFN-γ, but not perforin, as an important mediator of protection against rSeV-env. IFN-γ has also been described as important for the CD4+ T cell-mediated clearance of wild type SeV, with the provision that regulatory signals from CD8+ T cells are additionally required.29,30 Of note, in some circumstances, CD8+ T cells have been shown to mediate virus (respiratory syncytial virus) control via an IFN-γ-dependent mechanism in the absence of perforin, CD95 ligand, or TNF,31 emphasizing that IFN-γ and perforin-dependent mechanisms need not be linked.32
What is the precise mechanism by which cells mediate virus clearance of rSeV-env? A trivial explanation for our results may be that rare conventional CD8+ T cells escape antibody depletion and kill virus-infected targets by mechanisms that are independent of perforin. This explanation cannot be ruled out, but is considered unlikely because the depletion of CD8+ T cells in μmt animals did not reduce protection, but rather showed a slight improvement in virus clearance.5 A second trivial explanation is that B cells may contribute to virus clearance in some circumstances. Again, this explanation cannot be ruled out, but is considered unlikely because (1) the model was designed to preclude expression of the HIV-1 envelope antigen on the surface of virus or virus-infected cells (the antigen is expressed in its secreted form), and (2) there was solid protection in μMT animals. A third consideration is that CD4+ T cells are cytotoxic for MHC class II-positive infected cells, but that a perforin-independent mechanism is used such as Fas-ligand-mediated kill.14
Possibly CD4+ T cells have the capacity to “help” innate immune cells as well as B cells and CD8+ T cells to limit virus growth. The interaction of CD4+ T cells and innate cells is well appreciated in the context of bacterial infections, but innate cells are often dubbed “a nuisance” in the context of virus infection due to their association with enhanced inflammation.33 In recent literature, the positive roles of innate cells as inhibitors of virus growth have been highlighted.34 IFN-γ is known to upregulate IFN-α and downstream effector molecules, autophagy,35 and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL6,36–43). These functions may inhibit virus growth directly and/or lend to the destruction of virus-infected cells to limit virus load (e.g. TRAIL can downregulate receptors for HIV- 1 and can contribute to enhanced influenza virus clearance35,44–49). Cells that associate with inflammation (e.g., killer DCs,44,48 macrophages,46 and eosinophils50) may each be considered as potential contributors to these processes. Future experiments are warranted for the better dissection of mechanisms associated with CD4+ T cells, IFN-γ production, innate cells, and virus inhibition.34
Do CD4+T cells protect against virus in humans without CD8+T cell or B cell function?
The study of human CD4+ T cell protector function is difficult in vivo, but numerous in vitro studies suggest that human CD4+ T cells also control virus without CD8+ T cell or B cell assistance.51 Cytotoxic T cell studies have long indicated that CD4+ T cells can kill labeled targets following activation in tissue culture. For example, Slobod et al. demonstrated killing of human parainfluenza virus-type 1-infected targets by CD4+ T cells.52 In addition, Casazza et al.15 demonstrated CMV- specific CD4+ T cell kill. In the latter case, the authors associated direct cytolytic activity with intracellular expression of perforin, but also suggested that other mediators may be active. CD4+ T cells are likely to control HIV-1 infections in the absence of CD8+ T cell and B cell partners, as they secrete chemokines and cytokines that limit virus growth in vitro and they interact with innate cells such as macrophages and dendritic cells that influence the growth and transport of infectious virions.53–56
In conclusion, the current model system has demonstrated that CD4+ T cells control rSeV-env in the absence of CD8+ T cells, B cells, or perforin, but that control is dependent on IFN-γ. Follow-up studies of the precise mechanisms responsible for virus control are now warranted. The robust nature of the CD4+ T cell-mediated virus protection in the current rSeV-env model may assist systematic analyses of interactions between adaptive and innate effectors. Results may reveal that the CD4+ T cells play a much greater and more complex role in prevention of nonhuman and human viral diseases than was originally envisioned.
Acknowledgments
We thank Tim Lockey, Brita Brown, Amy Zirkel, Bob Sealy, Ruth Ann Scroggs, and Pam Freiden for assistance with the preparation of reagents. We thank the World Health Organization and Dr. James Bradac (AIDS Research and Reference Reagent Repository, Rockville, MD) for virus UG92005, from which a DNA sequence was derived for the preparation of vaccine and challenge virus. The expression cassette, with which the DNA vaccine was made, was kindly provided by Drs. James Mullins and Harriet Robinson. This work was supported in part by NIH NIAID P01-AI45142, R21-AI056974, R01-AI078819, NCI Cancer Center Support Core Grant P30-CA21765, the Carl C. Anderson Sr. and Marie Joe Anderson Charitable Foundation, James B. Pendleton Charitable Trust, the Pioneer Fund, the Mitchell Fund, the Federated Department Stores, and the American Lebanese Syrian Associated Charities (ALSAC).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.MacLennan IC. Liu YJ. Johnson GD. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol Rev. 1992;126:143–161. doi: 10.1111/j.1600-065x.1992.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 2.Chan WL. Lukig ML. Liew FY. Helper T cells induced by an immunopurified herpes simplex virus type I (HSV-I) 115 kilodalton glycoprotein (gB) protect mice against HSV-I infection. J Exp Med. 1985;162(4):1304–1318. doi: 10.1084/jem.162.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams MA. Holmes BJ. Sun JC. Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark EA. Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367(6462):425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 5.Brown SA. Hurwitz JL. Zirkel A, et al. A recombinant Sendai virus is controlled by CD4+ effector T cells responding to a secreted human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 2007;81(22):12535–12542. doi: 10.1128/JVI.00197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen JP. Cardin RD. Branum KC. Doherty PC. CD4(+) T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc Natl Acad Sci USA. 1999;96(9):5135–5140. doi: 10.1073/pnas.96.9.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreansky S. Liu H. Adler H, et al. The limits of protection by “memory” T cells in Ig-/- mice persistently infected with a gamma-herpesvirus. Proc Natl Acad Sci USA. 2004;101(7):2017–2022. doi: 10.1073/pnas.0307320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown SA. Stambas J. Zhan X, et al. Clustering of Th cell epitopes on exposed regions of HIV envelope despite defects in antibody activity. J Immunol. 2003;171(8):4140–4148. doi: 10.4049/jimmunol.171.8.4140. [DOI] [PubMed] [Google Scholar]
- 9.Surman S. Lockey TD. Slobod KS, et al. Localization of CD4+ T cell epitope hotspots to exposed strands of HIV envelope glycoprotein suggests structural influences on antigen processing. Proc Natl Acad Sci USA. 2001;98:4587–4592. doi: 10.1073/pnas.071063898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown SA. Lockey TD. Slaughter C, et al. T cell epitope “hotspots” on the HIV Type 1 gp120 envelope protein overlap with tryptic fragments displayed by mass spectrometry. Aids Res Hum Retroviruses. 2005;21(2):165–170. doi: 10.1089/aid.2005.21.165. [DOI] [PubMed] [Google Scholar]
- 11.Jellison ER. Kim SK. Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174(2):614–618. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 12.Nikiforow S. Bottomly K. Miller G. Munz C. Cytolytic CD4(+)-T-cell clones reactive to EBNA1 inhibit Epstein-Barr virus-induced B-cell proliferation. J Virol. 2003;77(22):12088–12104. doi: 10.1128/JVI.77.22.12088-12104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DM. Dilzer AM. Meents DL. Swain SL. CD4 T cell-mediated protection from lethal influenza: Perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177(5):2888–2898. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 14.Brown DM. Kamperschroer C. Dilzer AM. Roberts DM. Swain SL. IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells. Cell Immunol. 2009;257(1–2):69–79. doi: 10.1016/j.cellimm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casazza JP. Betts MR. Price DA, et al. Acquisition of direct antiviral effector functions by CMV- specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203(13):2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tay SS. McCormack A. Lawson C. Rose ML. IFN-gamma reverses the stop signal allowing migration of antigen-specific T cells into inflammatory sites. J Immunol. 2003;170(6):3315–3322. doi: 10.4049/jimmunol.170.6.3315. [DOI] [PubMed] [Google Scholar]
- 17.Mikloska Z. Kesson AM. Penfold ME. Cunningham AL. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J Infect Dis. 1996;173(1):7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 18.Berman JS. Beer DJ. Theodore AC, et al. Lymphocyte recruitment to the lung. Am Rev Respir Dis. 1990;142(1):238–257. doi: 10.1164/ajrccm/142.1.238. [DOI] [PubMed] [Google Scholar]
- 19.Kato A. Sakai Y. Shioda T, et al. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells. 1996;1(6):569–579. doi: 10.1046/j.1365-2443.1996.d01-261.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakai Y. Kiyotani K. Fukumura M, et al. Accommodation of foreign genes into the Sendai virus genome: Sizes of inserted genes and viral replication. FEBS Lett. 1999;456(2):221–226. doi: 10.1016/s0014-5793(99)00960-6. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto T. Hurwitz JL. Coleclough C, et al. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol. 2004;78(11):6043–6047. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou S. Doherty PC. Zijlstra M. Jaenisch R. Katz JM. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 23.Sarmiento M. Glasebrook AL. Fitch FW. IgG and IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt-2 antigen block T-cell mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–2672. [PubMed] [Google Scholar]
- 24.Mahy BWJ. Kangro HO. Virology Methods Manual. San Diego, CA: 1996. [Google Scholar]
- 25.Lehner T. Tao L. Panagiotidi C, et al. Mucosal model of genital immunization in male rhesus macaques with a recombinant simian immunodeficiency virus p27 antigen. J Virol. 1994;68:1624. doi: 10.1128/jvi.68.3.1624-1632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belyakov IM. Hel Z. Kelsall B, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7(12):1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 27.Belyakov IM. Derby MA. Ahlers JD, et al. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci USA. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogers WM. Bergmeier LA. Ma J, et al. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. AIDS. 2004;18(1):25–36. doi: 10.1097/00002030-200401020-00003. [DOI] [PubMed] [Google Scholar]
- 29.Zhong W. Marshall D. Coleclough C. Woodland DL. CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J Immunol. 2000;164(6):3274–3282. doi: 10.4049/jimmunol.164.6.3274. [DOI] [PubMed] [Google Scholar]
- 30.Zhong W. Roberts AD. Woodland DL. Antibody-independent antiviral function of memory CD4+ T cells in vivo requires regulatory signals from CD8+ effector T cells. J Immunol. 2001;167(3):1379–1386. doi: 10.4049/jimmunol.167.3.1379. [DOI] [PubMed] [Google Scholar]
- 31.Ostler T. Davidson W. Ehl S. Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur J Immunol. 2002;32(8):2117–2123. doi: 10.1002/1521-4141(200208)32:8<2117::AID-IMMU2117>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.de Alencar BC. Persechini PM. Haolla FA, et al. Perforin and interferon-{gamma} expression are required for CD4+ and CD8+ T cell-dependent protective immunity against a human parasite (Trypanosoma cruzi) elicited by a heterologous plasmid DNA prime-recombinant adenovirus 5 boost vaccination. Infect Immun. 2009;77(10):4383–4395. doi: 10.1128/IAI.01459-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castilow EM. Meyerholz DK. Varga SM. IL-13 is required for eosinophil entry into the lung during respiratory syncytial virus vaccine-enhanced disease. J Immunol. 2008;180(4):2376–2384. doi: 10.4049/jimmunol.180.4.2376. [DOI] [PubMed] [Google Scholar]
- 34.McGill J. Heusel JW. Legge KL. Innate immune control and regulation of influenza virus infections. J Leukoc Biol. 2009;86:803–812. doi: 10.1189/jlb.0509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hildeman D. Janssen E. IFN-gamma and self-absorbed CD4+ T cells: A regulatory double negative. Nat Immunol. 2008;9(11):1210–1212. doi: 10.1038/ni1108-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamai L. Ahmad M. Bennett IM, et al. Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188(12):2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karupiah G. Chen JH. Nathan CF. Mahalingam S. MacMicking JD. Identification of nitric oxide synthase 2 as an innate resistance locus against ectromelia virus infection. J Virol. 1998;72(9):7703–7706. doi: 10.1128/jvi.72.9.7703-7706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogdan C. Rollinghoff M. Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12(1):64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 39.MacLean A. Wei XQ. Huang FP, et al. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J Gen Virol. 1998;79(Pt 4):825–830. doi: 10.1099/0022-1317-79-4-825. [DOI] [PubMed] [Google Scholar]
- 40.Walzer T. Jaeger S. Chaix J. Vivier E. Natural killer cells: From CD3(-)NKp46(+) to post-genomics meta-analyses. Curr Opin Immunol. 2007;19(3):365–372. doi: 10.1016/j.coi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Wesa AK. Storkus WJ. Killer dendritic cells: Mechanisms of action and therapeutic implications for cancer. Cell Death Differ. 2008;15(1):51–57. doi: 10.1038/sj.cdd.4402243. [DOI] [PubMed] [Google Scholar]
- 42.Junt T. Moseman EA. Iannacone M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450(7166):110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 43.Schoenborn JR. Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 44.Hardy AW. Graham DR. Shearer GM. Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci USA. 2007;104(44):17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talloczy Z. Virgin HW. Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy. 2006;2(1):24–29. doi: 10.4161/auto.2176. [DOI] [PubMed] [Google Scholar]
- 46.Herold S. Steinmueller M, et al. Lung epithelial apoptosis in influenza virus pneumonia: The role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205(13):3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wurzer WJ. Ehrhardt C. Pleschka S, et al. NF-kappaB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J Biol Chem. 2004;279(30):30931–30937. doi: 10.1074/jbc.M403258200. [DOI] [PubMed] [Google Scholar]
- 48.Chaperot L. Blum A. Manches O, et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176(1):248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 49.Lee HK. Lund JM. Ramanathan B. Mizushima N. Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315(5817):1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg HF. Dyer KD. Domachowske JB. Respiratory viruses and eosinophils: Exploring the connections. Antiviral Res. 2009;83(1):1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Appay V. Zaunders JJ. Papagno L, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168(11):5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 52.Slobod KS. Allan JE. Parainfluenza type 1 virus-infected cells are killed by both CD8+ and CD4+ cytotoxic T cell precursors. Clin Exp Immunol. 1993;93:363–369. doi: 10.1111/j.1365-2249.1993.tb08186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur G. Tuen M. Virland D, et al. Antigen stimulation induces HIV envelope gp120-specific CD4(+) T cells to secrete CCR5 ligands and suppress HIV infection. Virology. 2007;369(1):214–225. doi: 10.1016/j.virol.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdelwahab SF. Cocchi F. Bagley KC, et al. HIV-1-suppressive factors are secreted by CD4+ T cells during primary immune responses. Proc Natl Acad Sci USA. 2003;100(25):15006–15010. doi: 10.1073/pnas.2035075100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardy GA. Sieg SF. Rodriguez B, et al. Desensitization to type I interferon in HIV-1 infection correlates with markers of immune activation and disease progression. Blood. 2009;113(22):5497–5505. doi: 10.1182/blood-2008-11-190231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy JA. Scott I. Mackewicz C. Protection from HIV/AIDS: The importance of innate immunity. Clin Immunol. 2003;108(3):167–174. doi: 10.1016/s1521-6616(03)00178-5. [DOI] [PubMed] [Google Scholar]