Abstract
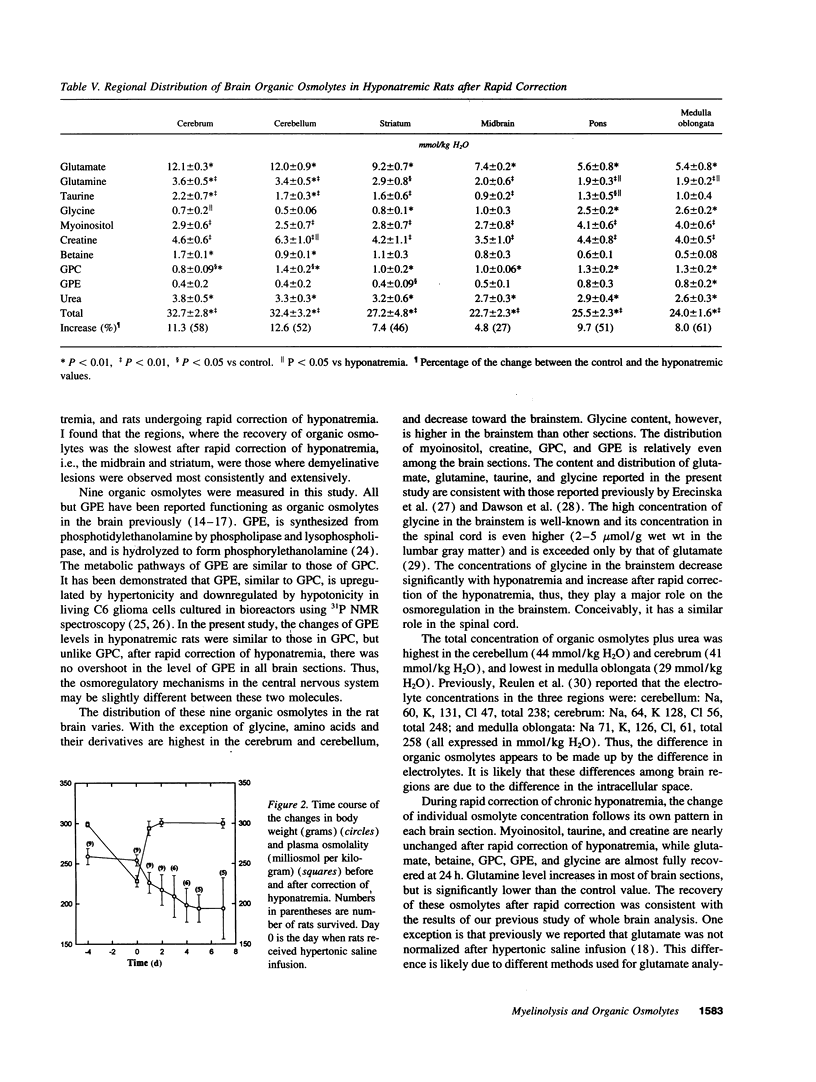
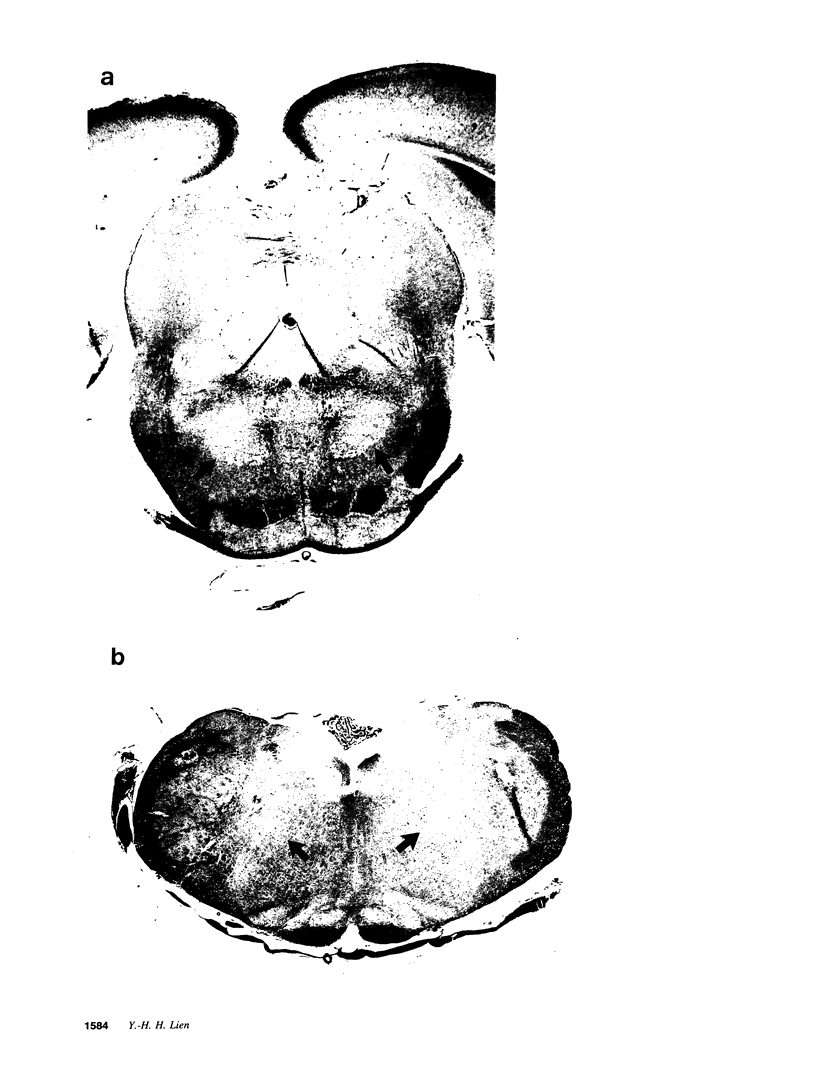
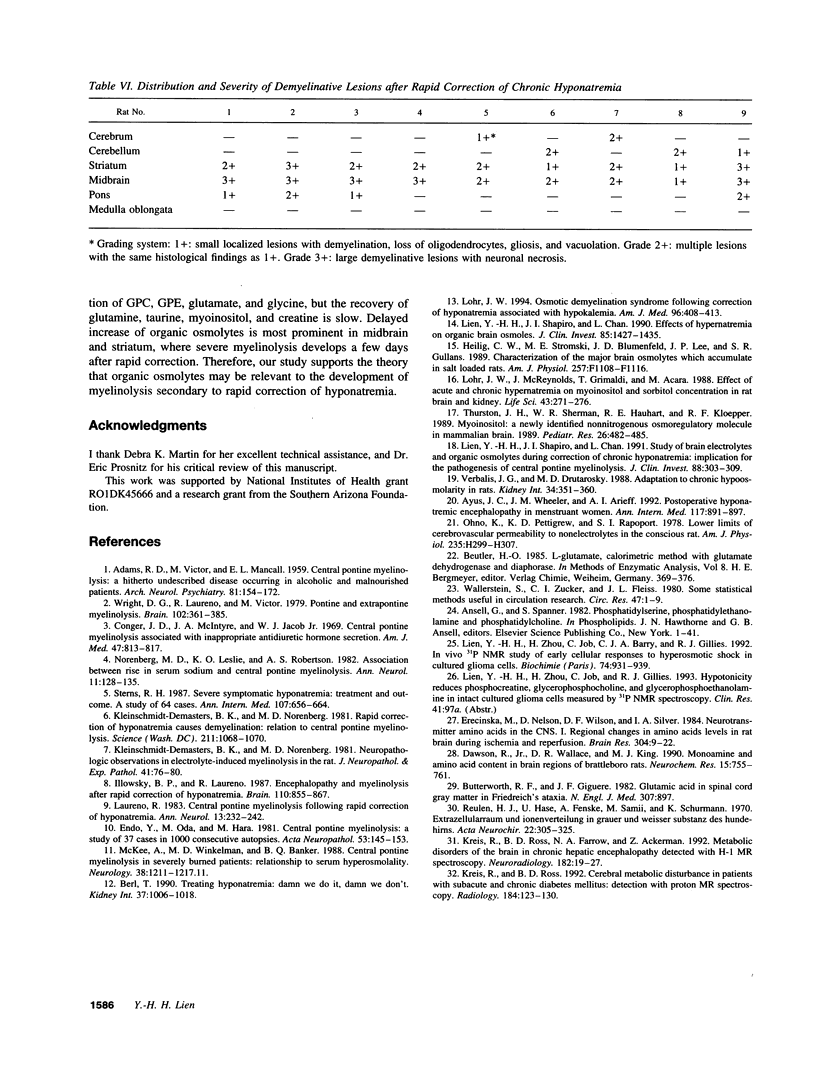
Organic osmolytes have been implicated in the pathogenesis of myelinolysis because some of them are accumulated slowly during correction of chronic hyponatremia. I investigated whether there was a topographic correlation between demyelinative lesions and the regional changes of organic osmolytes after rapid correction of chronic hyponatremia. In normal female Sprague-Dawley rats, concentrations of glutamate, glutamine, taurine, and betaine were highest in the cerebral cortex and decreased toward the brain stem. Conversely, glycine level was highest in the brainstem, and decreased toward the cortex. Myoinositol, glycerophosphorylcholine, glycerophosphorylethanolamine, and creatine were distributed more evenly. In chronic hyponatremic rats (plasma Na 110 +/- 4 meq/liter), organic osmolytes decreased globally with the total loss ranging from 13 (medulla) to 24 (cerebellum) mmol/kg H2O. After rapid correction with intraperitoneal injection of hypertonic saline, the recovery of the loss of organic osmolytes was 48% in the cerebral cortex, cerebellum, and medulla oblongata, 44% in pons, but only 17% in midbrain and 36% in striatum. Histopathology of the brain was examined in nine rats 2-7 d after correction of hyponatremia. Large demyelinative lesions were seen persistently in the midbrain and striatum, and smaller lesions in cerebrum, cerebellum, and pons were found less frequently. This is the first report of regional distribution of brain organic osmolytes. After rapid correction of chronic hyponatremia, a topographic correlation between demyelination lesions and delayed accumulation of organic osmolytes exists.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS R. D., VICTOR M., MANCALL E. L. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959 Feb;81(2):154–172. [PubMed] [Google Scholar]
- Ayus J. C., Wheeler J. M., Arieff A. I. Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med. 1992 Dec 1;117(11):891–897. doi: 10.7326/0003-4819-117-11-891. [DOI] [PubMed] [Google Scholar]
- Berl T. Treating hyponatremia: damned if we do and damned if we don't. Kidney Int. 1990 Mar;37(3):1006–1018. doi: 10.1038/ki.1990.78. [DOI] [PubMed] [Google Scholar]
- Butterworth R. F., Giguère J. F. Glutamic acid in spinal-cord gray matter in Friedreich's ataxia. N Engl J Med. 1982 Sep 30;307(14):897–897. doi: 10.1056/NEJM198209303071421. [DOI] [PubMed] [Google Scholar]
- Conger J. D., McIntyre J. A., Jacoby W. J., Jr Central pontine myelinolysis associated with inappropriate antidiuretic hormone secretion. Am J Med. 1969 Nov;47(5):813–817. doi: 10.1016/0002-9343(69)90175-2. [DOI] [PubMed] [Google Scholar]
- Dawson R., Jr, Wallace D. R., King M. J. Monoamine and amino acid content in brain regions of Brattleboro rats. Neurochem Res. 1990 Jul;15(7):755–761. doi: 10.1007/BF00973658. [DOI] [PubMed] [Google Scholar]
- Endo Y., Oda M., Hara M. Central pontine myelinolysis. A study of 37 cases in 1,000 consecutive autopsies. Acta Neuropathol. 1981;53(2):145–153. doi: 10.1007/BF00689995. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Nelson D., Wilson D. F., Silver I. A. Neurotransmitter amino acids in the CNS. I. Regional changes in amino acid levels in rat brain during ischemia and reperfusion. Brain Res. 1984 Jun 18;304(1):9–22. doi: 10.1016/0006-8993(84)90857-6. [DOI] [PubMed] [Google Scholar]
- Heilig C. W., Stromski M. E., Blumenfeld J. D., Lee J. P., Gullans S. R. Characterization of the major brain osmolytes that accumulate in salt-loaded rats. Am J Physiol. 1989 Dec;257(6 Pt 2):F1108–F1116. doi: 10.1152/ajprenal.1989.257.6.F1108. [DOI] [PubMed] [Google Scholar]
- Illowsky B. P., Laureno R. Encephalopathy and myelinolysis after rapid correction of hyponatraemia. Brain. 1987 Aug;110(Pt 4):855–867. doi: 10.1093/brain/110.4.855. [DOI] [PubMed] [Google Scholar]
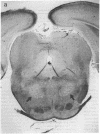
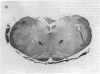
- Kleinschmidt-DeMasters B. K., Norenberg M. D. Neuropathologic observations in electrolyte-induced myelinolysis in the rat. J Neuropathol Exp Neurol. 1982 Jan;41(1):67–80. doi: 10.1097/00005072-198201000-00007. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters B. K., Norenberg M. D. Rapid correction of hyponatremia causes demyelination: relation to central pontine myelinolysis. Science. 1981 Mar 6;211(4486):1068–1070. doi: 10.1126/science.7466381. [DOI] [PubMed] [Google Scholar]
- Kreis R., Ross B. D. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology. 1992 Jul;184(1):123–130. doi: 10.1148/radiology.184.1.1319074. [DOI] [PubMed] [Google Scholar]
- Kreis R., Ross B. D., Farrow N. A., Ackerman Z. Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology. 1992 Jan;182(1):19–27. doi: 10.1148/radiology.182.1.1345760. [DOI] [PubMed] [Google Scholar]
- Laureno R. Central pontine myelinolysis following rapid correction of hyponatremia. Ann Neurol. 1983 Mar;13(3):232–242. doi: 10.1002/ana.410130303. [DOI] [PubMed] [Google Scholar]
- Lien Y. H., Shapiro J. I., Chan L. Effects of hypernatremia on organic brain osmoles. J Clin Invest. 1990 May;85(5):1427–1435. doi: 10.1172/JCI114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien Y. H., Shapiro J. I., Chan L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. J Clin Invest. 1991 Jul;88(1):303–309. doi: 10.1172/JCI115292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien Y. H., Zhou H. Z., Job C., Barry J. A., Gillies R. J. In vivo 31P NMR study of early cellular responses to hyperosmotic shock in cultured glioma cells. Biochimie. 1992 Sep-Oct;74(9-10):931–939. doi: 10.1016/0300-9084(92)90077-r. [DOI] [PubMed] [Google Scholar]
- Lohr J. W., McReynolds J., Grimaldi T., Acara M. Effect of acute and chronic hypernatremia on myoinositol and sorbitol concentration in rat brain and kidney. Life Sci. 1988;43(3):271–276. doi: 10.1016/0024-3205(88)90317-7. [DOI] [PubMed] [Google Scholar]
- Lohr J. W. Osmotic demyelination syndrome following correction of hyponatremia: association with hypokalemia. Am J Med. 1994 May;96(5):408–413. doi: 10.1016/0002-9343(94)90166-x. [DOI] [PubMed] [Google Scholar]
- McKee A. C., Winkelman M. D., Banker B. Q. Central pontine myelinolysis in severely burned patients: relationship to serum hyperosmolality. Neurology. 1988 Aug;38(8):1211–1217. doi: 10.1212/wnl.38.8.1211. [DOI] [PubMed] [Google Scholar]
- Norenberg M. D., Leslie K. O., Robertson A. S. Association between rise in serum sodium and central pontine myelinolysis. Ann Neurol. 1982 Feb;11(2):128–135. doi: 10.1002/ana.410110204. [DOI] [PubMed] [Google Scholar]
- Ohno K., Pettigrew K. D., Rapoport S. I. Lower limits of cerebrovascular permeability to nonelectrolytes in the conscious rat. Am J Physiol. 1978 Sep;235(3):H299–H307. doi: 10.1152/ajpheart.1978.235.3.H299. [DOI] [PubMed] [Google Scholar]
- Reulen H. J., Hase U., Fenske A., Samii M., Schürmann K. Extrazellulärraum und Ionenverteilung in grauer und weisser Substanz des Hundehirns. Acta Neurochir (Wien) 1970;22(4):305–325. doi: 10.1007/BF01402997. [DOI] [PubMed] [Google Scholar]
- Sterns R. H. Severe symptomatic hyponatremia: treatment and outcome. A study of 64 cases. Ann Intern Med. 1987 Nov;107(5):656–664. doi: 10.7326/0003-4819-107-5-656. [DOI] [PubMed] [Google Scholar]
- Thurston J. H., Sherman W. R., Hauhart R. E., Kloepper R. F. myo-inositol: a newly identified nonnitrogenous osmoregulatory molecule in mammalian brain. Pediatr Res. 1989 Nov;26(5):482–485. doi: 10.1203/00006450-198911000-00024. [DOI] [PubMed] [Google Scholar]
- Verbalis J. G., Drutarosky M. D. Adaptation to chronic hypoosmolality in rats. Kidney Int. 1988 Sep;34(3):351–360. doi: 10.1038/ki.1988.188. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Laureno R., Victor M. Pontine and extrapontine myelinolysis. Brain. 1979 Jun;102(2):361–385. doi: 10.1093/brain/102.2.361. [DOI] [PubMed] [Google Scholar]