Abstract
NY-ESO-1 is a cancer-testis antigen and an attractive target for immunotherapy in patients with different malignancies. Here we report the results of a phase I clinical study of intensive course NY-ESO-1 peptide vaccination, evaluating the safety, immunogenicity and clinical response in HLA-A2 positive patients with NY-ESO-1 expressing cancers. Of 20 patients enrolled in the trial, 14 completed at least 2 cycles of immunization and were evaluable for clinical and immunological response. Five of these evaluable patients were treated in cohort 1 (baseline seropositive) and 9 patients were treated in cohort 2 (baseline seronegative). During vaccination, NY-ESO-1-specific CD8+ T-cells were induced in 3 of 9 baseline seronegative patients. In patients with pre-existing antigen-specific CD8+ T-cells, their number increased or remained stable. In contrast to previous immunization protocols with less intensive immunization schedules, we observed a rapid induction of high magnitude NY-ESO-1 peptide-specific T-cell responses detectable already on day 15-22 of immunization. A specific immune response of high magnitude and early onset may be more effective in eliminating minimal residual disease in adjuvant treatment situations and in preventing tumor progression due to immune escape mechanisms.
Keywords: phase I clinical trial, cancer, NY-ESO-1, peptides, vaccination, immunological monitoring
Introduction
NY-ESO-1 is a cancer antigen of high immunogenicity leading to spontaneous integrated immune responses in a large proportion of patients with NY-ESO-1 expressing cancer (1-6). A number of clinical studies have been completed which evaluated the immunogenicity of NY-ESO-1 in vivo. Peptide-based vaccines have been shown to induce NY-ESO-1-specific cytotoxic CD8+ or CD4+ T-cells in different clinical settings (7-12). In parallel, immunological monitoring procedures, including ELISPOT, tetramer and chromium-release assays as well as serological testing, have been standardized and proven to be reliable to detect NY-ESO-1-specific immune responses following vaccination (7, 9, 13-15).
Previous NY-ESO-1 peptide-based studies were designed to evaluate the toxicity, immunogenicity and clinical efficacy of peptide vaccination. The first study performed with HLA-A2 binding NY-ESO-1 peptides p157-167 and p157-165 injected intradermally at weekly intervals and combined with GM-CSF as a systemic adjuvant in a later phase of the study showed that epitope-specific CD8+ T-cell responses were induced in 4 of 7 NY-ESO-1 seronegative patients. Skin reactions observed at the site of peptide inoculation were documented in 4 of 7 NY-ESO-1 antibody-negative patients and in 5 of 5 antibody-positive patients. The intensity of DTH reactions was found to correlate with the frequency of peptide-specific CD8+ T-cells detectable in the peripheral blood of patients. The onset and magnitude of peptide-specific CD8+ T-cell responses was assessed and correlated with the clinical development of individual patients. Notably, patients with detectable NY-ESO-1 immune responses induced by vaccination had a better survival as compared to patients with no immune response or with pre-existing NY-ESO-1-specific T-cell responses. Based on these observations, subsequent studies were initiated to evaluate different doses of immunogens, different NY-ESO-1 peptide epitopes and a variety of adjuvants to further enhance vaccine-induced immune responses for an improved clinical efficacy (10).
Modified protocols using high-dose and intensive course immunization schedules with NY-ESO-1 peptides have demonstrated enhanced immune responses (16, 17). Previous peptide-based cancer vaccine studies targeting other antigens evaluated single or multiple peptides, injected alone or mixed with adjuvants, for their immunogenicity (10, 18-23). The present clinical study was initiated to study the safety, kinetics and magnitude of immune responses, as well as the clinical benefit of an intensive course peptide immunization in HLA-A2 positive patients with advanced stage III and IV cancers expressing NY-ESO-1/LAGE.
Results
Patient demographics and disposition
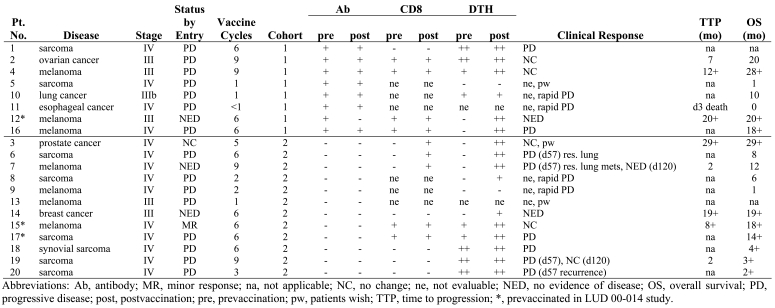
Twenty patients were enrolled in the study. Eight patients were enrolled in the baseline seropositive cohort (cohort 1) and 12 patients were enrolled in the baseline seronegative cohort (cohort 2). All patients had advanced malignancies. There were 8 patients with different types of sarcoma, 7 patients with melanoma, 1 with non-small cell lung cancer, 1 with esophageal cancer, 1 with breast cancer, 1 with ovarian cancer, and 1 patient with prostate cancer (Table 1).
Table 1.
Patient characteristics.
Nineteen patients received at least 1 cycle of vaccination and were considered evaluable for toxicity. Fourteen patients remained in the study until day 43 and were considered evaluable for immunological and clinical response. Four patients continued injections for a total of 9 cycles. Of 8 patients who withdrew from the study before completing 6 cycles, 3 patients withdrew voluntarily and 5 patients were withdrawn due to disease progression. Of the 5 patients with disease progression, one patient was withdrawn before completion of the first cycle, 3 patients before completion of 2 cycles, and one after 3 cycles. Twelve patients completed at least 6 cycles of vaccination and one patient received 5 cycles. Two seronegative patients (patients 15 and 17) and one seropositive patient (patient 12), had received vaccination with recombinant NY-ESO-1 viral constructs in a previous investigational study (24). These patients were enrolled after treatment-free intervals of >4 weeks (Table 2).
Table 2.
Patients' disposition.
Toxicity
Nineteen patients were evaluable for toxicity. Transient Common Toxicity Criteria (CTC) Grade I and II toxicities were experienced by the majority of patients. Most of these were local skin reactions at the sites of peptide or GM-CSF injection. There was no treatment-limiting toxicity.
NY-ESO-1 antibody
Seroconversion was not observed in any of the baseline NY-ESO-1 seronegative patients. One patient (patient 12) who was seropositive at baseline turned seronegative during vaccination.
NY-ESO-1-specific CD8+ T-cell responses
During the course of the study, results of a separate vaccination study utilizing the NY-ESO-1a peptide demonstrated the induction of immune responses against the cryptic epitope, NY-ESO-1 p159-167 (LMWITQCFL), a non-relevant epitope not presented on the surface of tumor cells (25). Consequently NY-ESO-1a was omitted from the protocol to prevent the induction of non-relevant immune responses against the cryptic epitope. Patients 18, 19, and 20 received NY-ESO-1b only, whereas patient 17 received 6 cycles of NY-ESO-1b and 1 cycle of NY-ESO-1a. All other patients received both peptides simultaneously. Both peptides were used to pre-sensitize CD8+ T-cells for the evaluation of the immune response. However, only results on T-cell reactivity against the relevant NY-ESO-1b (p157-165) epitope are shown
Cohort 1
Five of 8 baseline antibody positive patients completed at least 2 cycles of immunization (until day 43) and were evaluable for NY-ESO-1 p157-165-specific CD8+ T-cell responses. Four of these patients had detectable NY-ESO-1 peptide-specific CD8+ T-cell reactivity at baseline (patients 2, 4, 12, and 16). During immunization, the magnitude of peptide-specific T-cell reactivity increased in one patient (patient 2) and remained stable in the other three (patients 4, 12, and 16). One baseline antibody positive patient (patient 1) showed no NY-ESO-1b-specific immune response at baseline and remained negative during vaccination. The administration of GM-CSF starting on day 55 of vaccination did not alter the immune response.
Cohort 2
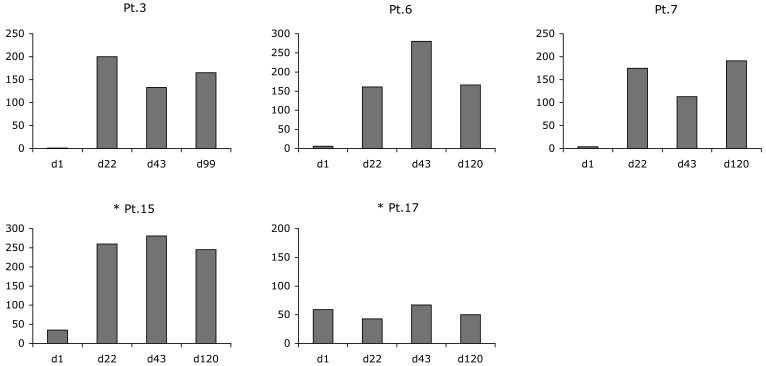
Nine of 12 baseline seronegative patients were evaluable for immune responses. Three patients who had no detectable CD8+ T-cells at baseline exhibited strong NY-ESO-1-specific CD8+ T-cell reactivity as early as day 15 or 22 after the onset of vaccination (patients 3, 6, and 7). Patients 15 and 17 had detectable NY-ESO-1b CD8+ T-cells at baseline that were related to previous vaccination in the LUD 00-014 trial. During vaccination, the magnitude of the NY-ESO-1-specific CD8+ T-cell response increased significantly in patient 15. Four seronegative patients who were negative for NY-ESO-1b CD8+ T-cells at baseline did not develop a detectable CD8+ T-cell response during vaccination (patients 14, 18, 19, and 20). Figure 1 displays the NY-ESO-1b (p157-165) T-cell reactivity assessed by ELISPOT. The administration of GM-CSF starting on day 55 of vaccination had no apparent impact on the intensity of NY-ESO-1-specific T-cell responses. No major differences were observed in the onset and magnitude of the CD8+ T-cell response between seropositive cohort 1 and seronegative cohort 2.
Figure 1.
NY-ESO-1b-specific immune response of baseline seronegative patients (cohort 2) at various time points. Bars show the number of spots per 25000 CD8+ cells in an ELISPOT assay after 6 days of prestimulation. *Indicates patients who had previously been vaccinated in the LUD 00-014 study.
DTH response and correlation with antigen-specific T-cells
Nineteen of twenty patients were evaluable for NY-ESO-1 peptide-specific DTH reactions during at least 1 complete cycle of immunization. Five of the 12 baseline seronegative patients (patients 15, 17, 18, 19, and 20) and 4 of the 8 baseline seropositive patients (patients 1, 2, 4, and 10) had positive DTH reactions at baseline. By study day 22, five patients of the seronegative cohort (patients 3, 6, 7, 8, and 14) and two patients of the seropositive cohort (patients 12 and 16) developed new DTH reactions (Table 3). The intensity of DTH reactions in antibody negative patients 3, 6, and 7 correlated with the frequency of peptide-specific CD8+ T-cells detectable in the peripheral blood of the patients as assessed by ELISPOT assay (Figure 1) and tetramer staining with HLA-A2/peptide complexes (Figure 2).
Table 3.
Characteristics and immune response of patients enrolled in the study.
Figure 2.
Development of the DTH and T-cell response in seronegative patient 3. A clear correlation between the DTH response on study day 5, 25, 66 (top, left to right) and HLA-A2/p157-165 tetramer staining on days 1, 22 and 64 (bottom, left to right) of immunization is documented. Classification of DTH reactions: 0, no local skin reaction of mild redness and/or induration of less than 4 mm in diameter; +, area of redness and induration of >4 mm in diameter; ++, enlarged area of redness and palpable induration of >8 mm in diameter and/or central necrosis.
Clinical response
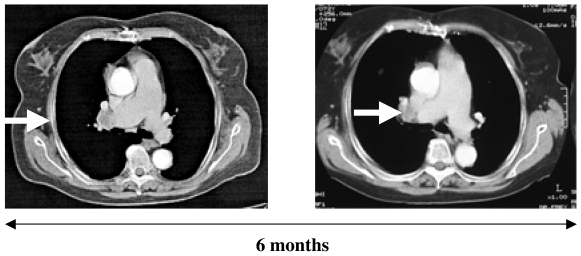
Patients were evaluable for clinical response if they had received at least 2 cycles of immunization and remained on study until day 43. Nine patients of the baseline seronegative cohort 2 were evaluable for clinical response. Seven patients completed 6 cycles (patients 6, 7, 14, 15, 17, 18, and 19) and 2 continued vaccination for a total of 9 cycles (patients 7 and 19). Patient 3 received 5 cycles. Patient 20 had evidence of progressive disease after 3 cycles of vaccination and was withdrawn from the study. Three additional patients (patients 6, 7, and 19) had progressive disease after completing 3 cycles. In two of them, patients 6 and 7, single new lung lesions were resected after study day 57 and vaccination was continued without evidence of disease for a total of 6 (patient 6) and 9 (patient 7) cycles. Both patients had developed an NY-ESO-1-specific CD8+ T-cell response at the time of surgery. The resected metastases did not express NY-ESO-1. Patient 19 continued and was stable until the sixth cycle. No evidence of disease progression was observed in 3 patients (patients 3, 14, and 15) with progression-free intervals of 29+, 19+ and 8+ months respectively. Figure 3 shows the course of disease in patient 15 as documented in computed tomography (CT) scans.
Figure 3.
CT scan of patient 15 showing stabilization of a mediastinal lymph node metastasis.
Five patients of the seropositive cohort were evaluable for clinical response and all 5 completed at least 6 cycles of immunization (patients 1, 2, 4, 12, and 16). Three of them (patients 2, 4, and 12) had no evidence of disease progression. Patients 1 and 16 developed progressive disease after 6 cycles.
Considering all patients, regardless of the presence of NY-ESO-1 serum antibody or prior exposure to NY-ESO-1 peptides, patients with detectable immune responses had extended overall survival times. The 5 seronegative patients who developed an antigen-specific immune response (patients 3, 6, 7, 15, and 17) had a prolonged overall survival compared with the 3 seronegative patients (patients 18, 19, and 20) who had no detectable immune response to vaccination. Patient 14 had no evidence of disease and a survival time of 19+ months without a measurable NY-ESO-1b-specific immune response. The 4 seropositive patients (patients 2, 4, 12, and 16) who showed a clear NY-ESO-1b-specific T-cell response had survival times of 20+, 28+, 20+ and 18+ months, respectively. Patient 1 had no detectable T-cell response and did not present for follow-up examinations (Table 3).
Impact of GM-CSF on NY-ESO-1-specific CD8+ T-cell responses
Five seropositive patients (patients 1, 2, 4, 12, and 16) and 5 seronegative patients (patients 3, 6, 7, 14, and 15) started injections of GM-CSF at the beginning of the third cycle. However, due to unavailability of the drug, 3 patients (patients 18, 19, and 20) did not receive GM-CSF at the start of the third treatment cycle and 1 patient (patient 17) received GM-CSF for one cycle only. All other patients who started with GM-CSF received the full course of injections.
The role of GM-CSF in this study is unclear. Three patients who did not receive GM-CSF had no CD8+ T-cell response (patients 18, 19, and 20). Patients who received vaccination with peptide alone, followed by peptide combined with GM-CSF, did not show significantly enhanced NY-ESO-1-specific immune responses.
Discussion
The immunogenicity of the cancer-testis antigen NY-ESO-1 has been demonstrated in several clinical settings and different strategies of specific vaccination have been shown to induce NY-ESO-1-specific T-cell responses in a number of clinical studies. In the present trial, a modified immunization protocol with intensive exposure of the patients to the vaccine over 5 consecutive days was used. The intensive course immunization schedule was shown to be safe and to have the potential of inducing high magnitude, NY-ESO-1-specific T-cell responses after a relatively short immunization period. The onset and intensity of immune responses was reflected in the strong and inflammatory DTH reactions. Five antibody negative patients had detectable vaccine-induced CD8+ T-cell responses that correlated with inflammatory DTH reactions. Survival times were longer for these patients as compared to patients who did not develop detectable CD8+ T-cell responses. Previous studies have shown an increase in dermal Langerhans cells induced by GM-CSF (26). In this study, the role of GM-CSF as an adjuvant is unclear, since NY-ESO-1 peptide-specific T-cell responses became detectable already before the onset of GM-CSF administration in baseline NY-ESO-1 seronegative patients. A synergistic effect mediated by an improved presentation of the vaccine antigen on the intensity of T-cell responses might play a role for vaccine protocols of extended duration. In NY-ESO-1 antibody positive patients, baseline CD8+ T-cell reactivity remained stable or was increased (patient 2) throughout the course of vaccination. Stabilization of disease was seen in 3 patients who had strong DTH reactions and detectable CD8+ T-cell responses.
Two patients of the seronegative cohort (patients 15 and 17) and one patient of the seropositive cohort (patient 12) had completed a previous NY-ESO-1 immunization study with recombinant vaccinia- and fowlpox-NY-ESO-1 constructs. All three patients had developed NY-ESO-1-specific T-cell responses, and one patient had shown an NY-ESO-1 seroconversion after 3 vaccinations (24). These patients were referred to the present study to possibly maintain the vaccine immune response by continued peptide vaccinations. HLA-A2-restricted NY-ESO-1 p157-165 specific immune responses were increased or maintained throughout 6 cycles of immunization within the intensive course NY-ESO-1 protocol and no signs of disease progression were noted. In the antibody positive patient, a decrease in NY-ESO-1 serum antibody was observed during vaccination which might be explained by the durable absence of NY-ESO-1-expressing tumor cells after resection of a single lymph node metastasis and the lack of appropriate antibody stimulation due to the use of short 9- and 10-mer peptides for vaccination.
The intensive course peptide vaccination protocol has shown an earlier onset and a higher magnitude of antigen-specific T-cell responses as compared to weekly immunization strategies (10, 26). To further improve immune responses to cancer vaccines, and in particular to NY-ESO-1, peptides with a higher binding affinity to HLA molecules and potent adjuvants are currently being evaluated in subsequent clinical trials (20, 27-31).
A phase I study evaluated the toxicity and immunogenicity of NY-ESO-1 p157-165 combined with MONTANIDE in patients with ovarian cancer. Peptide-specific CD8+ T-cell responses were observed in the majority of patients (7/9 patients). In a subsequent study with NY-ESO-1 p157-165 combined with CpG and MONTANIDE, CD8+ T-cell responses against the immunizing peptide were induced in 7 of 11 evaluable patients (unpublished results). In contrast to the results of this study, the majority of responding patients developed detectable T-cell responses only after prolonged immunization over 2 cycles (6 months) of immunization, which indicates that extended immunization may be essential to induce, and possibly maintain, specific immune responses. However, the rapid induction of high magnitude immune responses might be achieved more effectively by intensive course immunization protocols.
Even though no major clinical responses were observed in the latter two studies, measurable intervals of stable disease were associated with detectable immune responses to the vaccine. In conclusion, the present report demonstrates that intensive course peptide vaccination is an effective way of generating NY-ESO-1-specific CD8+ T-cell responses. Vaccine-induced NY-ESO-1 p157-165 specific T-cells from two patients (patients 7 and 16) were shown to be tumor-reactive because recognition of both NY-ESO-1 p157-165 peptide, as well as NY-ESO-1+/HLA-A2+ tumor cell lines, was demonstrated at the clonal level (32). However, these peptide-induced CD8+ T-cell responses did not correlate with significant clinical benefit in the respective patients. Tumor-related parameters, such as HLA class I/II expression and the distribution and composition of T-cell infiltrates within or around tumor tissues, might be predictive for possible clinical effects of antigen-specific vaccines and therefore warrant evaluation as prognostic parameters in future clinical vaccine studies. The simultaneous stimulation of specific CD4+ helper T-cells and inhibition of CD4+ suppressor T-cells by simultaneous administration of cytoreductive agents (11, 31, 33, 34) could improve the therapeutic capacity of peptide-based vaccines. Prime-boost strategies of vaccination are promising concepts to induce integrated immune responses including antigen-specific CD8+, CD4+ T-cells and antibodies. Based on the individual pattern and specificity of vaccine-induced immune responses to the full-length vaccine antigen, tailored peptide epitopes could be used for the specific maintenance and amplification of the vaccine-induced immune response (19, 24, 28, 30, 35, 36).
Acknowledgements
This work is part of the doctoral thesis of Melina Biskamp. This work was supported by the Cancer Vaccine Collaborative of the Cancer Research Institute and the Ludwig Institute for Cancer Research.
References
- 1.Gnjatic S, Atanackovic D, Jäger E, Matsuo M, Selvakumar A, Altorki NK, Maki RG, Dupont B, Ritter G, Chen YT, Knuth A, Old LJ. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc Natl Acad Sci U S A. 2003;100:8862–8867. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jäger E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jäger D, Arand M, Ritter G, Old LJ, Knuth A. Humoral immune responses of cancer patients against "Cancer-Testis" antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Stockert E, Jäger E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugita Y, Wada H, Fujita S, Nakata T, Sato S, Noguchi Y, Jungbluth AA, Yamaguchi M, Chen YT, Stockert E, Gnjatic S, Williamson B, Scanlan MJ, Ono T, Sakita I, Yasui M, Miyoshi Y, Tamaki Y, Matsuura N, Noguchi S, Old LJ, Nakayama E, Monden M. NY-ESO-1 expression and immunogenicity in malignant and benign breast tumors. Cancer Res. 2004;64:2199–2204. doi: 10.1158/0008-5472.can-03-3070. [DOI] [PubMed] [Google Scholar]
- 5.Valmori D, Dutoit V, Lienard D, Rimoldi D, Pittet MJ, Champagne P, Ellefsen K, Sahin U, Speiser D, Lejeune F, Cerottini JC, Romero P. Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Res. 2000;60:4499–4506. [PubMed] [Google Scholar]
- 6.Wang Y, Wu XJ, Zhao AL, Yuan YH, Chen YT, Jungbluth AA, Gnjatic S, Santiago D, Ritter G, Chen WF, Old LJ, Ji JF. Cancer/testis antigen expression and autologous humoral immunity to NY-ESO-1 in gastric cancer. Cancer Immun. 2004;4:11. http://www.cancerimmunity.org/v4p11/040812.htm [PubMed] [Google Scholar]
- 7.Baumgaertner P, Rufer N, Devevre E, Derre L, Rimoldi D, Geldhof C, Voelter V, Lienard D, Romero P, Speiser DE. Ex vivo detectable human CD8 T-cell responses to cancer-testis antigens. Cancer Res. 2006;66:1912–1916. doi: 10.1158/0008-5472.CAN-05-3793. [DOI] [PubMed] [Google Scholar]
- 8.Dutoit V, Taub RN, Papadopoulos KP, Talbot S, Keohan ML, Brehm M, Gnjatic S, Harris PE, Bisikirska B, Guillaume P, Cerottini JC, Hesdorffer CS, Old LJ, Valmori D. Multiepitope CD8(+) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting. J Clin Invest. 2002;110:1813–1822. doi: 10.1172/JCI200216428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gnjatic S, Nagata Y, Jäger E, Stockert E, Shankara S, Roberts BL, Mazzara GP, Lee SY, Dunbar PR, Dupont B, Cerundolo V, Ritter G, Chen YT, Knuth A, Old LJ. Strategy for monitoring T cell responses to NY-ESO-1 in patients with any HLA class I allele. Proc Natl Acad Sci U S A. 2000;97:10917–10922. doi: 10.1073/pnas.97.20.10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jäger E, Gnjatic S, Nagata Y, Stockert E, Jäger D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G, Hoffman E, Arand M, Old LJ, Knuth A. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci U S A. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa H, Qian F, Tsuji T, Ritter G, Old LJ, Gnjatic S, Odunsi K. Influence of CD4+CD25+ regulatory T cells on low/high-avidity CD4+ T cells following peptide vaccination. J Immunol. 2006;176:6340–6346. doi: 10.4049/jimmunol.176.10.6340. [DOI] [PubMed] [Google Scholar]
- 12.Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, Intengan M, Beck A, Keitz B, Santiago D, Williamson B, Scanlan MJ, Ritter G, Chen YT, Driscoll D, Sood A, Lele S, Old LJ. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- 13.Ayyoub M, Souleimanian NE, Godefroy E, Scotto L, Hesdorffer CS, Old LJ, Valmori D. A phenotype based approach for the immune monitoring of NY-ESO-1-specific CD4+ T cell responses in cancer patients. Clin Immunol. 2006;118:188–194. doi: 10.1016/j.clim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Jäger E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jäger D, Arand M, Ritter G, Cerundolo V, Dupont B, Chen YT, Old LJ, Knuth A. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci U S A. 2000;97:4760–4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purbhoo MA, Sutton DH, Brewer JE, Mullings RE, Hill ME, Mahon TM, Karbach J, Jäger E, Cameron BJ, Lissin N, Vyas P, Chen JL, Cerundolo V, Jakobsen BK. Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J Immunol. 2006;176:7308–7316. doi: 10.4049/jimmunol.176.12.7308. [DOI] [PubMed] [Google Scholar]
- 16.Cai Z, Sprent J. Influence of antigen dose and costimulation on the primary response of CD8+ T cells in vitro. J Exp Med. 1996;183:2247–2257. doi: 10.1084/jem.183.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kundig TM, Bachmann MF, Oehen S, Hoffmann UW, Simard JJ, Kalberer CP, Pircher H, Ohashi PS, Hengartner H, Zinkernagel RM. On the role of antigen in maintaining cytotoxic T-cell memory. Proc Natl Acad Sci U S A. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulie PG, Karanikas V, Lurquin C, Colau D, Connerotte T, Hanagiri T, Van Pel A, Lucas S, Godelaine D, Lonchay C, Marchand M, Van Baren N, Boon T. Cytolytic T-cell responses of cancer patients vaccinated with a MAGE antigen. Immunol Rev. 2002;188:33–42. doi: 10.1034/j.1600-065x.2002.18804.x. [DOI] [PubMed] [Google Scholar]
- 19.Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, Tsang KY, Panicali D, Schlom J, Kufe DW. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 20.Godelaine D, Carrasco J, Lucas S, Karanikas V, Schuler-Thurner B, Coulie PG, Schuler G, Boon T, Van Pel A. Polyclonal CTL responses observed in melanoma patients vaccinated with dendritic cells pulsed with a MAGE-3.A1 peptide. J Immunol. 2003;171:4893–4897. doi: 10.4049/jimmunol.171.9.4893. [DOI] [PubMed] [Google Scholar]
- 21.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, Noda S. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 22.Thurner B, Haendle I, Röder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Bröcker EB, Steinman RM, Enk A, Kämpgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Teates D, Neese P, Grosh WW, Petroni G, Engelhard VH, Slingluff CL Jr. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int J Cancer;2001;92:703–711. doi: 10.1002/1097-0215(20010601)92:5<703::aid-ijc1250>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Jäger E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, Ayyoub M, Ritter E, Ritter G, Jäger D, Panicali D, Hoffman E, Pan L, Oettgen H, Old LJ, Knuth A. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci U S A. 2006;103:14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gnjatic S, Jäger E, Chen W, Altorki NK, Matsuo M, Lee SY, Chen Q, Nagata Y, Atanackovic D, Chen YT, Ritter G, Cebon J, Knuth A, Old LJ. CD8(+) T cell responses against a dominant cryptic HLA-A2 epitope after NY-ESO-1 peptide immunization of cancer patients. Proc Natl Acad Sci U S A. 2002;99:11813–11818. doi: 10.1073/pnas.142417699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jäger E, Ringhoffer M, Dienes HP, Arand M, Karbach J, Jäger D, Ilsemann C, Hagedorn M, Oesch F, Knuth A. Granulocyte-macrophage-colony-stimulating factor enhances immune responses to melanoma-associated peptides in vivo. Int J Cancer. 1996;67:54–62. doi: 10.1002/(SICI)1097-0215(19960703)67:1<54::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Chen JL, Dunbar PR, Gileadi U, Jäger E, Gnjatic S, Nagata Y, Stockert E, Panicali DL, Chen YT, Knuth A, Old LJ, Cerundolo V. Identification of NY-ESO-1 peptide analogues capable of improved stimulation of tumor-reactive CTL. J Immunol. 2000;165:948–955. doi: 10.4049/jimmunol.165.2.948. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, Ritter G, Jäger E, Nomura H, Kondo S, Tawara I, Kato T, Shiku H, Old LJ, Galán JE, Gnjatic S. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slingluff CL Jr, Yamshchikov G, Neese P, Galavotti H, Eastham S, Engelhard VH, Kittlesen D, Deacon D, Hibbitts S, Grosh WW, Petroni G, Cohen R, Wiernasz C, Patterson JW, Conway BP, Ross WG. Phase I trial of a melanoma vaccine with gp100(280-288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- 30.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci U S A. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ, Kalinski P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karbach J, Gnjatic S, Pauligk C, Bender A, Maeurer M, Schultze J, Nadler K, Wahle C, Knuth A, Old LJ, Jäger E. Tumor-reactive CD8+ T-cell clones in patients after NY-ESO-1 peptide vaccination. Int J Cancer. 2007 doi: 10.1002/ijc.22957. in press. [DOI] [PubMed] [Google Scholar]
- 33.La Cava A, Van Kaer L, Shi FD. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–327. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 35.Coupar BE, Purcell DF, Thomson SA, Ramshaw IA, Kent SJ, Boyle DB. Fowlpox virus vaccines for HIV and SHIV clinical and pre-clinical trials. Vaccine. 2006;24:1378–1388. doi: 10.1016/j.vaccine.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 36.Davis ID, Chen Q, Morris L, Quirk J, Stanley M, Tavarnesi ML, Parente P, Cavicchiolo T, Hopkins W, Jackson H, Dimopoulos N, Tai TY, MacGregor D, Browning J, Svobodova S, Caron D, Maraskovsky E, Old LJ, Chen W, Cebon J. Blood dendritic cells generated with Flt3 ligand and CD40 ligand prime CD8+ T cells efficiently in cancer patients. J Immunother. 2006;29:499–511. doi: 10.1097/01.cji.0000211299.29632.8c. [DOI] [PubMed] [Google Scholar]
- 37.Common Toxicity Criteria. http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf Version 2.0; dated April 30, 1999.
Materials and methods
Investigational agents
HLA-A2 restricted NY-ESO-1 peptides NY-ESO-1a (p157-167; SLLMWITQCFL) and NY-ESO-1b (p157-165; SLLMWITQC) with a purity of >90% were manufactured by Multiple Peptide Systems, San Diego, CA according to current good manufacturing practice guidelines. Each peptide was solubilized in 100% DMSO and diluted with phosphate buffered saline (PBS) to a final concentration of 33% DMSO. Each peptide was administered intradermally in 3 individual injections of 0.3 ml containing 33.3 µg of peptide, for a total of 100 µg per peptide.
GM-CSF (molgramostim; LEUCOMAX®) (150 µg) was reconstituted aseptically with 1 ml of sterile water from the supplied vial of diluent. Patients were instructed on the proper procedure for preparation and use of GM-CSF.
Study design
This study was an open-label, phase I cohort study of intensive course immunization with NY-ESO-1a and NY-ESO-1b peptides simultaneously, first alone and later combined with systemic GM-CSF. Based on their baseline NY-ESO-1 serum antibody status, patients were assigned to cohort 1 (antibody positive) or cohort 2 (antibody negative). Peptides NY-ESO-1a (p157-167) and NY-ESO-1b (p157-165) were administered intradermally at a dose of 100 µg daily for 5 consecutive days beginning on study day 1 and repeated every three weeks for a total of 6 cycles. Injections were given at separate sites to the deltoid region of both arms and to the anterior aspect of the thigh, avoiding extremities where draining lymph nodes had been resected. During the study, new data suggested that NY-ESO-1b represents the naturally processed epitope presented by tumor cells. Therefore NY-ESO-1a was omitted from the protocol for subsequent patients and injections. GM-CSF was administered during the third and subsequent cycles of peptide immunization at sites separate from the peptide injections, consisting of one subcutaneous injection of 75 µg GM-CSF per day for 10 consecutive days starting 3 days before the first day of peptide immunization until 2 days after the last day of peptide immunization for a total of 4 cycles. The last three patients enrolled into the study received peptide alone because GM-CSF (LEUCOMAX®) was not available anymore. Safety was assessed according to the National Cancer Institute CTC Scale (37).
Immunological endpoints of the study were NY-ESO-1-specific antibody titers, the frequency of NY-ESO-1-specific CD8+ T-cells and DTH skin reactions before and after vaccination.
Clinical assessments were performed according to WHO criteria by standard imaging methods (X-ray, CT scan, ultrasound) every 3 cycles. WHO criteria are as follows: complete remission (CR): complete regression of the tumor mass; partial remission (PR): >50% regression of the tumor mass; minor remission (MR): 25 to 50% regression of the tumor mass; stable disease (SD): ±25% regression or progression of the tumor mass; progressive disease (PD): >25% progression of the tumor mass or the occurrence of new lesions. Patients without evidence of disease progression after 6 cycles were allowed to continue immunization.
Patients
Patients were eligible for the study if they had UICC stage III or IV cancers, were HLA-A2 positive, had NY-ESO-1- or LAGE-expressing tumors as determined by RT-PCR typing or immunohistochemical analysis and had an ECOG performance status of 0 to 1. Additional criteria were: absolute neutrophil counts of >2000/µl, >100000/µl platelets, >500/µl lymphocytes, >9 g/dl hemoglobin, <2.5x the upper limit of normal levels for aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin and alkaline phosphatase, and <2 mg/dl serum creatinine. Patients with brain metastases were allowed to participate when they were asymptomatic or successfully treated by surgery or radiation therapy. Patients were excluded if they had received cytotoxic chemotherapy or radiotherapy within the preceding 4 weeks, had known or suspected allergies to any component of the vaccine, had untreatable tumor-related symptoms for which chemotherapy or radiation therapy was anticipated within 2 months of entering the study, were pregnant or had NYHA class 3 or 4 heart disease. No concomitant treatment with steroids and/or anti-histaminic drugs was allowed. The study was approved by the local Institutional Review Board at Landesärztekammer Hessen, Frankfurt and all patients had given written informed consent.
Monitoring of immune response
Delayed type hypersensitivity
DTH reactions were assessed at vaccination sites 48 hours after the initial immunization and after the first day of each 5-day course of peptide immunization and were classified according to the scoring system for DTH reactions as described (10). The comparative development of DTH reactions was assessed in patients who had completed at least 2 cycles of treatment.
NY-ESO-1 antibody
NY-ESO-1-specific antibodies were measured in the serum before vaccination and one week after each vaccination cycle by standard Western blot analysis using recombinant NY-ESO-1 protein purified from Escherichia coli as described (3, 14).
NY-ESO-1-specific T-cells
PBLs were collected on the first day and 10 days after each 5-day course of peptide vaccination. Purified CD8+ T-cells were presensitized in vitro with NY-ESO-1 p157-165 peptide-pulsed irradiated autologous PBMCs depleted of CD4+ and CD8+ T-cells as described (9). Presensitized CD8+ T-cells were used as effector cells on day 6 in ELISPOT assays for recognition of peptide-pulsed T2 cells and in flow cytometry for binding of HLA-A2/peptide tetramer complexes.
ELISPOT assay
The frequency of NY-ESO-1-specific CD8+ T-cells in the peripheral blood of patients was assessed by ELISPOT as previously described (9). Briefly, T2 cells were pulsed with NY-ESO-1a and NY-ESO-1b peptides and used as target cells. The number of blue spots per well was determined and the results recorded as the average of duplicate wells. Background spots from T-cells on unpulsed T2 target cells were subtracted from the number of specific spots.
HLA-A2/ peptide multimers and flow cytometry
PE-conjugated multimeric HLA-A2/peptide complexes containing NY-ESO-1b peptide were synthesized by the Ludwig Institute of Cancer Research, Epalinges, Switzerland and used as described (9).