Abstract
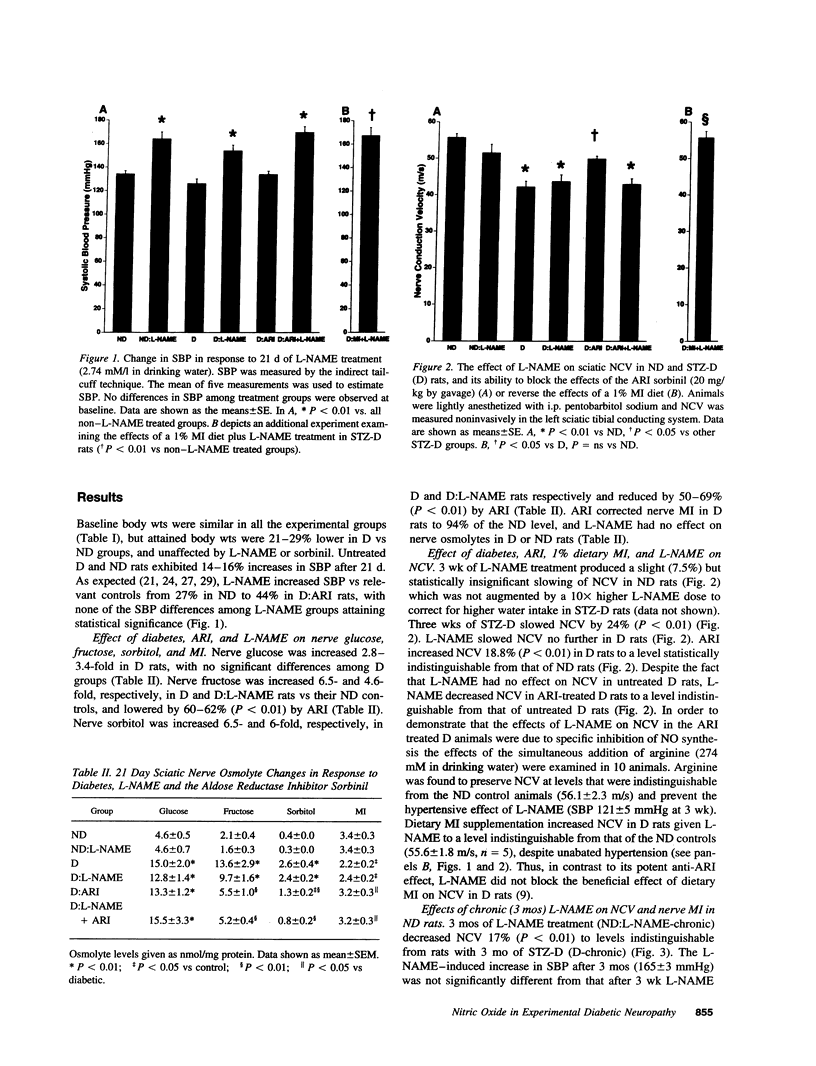
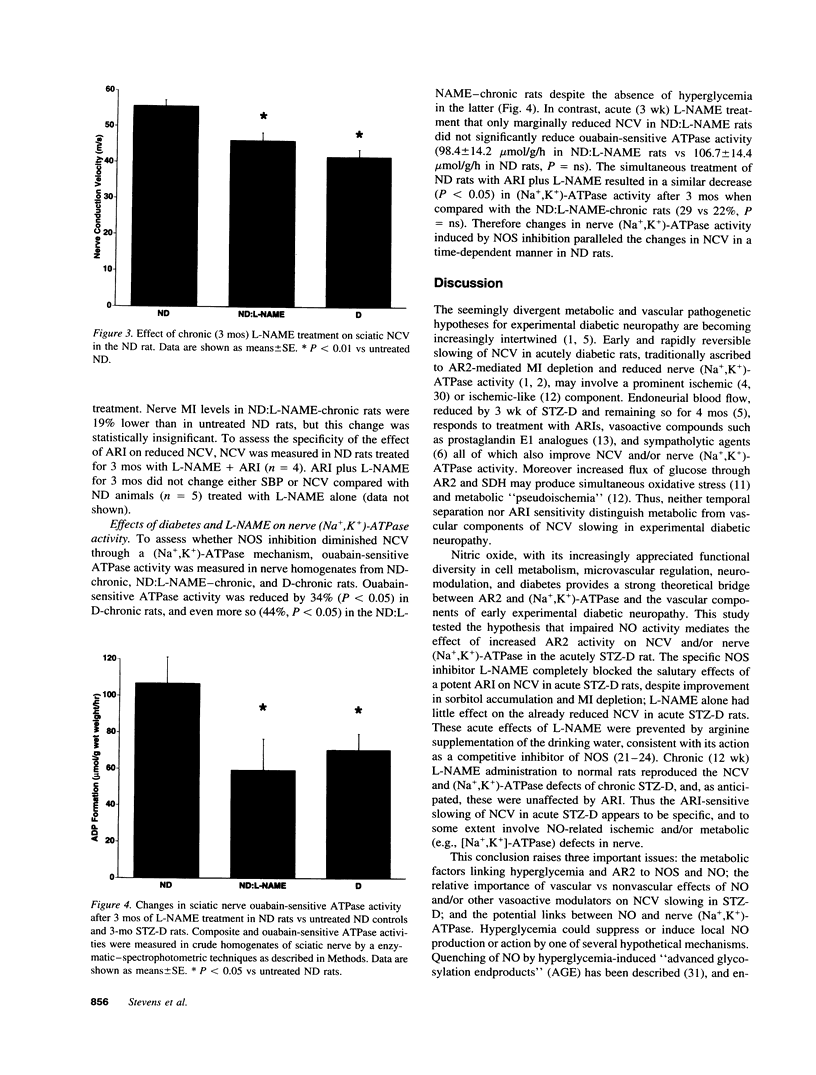
Metabolic and vascular factors have been invoked in the pathogenesis of diabetic neuropathy but their interrelationships are poorly understood. Both aldose reductase inhibitors and vasodilators improve nerve conduction velocity, blood flow, and (Na+,K+)-ATPase activity in the streptozotocin diabetic rat, implying a metabolic-vascular interaction. NADPH is an obligate cofactor for both aldose reductase and nitric oxide synthase such that activation of aldose reductase by hyperglycemia could limit nitric oxide synthesis by cofactor competition, producing vasoconstriction, ischemia, and slowing of nerve conduction. In accordance with this construct, N-nitro-L-arginine methyl ester, a competitive inhibitor of nitric oxide synthase reversed the increased nerve conduction velocity afforded by aldose reductase inhibitor treatment in the acutely diabetic rat without affecting the attendant correction of nerve sorbitol and myo-inositol. With prolonged administration, N-nitro-L-arginine methyl ester fully reproduced the nerve conduction slowing and (Na+,K+)-ATPase impairment characteristic of diabetes. Thus the aldose reductase-inhibitor-sensitive component of conduction slowing and the reduced (Na+,K+)-ATPase activity in the diabetic rat may reflect in part impaired nitric oxide activity, thus comprising a dual metabolic-ischemic pathogenesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aanderud S., Krane H., Nordøy A. Influence of glucose, insulin and sera from diabetic patients on the prostacyclin synthesis in vitro in cultured human endothelial cells. Diabetologia. 1985 Sep;28(9):641–644. doi: 10.1007/BF00291967. [DOI] [PubMed] [Google Scholar]
- Bazán N. G., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970 Oct 6;218(1):1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Calver A., Collier J., Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992 Dec;90(6):2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A., Ferguson K., Robertson S., Radcliffe M. A. Effects of chronic alpha-adrenergic receptor blockade on peripheral nerve conduction, hypoxic resistance, polyols, Na(+)-K(+)-ATPase activity, and vascular supply in STZ-D rats. Diabetes. 1991 Dec;40(12):1652–1658. doi: 10.2337/diab.40.12.1652. [DOI] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A. Impaired contraction and relaxation in aorta from streptozotocin-diabetic rats: role of polyol pathway. Diabetologia. 1992 Nov;35(11):1011–1019. doi: 10.1007/BF02221675. [DOI] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A., Low P. A. Nerve blood flow in early experimental diabetes in rats: relation to conduction deficits. Am J Physiol. 1991 Jul;261(1 Pt 1):E1–E8. doi: 10.1152/ajpendo.1991.261.1.E1. [DOI] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A., Robertson S. Angiotensin converting enzyme inhibition prevents development of muscle and nerve dysfunction and stimulates angiogenesis in streptozotocin-diabetic rats. Diabetologia. 1992 Jan;35(1):12–18. doi: 10.1007/BF00400846. [DOI] [PubMed] [Google Scholar]
- Carroll P. B., Thornton B. M., Greene D. A. Glutathione redox state is not the link between polyol pathway activity and myo-inositol-related Na+-K+-ATPase defect in experimental diabetic neuropathy. Diabetes. 1986 Nov;35(11):1282–1285. doi: 10.2337/diab.35.11.1282. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Sima A. A., Nakajima T., Yagihashi S., Greene D. A. Aldose reductase in the BB rat: isolation, immunological identification and localization in the retina and peripheral nerve. Diabetologia. 1987 Apr;30(4):244–251. doi: 10.1007/BF00270423. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Davidson C. M., DeRubertis F. R. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990 Jun;39(6):667–674. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Protein kinase C is activated in glomeruli from streptozotocin diabetic rats. Possible mediation by glucose. J Clin Invest. 1989 May;83(5):1667–1675. doi: 10.1172/JCI114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dananberg J., Sider R. S., Grekin R. J. Sustained hypertension induced by orally administered nitro-L-arginine. Hypertension. 1993 Mar;21(3):359–363. doi: 10.1161/01.hyp.21.3.359. [DOI] [PubMed] [Google Scholar]
- Durante W., Sen A. K., Sunahara F. A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol. 1988 Jun;94(2):463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Regional haemodynamic changes during oral ingestion of NG-monomethyl-L-arginine or NG-nitro-L-arginine methyl ester in conscious Brattleboro rats. Br J Pharmacol. 1990 Sep;101(1):10–12. doi: 10.1111/j.1476-5381.1990.tb12079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983 Sep;72(3):1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Sima A. A., Stevens M. J., Feldman E. L., Lattimer S. A. Complications: neuropathy, pathogenetic considerations. Diabetes Care. 1992 Dec;15(12):1902–1925. doi: 10.2337/diacare.15.12.1902. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Gupta S., Sussman I., McArthur C. S., Tornheim K., Cohen R. A., Ruderman N. B. Endothelium-dependent inhibition of Na(+)-K+ ATPase activity in rabbit aorta by hyperglycemia. Possible role of endothelium-derived nitric oxide. J Clin Invest. 1992 Sep;90(3):727–732. doi: 10.1172/JCI115944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M., Cerami A., Bucala R. Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide. Role in the vascular and renal complications of diabetes mellitus. J Clin Invest. 1992 Sep;90(3):1110–1115. doi: 10.1172/JCI115928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol. 1989 Jun;97(2):614–618. doi: 10.1111/j.1476-5381.1989.tb11993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Miranda-Guardiola F., Moncada S., Romero J. C. Effects of NG-nitro-L-arginine methyl ester on renal function and blood pressure. Am J Physiol. 1991 Dec;261(6 Pt 2):F1033–F1037. doi: 10.1152/ajprenal.1991.261.6.F1033. [DOI] [PubMed] [Google Scholar]
- Lou M. F., Dickerson J. E., Jr, Garadi R., York B. M., Jr Glutathione depletion in the lens of galactosemic and diabetic rats. Exp Eye Res. 1988 Apr;46(4):517–530. doi: 10.1016/s0014-4835(88)80009-5. [DOI] [PubMed] [Google Scholar]
- Lou M. F., Dickerson J. E., Jr, Garadi R., York B. M., Jr Glutathione depletion in the lens of galactosemic and diabetic rats. Exp Eye Res. 1988 Apr;46(4):517–530. doi: 10.1016/s0014-4835(88)80009-5. [DOI] [PubMed] [Google Scholar]
- Low P. A., Lagerlund T. D., McManis P. G. Nerve blood flow and oxygen delivery in normal, diabetic, and ischemic neuropathy. Int Rev Neurobiol. 1989;31:355–438. doi: 10.1016/s0074-7742(08)60283-4. [DOI] [PubMed] [Google Scholar]
- Mahadik S. P., Bharucha V. A., Stadlin A., Ortiz A., Karpiak S. E. Loss and recovery of activities of alpha+ and alpha isozymes of (Na(+) + K+)-ATPase in cortical focal ischemia: GM1 ganglioside protects plasma membrane structure and function. J Neurosci Res. 1992 Jun;32(2):209–220. doi: 10.1002/jnr.490320210. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G. Impairment of endothelium-dependent dilatation of cerebral arterioles during diabetes mellitus. Am J Physiol. 1989 Mar;256(3 Pt 2):H621–H625. doi: 10.1152/ajpheart.1989.256.3.H621. [DOI] [PubMed] [Google Scholar]
- Mayhan W. G., Simmons L. K., Sharpe G. M. Mechanism of impaired responses of cerebral arterioles during diabetes mellitus. Am J Physiol. 1991 Feb;260(2 Pt 2):H319–H326. doi: 10.1152/ajpheart.1991.260.2.H319. [DOI] [PubMed] [Google Scholar]
- Mayhew J. A., Gillon K. R., Hawthorne J. N. Free and lipid inositol, sorbitol and sugars in sciatic nerve obtained post-mortem from diabetic patients and control subjects. Diabetologia. 1983 Jan;24(1):13–15. doi: 10.1007/BF00275940. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nukada H., Dyck P. J. Microsphere embolization of nerve capillaries and fiber degeneration. Am J Pathol. 1984 May;115(2):275–287. [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pieper G. M., Gross G. J. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1988 Oct;255(4 Pt 2):H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- Radoff S., Cerami A., Vlassara H. Isolation of surface binding protein specific for advanced glycosylation end products from mouse macrophage-derived cell line RAW 264.7. Diabetes. 1990 Dec;39(12):1510–1518. doi: 10.2337/diab.39.12.1510. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Shindo H., Tawata M., Aida K., Onaya T. The role of cyclic adenosine 3',5'-monophosphate and polyol metabolism in diabetic neuropathy. J Clin Endocrinol Metab. 1992 Feb;74(2):393–398. doi: 10.1210/jcem.74.2.1370506. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Hay K. Functional aspects and pathogenetic considerations of the neuropathy in the spontaneously diabetic BB-Wistar rat. Neuropathol Appl Neurobiol. 1981 Sep-Oct;7(5):341–350. doi: 10.1111/j.1365-2990.1981.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Simmons D. A., Winegrad A. I. Mechanism of glucose-induced (Na+, K+)-ATPase inhibition in aortic wall of rabbits. Diabetologia. 1989 Jul;32(7):402–408. doi: 10.1007/BF00271258. [DOI] [PubMed] [Google Scholar]
- Smith S. S., Li J. Novel action of nitric oxide as mediator of N-methyl-D-aspartate-induced phosphatidylinositol hydrolysis in neonatal rat cerebellum. Mol Pharmacol. 1993 Jan;43(1):1–5. [PubMed] [Google Scholar]
- Smits P., Kapma J. A., Jacobs M. C., Lutterman J., Thien T. Endothelium-dependent vascular relaxation in patients with type I diabetes. Diabetes. 1993 Jan;42(1):148–153. doi: 10.2337/diab.42.1.148. [DOI] [PubMed] [Google Scholar]
- Sonobe M., Yasuda H., Hisanaga T., Maeda K., Yamashita M., Kawabata T., Kikkawa R., Taniguchi Y., Shigeta Y. Amelioration of nerve Na(+)-K(+)-ATPase activity independently of myo-inositol level by PGE1 analogue OP-1206.alpha-CD in streptozocin-induced diabetic rats. Diabetes. 1991 Jun;40(6):726–730. doi: 10.2337/diab.40.6.726. [DOI] [PubMed] [Google Scholar]
- Stevens M. J., Lattimer S. A., Kamijo M., Van Huysen C., Sima A. A., Greene D. A. Osmotically-induced nerve taurine depletion and the compatible osmolyte hypothesis in experimental diabetic neuropathy in the rat. Diabetologia. 1993 Jul;36(7):608–614. doi: 10.1007/BF00404069. [DOI] [PubMed] [Google Scholar]
- Takeuchi M., Low P. A. Dynamic peripheral nerve metabolic and vascular responses to exsanguination. Am J Physiol. 1987 Oct;253(4 Pt 1):E349–E353. doi: 10.1152/ajpendo.1987.253.4.E349. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B., Jakubowski J. A., Cohen R. A. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989 Nov;257(5 Pt 2):H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Tilton R. G., Baier L. D., Harlow J. E., Smith S. R., Ostrow E., Williamson J. R. Diabetes-induced glomerular dysfunction: links to a more reduced cytosolic ratio of NADH/NAD+. Kidney Int. 1992 Apr;41(4):778–788. doi: 10.1038/ki.1992.121. [DOI] [PubMed] [Google Scholar]
- Tsou K., Snyder G. L., Greengard P. Nitric oxide/cGMP pathway stimulates phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, in the substantia nigra. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3462–3465. doi: 10.1073/pnas.90.8.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Ward K. K., Low P. A., Schmelzer J. D., Zochodne D. W. Prostacyclin and noradrenaline in peripheral nerve of chronic experimental diabetes in rats. Brain. 1989 Feb;112(Pt 1):197–208. doi: 10.1093/brain/112.1.197. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Chang K., Frangos M., Hasan K. S., Ido Y., Kawamura T., Nyengaard J. R., van den Enden M., Kilo C., Tilton R. G. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993 Jun;42(6):801–813. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- Wolfe L. S. Eicosanoids: prostaglandins, thromboxanes, leukotrienes, and other derivatives of carbon-20 unsaturated fatty acids. J Neurochem. 1982 Jan;38(1):1–14. doi: 10.1111/j.1471-4159.1982.tb10847.x. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Sonobe M., Yamashita M., Terada M., Hatanaka I., Huitian Z., Shigeta Y. Effect of prostaglandin E1 analogue TFC 612 on diabetic neuropathy in streptozocin-induced diabetic rats. Comparison with aldose reductase inhibitor ONO 2235. Diabetes. 1989 Jul;38(7):832–838. doi: 10.2337/diab.38.7.832. [DOI] [PubMed] [Google Scholar]
- de la Rubia G., Oliver F. J., Inoguchi T., King G. L. Induction of resistance to endothelin-1's biochemical actions by elevated glucose levels in retinal pericytes. Diabetes. 1992 Dec;41(12):1533–1539. doi: 10.2337/diabetes.41.12.1533. [DOI] [PubMed] [Google Scholar]


