Abstract
The adrenal zona glomerulosa (ZG) secretes aldosterone to regulate sodium balance. Chronic sodium restriction increases aldosterone accompanied by ZG expansion. The ZG is innervated by sympathetic, vasoactive intestinal polypeptide (VIP) and neuropeptide tyrosine (NPY), and sensory, calcitonin gene-related peptide, nerves. It is unclear whether innervation is affected by ZG growth. Therefore, we measured neurite outgrowth in the ZG of adult male rats after dietary sodium manipulation. In response to 1 wk sodium restriction, VIP and NPY fibers elongated in parallel with expansion of the ZG, shown by aldosterone synthase (AS) expression, but calcitonin gene-related peptide fibers were not affected. Sodium repletion resulted in parallel regression in VIP and NPY fiber length and AS expression. These results show that sympathetic, but not sensory, innervation is coordinated with ZG growth. Mediators underlying changes in innervation are unknown; therefore, we characterized a novel gene TMEM35 [termed the unknown factor-1 (TUF1) due to its unknown function] that shows extensive overlap with AS in ZG. After sodium restriction, TUF1 expanded in parallel with the ZG. TUF1 bound the low-affinity neurotrophin receptor, p75NTR, which was expressed in NPY fibers and showed a response similar to TUF1 after sodium manipulation. TUF1- p75NTR binding was competitively displaced by nerve growth factor but not by TUF1 lacking the p75NTR binding motif. Moreover, TUF1 mRNA in rat ZG cells increased after angiotensin II exposure in vitro. Collectively, these findings suggest that TMEM35/TUF1 is a candidate for modulating neurite outgrowth in the ZG after sodium depletion.
TUF1 is a novel peptide that binds p75NTR and may mediate adrenal zona glomerulosa sympathetic fiber (NPY and VIP) plasticity in response to sodium manipulation.
The mammalian adrenal cortex shows a profound capacity for tissue growth in response to physiological challenges that require increased corticosteroid secretion (1,2). The adrenal cortex is comprised of distinct functional zones formed by zone-specific expression of steroidogenic enzymes. The outer zona glomerulosa expresses P450 aldosterone synthase (AS), required for aldosterone production, whereas the inner zona fasciculata expresses P450 11β-hydroxylase, required to synthesize glucocorticoids (3,4,5). Each cortical zone responds with some specificity to experimental manipulation. Compensatory adrenal growth after unilateral adrenalectomy and adrenal regeneration induced by enucleation occurs primarily via expansion of the zona fasciculata (6,7). In contrast, sodium depletion is a potent stimulus for growth of the zona glomerulosa (8,9). Activation of the renin-angiotensin system results in angiotensin II (Ang II)-induced stimulation of aldosterone secretion (10). Ang II also can stimulate (11) or inhibit (12) glomerulosa cell proliferation, responses that likely are dependent on cell-cell (11) and cell-extracellular matrix interactions (12). The zona glomerulosa is highly innervated by postganglionic sympathetic nerves that synthesize tyrosine hydroxylase (13) and numerous neuropeptides including vasoactive intestinal polypeptide (VIP) and neuropeptide Y (NPY) (14), and these neurotransmitters have been implicated in affecting glomerulosa cell proliferation and aldosterone secretion (15).
Our previous work has shown that growth and functional recovery of fasciculata cells after adrenal enucleation is associated with hyperinnervation by sympathetic nerves (16). Because enucleation consists of adrenal injury, it is likely that the neurotrophic response results in part from local inflammatory mediators as observed in other injury models (17,18). After sodium depletion, VIP content increases in the rat adrenal glomerulosa (19), suggesting that nerve growth may occur under physiological conditions in parallel with adrenal growth. We tested this hypothesis and observed that both VIPergic and NPYergic fibers lengthened in parallel with expansion of the zona glomerulosa after sodium depletion and retracted after sodium repletion. These novel findings led us to evaluate a potential neurotrophic factor for adrenal nerve growth after sodium depletion.
Neurotrophic factors including glial-derived neurotrophic factor and neurotrophin (NT)-4 and their receptors are present in the adrenal medulla and contribute to preganglionic sympathetic innervation of chromaffin cells (20,21,22). In contrast, whereas brain-derived neurotrophic factor (BDNF) has been identified in the inner zona fasciculata/reticularis (23), NT-3 is expressed in the zona reticularis and the outer zona glomerulosa (24). The cognate tyrosine kinase (Trk) receptors for BDNF and NT-3, TrkB and TrkC, respectively, are expressed in the inner cortex along with the low-affinity p75 NT receptor (p75NTR) (25). Despite the paucity of neurotrophin expression, there is extensive innervation of the outer zona glomerulosa by sympathetic nerves (26,27). This apparent discrepancy led us to characterize the adrenal expression of a recently identified hypothalamic protein, derived from the transmembrane protein 35 (TMEM35) gene (28); although the precise function of TMEM35 remains unknown, an in silico analysis revealed a potentially secreted peptide containing a highly conserved motif required for classical nerve growth factors including nerve growth factor (NGF), BDNF, and NT-3 to bind the p75NTR (29). We found that this protein, termed the unknown factor-1 (TUF1) for its unknown function, colocalizes extensively with AS in the zona glomerulosa. In addition, TUF1 expression changes in parallel with expansion of the zona glomerulosa and p75NTR neurite outgrowth in response to a low-sodium diet. Based on in vitro experiments showing that TUF1 peptide binding to p75NTR can be competitively displaced by NGF and that TUF1 mRNA increases in adrenal cells stimulated by Ang II stimulation, we propose that TUF1 is a potential candidate for modulating neurite outgrowth in the zona glomerulosa after sodium depletion.
Materials and Methods
Animals
Male Sprague Dawley rats (250–280 g) were purchased from Charles River (Wilmington, MA), maintained in a 12-h light, 12-h dark cycle, and were given free access to food and water. All animal protocols were approved by the University of Minnesota Animal Care and Use Committee.
Sodium manipulation
Rats were separated into three groups: control, sodium-restricted, and sodium-repleted (n = 6). The control group was fed a diet containing 0.49% sodium (TD.96208; Harlan Teklad, Madison, WI), whereas the sodium-restricted group was fed a diet containing 0.01–0.02% sodium (TD.90228; Harland Teklad) for 1 wk. The sodium-repleted group was fed a sodium-deficient diet for 1 wk followed by a control diet for 1 wk. At the end of experimental protocols, rats were killed by decapitation, and adrenals were collected.
Tissue collection
Dissected adrenal glands were fixed in 4% (wt/vol) paraformaldehyde diluted in PBS overnight at 4 C, cryoprotected in 30% (wt/vol) sucrose/PBS, embedded in a frozen section medium (Neg-50; Richard-Allan Scientific, Kalamazoo, MI), sectioned at 20 μm using a Leica cryostat (VT1000), and stored at −20 C until further use.
Immunohistology
Adrenal sections were equilibrated to room temperature for 10 min and rehydrated in Tris-buffered saline (pH 7.4) (TBS) for 10 min. Sections were immersed in 85 C 20 mm sodium citrate (pH 8.0) and cooled to room temperature to unmask antigen. Sections were then permeabilized for 1 h in 0.2% (vol/vol) Triton X-100 diluted in TBS, rinsed in TBS, blocked in BSA (10 mg/ml; Sigma Chemical Co., St. Louis, MO) for 30 min, and incubated in primary antibody diluted in BSA (1 mg/ml) overnight at 4 C. Excess antibody was removed with TBS rinses. Sections were then incubated overnight with Alexa-488- or -548-conjugated secondary antibody (Invitrogen, Eugene, OR) diluted 1:200 (vol/vol) with BSA (1 mg/ml). Excess antibody was removed with TBS rinses, and sections were mounted in aqueous medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc., Burlingame, CA). Primary antibodies included mouse anti-P450 aldosterone synthase (1:100 dilution, a gift of Dr. Celso Gomez-Sanchez), rabbit anti-calcitonin gene-related peptide (anti-CGRP) (1:1000; ImmunoStar, Inc., Hudson, WI), rabbit anti-NPY (1:200; ImmunoStar), rabbit anti-VIP (1:200; ImmunoStar), rabbit anti-p75NTR (1:100; a gift of Dr. Louis Reichardt), and rabbit anti-TUF1 (1:100, directed against TUF1152–167, generated by Sigma Genosys, Woodlands, TX).
Colabeling for p75 and NPY expression
Sections were first incubated overnight in rabbit anti-p75 followed by incubation with Alexa-548 goat antirabbit secondary as described above. Sections were then blocked with excess unlabeled goat antirabbit IgG (0.13 mg/ml) for 1 h at room temperature and rinsed with PBS plus 0.05% Tween 20. Sections were then incubated in rabbit anti-NPY overnight at 4 C followed by incubation in Alexa-488 donkey antirabbit antibody. As a control, the anti-NPY antibody was omitted; in the absence of this second primary antibody, no labeling was observed after incubation with the Alexa-488 donkey antirabbit secondary.
Adrenal glomerulosa cell dispersion
Adrenal glands were dissected from adult Sprague Dawley rats (280–300 g) and kept in cold PBS. After fat removal, adrenal capsules were detached and placed into a few drops of dispersion medium [DMEM, 0.32% collagenase (type I; Life Technologies, Inc., Carlsbad, CA), 4% BSA (Sigma), and 0.1% deoxyribonuclease (Sigma)]. Adrenal capsules were then minced with surgical scissors, transferred to dispersion medium, and incubated for 90 min at 37 C and 10% CO2 in a cell culture incubator with trituration at 15-min intervals. Dispersed cells were filtered through a 100-μm wire mesh into wash medium (DMEM, 0.4% BSA, and 0.28% HEPES) and centrifuged at 200 × g for 5 min. After supernatant removal, cells were rinsed in wash medium and resuspended in incubation medium (wash medium plus 7.65 mm CaCl2). Dissociated cells were seeded at 75,000 cells per well in a 24-well plate and incubated at 37 C and 10% CO2 for 2 h before stimulation. Cells were stimulated with Ang II (Sigma) or K+ and incubated overnight. After overnight incubation, medium was collected for aldosterone measurement and cells were lysed for RNA isolation. Experiments were performed three times, and mean responses were calculated for statistical analysis.
Quantitative real-time PCR (qPCR)
Total RNA was isolated from capsular/glomerulosa cells using an RNA isolation kit (Zymo Research, Orange, CA), and concentrations were measured by absorbance at 260 nm (A260) using a NanoDrop ND-1000 (NanoDrop Technologies, Inc., Wilmington, DE). cDNA was generated from 100 ng total RNA using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) per manufacturer recommendation. The resulting cDNA was diluted 2-fold to give a final volume of 40 μl. All qPCR experiments were performed with one half the manufacturer recommended volume (Applied Biosystems) consisting of 4 μl diluted cDNA, 5 μl 2× TaqMan qPCR universal mix, and 0.5 μl 20× TaqMan gene expression assay primer/probe mix (CYP11B2, TUF1, and ribosomal protein S18). Thermocyling was carried out according to the manufacturer’s protocol (Applied Biosystems) using a MX3000P instrument (Stratagene, La Jolla, CA).
Aldosterone RIA
Media aldosterone was determined using a commercially available RIA Coat-A-Count kit (Diagnostic Products Corp., Los Angeles, CA).
Binding assay for TUF143-54 and p75NTR
COS7 cells were seeded at 10,000 cells per well onto an 18-mm coverslip in a 12-well plate. Cells were allowed to settle overnight in a cell culture incubator (NuAire NU-8600, NuAire Inc., Plymouth, MN) set at 37 C and 5% CO2. Cells were transfected with pCMV-eGFP (Clontech, Palo Alto, CA) or pCMV-SPORT6-p75 (American Type Culture Collection, Manassas, VA) using Fugene HD (Roche, Indianapolis, IN) and were incubated overnight. pCMV-eGFP was used to control for transfection efficiency and nonspecific binding. The binding assays were carried out according to Horton et al. (30) with modifications. Synthetic TUF1 peptides (TUF143-54 and TUF124-38, NCBI reference no. NP_001001799) were generated (via Sigma Genosys) and were conjugated per manufacturer’s recommendation with DyLight 549 NHS Ester (Thermo Scientific, Rockford, IL). Unconjugated dye was removed with a dye removal column (Thermo Scientific). Labeled peptides were diluted to 5 nm in 0.15 m sucrose/PBS and added (1.0 ml/well) to transfected COS7 cells. The binding reaction was incubated in the dark at room temperature for 90 min. Cells were rinsed thoroughly with PBS/0.05% Tween 20 to remove unbound peptide. Cells were then fixed with 4% paraformaldehyde (5 min). After fixation, cells were permeabilized in PBS/0.1% Tween 20 for 10 min, rinsed with PBS, and blocked in BSA (10 mg/ml) for 10 min. Cells were incubated with rabbit anti-p75NTR (1:10,000) for 30 min, and excess antibody was removed with PBS washes. Cells were then incubated with Alexa-488-goat antirabbit (1:500 dilution; Invitrogen) for 30 min, rinsed with PBS and mounted in Vectashield mounting media plus DAPI (Vector).
Competitive binding assays
Binding assays were carried out according to Horton et al. (30) with modifications. Immulon II 96-well microtiter plates (Fisher Scientific, Pittsburgh, PA) were coated with 100 μl (1ng/μL) goat F(ab′)2 fragment of antihuman IgG (ImmunoResearch, Inc., West Grove, PA) diluted in PBS overnight at 4 C with shaking. After PBS rinses, 10 ng extracellular domain of mouse p75-human IgG Fc hybrid protein (R&D Systems, Minneapolis, MN) diluted in binding buffer [L15 medium, 20 mm HEPES (pH 7.2), 1 mg/ml fraction V BSA (Sigma), and 0.5 mg/ml cytochrome C (Sigma)] was added to each well and incubated at room temperature for 90 min. Unbound hybrid peptide was removed with PBS rinses. Competitive binding was carried out with recombinant mouse NGF-β (R&D Systems), unlabeled TUF143-54, and unlabeled TUF143-54[S46A] in the presence of 200 nm biotin-conjugated TUF143-54; peptide conjugation was performed using EZ-link NHS-LC-biotin (Pierce, Rockford, IL). Binding was performed in binding buffer for 90 min at room temperature. Unbound peptide was removed with PBS plus 0.1% Tween 20 rinses. Bound biotin-TUF143-54 was detected by incubation with 100 μl horseradish peroxidase-avidin solution (Vector) for 30 min and developed using 100 μl 3,3′,5,5′-Tetramethylbenzidine (TMB) Liquid Substrate System for ELISA (Sigma). Data were analyzed for best-fit curve using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Image collection and analysis
Images were collected with a Nikon confocal microscope (Digital-Eclipse C1 system; Nikon Instruments Inc., Melville, NY) or a Nikon E600 microscope equipped with a CCD camera and processed using Photoshop (CS3; Adobe System Inc., San Jose, CA). Adjacent adrenal sections were labeled for AS, NPY, VIP, CGRP, TUF1, and p75NTR. Colabeling experiments were carried out for AS and NPY and for AS and TUF1. Four to six sections separated by 200 μm were quantified for each adrenal. Each adrenal section was subdivided into four quadrants. An average of three or four measurements was obtained per quadrant. The average of all quadrants was used to represent the mean value per section. The mean value of four to six sections of each adrenal gland was collected to represent a single rat.
Statistical analysis
Data are presented as the mean ± sem (n = 4 per group). Treatment effects were determined using a one-way ANOVA followed by a Bonferroni’s post hoc analysis to identify differences between groups. Differences were considered statistically significant when the test yielded a P < 0.05. Statistical analyses were performed using GraphPad Prism.
Results
Sodium manipulation modulates neurite outgrowth in the adrenal zona glomerulosa
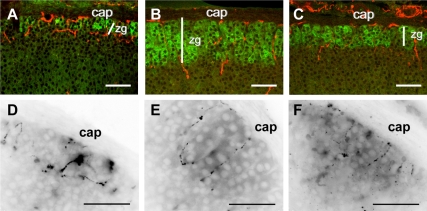
Consistent with previous results (14,16), nerve fibers immunoreactive for NPY or VIP were observed in the adrenal capsule with extensive branching in the zona glomerulosa [Fig. 1, A (NPY) and D (VIP)], defined by AS expression. Rats fed a sodium-deficient diet for 1 wk showed an inward expansion of the zona glomerulosa (Table 1) that was paralleled by elongation of NPYergic (Fig. 1B) and VIPergic (Fig. 1E) nerve fibers. Rats fed a control diet for 1 wk after the sodium-deficient diet showed a regression of the zona glomerulosa accompanied by retraction of NPYergic (Fig. 1C) and VIPergic (Fig. 1F) fibers, although zonation and innervation was still expanded relative to the control group (Table 1). In contrast, sodium depletion did not result in elongation of sensory CGRPergic nerve fibers (Table 1).
Figure 1.
Low-sodium diet induces growth of the zona glomerulosa and sympathetic nerve fibers. Adrenal sections from control (A and D), low-sodium diet (B and E), and sodium-repleted (C and F) rats labeled for AS (green) and NPY (red) (A–C) or for VIP (D–F). Adrenal capsule (cap) and zona glomerulosa (zg) are indicated. Width of zona glomerulosa (white line) is based on AS expression. Scale bar, 50 μm.
Table 1.
Protein expression and nerve fiber length in the outer adrenal cortex after sodium depletion and repletion in rats
| Protein | Distance from adrenal capsule (μm)
|
ANOVA P value | ||
|---|---|---|---|---|
| Control | Low-Na diet | Low Na/repletion | ||
| P450 AS | 35.4 ± 4.0a | 88.3 ± 3.1a | 66.4 ± 11.6a | <0.01 |
| NPY | 55.9 ± 15.7a | 81.7 ± 3.5a | 72.4 ± 2.1a | 0.04 |
| VIP | 37.4 ± 4.0a | 68.9 ± 1.2a | 45.5 ± 1.9a | <0.01 |
| CGRP | 12.9 ± 3.2a | 11.4 ± 2.5a | ND | ND |
| p75NTR | 49.1 ± 1.5a | 83.6 ± 3.3a | 75.8 ± 6.4a | <0.01 |
| TUF1 | 54.6 ± 9.4a | 90.8 ± 6.9a | 82.7 ± 6.3a | <0.01 |
Values are mean ± sd (n = 4). ND, Not determined.
Values without a common letter differ with a P < 0.05.
TUF1 expression colocalizes with AS in zona glomerulosa and is altered by sodium manipulation
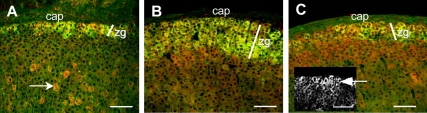
TUF1 expression was detected in the glomerulosa colocalizing with AS (Fig. 2A) and in clusters of fasciculata cells (Fig. 2A, arrow) but not in the adrenal medulla (data not shown). Rats fed a sodium-deficient diet showed a parallel expansion of AS and TUF1 (Fig. 2B and Table 1). In comparison with AS, sodium repletion did not result in marked regression of TUF1 expression (Fig. 2C and Table 1). However, a gradient of TUF1 expression was observed after sodium repletion with TUF1 expression remaining elevated in glomerulosa cells (Fig. 2C inset, arrow) relative to underlying fasciculata cells.
Figure 2.
Low-sodium diet expands TUF1 expression in the zona glomerulosa. A–C, Adrenal sections from control (A), low-sodium diet (B), and sodium-repleted (C) rats labeled for AS (green) and TUF1 (red). In addition to the zona glomerulosa, TUF1 was expressed in cell clusters in the zona fasciculata of control adrenals (A, arrow) and in the outer fasciculata after sodium repletion (C); after sodium repletion, TUF1 expression remained elevated in the glomerulosa (C, inset, arrow) relative to the underlying fasciculata. Adrenal capsule (cap) and zona glomerulosa (zg) are indicated. Width of zona glomerulosa (white line) is based on AS expression. Scale bar, 50 μm.
Nerve fibers expressing p75NTR innervate the zona glomerulosa and are regulated by sodium manipulation
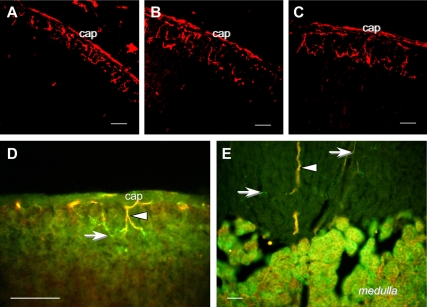
Nerve fibers expressing p75NTR were observed in the adrenal capsule and zona glomerulosa (Fig. 3). Compared with controls, p75NTR-positive fibers expanded with the zona glomerulosa after sodium depletion but showed limited regression in response to sodium repletion (Fig. 3, A–C, and Table 1).
Figure 3.
Low-sodium diet modifies p75NTR expression in the rat adrenal. A–C, Adrenal glomerulosa expression of p75NTR is shown for control (A), low-sodium diet (B), and sodium-repleted (C) rats. D–E, Adrenal sections labeled for p75NTR (red) and NPY (green) showed nerve fibers in the zona glomerulosa (D) and extending into the zona reticularis (E) that coexpressed p75NTR and NPY (yellow, arrowhead) or expressed only NPY (arrows). Chromaffin cells (medulla) also coexpress p75NTR and NPY. Adrenal capsule (cap) is indicated. Scale bar, 50 μm.
Some fibers in the capsule, glomerulosa and extending toward the medulla showed coexpression of p75NTR and NPY (Fig. 3, D and E, arrowheads), other fibers expressed only NPY (Fig. 3, D and E, arrows). Coexpression of p75NTR and NPY was also observed in medullary chromaffin cells (Fig. 3E).
TUF142–53 peptide binds p75NTR in vitro
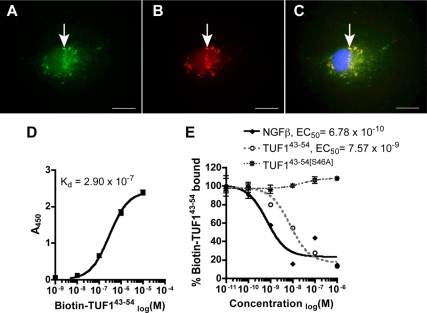
TUF1 contains a conserved domain (SYVRAL) of neurotrophic factors that is necessary to bind p75NTR (29). We found that the TUF143-54 peptide containing the conserved p75-binding motif bound readily to COS7 cells expressing p75NTR (Fig. 4, A–C). No binding was detected in cells transfected with pCMV-eGFP or when labeled TUF124-38 was used in COS7 cells expressing p75NTR (data not shown). Using a p75NTR immunoadhesion assay, TUF143-54 binds p75NTR with a low affinity (dissociation constant Kd = 2.90 × 107) (Fig. 4D). In addition, binding of p75NTR by TUF143-54 is displaced competitively by unlabeled TUF143-54 (EC50 = 7.6 × 10−9) and by NGF-β (EC50 = 6.8 × 10−10) but not by TUF143-54[S46A] [TUF143-54 with serine-46 replaced with alanine (46AYVRAL)] (Fig. 4E).
Figure 4.
TUF143-54 peptide binds p75NTR in vitro. A and B, pCMV-SPORT6–p75-transfected COS7 cell (A) showed TUF143-54 peptide binding (B); C, merged image shows colocalization of p75NTR and TUF143-54 peptide (arrow) with DAPI nuclear stain (blue). Scale bar, 50 μm. D and E, Binding assays using p75NTR immunoadhesion protein are shown for TUF143-54 peptide alone (D) and after competition with unlabeled TUF143-54, TUF143-54 with serine46 replaced with alanine (TUF143-54[S46A]) and NGFβ (E).
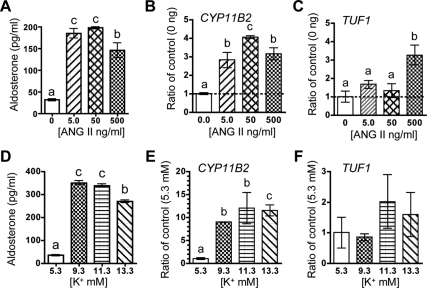
Ang II stimulates TUF1 expression in rat dissociated glomerulosa cells
We examined the possibility that known secretagogues for glomerulosa cell function like Ang II and potassium would regulate TUF1 expression. Acutely dissociated cells from the adrenal capsule/glomerulosa incubated with Ang II showed a dose-related increase in the expression of both CYP11B2 mRNA and aldosterone secretion (Fig. 5, A and B). The highest dose of Ang II (500 ng/ml or 480 pm) was required to stimulate TUF1 mRNA (Fig. 5C). Increasing K+ concentrations induced aldosterone secretion and CYP11B2 mRNA (Fig. 5, D and E) but failed to stimulate TUF1 expression (Fig. 5F).
Figure 5.
Ang II induces TUF1 expression in dissociated rat zona glomerulosa cells. A and D, Aldosterone responses to Ang II and K+ in growth medium; B and C, ratio of CYP11B2 (B) and TUF1 (C) mRNA normalized to control (0 μmol/liter) in glomerulosa cells stimulated with Ang II; E and F, ratio of CYP11B2 (E) and TUF1 (F) mRNA normalized to control in glomerulosa cells stimulated with K+. Values are means ± sem; n = 3 per group. Means without a common letter differ significantly (P < 0.05).
Discussion
The zona glomerulosa is densely innervated by sympathetic nerve fibers containing VIP, NPY, and catecholamines, and each has been implicated in controlling aldosterone secretion (reviewed in Ref. 31). Neuropeptides stimulate glomerulosa cell secretion directly (32,33) or stimulate local release of norepinephrine that acts via β-adrenergic receptors to augment aldosterone secretion (34,35). The observation that chronic sodium depletion in rats increases VIP content in capsular/glomerulosa tissue (19) prompted our examination of peptidergic innervation during glomerulosa expansion. Our findings show that VIP- and NPYergic fibers elongate and retract during sodium depletion and repletion, respectively, demonstrating a clear relationship between glomerulosa cell and nerve growth. Although activation of the renin-angiotensin system is the primary drive for aldosterone secretion during low sodium intake (10), glomerulosa cell responsiveness to neuropeptides are affected (36,37). Aldosterone secretory responses to VIP are enhanced in adrenal cells collected from sodium-depleted rats (36), whereas aldosterone responses to NPY are augmented when coincubated with Ang II (37). Our results indicate that increased sympathetic innervation may provide yet another mechanism for enhancing functional adrenal responses to sodium depletion. To meet physiological demands, functional plasticity of sympathetic nerves may be a common mechanism that is induced by chronic stimulation of target tissues. For example, chronic electrical stimulation of the stellate ganglion in dogs increased nerve sprouting in the cardiac atria (38), prolonged fasting in rats increases sympathetic innervation of white adipose tissue (39), and chronic exposure of mice to a cold environment increases the branching of sympathetic nerves in brown adipose tissue to promote heat production (40).
By examining adrenals from sodium-restricted rats, we identified TMEM35/TUF1 as a novel factor that colocalized with and expanded in parallel with AS in glomerulosa cells. TUF1 was initially identified as an enriched member of the mouse ventromedial hypothalamic transcriptome (28) that is expressed in multiple brain sites (Allen Brain Atlas; http://www.brain-map.org). The function of TUF1 is unknown, but an in silico analysis showed a small protein with potentially secreted peptide containing a highly conserved motif required for binding the p75NTR (Supplemental Fig. 1) (29). Experiments were done to examine a potential link between TUF1 and p75NTR. By assessing adrenal expression of p75NTR, we found that p75NTR-positive nerve fibers in the zona glomerulosa expanded inward after sodium restriction. Some of the NPYergic fibers coexpressed p75NTR, supporting a potential role for p75NTR in modulating neurite outgrowth as the glomerulosa expands. After 1 wk of sodium repletion, as NPY and VIP neurites retracted and a TUF1 gradient developed with increased expression in glomerulosa cells, the elongation of p75NTR fibers persisted. The apparent mismatch between sympathetic neuronal markers p75NTR and TUF1 during sodium repletion likely reflects differences in the rates of synthesis and degradation of these factors. Additional in vivo experiments are required to determine whether p75NTR or TUF1 are required for neurite expansion and retraction in the adrenal after sodium manipulation. However, to establish whether TUF1 could signal via p75NTR, in vitro experiments were done to assess receptor binding. We demonstrated that TUF143-54, a peptide containing the conserved p75-binding motif, binds specifically to COS7 cells ectopically expressed p75NTR. Using a more quantitative immunoadhesion assay (30), TUF1 bound dose-dependently to p75NTR with low affinity (Kd = 2.90 × 107). Unlabeled-TUF143-54, but not TUF143-54[S46A] (a TUF143-54 peptide modified to alter motif required for p75NTR binding) competitively displaced labeled TUF1 (EC50 = 7.6 × 10−9). Additionally, NGF-β displaced TUF1 binding with relatively high affinity (EC50 = 6.8 × 10−10), demonstrating that TUF1 could compete with known neurotrophins for binding to the p75NTR. These results establish a basis for implicating TUF1 as a neurotrophic factor that could signal via the p75NTR in the adrenal. Although TUF1 binding to high-affinity Trk receptors was not tested, it is unlikely that binding would occur because TUF1 lacks sequence homology to the specific binding domains of known neurotrophins. However, it is possible that in addition to low-affinity binding to p75NTR, TUF1 interacts with a high-affinity cognate receptor that remains to be identified.
The finding that sodium depletion results in elongation of sympathetic nerves clearly implicates the action of a neurotrophic factor in the zona glomerulosa. Although axonal extension of sympathetic nerves for most rodent tissues is NGF dependent, the requirement of NGF for adrenal innervation has not been established (41); also, neither NGF (25) nor TrkA receptors (22) are highly expressed in the adrenal cortex, implicating other neurotrophins in adrenocortical innervation. Both BDNF (42) and NT-3 (24) and their cognate receptors, TrkB (42) and TrkC (25), respectively, are expressed in the rodent adrenal cortex and could participate in adrenal nerve elongation. The p75NTR does not possess inherent Trk activity (43), but binding by neurotrophins clearly influences sympathetic axonal growth (44). Binding to p75NTR has been implicated both in promoting axonal extension by guiding axons to intermediate targets (45) and in retraction via axonal degeneration (46). During development of sympathetic innervation, multiple neurotrophins are involved in insuring precise guidance of axons to their final targets by competing for binding to Trk receptors and to p75NTR (47). Our observation that TUF1 can bind p75NTR and expand its expression region in parallel with sympathetic and p75NTR fibers as the glomerulosa grows implicates TUF1 in neurite outgrowth. However, it is likely that one or more of the known neurotrophins is required for any neurotrophic effects mediated by TUF1. Recent work has shown that Ang II stimulates BDNF expression in human H295R and rat glomerulosa cells (42). TUF1 mRNA expression also can be induced by Ang II in vitro; its relative insensitivity to Ang II and potassium compared with aldosterone secretion and CYP11B2 mRNA may stem from in vitro conditions that do not adequately mimic the in vivo environment produced by chronic sodium restriction (e.g., the requirement for multiple stimuli and/or long-term stimulation). Additional experiments are required to test the interesting possibility that TUF1 and BDNF may interact to provide neurotrophic signaling for sympathetic neurite growth after chronic activation of the renin-angiotensin system.
A hallmark of the adrenal response to chronic sodium depletion is the expansion of the zona glomerulosa resulting in increased capacity for aldosterone secretion and the maintenance of sodium balance. The present results show that adrenal nerve fibers expressing VIP or NPY elongate and retract as changes in adrenal growth are induced by sodium manipulation. Our experiments also identify TUF1 as a novel peptide localized in the adrenal cortex that changes its expression pattern in parallel with growth of the zona glomerulosa and p75NTR-expressing nerve fibers. The finding that TUF1 binds the p75NTR and responds to Ang II stimulation in vitro supports a potential role for TUF1 as a neurotrophic factor contributing to neurite outgrowth in the zona glomerulosa after sodium depletion.
Salt sensitivity remains a major cause of hypertension in humans (48). Dysregulation of zona glomerulosa responsiveness to sodium states has been linked to hypertensive pathology given the role of aldosterone in regulation of blood pressure through the renin-angiotensin system. In low-renin salt-sensitive hypertension, elevated plasma aldosterone has been associated with variants of the β2 adrenergic receptor gene, leading to speculation that increased adrenal β2 adrenergic activity interacts with sodium to enhance aldosterone secretion (49). Thus, the discovery of neuropeptides that regulate innervation of the zona glomerulosa in response to sodium status may be clinically relevant, particularly if their dysregulation leads to abnormal blood pressure control. The finding that the newly discovered TUF1 peptide varies its expression and location as a function of sodium status provides a potential target for therapeutic intervention in the future.
Supplementary Material
Acknowledgments
We thank Michelle Arnhold and J. Marina Yoder for their technical assistance and Dr. Celso Gomez-Sanchez (University of Mississippi Medical Center) and Dr. Louis Reichardt (University of California San Francisco) for their generous gift of antisera. We also are grateful for the expertise provided by Drs. Matilde Holzwarth (University of Illinois) and William Kennedy (Department of Neurology, University of Minnesota) during the initial stages of these experiments.
Footnotes
This work was supported by National Institute of Mental Health Training Grant T32MH073129 and Minnesota Medical Foundation Grant to P.V.T. and NSF IOB-0548584 to W.C.E.
Disclosure Summary: P.V.T. is an inventor on a U.S. patent application (PCT/US2009/069454). M.K.G. and W.C.E have nothing to declare.
First Published Online August 4, 2010
Abbreviations: Ang II, Angiotensin II; AS, aldosterone synthase; BDNF, brain-derived neurotrophic factor; CGRP, calcitonin gene-related peptide; DAPI, 4′,6-diamidino-2-phenylindole; NGF, nerve growth factor; NPY, neuropeptide Y; NT, neurotrophin; p75NTR, p75 NT receptor; qPCR, quantitative real-time PCR; TBS, Tris-buffered saline; Trk, tyrosine kinase receptor; TUF1, the unknown factor-1; VIP, vasoactive intestinal polypeptide.
References
- Bland ML, Desclozeaux M, Ingraham HA 2003 Tissue growth and remodeling of the embryonic and adult adrenal gland. Ann NY Acad Sci 995:59–72 [DOI] [PubMed] [Google Scholar]
- Hoeflich A, Bielohuby M 2009 Mechanisms of adrenal gland growth: signal integration by extracellular signal regulated kinases1/2. J Mol Endocrinol 42:191–203 [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Nonaka Y, Ying Z, Higaki J, Ogihara T, Okamoto M 1990 Molecular cloning and expression of cDNAs encoding rat aldosterone synthase: variants of cytochrome P-450 11β. Biochem Biophys Res Commun 169:245–252 [DOI] [PubMed] [Google Scholar]
- Nonaka Y, Matsukawa N, Morohashi, Omura T, Ogihara T, Teraoka H, Okamoto M 1989 Molecular cloning and sequence analysis of cDNA encoding rat adrenal cytochrome P-450 11B. FEBS Lett 255:21–26 [DOI] [PubMed] [Google Scholar]
- Ogishima T, Suzuki H, Hata J, Mitani F, Ishimura Y 1992 Zone-specific expression of aldosterone synthase cytochrome P-450 and cytochrome P-45011B in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology 130:2971–2977 [DOI] [PubMed] [Google Scholar]
- Engeland WC, Ennen WB, Elayaperumal A, Durand DA, Levay-Young BK 2005 Zone-specific cell proliferation during compensatory adrenal growth in rats. Am J Physiol Endocrinol Metab 288:E298–E306 [DOI] [PubMed] [Google Scholar]
- Ennen WB, Levay-Young BK, Engeland WC 2005 Zone-specific cell proliferation during adrenocortical regeneration after enucleation in rats. Am J Physiol Endocrinol Metab 289:E883–E891 [DOI] [PubMed] [Google Scholar]
- Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y 1994 A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology 135:431–438 [DOI] [PubMed] [Google Scholar]
- McEwan PE, Lindop GB, Kenyon CJ 1996 Control of cell proliferation in the rat adrenal gland in vivo by the renin-angiotensin system. Am J Physiol 271:E192–E198 [DOI] [PubMed] [Google Scholar]
- Aguilera G, Hauger RL, Catt KJ 1978 Control of aldosterone secretion during sodium restriction: adrenal regulation and increased adrenal sensitivity to angiotensin II. Proc Natl Acad Sci USA 75:975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H, Whitworth E, Vinson GP, Hinson JP 2005 Distribution of extracellular signal-regulated protein kinases 1 and 2 in the rat adrenal and their activation by angiotensin II. J Endocrinol 187:149–157 [DOI] [PubMed] [Google Scholar]
- Otis M, Campbell S, Payet MD, Gallo-Payet N 2008 In adrenal glomerulosa cells, angiotensin II inhibits proliferation by interfering with fibronectin-integrin signaling. Endocrinology 149:3435–3445 [DOI] [PubMed] [Google Scholar]
- Tóth IE, Vizi ES, Hinson JP, Vinson GP 1997 Innervation of the adrenal cortex, its physiological relevance, with primary focus on the noradrenergic transmission. Microsc Res Tech 36:534–545 [DOI] [PubMed] [Google Scholar]
- Kondo H 1985 Immunohistochemical analysis of the localization of neuropeptides in the adrenal gland. Arch Histol Jpn 48:453–481 [DOI] [PubMed] [Google Scholar]
- Malendowicz LK 1993 Involvement of neuropeptides in the regulation of growth, structure and function of the adrenal cortex. Histol Histopathol 8:173–186 [PubMed] [Google Scholar]
- Ulrich-Lai YM, Engeland WC 2000 Hyperinnervation during adrenal regeneration influences the rate of functional recovery. Neuroendocrinology 71:107–123 [DOI] [PubMed] [Google Scholar]
- Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM 2006 Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience 142:809–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan W, Jama A, Donohue T, Wernli G, Onyszchuk G, Al-Hafez B, Bilgen M, Smith PG 2006 Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res 1124:142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JP, Renshaw D, Carroll M, Kapas S 2001 Regulation of rat adrenal vasoactive intestinal peptide content: effects of adrenocorticotropic hormone treatment and changes in dietary sodium intake. J Neuroendocrinol 13:769–773 [DOI] [PubMed] [Google Scholar]
- Schober A, Wolf N, Huber K, Hertel R, Krieglstein K, Minichiello L, Kahane N, Widenfalk J, Kalcheim C, Olson L, Klein R, Lewin GR, Unsicker K 1998 TrkB and neurotrophin-4 are important for development and maintenance of sympathetic preganglionic neurons innervating the adrenal medulla. J Neurosci 18:7272–7284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A, Hertel R, Arumäe U, Farkas L, Jaszai J, Krieglstein K, Saarma M, Unsicker K 1999 Glial cell line-derived neurotrophic factor rescues target-deprived symapthetic spinal cord neurons but requires transforming growth factor-ß as cofactor in vivo. J Neurosci 19:2008–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV 1996 Expression of trkA and p75 nerve growth factor receptors in the adrenal gland. Neuroreport 7:1617–1622 [DOI] [PubMed] [Google Scholar]
- Schober A, Wolf N, Kahane N, Kalcheim C, Krieglstein K, Unsicker K 1999 Expression of neurotrophin receptors trkB and trkC and their ligands in rat adrenal gland and the intermediolateral column of the spinal cord. Cell Tissue Res 296:271–279 [DOI] [PubMed] [Google Scholar]
- Zhou XF, Rush RA 1993 Localization of neurotrophin-3-like immunoreactivity in peripheral tissues of the rat. Brain Res 621:189–199 [DOI] [PubMed] [Google Scholar]
- Suter-Crazzolara C, Lachmund A, Arab SF, Unsicker K 1996 Expression of neurotrophins and their receptors in the developing and adult rat adrenal gland. Brain Res Mol Brain Res 43:351–355 [DOI] [PubMed] [Google Scholar]
- Holzwarth MA 1984 The distribution of vasoactive intestinal peptide in the rat adrenal cortex and medulla. J Auton Nerv Syst 11:269–283 [DOI] [PubMed] [Google Scholar]
- Kleitman N, Holzwarth MA 1985 Catecholaminergic innervation of the rat adrenal cortex. Cell Tissue Res 241:139–147 [DOI] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA 2007 The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci 27:13624–13634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann C, Ultsch MH, Bass SH, de Vos AM 1999 Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature 401:184–188 [DOI] [PubMed] [Google Scholar]
- Horton A, Laramee G, Wyatt S, Shih A, Winslow J, Davies AM 1997 NGF binding to p75 enhances the sensitivity of sensory and sympathetic neurons to NGF at different stages of development. Mol Cell Neurosci 10:162–172 [DOI] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP 1998 Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev 19:101–143 [DOI] [PubMed] [Google Scholar]
- Bernet F, Maubert E, Bernard J, Montel V, Dupouy JP 1994 In vitro steroidogenic effects of neuropeptide Y (NPY 1–36), Y1 and Y2 receptor agonists (Leu31-Pro34 NPY, NPY18–36) and peptide YY (PYY) on rat adrenal capsule/zona glomerulosa. Regul Pept 52:187–193 [DOI] [PubMed] [Google Scholar]
- Cunningham LA, Holzwarth MA 1988 Vasoactive intestinal peptide stimulates adrenal aldosterone and corticosterone secretion. Endocrinology 122:2090–2097 [DOI] [PubMed] [Google Scholar]
- Renshaw D, Thomson LM, Carroll M, Kapas S, Hinson JP 2000 Actions of neuropeptide Y on the rat adrenal cortex. Endocrinology 141:169–173 [DOI] [PubMed] [Google Scholar]
- Bernet F, Bernard J, Laborie C, Montel V, Maubert E, Dupouy JP 1994 Neuropeptide Y (NPY)- and vasoactive intestinal peptide (VIP)-induced aldosterone secretion by rat capsule/glomerular zone could be mediated by catecholamines via β1 adrenergic receptors. Neurosci Lett 166:109–112 [DOI] [PubMed] [Google Scholar]
- Hinson JP, Kapas S 1995 Effects of sodium depletion on the response of rat adrenal zona glomerulosa cells to stimulation by neuropeptides: actions of vasoactive intestinal peptide, enkephalin, substance P, neuropeptide Y and corticotrophin-releasing hormone. J Endocrinol 146:209–214 [DOI] [PubMed] [Google Scholar]
- Hinson JP, Cameron LA, Kapas S 1995 Neuropeptide Y modulates the sensitivity of the rat adrenal cortex to stimulation by ACTH. J Endocrinol 145:283–289 [DOI] [PubMed] [Google Scholar]
- Swissa M, Zhou S, Tan AY, Fishbein MC, Chen PS, Chen LS 2008 Atrial sympathetic and parasympathetic nerve sprouting and hyperinnervation induced by subthreshold electrical stimulation of the left stellate ganglion in normal dogs. Cardiovasc Pathol 17:303–308 [DOI] [PubMed] [Google Scholar]
- Giordano A, Frontini A, Murano I, Tonello C, Marino MA, Carruba MO, Nisoli E, Cinti S 2005 Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem 53:679–687 [DOI] [PubMed] [Google Scholar]
- Murano I, Barbatelli G, Giordano A, Cinti S 2009 Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J Anat 214:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD 2004 Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci 24:743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Nádasy GL, Turu G, Süpeki K, Szidonya L, Buday L, Chaplin T, Clark AJ, Hunyady L 2010 Angiotensin II-induced expression of brain-derived neurotrophic factor in human and rat adrenocortical cells. Endocrinology 151:1695–1703 [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA 2002 Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67:203–233 [DOI] [PubMed] [Google Scholar]
- Lee KF, Bachman K, Landis S, Jaenisch R 1994 Dependence on p75 for innervation of some sympathetic targets. Science 263:1447–1449 [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD 2004 A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118:243–255 [DOI] [PubMed] [Google Scholar]
- Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD 1998 The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol 140:911–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD 1999 Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci 19:5393–5408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco V, Oparil S 2006 Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr 25:247S–255S [DOI] [PubMed] [Google Scholar]
- Pojoga L, Kolatkar NS, Williams JS, Perlstein TS, Jeunemaitre X, Brown NJ, Hopkins PN, Raby BA, Williams GH 2006 β-2 adrenergic receptor diplotype defines a subset of salt-sensitive hypertension. Hypertension 48:892–900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.