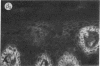
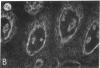
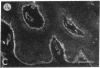
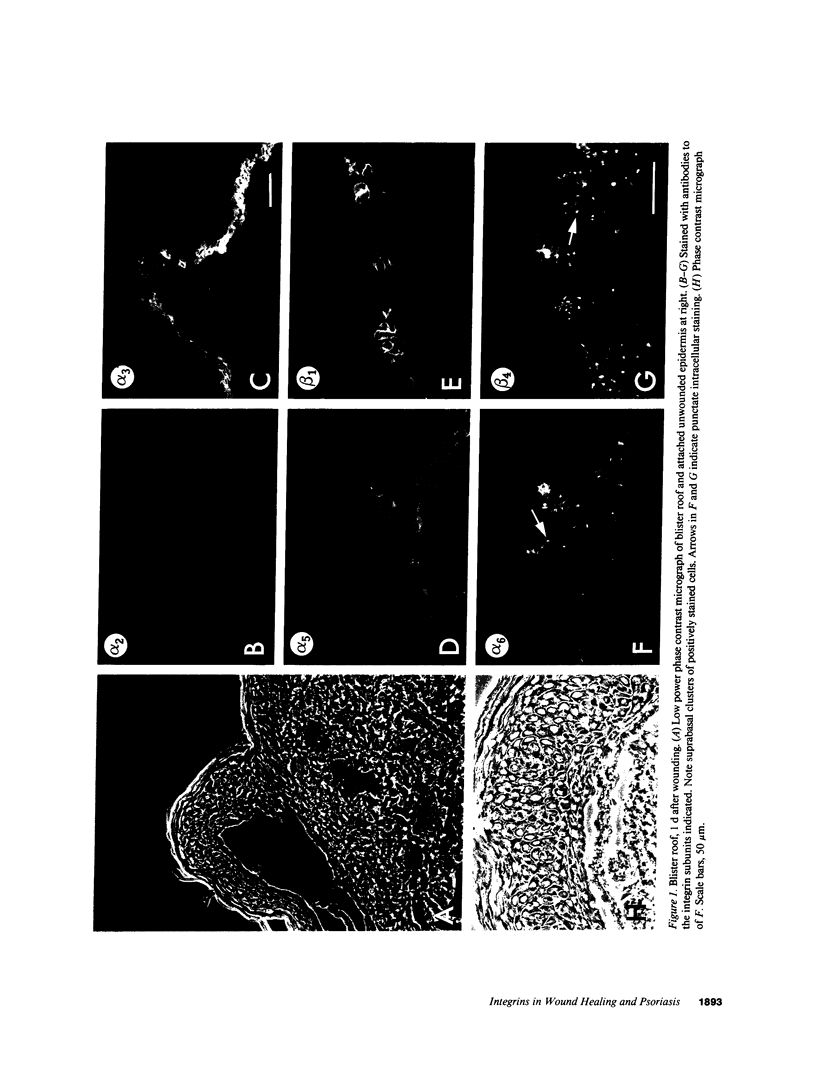
Abstract
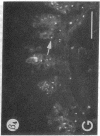
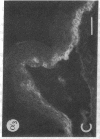
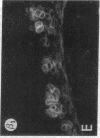
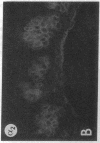
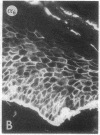
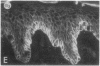
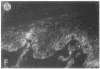
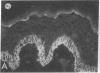
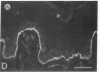
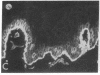
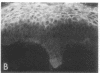
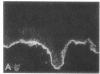
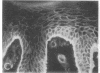
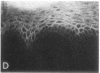
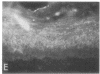
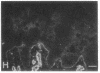
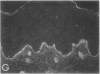
We have examined integrin expression during the remodeling of the epidermis that takes place during wound healing, using a suction blister model in which the epidermis is detached from the dermis, leaving the basement membrane intact. By immunofluorescence microscopy, we found that the same integrin subunits were expressed during wound healing as in normal epidermis with very little change in the relative intensity or distribution of staining at the leading edge of the migrating epidermis. However, at the time of wound closure, when the epidermis is still hyperproliferative, alpha 2, alpha 3, alpha 6, and beta 1 were no longer confined to the basal layer, as in normal epidermis, but were also found in all the living suprabasal cell layers, coexpressed with the terminal differentiation markers involucrin, keratin 10, and keratin 16. Strong suprabasal staining for alpha v was also found in one specimen. beta 4, which normally forms a heterodimer with alpha 6, and alpha 5 remained predominantly basal. Three of the integrin ligands, fibronectin, type IV collagen, and laminin, remained largely confined to the basement membrane zone and dermis. By 14 d after wounding, the integrins were once more restricted to the basal layer. Suprabasal integrin expression was also observed in involved psoriatic lesions. Thus, in two situations in which the epidermis is hyperproliferative, there is a failure to downregulate integrin expression on initiation of terminal differentiation. The functional consequences of this aberrant integrin expression remain to be explored.
Full text
PDF
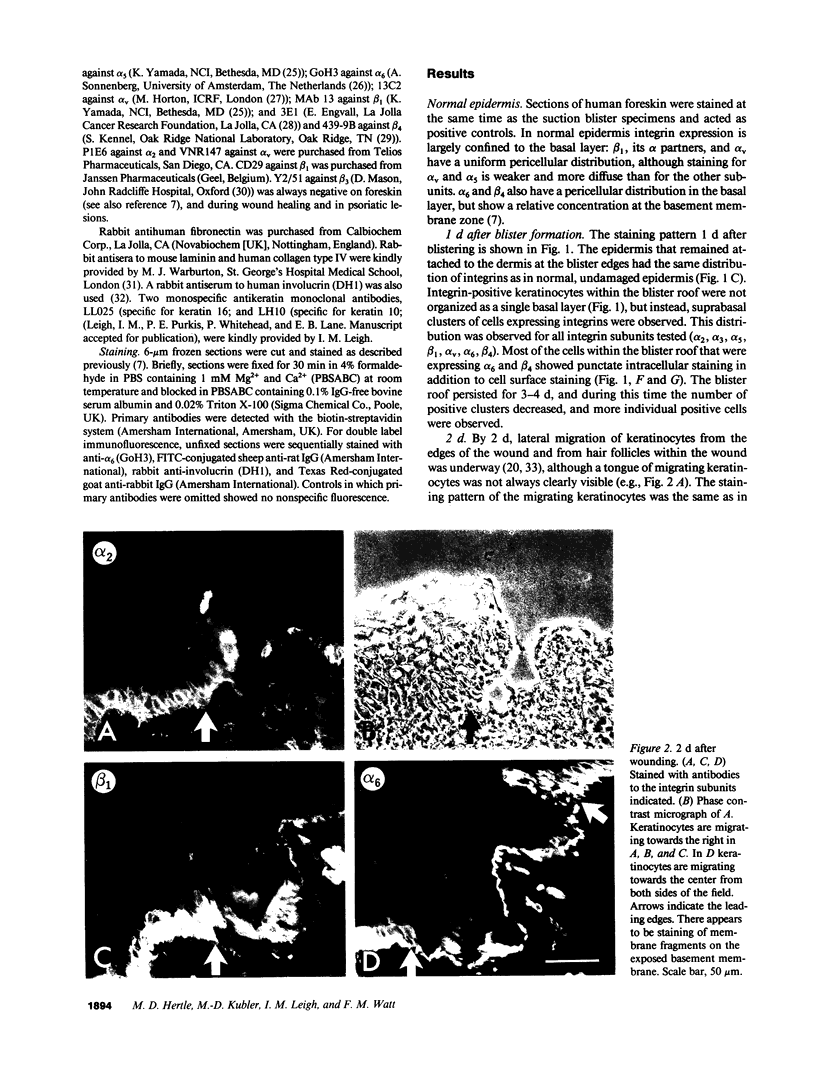
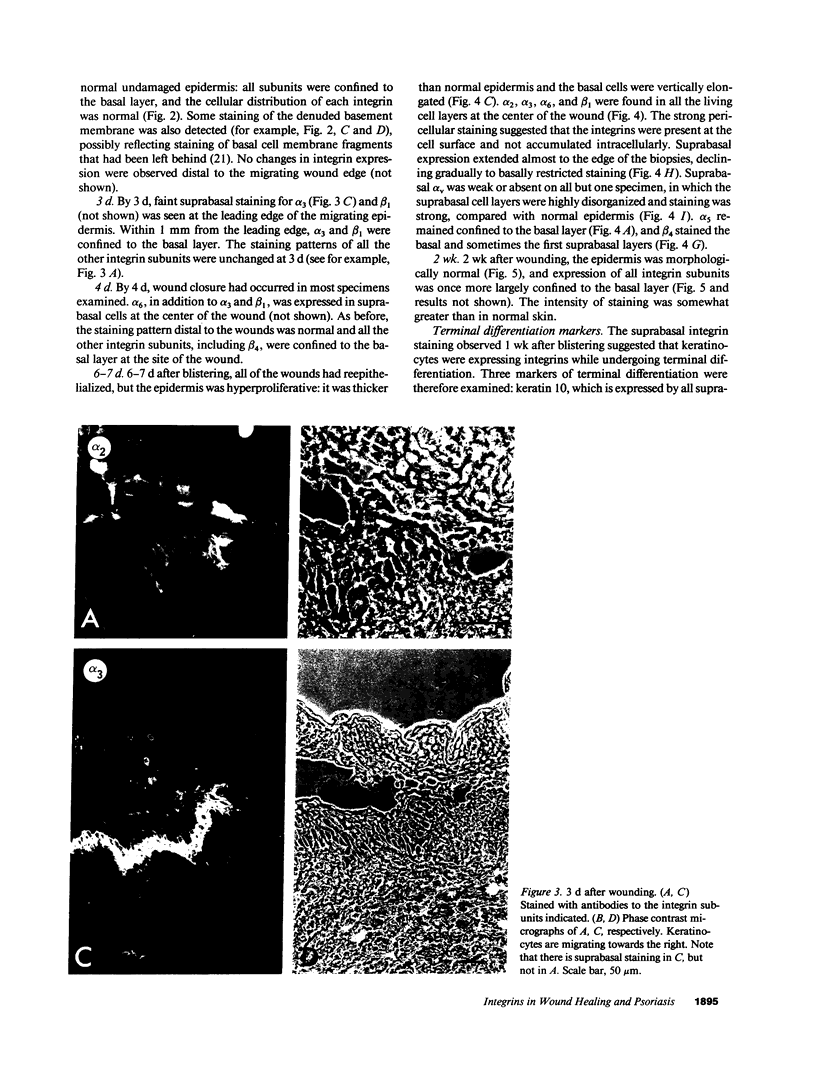
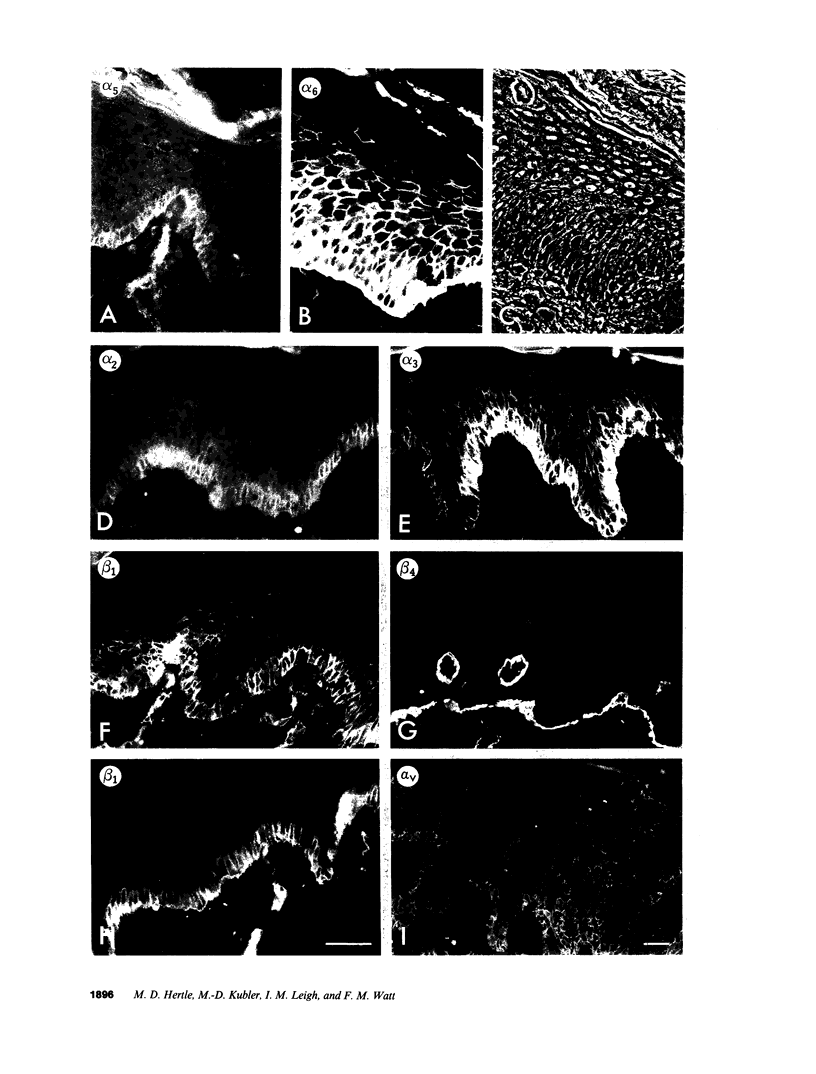
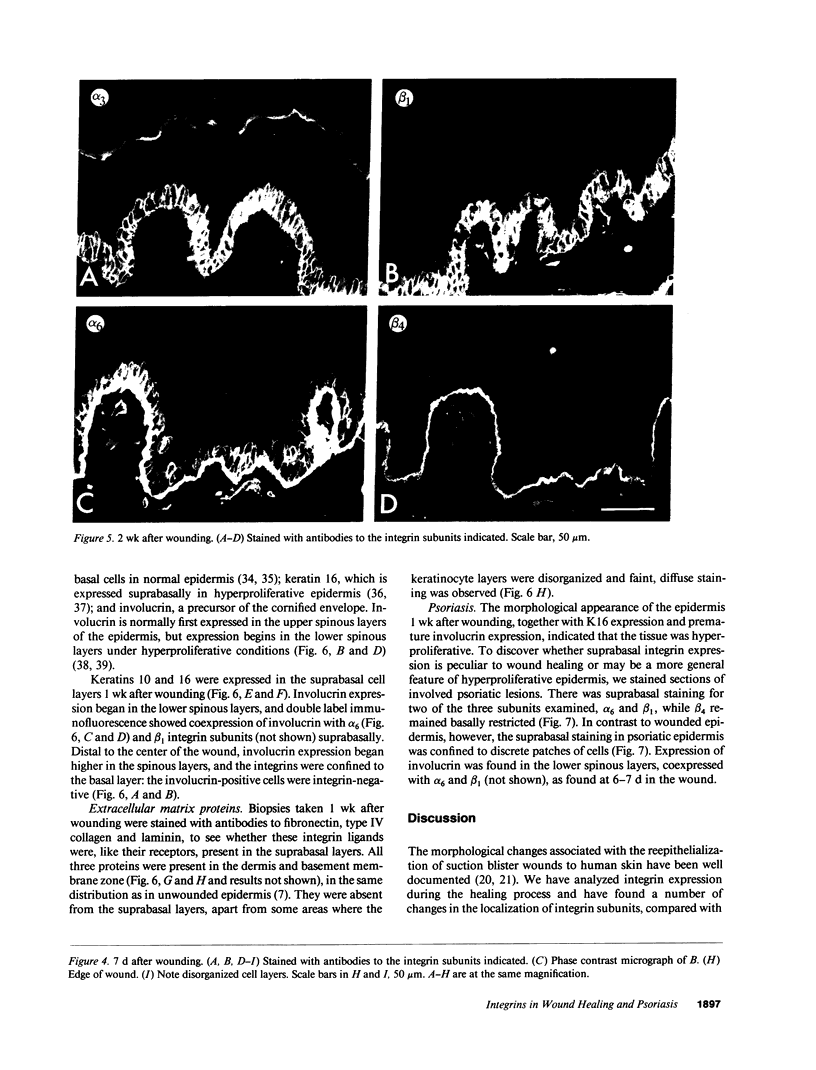
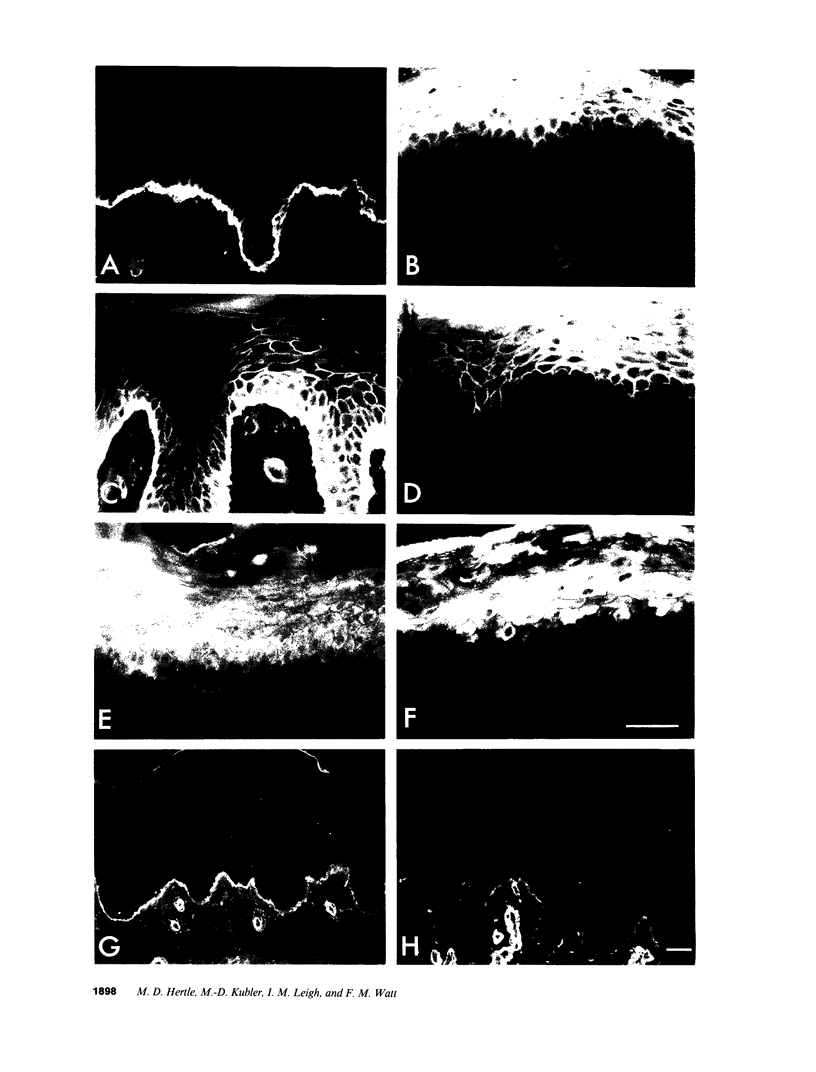
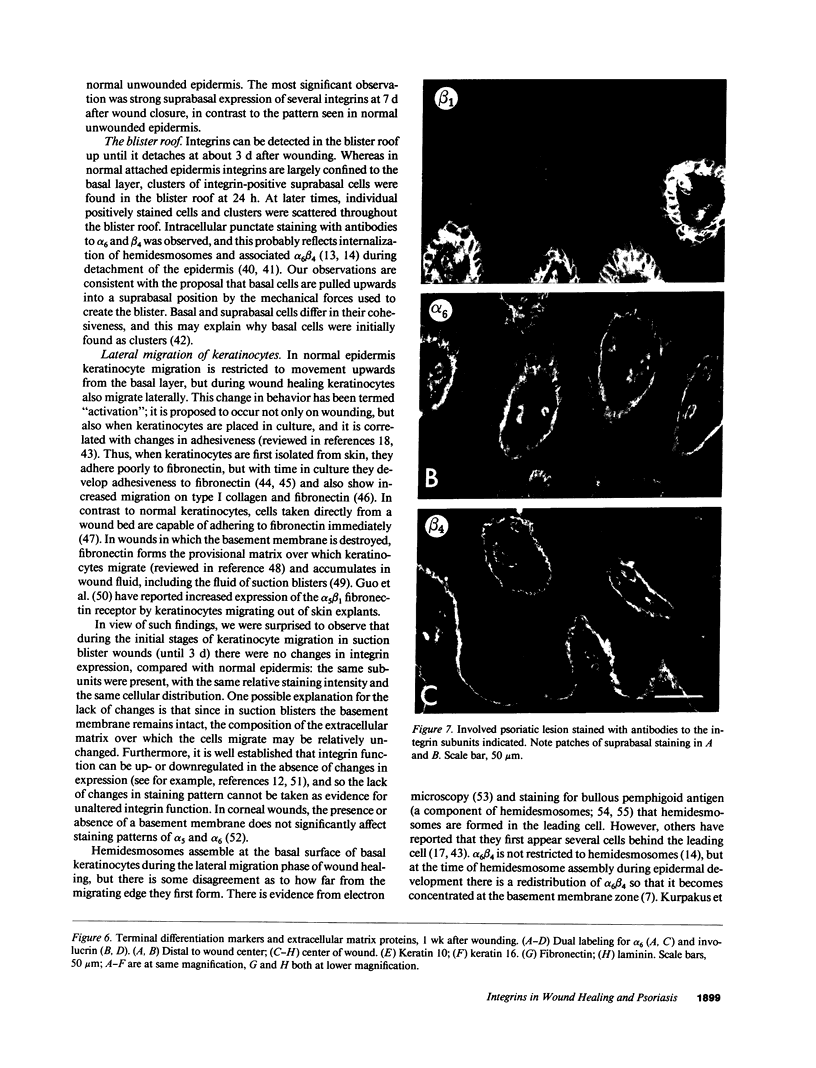


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Adams J. C., Watt F. M. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991 Nov;115(3):829–841. doi: 10.1083/jcb.115.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. C., Watt F. M. Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature. 1989 Jul 27;340(6231):307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989 Aug;109(2):863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H., Kligman A. M. Technique for estimating turnover time of human stratum corneum. Arch Dermatol. 1967 Apr;95(4):408–411. [PubMed] [Google Scholar]
- Bernard B. A., Reano A., Darmon Y. M., Thivolet J. Precocious appearance of involucrin and epidermal transglutaminase during differentiation of psoriatic skin. Br J Dermatol. 1986 Mar;114(3):279–283. doi: 10.1111/j.1365-2133.1986.tb02818.x. [DOI] [PubMed] [Google Scholar]
- Bodary S. C., Napier M. A., McLean J. W. Expression of recombinant platelet glycoprotein IIbIIIa results in a functional fibrinogen-binding complex. J Biol Chem. 1989 Nov 15;264(32):18859–18862. [PubMed] [Google Scholar]
- Carter W. G., Kaur P., Gil S. G., Gahr P. J., Wayner E. A. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990 Dec;111(6 Pt 2):3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol. 1990 Jun;94(6 Suppl):128S–134S. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- De Luca M., Tamura R. N., Kajiji S., Bondanza S., Rossino P., Cancedda R., Marchisio P. C., Quaranta V. Polarized integrin mediates human keratinocyte adhesion to basal lamina. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6888–6892. doi: 10.1073/pnas.87.17.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover R., Watt F. M. Measurement of the rate of epidermal terminal differentiation: expression of involucrin by S-phase keratinocytes in culture and in psoriatic plaques. J Invest Dermatol. 1987 Oct;89(4):349–352. doi: 10.1111/1523-1747.ep12471751. [DOI] [PubMed] [Google Scholar]
- Du X. P., Plow E. F., Frelinger A. L., 3rd, O'Toole T. E., Loftus J. C., Ginsberg M. H. Ligands "activate" integrin alpha IIb beta 3 (platelet GPIIb-IIIa). Cell. 1991 May 3;65(3):409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Galvin S., Loomis C., Manabe M., Dhouailly D., Sun T. T. The major pathways of keratinocyte differentiation as defined by keratin expression: an overview. Adv Dermatol. 1989;4:277–300. [PubMed] [Google Scholar]
- Gottlieb A. B. Immunologic mechanisms in psoriasis. J Invest Dermatol. 1990 Nov;95(5 Suppl):18S–19S. doi: 10.1111/1523-1747.ep12505675. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Toda K., Takashima A. Activation of keratinocyte fibronectin receptor function during cutaneous wound healing. J Cell Sci Suppl. 1987;8:199–209. doi: 10.1242/jcs.1987.supplement_8.11. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992 Jan;101(Pt 1):1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- Grushkin-Lerner L. S., Trinkaus-Randall V. Localization of integrin and syndecan in vivo in a corneal epithelial abrasion and keratectomy. Curr Eye Res. 1991 Jan;10(1):75–85. doi: 10.3109/02713689109007612. [DOI] [PubMed] [Google Scholar]
- Guo M., Kim L. T., Akiyama S. K., Gralnick H. R., Yamada K. M., Grinnell F. Altered processing of integrin receptors during keratinocyte activation. Exp Cell Res. 1991 Aug;195(2):315–322. doi: 10.1016/0014-4827(91)90379-9. [DOI] [PubMed] [Google Scholar]
- Guo M., Toda K., Grinnell F. Activation of human keratinocyte migration on type I collagen and fibronectin. J Cell Sci. 1990 Jun;96(Pt 2):197–205. doi: 10.1242/jcs.96.2.197. [DOI] [PubMed] [Google Scholar]
- Halprin K. M. Epidermal "turnover time"--a re-examination. Br J Dermatol. 1972 Jan;86(1):14–19. doi: 10.1111/j.1365-2133.1972.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hertle M. D., Adams J. C., Watt F. M. Integrin expression during human epidermal development in vivo and in vitro. Development. 1991 May;112(1):193–206. doi: 10.1242/dev.112.1.193. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Hunter J. A., McVittie E., Comaish J. S. Light and electron microscopic studies of physical injury to the skin. I. Suction. Br J Dermatol. 1974 May;90(5):481–490. doi: 10.1111/j.1365-2133.1974.tb06442.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kantor R. R., Mattes M. J., Lloyd K. O., Old L. J., Albino A. P. Biochemical analysis of two cell surface glycoprotein complexes, very common antigen 1 and very common antigen 2. Relationship to very late activation T cell antigens. J Biol Chem. 1987 Nov 5;262(31):15158–15165. [PubMed] [Google Scholar]
- Kaufmann Y., Tseng E., Springer T. A. Cloning of the murine lymphocyte function-associated molecule-1 alpha-subunit and its expression in COS cells. J Immunol. 1991 Jul 1;147(1):369–374. [PubMed] [Google Scholar]
- Kennel S. J., Foote L. J., Falcioni R., Sonnenberg A., Stringer C. D., Crouse C., Hemler M. E. Analysis of the tumor-associated antigen TSP-180. Identity with alpha 6-beta 4 in the integrin superfamily. J Biol Chem. 1989 Sep 15;264(26):15515–15521. [PubMed] [Google Scholar]
- Kiistala U., Mustakallio K. K. Dermo-epidermal separation with suction. Electron microscopic and histochemical study of initial events of blistering on human skin. J Invest Dermatol. 1967 May;48(5):466–477. [PubMed] [Google Scholar]
- Kurpakus M. A., Quaranta V., Jones J. C. Surface relocation of alpha 6 beta 4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991 Dec;115(6):1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe L. B., Jr, van der Leun J. C. Suction blisters and dermal-epidermal adherence. J Invest Dermatol. 1968 Apr;50(4):308–314. [PubMed] [Google Scholar]
- Mansbridge J. N., Knapp A. M. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987 Sep;89(3):253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- Marchisio P. C., Bondanza S., Cremona O., Cancedda R., De Luca M. Polarized expression of integrin receptors (alpha 6 beta 4, alpha 2 beta 1, alpha 3 beta 1, and alpha v beta 5) and their relationship with the cytoskeleton and basement membrane matrix in cultured human keratinocytes. J Cell Biol. 1991 Feb;112(4):761–773. doi: 10.1083/jcb.112.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay I. A., Leigh I. M. Epidermal cytokines and their roles in cutaneous wound healing. Br J Dermatol. 1991 Jun;124(6):513–518. doi: 10.1111/j.1365-2133.1991.tb04942.x. [DOI] [PubMed] [Google Scholar]
- McKenzie R. C., Sauder D. N. Keratinocyte cytokines and growth factors. Functions in skin immunity and homeostasis. Dermatol Clin. 1990 Oct;8(4):649–661. [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson L. J., Watt F. M. Decreased expression of fibronectin and the alpha 5 beta 1 integrin during terminal differentiation of human keratinocytes. J Cell Sci. 1991 Feb;98(Pt 2):225–232. doi: 10.1242/jcs.98.2.225. [DOI] [PubMed] [Google Scholar]
- Odland G., Ross R. Human wound repair. I. Epidermal regeneration. J Cell Biol. 1968 Oct;39(1):135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortonne J. P., Löning T., Schmitt D., Thivolet J. Immunomorphological and ultrastructural aspects of keratinocyte migration in epidermal wound healing. Virchows Arch A Pathol Anat Histol. 1981;392(2):217–230. doi: 10.1007/BF00430822. [DOI] [PubMed] [Google Scholar]
- Peltonen J., Larjava H., Jaakkola S., Gralnick H., Akiyama S. K., Yamada S. S., Yamada K. M., Uitto J. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989 Dec;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo M. A., Kim S. C., Korman N. J., Stanley J. R., Labib R. S., Futamura S., Anhalt G. J. Studies of the relationship of the 230-kD and 180-kD bullous pemphigoid antigens. J Invest Dermatol. 1990 Jun;94(6):793–797. doi: 10.1111/1523-1747.ep12874652. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryynänen J., Jaakkola S., Engvall E., Peltonen J., Uitto J. Expression of beta 4 integrins in human skin: comparison of epidermal distribution with beta 1-integrin epitopes, and modulation by calcium and vitamin D3 in cultured keratinocytes. J Invest Dermatol. 1991 Sep;97(3):562–567. doi: 10.1111/1523-1747.ep12481896. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Calafat J., Janssen H., Daams H., van der Raaij-Helmer L. M., Falcioni R., Kennel S. J., Aplin J. D., Baker J., Loizidou M. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991 May;113(4):907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Daams H., Van der Valk M. A., Hilkens J., Hilgers J. Development of mouse mammary gland: identification of stages in differentiation of luminal and myoepithelial cells using monoclonal antibodies and polyvalent antiserum against keratin. J Histochem Cytochem. 1986 Aug;34(8):1037–1046. doi: 10.1177/34.8.2426332. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Daams J. H., Kennel S. J. The alpha 6 beta 1 (VLA-6) and alpha 6 beta 4 protein complexes: tissue distribution and biochemical properties. J Cell Sci. 1990 Jun;96(Pt 2):207–217. doi: 10.1242/jcs.96.2.207. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Alvarez O. M., Bere E. W., Jr, Eaglstein W. H., Katz S. I. Detection of basement membrane zone antigens during epidermal wound healing in pigs. J Invest Dermatol. 1981 Aug;77(2):240–243. doi: 10.1111/1523-1747.ep12480082. [DOI] [PubMed] [Google Scholar]
- Staquet M. J., Levarlet B., Dezutter-Dambuyant C., Schmitt D., Thivolet J. Identification of specific human epithelial cell integrin receptors as VLA proteins. Exp Cell Res. 1990 Apr;187(2):277–283. doi: 10.1016/0014-4827(90)90092-o. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I. K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Mutasim D. F., Patel H. P., Anhalt G. J., Labib R. S., Diaz L. A. The use of human pemphigoid autoantibodies to study the fate of epidermal basal cell hemidesmosomes after trypsin dissociation. J Invest Dermatol. 1985 Oct;85(4):309–313. doi: 10.1111/1523-1747.ep12276893. [DOI] [PubMed] [Google Scholar]
- Takashima A., Billingham R. E., Grinnell F. Activation of rabbit keratinocyte fibronectin receptor function in vivo during wound healing. J Invest Dermatol. 1986 May;86(5):585–590. doi: 10.1111/1523-1747.ep12355243. [DOI] [PubMed] [Google Scholar]
- Takashima A., Grinnell F. Fibronectin-mediated keratinocyte migration and initiation of fibronectin receptor function in vitro. J Invest Dermatol. 1985 Oct;85(4):304–308. doi: 10.1111/1523-1747.ep12276880. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Ferns S. A., Rudland P. S. Enhanced synthesis of basement membrane proteins during the differentiation of rat mammary tumour epithelial cells into myoepithelial-like cells in vitro. Exp Cell Res. 1982 Feb;137(2):373–380. doi: 10.1016/0014-4827(82)90038-6. [DOI] [PubMed] [Google Scholar]
- Watt F. M., Boukamp P., Hornung J., Fusenig N. E. Effect of growth environment on spatial expression of involucrin by human epidermal keratinocytes. Arch Dermatol Res. 1987;279(5):335–340. doi: 10.1007/BF00431227. [DOI] [PubMed] [Google Scholar]
- Watt F. M. Selective migration of terminally differentiating cells from the basal layer of cultured human epidermis. J Cell Biol. 1984 Jan;98(1):16–21. doi: 10.1083/jcb.98.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsteed E. M., Bhogal B. S., Das A., Bekir S. S., Wojnarowska F., Black M. M., Mckee P. H. An ultrastructural comparison of dermo-epidermal separation techniques. J Cutan Pathol. 1991 Feb;18(1):8–12. doi: 10.1111/j.1600-0560.1991.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Wysocki A. B., Grinnell F. Fibronectin profiles in normal and chronic wound fluid. Lab Invest. 1990 Dec;63(6):825–831. [PubMed] [Google Scholar]
- Zylstra S., Chen F. A., Ghosh S. K., Repasky E. A., Rao U., Takita H., Bankert R. B. Membrane-associated glycoprotein (gp 160) identified on human lung tumors by a monoclonal antibody. Cancer Res. 1986 Dec;46(12 Pt 1):6446–6451. [PubMed] [Google Scholar]