Abstract
Study Objectives:
Hypocretin-1/orexin A administered directly into the oral part of rat pontine reticular formation (PnO) causes an increase in wakefulness and extracellular γ-aminobutyric acid (GABA) levels. The receptors in the PnO that mediate these effects have not been identified. Therefore, this study tested the hypothesis that the increase in wakefulness caused by administration of hypocretin-1 into the PnO occurs via activation of GABAA receptors and hypocretin receptors.
Design:
Within/between subjects.
Setting:
University of Michigan.
Patients or Participants:
Twenty-three adult male Crl:CD*(SD) (Sprague Dawley) rats.
Interventions:
Microinjection of hypocretin-1, bicuculline (GABAA receptor antagonist), SB-334867 (hypocretin receptor-1 antagonist), and Ringer solution (vehicle control) into the PnO.
Measurements and Results:
Hypocretin-1 caused a significant concentration-dependent increase in wakefulness and decrease in rapid eye movement (REM) sleep and non-REM (NREM) sleep. Coadministration of SB-334867 and hypocretin-1 blocked the hypocretin-1–induced increase in wakefulness and decrease in both the NREM and REM phases of sleep. Coadministration of bicuculline and hypocretin-1 blocked the hypocretin-1–induced increase in wakefulness and decrease in NREM sleep caused by hypocretin-1.
Conclusion:
The increase in wakefulness caused by administering hypocretin-1 to the PnO is mediated by hypocretin receptors and GABAA receptors in the PnO. These results show for the first time that hypocretinergic and GABAergic transmission in the PnO can interact to promote wakefulness.
Citation:
Brevig HN; Watson CJ; Lydic R; Baghdoyan HA. Hypocretin and GABA interact in the pontine reticular formation to increase wakefulness. SLEEP 2010;33(10):1285-1293.
Keywords: Orexin, bicuculline, SB-334867, microinjection
THE NEUROPEPTIDES HYPOCRETIN-1 AND HYPOCRETIN-2 (OREXIN A AND OREXIN B) ARE SYNTHESIZED EXCLUSIVELY BY NEURONS IN THE LATERAL hypothalamic area.1–3 Hypocretin deficiency in humans underlies the pathophysiology of narcolepsy,4,5 and disruption of hypocretin signaling in mouse,6 rat,7,8 and dog9 leads to narcolepsy-cataplexy. Hypocretinergic neurons project to multiple areas of the brain, including those important for regulating sleep and wakefulness.1 One such area is the pontine reticular nucleus, oral part (PnO).1,10 The PnO is the rostral portion of the rodent pontine reticular formation11 and contributes to the generation of wakefulness and rapid eye movement (REM) sleep.12 Microinjection of hypocretin-1 into rat PnO causes an increase in wakefulness,13 and microinjection of hypocretin-1 into cat pontine reticular formation increases the cortically activated states of REM sleep14 or wakefulness.15,16
Administering hypocretin-1 into the PnO may increase wakefulness by modulating the release of arousal-promoting neurotransmitters within the PnO. Direct administration of hypocretin-1 to the PnO of isoflurane-anesthetized rat causes a concentration-dependent increase in both acetylcholine (ACh) release17 and GABA levels13 within the PnO. Extracellular recording studies of PnO neurons in urethane-anesthetized rat show that iontophoretic application of hypocretin-1 causes a hyperpolarization that is blocked by prior application of bicuculline.10 This finding indicates that the hypocretin-1–induced inhibition of PnO neurons is mediated by GABAA receptors. Identified GABAergic neurons in brainstem slices of mouse PnO have been shown to be excited by hypocretin-1,18 and intracellular recording studies in halothane-anesthetized cat show that hypocretin-1 can also cause direct depolarization of PnO neurons and an increase in PnO neuronal firing rate.14 Numerous studies have demonstrated that GABAergic transmission in the PnO increases wakefulness and inhibits REM sleep.13,19–25 The present study provides the first test of the hypothesis that the wakefulness-promoting effects of delivering hypocretin-1 into the PnO are mediated by GABAA receptors as well as by hypocretin receptors. This hypothesis was evaluated by determining whether (1) microinjection of hypocretin-1 into the PnO causes a concentration-dependent increase in wakefulness, (2) this increase in wakefulness is blocked by coadministration of the hypocretin receptor-1 (hcrt-r1) antagonist SB-334867, and (3) coadministration of the GABAA receptor antagonist bicuculline also blocks the wakefulness response to hypocretin-1. Portions of these data have been presented as abstracts.26,27
MATERIALS AND METHODS
Chemicals and Drug Solutions
Human hypocretin-1 was purchased from California Peptide Research, Inc. (Napa, CA). Bicuculline methiodide was purchased from Sigma Aldrich (St. Louis, MO) and N-(2-Methyl-6-benzoxazolyl)-N2-1,5-naphthyridin-4-yl urea (SB-334867) was obtained from Tocris Bioscience (Ellisville, MO). Chemicals for Ringer solution (147.0 mM NaCl, 2.4 mM CaCl2, 4.0 mM KCl, 1.0 mM MgSO4, pH 6.0) were acquired from Fisher Scientific (Pittsburgh, PA). All drugs used for the antagonist-blocking studies were dissolved in Ringer solution containing 2% dimethyl sulfoxide, which was purchased from Sigma Aldrich. Drug solutions used for intracranial microinjections were made immediately before use.
Animals, Surgery, and Conditioning of Behavior
Animal experiments were approved by the University of Michigan Committee on Use and Care of Animals and performed in accordance with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Institutes of Health Publication 80-23). Adult (235- to 310-g) male Crl:CD*(SD) (Sprague Dawley) rats (n = 23; Charles River Laboratories, Wilmington, MA) were housed with unlimited access to food and water and kept on a 12-hour light/dark cycle (lights on at 06:00).
Procedures for surgical implantation of recording electrodes and a microinjection guide tube have been described in detail.13,28 Briefly, rats were anesthetized with isoflurane (Abbott Laboratories, North Chicago, IL) and implanted with 3 screw electrodes (8IE36320SPCE, Plastics One, Roanoke, VA) for recording the cortical electroencephalogram (EEG). Three pair of EEG electrodes were placed using the following stereotaxic coordinates relative to bregma: 1.0 mm anterior and 1.5 mm lateral, 2.0 mm posterior and 1.5 mm lateral, and 2.0 mm posterior and 1.27 mm lateral. Two electrodes for recording the electromyogram (EMG) were implanted bilaterally in the dorsal neck muscles. EMG electrodes were assembled from AS632 biomed wire (6 cm length; Cooner Wire Company, Chatsworth, CA) and electrical gold socket contacts (8IE3630XXXXE, Plastics One). A guide cannula (8IC315GSPCXC, Plastics One) containing a stylet (8IC315DCXXXC, Plastics One) was aimed 3 mm above the left PnO at 8.40 mm posterior to bregma, 1.0 mm lateral to the midline, and 6.2 mm below the skull surface.11 Electrode leads were routed into a 6-pin electrode pedestal (MS363, Plastics One). Dental acrylic (Lang Dental Manufacturing Company, Inc., Wheeling, IL) and 3 anchor screws (MPX-0080-02PC-C, Small Parts, Inc., Miami Lakes, FL) were used to adhere the guide cannula, electrodes, and pedestal to the skull.
Rats recovered from surgery for a minimum of 7 days, during which time they were conditioned to being handled for microinjections and to being tethered (363-441/6, Plastics One) in a recording chamber (Raturn; Bioanalytical Systems, West Lafayette, IN). To determine whether rats were adequately conditioned, a mock microinjection was performed by inserting and removing a microinjector (8IC315IXXXXC, Plastics One). EEG and EMG signals then were recorded for 2 hours. Rats were considered ready to enter the microinjection protocol when the latency to non-REM (NREM) sleep was less than 30 minutes.
Microinjections and Quantification of Arousal States
The day before each microinjection, rats were placed in the recording chambers and tethered overnight. All microinjections were made between 09:30 and 10:30, and microinjections into the same rat were separated by a minimum of 7 days. Microinjection volume (100 nL) and duration (1 min) were held constant for all drugs, and 2-hour electrophysiologic recordings were started immediately after injection. Two groups of rats were used for this study. The first group (n = 14) received 0, 0.1, 1, 10, and 100 pmol hypocretin-1 (0, 0.36, 3.56, 35.6, and 356 ng, respectively). The order in which the different concentrations of hypocretin-1 were administered was randomized, and not all animals received all concentrations. The second group of rats (n = 9) was microinjected with Ringer solution containing 2% dimethyl sulfoxide (vehicle control), hypocretin-1 (10 pmol), bicuculline (0.2 pmol; 0.1 ng) in combination with hypocretin-1 (10 pmol), or SB-334867 (10 pmol; 3.4 ng) in combination with hypocretin-1 (10 pmol). All rats in the second group received all 4 drug treatments.
EEG and EMG recordings were scored manually in 10-second bins as wakefulness, NREM sleep, or REM sleep using Icelus Acquisition software.29 Previously described methods were used for amplification, digitization, and fast Fourier transform (FFT) analysis of signals.13,28 Briefly, FFT plots were constructed by analyzing EEG signals in 0.5-Hz increments every 2 seconds for frequencies ranging from 0.5 to 25.0 Hz. Five consecutive 2-second bins were averaged to produce 1 FFT for each 10-second epoch. Sample bins of 10 seconds in duration were averaged over five 1-minute intervals in the first hour following microinjection of Ringer solution and hypocretin-1 (100 pmol/100 nL). Sleep records were scored by 2 investigators, 1 of which was blinded to the treatment condition. Agreement between the 2 scores of greater than 90% was achieved for all records. Dependent measures included the percentage of time spent in wakefulness, NREM sleep, and REM sleep; the average duration of the longest wakefulness episode from each recording; the average duration of NREM sleep and REM sleep episodes; the number of wakefulness, NREM sleep, and REM sleep episodes; the number of transitions; and the latency to onset of the first episode of NREM sleep and REM sleep.
Histologic Verification of Microinjection Sites
Rats were deeply anesthetized with isoflurane (5%) and decapitated. Brains were immediately removed, blocked, and frozen. The brainstem block was sectioned coronally from caudal to rostral using a cryostat (Leica Microsystems, Nussloch, Germany). Serial sections (40 microns thick) were slide mounted, dried, fixed in paraformaldehyde vapor (80°C), and stained with cresyl violet. Stained tissue sections and a 1-mm calibration bar were digitized using a Super Nikon Coolscan 4000 ED Film Scanner (Nikon Inc, Melville, NY). Microinjection sites were identified and assigned stereotaxic coordinates by comparison with a rat brain atlas.11
Statistical Analyses
Data sets for all dependent measures were normally distributed. Therefore, data were evaluated by parametric tests and are reported as mean ± SEM. For the concentration-response study, the effects of hypocretin-1 on sleep and wakefulness were determined using a linear mixed model for a randomized incomplete block design. Concentration response data were fit to the equation Y = B+(T-B)/(1+10∧((LogEC50-X)*HillSlope)), where B represents the lower limit for the dependent variable (e.g., percentage of wakefulness), T is the upper limit for the dependent variable, X is the logarithm of the hypocretin-1 concentration, and Y is the dependent variable. Regression analyses were used to obtain the coefficient of determination (r2) and calculate the percentage of the response accounted for by the concentration of hypocretin-1 (r2). For the antagonist-blocking study, drug effects on sleep and wakefulness were determined by 1-way analysis of variance (ANOVA) for repeated measures and Dunnett posthoc multiple comparisons test. Because there were so few REM sleep episodes in the first hour after injection, a Poisson regression with a Generalized Estimating Equations model was used to test for drug effects on the number of REM episodes. This approach takes into account the correlation among repeated observations from the same rat. Z-tests were used to determine significant effects, and Bonferroni correction was used for multiple comparisons between drug treatments. Statistical programs used included SAS (release 9.1.3, SAS Institute, Cary, NC) and GraphPad Prism v4.0c for Macintosh. Statistical significance required a P value ≤ 0.05.
RESULTS
Microinjection of Hypocretin-1 into the PnO Caused a Concentration-Dependent Increase in Wakefulness and Decrease in Sleep
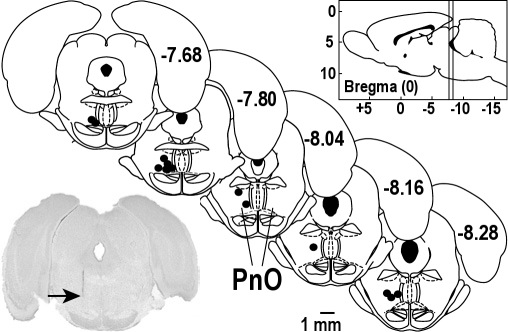
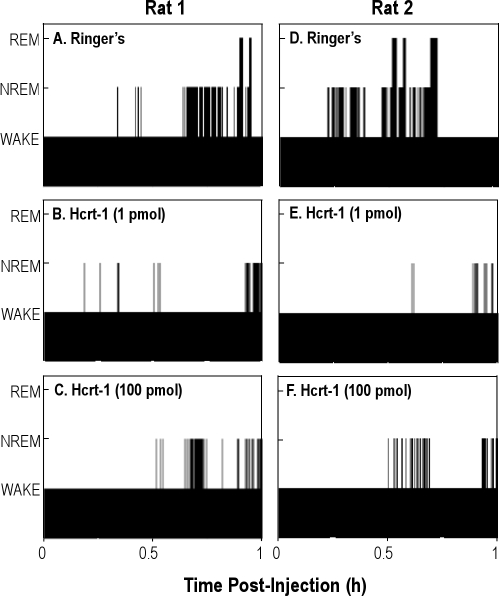
To determine whether the hypocretin-1–induced increase in wakefulness was mediated by hypocretin receptors, increasing concentrations of hypocretin-1 were microinjected into the PnO and the effects on sleep and wakefulness were quantified. Figure 1 shows that all microinjection sites used for the concentration-response study were localized to the PnO. The average stereotaxic coordinates11 for these injection sites were 7.9 ± 0.1 mm posterior to bregma, 8.6 ± 0.1 mm ventral to the top of the skull, and 1.1 ± 0.1 mm from the midline. Figure 2 illustrates the sequence of sleep and wakefulness from 2 representative rats in the first hour after PnO microinjection of 2 concentrations of hypocretin-1 relative to control (microinjection of Ringer solution). Hypocretin-1 increased the amount of time spent in wakefulness, decreased NREM sleep, and abolished REM sleep.
Figure 1.
All concentrations of hypocretin-1 were injected into the pontine reticular nucleus, oral part (PnO). Microinjection sites (n = 14) from the concentration-response experiments are represented as black dots on 5 coronal atlas plates (modified from11). Numbers on the right side of each plate indicate mm posterior to bregma. The sagittal drawing of the rat brain (upper right) contains vertical lines that designate the anterior to posterior range of the microinjection sites, which spanned from 7.6 to 8.3 mm posterior to bregma. The digitized image of a cresyl violet stained section from 1 rat shows a typical microinjection site (arrow) in the PnO.
Figure 2.
Representative time-course plots of wakefulness and sleep are shown for the first hour after microinjection of Ringer solution (vehicle control) or hypocretin-1 (Hcrt-1) into the pontine reticular nucleus, oral part (PnO). The height of the bars indicates the sequence of wakefulness (lowest bars), non-rapid eye movement sleep (NREM) (intermediate bars), and rapid eye movement sleep (REM) (highest bars). Each column shows data from 1 rat.
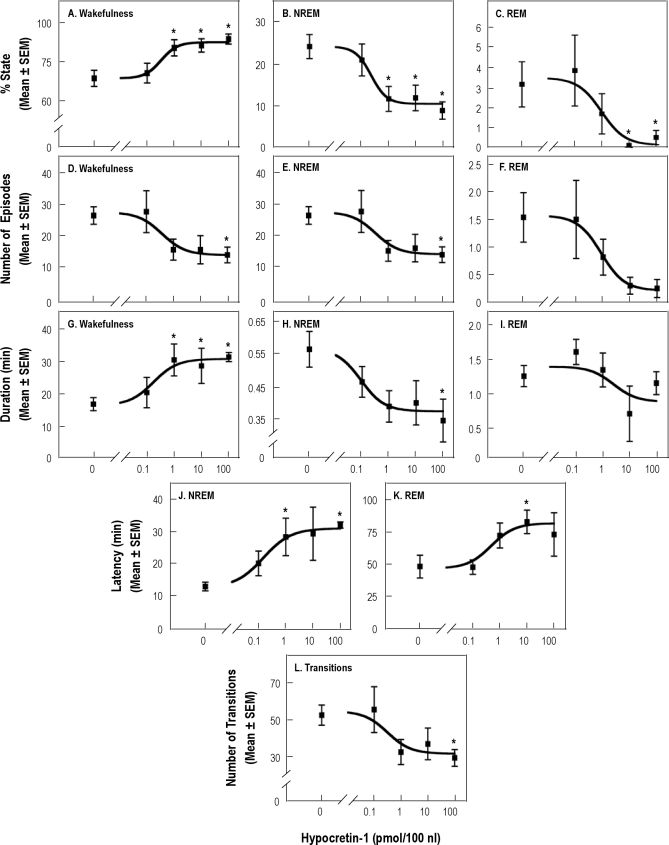
Figure 3 summarizes the group data for the first hour after injection. ANOVA revealed a significant concentration main effect of hypocretin-1 on the percentage of time spent in wakefulness (Figure 3A; F4,32 = 6.51; P = 0.0006), NREM sleep (Figure 3B; F4,32 = 6.37; P = 0.0007), and REM sleep (Figure 3C; F4,32 = 4.68; P = 0.004) and on the number of episodes of wakefulness (Figure 3D; F4,32 = 3.33; P = 0.02) and NREM sleep (Figure 3E; F4,32 = 3.38; P = 0.02). The effect of hypocretin-1 on the number of REM sleep episodes approached significance (Figure 3F; z = 9.04, P = 0.06). There was a significant concentration main effect on the duration of the longest wakefulness episode (Figure 3G; F4,32 = 6.85; P = 0.0004) and on the average duration of NREM sleep episodes (Figure 3H; F4,30 = 2.75; P = 0.046). There was no significant change in the average duration of REM sleep episodes (Figure 3I). Microinjection of hypocretin-1 into the PnO caused a significant concentration-dependent effect on the latency to onset of NREM sleep (Figure 3J; F4,32 = 29.81; P < 0.0001) and REM sleep (Figure 3K; F4,21 = 2.97; P = 0.043). Consistent with these effects, the number of state transitions varied significantly as a function of hypocretin-1 concentration (Figure 3L; F4,32 = 3.38; P = 0.02). All dependent measures except for the percentage of time spent in REM sleep returned to control levels in the second hour after injection (data not plotted). REM sleep remained decreased (F4,32 = 2.75; P = 0.048) following microinjection of 100 pmol of hypocretin (P < 0.01). FFT analysis of EEG signals recorded from 8 rats during the first hour after microinjection showed that, compared with an injection of Ringer solution, hypocretin-1 (100 pmol/100 nL) did not alter EEG power at any frequency.
Figure 3.
Significant concentration-dependent changes in sleep and wakefulness were caused by microinjection of hypocretin-1 into the pontine reticular nucleus, oral part (PnO). Data from 14 rats are plotted for the first hour after injection. Dunnett multiple comparisons tests indicated significant (* P ≤ 0.05) differences from control (0 pmol/100 nL). For all data sets except rapid eye movement sleep (REM) duration (I), coefficients of determination (r2) indicated the percentage of the variability accounted for by the concentration of hypocretin-1 was: (A) 98%; (B) 97%; (C) 93%; (D) 91%; (E) 90%; (F) 99%; (G) 95%; (H) 94%; (J) 90%; (K) 93%; (L) 84%. NREM refers to non-rapid eye movement sleep.
Both the Hypocretin Receptor-1 Antagonist SB-334867 and the GABAA Receptor Antagonist Bicuculline Blocked the Hypocretin-1–Induced Increase in Wakefulness
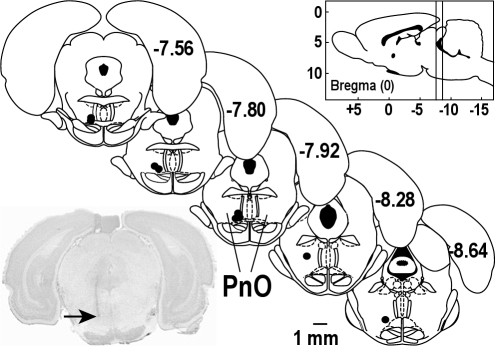
To further investigate whether the hypocretin-1–induced increase in wakefulness was mediated by hypocretin receptors, sleep and wakefulness were quantified after coadministering SB-334867 and hypocretin-1. This study also coadministered bicuculline and hypocretin-1 to determine whether GABAergic transmission in the PnO contributed to the hypocretin-1–induced increase in wakefulness. Figure 4 documents that all microinjection sites used for the blocking studies were localized to the PnO. The mean stereotaxic coordinates were 7.9 ± 0.1 mm posterior to bregma, 8.9 ± 0.1 mm ventral to the top of the skull, and 1.0 ± 0.1 from the midline. There was no significant difference between the stereotaxic coordinates of injection sites used for the concentration-response study (Figure 1) and the receptor-antagonist study (Figure 4).
Figure 4.
SB-334867 and bicuculline were injected into the pontine reticular nucleus, oral part (PnO). Microinjection sites (n = 9) from the antagonist-blocking studies are represented as black dots on 5 coronal atlas plates (modified from11). Numbers on the right side of each plate specify mm posterior to bregma. The vertical lines in the sagittal drawing (upper right) indicate that the microinjection sites spanned from 7.5 to 8.6 mm posterior to bregma. One representative microinjection site (arrow) in the PnO is shown in the tissue section at lower left.
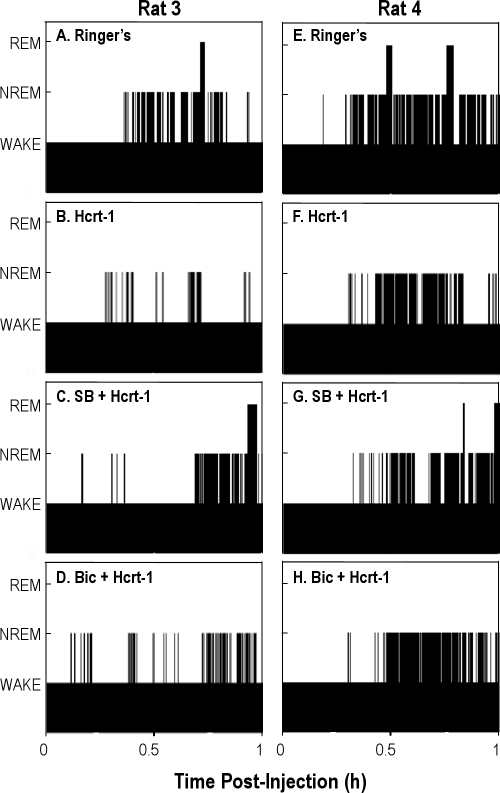
Figure 5 plots the time course of wakefulness and sleep recorded from 2 representative rats during the first hour after microinjection of Ringer solution (Figure 5A and 5E), hypocretin-1 (10 pmol) (Figure 5B and 5F), SB-334867 (10 pmol) + hypocretin-1 (10 pmol) (Figure 5C and 5G), and bicuculline (0.2 pmol) + hypocretin-1 (10 pmol) (Figure 5D and 5H). The hypocretin-1–induced increase in wakefulness and decrease in NREM sleep (Figure 5B and 5F) were blocked by coadministration of SB-334867 (Figure 5C and 5G) and by coadministration of bicuculline (Figure 5D and 5H). SB-334867 also antagonized the decrease in REM sleep (Figure 5C and 5G), whereas bicuculline did not block the REM-sleep inhibition caused by hypocretin-1 (Figure 5D and 5H).
Figure 5.
Temporal organization of wakefulness and sleep in the first hour after microinjection of Ringer solution (A and E), 10 pmol of hypocretin-1 (B and F), 10 pmol of SB-334867 (SB) + 10 pmol of hypocretin-1 (Hcrt-1) (C and G), and 0.2 pmol of bicuculline (Bic) + 10 pmol of Hcrt-1 (D and H) into the pontine reticular nucleus, oral part (PnO). Each column shows data from 1 rat. Bar height indicates wakefulness (lowest bars), non-rapid eye movement sleep (NREM) (intermediate bars), and rapid eye movement sleep (REM) (highest bars).
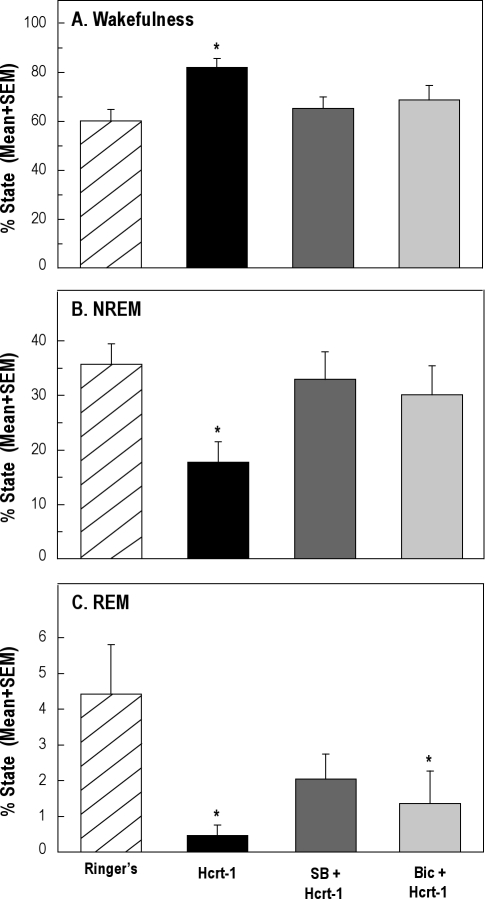
The group data are summarized in Figure 6. ANOVA revealed that, in the first hour after injection, the percentage of time spent in wakefulness (Figure 6A, F3,24 = 5.44; P = 0.005) and NREM sleep (Figure 6B, F3,24 = 4.44; P = 0.01) varied as a function of drug treatment. The hypocretin-1–induced increase in the percentage of time spent in wakefulness was blocked by coadministration of either SB-334867 or bicuculline (Figure 6A). The decrease in NREM sleep time was reversed by SB-334867 and by bicuculline (Figure 6B). A repeated-measures ANOVA adjusted for unequal variances revealed that the percentage of time spent in REM sleep varied significantly as a function of drug treatment (Figure 6C, F3,24 = 6.03; P = 0.003). The hypocretin-1–induced decrease in REM sleep was partially reversed by SB-334867 but was not blocked by bicuculline (Figure 6C).
Figure 6.
Coadministration of SB-334867 (SB) or bicuculline (Bic) blocked the increase in wakefulness and decrease in non-rapid eye movement (NREM) sleep caused by hypocretin-1 (Hcrt-1). Data represent measures from 9 rats during the first hour after injection. Dunnett multiple comparisons tests indicated significant (*P ≤ 0.05) differences from control (microinjection of Ringer solution).
DISCUSSION
The present study reports, for the first time, that microinjection of hypocretin-1 into the PnO of awake rat caused a concentration-dependent increase in wakefulness that was blocked by coadministration of a hcrt-r1 antagonist. These findings demonstrate that the PnO is one brain region where activating hypocretin receptors can promote wakefulness. An additional novel finding is that the hypocretin-1–induced increase in wakefulness was prevented by administering the GABAA receptor antagonist bicuculline into the PnO. Considered with data showing that delivering hypocretin-1 to the PnO increases GABA levels in the PnO13 and causes inhibition of some PnO neurons,10 and with evidence that GABAergic transmission in the PnO is wakefulness promoting,13,19–25 the present results support the interpretation that increasing GABAergic transmission in the PnO is one mechanism by which hypocretin-1 increases wakefulness. The results are discussed below relative to the functional roles of hypocretin-1 in rat PnO and the mechanisms by which hypocretin-1 administered to the PnO causes an increase in wakefulness. Limitations of the present study are also considered.
What are the Functional Roles of Hypocretin-1 in the PnO?
The hypocretin peptides have multiple functional roles, one of which is to maintain wakefulness.30 PnO neurons participate in generating the cortical activation of wakefulness and REM sleep,31 and microinjection of hypocretin-1 into rat PnO was recently shown to increase the amount of wakefulness.13 Determining concentration dependence and antagonist blocking are established approaches for revealing whether or not responses are receptor mediated.32 The present study provides data fulfilling the criteria of concentration dependence (Figure 3) and antagonist blocking (Figure 6), indicating that hypocretin-1 acts at its receptors in the PnO to increase wakefulness.
Efforts to elucidate functions of hypocretin-1 also have found that microinjecting hypocretin-1 into rat PnO causes an antinociceptive response that is blocked by coadministration of the hcrt-r1 antagonist SB-334867.33 This result demonstrates that the antinociceptive response is mediated by hypocretin receptors. Furthermore, blocking hypocretin receptors in the PnO with SB-334867 in the absence of exogenous hypocretin-1 increases nociceptive responsiveness to a thermal stimulus.33 This finding means that endogenous hypocretin-1 in rat PnO is antinociceptive. Microdialysis delivery of hypocretin-1 to rat PnO increases ACh release,17 and ACh in the PnO of cat34 and mouse35 is antinociceptive. Future studies can determine whether the antinociceptive effects of hypocretin-1 in rat PnO depend on cholinergic transmission.
By what Mechanisms does Hypocretin in the PnO Increase Wakefulness?
Hypocretins are excitatory,3 and one mechanism by which these peptides are thought to enhance arousal is to activate neurons that drive wakefulness,36 thus increasing the release of wakefulness-generating neurotransmitters. Hypocretin-1 delivered to the PnO increases local GABA levels, and GABA in the PnO increases wakefulness (reviewed in37). Pharmacologically increasing GABA levels in the PnO increases wakefulness,13 and endogenous GABA levels in the PnO are significantly greater during wakefulness than during REM sleep.38 GABA levels in the PnO are also greater during wakefulness than during the loss of consciousness produced by the general anesthetic isoflurane.24 Furthermore, loss of endogenous hypocretin increases recovery time from isoflurane anesthesia.39 The present finding that the hypocretin-1–induced increase in wakefulness was blocked by coadministration of bicuculline (Figure 6) demonstrates that the wakefulness response is mediated by GABAA receptors. This finding, therefore, supports the interpretation that the hypocretin-1–induced increase in wakefulness (Figure 3) results from enhanced GABAergic transmission in the PnO.
When given systemically to humans or animals, drugs that increase GABAergic transmission produce behavioral states such as sleep40 or general anesthesia.41 The mechanisms by which GABAergic drugs cause a loss of waking consciousness are not fully understood but are likely to include inhibition of wakefulness-promoting neurons. For example, the hypnotic eszopiclone may cause sleep by potentiating transmission at GABAA receptors on pedunculopontine tegmental neurons.42 The mechanisms by which enhancing GABAergic transmission locally in the PnO causes an increase in wakefulness remain to be elucidated. Increased wakefulness is also caused by administering GABAergic drugs directly into the preoptic area/anterior hypothalamus43 or the midbrain reticular formation.44 Increased sleep, however, is caused by delivering a GABAA receptor agonist directly into the posterior hypothalamus.43 Therefore, GABAmimetics have opposite effects on sleep and wakefulness depending upon their site of action in the brain.13
Hypocretin-1 increases ACh release in the PnO,17 and increasing cholinergic transmission in the PnO may contribute to the mechanism by which PnO administration of hypocretin-1 increases wakefulness. Cholinergic transmission in the PnO promotes the EEG activation characteristic of both wakefulness and REM sleep.12,31 Future studies are required to determine whether the increase in wakefulness caused by delivering hypocretin-1 into the PnO can be blocked by coadministering a cholinergic receptor antagonist.
The hypocretin-1–induced increase in wakefulness was comprised of an increase in the duration of the longest wakefulness episode and a decrease in the number of wakefulness episodes (Figure 3). The increase in wakefulness was accompanied by a decrease in NREM sleep and REM sleep. EEG power was not altered by hypocretin-1, demonstrating similarity to spontaneously occurring wakefulness, NREM sleep, and REM sleep with respect to the trait of EEG activity. Hypocretin-1 decreased the percentage of time spent in NREM sleep by decreasing both the number and duration of NREM sleep episodes. The hypocretin-1–induced consolidation of wakefulness into longer episodes and the decrease in the number of state transitions is consistent with the finding that narcoleptic patients45,46 and mice lacking hypocretin47,48 show fragmentation of sleep and wakefulness. These results also support a recent modeling study predicting that hypocretin-1 preferentially acts on long episodes of wakefulness.49
Effects of Hypocretin-1 on Wakefulness Vary with Behavioral State and Microinjection Site
The sleep-wake response to microinjecting hypocretin-1 into cat pontine reticular formation depends on the behavior state of the cat when the drug is administered, the site of injection within the pontine brainstem, and the amount of hypocretin-1 injected. Microinjecting hypocretin-1 (125 pmol/250 nL; 450 ng) into the oral part of cat pontine reticular formation during NREM sleep increases REM sleep,50 whereas administering hypocretin-1 (125 pmol/250 nL; 450 ng) into the same region during wakefulness produces an increase in wakefulness.16 Microinjection of hypocretin-1 (2 to 20 pmol/20 nL; 7 to 71 ng) into a more dorsal region of cat pontine reticular formation (referred to as peri-locus coeruleus α) during wakefulness causes a concentration-dependent increase in wakefulness and decrease in NREM sleep and REM sleep, whereas the same concentrations of hypocretin-1 delivered to the ventral part of cat pontine reticular formation cause a selective inhibition of REM sleep and no change in the amount of wakefulness or NREM sleep.15 The dissimilar findings between these 2 studies in cat are reconciled by noting that different brain regions were microinjected with different amounts of hypocretin-1 in different microinjection volumes.15,50
Species-specific responses to PnO microinjection of hypocretin-1 in rat and cat have been discussed in detail.13 For the present report and a previous study13 using rat, all microinjections were made during wakefulness, and hypocretin-1 caused an increase in wakefulness (Figure 3). Rat microinjection sites in the present study (Figures 1 & 4) are in homologous regions of cat pontine reticular formation that produced either increases in wakefulness when hypocretin-1 was injected during wakefulness16 or selective decreases in REM sleep.15 Hypocretin-1 did significantly decrease REM sleep in the present study and also decreased NREM sleep (Figure 3). The reasons for these differences are not known but could include differences in microinjection sites, hypocretin receptors, and afferent hypocretin terminals, in addition to the use of different microinjection volumes and amounts of hypocretin-1.
Limitations, Conclusions, and Potential Clinical Significance
One unexplained finding of the present study is that bicuculline did not block the hypocretin-1–induced decrease in the amount of REM sleep (Figure 6C). This finding was unexpected because bicuculline alone delivered to the PnO of rat,22,23 cat,25,51 and mouse20 increases REM sleep. Pontine reticular formation administration of bicuculline increases local ACh release,51 and the increase in REM sleep that occurs by administering GABAA receptor antagonists to the PnO is blocked by the muscarinic cholinergic antagonist atropine.22 Hypocretin-1 also increases ACh release in the PnO,17 and PnO administration of cholinomimetics significantly increases REM sleep (reviewed in52). These data support the interpretation that GABAergic transmission in the PnO inhibits REM sleep, in part, by inhibiting ACh release. A higher concentration of bicuculline or a GABAB receptor antagonist may be required to reverse the hypocretin-1–induced decrease in REM sleep.
The experiments reported here did not identify the hypocretin receptor subtype or subtypes in the PnO that mediate the wakefulness response to PnO administration of hypocretin-1 (Figure 3). Hypocretins signal through two subtypes of G protein-coupled receptors, hcrt-r1 and hcrt-r2, also called OX1R and OX2R (reviewed in30). Hcrt-r1 is selective for hypocretin-1, whereas hcrt-r2 does not distinguish between hypocretin-1 and hypocretin-2.2 Rat PnO contains both hypocretin receptor subtypes,53 and hcrt-r1 and hcrt-r2 in the PnO each contribute to the hypocretin-induced increase in ACh release within the PnO.17 Hcrt-r2 is present on GABAergic neurons in rat PnO,54 suggesting that hcrt-r2 mediates the increase in PnO GABA levels caused by hypocretin-1,13 as well as the hypocretin-1–induced hyperpolarization of PnO neurons.10 SB-334867 has a 50-fold higher affinity for hcrt-r1 than for hcrt-r2,55 and the finding that the hypocretin-1–induced increase in wakefulness was blocked by SB-334867 (Figure 6A) supports the interpretation that the wakefulness response was mediated by hcrt-r1. Coadministration of SB-334867 did not return REM sleep to control levels (Figure 6C), suggesting that hcrt-r2 may mediate the decrease in REM sleep.
Data from the present study (Figures 3 & 6) demonstrate that exogenous hypocretin-1 activates hypocretin receptors in rat PnO to increase and consolidate wakefulness. Whether endogenous hypocretin in rat PnO normally functions to promote wakefulness awaits the demonstration that antagonizing hypocretin receptors in the PnO causes a concentration-dependent decrease in wakefulness.
Despite the limitations discussed above, the present data show for the first time that activating hypocretin receptors in rat PnO causes an increase in wakefulness that is mediated by GABAergic transmission at GABAA receptors. This novel finding demonstrates that hypocretinergic and GABAergic transmission can interact in the pontine reticular formation to increase wakefulness. An extensive body of evidence supports the interpretation that one physiologic role of hypocretins is to maintain wakefulness (see review30). Neuronal systems regulating sleep and wakefulness are anatomically distributed and neurochemically heterogeneous, and the present data suggest that the PnO is one region where hypocretin-1 and GABA interact to increase periods of uninterrupted wakefulness and inhibit sleep. In addition to its wakefulness-promoting actions, hypocretin-1 has antinociceptive effects,56 which also can be evoked from the PnO.33 Opioids are the most widely used drugs for the treatment of pain, and negative side effects include disruption of the sleep-wake cycle.57 GABA levels in the PnO are decreased by opioids28 and increased by hypocretin-1.13 Studies of GABAA-receptor point-mutated mice showing that systemic alterations of transmission at GABAA receptors can be antinociceptive58 encourage continuing efforts to localize GABAergic modulation of nociception to specific brain regions. The present demonstration of a wakefulness-promoting interaction between hypocretinergic and GABAergic neurotransmission in the PnO indicates the importance of determining whether coadministering hypocretin receptor agonists with opioids can provide analgesia without disrupting wakefulness.
DISCLOSURE STATEMENT
This was not an industry-supported study. Ms. Brevig and Dr. Watson have indicated no financial conflicts of interest. Drs. Lydic and Baghdoyan have received support from Sepracor, Inc.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants MH45361, HL40881, HL65272, and by the Department of Anesthesiology. We thank the Pharmacological Sciences Training Program (GM007767) for support of HNB's coursework. We also thank EA Gauthier, MA Norat, and S Jiang for expert assistance. Statistical analysis was carried out in consultation with K Welch at the Center for Statistical Consultation and Research at the University of Michigan, Ann Arbor.
ABBREVIATIONS
- ANOVA
analysis of variance
- EEG
electroencephalogram
- EMG
electromyogram
- GABA
γ-aminobutyric acid
- Hcrt-r1
hypocretin receptor-1
- Hcrt-r2
hypocretin receptor-2
- LC
locus coeruleus
- LDT
laterodorsal tegmental nucleus
- NREM
non-rapid eye movement
- PPT
pedunculopontine tegmental nucleus
- PnO
pontine reticular nucleus, oral part
- REM
rapid eye movement
- SB-334867
N-(2-Methyl-6-benzoxazolyl)-N″-1,5-naphthyridin-4-yl urea
REFERENCES
- 1.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 3.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 5.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 7.Gerashchenko D, Kohls MD, Greco M, et al. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–83. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beuckmann CT, Sinton CM, Williams SC, et al. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24:4469–77. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 10.Nuñez A, Moreno-Balandrán ME, Rodrigo-Angulo ML, Garzón M, De Andrés I. Relationship between the perifornical hypothalamic area and oral pontine reticular nucleus in the rat. Possible implication of the hypocretinergic projection in the control of rapid eye movement sleep. Eur J Neurosci. 2006;24:2834–42. doi: 10.1111/j.1460-9568.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. London: Elsevier Press; 2007. [Google Scholar]
- 12.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–95. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–64. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–8. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Balandrán E, Garzón M, Bodaló C, Reinoso-Suárez F, de Andrés I. Sleep-wakefulness effects after microinjections of hypocretin 1 (orexin A) in cholinoceptive areas of the cat oral pontine tegmentum. Eur J Neurosci. 2008;28:331–41. doi: 10.1111/j.1460-9568.2008.06334.x. [DOI] [PubMed] [Google Scholar]
- 16.Xi M, Chase M. Hypocretin induces either active (REM) sleep or wakefulness depending on the state of the animal at the time of administration. Sleep. 2009;32(Suppl):0012. Abstract. [Google Scholar]
- 17.Bernard R, Lydic R, Baghdoyan HA. Hypocretin (orexin) receptor subtypes differentially enhance acetylcholine release and activate G protein subtypes in rat pontine reticular formation. J Pharmacol Exp Ther. 2006;317:163–71. doi: 10.1124/jpet.105.097071. [DOI] [PubMed] [Google Scholar]
- 18.Brown RE, McKenna JT, Winston S, et al. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci. 2008;27:352–63. doi: 10.1111/j.1460-9568.2008.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camacho-Arroyo I, Alvarado R, Manjarrez J, Tapia R. Microinjections of muscimol and bicuculline into the pontine reticular formation modify the sleep-waking cycle in the rat. Neurosci Lett. 1991;129:95–7. doi: 10.1016/0304-3940(91)90728-c. [DOI] [PubMed] [Google Scholar]
- 20.Chang T, Vihtelic CM, Gold C, Lydic R, Baghdoyan HA. Microinjection of the GABAA receptor antagonist bicuculline into the pontine reticular formation of C57BL/6J mouse decreases wakefulness and increases sleep. Sleep. 2006;29(Suppl):0022. Abstr. [Google Scholar]
- 21.Flint RR, Lydic R, Baghdoyan HA. Microinjection of the GABAA receptor agonist muscimol into the pontine reticular nucleus, oral part (PnO) of C57BL/6J (B6) mouse causes a concentration dependent increase in wakefulness and decrease in sleep. Neuroscience Meeting Planner Online. 2007 Program No. 631.5. [Google Scholar]
- 22.Marks GA, Sachs OW, Birabil CG. Blockade of GABA, type A, receptors in the rat pontine reticular formation induces rapid eye movement sleep that is dependent upon the cholinergic system. Neuroscience. 2008;156:1–10. doi: 10.1016/j.neuroscience.2008.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–45. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- 24.Vanini G, Watson CJ, Lydic R, Baghdoyan HA. GABA-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–88. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–9. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]
- 26.Brevig HN, Watson CJ, Lydic R, Baghdoyan HA. The hypocretin-1 (hcrt-1)-induced increase in wakefulness and decrease in sleep is blocked by bicuculline or the hypocretin receptor-1 (hcrt-r1) antagonist SB-334867. Neuroscience Meeting Planner Online. 2008 Program Number 285.14. [Google Scholar]
- 27.Brevig HN, Watson CJ, Lydic R, Baghdoyan HA. Hypocretin-1 microinjected into the pontine reticular nucleus, oral part (PnO) of Sprague Dawley rat causes a concentration dependent increase in wakefulness and decrease in sleep. Sleep. 2009;32(Suppl):0018. Abstr. [Google Scholar]
- 28.Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience. 2007;144:375–86. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opp MR. Rat strain differences suggest a role for corticotropin-releasing hormone in modulating sleep. Physiol Behav. 1998;63:67–74. doi: 10.1016/s0031-9384(97)00390-9. [DOI] [PubMed] [Google Scholar]
- 30.Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. 2nd ed. New York: Kluwer Academic/Plenum Press; 2005. [Google Scholar]
- 32.Norman J. Drug-receptor reactions. Br J Anaesth. 1979;51:595–601. doi: 10.1093/bja/51.7.595. [DOI] [PubMed] [Google Scholar]
- 33.Watson SL, Watson CJ, Baghdoyan HA, Lydic R. Thermal nociception is decreased by hypocretin-1 and an adenosine A1 receptor agonist microinjected into the pontine reticular formation of Sprague Dawley rat. J Pain. 2010;11:535–44. doi: 10.1016/j.jpain.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kshatri AM, Baghdoyan HA, Lydic R. Cholinomimetics, but not morphine, increase antinociceptive behavior from pontine reticular regions regulating rapid-eye-movement sleep. Sleep. 1998;21:677–85. doi: 10.1093/sleep/21.7.677. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Baghdoyan HA, Lydic R. Leptin replacement restores supraspinal cholinergic antinociception in leptin deficient obese mice. J Pain. 2009;10:836–43. doi: 10.1016/j.jpain.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–76. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 37.Vanini G, Lydic R, Baghdoyan HA. GABAergic modulation of REM sleep. In: Mallick BN, Pandi-Perumal SR, McCarley RW, Morrison AR, editors. Rapid Eye Movement Sleep — Regulation and Function. New York, NY: Cambridge University Press; 2010. in press. [Google Scholar]
- 38.Vanini G, Wathen BL, Lydic R, Baghdoyan HA. GABA levels in cat pontine reticular formation (PRF) are lower during rapid eye movement (REM) sleep and the neostigmine-induced REM sleep-like state (REM-Neo) than during wakefulness. Sleep. 2009;32(Suppl):0011. Abstr. [Google Scholar]
- 39.Kelz MB, Sun Y, Chen J, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–14. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009;29:1779–94. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- 41.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 42.Ye M, Garcia-Rill E. Potentiating effect of eszopiclone on GABAA receptor-mediated responses in pedunculopontine neurons. Sleep. 2009;32:879–87. doi: 10.1093/sleep/32.7.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–40. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 44.Tsuchiya T, Fukushima H. Effects of benzodiazepines on PGO firings and multiple unit activity in the midbrain reticular formation in cats. Electroencephalogr Clin Neurophysiol. 1977;43:700–6. doi: 10.1016/0013-4694(77)90085-2. [DOI] [PubMed] [Google Scholar]
- 45.Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–66. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- 46.Taheri S, Zeiter JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- 47.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakai K, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:649–63. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581:649–63. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diniz Behn CG, Kopell N, Brown EN, Mochizuki T, Scammell TE. Delayed orexin signaling consolidates wakefulness and sleep: physiology and modeling. J Neurophysiol. 2008;99:3090–103. doi: 10.1152/jn.01243.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xi M, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–8. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- 51.Vazquez J, Baghdoyan HA. GABAA receptors inhibit acetylcholine release in cat pontine reticular formation: implications for REM sleep regulation. J Neurophysiol. 2004;92:2198–206. doi: 10.1152/jn.00099.2004. [DOI] [PubMed] [Google Scholar]
- 52.Lydic R, Baghdoyan HA. Acetylcholine modulates sleep and wakefulness: a synaptic perspective. In: Monti JM, Pandi-Perumal SR, Sinton CM, editors. Neurochemistry of Sleep and Wakefulness. New York, NY: Cambridge University Press; 2008. pp. 109–43. [Google Scholar]
- 53.Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res Mol Brain Res. 2001;88:176–82. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 54.Brischoux F, Mainville L, Jones BE. Muscarinic-2 and orexin-2 receptors on GABAergic and other neurons in the rat mesopontine tegmentum and their potential role in sleep-wake state control. J Comp Neurol. 2008;510:607–30. doi: 10.1002/cne.21803. [DOI] [PubMed] [Google Scholar]
- 55.Duxon MS, Stretton J, Starr K, et al. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–9. doi: 10.1007/s002130000550. [DOI] [PubMed] [Google Scholar]
- 56.Bingham S, Davey PT, Babbs AJ, et al. Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 2001;92:81–90. doi: 10.1016/s0304-3959(00)00470-x. [DOI] [PubMed] [Google Scholar]
- 57.Lydic R, Baghdoyan HA. Neurochemical mechanisms mediating opioid-induced REM sleep disruption. In: Lavigne G, Sessle BJ, Choiniere M, Soja PJ, editors. Sleep and Pain. Seattle, WA: IASP Press; 2007. pp. 99–122. [Google Scholar]
- 58.Zeilhofer HU, Mohler H, Lio AD. GABAergic analgesia: new insights from mutant mice and subtype-selective agonists. Trends Pharmacol Sci. 2009;30:397–402. doi: 10.1016/j.tips.2009.05.007. [DOI] [PubMed] [Google Scholar]