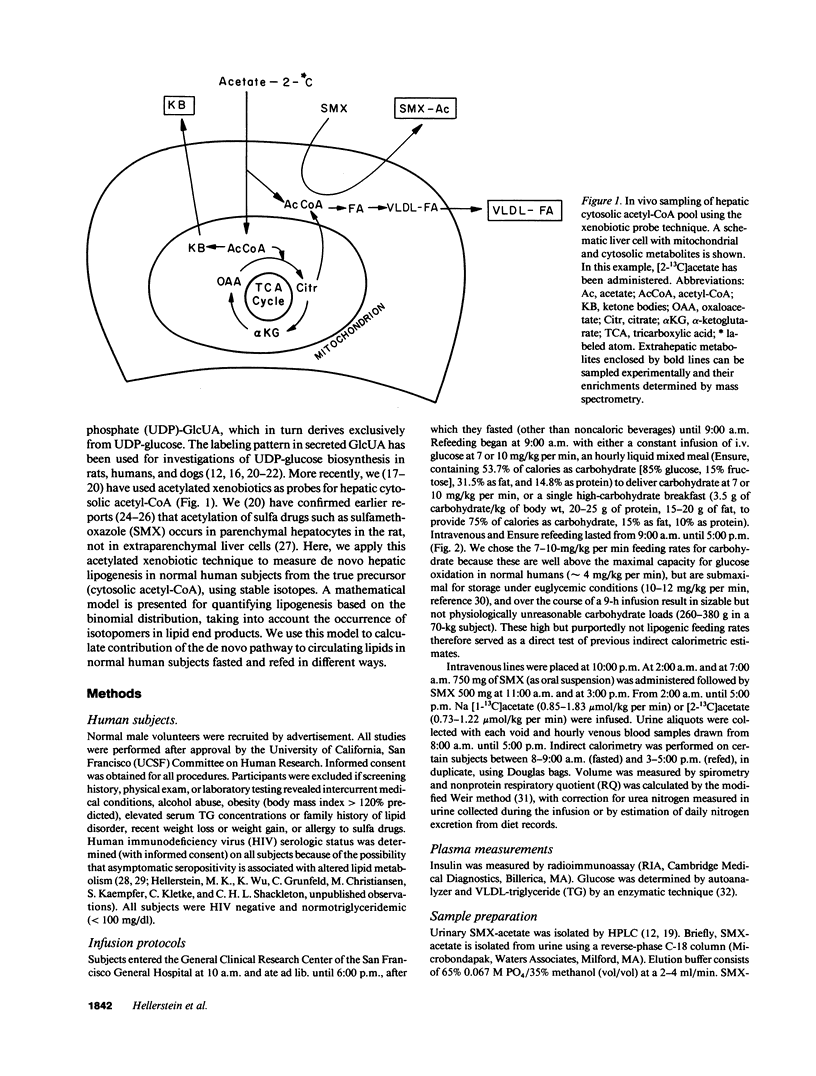
Abstract
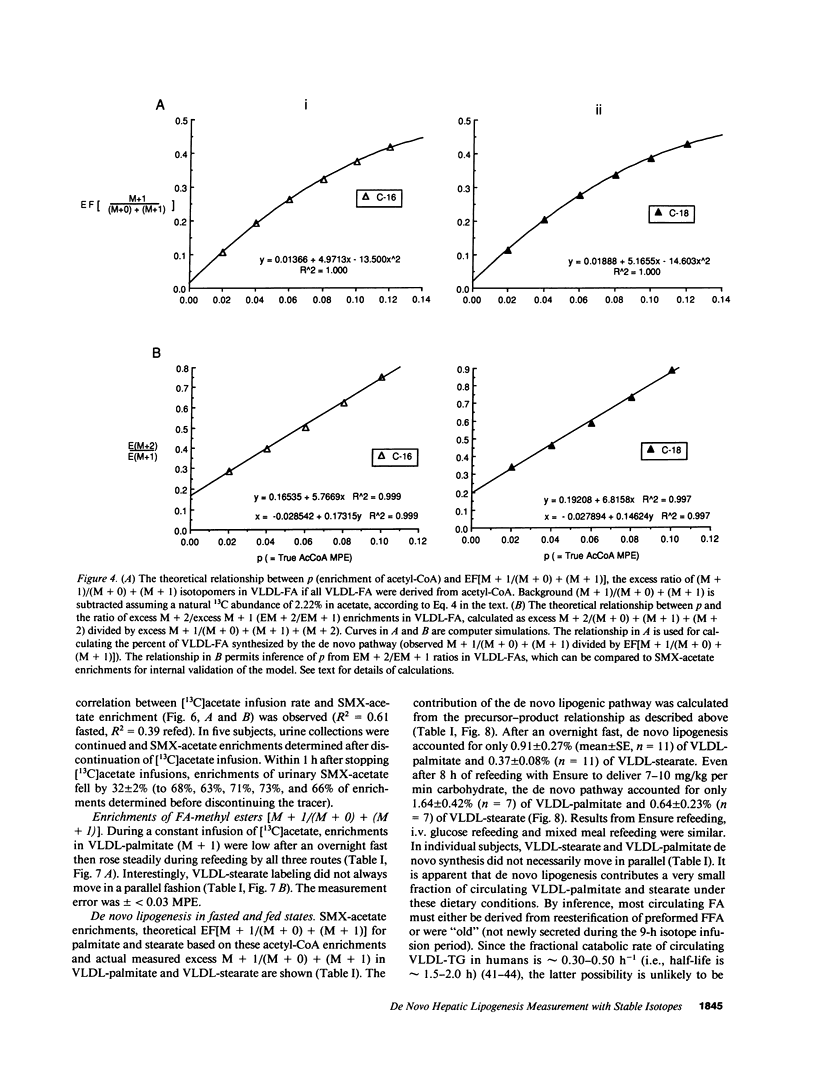
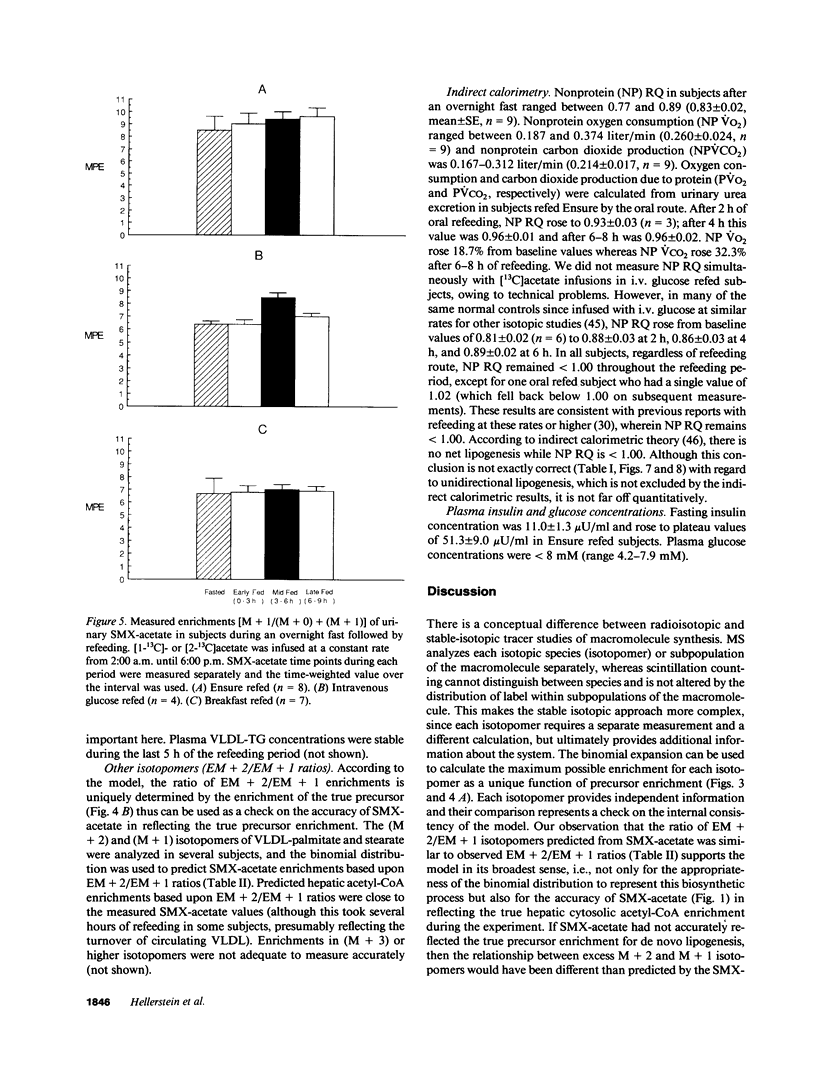
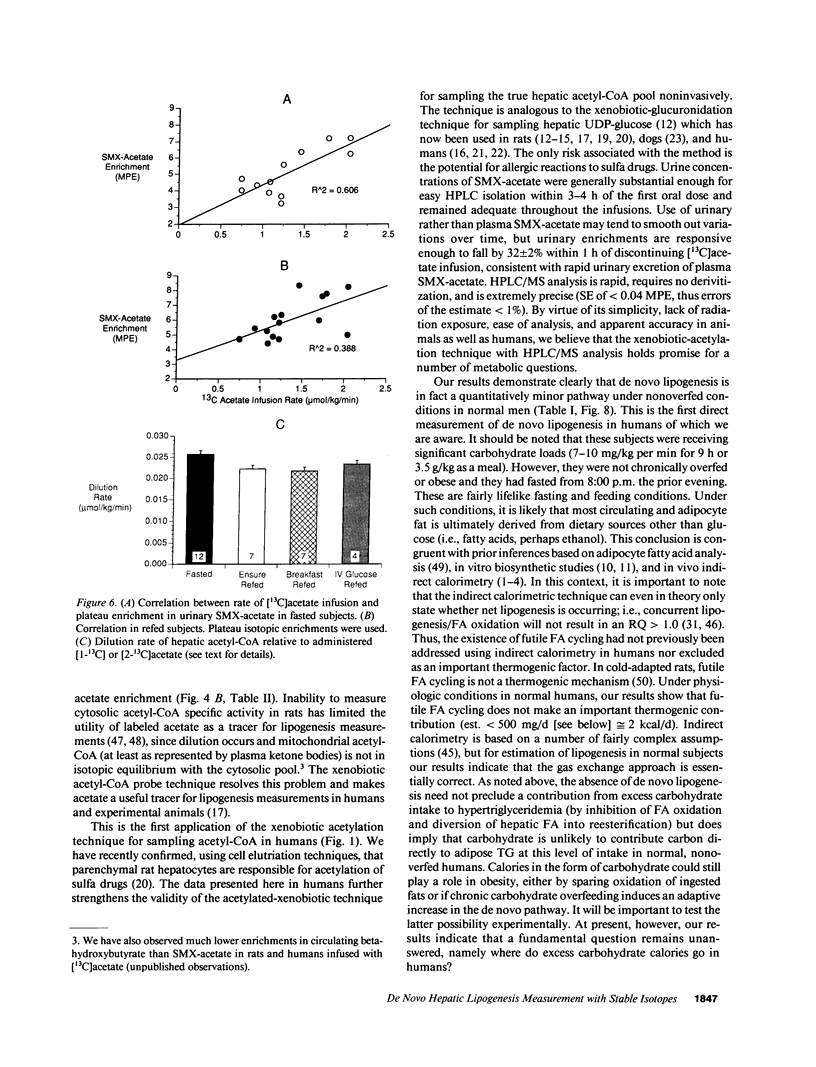
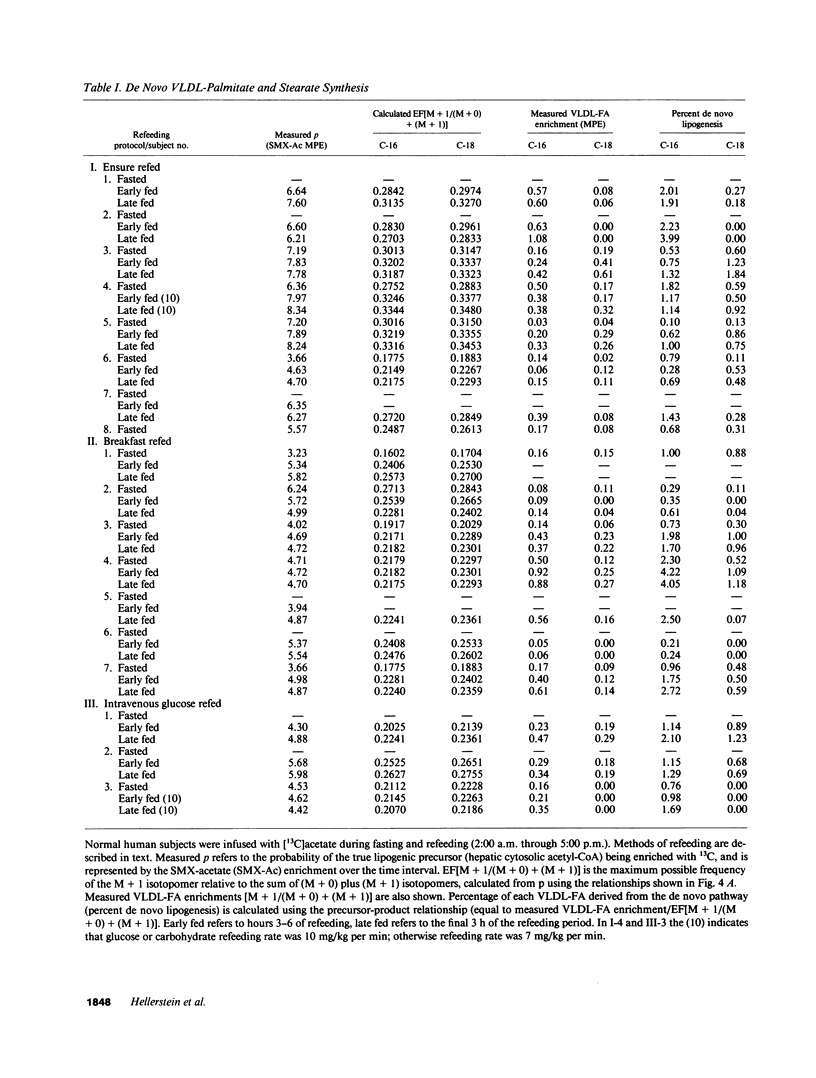
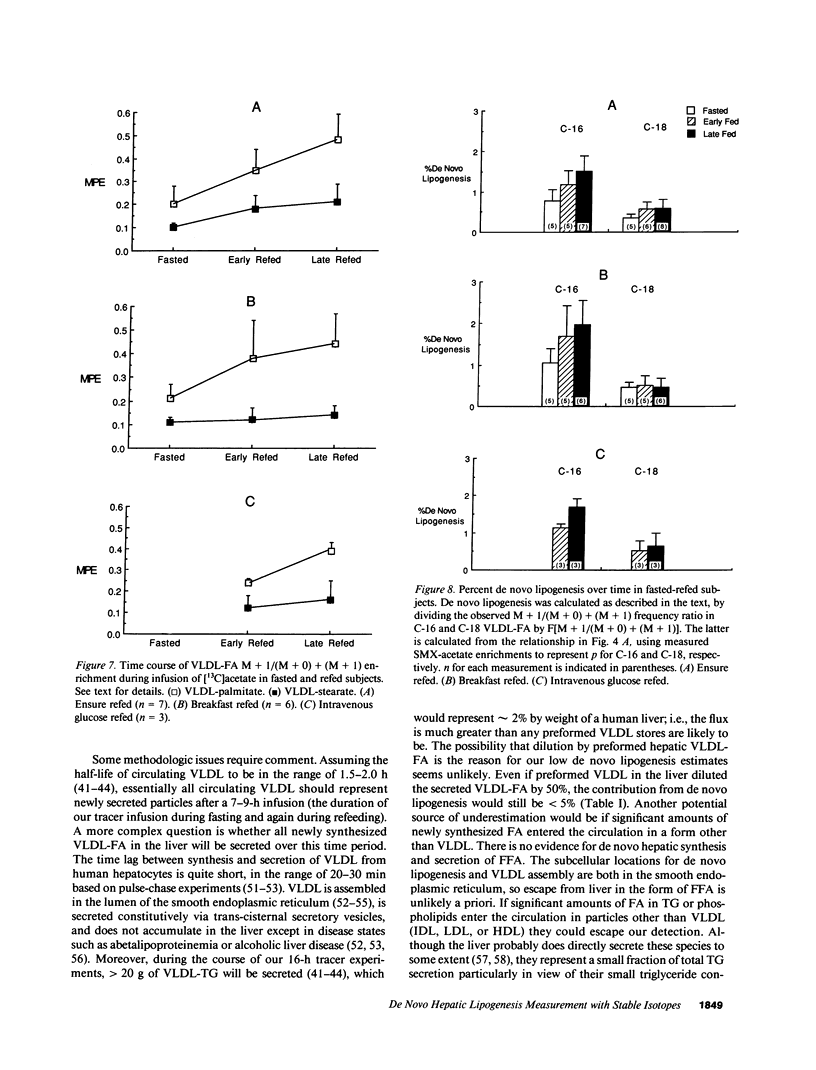
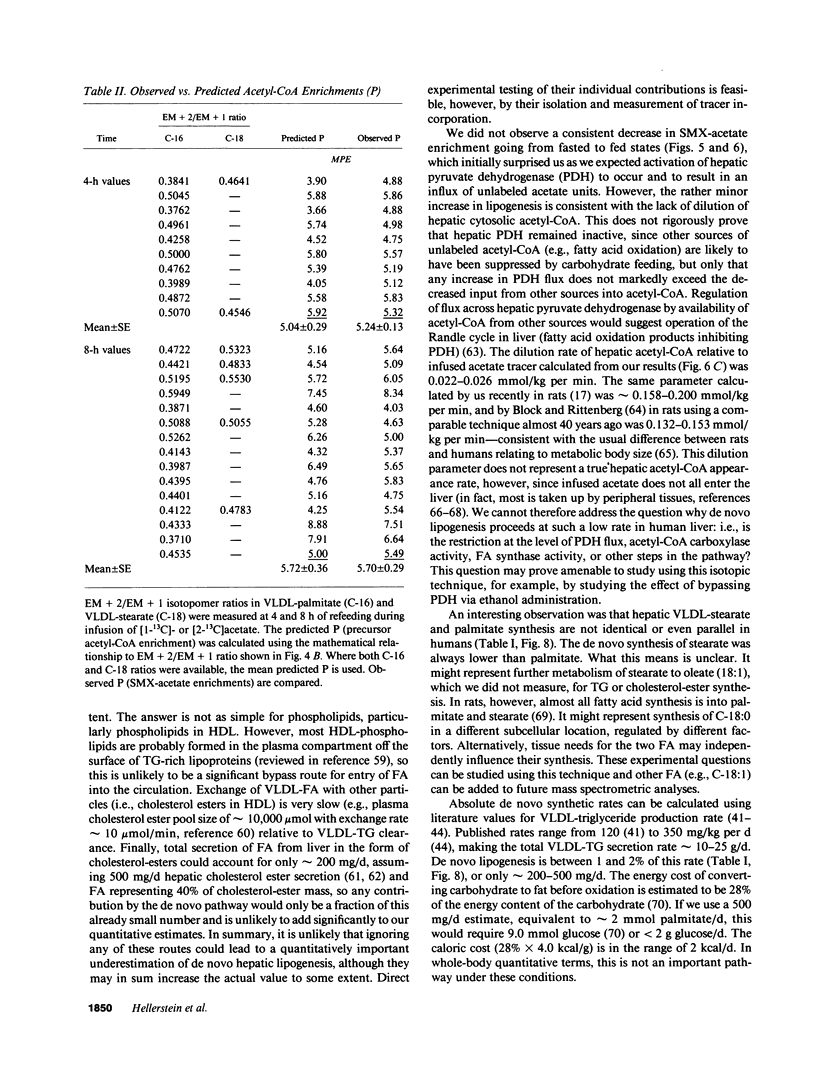
Direct measurement of de novo lipogenesis has not previously been possible in humans. We measured de novo hepatic lipogenesis in normal men by means of stable isotopes and by combining the acetylated-xenobiotic probe technique with mass isotopomer analysis of secreted very low density lipoprotein-fatty acids (VLDL-FA). Sulfamethoxazole (SMX) was administered with [13C]acetate during an overnight fast followed by refeeding with intravenous glucose (7-10 mg/kg of weight per min), oral Ensure (7-10 mg of carbohydrate/kg of weight per min), or a high-carbohydrate mixed-meal breakfast (3.5 g of carbohydrate/kg of weight). Respiratory quotients remained less than 1.0. High-performance liquid chromatography/mass spectrometry-determined enrichments in SMX-acetate attained stable plateau values, and hepatic acetyl-coenzyme A (CoA) dilution rate did not increase with refeeding (approximately 0.024 mmol/kg per min). The fraction of VLDL-palmitate derived from de novo lipogenesis was only 0.91 +/- 0.27% (fasted) and 1.64-1.97% (fed). For stearate, this was 0.37 +/- 0.08% and 0.47-0.64%. Precursor enrichments predicted from isotopomer ratios were close to measured SMX-acetate enrichments, indicating that SMX-acetate samples the true lipogenic acetyl-CoA pool. Stearate synthesis was less than palmitate and the two did not move in parallel. Estimated total VLDL-FA synthesis is less than 500 mg/day. Thus, de novo hepatic lipogenesis is a quantitatively minor pathway, consistent with gas exchange estimates; fatty acid futile cycling (oxidation/resynthesis) is not thermogenically significant; and synthesis rates of different nonessential fatty acids by human liver are not identical in nonoverfed normal men. The contribution and regulation of de novo lipogenesis in other settings can be studied using this technique.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. G., Boyce V. L., Grundy S. M., Howard B. V. Effects of replacing saturated fat with complex carbohydrate in diets of subjects with NIDDM. Diabetes Care. 1989 Feb;12(2):102–107. doi: 10.2337/diacare.12.2.102. [DOI] [PubMed] [Google Scholar]
- Abrams J. J., Ginsberg H., Grundy S. M. Metabolism of cholesterol and plasma triglycerides in nonketotic diabetes mellitus. Diabetes. 1982 Oct;31(10):903–910. doi: 10.2337/diab.31.10.903. [DOI] [PubMed] [Google Scholar]
- Acheson K. J., Flatt J. P., Jéquier E. Glycogen synthesis versus lipogenesis after a 500 gram carbohydrate meal in man. Metabolism. 1982 Dec;31(12):1234–1240. doi: 10.1016/0026-0495(82)90010-5. [DOI] [PubMed] [Google Scholar]
- Acheson K. J., Schutz Y., Bessard T., Ravussin E., Jéquier E., Flatt J. P. Nutritional influences on lipogenesis and thermogenesis after a carbohydrate meal. Am J Physiol. 1984 Jan;246(1 Pt 1):E62–E70. doi: 10.1152/ajpendo.1984.246.1.E62. [DOI] [PubMed] [Google Scholar]
- Ballard F. J. Supply and utilization of acetate in mammals. Am J Clin Nutr. 1972 Aug;25(8):773–779. doi: 10.1093/ajcn/25.8.773. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Sjöström L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism. 1978 Dec;27(12 Suppl 2):1853–1865. doi: 10.1016/s0026-0495(78)80004-3. [DOI] [PubMed] [Google Scholar]
- Cohn J. S., Wagner D. A., Cohn S. D., Millar J. S., Schaefer E. J. Measurement of very low density and low density lipoprotein apolipoprotein (Apo) B-100 and high density lipoprotein Apo A-I production in human subjects using deuterated leucine. Effect of fasting and feeding. J Clin Invest. 1990 Mar;85(3):804–811. doi: 10.1172/JCI114507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulston A. M., Hollenbeck C. B., Swislocki A. L., Reaven G. M. Persistence of hypertriglyceridemic effect of low-fat high-carbohydrate diets in NIDDM patients. Diabetes Care. 1989 Feb;12(2):94–101. doi: 10.2337/diacare.12.2.94. [DOI] [PubMed] [Google Scholar]
- Danforth E., Jr Diet and obesity. Am J Clin Nutr. 1985 May;41(5 Suppl):1132–1145. doi: 10.1093/ajcn/41.5.1132. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Brown M. S. Effect of alterations of the specific activity of the intracellular acetyl CoA pool on apparent rates of hepatic cholesterogenesis. J Lipid Res. 1974 Sep;15(5):508–516. [PubMed] [Google Scholar]
- Dietschy J. M., McGarry J. D. Limitations of acetate as a substrate for measuring cholesterol synthesis in liver. J Biol Chem. 1974 Jan 10;249(1):52–58. [PubMed] [Google Scholar]
- FOSTER D. W., BLOOM B. The synthesis of fatty acids by rat liver slices in tritiated water. J Biol Chem. 1963 Mar;238:888–892. [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Govier W. C. Reticuloendothelial cells as the site of sulfanilamide acetylation in the rabbit. J Pharmacol Exp Ther. 1965 Nov;150(2):305–308. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res. 1969 May;10(3):304–315. [PubMed] [Google Scholar]
- Grunfeld C., Kotler D. P., Hamadeh R., Tierney A., Wang J., Pierson R. N. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989 Jan;86(1):27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- Hellerstein M. K., Greenblatt D. J., Munro H. N. Glycoconjugates as noninvasive probes of intrahepatic metabolism: I. Kinetics of label incorporation with evidence of a common precursor UDP-glucose pool for secreted glycoconjugates. Metabolism. 1987 Oct;36(10):988–994. doi: 10.1016/0026-0495(87)90138-7. [DOI] [PubMed] [Google Scholar]
- Hellerstein M. K., Greenblatt D. J., Munro H. N. Glycoconjugates as noninvasive probes of intrahepatic metabolism: pathways of glucose entry into compartmentalized hepatic UDP-glucose pools during glycogen accumulation. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7044–7048. doi: 10.1073/pnas.83.18.7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstein M. K., Munro H. N. Glycoconjugates as noninvasive probes of intrahepatic metabolism: II. Application to measurement of plasma alpha 1-acid glycoprotein turnover during inflammation. Metabolism. 1987 Oct;36(10):995–1000. doi: 10.1016/0026-0495(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Hellerstein M. K., Munro H. N. Glycoconjugates as noninvasive probes of intrahepatic metabolism: III. Application to galactose assimilation by the intact rat. Metabolism. 1988 Apr;37(4):312–317. doi: 10.1016/0026-0495(88)90129-1. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Perez G., Vranic M. Turnover and precursor-product relationships of nonlipid metabolites. Physiol Rev. 1983 Apr;63(2):606–667. doi: 10.1152/physrev.1983.63.2.606. [DOI] [PubMed] [Google Scholar]
- Kesäniemi Y. A., Beltz W. F., Grundy S. M. Comparisons of metabolism of apolipoprotein B in normal subjects, obese patients, and patients with coronary heart disease. J Clin Invest. 1985 Aug;76(2):586–595. doi: 10.1172/JCI112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H., Alfarsi S., Evans D. J., Adams P. W. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in non-insulin-dependent diabetes mellitus. Diabetes. 1982 Mar;31(3):217–225. doi: 10.2337/diab.31.3.217. [DOI] [PubMed] [Google Scholar]
- Lackner K. J., Monge J. C., Gregg R. E., Hoeg J. M., Triche T. J., Law S. W., Brewer H. B., Jr Analysis of the apolipoprotein B gene and messenger ribonucleic acid in abetalipoproteinemia. J Clin Invest. 1986 Dec;78(6):1707–1712. doi: 10.1172/JCI112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I., Chandramouli V., Schumann W. C., Kumaran K., Wahren J., Landau B. R. Pathways of hepatic glycogen formation in humans following ingestion of a glucose load in the fed state. Metabolism. 1989 Jun;38(6):583–585. doi: 10.1016/0026-0495(89)90221-7. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Chandramouli V., Schumann W. C., Kumaran K., Wahren J., Landau B. R. Quantitation of the pathways of hepatic glycogen formation on ingesting a glucose load. J Clin Invest. 1987 Dec;80(6):1748–1754. doi: 10.1172/JCI113267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musliner T. A., Krauss R. M. Lipoprotein subspecies and risk of coronary disease. Clin Chem. 1988;34(8B):B78–B83. [PubMed] [Google Scholar]
- Nestel P. J. Cholesterol turnover in man. Adv Lipid Res. 1970;8:1–39. doi: 10.1016/b978-0-12-024908-4.50008-8. [DOI] [PubMed] [Google Scholar]
- Nägele U., Hägele E. O., Sauer G., Wiedemann E., Lehmann P., Wahlefeld A. W., Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984 Feb;22(2):165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- Olofsson S. O., Boström K., Carlsson P., Borén J., Wettesten M., Bjursell G., Wiklund O., Bondjers G. Structure and biosynthesis of apolipoprotein B. Am Heart J. 1987 Feb;113(2 Pt 2):446–452. doi: 10.1016/0002-8703(87)90612-0. [DOI] [PubMed] [Google Scholar]
- Olsen H. Interaction between drug acetylation and ethanol, acetate, pyruvate, citrate, and L(-) carnitine in isolated rat liver parenchymal cells. Acta Pharmacol Toxicol (Copenh) 1982 Jan;50(1):67–74. doi: 10.1111/j.1600-0773.1982.tb00941.x. [DOI] [PubMed] [Google Scholar]
- Olsen H., Mørland J. Ethanol interaction with drug acetylation in vivo and in vitro. Pharmacol Biochem Behav. 1983;18 (Suppl 1):295–300. doi: 10.1016/0091-3057(83)90189-2. [DOI] [PubMed] [Google Scholar]
- Olsen H., Mørland J. Ethanol-induced increase in drug acetylation in man and isolated rat liver cells. Br Med J. 1978 Nov 4;2(6147):1260–1262. doi: 10.1136/bmj.2.6147.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSMORE R., SWINDELLS Y. E. OBSERVATIONS ON THE RESPIRATORY QUOTIENTS AND WEIGHT GAIN OF MAN AFTER EATING LARGE QUANTITIES OF CARBOHYDRATE. Br J Nutr. 1963;17:331–339. doi: 10.1079/bjn19630036. [DOI] [PubMed] [Google Scholar]
- PATKIN J. K., MASORO E. J. FATTY ACID SYNTHESIS IN NORMAL AND COLD-ACCLIMATED RATS. Can J Physiol Pharmacol. 1964 Jan;42:101–107. doi: 10.1139/y64-012. [DOI] [PubMed] [Google Scholar]
- Randle P. J. Fuel selection in animals. Biochem Soc Trans. 1986 Oct;14(5):799–806. doi: 10.1042/bst0140799. [DOI] [PubMed] [Google Scholar]
- Reid S., Shackleton C., Wu K., Kaempfer S., Hellerstein M. K. Liquid chromatography/mass spectrometry of plasma glucose and secreted glucuronate for metabolic studies in humans. Biomed Environ Mass Spectrom. 1990 Sep;19(9):535–540. doi: 10.1002/bms.1200190903. [DOI] [PubMed] [Google Scholar]
- Shrago E., Glennon J. A., Gordon E. S. Comparative aspects of lipogenesis in mammalian tissues. Metabolism. 1971 Jan;20(1):54–62. doi: 10.1016/0026-0495(71)90059-x. [DOI] [PubMed] [Google Scholar]
- Shrago E., Spennetta T., Gordon E. Fatty acid synthesis in human adipose tissue. J Biol Chem. 1969 May 25;244(10):2761–2766. [PubMed] [Google Scholar]
- Sjöström L. Fatty acid synthesis de novo in adipose tissue from obese subjects on a hypercaloric high-carbohydrate diet. Scand J Clin Lab Invest. 1973 Dec;32(4):339–349. doi: 10.3109/00365517309084357. [DOI] [PubMed] [Google Scholar]
- Streja D. A., Marliss E. B., Steiner G. The effects of prolonged fasting on plasma triglyceride kinetics in man. Metabolism. 1977 May;26(5):505–516. doi: 10.1016/0026-0495(77)90094-4. [DOI] [PubMed] [Google Scholar]
- Tall A. R. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J Clin Invest. 1990 Aug;86(2):379–384. doi: 10.1172/JCI114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinen M. R., Beltz W. F., Harper I., Fields R. M., Schonfeld G., Grundy S. M., Howard B. V. Effects of NIDDM on very-low-density lipoprotein triglyceride and apolipoprotein B metabolism. Studies before and after sulfonylurea therapy. Diabetes. 1986 Nov;35(11):1268–1277. doi: 10.2337/diab.35.11.1268. [DOI] [PubMed] [Google Scholar]
- Thiebaud D., Jacot E., DeFronzo R. A., Maeder E., Jequier E., Felber J. P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes. 1982 Nov;31(11):957–963. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- von Schacky C., Fischer S., Weber P. C. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J Clin Invest. 1985 Oct;76(4):1626–1631. doi: 10.1172/JCI112147. [DOI] [PMC free article] [PubMed] [Google Scholar]