Summary
FAS (also known as APO-1 or CD95) belongs to the subgroup of the tumor necrosis factor receptor (TNF-R) family that contain an intra-cellular ‘death domain’ and can trigger apoptosis. Its physiological ligand, FASL (CD95L), is a member of the corresponding TNF cytokine family. Studies with spontaneous mutant mice, gene-targeted mice and cells from human patients have shown that FAS and FASL play critical roles in the immune system, in particular in the killing of pathogen infected target cells and the death of no longer needed, potentially deleterious as well as autoreactive lymphocytes. This ligand-receptor pair thereby functions as a guardian against autoimmunity and tumor development. FASL-FAS signaling triggers apoptosis through FADD (Fas-associated protein with death domain, also called MORT1) adaptor protein-mediated recruitment and activation of the aspartate-specific cysteine protease, caspase-8. In certain cells such as hepatocytes, albeit not in lymphocytes, FAS-induced apoptosis signaling requires amplification through proteolytic activation of the pro-apoptotic BCL-2 family member BID. Curiously, several components of the FAS signaling machinery have been implicated in non-apoptotic processes, including cellular activation, differentiation and proliferation. Here we describe current knowledge of the roles of FASL and FAS in the immune system, discuss important unresolved issues and propose experimental approaches to address them.
Keywords: FAS, FAS ligand, apoptosis, death receptors, immune system
Introduction
The cell surface-bound receptor FAS (also called APO-1 or CD95) was originally discovered as the target of two monoclonal antibodies that trigger apoptotic cell death in certain human tumor derived cell lines in culture or as xeno-transplants in immuno-deficient mice (Trauth et al., 1989; Yonehara et al., 1989). Cloning of the gene encoding FAS revealed that it is a member of the tumor necrosis factor receptor (TNF-R) family (Itoh et al., 1991), which also includes the receptors for TNF, TRAIL (TNF-related apoptosis-inducing ligand) and those for other members of the TNF cytokine family. Accordingly, identification of the physiological ligand for FAS, called FAS ligand (FASL or CD95L) (Suda et al., 1993), through expression cloning demonstrated that it belongs to the TNF cytokine family. The discovery that certain spontaneous mutant mouse strains that develop lymphadenopathy and SLE (systemic lupus erythematosus)-like autoimmune disease carry homozygous defects in the genes encoding FAS (Faslpr/lpr or Faslprcg/lprcg) (Watanabe-Fukunaga et al., 1992) or FASL (Faslgld/gld) (Takahashi et al., 1994) and the realization that a large fraction of human ALPS (autoimmune lympho-proliferative syndrome) patients have heterozygous inherited mutations in the FAS gene (Drappa et al., 1996; Fisher et al., 1995; Rieux-Laucat et al., 1995) demonstrated that FASL-FAS signaling plays a critical role in the control of the immune system. ALPS patients (Straus et al., 2001) as well as the FAS- or FASL-deficient mice (Davidson et al., 1998) have an abnormally increased predisposition to lymphoma development, demonstrating that FASL-FAS signaling is also critical for tumor suppression, at least within the lymphoid compartment. FASL expressed on activated T lymphocytes or natural killer (NK) contributes to their ability to kill target cells, such as virus infected or damaged cells (Trambas and Griffiths, 2003). Abnormally increased FASL-mediated killing of healthy (FAS+) bystander cells has been implicated in certain immuno-pathological states, such as hepatitis induced by extensive T cell activation (Ksontini et al., 1998; Tagawa et al., 1998). Pharmacological modulation of the FASL-FAS signaling machinery may therefore be a useful strategy for therapeutic intervention in certain diseases, but caution is warranted because administration of agonistic FAS-specific antibodies (Ogasawara et al., 1993) or FASL (Huang et al., 1999; Shiraishi et al., 2004) cause extensive hepatocyte apoptosis and fatal hepatitis in mice. Detailed understanding of FASL-FAS signaling may allow the development of more subtle intervention strategies and, interestingly, one FAS-specific antibody was shown to cure Faslgld/gld mutant mice from their lymphadenopathy without causing liver toxicity (Ichikawa et al., 2000).
Mechanisms of FASL-FAS signaling induced apoptosis
It has been argued that researchers readily observe that FAS stimulation causes apoptosis because activators of FAS (agonistic antibodies or recombinantly produced forms of FASL) have been selected for this property and that we may therefore have overly emphasized this function of FASL-FAS signaling while under-estimating other activities, such as induction of cellular proliferation or differentiation (Peter et al., 2007). However, activation of FAS by its physiological ligand, FASL, has certainly been proven to trigger apoptosis (Krammer, 2000; Nagata, 1997; Trambas and Griffiths, 2003). For example, several studies have shown that cytotoxic T cells, which express FASL in membrane-bound form (mFASL) on their surface, can kill FAS+ target cells and neutralization of FASL with specific antibodies or FAS-Fc fusion proteins proved that this killing is mediated by FASL-induced activation of FAS (Krammer, 2000; Nagata, 1997). Moreover, re-stimulation of already activated normal T cells or T lymphoma-derived cell lines via their TCR (T cell antigen receptor) complex causes extensive suicide or fratricide, a process called activation induced cell death (AICD). (It has recently been proposed to re-name this process ‘Re-stimulation Induced Cell Death’ (RICD) in order to distinguish it more clearly from the cell death that can occur when naïve T lymphocytes are stimulated for the first time with antigen or mitogen (Ramaswamy and Siegel, 2007)). Experiments using Faslpr/lpr or Faslgld/gld mice or the aforementioned FASL blockers showed that this apoptosis is caused (at least in part) by physiological FASL-FAS signaling (Alderson et al., 1995; Brunner et al., 1995; Dhein et al., 1995; Russell et al., 1993; Russell and Wang, 1993). It is widely believed that defects in this process underlie the lymphadenopathy and autoimmune disease that develop in mice and humans that have abnormalities in their genes encoding FAS or FASL (Krammer, 2000; Nagata, 1997; Strasser, 1995).
Given that physiological FASL-FAS signaling can induce apoptosis we first describe the mechanisms of this process, but non-apoptotic processes that are reportedly activated by FAS will also be discussed. Elegant biochemical studies have shown that ligation of FAS rapidly causes assembly of an intra-cellular ‘death inducing signaling complex’ (DISC) (Kischkel et al., 1995), which was shown to contain the aspartate specific cysteine protease, caspase-8 (Boldin et al., 1996; Medema et al., 1997; Muzio et al., 1996), its adaptor/activator FADD (Boldin et al., 1995; Chinnaiyan et al., 1995) and its modulator c-FLIP (FLICE (i.e. caspase-8) inhibitory protein) (Irmler et al., 1997). The interaction between FAS and FADD requires homotypic interaction of ‘death domains’ (DD) (Boldin et al., 1995; Chinnaiyan et al., 1995), which are present in both the intra-cellular region of FAS and FADD (Figure 1). The recruitment of caspase-8 by FADD is mediated (at least in part) by homotypic interaction of ‘death effector domains’ (DED) (Boldin et al., 1996; Medema et al., 1997; Muzio et al., 1996), which are present in both proteins (Figure 1). Studies with gene-targeted mice and transgenic mice expressing a dominant negative mutant of FADD (FADD-DN) or a viral enzymatic inhibitor of caspase-8 (CRMA: cytokine response modifier A) demonstrated that FADD mediated activation of the proteolytic activity of caspase-8 is essential for FAS-induced apoptosis in many (possibly all) cell types (including lymphoid and other hemopoietic ones) both in vitro and within the whole animal (Kang et al., 2004; Kang et al., 2008; Newton et al., 1998; Salmena et al., 2003; Smith et al., 1996; Varfolomeev et al., 1998; Zhang et al., 1998). It is noteworthy that in human cells FAS activation also causes recruitment of caspase-10 into the DISC and in certain cell lines caspase-10 activation was reported to contribute to apoptosis signaling (Kischkel et al., 2001; Sprick et al., 2002). Mice do not have a gene encoding caspase-10 and because the activation and function of caspase-10 are thought to be very similar to those of caspase-8, we will not deal with caspase-10 in detail in this review (for further information refer to (Boatright et al., 2004)). Caspase-8 activation within the DISC occurs in two steps. Firstly, recruitment of FADD to the intra-cellular region of FAS promotes dimerization and a conformational change in caspase-8 within the DISC that allows caspase-8 to gain full enzymatic activity (Boatright et al., 2003). Secondly, active caspase-8 undergoes auto-proteolytic processing, which allows the enzyme to leave the DISC and gain access to substrates in other cellular compartments (Boatright et al., 2003). Experiments with caspase-8 deficient (Casp8−/−) mice carrying a BAC transgene encoding WT caspase-8 or a mutant of caspase-8 that has full enzymatic activity but cannot cleave itself demonstrated that auto-proteolysis of caspase-8 is essential for FAS-induced apoptosis, at least in lymphoid cells and hepatocytes (Kang et al., 2008). This indicates that upon activation, caspase-8 must leave the DISC to be able to trigger apoptosis, probably so that it can gain access to critical proteolytic substrates. Active caspase-8 can proteolytically activate downstream effector caspases, such as caspase-3 and caspase-7 (Figure 1), but (at least theoretically) this may also directly contribute to cell demolition by proteolysis of certain cellular proteins (Stennicke et al., 1998). During the later stages of apoptosis, effector caspases perform the bulk of the proteolysis of vital cellular proteins, such as structural components (e.g. lamins, gelsolin), and they can also activate certain processes that dismantle non-proteinaceaous cellular constituents (Salvesen and Dixit, 1997). For example, effector caspase-mediated cleavage of ICAD (inhibitor of caspase activated DNase), which acts as both a chaperone and inhibitor of CAD (caspase activated DNase), is critical for inter-nucleosomal DNA cleavage, a hallmark of apoptosis (Enari et al., 1998).
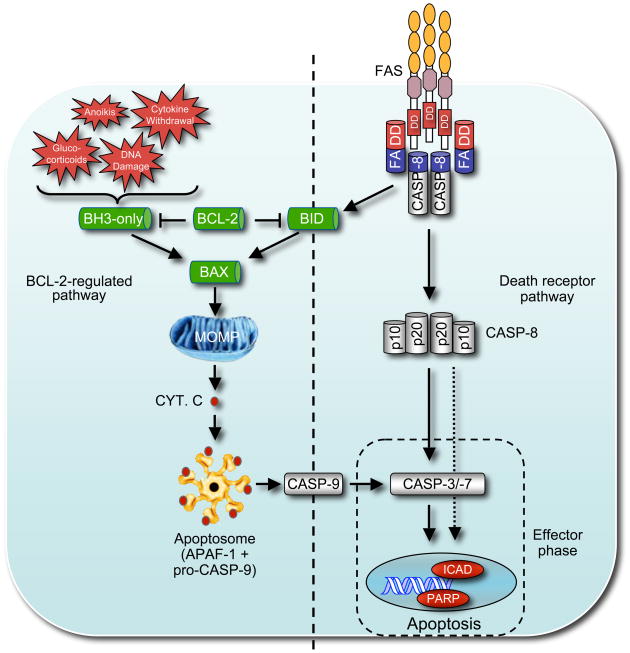
Figure 1.
This figure shows the two distinct but ultimately converging apoptosis signaling pathways in mammals. One is activated by so-called ‘death receptors’, members of the tumor necrosis factor receptor (TNF-R) family with an intra-cellular ‘death domain’ and requires FADD-mediated activation of caspase-8 (shown on the right). Auto-processing of caspase-8 results in a hetero-tetrameric form of the protein that is able to activate the zymogens of ‘effector’ caspases, caspase-3 and caspase-7, eventually leading to cleavage of vital proteins within the cell, such as PARP or lamins, or activation of the DNAse CAD (caspase-activated DNAse) by cleavage of its inhibitor, ICAD (inhibitor of CAD). It is possible that caspase-8 may also proteolyze to a certain extent some of the classical substrates of effector caspases. The ‘BCL-2-regulated’ pathway (shown on the left) is arbitrated by the complex interplay between pro- and anti-apoptotic members of the BCL-2 protein family and involves mitochondrial outer membrane permeabilization (MOMP). MOMP results in release of cytochrome C from the inter-membrane space of the mitochondria into the cytosol to initiate apoptosome formation resulting in APAF-1-mediated activation of the ‘initiator’ caspase, caspase-9, further leading to proteolytic activation of the downstream ‘effector’ caspases, caspase-3 and caspase-7. Abbreviations: BH3-only: BCL-2 homology domain 3 only protein; MOMP: mitochondrial outer membrane permeabilization; Cyt. C: cytochrome C. ICAD: Inhibitor of caspase activated DNAse (CAD); PARP: Poly (ADP-ribose) polymerase.
The c-FLIP protein, which structurally resembles caspase-8 (presence of DED but no enzymatic activity), appears to affect FAS-induced apoptosis in opposite ways depending on the extent of its expression (Figure 1). At low concentration it is thought to promote caspase-8 activation, possibly because caspase-8 binds to c-FLIP with higher affinity than to itself (i.e. in caspase-8 homo-dimerization). In contrast, at high concentration c-FLIP reduces the proteolytic activity of caspase-8, possibly by competing for binding to FADD, and thereby inhibits apoptosis (Boatright et al., 2004; Chang et al., 2002; Micheau et al., 2002). Studies with gene-targeted mice confirmed that c-FLIP has a dual role, since its selective loss in T lymphocytes or fibroblasts accelerated their Fas-induced apoptosis (Zhang and He, 2005) while loss of c-FLIP in all tissues pheno-copied the embryonic lethality seen in Casp8−/− mice (Yeh et al., 2000), which is caused by loss of a non-apoptotic function of caspase-8 (Varfolomeev et al., 1998).
The pro-apoptotic BH3-only family member BID is a critical substrate of caspase-8. Its proteolysis allows the truncated BID (tBID) to translocate from the cytosol to the outer mitochondrial membrane, thereby unleashing its pro-apoptotic activity (Figure 1) (Li et al., 1998; Luo et al., 1998). BH3-only proteins (BID, BAD, BIK (also called BLK or NBK), HRK (also called DP5), BIM (also called BOD), NOXA, Puma (also called BBC3) and BMF) form one of the two pro-apoptotic sub-groups of the BCL-2 protein family. Their name derives from the fact that they share with each other and the BCL-2 family at large only the ~16-24 aa BH3 region (Huang and Strasser, 2000). The BH3-only proteins are essential for initiation of developmentally programmed cell death and stress-induced apoptosis and they are activated in a death stimulus- as well as cell type-specific manner (Huang and Strasser, 2000; Puthalakath and Strasser, 2002). BAX, BAK and BOK belong to the second pro-apoptotic (often called the ‘BAX-BAK’ or ‘multi-BH domain’) subgroup of the BCL-2 family. These proteins contain three BH (BCL-2 homology) regions and although they share surprisingly extensive structural similarity to their pro-survival relatives (Suzuki et al., 2000), they are required for mitochondrial outer membrane permeabilization (MOMP) and activation of the caspase cascade in the ‘BCL-2-regulated’ (also called ‘mitochondrial’ or ‘intrinsic’) apoptosis signaling pathway (Green and Kroemer, 2004). The anti-apoptotic BCL-2 family members – BCL-2, BCL-XL, BCL-W, A1 and MCL-1 – share with each other up to four regions of homology (BH1, 2, 3 and 4) and are essential for cell survival, functioning in a cell type-specific manner (Youle and Strasser, 2008). There are currently two models to explain the functional interactions of the three BCL-2 sub-groups in apoptosis signaling. The ‘direct model’ suggests that the BH3-only proteins bind and thereby activate BAX and/or BAK directly and that the pro-survival relatives function to bind and sequester the BH3-only proteins (Green and Kroemer, 2004). The ‘indirect model’ proposes that in healthy cells BAX and BAK are kept in check by the pro-survival BCL-2 family members and that BH3-only proteins, when induced by apoptotic stimuli, bind with higher affinity to the pro-survival BCL-2-like proteins, thereby unleashing BAX and/or BAK (Youle and Strasser, 2008). Regardless, experiments with gene-targeted mice and biochemical studies have shown that caspase-8 mediated proteolytic activation of BID is essential for FAS-induced killing in hepatocytes (within the whole animal) (Kaufmann et al., 2007; Yin et al., 1999) and pancreatic β cells (in culture) (McKenzie et al., 2008). In contrast, BID is dispensable for Fas-induced apoptosis in lymphoid cells (both in culture and within the whole animal) and accordingly, in contrast to FAS- or FASL-deficient mice, Bid−/− mice do not develop lymphadenopathy or autoimmunity (Kaufmann et al., 2009; Kaufmann et al., 2007). The reasons for this discrepancy in FAS-induced apoptosis signaling between so-called type 1 (e.g. lymphocytes) and type 2 cells (e.g. hepatocytes) are presently unclear (Scaffidi et al., 1998), but they may be due to differences in FAS aggregation or internalization or differences in the extent of caspase activation or the amount of substrates that must be proteolyzed for the cells to die (Peter et al., 2007).
Some time ago, it was proposed that FAS-induced apoptosis occurs not only when FASL expressing killer cells engage FAS+ target cells, but also when cells are treated with certain chemotherapeutic drugs or γ-radiation (Bennett et al., 1998; Debatin and Krammer, 2004; Friesen et al., 1996). It was suggested that these cytotoxic stimuli, induce FASL expression (via ceramide induction) (Herr et al., 1997) and/or increase surface membrane deposition of Fas via a P53 dependent mechanism, both processes ultimately leading to autocrine or paracrine FASL-FAS-induced apoptosis (Bennett et al., 1998). Studies with cells from Faslpr/lpr mice did, however, show that γ-irradiation and chemotherapeutic drugs do not require Fas to trigger apoptosis (Newton and Strasser, 2000; O’Connor and Strasser, 1999). Moreover, experiments with primary cells from mutant mice lacking FADD (Newton et al., 1998; Newton and Strasser, 2000; Yeh et al., 1998; Zhang et al., 1998) or caspase-8 function (Salmena et al., 2003; Smith et al., 1996; Varfolomeev et al., 1998) demonstrated that ‘death receptor’ signaling in toto is not required for these pathways to apoptosis. Although we cannot rule out the possibility that the mechanisms regulating apoptosis differ markedly among distinct tumor cells, expression of dominant-negative FADD, CRMA or c-FLIP had no impact on chemotherapeutic drug-induced killing of a collection of lymphoma-derived cell lines, demonstrating that FADD and caspase-8 are dispensable for this process in at least certain cancers (Kataoka et al., 1998; Strasser et al., 1995; Strasser et al., 2000). We surmise that increased expression of FAS, FASL and certain other members of these receptor and ligand families in chemotherapeutic drug-treated or γ-irradiated cells is a consequence of cellular stress. Although this process is not required for the death of these cells, it may be exploited therapeutically as illustrated by the synergistic effects of TRAIL (APO-2L, a TNF cytokine family member) and certain chemotherapeutic drugs (e.g. 5-fluoro-uracil) in the killing of certain tumor cells (Ashkenazi et al., 1999). Collectively, these observations demonstrate that FADD-mediated recruitment and activation of caspase-8 are essential for FAS- and all ‘death receptor’-induced apoptosis and that the BH3-only protein BID is critical for this cell killing in some, albeit not all, cell types.
The role of FASL-FAS induced apoptosis in normal as well as pathological killing of target cells
Both classical CD8+ cytotoxic T cells as well as a portion of activated CD4+ T cells express FASL on their surface in membrane-bound form (mFASL) (Trambas and Griffiths, 2003). Cleavage by a metallo-protease causes shedding of FASL and the resulting soluble FASL, sFASL, can still bind FAS (Tanaka et al., 1998; Tanaka et al., 1995). Studies with various forms of FASL that were produced in E. coli or transfected mammalian cell lines have indicated that mFASL kills target cells via FAS activation much more potently than sFASL (Hohlbaum et al., 2000; Schneider et al., 1998; Suda et al., 1997; Tanaka et al., 1998). This is consistent with the notion that extensive aggregation of multiple pre-assembled FAS trimers and not only FASL trimer-FAS trimer interaction is required for apoptosis induction (Siegel et al., 2000). There is also some confusion in the literature based on the findings that some cultured cell lines shed mFASL-bearing vesicles into the supernatant. Although such vesicles can kill FAS+ cells in vitro (Schneider et al., 1998), the physiological relevance of this process is currently unclear.
Comparison of T cells lacking both functional FASL and perforin (Faslgld/gldPrf1−/−) with Prf1−/− T cells demonstrated that these two cell death inducing proteins account for most killing activity of cytotoxic T cells and NK cells (Kägi et al., 1994; Kojima et al., 1994; Lowin et al., 1994), possibly with some additional contribution by TNFα. While FASL normally contributes to the killing of virus-infected, damaged or excess cells, abnormally increased FASL-induced apoptosis of cells that should not be killed has been implicated as a cause of certain immuno-pathological disorders. For example, it has been shown that acute graft-versus-host (GvH) disease in mice can be prevented by combined treatment with neutralizing antibodies to FASL plus TNFα (Hattori et al., 1998; Mori et al., 1998). Moreover, FASL was found to be critical for the pathological killing of hemopoietic progenitors during cytomegalovirus (CMV) infection (Mori et al., 1997). Interestingly, transfer of lymphocytes from Faslpr/lpr mutant mice into congenic recipients elicits fatal GvH (Theofilopoulos et al., 1985). This is due to the fact that Fas-deficient T cells produce abnormally high amounts of mFASL, which kill FAS+ target cells within the host (Chu et al., 1995; Watanabe et al., 1995), because transfer of lymphocytes from FASL-FAS doubly deficient (Faslgld/gldFaslpr/lpr) mice does not cause GvH disease (Zhu et al., 2000). When the Faslpr mutation was crossed onto the NOD background, it was observed that such NOD-Faslpr/lpr mice do not develop diabetes and therefore concluded that FASL-FAS signaling is critical for the pathological destruction of islet β cells in type I diabetes (Chervonsky et al., 1997). The interpretation of this finding is, however, confounded by the complication that these animals rapidly develop profound lymphadenopathy and that Faslpr/lpr (and Faslgld/gld) mice actually become highly immuno-compromised when they develop lymphadenopathy (Cohen and Eisenberg, 1991).
Indeed, experiments in which islets from NOD-Faslpr/lpr mice were transplanted into NOD mice demonstrated that FASL-FAS signaling played only a minor role in β cell destruction in these diabetes-prone animals (Allison and Strasser, 1998). Conversely, experiments in which diabetogenic T cells from NOD mice were injected into NOD-Faslpr/lprscid/scid recipients (precluding excess FASL expression in the host) showed that FAS contributes to β cell destruction, at least within this context (Su et al., 2000). Analysis of perforin-deficient NOD mice revealed that as for the physiological killing of virus-infected target cells, the perforin-granzyme system plays a major role in the pathological killing of β cells in type I diabetes (Kägi et al., 1997). On the basis of all these data we conclude that perforin plays the principal and FASL a contributory role in β cell destruction during the development of type I diabetes.
Although FASL has been widely reported to be expressed predominantly (perhaps exclusively) in antigen-stimulated T lymphocytes (both CD8+ and also some CD4+ T cells) and NK cells (Krammer, 2000; Mabrouk et al., 2008; Nagata, 1997; Trambas and Griffiths, 2003), some reports indicated that in certain tissues, such as the testis, thyroid or the eye, non-hemopoietic cells can express FASL and thereby kill infiltrating T cells to establish an immune privileged niche (Bellgrau et al., 1995; Griffith et al., 1995). However, subsequent studies using transplantation of pancreatic islets transgenically engineered to express FASL ectopically indicated that FASL expressed on non-hemopoietic cells may not be critical to establish immune privilege (Allison et al., 1997; Kang et al., 1997). It has also been proposed that FASL expression on cancer cells may render them refractory to immune attack by engendering them with the ability to kill FAS+ tumor infiltrating lymphoid and myeloid cells, but this so-called “tumor counter-attack” hypothesis has also been questioned (Restifo, 2000). Indeed, enforced expression of FASL on certain tumour cells was found to enhance rather than delay their destruction in transplant recipients (Igney et al., 2000). At least some of the confusion concerning the expression of FASL may be due to the use of unreliable reagents for its detection (Fiedler et al., 1998; Smith et al., 1998; Stokes et al., 1998). The generation of gene-targeted knock-in mice (and tumors derived from them) with inducible deletion of the Fas (Hao et al., 2004) or Fasl (Mabrouk et al., 2008) genes are expected to resolve some of these controversies.
The role of FASL-FAS induced apoptosis in lymphocyte homeostasis
Mice lacking FAS or FASL develop progressive lymphadenopathy, which predominantly involves the so-called ‘unusual’ (Thy-1+CD4−CD8−TCRα/β+B220+) T cells, which are thought to derive from conventional T cells that have been repeatedly activated in vivo via their TCR complex (Renno et al., 1996). These mutant mice do, however, also accumulate several-fold increases in conventional CD4+ as well as CD8+ T lymphocytes and B lymphocytes (Cohen and Eisenberg, 1991; Krammer, 2000; Nagata, 1997). This led to the conclusion that FASL-FAS signaling plays a critical role in the homeostasis of the lymphoid system, most likely by killing unwanted cells at one or several developmental checkpoints. During both B as well as T lymphopoiesis in primary lymphoid organs apoptosis plays a critical role in the killing of cells that are either ‘useless’ or ‘potentially dangerous’ (Marsden and Strasser, 2003; Opferman, 2007; Strasser, 2005). For example, immature lymphoid cells that failed to productively rearrange the V, (D) and J gene segments encoding one of their IG or TCR chains and therefore cannot express surface antigen receptors (BCR or TCR) are deleted as are those expressing antigen receptors that bind self-antigens with high affinity (Marsden and Strasser, 2003; Opferman, 2007; Strasser, 2005). Because TCR stimulation induced apoptosis (AICD) of T lymphoma derived cell lines requires FASL-FAS signaling (Brunner et al., 1995; Dhein et al., 1995; Strasser, 1995), it was hypothesized that FAS-induced apoptosis may be critical for the deletion of autoreactive thymocytes and immature B cells in the bone marrow. Although some studies provided evidence that FAS is essential for the deletion of thymocytes specific for so-called endogenous or experimentally applied super-antigens (antigens that are presented by antigen presenting cells (APC) to T cells as full proteins associated with MHC molecules and not like conventional antigens as peptide fragments embedded within the MHC) (Kishimoto et al., 1998), this is controversial (Villunger et al., 2004). More definitive experiments using TCR or BCR transgenic mouse models confirmed that FASL and FAS are dispensable for the deletion of auto-reactive thymocytes (Sidman et al., 1992; Singer and Abbas, 1994) as well as auto-reactive B cells developing in the bone marrow (Rubio et al., 1996), respectively. Further studies with TCR transgenic mice showed that FAS is also not required for the rapid death of naïve T cells that occurs upon their transfer into hosts expressing the cognate antigen (Davey et al., 2002). In fact, studies using transgenic or gene-targeted mice that lack FADD (Newton et al., 1998) or caspase-8 function (Salmena et al., 2003; Smith et al., 1996) selectively in T lymphoid cells, showed that ‘death receptor’-induced apoptosis signaling in toto is not required for deletion of auto-reactive thymocytes. Instead the process for killing auto-reactive T and B cells during their development and even in their mature state relies on the ‘BCL-2 regulated’ apoptotic pathway (Hartley et al., 1993; Strasser et al., 1991a; Strasser et al., 1991b) and is initiated by the pro-apoptotic BH3-only protein BIM (Bouillet et al., 2002; Davey et al., 2002; Enders et al., 2003).
Programmed cell death also plays a role in the shut-down of cellular as well as humoral immune responses (Sprent and Tough, 2001; Strasser and Pellegrini, 2004) (Figure 2). In the case of acute immune responses, such as infection with a non-persisting pathogen (or injection with a degradable antigen), foreign antigen-specific T and B cells can expand several hundred-fold in numbers (Sprent and Tough, 2001). Most differentiate into effector cells (cytotoxic as well as helper T cells and antibody producing plasma cells), which help overcome the pathogens by killing them, either directly or indirectly through cytokine-mediated activation of cells of the innate immune system. A small number of activated B and T cells differentiate into so-called ‘memory’ cells, which are long-lived and able to respond rapidly in response to challenge with the same pathogen, thereby providing the organism with long-lasting immunity (Sprent and Tough, 2001). After pathogens have been defeated, effector cells are no longer needed and in fact potentially dangerous, so most undergo programmed cell death (Sprent and Tough, 2001; Strasser and Pellegrini, 2004). This apoptosis restores normal cellularity in peripheral lymphoid organs, thereby making space for the development of immune responses to subsequent infectious challenges. In addition, apoptosis of activated lymphocytes minimizes the collateral damage to healthy tissues that can be caused by the immune effector molecules (e.g. inflammatory cytokines, immune complexes, perforin-granzymes) (Sprent and Tough, 2001; Strasser and Pellegrini, 2004). In the case of chronic infections with persistent antigenic challenge, the killing of activated lymphocytes is critical to achieve a state of co-habitation between the pathogen and the host, so that the numbers of pathogens are kept at an acceptable titre and at the same limiting immune activation to minimize inadvertent destruction of vital tissues (Sprent and Tough, 2001; Strasser and Pellegrini, 2004).
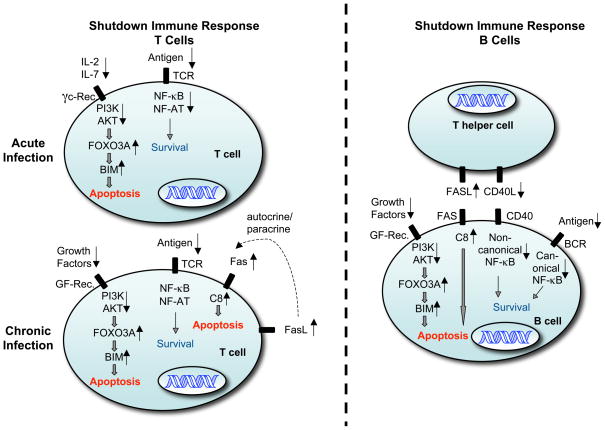
Figure 2.
This figure shows the mechanisms that control FASL-FAS induced apoptosis and BIM-dependent ‘BCL-2-regulated’ apoptosis in activated T and B cells during shutdown of acute or chronic immune responses.
During shutdown of an acute T cell immune response (left side, top panel), the reduction in the concentrations of cytokines, such as IL-2 and IL-7, leads to a reduction in PI3K and AKT. This causes activation of the transcription factor FOXO3A, which promotes transcriptional induction of the pro-apoptotic BH3-only protein BIM and consequently activation of the ‘BCL-2-regulated’ apoptotic pathway. Furthermore, reduced antigen concentration leads to diminished TCR stimulation and consequently reduced activation of the REL-NF-κB and NF-AT transcription factors, which are known to promote expression of pro-survival proteins, such as BCL-2 or BCL-XL.
As in shutdown of acute T cell immune responses, during shutdown of a chronic T cell response (left side, bottom panel) the availability of growth factors is also reduced, leading to the activation of BIM and initiation of the ‘BCL-2-regulated’ apoptotic pathway as described above. In addition, repeated TCR ligation triggers FASL expression on T cells leading to autocrine and/or paracrine stimulation of FAS, caspase-8 activation, activation of ‘effector’ caspases and consequently apoptosis as shown in Figures 1.
During shutdown of a B cell immune response (left side) FAS is induced on B cells and can be activated by FASL presented on T helper cells. This leads to B cell apoptosis when CD40 signaling is diminished due to reduced expression of CD40L on T helper cells, because this causes a reduction in the activity of the REL/NF-κB and NF-AT transcription factors, which normally promote expression of pro-survival proteins, such as c-FLIP. In addition, a reduction in the concentration of growth factors can lead to BIM up-regulation as described above.
Abbreviations: γc Receptors: common gamma chain containing cytokine receptors (e.g. receptors for IL-2, IL-4, IL-7, IL-15); DISC: death inducing signaling complex; GF-Rec.: Growth factor receptors; TCR: T cell antigen receptor; PI3K: Phosphoinositide 3-kinases.
When FASL and FAS were discovered to be critical for TCR stimulation induced AICD of normal cycling T lymphoblasts (Alderson et al., 1995; Russell et al., 1993; Russell and Wang, 1993) and transformed T lymphoma-derived cell lines (Brunner et al., 1995; Dhein et al., 1995), it was widely speculated that FASL-FAS induced apoptosis would also prove to be essential for the death of antigen-activated T and B cells during shutdown of immune responses. For the removal of activated T cells during an acute immune response this model makes, however, little sense. The induction of FASL on T lymphoid cells undergoing AICD requires TCR stimulation, but during termination of acute immune responses T cells die after pathogens have been expelled, a time when no antigen is present to activate the TCR (Strasser and Pellegrini, 2004). Indeed, although it was initially reported that FASL and FAS are critical for the killing of activated T cells during termination of acute immune responses (e.g. in TCR transgenic mice injected with the cognate peptide; reviewed in (Van Parijs and Abbas, 1998)), later experiments with mice injected with a single dose of the super-antigen staphylococcus enterotoxin B (SEB) (Hildeman et al., 2002) or challenged with herpes simplex virus (HSV, causing nonpersistent infection) (Pellegrini et al., 2003) demonstrated that FAS and TNF-R1 ‘death receptor’-signaling are dispensable for this process. In fact the killing of antigen-activated T cells during termination of acute immune responses appears to be initiated by a decline in the levels of cytokines that promote their survival (e.g. IL-2, IL-7) (Vella et al., 1998), which triggers the ‘BCL-2-regulated’ apoptotic pathway (Strasser et al., 1991a) through activation of the BH3-only protein BIM (Figure 2) (Hildeman et al., 2002; O’Connor et al., 1998; Pellegrini et al., 2003) and to a lesser extent PUMA (Fischer et al., 2008). In contrast to acute immune responses, pathogens and their antigens persist during chronic immune responses, thereby potentially facilitating repeated stimulation of already activated T lymphocytes through their TCR, which would then lead to FASL up-regulation and their FAS-induced suicide or fratricide (Krammer, 2000; Nagata, 1997; Strasser, 1995). Indeed, the first clear demonstration for a role of FAS-induced apoptosis in the shut-down of a T cell immune response came from studies in which mice were repeatedly injected with SEB (Kawabe and Ochi, 1991; Strasser et al., 1995), thereby mimicking a chronic infection. More recently, experiments with mice infected with persistent viruses (e.g. LCMV or mouse gamma herpes virus) showed that the killing of activated T cells during the ensuing chronic immune response requires both FASL-FAS and the pro-apoptotic BH3-only BCL-2 family member BIM (Figure 2) (Hughes et al., 2008; Hutcheson et al., 2008; Weant et al., 2008). Thus, their death appears to be caused by FASL-FAS signaling, triggered by repeated TCR stimulation, and by the ‘BCL-2-regulated’ apoptotic pathway, which presumably is initiated by growth factor deprivation-induced induction of BIM.
B lymphoid cells express Fas on their surface and can be killed by treatment with FASL or agonistic FAS-specific antibodies (Krammer, 2000; Nagata, 1997) and, accordingly, FASL-FAS signaling was found to play a critical role in the control of humoral immune responses (Figure 2). The consequences of FAS activation in B cells are critically influenced by the activity of other signaling pathways. BCR crosslinking or CD40 stimulation, particularly their combination, protect B lymphocytes from FAS-induced apoptosis (Lagresle et al., 1996; Rothstein et al., 1995). This protection appears to be mediated by activation of REL-NF-κB transcription factors (Schram and Rothstein, 2003), which activate expression of c-FLIP (Hennino et al., 2001). In fact, in combination with BCR-crosslinking and CD40 activation, FAS ligation may actually contribute to activation, proliferation and differentiation of B cells, rather than trigger their apoptosis (Figure 2, and see below).
Germinal centers are anatomical niches within secondary lymphoid organs composed of B cells, specific CD4+ T helper subsets and follicular dendritic cells (Tarlinton, 1998). In these locations, B cells and intra-follicular CD4+ T cells recognizing the same antigenic compound are activated by interaction with each other and antigen retained on the follicular dendritic cells. This results in stimulation of B cells leading to their proliferation, hyper-mutation of the rearranged and expressed Ig variable region genes and differentiation into antibody-secreting plasma cells on one hand and long-lived memory B cells on the other (Tarlinton, 1998). During and after the germinal center reaction, activated B lymphocytes are subject to stringent selection. Only those B cells expressing high-affinity antibodies for the antigen (due to Ig V gene somatic hyper-mutation) can compete successfully for the limiting amount of antigen and T cell help (CD40L) and therefore gain survival signals through their BCR and CD40 – low affinity antibody expressing B cells die by neglect (Tarlinton, 1998). Theoretically, IGV gene hyper-mutation can give rise to B cells expressing BCRs specific to self-antigens and experiments with BCR transgenic mice showed that such auto-reactive B cells are deleted by apoptosis (Pulendran et al., 1995; Shokat and Goodnow, 1995). FASL and FAS play critical roles in the control of B lymphocyte survival during the germinal center reaction (Figure 2) (Krammer, 2000; Nagata, 1997). Activated B lymphocytes bearing low-affinity BCR are killed when FAS on their surface is ligated by FASL expressed on intra-follicular CD4+ T cells and they fail to receive a pro-survival signal through their BCR and CD40 (Figure 2) (Rathmell et al., 1996). In experimental models for deletion of auto-reactive B lymphocytes within germinal centers, it was found that both FAS ‘death receptor’-signaling (Rathmell et al., 1995) as well as the ‘BCL-2-regulated’ apoptotic pathway (Pulendran et al., 1995) contribute to B cell killing (Figure 2). This is reminiscent of the finding that both of these pathways (the latter initiated by the pro-apoptotic BH3-only protein BIM) contribute to the killing of T cells that are chronically activated by pathogen-derived antigens or self-antigens (Hughes et al., 2008; Hutcheson et al., 2008; Weant et al., 2008). In further support of a collaboration of the FAS ‘death receptor’ and the ‘BCL-2-regulated’ apoptotic pathways in the deletion of auto-reactive B and T cells in peripheral lymphoid organs and establishment of immunological tolerance, it was found that BCL-2 over-expression (Reap et al., 1995; Strasser et al., 1995; Tamura et al., 1996) or loss of BIM (Hughes et al., 2008; Hutcheson et al., 2008; Weant et al., 2008) greatly accelerate and enhance lymphadenopathy and autoimmunity in FAS- or FASL-deficient mice. BIM was shown to play a critical role in the killing of activated B cells and IG-secreting plasma cells during shut-down of acute humoral immune responses (Fischer et al., 2007), and this BH3-only protein is probably activated by the decline in the concentration of growth factors (Figure 2).
Collectively, these results demonstrate that FAS- and ‘death receptor’-signaling in toto play no role in the deletion of autoreactive B or T cells during their development in primary lymphoid organs. Instead these pathways are critical, either alone or in combination with the ‘BCL-2-regulated’ apoptotic pathway, for the killing of antigen-activated lymphocytes during termination of immune responses.
Unexpected lessons from deletion of Fas in select cell types
Because FAS is expressed on a broad range of lymphoid, myeloid and also non-hemopoietic cell types, it is not immediately clear in which cells FAS function must be lost to cause the lymphadenopathy and autoimmunity observed in FAS-deficient mice and humans (Krammer, 2000; Nagata, 1997). This important question has been addressed by generating FAS-deficient mice (Faslpr/lpr) that express a FAS transgene in either B and/or T cells (Fukuyama et al., 1998, 2002; Komano et al., 1999) or mice in which the sequences within the Fas gene that encode the ‘death domain’ have been flanked by loxP sites and can therefore be deleted in a cell type-specific or inducible manner (using transgenic mice expressing the CRE recombinase under control of a cell type-specific or inducible promoter) (Hao et al., 2008; Hao et al., 2004). When interpreting data from the latter mice (and the Fas−/− mice (Adachi et al., 1995)), it is important to remember that cells that have undergone CRE-mediated deletion of these sequences can still express FAS (devoid of the ‘death domain’) on their surface. It has been speculated that such a truncated FAS may still be able to exert some non-apoptotic functions either directly, or indirectly by so-called ‘reverse signaling’ via FASL ((Peter et al., 2007); see also below). It must also be borne in mind that several of these experiments were performed on different inbred or even mixed genetic backgrounds, so the results may not always be directly comparable, because the impact of the Faslpr and the Faslgld mutations are greatly affected by genetic background (Cohen and Eisenberg, 1991). Nonetheless, these studies revealed many interesting aspects of the role of FASL-FAS signaling in the control of the normal immune system and prevention of autoimmunity (see references above). Transgenic expression of normal amounts of FAS in T cells of Faslpr/lpr mice prevented lymphadenopathy, in particular the accumulation of the ‘unusual’ TCRα/β+CD3+CD4−CD8−B220+ T cells, but these animals still produced auto-antibodies and developed SLE-like glomerulonephritis (Fukuyama et al., 1998). In contrast, transgenic expression of FAS on B cells protected Faslpr/lpr mutant mice from SLE-like autoimmunity although abnormal accumulation of T cells, including TCRα/β+CD3+CD4−CD8−B220+ ones, was not affected (Komano et al., 1999). Remarkably, with increasing age these mice developed a severe deficit in B cells and antibody-secreting plasma cells and this appeared to be due to the excess FASL produced by the abnormally accumulating T cells (Chu et al., 1995; Watanabe et al., 1995) triggering apoptosis of B lymphoid cells via the transgene-encoded FAS (Komano et al., 1999). Generation of Faslpr/lpr mutant mice that lack B cells because they carry a homozygous deletion of the JH gene locus (precluding IGH expression) showed that B cells are required for the SLE-like autoimmunity but dispensable for the T cell associated lymphadenopathy that is caused by defects in FAS (Shlomchik et al., 1994).
Selective loss of FAS on T cells (in Cd4-Cre or lck-Cre transgenic FasloxP/loxP mice) caused progressive lymphopoenia on the C57BL/6 background albeit to a lesser extent on a (C57BL/6xMRL) F1 background (Hao et al., 2004). This cell loss was mediated by the excessive FASL produced by the FAS-deficient T lymphocytes (Chu et al., 1995; Watanabe et al., 1995), because it could be prevented by injection with neutralizing antibodies to FasL (Hao et al., 2004). B lymphocyte deletion in these animals was probably mediated directly, as they express FAS on their surface, but such a process cannot explain the loss of T cells, since they lack FAS. Their progressive disappearance may be due to an indirect mechanism, which involves FASL-mediated killing of FAS+ cells that deliver critical survival signals to T lymphocytes. B lymphocytes account for some, albeit clearly not all, of these survival signals, since T cell lymphadenopathy with CD4+, CD8+ as well as the ‘unusual’ TCRα/β+CD3+CD4−CD8−B220+ T cells is substantially diminished (although not abrogated) in B cell-deficient Faslpr/lpr mutant mice compared to control Faslpr/lpr animals (Shlomchik et al., 1994).
Loss of FAS on B cells or on both B cells plus T cells (in Cd19-Cre transgenic or Cd19-Cre-Cd4-Cre bi-transgenic FasloxP/loxP mice) elicited lymphadenopathy, hyper-gammaglobulinemia and auto-antibody production (Hao et al., 2008; Hao et al., 2004). All of these abnormalities developed in these animals, however, to a considerably lower extent and more slowly compared to animals with loss of FAS in all tissues or in all hemopoietic cells (Mx-Cre transgenic FasloxP/loxP mice injected with poly-IC). Interestingly, specific loss of FAS-mediated apoptosis in dendritic cells (using CD11c-CRE transgenic FasloxP/loxP mice) also elicits certain features of autoimmunity, such as lymphoid hyperplasia and production of anti-nuclear auto-antibodies (Stranges et al., 2007). Collectively, these results demonstrate that FAS imposes a barrier against lymphadenopathy and autoimmunity by acting not only in B and T cells but by functioning also in certain other hemopoietic and possibly non-hemopoietic cell types. A recent study (using IgHg1-Cre transgenic FasloxP/loxP mice, in which the Fas gene is deleted only in B cells that have switched from IGM to IGG production) has shown that FAS plays a particularly prominent role in the control of B as well as T cell homeostasis and auto-antibody production in germinal center B cells (Hao et al., 2008). All immune defects caused by loss of FAS in B cells could be prevented by loss of T cells (by deletion of the locus encoding the β chain of the TCR) or by blockade of the mutual co-stimulatory signals that are exchanged between activated B cells and activated CD4+ T cells within germinal centers (by deletion of the Cd28 gene) (Hao et al., 2008). These findings are consistent with the model (see also above) that antigen-stimulated B cells in germinal centers, which as a result of IGV gene somatic hyper-mutation express either a self-antigen specific BCR or a BCR with low affinity for the immunogen, are killed by encountering FASL on activated intra-follicular CD4+ T cells. It will be interesting to test this hypothesis by generating mutant mice that lack FASL exclusively within this T cell subset, and to examine how T cells recognize autoreactive B cells to kill them via FASL-FAS signaling.
Non-apoptotic activities of components the ‘death receptor’ signaling machinery
It is firmly established that several members of the TNF-R family, including some that are classified as ‘death receptors’ (e.g. TNF-R1), and their corresponding ligands exert (either exclusively or in addition to their pro-death activity) non-apoptotic functions, such as the induction of cellular activation, proliferation, differentiation or migration (Aggarwal, 2003; Ashkenazi and Dixit, 1998; Krammer, 2000; Nagata, 1997; Peter et al., 2007; Smith et al., 1994; Wallach, 1996) (Figure 3). Also for FAS activation, several investigations (even quite early ones in the course of studies of this receptor) have observed non-apoptotic consequences in a range of cell types. For example, FAS was reported to promote proliferation of human T lymphocytes (Alderson et al., 1993) as well as growth factor-deprived fibroblasts (Aggarwal et al., 1995) and maturation of dendritic cells (Rescigno et al., 2000) in culture. Perhaps most impressively (and intriguingly), although injection of agonistic FAS-specific antibodies (Ogasawara et al., 1993) or FASL causes fatal hepatitis in mice (Huang et al., 1999), FAS stimulation (using agonistic FAS-specific antibodies) was reported to accelerate liver regeneration in mice subjected to partial hepatectomy (Desbarats and Newell, 2000). Interestingly, a delay in liver regeneration was seen in Faslpr/lpr mutant mice (Desbarats and Newell, 2000) as well as TNF-R1-deficient animals (Yamada et al., 1997), implicating both of these ‘death receptors’ in this process. The mechanisms by which FAS ligation stimulates cell proliferation and/or maturation are presently unclear, but the REL/NF-κB and MAP kinase signaling pathways have both been implicated (Aggarwal, 2003; Peter et al., 2007). Interestingly, normal liver regeneration after partial hepatectomy was seen in Faslpr(cg)/lpr(cg) mutant mice although Faslpr/lpr mice exhibited a significant delay (Desbarats and Newell, 2000). The Faslpr(cg) mutation causes an amino acid substitution within the ‘death domain’ of FAS (Watanabe-Fukunaga et al., 1992) that is thought to prevent recruitment of FADD into the DISC. This may indicate that FAS induced cell growth, at least in hepatocytes, occurs by a mechanism that is independent of FADD and therefore probably also caspase-8. This idea is, however, contradicted by the finding that selective loss of caspase-8 in hepatocytes (in Alb-Cre transgenic Casp8loxP/loxP mice) impairs liver regeneration after partial resection (Ben Moshe et al., 2007).
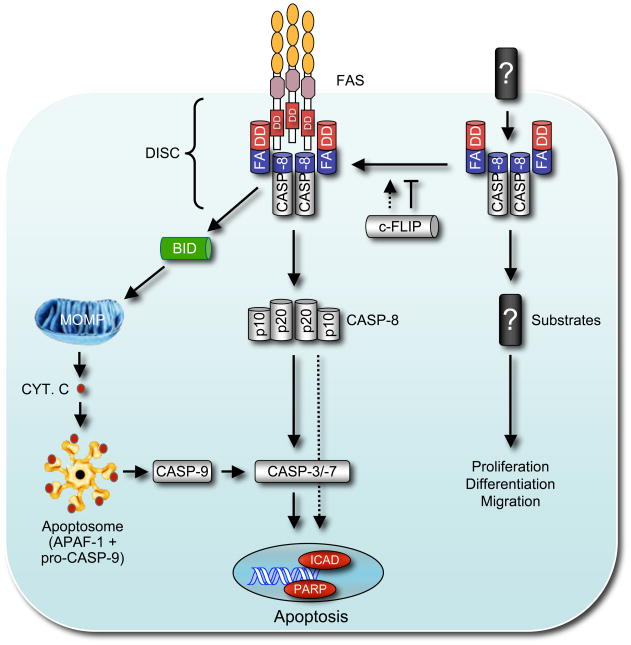
Figure 3.
This figure shows the two distinct modes of caspase-8 activation – the well-known ‘death receptor’ apoptotic pathway involving caspase-8 auto-processing (shown on the left) followed by either direct activation of effector caspases or proteolytc activation of the BH3-only protein BID to activate the ‘BCL-2-regulated’ apoptotic pathway (see also Figure 1). The caspase-8 related protein c-FLIP mainly acts as a catalytically inactive caspase-8 substitute competing for the binding to FADD, thereby limiting recruitment and activation of caspase-8 and thus blocking apoptosis initiation. However, c-FLIP has also been shown to promote caspase-8 recruitment and activation in certain circumstances. The right side illustrates the presently only poorly understood pathway by which FADD and caspase-8 promote cellular activation, proliferation and differentiation without the need of self-processing. For this pathway, neither the upstream activators (‘death receptors’?) nor the substrates of caspase-8 are identified. Abbreviations: DISC: death inducing signaling complex; MOMP: mitochondrial outer membrane permeabilization; Cyt. C: cytochrome C; c-FLIP: cellular form of FADD-like IL-1β-converting enzyme-inhibitory protein.
Although the physiological importance of non-apoptotic signaling pathways activated by Fas remains controversial (Aggarwal, 2003; Krammer, 2000; Nagata, 1997; Peter et al., 2007), it is now widely accepted that essential components of the ‘death receptor’ machinery also perform critical non-apoptotic roles (Newton and Strasser, 2003) (Figure 3). This was first discovered when it was observed that blocking the function of FADD does not only inhibit FASL-induced apoptosis of T lymphocytes but also impairs their activation and proliferation in response to mitogenic or antigenic stimulation (Newton et al., 1998; Newton et al., 2001; Zhang et al., 1998). Subsequently, it was found that FADD is also critical for pre-TCR-induced proliferation of T cell progenitors (pro-T3 and/or pro-T4 cells) in the thymus (Newton et al., 2000), TLR (Toll-like receptor)-mediated innate immune responses (Balachandran et al., 2004) (a process that notably also requires FADD in Drosophila (Hoffmann, 2003)), TLR-induced proliferation of B cells (Imtiyaz et al., 2006) and cytokine-induced proliferation of myeloid progenitors (Pellegrini et al., 2005). Experiments with gene-targeted mice in which the Casp8 gene was deleted in a cell type specific manner (using crosses with appropriate Cre transgenic mice) confirmed that most of these processes require not only FADD but also caspase-8 (Kang et al., 2004; Salmena et al., 2003). In addition, caspase-8 was found to be essential for cytokine-induced monocyte differentiation in culture (Kang et al., 2004) and, as already discussed above, for liver regeneration within the whole animal after partial hepatectomy (Ben Moshe et al., 2007). Remarkably, loss of caspase-8 (Varfolomeev et al., 1998), its activator FADD (Yeh et al., 1998) or its modulator c-FLIP (Yeh et al., 2000) in all tissues causes early embryonic lethality (~E10.5) due to non-apoptotic defects in vascular development and early hemopoiesis. It is presently not fully resolved whether these non-apoptotic processes require the enzymatic activity of caspase-8 or some other function of this protease, but there is evidence that the former may well be the case, at least for some. For example, in tissue culture enzymatic inhibitors of caspase-8 were found to impair mitogen- or antigen-induced T cell proliferation (Kennedy et al., 1999) and cytokine-induced proliferation of myeloid progenitors (Pellegrini et al., 2005). We anticipate that the generation of gene-targeted mice that contain a mutation that disables the catalytic activity of caspase-8 may resolve this issue.
Accepting that FADD-mediated activation of caspase-8 is essential for the aforementioned non-apoptotic processes, one must ask the following two questions: (1) how are FADD and caspase-8 activated during these processes? and (2) what intra-cellular non-apoptotic signaling pathways are triggered by caspase-8 (Figure 3)? Both of these important questions are currently unresolved and are considered by some as a ‘holy grail’. ‘Death receptors’ are the only presently known activators of FADD and caspase-8, and it is interesting to contemplate that low level (‘tonic’) autocrine or paracrine ‘death ligand’-’death receptor’ signaling may play a critical role in cell fate determination during embryogenesis and in other scenarios (see above). Such ‘tonic’ autocrine and/or paracrine TNF-TNF-R1 signaling has recently been found to play an essential role in the response of tumor cells to SMAC (second mitochondria-derived activator of caspases, also called DIABLO: direct inhibitor of apoptosis protein binding-protein with low pI) mimetic IAP (inhibitor of apoptosis protein) inhibitory drugs (Gaither et al., 2007; Petersen et al., 2007; Varfolomeev et al., 2007; Vince et al., 2007). There is, however, currently no evidence that ‘death receptors’ and their ligands are essential for their established non-apoptotic functions. None of gene-targeted mice lacking one or even two ‘death receptors’ (FAS, TNF-R1, DR3 or TRAIL-R) or ‘death ligands’ exhibit early embryonic lethality or defects in mitogen-induced activation and proliferation of B and/or T lymphocytes (reviewed in (Aggarwal, 2003; Newton and Strasser, 2003; Peter et al., 2007)). It remains, however, possible that there is greater functional overlap between ‘death receptors’ than currently anticipated and defects in vascular development during embryogenesis or mitogen-induced T cell proliferation, akin to those caused by loss of FADD or caspase-8, may only become apparent in mice lacking a combination of three or all four of these receptors or their ligands. Alternatively, there may be mechanisms for FADD and caspase-8 activation that are independent of ‘death receptors’ (Figure 3). Perhaps biochemical studies, using pull-down of FADD and/or caspase-8 containing complexes from cells in which these proteins fulfil a non-apoptotic function will be able to identify the mechanisms of their activation.
There is increasing evidence that FADD and caspase-8 must undergo different post-translational modifications and must be localized to different sub-cellular compartments depending on whether they mediate cell death (e.g. after treatment of T cells with FASL) or transduce cellular activation signals (e.g. after mitogenic stimulation of T cells) (O’Reilly et al., 2004). For example, a single amino acid within FADD was reported to be critical for its action in cell proliferation but apparently has no role in FADD-mediated apoptosis (Hua et al., 2003). Interestingly, T cells undergoing FASL-induced apoptosis contain a large amount of caspase-8 activity and most of it is found in the cytosol, whereas mitogenically activated T cells have considerably lower levels of caspase-8 activity, which is mostly concentrated in discrete foci at the plasma membrane (Koenig et al., 2008). Studies with mice expressing mutant forms of caspase-8 have shown that whilst auto-proteolysis is required for the ability of this caspase to mediate FASL-induced cell killing, this processing is dispensable for its non-apoptotic functions (Kang et al., 2008) (Figure 3). This is consistent with the notion that caspase-8 must be released from the DISC to gain access to critical substrates (i.e. the zymogens of the ‘effector’ caspases) within the cytosol to effect cell killing, but must be retained at the plasma membrane to mediate its non-apoptotic functions. Identification of the substrates that are cleaved by caspase-8 near the plasma membrane is expected to greatly advance our understanding of its function in non-apoptotic processes. Although one study reported that caspase-8 is essential for TCR-ligation induced REL/NF-κB activation (Su et al., 2005), others found that REL/NF-κB, MAP kinase and NFAT activation all occur normally in the absence of FADD or caspase-8 function in TCR- or TLR-stimulated T and B cells, respectively (Beisner et al., 2003; Imtiyaz et al., 2006; Newton et al., 2001; Salmena et al., 2003). It is also noteworthy, that for at least some of the processes in which FADD and caspase-8 play an essential non-apoptotic function, such as vascular development during embryogenesis, there is no evidence that REL/NF-κB plays a critical role. We find it remarkable how diverse the processes actually are in which FADD and caspase-8 play a critical non-apoptotic function, impacting on the responses of cells to ligation of cytokine receptors, antigen receptors or TLRs. Interestingly, many of the non-apoptotic processes in which FADD and caspase-8 play a role involve transition of cells from the quiescent (G0) into the cycling state. Therefore, and because pathways that are critical for cellular activation and proliferation (e.g. activation of REL/NF-κB, MAPK, NFAT) occur normally in their absence, we speculate that FADD and caspase-8 trigger a non-apoptotic function that modifies or facilitates the responses of other signaling pathways within cells. Alternatively (and not mutually exclusive), it is possible that FADD and caspase-8 play a critical role in the regulation of autophagy (Bell et al., 2008), a mechanism for procurement of energy and metabolites (e.g. in starved or stressed cells) that impacts on many cellular and developmental processes (Mizushima, 2005). As mentioned above, identification of binding partners for FADD and substrates of caspase-8 within mitogenically activated T cells or cytokine-stimulated monocytes may open an entire new area of investigation.
Collectively, these observations demonstrate that FADD and caspase-8 are not only essential for ‘death receptor’-induced apoptosis signaling but also have critical, albeit biochemically still ill-defined, roles in cellular activation, proliferation and differentiation.
Conclusions and Perspectives
We now have a very good framework of understanding of the role of FASL-FAS induced apoptosis in the control of the immune system and its critical function as a guardian against autoimmune disease and certain lymphoid malignancies. This knowledge is being exploited to develop novel cancer therapies, such as TRAIL-R-specific agonists or SMAC/DIABLO mimetics. Since many anti-cancer drugs have proven to be efficacious in the treatment of (certain) autoimmune diseases (e.g. Rituximab, CD20-specific antibodies), it is possible that the aforementioned compounds that are currently being developed, may also become useful in the treatment of such diseases.
Acknowledgments
Work in our laboratories was supported by fellowships and grants from the National Health and Medical Research Council (Australia; #257502, #461263 and #356214), the Leukemia and Lymphoma Society (New York; SCOR grant #7015) and the National Cancer Institute (NIH, US; CA 80188 and CA 43540), the Mildred Scheel-Stiftung/Deutsche Krebshilfe, the Ministry of Education, Science, Sports, and Culture in Japan (Specially Promoted Research) and the Japan Science and Technology Agency (SORST).
References
- Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nature Genetics. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Singh S, LaPushin R, Totpal K. Fas antigen signals proliferation of normal human diploid fibroblast and its mechanism is different from tumor necrosis factor receptor. FEBS Letters. 1995;364:5–8. doi: 10.1016/0014-5793(95)00339-b. [DOI] [PubMed] [Google Scholar]
- Alderson M, Armitage RJ, Maraskovsky E, Tough TW, Roux E, Schooley K, Ramsdell F, Lynch DH. Fas transduces activation signals in normal human T lymphocytes. Journal of Experimental Medicine. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. Journal of Experimental Medicine. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J, Georgiou HM, Strasser A, Vaux DL. Transgenic expression of CD95 ligand on islet β cells induces a granulocytic infiltration, but does not confer immune privilege upon islet allografts. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3943–3947. doi: 10.1073/pnas.94.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J, Strasser A. Mechanisms of β cell death in diabetes: a minor role for CD95. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13818–13822. doi: 10.1073/pnas.95.23.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. Journal of Clinical Investigation. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432:401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- Beisner DR, Chu IH, Arechiga AF, Hedrick SM, Walsh CM. The requirements for Fas-associated death domain signaling in mature T cell activation and survival. Journal of Immunology. 2003;171:247–256. doi: 10.4049/jimmunol.171.1.247. [DOI] [PubMed] [Google Scholar]
- Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA, Morrissette NS, Walsh CM. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- Ben Moshe T, Barash H, Kang TB, Kim JC, Kovalenko A, Gross E, Schuchmann M, Abramovitch R, Galun E, Wallach D. Role of caspase-8 in hepatocyte response to infection and injury in mice. Hepatology. 2007;45:1014–1024. doi: 10.1002/hep.21495. [DOI] [PubMed] [Google Scholar]
- Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Deis C, Denault JB, Sutherlin DP, Salvesen GS. Activation of caspases-8 and -10 by FLIP(L) Biochem J. 2004;382:651–657. doi: 10.1042/BJ20040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Molecular Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. Journal of Biological Chemistry. 1995;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X. c-FLIPL is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO Journal. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervonsky AV, Wang Y, Wong FS, Visintin I, Flavell RA, Janeway CA, Jr, Matis LA. The role of Fas in autoimmune diabetes. Cell. 1997;89:17–24. doi: 10.1016/s0092-8674(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chu JL, Ramos P, Rosendorff A, Nikolic-Zugic J, Lacy E, Matsuzawa A, Elkon KB. Massive upregulation of the Fas ligand in lpr and gld mice: implications for Fas regulation and the graft-versus-host disease-like wasting syndrome. J Exp Med. 1995;181:393–398. doi: 10.1084/jem.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Davey GM, Kurts C, Miller JF, Bouillet P, Strasser A, Brooks AG, Carbone FR, Heath WR. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. Journal of Experimental Medicine. 2002;196:947–955. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WF, Giese T, Fredrickson TN. Spontaneous development of plasmacytoid tumors in mice with defective Fas-Fas ligand interactions. Journal of Experimental Medicine. 1998;187:1825–1838. doi: 10.1084/jem.187.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- Desbarats J, Newell MK. Fas engagement accelerates liver regeneration after partial hepatectomy. Nature Medicine. 2000;6:920–923. doi: 10.1038/78688. [DOI] [PubMed] [Google Scholar]
- Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Drappa J, Vaishnaw AK, Sullivan KE, Chu JL, Elkon KB. Fas gene mutations in the Canale-Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. New England Journal of Medicine. 1996;335:1643–1649. doi: 10.1056/NEJM199611283352204. [DOI] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreative B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler P, Schaetzlein CE, Eibel H. Constitutive expression of Fas ligand in thyrocytes. Science. 1998;279 http://www.sciencemag.org/content/vol279/issue5359/
- Fischer SF, Belz G, Strasser A. BH3-only protein puma contributes to death of antigen-specific T cells during shut-down of an immune response to acute viral infection. Proc Natl Acad Sci U S A. 2008;105:3035–3040. doi: 10.1073/pnas.0706913105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SF, Bouillet P, O’Donnell K, Light A, Tarlinton DM, Strasser A. Pro-apoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody forming cells. Blood. 2007;110:3978–3984. doi: 10.1182/blood-2007-05-091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middelton LA, Lin AY, Strober W, Lenardo MJ, Puck JM. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Friesen C, Herr I, Krammer PH, Debatin KM. Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Medicine. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Adachi M, Suematsu S, Miwa K, Suda T, Yoshida N, Nagata S. Transgenic expression of Fas in T cells blocks lymphoproliferation but not autoimmune disease in MRL-lpr mice. Journal of Immunology. 1998;160:3805–3811. [PubMed] [Google Scholar]
- Fukuyama H, Adachi M, Suematsu S, Miwa K, Suda T, Yoshida N, Nagata S. Requirement of Fas expression in B cells for tolerance induction. European Journal of Immunology. 2002;32:223–230. doi: 10.1002/1521-4141(200201)32:1<223::AID-IMMU223>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J, Sage D, Slisz J, Tran M, Straub C, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- Hao Z, Duncan GS, Seagal J, Su YW, Hong C, Haight J, Chen NJ, Elia A, Wakeham A, Li WY, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Hampel B, Yagita H, Rajewsky K. T Cell-specific Ablation of Fas Leads to Fas Ligand-mediated Lymphocyte Depletion and Inflammatory Pulmonary Fibrosis. J Exp Med. 2004;199:1355–1365. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Hattori K, Hirano T, Miyajima H, Yamakawa N, Tateno M, Oshimi K, Kayagaki N, Yagita H, Okumura K. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood. 1998;91:4051–4055. [PubMed] [Google Scholar]
- Hennino A, Bérard M, Krammer PH, Defrance T. FLICE-inhibitory protein is a key regulator of germinal center B cell apoptosis. Journal of Experimental Medicine. 2001;193:447–458. doi: 10.1084/jem.193.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr I, Wilhelm D, Böhler T, Angel P, Debatin KM. Activation of CD95 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO Journal. 1997;16:6200–6208. doi: 10.1093/emboj/16.20.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Hohlbaum AM, Moe S, Marshak-Rothstein A. Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. Journal of Experimental Medicine. 2000;191:1209–1219. doi: 10.1084/jem.191.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua ZC, Sohn SJ, Kang C, Cado D, Winoto A. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity. 2003;18:513–521. doi: 10.1016/s1074-7613(03)00083-9. [DOI] [PubMed] [Google Scholar]
- Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, Newton K, Tschopp J, Strasser A. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-xL. Proc Natl Acad Sci U S A. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DCS, Strasser A. BH3-only proteins – essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- Hughes PD, Belz GT, Fortner K, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, 3rd, Wu T, Li QZ, Davis LS, Mohan C, Perlman H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Ichikawa K, Yoshida-Kato H, Ohtsuki M, Ohsumi J, Yamaguchi J, Takahashi S, Tani Y, Watanabe M, Shiraishi A, Nishioka K, et al. A novel murine anti-human fas mAb which mitigates lymphadenopathy without hepatotoxicity. International Immunology. 2000;12:555–562. doi: 10.1093/intimm/12.4.555. [DOI] [PubMed] [Google Scholar]
- Igney FH, Behrens CK, Krammer PH. Tumor counterattack - concept and reality. European Journal of Immunology. 2000;30:725–731. doi: 10.1002/1521-4141(200003)30:3<725::AID-IMMU725>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Imtiyaz HZ, Rosenberg S, Zhang Y, Rahman ZS, Hou YJ, Manser T, Zhang J. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol. 2006;176:6852–6861. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–194. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima SI, Samashima M, Hase A, Seta Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;65:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kägi D, Odermatt B, Seiler P, Zinkernagel RM, Mak TW, Hengartner H. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. Journal of Experimental Medicine. 1997;186:989–997. doi: 10.1084/jem.186.7.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, Baekkeskov S. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nature Medicine. 1997;3:738–743. doi: 10.1038/nm0797-738. [DOI] [PubMed] [Google Scholar]
- Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- Kang TB, Oh GS, Scandella E, Bolinger B, Ludewig B, Kovalenko A, Wallach D. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol. 2008;181:2522–2532. doi: 10.4049/jimmunol.181.4.2522. [DOI] [PubMed] [Google Scholar]
- Kataoka T, Schroter M, Hahne M, Schneider P, Irmler M, Thome M, Froelich CJ, Tschopp J. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. Journal of Immunology. 1998;161:3936–3942. [PubMed] [Google Scholar]
- Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, Cretney E, Smyth MJ, Silke J, Hakem R, et al. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Tai L, Ekert PG, Huang DC, Norris F, Lindemann RK, Johnstone RW, Dixit VM, Strasser A. The BH3-Only Protein Bid Is Dispensable for DNA Damage- and Replicative Stress-Induced Apoptosis or Cell-Cycle Arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+CD4− T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. Journal of Experimental Medicine. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95) - associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO Journal. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, Gazdar A, Blenis J, Arnott D, Ashkenazi A. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. Journal of Biological Chemistry. 2001;276:46639–46646. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- Kishimoto H, Surh CD, Sprent J. A role for Fas in negative selection of thymocytes in vivo. Journal of Experimental Medicine. 1998;187:1427–1438. doi: 10.1084/jem.187.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig A, Russell JQ, Rodgers WA, Budd RC. Spatial differences in active caspase-8 defines its role in T-cell activation versus cell death. Cell Death Differ. 2008;15:1701–1711. doi: 10.1038/cdd.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Komano H, Ikegami Y, Yokoyama M, Suzuki R, Yonehara S, Yamasaki Y, Shinohara N. Severe impairment of B cell function in lpr/lpr mice expressing transgenic Fas selectively on B cells. International Immunology. 1999;11:1035–1042. doi: 10.1093/intimm/11.7.1035. [DOI] [PubMed] [Google Scholar]
- Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- Ksontini R, Colagiovanni DB, Josephs MD, Edwards CK, III, Tannahill CL, Solorzano CC, Norman J, Denham W, Clare-Salzler M, MacKay SLD, Moldawer LL. Disparate roles for TNF-α and Fas ligand in concanavalin A-induced hepatitis. Journal of Immunology. 1998;160:4082–4089. [PubMed] [Google Scholar]
- Lagresle C, Mondière P, Bella C, Krammer PH, Defrance T. Concurrent engagement of CD40 and the antigen receptor protects naive and memory human B cells from APO-1/Fas-mediated apoptosis. Journal of Experimental Medicine. 1996;183:1377–1388. doi: 10.1084/jem.183.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Luo X, Budlhardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Mabrouk I, Buart S, Hasmim M, Michiels C, Connault E, Opolon P, Chiocchia G, Levi-Strauss M, Chouaib S, Karray S. Prevention of autoimmunity and control of recall response to exogenous antigen by Fas death receptor ligand expression on T cells. Immunity. 2008;29:922–933. doi: 10.1016/j.immuni.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Marsden V, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Ann Rev of Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- McKenzie MD, Carrington EM, Kaufmann T, Strasser A, Huang DC, Kay TW, Allison J, Thomas HE. Proapoptotic BH3-only protein Bid is essential for death receptor-induced apoptosis of pancreatic beta-cells. Diabetes. 2008;57:1284–1292. doi: 10.2337/db07-1692. [DOI] [PubMed] [Google Scholar]
- Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO Journal. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grütter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. Journal of Biological Chemistry. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–1541. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- Mori T, Ando K, Tanaka K, Ikeda Y, Koga Y. Fas-mediated apoptosis of the hematopoietic progenitor cells in mice infected with murine cytomegalovirus. Blood. 1997;89:3565–3573. [PubMed] [Google Scholar]
- Mori T, Nishimura T, Ikeda Y, Hotta T, Yagita H, Ando K. Involvement of Fas-mediated apoptosis in the hematopoietic progenitor cells of graft-versus-host reaction-associated myelosuppression. Blood. 1998;92:101–107. [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Newton K, Harris AW, Bath ML, Smith KGC, Strasser A. A dominant interfering mutant of FADD/Mort1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO Journal. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Harris AW, Strasser A. FADD/MORT1 regulates the pre-TCR checkpoint and can function as a tumour suppressor. EMBO Journal. 2000;19:931–941. doi: 10.1093/emboj/19.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Kurts C, Harris AW, Strasser A. Effects of a dominant interfering mutant of FADD on signal transduction in activated T cells. Current Biology. 2001;11:273–276. doi: 10.1016/s0960-9822(01)00067-7. [DOI] [PubMed] [Google Scholar]
- Newton K, Strasser A. Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of fas or FADD/MORT1 signaling: implications for cancer therapy. Journal of Experimental Medicine. 2000;191:195–200. doi: 10.1084/jem.191.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Strasser A. Caspases signal not only apoptosis but also antigen-induced activation in cells of the immune system. Genes and Development. 2003;17:819–825. doi: 10.1101/gad.1077403. [DOI] [PubMed] [Google Scholar]
- O’Connor L, Strasser A. Fas, p53, and apoptosis. Science. 1999;284:1430. [Google Scholar]
- O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, Huang DCS. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly LA, Divisekera U, Newton K, Scalzo K, Kataoka T, Puthalakath H, Ito M, Huang DC, Strasser A. Modifications and intracellular trafficking of FADD/MORT1 and caspase-8 after stimulation of T lymphocytes. Cell Death Differ. 2004;11:724–736. doi: 10.1038/sj.cdd.4401408. [DOI] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Opferman JT. Life and death during hematopoietic differentiation. Current Opinion in Immunology. 2007;19:497–502. doi: 10.1016/j.coi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Bath S, Marsden VS, Huang DC, Metcalf D, Harris AW, Strasser A. FADD and Caspase-8 are required for cytokine-induced proliferation of hemopoietic progenitor cells. Blood. 2005;106:1581–1589. doi: 10.1182/blood-2005-01-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Belz G, Bouillet P, Strasser A. Shut down of an acute T cell immune response to viral infection is mediated by the pro-apoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci U S A. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Kannourakis G, Nouri S, Smith KG, Nossal GJ. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995;375:331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- Ramaswamy M, Siegel RM. A FAScinating receptor in self-tolerance. Immunity. 2007;26:545–547. doi: 10.1016/j.immuni.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- Reap EA, Felix NJ, Wolthusen PA, Kotzin BL, Cohen PL, Eisenberg RA. bcl-2 transgenic Lpr mice show profound enhancement of lymphadenopathy. Journal of Immunology. 1995;155:5455–5462. [PubMed] [Google Scholar]
- Renno T, Hahne M, Tschopp J, MacDonald HR. Peripheral T cells undergoing superantigen-induced apoptosis in vivo express B200 and upregulate Fas and Fas ligand. J Exp Med. 1996;183:431–437. doi: 10.1084/jem.183.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Piguet V, Valzasina B, Lens S, Zubler R, French L, Kindler V, Tschopp J, Ricciardi-Castagnoli P. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1β, and the production of interferon γ in the absence of IL-12 during DC-T cell cognate interaction: a new role for Fas ligand in inflammatory responses. Journal of Experimental Medicine. 2000;192:1661–1668. doi: 10.1084/jem.192.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo NP. Not so fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nature Medicine. 2000;6:493–495. doi: 10.1038/74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IAG, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Rothstein TL, Wang JKM, Panka DJ, Foote LC, Wang Z, Stanger B, Cui H, Ju ST, Marshak-Rothstein A. Protection against Fas-dependent Th1-mediated apoptosis by antigen receptor engagement in B cells. Nature. 1995;374:163–165. doi: 10.1038/374163a0. [DOI] [PubMed] [Google Scholar]
- Rubio CF, Kench J, Russell DM, Yawger R, Nemazee D. Analysis of central B cell tolerance in autoimmune-prone MRL/lpr mice bearing autoantibody transgenes. Journal of Immunology. 1996;157:65–71. [PubMed] [Google Scholar]
- Russell JH, Rush B, Weaver C, Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proceedings of the National Academy of Sciences of the USA. 1993;90:4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JH, Wang R. Autoimmune gld mutation uncouples suicide and cytokine/proliferation pathways in activated, mature T cells. European Journal of Immunology. 1993;23:2379–2382. doi: 10.1002/eji.1830230951. [DOI] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au B, Berry DM, Tamblyn L, Shehabeldin EM, Migon E, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes and Development. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO Journal. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. Journal of Experimental Medicine. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram BR, Rothstein TL. NF-κB is required for surface Ig-induced Fas resistance in B cells. Journal of Immunology. 2003;170:3118–3124. doi: 10.4049/jimmunol.170.6.3118. [DOI] [PubMed] [Google Scholar]
- Shiraishi T, Suzuyama K, Okamoto H, Mineta T, Tabuchi K, Nakayama K, Shimizu Y, Tohma J, Ogihara T, Naba H, et al. Increased cytotoxicity of soluble Fas ligand by fusing isoleucine zipper motif. Biochem Biophys Res Commun. 2004;322:197–202. doi: 10.1016/j.bbrc.2004.07.098. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. Journal of Experimental Medicine. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokat KM, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334–338. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- Sidman CL, Marshall JD, von Boehmer H. Transgenic T cell receptor interactions in the lymphoproliferative and autoimmune syndromes of lpr and gld mutant mice. Eur J Immunol. 1992;22:499–504. doi: 10.1002/eji.1830220231. [DOI] [PubMed] [Google Scholar]
- Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, Lenardo MJ. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Smith D, Sieg S, Kaplan D. Technical note: aberrant detection of cell surface Fas ligand with anti-peptide antibodies. Journal of Immunology. 1998;160:4159–4160. [PubMed] [Google Scholar]
- Smith KGC, Strasser A, Vaux DL. CrmA expression in T lymphocytes of transgenic mice inhibits CD95 (Fas/APO-1)-transduced apoptosis, but does not cause lymphadenopathy or autoimmune disease. EMBO Journal. 1996;15:5167–5176. [PMC free article] [PubMed] [Google Scholar]
- Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- Sprick MR, Rieser E, Stahl H, Grosse-Wilde A, Weigand MA, Walczak H. Caspase-10 is recruited to and activated at the native TRAIL and CD95 death-inducing signalling complexes in a FADD-dependent manner but can not functionally substitute caspase-8. Embo Journal. 2002;21:4520–4530. doi: 10.1093/emboj/cdf441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennicke HR, Jürgensmeier JM, Shin H, Deveraux Q, Wolf BB, Yang X, Zhou Q, Ellerby HM, Ellerby LM, Bredesen D, et al. Pro-caspase-3 is a major physiologic target of caspase-8. Journal of Biological Chemistry. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- Stokes TA, Rymaszewski M, Arscott PL, Wang SH, Bretz JD, Bartron J, Baker JRJ. Constitutive expression of Fas ligand in thyrocytes. Science. 1998;279 http://www.sciencemag.org/content/vol279/issue5359/
- Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. Death of a T cell. Nature. 1995;373:385–386. doi: 10.1038/373385a0. [DOI] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991a;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Ann Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991b;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- Su X, Hu Q, Kristan JM, Costa C, Shen Y, Gero D, Matis LA, Wang Y. Significant role for Fas in the pathogenesis of autoimmune diabetes. J Immunol. 2000;164:2523–2532. doi: 10.4049/jimmunol.164.5.2523. [DOI] [PubMed] [Google Scholar]
- Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. Journal of Experimental Medicine. 1997;186:2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Tagawa YI, Kakuta S, Iwakura Y. Involvement of Fas/Fas ligand system-mediated apoptosis in the development of concanavalin A-induced hepatitis. European Journal of Immunology. 1998;28:4105–4113. doi: 10.1002/(SICI)1521-4141(199812)28:12<4105::AID-IMMU4105>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tamura A, Katsumata M, Greene MI, Yui K. Inhibition of apoptosis and augmentation of lymphoproliferation in bcl-2 transgenic Fas/Fas ligand-defective mice. Cellular Immunol. 1996;168:220–228. doi: 10.1006/cimm.1996.0069. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nature Medicine. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. Embo J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D. Germinal centers: form and function. Current Opinion in Immunology. 1998;10:245–251. doi: 10.1016/s0952-7915(98)80161-1. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Balderas RS, Gozes Y, Aguado MT, Hang LM, Morrow PR, Dixon FJ. Association of lpr gene with graft-vs.-host disease-like syndrome. J Exp Med. 1985;162:1–18. doi: 10.1084/jem.162.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trambas CM, Griffiths GM. Delivering the kiss of death. Nature Immunology. 2003;4:399–403. doi: 10.1038/ni0503-399. [DOI] [PubMed] [Google Scholar]
- Trauth BC, Klas C, Peters AMJ, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, et al. IAP Antagonists Induce Autoubiquitination of c-IAPs, NF-kappaB Activation, and TNFalpha-Dependent Apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A, Marsden VS, Zhan Y, Erlacher M, Lew AM, Bouillet P, Berzins S, Godfrey DI, Heath WR, Strasser A. Negative selection of semimature CD4(+)8(-)HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc Natl Acad Sci U S A. 2004;101:7052–7057. doi: 10.1073/pnas.0305757101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, et al. IAP Antagonists Target cIAP1 to Induce TNFalpha-Dependent Apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Wallach D. Suicide by order: some open questions about the cell-killing activities of the TNF ligand and receptor families. Cytokine and Growth Factor Reviews. 1996;7:211–221. doi: 10.1016/s1359-6101(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Suda T, Hashimoto H, Nagata S. Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. EMBO Journal. 1995;14:12–18. doi: 10.1002/j.1460-2075.1995.tb06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, Mak TW. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, Ng M, Wakeham A, Khoo W, Mitchell K, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J Exp Med. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Beaudette BC, Rifkin IR, Marshak-Rothstein A. Double mutant MRL-lpr/lpr-gld/gld cells fail to trigger lpr-graft- versus-host disease in syngeneic wild-type recipient mice, but can induce wild-type B cells to make autoantibody. European Journal of Immunology. 2000;30:1778–1784. doi: 10.1002/1521-4141(200006)30:6<1778::AID-IMMU1778>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]