Abstract
Animals perceive and discriminate among a vast array of sensory cues in their environment. Both genetic and environmental factors contribute to individual variation in behavioral responses to these cues. Here, we asked to what extent sequence variants in six Drosophila melanogaster odorant receptor (Or) genes are associated with variation in behavioral responses to benzaldehyde by sequencing alleles from a natural population. Sequence analyses showed signatures of deviations from neutrality for Or42b and Or85f, and linkage disequilibrium analyses showed a history of extensive recombination between polymorphic markers for all six Or genes. We identified polymorphisms in Or10a, Or43a, and Or67b that were significantly associated with variation in response to benzaldehyde. To verify these associations, we repeated the analyses with an independent set of behavioral measurements of responses to a structurally similar odorant, acetophenone. Association profiles for both odorants were similar with many polymorphisms and haplotypes associated with variation in responsiveness to both odorants. Some polymorphisms, however, were associated with one, but not the other odorant. We also observed a correspondence between behavioral response to benzaldehyde and differences in Or10a and Or43a expression. These results illustrate that sequence variants that arise during the evolution of odorant receptor genes can contribute to individual variation in olfactory behavior and give rise to subtle shifts in olfactory perception.
RESEARCHERS in many scientific fields have long appreciated that different animal species perceive the world differently. In fact, these differences are so striking that new disciplines have arisen to study the adaptations of sense organs to the environment (e.g., Ali 1978; Lythgoe 1979; Dusenbery 1992). Differences in sensory perception exist not only between species, but also between populations of a single species and between individuals within a population. What is the underlying genetic architecture for individual variation in sensory perception?
Olfaction provides an excellent model for examining the underlying genetic mechanisms that result in variation in behavior. In both vertebrates and invertebrates, odorants are detected by families of odorant receptors expressed in populations of olfactory receptor neurons (ORNs), whose activation elicits a distinct spatial pattern of glomerular activity in the brain (Buck and Axel 1991; Vassar et al. 1994; Mombaerts et al. 1996; Laissue et al. 1999; Gao et al. 2000; Vosshall et al. 2000; Bhalerao et al. 2003; Wang et al. 2003). This combinatorial code allows for discrimination of a diverse repertoire of odorants.
Drosophila melanogaster has a relatively simple olfactory system with only 60 odorant receptor (Or) genes (Vosshall and Stocker 2007) compared to ∼1000 in the mouse (Zhang and Firestein 2002; Zhang et al. 2004). The 60 genes are located throughout the genome, and 2 of these genes are alternatively spliced for a total of 62 identified proteins (Clyne et al. 1999; Gao and Chess 1999; Vosshall et al. 1999; Robertson et al. 2003). Furthermore, clusters of Ors throughout the genome suggest several recent gene duplication events (Robertson et al. 2003).
The response spectra of individual ORNs have been extensively characterized using extracellular electrophysiological recordings from single sensilla on the antennae and maxillary palps. Recordings from basiconic sensilla on the antenna identified classes of neurons with distinct olfactory response profiles organized as two to four neurons in each sensillum with specific neuronal combinations occurring in distinct spatial regions of the antenna (de Bruyne et al. 1999, 2001).
The majority of ORNs express a unique odorant receptor in addition to the highly conserved coreceptor, Or83b (Jones et al. 2005). Studies of a null mutant of Or83b implicated this receptor in positioning odorant receptor proteins in the sensory dendrites (Larsson et al. 2004; Benton et al. 2006). Odorant receptors in Drosophila have an atypical membrane topology with a cytoplasmic N terminus and an extracellular C terminus (Benton et al. 2006). Specific domains in the third cytoplasmic loops of two odorant receptors, Or22a and Or43a, have been implicated to interact with the third loop of Or83b (Benton et al. 2006). Drosophila odorant receptors act as ligand-gated nonselective cation channels formed by a dimeric complex between a unique Or and the Or83b coreceptor (Sato et al. 2008; Wicher et al. 2008).
Several studies have examined ligand specificities of individual odorant receptor proteins and demonstrated that they respond to diverse and overlapping suites of ligands. Response profiles for many receptors have been characterized using the Gal4/UAS system to drive expression of individual odorant receptors in a mutant ORN lacking expression of its endogeneous receptor, followed by electrophysiological recording (Dobritsa et al. 2003; Hallem et al. 2004; Hallem and Carlson 2006). In addition, misexpression studies of Or43a resulted in a reduction of behavioral avoidance responses to benzaldehyde (Stortkuhl et al. 2005). This result combined with electrophysiological recordings from ORNs and heterologous expression in Xenopus oocytes further functionally characterized the odorant response profiles of this receptor (Wetzel et al. 2001) and identified several Or43a ligands, such as fruit- derived odorants benzaldehyde, cyclohexanone, cyclohexanol, and benzyl alcohol (Stortkuhl and Kettler 2001; Hallem et al. 2004).
Despite advances in our understanding of odor coding, the molecular mechanisms responsible for variation in olfactory perception remain poorly understood. D. melanogaster is especially amenable to conducting such studies given its quantitatively simple olfactory system and since large numbers of genetically identical individuals can be reared in a common environment and these individuals can be subjected to simple, rapid, and highly reproducible quantitative behavioral assays Anholt and Mackay 2004). Here, we examine how molecular variation in odorant receptors contributes to variation in olfactory behavior in inbred lines derived from a natural population of D. melanogaster. We focused our analyses on six odorant receptors, Or7a, Or10a, Or42b, Or43a, Or67b, and Or85f, which have been shown by electrophysiology (Stortkuhl and Kettler 2001; Hallem et al. 2004; Stortkuhl et al. 2005; Hallem and Carlson 2006), through heterologous expression systems (Wetzel et al. 2001), or by calcium imaging studies (Wang et al. 2003) to respond to benzaldehyde. Significant variation in behavioral responses to benzaldehyde has been observed previously in this population and was normally distributed as is typical for a quantitative trait influenced by multiple genes (Wang et al. 2007). Here, we report associations between olfactory behavior and sequence variants in three Or genes. To validate the reliability of these associations we measured responses to a structurally similar odorant, acetophenone, in the same population, and showed that the associations with variation in responses to both odorants are largely similar with occasional molecular polymorphisms associated with variation in response to only one, but not the other odorant. These observations illustrate how sequence variants that arise during the evolution of Or genes can contribute to individual variation in olfactory behavior, how polymorphisms can give rise to subtle shifts in olfactory perception, and how naturally arising mutations within a population can combine to generate broad individual variation in sensory perception.
MATERIALS AND METHODS
Drosophila stocks:
D. melanogaster isofemale lines were established from flies collected from a natural population in Raleigh, North Carolina. Each isofemale line was subsequently inbred by 20 generations of full-sib mating to generate wild-derived inbred lines (Ayroles et al. 2009). All flies were reared on standard agar–yeast–molasses medium at 25° under a 12-h light/dark cycle. These are the same lines used to examine phenotypic variation in odor-mediated behavior by (Wang et al. 2007, 2010).
Behavioral assay:
Variation in odor-mediated behavioral responses to benzaldehyde and acetophenone was measured by Wang et al. (2007) and Wang et al. (2010), respectively, using the behavioral assay of Anholt et al. (1996). Dose-response experiments were conducted to determine the optimal concentration of benzaldehyde and acetophenone needed to maximally resolve variation among the wild-derived lines (Wang et al. 2007, 2010). Briefly, five flies of a single sex were placed in a vial demarcated into two equal compartments. The odorant, 3.5% (v/v) benzaldehyde or 3.5% (v/v) acetophenone, was then introduced on a cotton swab and the number of flies in the distal-most compartment of the vial were scored every 5 sec for 1 min. An average score was recorded and the assay was repeated 10 times for each sex for each genotype. The score for each line was the average of these 10 replicates, with a score of 0 indicating maximal attraction to the odorant and a score of 5 indicating maximum repulsion.
Identification of odorant receptor polymorphisms:
Genomic DNA was extracted using Gentra DNA isolation kits (Gentra Systems, Minneapolis, MN). Primers were designed approximately every 500 bp from the published DNA sequence (Drysdale et al. 2005) for each odorant receptor gene (Or7a, Or10a, Or42b, Or43a, Or67b, and Or85f) to obtain overlapping fragments resulting in full-length sequencing of the coding and non coding regions, as well as 5′- and 3′-untranslated regions. PCR products were amplified and sequenced directly using ABI big dye terminator cycle sequencing chemistry [Applied Biosystems (ABI), Foster City, CA] with the original PCR primers as well as internal sequencing primers. Sequences were aligned using Vector NTI Suite 11 software (Informax, Frederick, MD) to identify polymorphic sites.
Population genetic analyses:
Population genetic analyses were conducted for each of the six odorant receptor loci using DnaSP 5.10.00 (Librado and Rozas 2009; http://www.ub.es/dnasp). Estimates of population genetic parameters π and θ, and Tajima's D (Tajima 1989), were calculated. Significant departures from neutrality were examined using the McDonald–Kreitman (MK) test (McDonald and Kreitman 1991). For the MK test, D. melanogaster population sequences were compared to D. simulans sequence (Release 1.3, Apr 2005, droSim1), with the exception of Or85f in which D. sechellia (Release 1.3, Oct 2005, droSec1) was used for comparison. A single allele of Or67b contained a premature stop codon and was not included in the MK analysis (interpretation of analyses did not differ with its inclusion). Linkage disequilibrium (LD) between markers was determined using Tassel 2.1 software (http://www.maizegenetics.net/tassel) and significant differences between polymorphic markers were examined using Fisher's exact test. Haplotypes were determined using SNAP software (Price and Carbone 2005).
Genotype–phenotype associations:
To assess the extent to which individual polymorphisms are associated with olfactory behavior, associations between molecular polymorphisms and behavior were determined using ANOVA according to the two-way factorial model y = μ + M + S + M × S + E, with the two main fixed effects being molecular marker (M) and sex (S), and where E indicates error. Differences in trait values among haplotypes were assessed by two-way factorial ANOVA with the model y = μ + H + S + H × S + E, with the two main fixed effects of haplotype (H) and sex (S), and where E indicates error. We accounted for multiple testing using permutation tests (Churchill and Doerge 1994). Phenotypic line means were randomly permuted with respect to marker data. A thousand permuted data sets were generated and the lowest P-value for each of the 1000 permuted data sets was recorded. A distribution of P-values was generated and significant genotype–phenotype associations were determined if the P-value in the nonpermuted data was in the lower 5% of the distribution of permuted values. Singletons were excluded from LD and genotype–phenotype association analyses. The numbers of sequenced alleles for association studies for benzaldehyde are as follows: Or10a, N = 177; Or43a, N = 152; Or67b, N = 136. For acetophenone the numbers of sequenced alleles are as follows: Or10a, N = 174; Or43a, N = 149; Or67b, N = 135.
Quantitative reverse transcriptase PCR:
Total RNA was isolated from wild-derived inbred lines that corresponded to either the haplotype with the highest response or the haplotype with the lowest response to benzaldehyde for each of the three genes, Or10a, Or43a, and Or67b. Four independent RNA samples were extracted for each line and sex using the RNAqueous kit (ABI). RNA samples were treated with DNase (Ambion, Austin, TX) and reverse transcribed using the High Capacity cDNA Reverse Transcription kit (ABI). Real-time quantitative PCR was performed using TaqMan reagents on an ABI PRISM 7500 system. Primer–probe sets were custom designed as follows: Or10a, forward (For) CCAACTGCTGGTTTATTGCTATGG, reverse (Rev) CGAGTCGACGTTGTTTAGGCTTAA, probe FAM-CACAGACCAGTGCTACTTT; Or43a, For CCTACTACAATCGGGCCAATGAAAT, Rev GTACCAGGGCACATTGTAAACAG, probe FAM-ATGCCTCGAGAACAAC; Or67b, For GTGGAGTACAGTGCCTATGCA, Rev AGGCGACGAGACTGTAGATTATACT, probe FAM-CAAAATGCGAGTTAATCG. Odorant receptor expression was normalized to the expression of ribosomal protein, rpl32 (ABI Assay ID: Dm02151827_g1). Relative expression was determined by comparison of dT values relative to rpl32 expression using the 2−ΔΔCT method (Livak and Schmittgen 2001). Three technical replicates were performed for each extract of each line and sex. Expression differences between the high and low responder haplotypes for each sex were determined by ANOVA.
RESULTS
Identification of polymorphisms in Odorant receptor genes:
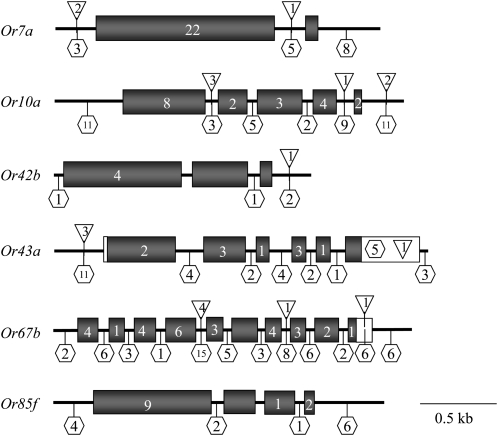
We sequenced six odorant receptor genes (Or7a, Or10a, Or42b, Or43a, Or67b, and Or85f) previously shown to respond to benzaldehyde (Stortkuhl and Kettler 2001; Wetzel et al. 2001; Wang et al. 2003; Hallem et al. 2004; Stortkuhl et al. 2005) in 50 wild-derived inbred lines from a single natural population (Ayroles et al. 2009). For each Or gene we identified single nucleotide polymorphisms (SNPs) and insertion/deletion polymorphisms (indels) that were present in more than one line(Figure 1; supporting information, Table S1). Polymorphisms in these receptors result in five amino acid substitutions in Or7a (A2T, R47Q, P245H, I282V, and A311G), five amino acid substitutions in Or10a (F18L, I264V, M347V, C350S, and G396D), two amino acid substitutions in Or42b (S159P, S206T), four amino acid substitutions in Or43a (I170M, V254A, L287M, and F333I), six amino acid substitutions in Or67b (Q4E, Y25H, G121D, Q124K, L221I, and R369K), and seven amino acid substitutions in Or85f (L47V, R96G, T164I, G186S, A228E, H253R, and V331A). There were also several amino acid substitutions that were represented in only one inbred line: Or10a (R109S and E228K), Or42b (L132Q, I276V, and Q352H), Or67b (K131N and L237F), and Or85f (H167Q).
Figure 1.—
Molecular variation in six Or genes. The gene structure for each receptor is schematically represented with a horizontal line denoting genomic DNA and exons represented by shaded boxes. The number of SNPs in the coding and noncoding regions is shown within each exon or within hexagons, respectively. Open boxes denote 5′-UTRs and 3′-UTRs. The number of indels is indicated by numbers within inverted triangles.
To assess the extent of nonrandom associations among polymorphic markers we conducted an LD analysis. For each odorant receptor, significant LD was commonly observed only between polymorphisms in close physical proximity (Figure 2). However, Or loci differed in the level and pattern of LD. Little LD was observed between polymorphisms in the ∼2-kb region of Or42b, while LD was observed among markers throughout the ∼2.5-kb region encompassing Or43a. In addition, we detected long-range linkage disequilibria in Or10a among polymorphic nucleotide marker C-375T and eight markers (T857A, C867T, T1346C, T1372A, G1373T, G1377A, G1550C, and 1606Del) spanning an ∼2-kb region. Also, in Or67b long- range LD was observed among nucleotide marker T1922A and five markers (G1506T, C1316T, 784Del, C662T, and C523T) (an ∼1.4-kb region).
Figure 2.—
Linkage disequilibrium between polymorphisms in each of six Or genes. Segments below and above diagonals reflect r2 values and P-values, respectively, for all possible marker pair combinations. P-values are determined by Fisher's exact test (not corrected for multiple tests).
Population genetic analyses:
Population genetic analyses of the sequences of these six Or genes revealed overall estimates of nucleotide diversity (π) between 0.0014 and 0.0122 and θw between 0.0015 and 0.0082 (Table 1). For all six loci, approximately the same sized (∼2.3-kb) genomic region was examined. Overall, higher nucleotide variation was observed in introns compared to coding regions, as is typical for coding sequences that are under selective constraint. Estimates of nucleotide diversity in Or67b may be influenced by the putative upstream regulatory region of a closely neighboring gene of unknown function (CG8336, ∼500 bp away). These population genetic parameters are consistent overall with average diversity values for D. melanogaster (Moriyama and Powell 1996; Andolfatto 2001) and similar to what has been observed for odorant-binding proteins (Wang et al. 2007). An exception is Or42b in which estimates of π were reduced (π = 0.0014) relative to other D. melanogaster Or loci.
TABLE 1.
Population genetic parameters
| Genes | No. sequences | Length (bp) | No. segregating sites | π | θw |
|---|---|---|---|---|---|
| Or7a | |||||
| CDS | 50 | 1254 | 26 (4)a | 0.0044 | 0.0047 |
| Intronic regions | 50 | 203 | 5 (0) | 0.0033 | 0.0055 |
| 5′-UTR | 50 | 426 | 4 (1) | 0.0023 | 0.0022 |
| 3′-UTR | 50 | 454 | 15 (7) | 0.0046 | 0.0074 |
| Combined | 50 | 2337 | 50 (12) | 0.0040 | 0.0048 |
| Or10a | |||||
| CDS | 197 | 1221 | 26 (7) | 0.0022 | 0.0036 |
| Intronic regions | 197 | 319 | 24 (3) | 0.0159 | 0.0139 |
| 5′-UTR | 197 | 488 | 12 (1) | 0.0021 | 0.0043 |
| 3′-UTR | 197 | 267 | 19 (6) | 0.0160 | 0.0123 |
| Combined | 197 | 2295 | 0.0056 | 0.0061 | |
| Or42b | |||||
| CDS | 41 | 1200 | 8 (3) | 0.0012 | 0.0016 |
| Intronic regions | 41 | 124 | 1 (0) | 0.0022 | 0.0020 |
| 5′-UTR | 41 | 359 | 2 (1) | 0.0015 | 0.0013 |
| 3′-UTR | 41 | 370 | 2 (0) | 0.0016 | 0.0014 |
| Combined | 41 | 2053 | 13 (4) | 0.0014 | 0.0015 |
| Or43a | |||||
| CDS | 145 | 1131 | 13 (2) | 0.0031 | 0.0021 |
| Intronic regions | 145 | 545 | 18 (3) | 0.0072 | 0.0060 |
| 5′-UTR | 145 | 435 | 13 (1) | 0.0066 | 0.0055 |
| 3′-UTR | 145 | 473 | 10 (3) | 0.0031 | 0.0038 |
| Combined | 145 | 2584 | 54 (9) | 0.0045 | 0.0038 |
| Or67b | |||||
| CDS | 148 | 1266 | 36 (8) | 0.0060 | 0.0051 |
| Intronic regions | 148 | 531 | 57 (5) | 0.0308 | 0.0194 |
| 5′-UTR | 148 | 188 | 3 (1) | 0.0051 | 0.0029 |
| 3′-UTR | 148 | 401 | 13 (1) | 0.0109 | 0.0058 |
| Combined | 148 | 2386 | 109 (15) | 0.0122 | 0.0082 |
| Or85f | |||||
| CDS | 36 | 1179 | 13 (1) | 0.0037 | 0.0027 |
| Intronic regions | 36 | 185 | 3 (0) | 0.0042 | 0.0039 |
| 5′-UTR | 36 | 290 | 8 (4) | 0.0057 | 0.0067 |
| 3′-UTR | 36 | 474 | 8 (2) | 0.0030 | 0.0041 |
| Combined | 36 | 2128 | 32 (7) | 0.0038 | 0.0036 |
Singletons are given in parentheses.
Next, we tested for deviations from neutrality using the MK test (McDonald and Kreitman 1991). The MK test compares within- species variation with between- species divergence. This method takes advantage of the expectation under the neutral mutation hypothesis that the ratio of nonsynonymous to synonymous (silent) differences between species should be the same as the ratio of nonsynonymous to silent polymorphisms within species. A significant deviation in ratios between species vs. within species rejects the neutral mutation hypothesis. We observed no departure from neutrality in the coding regions of four of six odorant receptor loci (Table 2).However, we detected significant deviations from neutrality for Or42b and Or85f that appear to be driven by a reduction in silent-site variation within species (Table 2). Calculations of Tajima's D revealed estimates for Or42b and Or85f to be −0.3185 and 0.2067, respectively. These estimates do not differ significantly from expectations under the neutral mutation model (Tajima 1989).
TABLE 2.
McDonald–Kreitman tests
| Synonymous substitutions |
Nonsynonymous substitutions |
||||
|---|---|---|---|---|---|
| Genes | Between species | Within species | Between species | Within species | P-value |
| Or7a | 35 | 21 | 16 | 5 | 0.2486 |
| Or10a | 40 | 19 | 16 | 7 | 0.8768 |
| Or42b | 33 | 3 | 3 | 5 | 0.0012* |
| Or43a | 27 | 10 | 5 | 4 | 0.3202 |
| Or67b | 43 | 29 | 7 | 8 | 0.3547 |
| Or85f | 35 | 4 | 8 | 8 | 0.0018* |
*0.001 < P < 0.01.
Associations between polymorphisms in Or genes and olfactory behavior:
To assess to what extent sequence variation in these odorant receptors may contribute to natural variation in olfactory behavior, we conducted first a preliminary analysis on ∼50 alleles from this wild-derived population for each of these six Or genes. This analysis was designed to identify loci in which more polymorphisms than expected by chance were associated with variation in odor-mediated behavior in response to benzaldehyde (Wang et al. 2007). On the basis of results of these analyses, we focused our investigation of genotype–phenotype relationships on three Or genes (Or10a, Or43a, and Or67b) and we obtained additional sequences of alleles for each of these loci.
For each polymorphic marker, the statistical association between the marker and the phenotypic means for olfactory behavior to benzaldehyde was determined using two-way factorial analysis of variance. We identified multiple polymorphisms in each of these three Or genes (Or10a, Or43a, and Or67b) that significantly contributed to variation in responsiveness to benzaldehyde in this population (Figure 3). Thirteen polymorphisms in Or10a contributed to variation in behavioral responses, exceeding the permutation threshold. It is of note that of those markers, C-375T and eight markers, T857A, C867T, T1346C, T1372A, G1373T, G1377A, G1550C, and 1606Del, were in complete LD (Figure 2). In the case of Or43a, 13 polymorphisms were significantly associated with variation in response to benzaldehyde (Figure 3). Markers C-167A and C-152T are in complete LD as well as marker group C1158T, T1167A, G1169A, and T1175A. Substantial LD (r2 > 0.87) was also observed between T576C, C685G and C1158T, T1167A, G1169A, and T1175A (Figure 2). Furthermore, of the significantly associated SNPs, one (C685G) resulted in an isoleucine to methionine (I170M) substitution that is located extracellularly. Finally, 12 polymorphisms in Or67b contributed to variation in behavior (Figure 3). Of those markers, there were three groups whose members were in complete LD: C523T, C662T, 784Del, C1316T, G1506T, and T1922A; G932A, G943A, and A944C; and T1475A and G1524T (Figure 2).
Figure 3.—
Associations between polymorphisms in each of three Or genes and variation in olfactory behavioral responses. Polymorphic markers are depicted on the x-axis, and log(1/P) values for marker-trait associations on the y-axis. Marker–trait associations for benzaldehyde and acetophenone are indicated by the red and gray lines, respectively. The dashed red and gray horizontal lines indicate the corresponding permutation thresholds.
As similar large collections of wild-derived inbred lines from other populations of D. melanogaster do not exist, we devised an alternative strategy to confirm the validity of the observed associations. To independently verify the observed associations while at the same time gaining additional insights into the relationship between variation in olfactory behavior and variation in Or gene polymorphisms, we repeated our association analyses with an independent set of behavioral measurements of responses to a structurally similar odorant, acetophenone, which differs from benzaldehyde only in the presence of a methyl group (Figure 3). Previous studies showed substantial correlation between behavioral responses to benzaldehyde and acetophenone (Wang et al. 2010). Thus, the prediction is that the association profiles from the analyses for both odorants would show global similarity, while sporadically uncovering individual polymorphisms that are associated with only one, but not the other odorant. Indeed, we found overlapping sets of SNPs contributing to variation in behavioral responses to both odorants. Fifteen polymorphisms in Or10a, 2 polymorphisms in Or43a, and 14 polymorphisms in Or67b were associated with variation in responses to acetophenone (Figure 3). Of those markers, many were identical to those contributing to variation in responses to benzaldehyde and the association profiles shown in Figure 3 show remarkable similarities.
As expected, there were also polymorphic markers associated with variation in behavior that were distinct for each odorant. For Or10a, C1636T was exclusively associated with variation in responsiveness to benzaldehyde and C-465A, C757T, and G806T with responsiveness to acetophenone. For Or43a, all but marker 12 were solely associated with variation in response to benzaldehyde. However, it should be noted that C-356T, C-167A, and C1158T were just below the permutation threshold of significance for responsiveness to acetophenone. Furthermore, one SNP (C670T) contributed only to variation in responses to acetophenone. Finally, in the case of Or67b, only G932A, G943A, and A944C were significantly associated with behavioral responses to both odorants. Eleven distinct SNPs contributed to variation in responses to acetophenone. Of those markers, we observed three discrete groups of markers (C695T and C728T, A799T and 803Del, and A1330C and T1335A) whose members were in complete LD. Thus, one might expect that among these three odorant receptors Or67b might be particularly well positioned to discriminate these two odorants.
We estimated the expected genetic variance contributed by each molecular polymorphism associated with behavioral responses to benzaldehyde and acetophenone in an outbred population with the same allele frequencies found in the inbred lines and assuming random mating (Table S2).Under an additive model, the genetic variance (VA) contributed by each biallelic locus is 2pqa2, where p and q are the allele frequencies and a is one-half the difference in the mean of the trait between the two homozygous marker genotype classes (Falconer and Mackay 1996). The estimate of the heritability attributable to each marker is VA/VP, where VP is the estimated phenotypic variance of the trait in this population (Wang et al. 2007, 2010). Each marker is expected to account for ∼1.6–3.2% of the total phenotypic variation in olfactory behavior. However, LD among the markers precludes summing the contribution from each marker to infer the total variance explained by each locus. These effect sizes are consistent with other studies in which polymorphisms in odorant-binding proteins contributed 3–6% to the total phenotypic variance in olfactory behavior (Wang et al. 2007, 2010).
As each Or gene contained multiple SNPs that were associated with variation in olfactory behavior, we examined their combined effects by conducting haplotype analyses. Analyses of the polymorphisms significantly associated with variation in responses to benzaldehyde identified 6 haplotypes for Or10a, 17 for Or43a, and 9 for Or67b (Figure 4; Table S3). For polymorphisms associated with variation in responsiveness to acetophenone, haplotype analyses identified 9 haplotypes for Or10a, 3 for Or43a, and 12 for Or67b (Figure 4; Table S3). The majority of haplotypes were present at low frequency. A notable exception was observed for Or43a in which there were two SNPs associated with variation in behavioral responses to acetophenone that formed 3 haplotypes (AT, CT, and CC) at individual frequencies of 0.207, 0.271, and 0.521, respectively. Of those haplotypes significant phenotypic differences were observed between the AT and CC haplotypes. The CC haplotype had a significant reduction in least-squares mean responses to acetophenone relative to the AT haplotype. In addition, we observed for all three Or genes significant differences among haplotypes in their responsiveness to both odorants (Figure 4; Table S4a). We estimated the contribution of each locus to variation in olfactory behavior in the population of inbred lines on the basis of haplotype analysis. Variation in olfactory behavior among haplotypes accounted for 10.1–16.3% of the total variance in response to benzaldehyde and 3.9–11.8% of the total variance in response to acetophenone (Table S4b).
Figure 4.—
Haplotype analysis of SNPs/indels significantly associated with variation in behavioral responses to either benzaldehyde (red bars) or acetophenone (gray bars) for Or genes Or10a, Or43a, and Or67b. Significant phenotypic differences among haplotypes were determined by ANOVA and haplotypes that differed significantly in olfactory behavior were determined by post hoc Tukey's tests at P < 0.05 and are indicated by different letters above the bars. Specific haplotype sequences for each of the odorant receptors and their frequencies are presented in Table S3.
Differences in odorant receptor gene expression among high- and low-responder haplotypes:
SNPs can influence protein structure and function (Hoekstra et al. 2006), regulate gene expression levels (Campbell et al. 2006; Wang et al. 2010), or affect the structure and stability of mRNA (Nackley et al. 2006; Wang et al. 2007). Nonsynonymous polymorphisms are likely to result in changes in protein structure/function, while SNPs in noncoding regions can result in changes in gene expression levels or mRNA stability. Although all may contribute to behavioral variation, the majority of SNPs associated with variation in behavioral responses in our study were in noncoding regions. To test if there was a correspondence between variation in behavioral response to benzaldehyde and expression differences in Or10a, Or43a, and Or67b, we selected wild-derived lines that corresponded to the haplotype with either the highest response or the lowest response to benzaldehyde for each gene and measured transcript abundance using quantitative PCR (Figure 5; Table S5). We observed that haplotypes associated with the highest response scores were associated with a decrease in Or10a expression in both sexes (P < 0.0001 for both males and females), with Or10a expression lower in high- responder males and females by ∼54 and 37%, respectively. We analyzed the sexes separately since we observed a significant haplotype-by-sex interaction. We observed a significant male-specific increase in Or43a expression (P < 0.0168) in which Or43a expression was ∼37% higher in males of the high-responder haplotype. We did not see a significant difference in Or67b gene expression between the high-responder and low-responder haplotypes.
Figure 5.—
Correspondence between behavioral response to benzaldehyde and differences in Or10a and Or43a expression. Expression of (a) Or10a and (b) Or43a in male and female high- and low-responder haplotypes is shown. Odorant receptor expression is shown relative to the expression in the low responder. Data are shown as mean ± SEM, *P < 0.05; ****P < 0.0001.
DISCUSSION
Chemical cues play a major role in environmental adaptation during evolution. Consequently, individual Or loci are likely subject to different evolutionary pressures. Indeed, our molecular evolutionary analysis of nucleotide variation in six Or genes revealed differences in their evolutionary dynamics, even though these odorant receptors have at least partially overlapping ligand specificities (Hallem et al. 2004; Fishilevich and Vosshall 2005; Hallem and Carlson 2006).This is suggested from differences in estimates of nucleotide diversity, especially for Or42b where π = 0.0014 (Table 1), and is evident from the results of our McDonald–Kreitman tests, which showed no departure from neutrality for the coding region of four of the six odorant receptors, but a significant deviation from neutral expectations for Or42b and Or85f (Table 2). The observed reduction in nucleotide variability of Or42b may be due to its location near the centromeric region of chromosome arm 2R, which shows reduced recombination (Ashburner 1989; Kliman and Hey 1993; Charlesworth 1996). Reduction in nucleotide diversity in this region suggests either a selective sweep or background selection (Begun and Aquadro 1992; Charlesworth et al. 1993). Three of the five replacement polymorphisms were represented in only one inbred line, but Tajima's D estimates suggest the frequency spectrum of polymorphisms is only slightly negatively skewed and not significantly different from neutral expectations (Tajima 1989). These observations suggest a model of background selection contributing to the observed reduction in synonymous polymorphism in the Or42b region (Braverman et al. 1995). Interestingly, Or42b has also been identified as one of the most conserved Or genes among Drosophila species (McBride et al. 2007 ). Or85f, however, is located more distally on chromosome arm 3R. Here, possible explanations for the reduction in synonymous polymorphisms include balancing selection in addition to background selection or a selective sweep. The lack of a significant Tajima's D, however, is not reflective of expectations under either a model of selective sweep or balancing selection in which we might predict to see a significant negative or positive Tajima's D, respectively. Thus, the selective pressures that give rise to deviations from neutrality at this locus remain unclear.
We identified molecular polymorphisms in Or10a, Or43a, and Or67b that are associated with behavioral variation in responses to both benzaldehyde and acetophenone. Our results are consistent with the combinatorial nature of odor coding in which odorant receptors overlap in their ligand response profiles and there is functional redundancy among receptors (Hallem et al. 2004; Hallem and Carlson 2006; Vosshall and Stocker 2007). It should be noted that we examined only six genes from the multigene family of odorant receptors and that additional Or genes may also contribute to variation in behavioral responses to these odorants. Furthermore, the statistical power for detecting associations between SNPs and variation in olfactory behavior increases as sample size increases. Increasing sample sizes within a population and surveying additional populations may identify more SNPs associated with variation in behavioral responses to both benzaldehyde and acetophenone.
Within each gene, multiple SNPs or indels were associated with variation in behavioral responses to both odorants. The majority of these polymorphisms were in noncoding regions. As postulated previously (Wang et al. 2007), mutations in noncoding regions that do not directly influence protein structure can be primary agents of behavioral variation by regulating gene expression or affecting the structure and stability of mRNA. Indeed, misexpression of Or43a resulted in changes in avoidance behavior to benzaldehyde (Stortkuhl and Kettler 2001; Stortkuhl et al. 2005).
We found that high- and low-responder haplotypes differed in the expression of Or10a and Or43a, with differences in direction and sex specificity. Closely related odorants, like benzaldehyde and acetophenone, are expected to interact with multiple, albeit overlapping, odorant receptors with different affinities. Reduction in the expression level of one receptor may have differential effects on recognition of these two odorants depending on their relative affinities for the receptor and for other cognate receptors. For example, in a case in which a receptor binds one odorant with high affinity and a structurally similar odorant with lower affinity, a small reduction in the expression level of the receptor could potentially affect the behavioral response to one odorant, but not the other. The contribution to odorant perception of these odorants hence is dependent on the relative weight of interactions with the specific receptor in the context of the entire combinatorial set of cognate odorant receptors. It is also possible that additional regulatory polymorphisms may be present in upstream or downstream flanking regions that extend beyond the sequenced regions. Such polymorphisms are not detected in our current study. Finally, it is possible that potential regulatory sites in introns might change the structure or stability of the mRNA, as previously suggested for intronic polymorphisms in odorant-binding proteins associated with olfactory behavior (Wang et al. 2007). Extensive phenotypic plasticity in the expression of chemosensory genes in D. melanogaster in response to different developmental, physiological, and social conditions has been documented (Zhou et al. 2009). A detailed analysis of the role of chemosensory gene regulation in modulating behavior awaits further study, but results from this study illustrate the complex relationship between the chemosensory receptor transcriptome and olfactory behavior.
Protein-coding changes can also influence variation in behavior by directly influencing protein structure and function. Amino acid substitutions in vertebrate chemosensory receptors can result in changes in ligand-binding affinity and confer differences in odorant sensitivity (Krautwurst et al. 1998; Feinstein and Mombaerts 2004; Reed et al. 2004; Nie et al. 2005; Abaffy et al. 2007; Keller et al. 2007). Thus, it is of particular interest that Or43a harbors an isoleucine to methionine substitution that contributes to variation in responsiveness to benzaldehyde. This amino acid residue is predicted to be located extracellularly and hence could influence ligand binding.
We observed that many of the SNPs and indels associated with variation in behavioral responses to benzaldehyde also contributed to variation in behavioral responses to acetophenone. These results are in line with previous electrophysiological studies in which Or10a was shown to respond strongly to aromatic compounds that have both a benzene ring and a carbonyl group (Fishilevich and Vosshall 2005; Hallem and Carlson 2006). These two odorants also act as ligands for Or67b (Fishilevich and Vosshall 2005). Furthermore, misexpression studies of Or43a identified benzaldehyde as a strong ligand (Stortkuhl et al. 2005) and in a screen of structurally similar chemicals suggest that ligands for Or43a should contain a benzene ring and a polar group (Stortkuhl and Kettler 2001; Wetzel et al. 2001). Finally, behavioral responses to acetophenone were strongly correlated with responses to benzaldehyde for this population (Wang et al. 2010). The overlapping set of SNPs contributing to variation in behavioral responses to both odorants provides independent validation for the reliability of our association tests. However, the fact that certain SNPs are associated with variation in response to one odorant but not the other, especially in the case of Or67b, provides a glimpse into the manner in which sequence variants that arise during evolution can potentially generate subtle shifts in odorant response profiles.
In addition to polymorphisms in Or genes that are associated with variation in responses to benzaldehyde and acetophenone, studies on Odorant binding protein (Obp) genes identified SNPs within the Obp99 gene cluster associated with differences in responses to these odorants (Wang et al. 2010). The functional relationships between sequence variants in Obp genes and in Or genes associated with responses to similar odorants, however, remain to be elucidated. Here, we have shown that sequence variants that arise during the evolution of Or genes can contribute to individual variation in olfactory behavior and give rise to subtle shifts in olfactory perception. It seems reasonable to predict that the combined effects of polymorphisms throughout the entire Or gene repertoire would generate broad individual variation in chemosensory perception.
Acknowledgments
We thank Peter Andolfatto, Mary Anna Carbone, Richard Kirby, John Layne, and Ken Petren for helpful discussions or technical assistance and Allison Weber for help with the permutation tests. This research was supported by grants from the National Institutes of Health to S.M.R. (GM080592), R.R.H.A. (GM059469), and T.F.C.M (GM045146).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.119446/DC1.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under the following accession nos.: Or7a,GU445973–GU446022; Or10a,GU446073–GU446269; Or42b, GU445928–GU445972; Or43a,GU446270–GU446436; Or67b, GU446437–GU446587; and Or85f, GU446023–U446072.
References
- Abaffy, T., A. Malhotra and C. W. Leutje, 2007. The molecular basis for ligand specificity in a mouse olfactory receptor. J. Biol. Chem. 282 1216–1224. [DOI] [PubMed] [Google Scholar]
- Andolfatto, P., 2001. Contrasting patterns of X-linked and autosomal nucleotide variation in Drosophila melanogaster and Drosophila simulans. Mol. Biol. Evol. 18 279–290. [DOI] [PubMed] [Google Scholar]
- Anholt, R. R. H., and T. F. C. Mackay, 2004. Quantitative genetic analyses of complex behaviours in Drosophila. Nat. Rev. Genet. 5 838–849. [DOI] [PubMed] [Google Scholar]
- Anholt, R. R. H., R. F. Lyman and T. F. C. Mackay, 1996. Effects of single P-element insertions on olfactory behavior in Drosophila melanogaster. Genetics 143 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, M. A., 1978. Sensory Ecology: Review and Perspectives. Plenum Press, New York.
- Ayroles, J. F., M. A. Carbone, E. A. Stone, K. W. Jordan, R. F. Lyman et al., 2009. Systems genetics of complex traits in Drosophila melanogaster. Nat. Genet. 41 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, M., 1989. Handbook on Drosophila. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N Y.
- Begun, D. J., and C. F. Aquadro, 1992. Levels of naturally occurring DNA polymorphism correlate with recombination rates in D. melanogaster. Nature 356 519–520. [DOI] [PubMed] [Google Scholar]
- Benton, R., S. Sachse, S. W. Michnick and L. B. Vosshall, 2006. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4 240–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao, S., A. Sen, R. Stocker and V. Rodrigues, 2003. Olfactory neurons expressing identified receptor genes project to subsets of glomeruli within the antennal lobe of Drosophila melanogaster. J. Neurobiol. 54 577–592. [DOI] [PubMed] [Google Scholar]
- Braverman, J. M., R. R. Hudson, N. L. Kaplan, C. H. Langley and W. Stephan, 1995. The hitchhiking effect on the site frequency spectrum of DNA polymorphism. Genetics 140 783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, L., and R. Axel, 1991. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65 175–187. [DOI] [PubMed] [Google Scholar]
- Campbell, D. B., J. S. Sutcliffe, P. J. Ebert, R. Militerni, C. Bravaccio et al., 2006. A genetic variant that disrupts MET transcription is associated with autism. Proc. Natl. Acad. Sci. USA 103 16834–16839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, B., 1996. Background selection and patterns of genetic diversity in Drosophila melanogaster. Genet. Res. 68 131–149. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., M. T. Morgan and D. Charlesworth, 1993. The effects of deleterious mutations on neutral molecular variation. Genetics 134 1289–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill, G. A., and R. W. Doerge, 1994. Empirical threshold values for quantitative trait mapping. Genetics 138 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne, P. J., C. G. Warr, M. R. Freeman, D. Lessing, J. H. Kim et al., 1999. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22 327–338. [DOI] [PubMed] [Google Scholar]
- de Bruyne, M., P. J. Clyne and J. R. Carlson, 1999. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J. Neurosci. 19 4520–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne, M., K. Foster and J. R. Carlson, 2001. Odor coding in the Drosophila antenna. Neuron 30 537–552. [DOI] [PubMed] [Google Scholar]
- Dobritsa, A. A., W. van der Goes van Naters, C. G. Warr, R. A. Steinbrecht and J. R. Carlson, 2003. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37 827–841. [DOI] [PubMed] [Google Scholar]
- Drysdale, R. A., M. A. Crosby and The FlyBase Consortium, 2005. Flybase genes and gene models. Nucleic Acids Res. 33 D390–D395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenbery, D. B., 1992. Sensory Ecology: How Organisms Acquire and Respond to Information. W. H. Freeman, New York.
- Falconer, D. S., and T. F. C. Mackay, 1996. Introduction to Quantitative Genetics, Ed. 4. Longmans Green, Harlow, Essex, UK.
- Feinstein, P., and P. Mombaerts, 2004. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell 117 817–831. [DOI] [PubMed] [Google Scholar]
- Fishilevich, E., and L. B. Vosshall, 2005. Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15 1548–1553. [DOI] [PubMed] [Google Scholar]
- Gao, Q., and A. Chess, 1999. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics 60 31–39. [DOI] [PubMed] [Google Scholar]
- Gao, Q., B. Yuan and A. Chess, 2000. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat. Neurosci. 3 780–785. [DOI] [PubMed] [Google Scholar]
- Hallem, E. A., and J. R. Carlson, 2006. Coding of odors by a receptor repertoire. Cell 125 143–160. [DOI] [PubMed] [Google Scholar]
- Hallem, E. A., M. G. Ho and J. R. Carlson, 2004. The molecular basis of odor coding in the Drosophila antenna. Cell 117 965–979. [DOI] [PubMed] [Google Scholar]
- Hoekstra, H. E., R. J. Hirschmann, R. A. Bundey, P. A. Insel and J. P. Crossland, 2006. A single amino acid mutation contributes to adaptive beach mouse color pattern. Science 313 101–104. [DOI] [PubMed] [Google Scholar]
- Kliman, R. M., and J. Hey, 1993. Reduced natural selection associated with low recombination in Drosophila melanogaster. Mol. Biol. Evol. 10 1239–1258. [DOI] [PubMed] [Google Scholar]
- Jones, W. D., T. A. Nguyen, B. Kloss, K. J. Lee and L. B. Vosshall, 2005. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr. Biol. 15 R119–R121. [DOI] [PubMed] [Google Scholar]
- Keller, A., H. Zhuang, Q. Chi, L. B. Vosshall and H. Matsunami, 2007. Genetic variation in a human odorant receptor alters odour perception. Nature 449 468–472. [DOI] [PubMed] [Google Scholar]
- Krautwurst, D., K.-W. Yau and R. R. Reed, 1998. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell 95 917–926. [DOI] [PubMed] [Google Scholar]
- Laissue, P. P., C. Reiter, P. R. Hiesinger, S. Halter, K. F. Fischbach et al., 1999. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J. Comp. Neurol. 405 543–552. [PubMed] [Google Scholar]
- Larsson, M. C., A. I. Domingos, W. D. Jones, M. E. Chiappe, H. Amrein et al., 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43 703–714. [DOI] [PubMed] [Google Scholar]
- Librado, P., and J. Rozas, 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25 1451–1452. [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and T. D. Schmittgen, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25 402–408. [DOI] [PubMed] [Google Scholar]
- Lythgoe, J. N., 1979. The Ecology of Vision. Clarendon Press, Oxford.
- McBride, C. S., J. R. Arguello and B. C. O'Meara, 2007. Five Drosophila genomes reveal nonneutral evolution and the signature of host specialization in the chemoreceptor superfamily. Genetics 177 1395–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. H., and M. Kreitman, 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351 652–654. [DOI] [PubMed] [Google Scholar]
- Mombaerts, P., F. Wang, C. Dulac, S. K. Chao, A. Nemes et al., 1996. Visualizing an olfactory sensory map. Cell 87 675–686. [DOI] [PubMed] [Google Scholar]
- Moriyama, E. N., and J. R. Powell, 1996. Intraspecific nuclear DNA variation in Drosophila. Mol. Biol. Evol. 13 261–277. [DOI] [PubMed] [Google Scholar]
- Nackley, A. G., S. A. Shabalina, I. E. Tchivileva, K. Satterfield, O. Korchynskyi et al., 2006. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science 314 1930–1933. [DOI] [PubMed] [Google Scholar]
- Nie, Y., S. Vigues, J. R. Hobbs, G. L. Conn and S. D. Munger, 2005. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr. Biol. 15 1948–1952. [DOI] [PubMed] [Google Scholar]
- Price, E. W., and I. Carbone, 2005. SNAP: workbench management tool for evolutionary population genetic analysis. Bioinformatics. 21 402–404. [DOI] [PubMed] [Google Scholar]
- Reed, D. R., S. Li, X. Li, L. Huang, M. G. Tordoff et al., 2004. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J. Neurosci. 24 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, H. M., C. G. Warr and J. R. Carlson, 2003. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 100 14537–14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., M. Pellegrino, T. Nakagawa, T. Nakagawa, L. B. Vosshall et al., 2008. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452 1002–1006. [DOI] [PubMed] [Google Scholar]
- Stortkuhl, K. F., and R. Kettler, 2001. Functional analysis of an olfactory receptor in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 98 9381–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stortkuhl, K. F., R. Kettler, S. Fischer and B. T. Hovemann, 2005. An increase receptive field of olfactory receptor Or43a in the antennal lobe of Drosophila reduces benzaldehyde-driven avoidance behavior. Chem. Senses 30 81–87. [DOI] [PubMed] [Google Scholar]
- Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar, R., S. K. Chao, J. M. Sitcheran, L. Nunez, L. B. Vosshall et al., 1994. Topographic organization of sensory projections to the olfactory bulb. Cell 79 981–992. [DOI] [PubMed] [Google Scholar]
- Vosshall, L. B., and R. F. Stocker, 2007. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30 505–533. [DOI] [PubMed] [Google Scholar]
- Vosshall, L. B., H. Amrein, P. S. Morozov, A. Rzhetsky and R. Axel, 1999. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 96 725–736. [DOI] [PubMed] [Google Scholar]
- Vosshall, L. B., A. M. Wong and R. Axel, 2000. An olfactory sensory map in the fly brain. Cell 102 147–159. [DOI] [PubMed] [Google Scholar]
- Wang, J. W., A. M. Wong, J. Flores, L. B. Vosshall and R. Axel, 2003. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112 271–282. [DOI] [PubMed] [Google Scholar]
- Wang, P., R. F. Lyman, S. A. Shabalina, T. F. C. Mackay and R. R. H. Anholt, 2007. Association of polymorphisms in odorant-binding protein genes with variation in olfactory response to benzaldehyde in Drosophila. Genetics 177 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, P., R. F. Lyman, T. F. C. Mackay and R. R. H. Anholt, 2010. Natural variation in odorant recognition among odorant binding proteins in Drosophila melanogaster. Genetics 184 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel, C. H., H. J. Behrendt, G. Gisselmann, K. F. Stortkuhl, B. Hovemann et al., 2001. Functional expression and characterization of a Drosophila odorant receptor in a heterologous cell system. Proc. Natl. Acad. Sci. USA 98 9377–9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher, D., R. Schafer, R. Bauernfeind, M. C. Stensmyr, R. Heller, et al., 2008. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452 1007–1011. [DOI] [PubMed] [Google Scholar]
- Zhang, X., and S. Firestein, 2002. The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. 5 124–133. [DOI] [PubMed] [Google Scholar]
- Zhang, X., I. Rodriguez, P. Mombaerts and S. Firestein, 2004. Odorant and vomeronasal receptor genes in two mouse genome assemblies. Genomics 83 802–811. [DOI] [PubMed] [Google Scholar]
- Zhou, S., E. A. Stone, T. F. C. Mackay and R. R. H Anholt, 2009. Plasticity of the chemoreceptor repertoire in Drosophila melanogaster. PLoS Genet. 5 e1000681. [DOI] [PMC free article] [PubMed] [Google Scholar]