Abstract
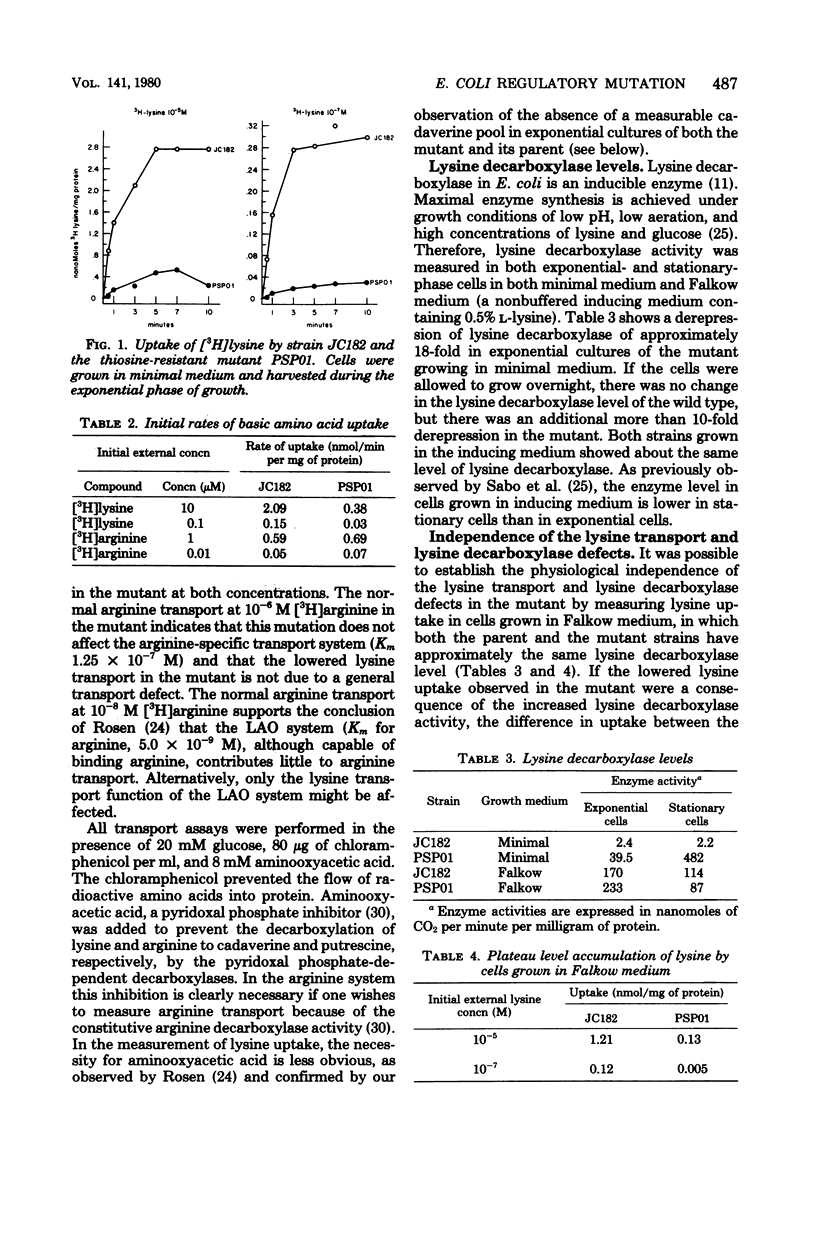
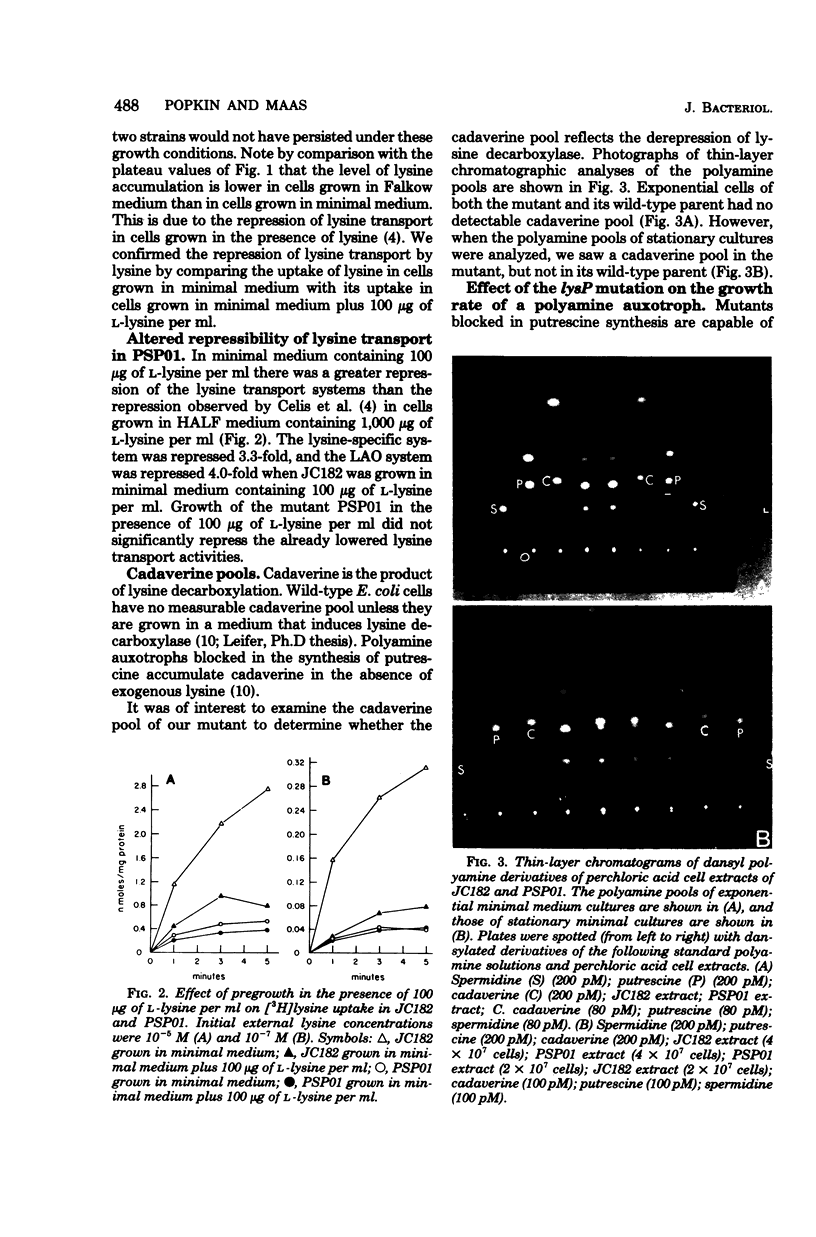
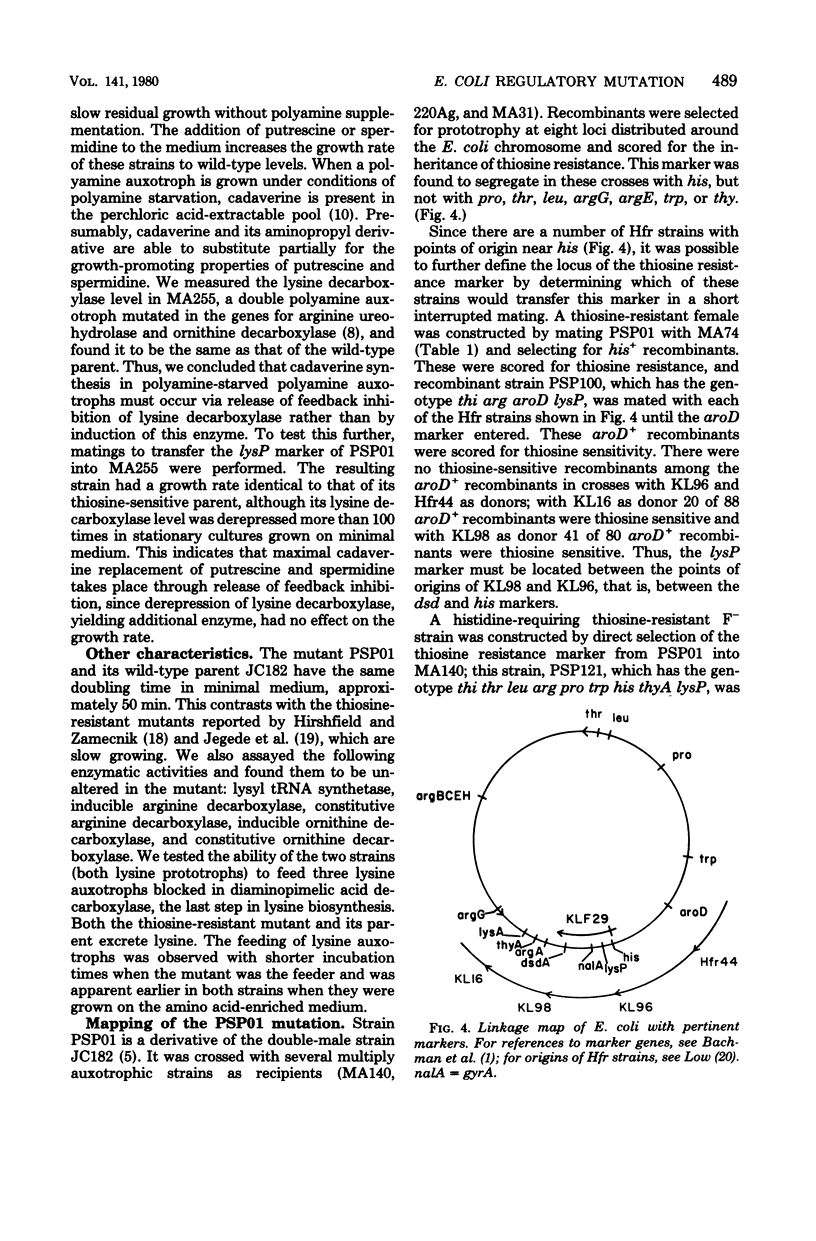
A spontaneous thiosine-resistant mutant of Escherichia coli was shown to have the following characteristics: lowered initial rate of lysine uptake and lowered plateau level of accumulation of exogenous lysine by both the lysine-specific and the general basic amino acid transport systems; altered repressibility of these two lysine transport systems; a derepressed level of lysine decarboxylase; normal growth rate; parental levels of lysyl-transfer ribonucleic acid synthetase and the inducible and constitutive arginine and ornithine decarboxylases. Both the mutant (lysP) and its parent (lysP+) feed a lysine auxotroph when they are plated in proximity on solid medium. However, the feeding response was observable after 1 day less of incubation when the mutant was the feeding strain. Despite the derepressed level of lysine decarboxylase in exponential cultures of the mutant extracts of these cultures had no detectable cadaverine pool. Conjugation experiments established the following gene order: gyrA (formerly nalA) lysP metG his. All thiosine-resistant recombinants assayed showed reduced lysine transport. In many of these recombinants the derepression of lysine decarboxylase was not expressed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois S., Cohn M., Orgel L. E. Suppression of and complementation among mutants of the regulatory gene of the lactose operon of Escherichia coli. J Mol Biol. 1965 Nov;14(1):300–302. doi: 10.1016/s0022-2836(65)80252-2. [DOI] [PubMed] [Google Scholar]
- CLARK A. J. Genetic analysis of a "double male" strain of Escherichia coli K-12. Genetics. 1963 Jan;48:105–120. doi: 10.1093/genetics/48.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis T. F., Rosenfeld H. J., Maas W. K. Mutant of Escherichia coli K-12 defective in the transport of basic amino acids. J Bacteriol. 1973 Nov;116(2):619–626. doi: 10.1128/jb.116.2.619-626.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles F. T., Brenchley J. E. Inhibition of growth and aspartokinase activity of Salmonella typhimurium by thialysine. Biochim Biophys Acta. 1976 May 28;428(3):647–655. doi: 10.1016/0304-4165(76)90194-x. [DOI] [PubMed] [Google Scholar]
- Coudert M., Vandecasteele J. P. Obtention de mutants de régulation de la voie de biosynthèse de la lysine chez une souche de Corynebacterium résistante aux analogues de la lysine. C R Acad Sci Hebd Seances Acad Sci D. 1973 Oct 1;277(13):1245–1248. [PubMed] [Google Scholar]
- Cunningham-Rundles S., Maas W. K. Isolation, characterization, and mapping of Escherichia coli mutants blocked in the synthesis of ornithine decarboxylase. J Bacteriol. 1975 Nov;124(2):791–799. doi: 10.1128/jb.124.2.791-799.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines in the synthesis of bacteriophage deoxyribonucleic acid. II. Requirement for polyamines in T4 infection of a polyamine auxotroph. J Virol. 1972 Mar;9(3):423–430. doi: 10.1128/jvi.9.3.423-430.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Epps H. M. Studies on bacterial amino-acid decarboxylases: 1. l(+)-lysine decarboxylase. Biochem J. 1944;38(3):232–242. doi: 10.1042/bj0380232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. II. Evidence for a specific lysine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):339–346. doi: 10.1016/0304-4165(66)90388-6. [DOI] [PubMed] [Google Scholar]
- Halsall D. M. Overproduction of lysine by mutant strains of Escherichia coli with defective lysine transport systems. Biochem Genet. 1975 Feb;13(1-2):109–124. doi: 10.1007/BF00486010. [DOI] [PubMed] [Google Scholar]
- Hermann M., Thevenet N. J., Coudert-Maratier M. M., Vandecasteele J. P. Consequences of lysine oversynthesis in Pseudomonas mutants insensitive to feedback inhibition. Lysine excretion or endogenous induction of a lysine-catabolic pathway. Eur J Biochem. 1972 Oct 17;30(1):100–106. doi: 10.1111/j.1432-1033.1972.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Yeh F. M., Sawyer L. E. Metabolites influence control of lysine transfer ribonucleic acid synthetase formation in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1364–1367. doi: 10.1073/pnas.72.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield I. N., Zamecnik P. C. Thiosine-resistant mutants of Escherichia coli K-12 with growth-medium-dependent lysl-tRNA synthetase activity. I. Isolation and physiological characterization. Biochim Biophys Acta. 1972 Feb 15;259(3):330–343. [PubMed] [Google Scholar]
- Jegede V. A., Spencer F., Brenchley J. E. Thialysine-resistant mutant of Salmonella typhimurium with a lesion in the thrA gene. Genetics. 1976 Aug;83(4):619–632. doi: 10.1093/genetics/83.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas W. K. Mapping of genes involved in the synthesis of spermidine in Escherichia coli. Mol Gen Genet. 1972;119(1):1–9. doi: 10.1007/BF00270439. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Sabo D. L., Boeker E. A., Byers B., Waron H., Fischer E. H. Purification and physical properties of inducible Escherichia coli lysine decarboxylase. Biochemistry. 1974 Feb 12;13(4):662–670. doi: 10.1021/bi00701a005. [DOI] [PubMed] [Google Scholar]
- Vold B., Szulmajster J., Carbone A. Regulation of dihydrodipicolinate synthase and aspartate kinase in Bacillus subtilis. J Bacteriol. 1975 Mar;121(3):970–974. doi: 10.1128/jb.121.3.970-974.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson O. H., Holden J. T. Arginine transport and metabolism in osmotically shocked and unshocked cells of Escherichia coli W. J Biol Chem. 1969 May 25;244(10):2737–2742. [PubMed] [Google Scholar]



