Introduction
Although viral and parasitic agents have been implicated in human cancers, gastric cancer is at the present time the only malignant neoplasia recognized as causally associated in humans with a bacterium. In 1994 the International Agency for Research on Cancer (IARC) concluded: “there is sufficient evidence in humans for the carcinogenicity of infection with Helicobacter pylori” [1]. At that time they concluded that “there is inadequate evidence in experimental animals for the carcinogenicity of infection with Helicobacter pylori”. Since then, experimental evidence of carcinogenicity has been documented, especially utilizing the Mongolian gerbil model [2]. In 2009 the evidence was reevaluated and confirmed by the IARC [3]. H. pylori is associated with causation of gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [3]. Since gastric adenocarcinomas account for more than 90% of all gastric malignancies [4], this review will focus on adenocarcinomas.
Although gastric cancer rates have been decreasing in many countries, this disease is the second most common cause of death from cancer worldwide and ranks fourth worldwide in cancer incidence (Table 1) [5]. Approximately one million new cases were estimated in 2007 [6]. There are marked differences in gastric cancer rates among populations worldwide. The highest incidence rates are in Japan, Korea, China, Eastern Europe and the Andean portions of Latin America. Lower rates are seen in Africa, Oceania, North America, and Brazil (Fig. 1). Despite the low overall rates in gastric cancer incidence and mortality in the United States, there are some ethnic groups at increased risk, including African Americans, Native Americans, and immigrants from East Asia and Latin America [7–9]. In addition, although overall incidence rates of gastric cancer have been steadily declining in the United States, a recent observational study based on data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results identified increasing rates of non-cardia cancer in white U.S. residents aged 25 to 39 years in the past 3 decades [10]. The causes of this phenomenon are unclear.
Table 1.
New cases and deaths by cancer site worldwide, 2002 [5].
| New cases | Deaths | |
|---|---|---|
| Lung | 1,352,132 | 1,178,918 |
| Breast | 1,151,298 | 410,712 |
| Colon and rectum | 1,023,152 | 528,978 |
| Stomach | 933,937 | 700,349 |
| Liver | 626,162 | 598,321 |
| Prostate | 679,023 | 221,002 |
| Cervix uteri | 493,243 | 273,505 |
| Esophagus | 462,117 | 385,892 |
| Bladder | 356,557 | 145,009 |
| Non-Hodgkin lymphoma | 300,571 | 171,820 |
| Leukemia | 300,522 | 222,506 |
| Pancreas | 232,306 | 227,023 |
| All sites but skin | 10,862,496 | 6,723,887 |
Figure 1.

Incidence of stomach cancer in males, worldwide (age-standardized rates). GLOBOCAN, 2000 (http://www-dep.iarc.fr/).
Agent-host-environment interactions
Infection with H. pylori is the strongest known risk factor for gastric cancer [1, 11–13]; however, only a small minority of people infected with H. pylori develops gastric cancer or gastric precancerous lesions. The epidemiologic triangle, a conceptual model that posits that the outcome depends on the complex interplay of the agent with environmental and host factors [14], can be applied to better understand the etiology of gastric cancer. Factors specific to the host, such as genetic background, diet, and smoking behavior, as well as factors related to the environment, including neighborhood socioeconomic status, parasites endemic to the region, and possibly even climate, play key roles in whether gastric cancer develops in a particular individual. There is clearly a strong environmental component that affects the cancer risk. Migrant populations from high-risk areas of the world show a decrease in risk in the second generation when they move to a lower-risk area [15]. Some of these factors work on both the individual and societal level, and can be viewed as factors associated with host, environment, or both, depending on the specific characteristic. A change in this precarious balance of agent, host, and environment – such as infection with a more virulent strain of H. pylori or increased salt intake – can affect the speed of the cascade of events that lead to the development of gastric cancer.
The infectious agent
H. pylori
H. pylori is a Gram-negative microaerophilic spiral bacterium that localizes mostly extracellular within the gastric lumen (Fig. 2). Identified and cultured for the first time in 1982 by Marshall and Warren [16], H. pylori is present in more than 50% of the human population [17] and is highly adapted to colonize the human stomach. It possesses a potent urease which allows it to live in the acid microenvironment of the gastric lumen. This is accomplished by hydrolyzing the urea which filters into the lumen resulting in an ammonium cloud that protects the bacterium from the acid pH. In the same reaction, carbon dioxide is produced and immediately eliminated with the exhaled air. Oral administration of 13C-urea is used as a diagnostic test since 13CO2 is exhaled if the infection is present. Other factors that contribute to the persistence of the bacterium in the stomach are certain characteristics of the lipopolysaccharide that reduce the intensity of the host immune response and the expression of adhesins that confer intimate adherence to the gastric epithelium [18, 19].
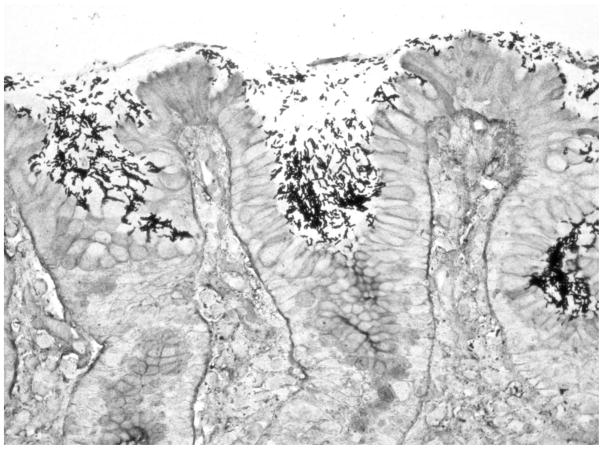
Figure 2.
Microphotograph of gastric mucosa colonized by abundant H. pylori organisms (modified Steiner silver stain, ×400).
H. pylori has been part of the native human flora since time immemorial. Both species migrated out of Africa some 60,000 years ago and have traveled together since then to other continents. Molecular microbiological studies have shown that the genome of the bacteria evolves frequently, mostly from recombination. Achtman and colleagues, using the multilocus sequence typing (MLST) of 7 housekeeping genes, have identified bacterial strains which traced their origin to specific populations of Africa (hpAfrica), Europe (hpEurope) and Asia (hpEAsia) [20–22]. The original Amerindian strains in the Americas, after being exposed to European strains, supposedly then acquired the cag pathogenicity island (cag PAI), a recognized virulence factor [23–26]. It is not clear if the Amerindian strains were totally replaced by European strains or if they acquired some of their genes by recombination [26]. Preliminary results from an ongoing study in Colombia show that H. pylori isolates from the high-gastric cancer-risk populations of the Andes Mountains, of mestizo extraction (mixed Amerindian and European ancestry), display European genotypes by MLST, presumably indicating the exposure of Amerindian strains to European strains. In contrast, inhabitants of the low-gastric cancer-risk area on the Pacific coast, of mixed African and European extraction, display heterogeneity of their H. pylori strains: some harbor West African genotypes and some harbor European genotypes (data not published). These findings suggest that the ancestry of the bacterial strains may be linked to cancer risk.
Despite the widespread dissemination of H. pylori infection, it is estimated that only a minute fraction of infected subjects will ever develop gastric adenocarcinoma. However, it is also estimated that 77% of the world’s non-cardia gastric cancer is attributable to H. pylori infection [17]. Several components of the H. pylori genome are linked to carcinogenicity. cag PAI, a major determinant of virulence, is a cluster of genes present in about 60% of H. pylori isolates from Western countries and in almost all of the isolates from East Asian countries [27]. One gene (cagA) in the H. pylori cag PAI encodes an effector protein (CagA) and others encode proteins for a type IV secretion apparatus that translocates CagA into gastric epithelial cells [23, 24]. Infection with cagA-positive H. pylori strains has been associated with increased risk for development of peptic ulcer [24, 28], gastric precancerous lesions and gastric adenocarcinoma [29–31]. cagA-positive strains are more prevalent in high-cancer-risk than in low-risk populations: approximately 90% in the Andes Mountains and 70% in the Pacific coast of Colombia [32]. The CagA protein is polymorphic, as shown by the sequences flanking the so-called EPIYA motifs. Most strains have EPIYA-A and EPIYA-B motifs. The EPIYA-C motif is characteristic of the Western strains while the EPIYA-D segment characterizes East Asian strains. These motifs become tyrosine phosphorylated when they enter the epithelial cells of the host, presumably starting the chain of events that may eventually result in neoplastic transformation [27]. Another virulence factor is a protein known as VacA, a multifunctional cytotoxin which causes intracellular vacuoles and form membrane channels in epithelial cells [33]. The vacA gene is present in all H. pylori strains, and comprises several variable loci (designed s, i, m). The combination of different alleles determines the production of cytotoxin and is associated with the pathogenicity of the bacterium [18, 33].
Despite the fact that both types of peptic ulcers (gastric and duodenal) are causally linked to H. pylori infection, it has been recognized that gastric peptic ulcer is associated with a high risk of gastric cancer, whereas duodenal ulcer is associated with a low risk when compared to the general population [13, 34]. Patients with gastric ulcers typically have multifocal atrophic gastritis. Patients with duodenal ulcers have antrum-predominant gastritis but none of the atrophic changes.
Epstein-Barr virus (EBV)
Increasing evidence indicates the possibility of a role of EBV in the etiology of some gastric cancers. Multiple studies around the world have found the presence of the EBV in 5–16% of gastric adenocarcinomas. A recent meta-analysis including 70 articles estimated that the overall EBV positivity was 8.7% among gastric cancer cases and that EBV-associated adenocarcinomas are more frequent in males than females, in gastric cardia or corpus than in antrum, and in tumors of postsurgical gastric stump/remnants [35]. In addition, a strong association (>90%) was confirmed between EBV and the uncommon histologic type lymphoepithelioma-like gastric carcinoma [35]. Several observations support the etiological involvement of EBV in some gastric cancers, including the uniform presence of clonal EBV in all malignant cells of EBV-positive tumors but not in surrounding normal epithelial cells [36]. However, the precise role of EBV in gastric carcinogenesis is still unclear.
The environment
Diet
In 2007 an expert panel from the World Cancer Research Fund released a report declaring that high intakes of vegetables and fruit probably decrease risk of gastric cancer, and that high intakes of salt and salty food probably increase risk of gastric cancer [37]. The majority of the evidence for these associations comes from case-control studies; cohort studies have been more inconsistent and have primarily found weaker, non-significant associations. The mechanism for the inverse association of gastric cancer risk with high vegetable and fruit intake has been hypothesized to be related to the presence of antioxidants, which protect against oxidative damage. The positive association with salt has been more clearly delineated, as salt acts directly on the stomach lining, destroying the mucosal barrier and causing gastritis, increasing epithelial proliferation [38]. A synergistic interaction between diet and H. pylori infection with risk of gastric cancer has been proposed [39], and studies on this topic have generally suggested a stronger effect among H. pylori-positive individuals [40, 41].
Smoking
Tobacco smoking is the risk factor associated with the largest number of cancer cases worldwide and the causal link with stomach cancer is recognized [42]. A recent meta-analysis of 32 studies, including 18 cohort studies, found significant positive associations of smoking with risk of both cardia and non-cardia gastric cancer among the majority of studies, overall increasing risk by 62% for male current smokers (95% CI: 1.50–1.75) and 20% for female current smokers (95% CI: 1.01–1.43) [43]. Tobacco smoke contains multiple well-known chemical carcinogens [44]. While the mechanisms by which smoking increases the risk of gastric cancer are not completely understood, it is possible that tobacco smoke carcinogens affect gastric cancer risk directly through contact with the stomach mucosa or indirectly through the blood flow [45].
Non-steroidal anti-inflammatory drugs
Observational studies have consistently found a protective effect of regular use of non-steroidal anti-inflammatory drugs (NSAIDs), particularly aspirin, on risk of gastric cancer. Specifically, a 2009 meta-analysis found that regular NSAID users had an 18%–20% reduced risk of gastric cardia adenocarcinoma and a 32%–36% reduced risk of distal gastric adenocarcinoma [46]. NSAIDs are seen as chemopreventive agents as they suppress the production of cyclooxygenase enzymes, which are involved in prostaglandin biosynthesis [47]. While clinical trials of NSAIDs and risk of colorectal adenoma among high-risk populations have mostly met with success [48], there has been only one clinical trial with gastric cancer or a gastric cancer precursor. In this randomized trial of the COX-2 inhibitor rofecoxib, the drug did not reduce risk of gastric intestinal metaplasia after a 2-year period [49]. It is possible, however, that the duration of drug use was not long enough, and/or that intervention with NSAIDs may be effective at a later stage of the carcinogenesis process. A recent international consensus statement on aspirin and cancer prevention concluded that future research should focus on high-risk individuals and aim to resolve the questions of optimal dose, age to begin therapy, and treatment duration [50].
Socioeconomic status
Lower socioeconomic status, whether measured by education and/or income, has been consistently associated with an at least two-fold greater risk of gastric cancer [51]. This gradient has been observed within both high-risk countries (such as Japan) and low-risk countries (including the United States) [52]. The actual factors creating this association are most likely characteristics related to low socioeconomic status that increase likelihood of transmission and re-infection with H. pylori, such as household crowding, large family size, poor household sanitation, and less frequent use of antibiotics. Additionally, low socioeconomic status could be an indicator of a diet lower in fresh fruits and vegetables. The high gastric cancer risk seen in a few countries with overall high socioeconomic status, such as Japan and South Korea, is not completely understood, but it is possibly due to the prevalence of highly virulent strains of H. pylori in these countries [53].
The African enigma
H. pylori infects more than half of the world’s population, with variable rates of prevalence across countries and among ethnic groups [17]. However, discordance between the high prevalence of H. pylori infection and the low rates of gastric cancer has been observed in some areas, especially in the African continent. This phenomenon has been called the “African enigma” [54]. Studies carried out in different regions of Africa have shown that the majority of populations are infected with H. pylori, with 61–80% showing evidence of antibodies to H. pylori, and that acquisition of the infection occurs at an early age [54, 55]. In sub-Saharan Africa, despite overall high H. pylori infection prevalence, gastric cancer incidence rates are relatively low [55]. A similar pattern has been found in other geographic regions. In Colombia, our group has identified a high-risk area for gastric cancer in the Andes Mountains and a low-risk area on the Pacific coast [56]. The two populations have similar prevalence of H. pylori infection in adult population (74% and 80%) [32] and a common pattern of very early age at infection [57]. In Costa Rica, marked regional heterogeneity in cancer incidence has been observed in spite of no significant variation in H. pylori infection prevalence [58]. In both Colombia and Costa Rica, greater prevalence of more virulent strains have been observed in the high-risk areas [32, 59, 60]. However, it is unlikely that these differences are large enough to completely explain the differences in gastric cancer risk.
The lack of correlation between gastric cancer incidence and H. pylori infection prevalence indicates that other factors, such as environmental factors, host genetic background, and co-infections, may modulate the outcome of the infection. One important factor is diet. High-risk populations tend to have excessive salt intake, while low-risk communities on the coastal regions of Colombia more frequently consume fish and seafood and fruits. Another factor is the type of immune response of the host to the H. pylori infection. Intestinal parasites, especially helminths, are more frequent in tropical climates and they tend to modulate the immune response towards a Th2 anti-inflammatory type [61, 62]. In Colombia, we observed that children in the low-risk area (on the coast) were more than twice as likely to be infected with helminths and both adults and children had serum IgE levels several times higher than those in the high-risk area (mountains) [62]. In addition, significantly greater eosinophilic infiltration of the gastric mucosa was observed in infected adult subjects of the low-risk area compared to subjects of the high-risk area [63]. In an animal model, supporting this hypothesis, concurrent helminth infection considerably reduced Helicobacter-associated gastric inflammatory cytokines and chemokines associated with a Th1 response and gastric atrophy [64]. Similar evidence from a Chinese population indicates that a concurrent helminth infection modifies the immune response to H. pylori and reduces the probability of developing gastric corpus atrophy [65]. These results suggest that early acquisition of the parasite induces an anti-inflammatory Th2 immune response against the H. pylori infection. This anti-inflammatory response may aid in ameliorating the chronic damage to the gastric mucosa, subsequently decreasing the risk of gastric cancer.
The host
Host genetics in H. pylori-induced gastric cancer
The association between chronic inflammation and cancer is well established and gastric adenocarcinoma is usually accompanied by an evident inflammatory infiltrate. The long-standing inflammatory response against H. pylori in the gastric mucosa may cause sustained tissue injury leading to the development of distal gastric cancer. Host genetic factors may influence the nature and intensity of the immune response to H. pylori. Polymorphisms in cytokine genes have shown to be associated with risk for gastric cancer. Biologically, the genetic polymorphisms are thought to modulate risk by increasing expression of pro-inflammatory factors that enhance and prolong the inflammatory response in gastric mucosa. El-Omar et al. [66, 67] were the first to show that polymorphisms in IL1B and IL1RN genes (IL1B encoding interleukin (IL)-1β and IL1RN encoding its naturally occurring receptor antagonist) were associated with elevated risk for hypochlorhydria and gastric cancer in subjects with H. pylori infection. IL-1β is a pro-inflammatory cytokine and a potent inhibitor of gastric acid secretion. It has been hypothesized that a profound acid secretion suppression promotes proliferation and dissemination of H. pylori from the antrum to the corpus, leading to a severe and more extensive gastritis that favors the development of atrophy and subsequently adenocarcinoma [68]. A meta-analysis concluded that IL1B-511T and IL1RN*2 polymorphisms are associated with gastric cancer in Caucasians but not in Asians, and that the association of IL1B-511T in Caucasians was stronger when intestinal-type and noncardia gastric cancer cases were examined [69]. Polymorphisms in other cytokine genes have also been associated with cancer risk, including tumor necrosis factor alpha [70–72] and IL-10 [70]. Studies combining host susceptibility and bacterial virulence factors have shown that gastric cancer risk is highest among those with both host and bacterial high-risk genotypes [73, 74]. An increasing amount of evidence shows the possible role of multiple other polymorphisms in genes mainly related to processes involved in carcinogenesis and/or cell defense and repair [75].
Pathology
Gastric adenocarcinomas are classified anatomically as proximal (cardia) and distal (non-cardia). Distal adenocarcinomas are commonly associated with H. pylori infection, but the association of this infection with cardia adenocarcinomas is less well defined. Gastric cardia adenocarcinomas are associated with gastroesophageal reflux disease [76]. Due to unclear reasons, the incidence of gastric cardia adenocarcinoma has been increasing during the last decades in conjunction with increase in esophageal adenocarcinoma, especially among white males [76–79]. In the United States, gastric cardia adenocarcinomas have lower overall 5-year survival rates than distal adenocarcinomas (14% vs. 26%) [76]. In addition to the problem of distinguishing gastric cardia from non-cardia adenocarcinomas, there is the difficulty in separating true cardia tumors from adenocarcinomas of the distal esophagus, frequently involving the gastroesophageal junction (GEJ). Thus, according to the Siewert and Stein classification, three types of carcinomas develop around the GEJ: 1) adenocarcinomas of the distal esophagus; 2) true cardia carcinomas, extending 1 cm above and 2 cm below the anatomic GEJ; and 3) subcardiac gastric cancers, tumors located more than 2 cm below the anatomic GEJ that may infiltrate the GEJ from below [80].
Carcinoma of the stomach may be detected either in an early stage or in an advanced stage. Early gastric cancer is defined as an adenocarcinoma confined to the gastric mucosa or submucosa regardless of lymph node metastasis [81]. The majority of patients with early gastric cancer are asymptomatic. Among symptomatic patients, dyspepsia and epigastric pain are the most common symptoms. The macroscopic appearance of advanced carcinomas may be polypoid, fungating, ulcerated or infiltrative (Borrmann’s classification), with occasional combination of types. Histologically, there are several classifications for gastric adenocarcinomas. The most widely used in the United States is the Lauren’s classification [82], which recognizes two main types: intestinal and diffuse (Fig. 3). Intestinal-type tumors predominate in geographic areas with a high incidence of gastric cancer, whereas diffuse-type tumors are found more uniformly throughout the world.
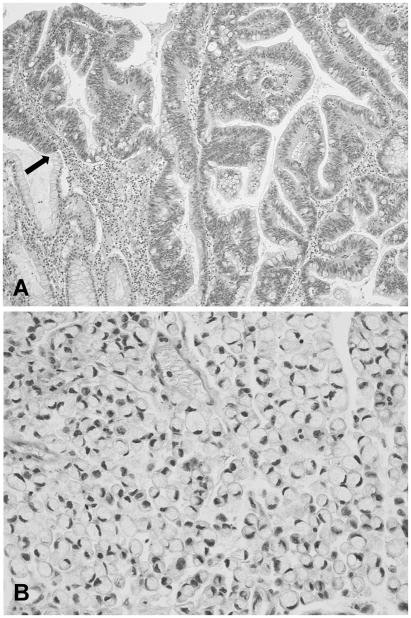
Figure 3.
Microphotographs of gastric adenocarcinoma. A) Intestinal type, showing tumor cells cohesively arranged forming irregular glandular structures. On the left lower corner there are few glands with intestinal metaplasia. An arrow shows the transition zone between intestinal metaplasia and adenocarcinoma (×100). B) Diffuse type, with tumor cells that show lack of cohesiveness infiltrating diffusely. In this subtype, the signet-ring adenocarcinoma, the nuclei are pushed to the periphery due to the abundant mucinous cytoplasmic content (×400).
The precancerous process
It is currently accepted that intestinal-type adenocarcinomas develop through a series of sequential lesions in the gastric mucosa (Fig. 4). This multistep precancerous process was described in 1975 [83, 84] based on observations in Colombian populations with high risk of gastric cancer [56, 85, 86], and before the identification of H. pylori infection as a carcinogen. The process starts when H. pylori colonizes the gastric mucosa, initially in the antropyloric region, avoiding the lower pH in the acid-producing areas (fundus and corpus) of the stomach. The immune response induced by the bacterium may vary in severity, but usually causes a non-atrophic chronic gastritis that may last decades, unless treatment eradicates the bacterium. Over time, the infection may spread proximally to the oxyntic mucosa, mainly in patients taking proton pump inhibitors. Prolonged and severe infection may eventually result in loss of glandular tissue (multifocal atrophic gastritis) and sometimes in gastric ulcers. The atrophic changes usually start in the incisura angularis and may extend to the antrum and the corpus mucosa, as the foci become progressively larger and coalesce. In some patients with multifocal atrophic gastritis, the lost glands are then replaced by glandular structures with intestinal phenotype, displaying characteristics of small intestine (complete intestinal metaplasia) or colonic epithelium (incomplete intestinal metaplasia). The complete type displays absorptive enterocytes with brush border, well-developed goblet cells, and Paneth cells. In incomplete intestinal metaplasia, there are goblet cells of variable size, absence of brush border and sometimes presence of sulfomucins. Evidence has supported that intestinal metaplasia of the incomplete type is associated with increased risk of gastric cancer [87, 88]. A small proportion of subjects with intestinal metaplasia eventually will progress further to dysplasia (synonyms: intraepithelial neoplasia, non-invasive neoplasia, adenoma), which is classified as low grade or high grade. A fraction of subjects with dysplasia will develop adenocarcinoma, defined as invasion of the lamina propria or beyond. H. pylori tends to disappear as intestinal metaplasia develops. Therefore, previous or current H. pylori infection may be underestimated in subjects with intestinal metaplasia or more advanced lesions.
Figure 4.
Model of sequential steps in the gastric precancerous process. Adapted from Correa P., et al. [83].
Intestinal-type adenocarcinoma
Besides H. pylori infection, other environmental factors including diet and smoking are recognized risk factors for intestinal-type adenocarcinoma. More recently (as described above), the etiopathogenic role of host genetic factors is increasingly recognized in this type of carcinoma. Most cases of intestinal-type adenocarcinomas are diagnosed between the ages of 50 and 70 years, and the incidence rate is approximately double in men compared to women. Microscopically, intestinal-type adenocarcinomas are formed by tumor cells arranged cohesively in irregular tubular or papillary structures infiltrating the stroma. Epithelium with intestinal metaplasia is frequently seen in neighboring mucosa (Fig. 3A). Based on architectural and cellular characteristics, the tumors have variable degree of differentiation. In the better differentiated adenocarcinomas, most of the cells are columnar and contain cytoplasmic mucin. Poorly differentiated adenocarcinomas have a predominantly solid pattern.
Diffuse type adenocarcinoma
Diffuse-type adenocarcinoma is relatively more frequent in populations at low risk for gastric cancer, in younger subjects, and environmental factors have been thought to play a less important role than genetic factors. Since atrophic changes are not severe in diffuse-type gastric cancer, it was previously considered to have little relation to H. pylori infection. However, epidemiologic and histopathological studies [30, 89] have shown that the development of diffuse-type cancer is also related to H. pylori infection. Gross alterations include thickening and rigidity of the gastric wall, a condition known as linitis plastica. Microscopically, the tumoral cells of the diffuse type are usually round and rather small, and are arranged as single cells with minimal or absence of intercellular cohesion (Fig. 3B).
Hereditary diffuse gastric cancer is an autosomal dominant disorder that accounts for less than 1% of all cases of gastric cancer. Mutations in E-cadherin gene (CDH1) are germline defects associated with this syndrome [90–92]. CDH1 encodes E-cadherin, a cell-to-cell adhesion molecule that plays a fundamental role in the maintenance of the normal architecture of epithelial tissues. Diffuse gastric cancer is the most important cause of cancer mortality in these families [93]. In addition to mutation, epigenetic inactivation of E-cadherin by promoter hypermethylation has been frequently reported in sporadic diffuse gastric cancer [94, 95].
Cancer control
The high mortality rate from gastric cancer is believed to be primarily due to late-stage diagnoses. In the United States, two thirds of gastric cancer cases are diagnosed when the tumor has invaded the muscularis propria and the overall 5-year survival rate is about 25% [96]. Early gastric cancer is generally small and asymptomatic, and surgery or endoscopic resection can offer the chance of a cure. In Japan, a country with one of the highest incidence rates of gastric cancer, over 50% of cases are diagnosed at an early stage due to a massive screening program. The 5-year survival rate in this group is over 90% [97]. A recent review article authored by gastric cancer experts from the Asia Pacific Working Group on Gastric Cancer recommended multistage screening using serum-pepsinogen testing (to determine the presence and extension of atrophic gastritis) and H. pylori serology to identify patients at high risk, who should then go on to endoscopic surveillance [98]. H. pylori eradication has also been proposed as a method of gastric cancer prevention. In studies of precancerous gastric lesions, H. pylori eradication generally reduced the rate of progression [99]. While individual randomized, controlled trials of H. pylori eradication on gastric cancer risk have generally found non-statistically significant suggestions of protection, a recent meta-analysis of these trials found that when considering these trials together (and thus increasing the power to observe an association), H. pylori eradication treatment does significantly reduce risk of gastric cancer [100]. Additionally, a recent retrospective cohort study in Taiwan concluded that for patients with peptic ulcers, early H. pylori eradication – defined as within one year of hospitalization for the ulcer – decreased the risk of gastric cancer [101]. However, large scale H. pylori eradication strategies face challenges such as development of antibiotic resistant strains. In the United States, due to the low incidence rates of gastric cancer, endoscopic surveillance is recommended only in subjects with low-grade dysplasia. Patients with high-grade dysplasia need to undergo endoscopic or surgical resection [102]. However, surveillance of subjects with gastric intestinal metaplasia should be considered in presence of risk factors for gastric cancer such as family history of gastric cancer, ethnicity, and extensive or incomplete-type intestinal metaplasia [88, 103, 104].
An H. pylori vaccine has been in development for years, but there has been little success and currently an efficacious human vaccine does not exist. Both H. pylori strategies of eradication and vaccine development have also been criticized due to the potential unexpected effects of H. pylori eradication, including increased risk of esophageal adenocarcinoma, gastro-esophageal reflux-related diseases, and possibly allergic and autoimmune diseases [105–108].
Epilogue
The role of infectious agents and chronic inflammation in carcinogenesis is being increasingly recognized. It has been estimated that about 18% of cancers are directly linked to infections, particularly gastric adenocarcinoma (H. pylori), cervical carcinoma (human papilloma viruses) and hepatocarcinoma (hepatitis B and C viruses) [17]. Multiple clinical trials of COX-2 inhibitors and anti-inflammatory agents have shown a beneficial effect on the development of very diverse tumors, such as those of the colon, prostate and breast [50]. However, their mechanism of action is not completely understood and may differ among the infectious agents and tumor types.
Acknowledgments
This work was supported by the grant P01-CA28842 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schistosomes, liver flukes and Helicobacter pylori. Lyon: International Agency for Research on Cancer; 1994. IARC Monographs on the evaluation of carcinogenic risks to humans; pp. 177–240. [PMC free article] [PubMed] [Google Scholar]
- 2.Honda S, Fujioka T, Tokieda M, et al. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58(19):4255–9. [PubMed] [Google Scholar]
- 3.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10(4):321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 4.Coleman MP, Esteve J, Damiecki P, et al. Trends in cancer incidence and mortality. Lyon: International Agency for Research on Cancer; 1993. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA: Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Thun MJ, DeLancey JO, Center MM, et al. The Global Burden of Cancer: Priorities for Prevention. Carcinogenesis. 2010;31(1):100–10. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Chen VW, Andrews PA, et al. Incidence of esophageal and gastric cancers among Hispanics, non-Hispanic whites and non-Hispanic blacks in the United States: subsite and histology differences. Cancer Causes Control. 2007;18(6):585–93. doi: 10.1007/s10552-007-9000-1. [DOI] [PubMed] [Google Scholar]
- 9.Wu X, Chen VW, Ruiz B, et al. Incidence of esophageal and gastric carcinomas among American Asians/Pacific Islanders, whites, and blacks: subsite and histology differences. Cancer. 2006;106(3):683–92. doi: 10.1002/cncr.21542. [DOI] [PubMed] [Google Scholar]
- 10.Anderson WF, Camargo MC, Fraumeni JF, Jr, et al. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303(17):1723–8. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49(3):347–53. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamangar F, Dawsey SM, Blaser MJ, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98(20):1445–52. doi: 10.1093/jnci/djj393. [DOI] [PubMed] [Google Scholar]
- 13.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 14.Leavell HR, Clark EG. Preventive medicine for the doctor in his community; an epidemiologic approach. New York: McGraw-Hill; 1965. [Google Scholar]
- 15.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40(1):43–68. [PubMed] [Google Scholar]
- 16.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 17.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 18.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136(6):1863–73. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208(2):233–48. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- 20.Achtman M, Azuma T, Berg DE, et al. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol Microbiol. 1999;32(3):459–70. doi: 10.1046/j.1365-2958.1999.01382.x. [DOI] [PubMed] [Google Scholar]
- 21.Falush D, Wirth T, Linz B, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299(5612):1582–5. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 22.Linz B, Balloux F, Moodley Y, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445(7130):915–8. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Censini S, Lange C, Xiang Z, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93(25):14648–53. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90(12):5791–5. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devi SM, Ahmed I, Khan AA, et al. Genomes of Helicobacter pylori from native Peruvians suggest admixture of ancestral and modern lineages and reveal a western type cag-pathogenicity island. BMC Genomics. 2006;7:191. doi: 10.1186/1471-2164-7-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominguez-Bello MG, Perez ME, Bortolini MC, et al. Amerindian Helicobacter pylori strains go extinct, as European strains expand their host range. PLoS One. 2008;3(10):e3307. doi: 10.1371/journal.pone.0003307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatakeyama M. Helicobacter pylori and gastric carcinogenesis. J Gastroenterol. 2009;44(4):239–48. doi: 10.1007/s00535-009-0014-1. [DOI] [PubMed] [Google Scholar]
- 28.Tham KT, Peek RM, Jr, Atherton JC, et al. Helicobacter pylori genotypes, host factors, and gastric mucosal histopathology in peptic ulcer disease. Hum Pathol. 2001;32(3):264–73. doi: 10.1053/hupa.2001.21136. [DOI] [PubMed] [Google Scholar]
- 29.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55(10):2111–5. [PubMed] [Google Scholar]
- 30.Parsonnet J, Friedman GD, Orentreich N, et al. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40(3):297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plummer M, van Doorn LJ, Franceschi S, et al. Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J Natl Cancer Inst. 2007;99(17):1328–34. doi: 10.1093/jnci/djm120. [DOI] [PubMed] [Google Scholar]
- 32.Bravo LE, van Doom LJ, Realpe JL, et al. Virulence-associated genotypes ofHelicobacter pylori: do they explain the African enigma? Am J Gastroenterol. 2002;97(11):2839–42. doi: 10.1111/j.1572-0241.2002.07031.x. [DOI] [PubMed] [Google Scholar]
- 33.Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3(4):320–32. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 34.Hansson LE, Nyren O, Hsing AW, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335(4):242–9. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 35.Murphy G, Pfeiffer R, Camargo MC, et al. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gatroenterology. 2009;137(3):824–33. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiba S, Koriyama C, Herrera-Goepfert R, et al. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. 2008;99(2):195–201. doi: 10.1111/j.1349-7006.2007.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 38.Fox JG, Dangler CA, Taylor NS, et al. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59(19):4823–8. [PubMed] [Google Scholar]
- 39.Yamaguchi N, Kakizoe T. Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. Lancet Oncol. 2001;2(2):88–94. doi: 10.1016/S1470-2045(00)00225-4. [DOI] [PubMed] [Google Scholar]
- 40.Epplein M, Nomura AM, Hankin JH, et al. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer Causes Control. 2008;19(8):869–77. doi: 10.1007/s10552-008-9149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez CA, Pera G, Agudo A, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int J Cancer. 2006;118(10):2559–66. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 42.Secretan B, Straif K, Baan R, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–4. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 43.Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19(7):689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 44.Tobacco smoke and involuntary smoking. Lyon: International Agency for Research on Cancer; 2004. IARC Monographs on the evaluation of carcinogenic risks to humans; pp. 59–94. [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez CA, Pera G, Agudo A, et al. Smoking and the risk of gastric cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Int J Cancer. 2003;107(4):629–34. doi: 10.1002/ijc.11426. [DOI] [PubMed] [Google Scholar]
- 46.Abnet CC, Freedman ND, Kamangar F, et al. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100(3):551–7. doi: 10.1038/sj.bjc.6604880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94(4):252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 48.Baron JA. Aspirin and NSAIDs for the prevention of colorectal cancer. Recent Results Cancer Res. 2009;181:223–9. doi: 10.1007/978-3-540-69297-3_21. [DOI] [PubMed] [Google Scholar]
- 49.Leung WK, Ng EK, Chan FK, et al. Effects of long-term rofecoxib on gastric intestinal metaplasia: results of a randomized controlled trial. Clin Cancer Res. 2006;12(15):4766–72. doi: 10.1158/1078-0432.CCR-06-0693. [DOI] [PubMed] [Google Scholar]
- 50.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10(5):501–7. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 51.Nyren O, Adami H-O. Stomach cancer. In: Adami H-O, Hunter D, Trichopoulos D, editors. Textbook of cancer epidemiology. New York: Oxford University Press; 2002. pp. 162–87. [Google Scholar]
- 52.Nomura A. Stomach cancer. In: Schottenfeld D, Fraumeni J, editors. Cancer epidemiology and prevention. 2. New York: Oxford University Press; 1996. pp. 707–24. [Google Scholar]
- 53.Nguyen LT, Uchida T, Murakami K, et al. Helicobacter pylori virulence and the diversity of gastric cancer in Asia. J Med Microbiol. 2008;57(Pt 12):1445–53. doi: 10.1099/jmm.0.2008/003160-0. [DOI] [PubMed] [Google Scholar]
- 54.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33(4):429–31. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segal I, Ally R, Mitchell H. Gastric cancer in sub-Saharan Africa. Eur J Cancer Prev. 2001;10(6):479–82. doi: 10.1097/00008469-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Correa P, Cuello C, Duque E, et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976;57(5):102735. doi: 10.1093/jnci/57.5.1027. [DOI] [PubMed] [Google Scholar]
- 57.Camargo MC, Yepez MC, Ceron C, et al. Age at acquisition of Helicobacter pylori infection: comparison of two areas with contrasting risk of gastric cancer. Helicobacter. 2004;9(3):262–70. doi: 10.1111/j.1083-4389.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 58.Tsuji S. The “Costa Rican enigma” of Helicobacter pylori CagA and gastric cancer. J Gastroenterol. 2006;41(7):716–7. doi: 10.1007/s00535-006-1824-z. [DOI] [PubMed] [Google Scholar]
- 59.Con SA, Valerin AL, Takeuchi H, et al. Helicobacter pylori CagA status associated with gastric cancer incidence rate variability in Costa Rican regions. J Gastroenterol. 2006;41(7):632–7. doi: 10.1007/s00535-006-1812-3. [DOI] [PubMed] [Google Scholar]
- 60.Sicinschi LA, Correa P, Peek RM, Jr, et al. Helicobacter pylori genotyping and sequencing using paraffin-embedded biopsies from residents of Colombian areas with contrasting gastric cancer risks. Helicobacter. 2008;13(2):135–45. doi: 10.1111/j.1523-5378.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell HM, Ally R, Wadee A, et al. Major differences in the IgG subclass response to Helicobacter pylori in the first and third worlds. Scand J Gastroenterol. 2002;37(5):517–22. doi: 10.1080/00365520252903044. [DOI] [PubMed] [Google Scholar]
- 62.Whary MT, Sundina N, Bravo LE, et al. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1464–9. doi: 10.1158/1055-9965.EPI-05-0095. [DOI] [PubMed] [Google Scholar]
- 63.Piazuelo MB, Camargo MC, Mera RM, et al. Eosinophils and mast cells in chronic gastritis: possible implications in carcinogenesis. Hum Pathol. 2008;39(9):1360–9. doi: 10.1016/j.humpath.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fox JG, Beck P, Dangler CA, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6(5):536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 65.Du Y, Agnew A, Ye XP, et al. Helicobacter pylori and Schistosoma japonicum co-infection in a Chinese population: helminth infection alters humoral responses to H. pylori and serum pepsinogen I/II ratio. Microbes Infect. 2006;8(1):52–60. doi: 10.1016/j.micinf.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 66.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 67.El-Omar EM, Carrington M, Chow WH, et al. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412(6842):99. doi: 10.1038/35083631. (erratum) [DOI] [PubMed] [Google Scholar]
- 68.El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48(6):743–7. doi: 10.1136/gut.48.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camargo MC, Mera R, Correa P, et al. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1674–87. doi: 10.1158/1055-9965.EPI-06-0189. [DOI] [PubMed] [Google Scholar]
- 70.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124(5):1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 71.Gorouhi F, Islami F, Bahrami H, et al. Tumour-necrosis factor-A polymorphisms and gastric cancer risk: a meta-analysis. Br J Cancer. 2008;98(8):1443–51. doi: 10.1038/sj.bjc.6604277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125(2):364–71. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 73.Figueiredo C, Machado JC, Pharoah P, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94(22):1680–7. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 74.Sicinschi LA, Lopez-Carrillo L, Camargo MC, et al. Gastric cancer risk in a Mexican population: role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and -10 genes. Int J Cancer. 2006;118(3):649–57. doi: 10.1002/ijc.21364. [DOI] [PubMed] [Google Scholar]
- 75.Correa P, Camargo MC, Piazuelo MB. Overwiew and pathology of gastric cancer. In: Wang TC, Fox JG, Giraud AS, editors. The biology of gastric cancers. New York: Springer; 2009. pp. 1–24. [Google Scholar]
- 76.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11(2):235–56. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 77.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287–9. [PubMed] [Google Scholar]
- 78.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–53. [PubMed] [Google Scholar]
- 79.Devesa SS, Fraumeni JF., Jr The rising incidence of gastric cardia cancer. J Natl Cancer Inst. 1999;91(9):747–9. doi: 10.1093/jnci/91.9.747. [DOI] [PubMed] [Google Scholar]
- 80.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85(11):1457–9. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 81.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd English Edition. Gastric Cancer. 1998;1(1):10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 82.Lauren P. The two histological main types of gastric carcinoma: diffuse and so called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 83.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735–40. [PubMed] [Google Scholar]
- 84.Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet. 1975;2(7924):58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 85.Correa P, Bolanos O, Garcia F, et al. The Cancer Registry of Cali, Colombia. Epidemiologic studies of gastric cancer. Recent Results Cancer Res. 1975;50:155–69. [Google Scholar]
- 86.Correa P, Cuello C, Duque E. Carcinoma and intestinal metaplasia of the stomach in Colombian migrants. J Natl Cancer Inst. 1970;44(2):297–306. [PubMed] [Google Scholar]
- 87.Filipe MI, Munoz N, Matko I, et al. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer. 1994;57(3):324–9. doi: 10.1002/ijc.2910570306. [DOI] [PubMed] [Google Scholar]
- 88.Tava F, Luinetti O, Ghigna MR, et al. Type or extension of intestinal metaplasia and immature/atypical “indefinite-for-dysplasia” lesions as predictors of gastric neoplasia. Hum Pathol. 2006;37(11):1489–97. doi: 10.1016/j.humpath.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 89.Kikuchi S, Wada O, Nakajima T, et al. Serum anti-Helicobacter pylori antibody and gastric carcinoma among young adults. Research Group on Prevention of Gastric Carcinoma among Young Adults. Cancer. 1995;75(12):2789–93. doi: 10.1002/1097-0142(19950615)75:12<2789::aid-cncr2820751202>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 90.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392(6674):402–5. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 91.Guilford PJ, Hopkins JB, Grady WM, et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999;14(3):249–55. doi: 10.1002/(SICI)1098-1004(1999)14:3<249::AID-HUMU8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 92.Lynch HT, Kaurah P, Wirtzfeld D, et al. Hereditary diffuse gastric cancer: diagnosis, genetic counseling, and prophylactic total gastrectomy. Cancer. 2008;112(12):2655–63. doi: 10.1002/cncr.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pharoah PD, Guilford P, Caldas C. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121(6):1348–53. doi: 10.1053/gast.2001.29611. [DOI] [PubMed] [Google Scholar]
- 94.Machado JC, Oliveira C, Carvalho R, et al. E-cadherin gene (CDH1) promotermethylation as the second hit in sporadic diffuse gastric carcinoma. Oncogene. 2001;20(12):1525–8. doi: 10.1038/sj.onc.1204234. [DOI] [PubMed] [Google Scholar]
- 95.Tamura G, Yin J, Wang S, et al. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst. 2000;92(7):569–73. doi: 10.1093/jnci/92.7.569. [DOI] [PubMed] [Google Scholar]
- 96.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 97.Sano T, Katai H, Sasako M, et al. The management of early gastric cancer. Surg Oncol. 2000;9(1):17–22. doi: 10.1016/s0960-7404(00)00018-9. [DOI] [PubMed] [Google Scholar]
- 98.Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9(3):279–87. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 99.Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54(11):1536–40. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med. 2009;151(2):121–8. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 101.Wu CY, Kuo KN, Wu MS, et al. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology. 2009;137(5):1641–8. doi: 10.1053/j.gastro.2009.07.060. [DOI] [PubMed] [Google Scholar]
- 102.Lauwers GY, Srivastava A. Gastric preneoplastic lesions and epithelial dysplasia. Gastroenterol Clin North Am. 2007;36(4):813–29. doi: 10.1016/j.gtc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 103.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: Clinical implications. Am J Gastroenterol. 2010;105(3):493–8. doi: 10.1038/ajg.2009.728. (updated) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Vries AC, Haringsma J, de Vries RA, et al. The use of clinical, histologic, and serologic parameters to predict the intragastric extent of intestinal metaplasia: a recommendation for routine practice. Gastrointest Endosc. 2009;70(1):18–25. doi: 10.1016/j.gie.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 105.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7(12):887–94. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167(8):821–7. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 107.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila Pa) 2008;1(5):32938. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2(1):28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]