Abstract
The hedgehog (Hh)/glioma-associated oncogene (GLI) signaling network is among the most important and fascinating signal transduction systems that provide critical functions in the regulation of many developmental and physiological processes. The coordinated spatiotemporal interplay of the Hh ligands and other growth factors is necessary for the stringent control of the behavior of diverse types of tissue-resident stem/progenitor cells and their progenies. The activation of the Hh cascade might promote the tissue regeneration and repair after severe injury in numerous organs, insulin production in pancreatic β-cells, and neovascularization. Consequently, the stimulation of the Hh pathway constitutes a potential therapeutic strategy to treat diverse human disorders, including severe tissue injuries; diabetes mellitus; and brain, skin, and cardiovascular disorders. In counterbalance, a deregulation of the Hh signaling network might lead to major tissular disorders and the development of a wide variety of aggressive and metastatic cancers. The target gene products induced through the persistent Hh activation can contribute to the self-renewal, survival, migration, and metastasis of cancer stem/progenitor cells and their progenies. Moreover, the pivotal role mediated through the Hh/GLI cascade during cancer progression also implicates the cooperation with other oncogenic products, such as mutated K-RAS and complex cross-talk with different growth factor pathways, including tyrosine kinase receptors, such as epidermal growth factor receptor (EGFR), Wnt/β-catenin, and transforming growth factor-β (TGF-β)/TGF-β receptors. Therefore, the molecular targeting of distinct deregulated gene products, including Hh and EGFR signaling components and other signaling elements that are frequently deregulated in highly tumorigenic cancer-initiating cells and their progenies, might constitute a potential therapeutic strategy to eradicate the total cancer cell mass. Of clinical interest is that these multitargeted approaches offer great promise as adjuvant treatments for improving the current antihormonal therapies, radiotherapies, and/or chemotherapies against locally advanced and metastatic cancers, thereby preventing disease relapse and the death of patients with cancer.
I. Introduction
The hedgehog (Hh1)/glioma-associated oncogene (GLI) developmental cascade is a highly evolutionarily conserved signaling pathway that serves critical functions in the regulation of the normal cell-fate specification, tissue polarity and patterning, and organogenesis during embryogenesis as well as the maintenance of the tissue homeostasis and repair after severe injuries in postnatal and adult life (Bak et al., 2003; McMahon et al., 2003; Cohen, 2003; Palma et al., 2005; Kasper et al., 2006a; Nielsen et al., 2008; Varjosalo and Taipale, 2008; Amankulor et al., 2009; Vaillant and Monard, 2009; Yauch et al., 2009). In particular, sonic hedgehog (SHH)/patched receptor 1 (PTCH1)/smoothened (SMO) coreceptor/GLI transcription factors are recognized as key players that provide a pivotal role in the stringent regulation of important cellular responses. The Hh signaling pathway, in conjunction with other developmental cascades, such as EGF/EGFR and Wnt/β-catenin, regulate the self-renewal ability versus differentiation; survival; intercellular and cell-matrix adhesion; and migration of diverse types of embryonic, fetal, and tissue-resident adult stem/progenitor cells and their progenies (Cohen, 2003; Beachy et al., 2004; Palma and Ruiz i Altaba, 2004; Palma et al., 2005; Katoh and Katoh, 2006; Liu et al., 2006; Sicklick et al., 2006; Zhou et al., 2006; Lin et al., 2007; Shi et al., 2008; Varjosalo and Taipale, 2008; Amankulor et al., 2009; Rittié et al., 2009). Conversely, the genetic abnormalities that belong to the Hh/GLI signaling pathway might result in an aberrant cell growth, differentiation, and migration concomitant with major tissue homeostatic imbalance and severe disorders (Bak et al., 2003; Cohen, 2003; Beachy et al., 2004; Kasper et al., 2006a; Liu et al., 2006; Varjosalo and Taipale, 2008; Vaillant and Monard, 2009). The disorders associated with inherited or somatic alterations in the Hh signaling network include holoprosencephaly, the embryonic defect most often seem in these disorders, in which the forebrain and the face fail to develop; congenital ataxia; microcephaly; mental retardation; brain, skin, ocular and pancreatic disorders; and pediatric and adult cancer development (Ming et al., 1998; Odent et al., 1999; Bale, 2002; Bak et al., 2003; Cohen, 2003; Beachy et al., 2004; Maity et al., 2005; Lau et al., 2006; Vaillant and Monard, 2009).
Numerous studies have shown that the genetic and/or epigenetic alterations leading to the enhanced expression levels and/or activities of Hh signaling elements in stem/progenitor cells commonly occur in a wide variety of human cancers during etiopathogenesis and disease progression to locally invasive and metastatic stages (Berman et al., 2003; Cohen, 2003; Beachy et al., 2004; Rubin and de Sauvage, 2006; Taniguchi et al., 2007; Bhattacharya et al., 2008; Mimeault and Batra, 2008a; Mimeault et al., 2008; Tada et al., 2008; Varjosalo and Taipale, 2008; Schnidar et al., 2009; Yang et al., 2010; Mimeault and Batra, 2010c). Human cancer types frequently harboring a deregulation in the Hh pathway include leukemia, multiple myeloma, and brain, skin, head and neck, lung, liver, gastrointestinal, colorectal, pancreatic, prostate, mammary, ovarian, and renal carcinomas (Berman et al., 2003; Thayer et al., 2003; Karhadkar et al., 2004; Oniscu et al., 2004; Sanchez et al., 2004; Sheng et al., 2004; Ohta et al., 2005; Datta and Datta, 2006; Douard et al., 2006; Mimeault et al., 2006, 2007a; Bian et al., 2007; Chen et al., 2007b; Stecca et al., 2007; Taniguchi et al., 2007; Bhattacharya et al., 2008; Hegde et al., 2008; Tada et al., 2008; Eichenmüller et al., 2009). More importantly, accumulating lines of evidence have also revealed that the persistent activation of the Hh cascade may represent a critical step in the malignant transformation of cancer stem/progenitor cells (also designated as cancer- and metastasis-initiating cells), treatment resistance, and disease relapse (Liu et al., 2006; Bar et al., 2007; Clement et al., 2007; Ehtesham et al., 2007; Mimeault et al., 2007b, 2008; Peacock et al., 2007; Mimeault and Batra, 2008a, 2010b,c; Xu et al., 2008; Kobune et al., 2009; Ward et al., 2009). The sustained activation of the Hh signal transduction pathway might lead, in an autocrine or a paracrine manner, to the modulation of the expression levels and/or activities of numerous target gene products (Cohen, 2003; Beachy et al., 2004; Douard et al., 2006; Eichberger et al., 2006; Kasper et al., 2006a; Li et al., 2006; Clement et al., 2007; Feldmann et al., 2007; Bhattacharya et al., 2008; Mimeault and Batra, 2008a; Mimeault et al., 2008; Varjosalo and Taipale, 2008; Klarmann et al., 2009; Laner-Plamberger et al., 2009; Liao et al., 2009; Park et al., 2009). These signaling elements can contribute to the proliferation, survival, migration, invasion, and metastasis of cancer cells. Moreover, multiple cross-talks between the Hh cascade and other tumorigenic signaling components, including receptor tyrosine kinases (RTKs) such as EGFR, can cooperate during cancer initiation and progression to aggressive, invasive, and metastatic disease stages (Xie et al., 2001; Bigelow et al., 2005; Palma et al., 2005; Kasper et al., 2006b; Mimeault et al., 2006; Riobo et al., 2006a; Stecca et al., 2007; Schnidar et al., 2009; Seto et al., 2009).
In this review article, the most recent advancements on the structural and functional characterization of diverse signal transduction elements of Hh signaling network and molecular mechanisms involved in their regulation are described. The physiological functions mediated through the Hh signaling pathway during embryonic development and adult life are reviewed. The frequent deregulations in the Hh signaling network associated with diverse diseases and cancer development, and potential interactive cross-talk with other developmental cascades, including EGFR, are also discussed. The results from recent studies underlining the therapeutic interest of cotargeting Hh and EGFR pathways and other oncogenic cascades for reversing treatment resistance, eradicating the cancer stem/progenitor cells and their progenies, and improving the current clinical therapies against aggressive and metastatic cancers are also reviewed.
II. The Hedgehog Signal Transduction Pathway and Regulatory Mechanisms
It is important to understand the regulation of the Hh signaling network at the molecular level in the normal adult stem/progenitor cells and their progenies as well as how it is deregulated during carcinogenesis. This knowledge will allow us to identify new drug targets and develop novel therapeutic strategies to block this tumorigenic cascade and thus improve the current cancer treatments. Many efforts made in the last few years have led to the structural and functional characterization of diverse Hh signaling components that can contribute in a cell type- and concentration-dependent manner to the signal transduction. Important information has also been obtained about complex regulatory mechanisms that modulate the Hh-induced cellular responses in normal and pathological conditions (McMahon et al., 2003; Cohen, 2003; Palma and Ruiz i Altaba, 2004; Palma et al., 2005; Kasper et al., 2006a; Varjosalo and Taipale, 2008). In this matter, we describe the structural features and functions of Hh ligands, PTCH1 receptor, SMO coreceptor, and GLI transcription factors in mediating the Hh signal transduction and cellular responses as well as the molecular mechanisms implicated in the regulation of their functions.
A. Structures and Mechanisms of Actions of Hedgehog Ligands
The Hh gene has been first identified to control the segmentation pattern of fruit fly Drosophila melanogaster during embryogenesis (Nüsslein-Volhard and Wieschaus, 1980). Subsequent investigations have led to the identification of three Hh homologous genes in mammalian tissues encoding three different Hh proteins (Marigo et al., 1995). The Hh proteins include SHH, Indian hedgehog (IHH), and Desert hedgehog (DHH) (Marigo et al., 1995). All three mammalian Hh proteins are able to specifically bind the PTCH1 receptor and activate the Hh pathway in a time- and concentration-dependent manner (Pathi et al., 2001). The specific and redundant biological functions of Hh proteins is governed in part by their expression patterns and diverse regulatory mechanisms in a given cell type (Pathi et al., 2001). Among them, the SHH protein is the most extensively studied and characterized ligand of the Hh signaling pathway. Human SHH protein shows a similarity of 92.4% in the amino acid sequence with its murine homolog (Marigo et al., 1995). It has been shown that the SHH protein plays key roles in controlling organogenesis and morphogenesis of a variety of tissues and organs and epithelial-mesenchymal interactions during the vertebrate embryonic development as well as in the regulation of adult stem/progenitor cell behavior (Cohen, 2003; Beachy et al., 2004; Palma and Ruiz i Altaba, 2004; Palma et al., 2005; Katoh and Katoh, 2006; Liu et al., 2006; Zhou et al., 2006; Lin et al., 2007; Shi et al., 2008; Varjosalo and Taipale, 2008; Amankulor et al., 2009; Rittié et al., 2009).
1. Structural Organization, Processing and Secretion of the Sonic Hedgehog Ligand.
The human Shh gene maps to chromosome 7 in the region 7q36 and consists of a DNA sequence of 9410 base pairs that encompass three exons (Marigo et al., 1995). Human SHH ligand is synthesized under a 462-amino acid (aa) protein precursor of approximately 45 kDa designated as preproprotein (Fig. 1) (Odent et al., 1999). The preproprotein is composed of a 23-aa signal peptide sequence, a 174-aa signaling domain, and a 265-aa autoprocessing domain endowed with an autoproteolysis activity and a cholesterol transferase activity (Fig. 1). During the post-translational processing of preproprotein in the endoplasmic reticulum, the short N-terminal hydrophobic signal peptide sequence is removed by a signal peptidase. The SHH precursor then undergoes an autocatalytic intramolecular cleavage at position 198 catalyzed by its C-terminal domain, yielding an N-terminal signaling product of approximately 19 kDa, which represents the mature and biologically active SHH form, and a C-terminal product with no known signaling function (Fig. 1) (Porter et al., 1995; Cohen, 2003; Varjosalo and Taipale, 2008). During this reaction, a cholesterol moiety is covalently attached at the C-terminal residue of the cleaved N-terminal signaling fragment of SHH (Cohen, 2003; Varjosalo and Taipale, 2008). Moreover, the N-terminal cysteine residue of the cleaved N-terminal signaling fragment of SHH is also modified by palmitoylation (Fig. 1) (Buglino and Resh, 2008). It has been reported that a stable attachment of a fatty acid [a palmitate molecule catalyzed by a palmitoylacyltransferase, Hh acyltransferase (Hhat)] might occur on both the SHH precursor and SHH protein along the secretory pathway (Fig. 1) (Buglino and Resh, 2008). These hydrophobic lipid modifications of mature SHH protein might promote its interaction with caveolin, tethering at the plasma membrane within caveolin- and cholesterol-enriched lipid raft microdomains, designated as caveolea, and thereby increase its local concentration and the efficiency of signal transduction (Karpen et al., 2001; Cohen, 2003; Mao et al., 2009). Hence, the mature and lipid-modified SHH protein resulting from intracellular processing may be secreted from cells into the extracellular compartment and mediated its biological effects on responsive cells (Fig. 1).
Fig. 1.
Schematic representation of the molecular events associated with cellular processing, lipid modification, and secretion of the SHH protein and the autocrine and paracrine actions of mature and secreted SHH protein. The scheme shows the molecular mechanisms associated with the cellular processing of the SHH protein precursor, including the cleavage of its N-terminal signal peptide fragment. The autocatalytic cleavage at the position 198 of SHH protein precursor catalyzed by its C-terminal fragment, which results in the release of cleaved N-terminal and C-terminal products, is also shown. Moreover, the lipid modifications of cleaved N-terminal SHH fragment via the attachment of a cholesterol moiety at the C-terminal position and a palmitate molecule at the N-terminal position catalyzed by the palmitoylacyltransferase Hhat are also indicated. The secretion of the mature and lipid-modified SHH protein into extracellular space as well as its diffusion and potential autocrine or paracrine action on secreting and neighboring responsive cells are also illustrated. In addition, the function of dispatched (DISP) transmembrane protein in the formation of large SHH oligomers and their secretion in extracellular compartment is illustrated. Moreover, the inhibitory effect on the SHH actions induced through the sequestration of cell surface-associated SHH molecules by HHIP is shown.
2. Autocrine and Paracrine Mechanisms of Actions of Hedgehog Ligands.
The secreted SHH ligand can act, in autocrine and paracrine manners, under the form of monomers and/or oligomers on producing cells and responsive cells localized near or at a distant localization of the secreting cells (Fig. 1). In fact, the secreted SHH protein and other Hh ligands, IHH and DHH, can diffuse and act as morphogens by forming a concentration gradient for short- and long-range actions (Fig. 1) (Cohen, 2003; Varjosalo and Taipale, 2008; Vyas et al., 2008). In this regard, the results from a recent study have also indicated that the full-length unprocessed SHH protein can traffic to the plasma membrane and thereby participate in a localized manner to certain short-range effects (Tokhunts et al., 2010). More specifically, the paracrine signals mediated through the Hh cascade require the release of the membrane-tethered Hh ligands from producing cells and their transport to the surrounding-responsive cells or more distant cells, including the stromal cells (Fig. 1) (Cohen, 2003; Vyas et al., 2008; Dierker et al., 2009a,b). This diffusion process, which is accomplished through the formation of large nanoscale oligomers by ligand molecules, might be modulated through different molecular mechanisms. In particular, the release of Hh ligand oligomers from producing cells may be promoted via their interaction with a 12-pass transmembrane protein known as dispatched, and cell-surface heparan sulfate proteoglycans (Fig. 1) (Cohen, 2003; Dierker et al., 2009a,b). The formation of large Hh ligand oligomers may permit their release into the extracellular compartment and transport via the lipoprotein carriers over a long distance, where they can act in a paracrine fashion on surrounding cells (Fig. 1) (Vyas et al., 2008; Dierker et al., 2009a,b). Conversely, a negative regulatory feedback loop might also be induced through the enhanced expression of an endogenous Hh inhibitor, hedgehog-interacting protein (HHIP), found at the plasma membrane, that can interact with a high affinity with the three Hh ligands (Chuang and McMahon, 1999; Cohen, 2003; Varjosalo and Taipale, 2008; Bosanac et al., 2009). This molecular event would impede the binding of Hh ligands to the PTCH1 receptor and inhibit the signal transduction (Fig. 1).
B. Hedgehog Signal Transduction
A simplified view of the stimulation of the Hh signaling network implicates the binding of a secreted Hh protein, including SHH, IHH, or DHH ligand to its cognate 12-pass transmembrane PTCH1 or PTCH2 receptor on responsive cells (Fig. 1) (Stone et al., 1996; Carpenter et al., 1998; Fuse et al., 1999; Kalderon, 2000; Taipale et al., 2002; Cohen, 2003). The PTCH1, which is the better characterized receptor of Hh ligands, displayed 54% sequence homology with PTCH2 protein. Despite the fact that all three Hh ligands can bind both PTCH1 and PTCH2, the specific functions of these receptors depend in part of their expression pattern (Carpenter et al., 1998; Cohen, 2003). In general, the binding of a Hh ligand to PTCH1 relieves the repressive effect induced by this receptor on the activity of its signaling partner, a seven-pass transmembrane coreceptor, SMO protein (Kalderon, 2000; Taipale et al., 2002; Cohen, 2003; Rubin and de Sauvage, 2006; Varjosalo and Taipale, 2008). The stimulation of the SMO signaling transduction element results in the activation of cytoplasmic GLIs and their translocation to the nucleus, where they participate with other transcription factors in the stringent regulation of the expression of numerous Hh target gene products (Fig. 1) (Cohen, 2003; Kasper et al., 2006a; Kasai et al., 2008; Varjosalo and Taipale, 2008; Jia et al., 2009; Yue et al., 2009).
Although work has been done to characterize the different signaling elements of the canonical Hh cascade, the molecular events and signaling molecules involved in the repressive effect induced through the PTCH1 receptor on SMO activity in the absence of the Hh ligand and stimulation of the SMO protein in the presence of the Hh ligand remain not precisely established. Different models of the molecular mechanisms of Hh signal transduction have been proposed to explain the repressive effect induced by PTCH1 in the absence of Hh ligand on SMO activity and the activation of SMO coreceptor after the formation of Hh ligand-PTCH1 complexes (Fig. 2) (Taipale et al., 2002; Rubin and de Sauvage, 2006; Rohatgi and Scott, 2007). In general, it has been proposed that the binding of the Hh ligand, including SHH protein, to PTCH1 might result in a SMO conformational change from inactive to active state (Taipale et al., 2002; Rubin and de Sauvage, 2006; Rohatgi and Scott, 2007). More specifically, the formation of the Hh ligand-PTCH1 receptor complexes might indirectly stimulate the SMO activity, possibly through the induction of membrane changes, activation of intracellular positive modulators, and/or stimulation of an endogenous SMO agonist (Fig. 2a and b) (Rosenbaum and Witman, 2002; Taipale et al., 2002; Bijlsma et al., 2006; Corcoran and Scott, 2006; Rubin and de Sauvage, 2006; Rohatgi and Scott, 2007; Rohatgi et al., 2007).
Fig. 2.
Proposed models of the molecular mechanisms involved in the regulation of ligand-dependent and independent-SMO activation and modulation by the pharmacological agents. a, the binding of SHH protein to the PTCH1 transmembrane receptor might lead to either membrane changes or activation of an endogenous SMO agonist. These molecular events, in turn, may result in the adoption of an active conformation by the SMO transmembrane protein and the stimulation of SMO-mediated cellular response. b, in the absence of SHH ligand, the PTCH1 receptor can act as a transporter and pump the endogenous cholesterol derivatives, such as oxysterols, out of the cells. The binding of the SHH protein to PTCH1 receptor, however, might inhibit the efflux of cholesterol derivatives such as oxysterols, and thereby promote the adoption of an active conformation by the SMO protein and SMO-induced cellular response. The occurrence of activating mutations in the SMO oncoprotein (c) or inactivating mutations in the PTCH1 tumor suppressor protein (d) might result in the adoption of an active conformation by SMO protein in the absence of SHH ligand and a sustained induction of a cellular response. In the same way, the exposure of cells to a pharmacological agent acting as a SMO agonist (e) also can induce the adoption of an active conformation by the SMO protein and a cellular response. In contrast, the exposure of cells to a chemical compound acting as a SMO antagonist (f), such as cyclopamine, KAAD-cyclopamine, IPI-269609, or GDC-0449, can inhibit the SHH protein-induced SMO activation and cellular response.
In view of the fact that the PTCH1 receptor contains a sterol-sensing domain and shows a structural homology with diverse family members of membrane transporters, such as Niemann-Pick C1 protein and bacterial proton-driven transmembrane molecular transporters, it has been proposed that the PTCH1 receptor can act as a transmembrane transporter of small molecules (Davies et al., 2000; Strutt et al., 2001; Taipale et al., 2002; Corcoran and Scott, 2006; Rubin and de Sauvage, 2006). Then, the PTCH1 transporter, unbound by Hh ligand, could pump the endogenous molecules, such as cholesterol derivatives, including oxysterols, out of cells (Fig. 2b). The binding of Hh ligand to PTCH1, however, could lead to the intracellular accumulation of endogenous molecules, including oxysterols, that, in turn, can positively modulate the SMO activity (Fig. 2b) (Corcoran and Scott, 2006; Dwyer et al., 2007).
Consistent with these models, it has been observed that the activating mutations in the SMO protein or inactivating mutations in the PTCH1 receptor might lead to the adoption of a constitutively active conformation by the SMO protein (Fig. 2, c and d) (Johnson et al., 1996; Raffel et al., 1997; Rubin and de Sauvage, 2006). Moreover, it has been observed that the sterol synthesis inhibitors reduced SHH induced-target gene transcription and blocked SHH pathway-dependent proliferation of medulloblastoma cells (Corcoran and Scott, 2006). The inhibitory effect induced by the sterol inhibitors, however, could be reversed by a treatment of medulloblastoma cells with exogenous cholesterol or specific oxysterols (Corcoran and Scott, 2006). In addition, different SMO full agonists (such as the synthetic chlorobenzothiophene-containing SMO agonist termed SAG) and antagonists (including a plant-derived steroidal alkaloid, cyclopamine) have been shown to specifically interact with the heptahelical bundle of the SMO protein, and thereby modulate its activity and cellular response (Fig. 2e and f) (Chen et al., 2002; Frank-Kamenetsky et al., 2002; Corcoran and Scott, 2006; Rubin and de Sauvage, 2006). It is noteworthy that recent accumulating lines of experimental evidence have also indicated that the primary cilium found in Hh responsive cells might play a critical role in the activation of Hh signal transduction in certain normal and cancer cell types.
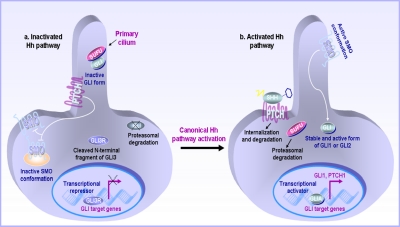
1. Roles of the Primary Cilium in the Hedgehog Signal Transduction Mechanism.
Recent studies have revealed that an extracellular projection found at the cell surface, designated as primary cilium, which is a microtubule-based organelle, constitutes a key specialized structure that is required to concentrate the Hh signaling components and trigger the SMO-mediated canonical pathway in certain types of SHH-responsive cells (Fig. 3) (Rosenbaum and Witman, 2002; Corbit et al., 2005; Haycraft et al., 2005; Rohatgi and Scott, 2007; Rohatgi et al., 2007; Han et al., 2008; Spassky et al., 2008; Bailey et al., 2009; Han et al., 2009; Veland et al., 2009; Wong et al., 2009). More specifically, it has been shown that the PTCH1 receptor unbound by the SHH ligand is localized at the base of the primary cilium and can prevent the SMO ciliary localization (Fig. 3) (Rohatgi et al., 2007). Moreover, all three full-length GLI proteins as well as the negative regulator of GLI activities, suppressor of fused (SUFU) are also colocalized at the distal tip of cilium in the absence of the Hh ligand (Fig. 3) (Haycraft et al., 2005; Rohatgi et al., 2007). The binding of secreted SHH protein to the PTCH1 receptor found at the primary cilium, however, might result in a decreased number of PTCH1 molecules in the primary cilium because of its re-localization at the cell surface out of the ciliary structure, its internalization in intracellular vesicles, and/or degradation (Rohatgi et al., 2007). Hence, the exclusion of PTCH1 molecules from the ciliary structure may allow SMO molecules to translocate to the plasma membrane in the primary cilium, and thereby lead to the activation of downstream GLI transcriptional factors and Hh target gene expression (Fig. 3) (Corbit et al., 2005; Rohatgi et al., 2007).
Fig. 3.
Schematic representation of molecular events associated with the repressive effect induced by PTCH1 receptor on the SMO activity and the activation of the Hh signaling pathway mediated by the SHH protein in the primary cilium. a, in the absence of the SHH ligand, PTCH1 is localized at the base of the ciliary structure and inhibits SMO protein translocation to the primary cilium and its activation. In the absence of activated SMO protein, the negative modulator of Hh cascade SUFU sequesters full-length GLI proteins in the cytoplasm and prevents their nuclear translocation, Hh target gene expression, and induction of a cellular response. Moreover, the cytoplasmic GLI proteins also may be degraded through the proteasomal pathway, and the GLI3 protein cleaved into a C-terminal fragment (GLI3R) that acts as a nuclear transcriptional repressor of Hh gene expression. b, the binding of the mature and lipid-modified SHH protein to the PTCH1 receptor in the primary cilium leads to its translocation out of the ciliary structure and retrieves its repressive effect on the SMO protein localized in intracellular vesicles or plasma membrane out of the primary cilium. These molecular events culminate in SMO translocation into the primary cilium and activation of downstream signaling elements, GLI proteins localized in the primary cilium. The negative modulator of GLI proteins, SUFU protein, is then degraded by the proteasomal pathway, and the activated GLI zinc-finger transcriptional activators, GLI1 or GLI2 molecules, are translocated to nucleus and participate to the up-regulation of Hh target gene expression, including GLI1 and PTCH1, and induction of a cellular response.
Although the molecular mechanisms by which the formation of Hh ligand-PTCH1 complexes results in an increase of SMO levels into the primary cilium are not precisely established, it has been reported that the C-terminal sequence motif of the SMO protein, which is constituted of hydrophobic and basic residues, is required for its transport to the primary cilium and activation in certain types of cultured cells (Corbit et al., 2005). In support with this, the occurrence of mutations in this C-terminal domain of SMO protein has been observed to prevent its ciliary translocation and subsequent GLI activation (Corbit et al., 2005). Moreover, it has been observed that the SMO full agonist, SAG, or small regulatory molecules, including oxysterols, can induce the SMO translocation to the primary cilium and activate the Hh signaling cascade (Dwyer et al., 2007; Rohatgi et al., 2007). Hence, the endogenous molecules, such as oxysterols (which are cholesterol derivatives), might represent important factors that indirectly regulate the Hh signaling transduction by modulating the ciliary translocation and activation of the SMO protein. Therefore, the use of specific pharmacological agents that are able to interfere with the sterol synthesis constitutes then a potential therapeutic approach to modulate the Hh pathway in pathophysiological conditions, including cancers (Corcoran and Scott, 2006).
Consistent with the critical role of primary cilium in the Hh signal transduction, it has been reported that the conditional ablation of primary cilium on the surface of granule cell progenitors in mice disrupted SHH-mediated expansion of granule cell precursors (GCPs) and cerebellar development (Han et al., 2008; Spassky et al., 2008). Moreover, the mutations in intraflagellar transport (IFT) proteins, which are essential for the primary cilium assembly, formation, and maintenance, also resulted in major defects in mouse neural tube patterning and reduced the expression of the Hh downstream genes GLI-1 and PTCH1 reminiscent of deregulated Hh signaling (Huangfu et al., 2003; Liu et al., 2005a). The loss of IFT proteins was also associated with alterations in the proteolytic processing of the GLI3 protein that abrogated its repressor function in mice (Haycraft et al., 2005; Liu et al., 2005a). Thus, IFT proteins seem to be able to provide different functions, including the maintenance of the ciliary structure as well as the regulation of processing, activator, and repressor activities of the GLI proteins in the Hh pathway.
It has also been observed that high levels of SMO and GLI2 and low levels of PTCH are detected in the primary cilium of PANC-1 and CFPAC-1 pancreatic cancer cells, whereas the nuclear level of the GLI3R repressor form is low, suggesting that an autonomous activation of Hh cascade might be prevalent in these cancer cells (Nielsen et al., 2008). Moreover, it has also been observed that the occurrence of activating mutations in the SMO protein might promote its translocation to the ciliary structure and GLI activation in the absence of the Hh ligand (Corbit et al., 2005). The SMO antagonist cyclopamine has also been shown to inhibit the translocation of the SMO protein from the intracellular compartment to the primary cilium, and thereby prevent the biological effects induced by downstream effectors, GLI proteins (Corbit et al., 2005). Hence, the relocalization of the SMO protein in appropriate subcellular compartments, such as the primary cilium, might be a determinant factor that governs the dynamic process of Hh pathway activation and GLI induced-target gene expression in certain Hh-responsive cells. In particular, the localization of the SMO protein in primary cilium may be an important factor that contributes to the aberrant activation of the Hh cascade in certain cancer cell types.
2. Functions of Glioma-Associated Oncogene Transcription Factors in Modulating Hedgehog Target Gene Expression.
The GLI family comprises three nuclear zing-finger transcription factors, GLI1, GLI2, and GLI3, that contain conserved C2-H2 zing finger domains and can specifically interact with the DNA sequences encompassing a GACCACCCA motif found in target gene promoters (Kinzler et al., 1987; Kinzler and Vogelstein, 1990; Ruppert et al., 1990; Matise and Joyner, 1999; Park et al., 2000; Cohen, 2003; Kasper et al., 2006a; Ruiz i Altaba et al., 2007; Varjosalo and Taipale, 2008; Tsanev et al., 2009). The GLI proteins can cooperate with distinct nuclear cofactors in the regulation of the expression levels of Hh target gene products. More particularly, the full-length GLI proteins can act as transcriptional activators and induce the expression of target genes, whereas the N-terminal fragment of GLI proteins, generated after processing and intracellular proteolytic cleavage, can act as a transcriptional repressor (Figs. 3 and 4) (Matise and Joyner, 1999; Sasaki et al., 1999; Cohen, 2003; Kasper et al., 2006a; Ruiz i Altaba et al., 2007; Varjosalo and Taipale, 2008; Tsanev et al., 2009). Because the full-length GLI1 transcription factor does not contain a repressor domain, it consequently acts as a strong transcriptional activator (Matise and Joyner, 1999; Kasper et al., 2006a; Ruiz i Altaba et al., 2007). Although GLI2 and GLI3 proteins contain both activator and repressor domains, GLI2 has been observed to act principally as a transcriptional activator, whereas GLI3 acts mainly as a repressor of the target gene expression (Matise and Joyner, 1999; Sasaki et al., 1999; Cohen, 2003; Kasper et al., 2006a; Ruiz i Altaba et al., 2007; Varjosalo and Taipale, 2008; Tsanev et al., 2009). The cellular processing of full-length 190-kDa GLI3 protein involves its phosphorylation by protein kinase A followed by an intracellular proteolytic cleavage that generates an 83-kDa N-terminal fragment of GLI3 that acts as a potent transcriptional repressor, GLI3R (Fig. 4) (Wang and Li, 2006). It has been observed that the processing of full-length GLI3 protein into its repressor form is promoted in the absence or at low levels of the SHH protein (Fig. 3) (Wang et al., 2000; Litingtung et al., 2002; Huangfu et al., 2003). Hence, the balance between the cellular levels of GLI3R repressor form versus GLI1 and GLI2 transactivators determines the final outcome on Hh target gene expression in a given cell type.
Fig. 4.
Schematic representation of structural features of human GLI3 protein and molecular events associated with its processing into a transcriptional repressor. The scheme shows the positions of the zinc finger DNA-binding domain (ZF), the six potential sites of the phosphorylation by protein kinase A (PKA) (asterisks) identified by mutagenesis analyses, and the intracellular cleavage site of the full-length GLI3 protein. The processing of the full-length 190-kDa GLI3 protein, which implicates its phosphorylation by PKA followed by its intracellular proteolytic cleavage, yielding an N-terminal fragment of GLI3, is also illustrated. The cleaved N-terminal fragment of GLI3 of approximately 83 kDa (GLI3R) can act as a transcriptional repressor and inhibit the Hh target gene expression.
In general, the Hh ligand-dependent activation of the Hh cascade leads to the inhibition of SUFU protein by SMO and the nuclear translocation of GLI1 and/or GLI2 transcriptional activators that, in turn, up-regulate the expression levels of numerous Hh target genes in a cell-type and context-dependent manner (Fig. 3) (Kinzler et al., 1987; Kinzler and Vogelstein, 1990; Ruppert et al., 1990; Cohen, 2003; Kasper et al., 2006a; Rahnama et al., 2006; Yue et al., 2009). The Hh target gene products include GLI1 as well as PTCH1 and HHIP, which constitute the positive and negative feedback mechanisms involved in the regulation of Hh cascade, respectively (Rahnama et al., 2006). Other up-regulated Hh gene products also comprise bone morphogenic protein-1, cyclins D1 and D2, JUN transcription factor, and polycomb ring finger oncogene, BMI-1, that can act by down-regulating p16INK4A and forkhead box M1 transcription factor, which in turn can contribute to the up-regulation of c-Myc and BMI-1 expression (Douard et al., 2006; Eichberger et al., 2006; Kasper et al., 2006a; Li et al., 2006; Clement et al., 2007; Feldmann et al., 2007; Bhattacharya et al., 2008; Varjosalo and Taipale, 2008; Laner-Plamberger et al., 2009; Park et al., 2009). Moreover, the Hh activation also results in an up-regulated expression of Wingless ligands (Wnts), Notch ligand JAG2, interleukin-1 receptor type 2, snail, antiapoptotic factors such as Bcl-2, ATP-binding cassette (ABC) multidrug transporters, and CXC chemokine receptor 4 (Eichberger et al., 2006; Kasper et al., 2006a; Clement et al., 2007; Sims-Mourtada et al., 2007; Katoh and Katoh, 2010). The regulation of the Hh cascade is also influenced by diverse external and internal stimuli and interactive cross-talk with other signaling pathways initiated by diverse growth factors.
C. Regulatory Mechanisms of the Hedgehog Ligand Expression, Glioma-Associated Oncogene Activities and Hedgehog Ligand-Dependent and -Independent Activation of the Hedgehog Pathway
The stimulation of different growth factor and cytokine pathways and genetic and epigenetic alterations during embryonic development; tissue regeneration; and repair after severe injury, chronic inflammation, and cancer development may activate different intracellular signaling elements (Hingorani et al., 2005; Koga et al., 2008). These signaling components include nuclear factor-κB (NF-κB), phosphatidylinositol 3′ kinase (PI3K)/Akt and/or K-RAS that can contribute to increase the cellular expression level of Hh ligands, including SHH protein, GLI activities and Hh signaling activation (Fig. 5) (Schmidt-Ullrich et al., 2006; Amankulor et al., 2009; Kasperczyk et al., 2009; Cui et al., 2010). More specifically, it has been shown that EGF can up-regulate SHH protein expression and secretion through the activation of PI3K/Akt signaling components in gastric parietal cells and thereby stimulate gastric acid secretion (Stepan et al., 2005). Moreover, the activation of macrophages caused by severe tissue injury and inflammation might result in the production of diverse pro-inflammatory cytokines that, in turn, can up-regulate SHH transcriptional expression (Amankulor et al., 2009). As a matter of fact, it has been shown that the activation of NF-κB might occur after inflammatory stimuli (such as tumor necrosis factor-α, interleukin-1α, and lipopolysaccharide) and during cancer progression (Nakashima et al., 2006; Schmidt-Ullrich et al., 2006; Kasperczyk et al., 2009; Cui et al., 2010). Thereby, the activated NF-κB can specifically interact with human SHH promoter, up-regulate the SHH expression level in a variety of normal and malignant cells, and contribute to their proliferation and survival in vitro and in animal models in vivo (Nakashima et al., 2006; Schmidt-Ullrich et al., 2006; Kasperczyk et al., 2009; Cui et al., 2010). A hypomethylation status of the SHH region promoter has also been associated with an up-regulation of its transcriptional expression during breast cancer progression suggesting a potential epigenetic regulation of the SHH expression under specific physiological and pathological conditions (Yakushiji et al., 2007; Cui et al., 2010).
Fig. 5.
Cellular events and signaling elements involved in the regulation of SHH expression, GLI activation, and mediation of the Hh activation-induced cellular response. The increase of the SHH expression, which might be induced during tissue regeneration in homeostatic conditions, and after an adaptive response to tissue injury, ischemia and hypoxia, chronic inflammation, and cancer progression, is indicated. The potential cellular signaling elements involved in the regulation of the SHH expression are also indicated. These intracellular signaling components include NF-κB, PI3K/Akt, and K-RAS, which might be induced through the stimulation of different growth factor and cytokine signaling pathways in normal and cancer cells. The possibility of SHH-dependent and -independent activation of GLI1 and GLI2 transcriptional activators by different growth factor pathways is also indicated. In addition, the potential biological effects induced through stimulation of GLI protein-induced Hh target gene expression in normal and cancer cells are also indicated. ER-α, estrogen receptor α; PDGF, platelet-derived growth factor; RORα, retinoid-related orphan receptor α; TNF-α, tumor necrosis factor-α.
Among other potential regulators of the SHH expression, the overexpression of the p63 protein, which is a homolog of the p53 tumor suppressor protein and is known to provide a regulatory role in the maintenance of epithelial stem cells and tumorigenesis, has been shown to interact with the SHH promoter and up-regulate its expression (Fig. 5) (Caserta et al., 2006). It has also been noticed that the transactivation of the SHH gene by p63 protein could be inhibited by p14ARF tumor suppressor protein (Caserta et al., 2006). Moreover, the orphan nuclear receptor RORα has been shown to be able to interact with the SHH promoter and to promote the recruitment of other coactivators, including β-catenin and p300 (Gold et al., 2003). Thereby, these nuclear factors can cooperate to up-regulate the SHH expression in GCPs and contribute to their proliferation during cerebellar development (Gold et al., 2003). The activation of estrogen/estrogen receptor-α axis has also been reported to up-regulate the SHH expression and proliferation of breast cancer cells (Koga et al., 2008).
In addition, a growing body of experimental evidence has revealed that different signaling elements might negatively or positively modulate the expression level, stability, and activity of GLI proteins and influence the cellular responses mediated through the canonical Hh cascade (Cohen, 2003; Haycraft et al., 2005; Kasper et al., 2006a; Ruiz i Altaba et al., 2007; Varjosalo and Taipale, 2008). These regulatory mechanisms include changes at the transcriptional and post-transcriptional levels, subcellular localization, phosphorylation status, and stability versus degradation of GLI proteins. Among the important negative regulators of the Hh pathway, SUFU protein can bind, stabilize, and retain the three GLI proteins in the cytoplasm in the absence of Hh ligands and thereby prevent their activation and nuclear translocation (Kogerman et al., 1999; Kasper et al., 2006a; Ruiz i Altaba et al., 2007). It has consistently been shown that the inactivation of SUFU by mutations, gene targeting, or small interference RNA (siRNA) is sufficient to up-regulate the GLI-induced Hh gene expression in normal or cancer cells (Taylor et al., 2002; Varjosalo et al., 2006). Moreover, the activation of the SHH signaling cascade also might promote the ubiquitination and proteasomal degradation of SUFU molecules in normal and cancer cells and thereby contribute to the Hh ligand-mediated cell growth (Yue et al., 2009). The negative regulation of GLI proteins is also regulated by protein kinase A and glycogen synthase kinase 3b (GSK3b) that can phosphorylate, destabilize, and inactivate GLI proteins (Mizuarai et al., 2009).
On the other hand, the positive regulatory mechanisms might be induced in the presence or absence of the Hh ligand. As mentioned previously, the activation of canonical Hh cascade might lead to a feedback loop in which the nuclear GLI2 or GLI3 transactivators can directly interact with the GLI1 promoters and up-regulate its expression (Dai et al., 1999; Cohen, 2003; Ikram et al., 2004; Haycraft et al., 2005; Kasper et al., 2006a; Varjosalo and Taipale, 2008). Likewise, the increase of GLI1 expression level may result in an up-regulation of the GLI2 level through an indirect mechanism that does not involve the transactivation of the GLI2 promoter by the GLI1 protein (Regl et al., 2002). In this regard, the results from recent studies have also revealed that the SUFU function and/or GLI expression, stability, and/or transcriptional activity in normal and cancer cells may be positively modulated via the persistent stimulation of different growth factor cascades. These signaling pathways include EGF/EGFR, Wnt/β-catenin, and the TGF-β1/TGF-βR system, which can cooperate with the canonical Hh ligand-induced signaling to activate GLI proteins and Hh target gene expression (Figs. 5 and 6) (Xie et al., 2001; Bigelow et al., 2005; Palma et al., 2005; Kasper et al., 2006b; Riobo et al., 2006a; Dennler et al., 2007, 2009; Stecca et al., 2007; Schnidar et al., 2009; Seto et al., 2009). For instance, it has been shown that TGF-β protein can up-regulate GLI1 and GLI2 expression and thereby contribute to the acquisition of a more malignant behavior by cancer cells (Dennler et al., 2007, 2009). More specifically, the activation of TGF-β/TGF-βR1-ALK5 system might result in the nuclear translocation of Smad3-Smad4 complexes that directly interact with the GLI2 promoter and promote the recruitment of β-catenin. Then, these nuclear factors can cooperate to up-regulate the GLI2 expression, which in turn can induce the transactivation of Hh target genes, including GLI1 (Fig. 6) (Dennler et al., 2009). Hence, the integration of these diverse mechanisms of negative and positive regulation of the Hh cascade determines the biological effect induced in a given cell type.
Fig. 6.
Scheme showing the signaling elements and frequent deregulations in the Hh signaling network and potential cross-talk with the EGFR signaling pathway involved in the malignant behavior of cancer cells. The molecular events associated with the cellular processing of the SHH precursor into a biologically active form via autocatalytic cleavage and lipid modifications are illustrated. Autocrine and paracrine stimulation of the cancer cells by the monomeric and multimeric SHH molecules is also illustrated. The repressive effect of SUFU on the GLI activity is shown. Moreover, the frequent deregulations, including overexpression of the SHH ligand; inactivating mutations in HHIP, PTCH, or SUFU; or activating mutations in SMO coreceptor, which may contribute to cancer development, are also indicated. The potential stimulatory effect induced by the activation of EGFR pathway and oncogenic mutations in K-RASmut and B-RAFmut on the GLI transcriptional activity is indicated. The target gene products induced through the activation of Hh and EGFR signaling pathways are also described. Moreover, the stimulatory effect induced through the activation of TGF-β/TGF-βR/Smad3-Smad4 and Wnt/β-catenin on the GLI2 expression is illustrated. In addition, the potential inhibitory effect induced by diverse pharmacological agents, such as a mAb directed against SHH ligand, EGF, EGFR, and Wnt, selective inhibitors of SMO (cyclopamine, KAAD-cyclopamine, IPI-269609, or GDC-0449), EGFR tyrosine kinase activity (gefitinib and erlotinib), TGF-β type I activin receptor-like kinase, and ALK5 (SB431542) are also indicated. COX2, cyclooxygenase 2; CXCR4, CXC chemokine receptor 4; FOXM1, forkhead box M1 transcription factor; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor.
III. Critical Functions of the Hedgehog Signaling Pathway during Embryonic and Postnatal Development and Adult Life and Their Therapeutic Implications
In mammals, the interplay of diverse growth factor pathways, including Hh/GLI, EGF/EGFR, Wnt/β-catenin, and TGF-β/TGF-β receptors, is involved in the stringent control of the tissue patterning and organogenesis during embryogenesis and fetal development as well as the tissue homeostasis and repair after severe injuries and inflammation in the postnatal period or adulthood (Cohen, 2003; Beachy et al., 2004; Palma and Ruiz i Altaba, 2004; Palma et al., 2005; Liu et al., 2006; Zhou et al., 2006; Shi et al., 2008; Varjosalo and Taipale, 2008; Amankulor et al., 2009). Among them, Hh proteins play critical roles by acting as potent morphogens, mitogens, and survival factors for a variety of cell types (including pluripotent embryonic stem/progenitor cells and multipotent tissue-resident adult stem/progenitor cells) in a time- and concentration-dependent manner (Cohen, 2003; Beachy et al., 2004; Palma and Ruiz i Altaba, 2004; Palma et al., 2005; Liu et al., 2006; Zhou et al., 2006; Shi et al., 2008; Varjosalo and Taipale, 2008; Amankulor et al., 2009). Despite the fact that complex molecular mechanisms are involved in the regulation of the Hh signaling network in normal physiological conditions, the inactivation or hyperactivation of the Hh cascade might lead to severe congenital diseases and hyperproliferative disorders in postnatal life, including cancer development (Ming et al., 1998; Odent et al., 1999; Bale, 2002; Bak et al., 2003; Cohen, 2003; Beachy et al., 2004; Maity et al., 2005; Vaillant and Monard, 2009).
Numerous loss- and gain-function studies of Hh signaling elements carried out with transgenic mice, animal models of diseases and cell cultures have provided important insights into the critical roles of Hh pathway during embryonic and postnatal development and along life span in adulthood. Analyses of SHH-null mice have particularly indicated that numerous defects occur in diverse structures during embryonic development and result in a rapid perinatal lethality of SHH mutants compared with wild-type mice (Chiang et al., 1996; Lu et al., 2000; Ishibashi and McMahon, 2002; Rallu et al., 2002). These defects consist of the absence of distal limb structures and the spinal column; ventral cell types within the neural tube; reduced size of dorsoventral structures of telencephalon and diencephalon; abnormalities in skin development, including hair follicle morphogenesis; and cyclopia, which refers to the presence of a single eye in the center of the face. In contrast, the conditional null alleles of the SHH or SMO genes resulted in only minor brain patterning abnormalities, whereas the number of neural progenitors in both the postnatal subventricular zone and the dentate gyrus of hippocampus was dramatically reduced and was associated with a marked increase in programmed cell death (Machold et al., 2003).
In addition, the gain-of-function approaches, in vitro and in vivo up-regulation, or exogenous application of the SHH protein have also given complementary information about SHH functions in the tissue patterning and the early and later stages of neurogenesis during embryogenesis until postnatal development and adult brain maturity (Gaiano et al., 1999; Lu et al., 2000; Charytoniuk et al., 2002b; Machold et al., 2003; Vaillant and Monard, 2009). Specifically, it has been observed that an up-regulation of SHH expression in mice promoted the proliferation of GCPs and oligodendrocyte specification in telencephalon and resulted in an enhanced number of differentiated oligodendrocytes in the embryonic and postnatal cerebellar region (Lu et al., 2000; Nery et al., 2001; Machold et al., 2003). In same way, the loss-of function mutations in PTCH tumor suppressor gene or activating mutations in SMO oncogene also led to phenotypic changes as observed for mice overexpressing SHH protein (Hahn et al., 1999, 2000). It has been observed that SHH target genes are aberrantly activated in heterozygous PTCH(−/+) knockout mice, and these mice have a higher tendency to develop nevoid basal cell carcinoma syndrome (NBCCS), and a variety of cancers including cerebellar and skin tumors compared with wild-type mice (Goodrich et al., 1997). In fact, the NBCCS, also known as Gorlin syndrome or basal cell nevus syndrome, is an autosomal-dominant disorder associated with inherited inactivating mutations in the PTCH receptor. Patients with NBCCS exhibit a variety of developmental defects accompanied by a predisposition to develop a variety of postnatal disorders and cancer types, such as basal cell carcinomas (BCCs), medulloblastoma, ovarian dermoid and fibroma, meningioma, fibrosarcoma, rhabdomyosarcoma, and cardiac fibroma (Hahn et al., 1996, 1999; Goodrich et al., 1997; Zurawel et al., 2000). Hence, these observations suggest that the SHH cascade supplies important roles for patterning of a variety tissues during embryonic development and maintenance of cellular functions and organ integrity after the birth, and more particularly in the embryonic and postnatal development of brain. Therefore, the alterations in the Hh cascade might cause severe human disorders and cancers in numerous tissues and organs.
A. Functions of the Hedgehog Cascade in Adult Tissues and Their Therapeutic Implications
The analyses of the expression patterns of human Hh proteins have revealed that SHH, IHH, and DHH are differently expressed in different adult tissues and organs, in homeostatic conditions, and during tissue regeneration and repair after severe injury (Pathi et al., 2001; Bak et al., 2003; Nielsen et al., 2004; Sicklick et al., 2005, 2006; Spicer et al., 2009; Vaillant and Monard, 2009). Consequently, they can contribute to the mediation of specific functions dependent of their expression levels in a given cell type. Among them, the SHH protein is required for the regulation of multiple key cellular events in a wide range of adult tissue and organ types including bone marrow (BM), central nervous system and peripheral nerves, cardiovascular system, and epithelial tissues such as skin, lung, liver, gastrointestinal tract, pancreas, prostate, breast, and ovary (McMahon et al., 2003; Nielsen et al., 2004; Paladini et al., 2005; Katoh and Katoh, 2006; Sicklick et al., 2006; Vaillant and Monard, 2009). It has been shown that Hh proteins can promote the proliferation of diverse multipotent tissue-resident adult stem/progenitor cells, including hematopoietic, neural, skin, cochlear, gastrointestinal, hepatic, pancreatic, and mammary stem/progenitor cells, and thereby participate in cell replenishment and tissue regeneration and repair after severe injuries (Bhardwaj et al., 2001; Machold et al., 2003; McMahon et al., 2003; Nielsen et al., 2004; Ahn and Joyner, 2005; Paladini et al., 2005; Sicklick et al., 2005; Katoh and Katoh, 2006; Lau et al., 2006; Liu et al., 2006; Sicklick et al., 2006; Lin et al., 2007; Mimeault and Batra, 2008c). Hence, the SHH protein plays important roles in the replenishment of cells that are lost during tissue turnover and injuries. For instance, the activation of the SHH pathway is required for the maintenance of hair follicle stem/progenitor cells found in bulge areas. The SHH protein can cooperate with EGF in controlling the follicular growth and cycling, including the transition from the resting phase (telogen) to the growth phase (anagen), and participate in the skin regeneration after injury (Paladini et al., 2005; Kasper et al., 2006b; Rittié et al., 2009). The stimulation of the SHH pathway might also contribute to the long-term repopulating of epidermal progenitors after severe wounding (Levy et al., 2007). Therefore, the activation of Hh cascade by topical application of exogenous SHH protein or its synthetic Hh agonist might represent a potential therapeutic strategy to treat diverse skin disorder associated with a decreased proliferation of epidermal and epithelial cells and hair cycle defects (Paladini et al., 2005).
On the other hand, the analyses of the expression levels of Hh signaling components in mice, rat, and bovine ovaries have also revealed that IHH and DHH RNAs were detected in granulosa cells (Spicer et al., 2009). In contrast, PTCH1 and SMO transcripts were detected in thecal-interstitial cells of small versus large follicles of cattle and PTCH1 and GLI expression levels enhanced after ligand stimulation, suggesting a paracrine mechanism of the Hh system in follicular development (Spicer et al., 2009). Furthermore, the activation of the Hh signaling pathway was also associated with an enhanced proliferation and steroidogenesis, including androgen production in mammalian ovarian cells (Spicer et al., 2009). In this matter, we are reporting in a more detailed manner the specific functions provided by the Hh proteins in other adult tissues, including the maintenance of the adult pancreas, BM, brain, and cardiovascular system and their therapeutic implications.
1. Functions of Hedgehog Proteins in the Pancreas and Their Therapeutic Implications.
The Hh proteins provide important functions in the regulation of pancreatic morphogenesis during embryonic development as well as the ductal epithelial cell regeneration and maintenance of the pancreatic β-cell mass and regulation of insulin production in adult pancreas (Thomas et al., 2000; Kim and Hebrok, 2001; Lau et al., 2006; Mimeault and Batra, 2008a; Parkin and Ingham, 2008). In particular, IHH and DHH, SMO, and PTCH1 are expressed in adult pancreatic islets of Langerhans, and the activation of Hh cascade in adult pancreatic β-cells may result in a transcriptional activation of islet duodenum homeobox-1, IDX-1, also designated as PDX-1, that in turn, can interact with the insulin promoter and up-regulate its expression (Thomas et al., 2000; Kim and Hebrok, 2001). Hence, the stimulation of the Hh cascade may represent a potential therapeutic strategy to up-regulate the IDX-1-induced insulin expression and maintain normal glucose homeostasis, and thereby treat diverse disorders, including diabetes mellitus. In counterbalance, the enhanced expression of SHH and IHH in pancreatic ductal epithelial cells in the exocrine compartment, which can be induced through the NF-κB activation during inflammation, however, might result in development of chronic pancreatitis and cancer (Kayed et al., 2003, 2004; Lau et al., 2006; Nakashima et al., 2006; Morton et al., 2007; Mimeault and Batra, 2008a; Parkin and Ingham, 2008). These observations support, then, the therapeutic interest of targeting the NF-κB or SHH pathway to treat inflammatory disorders of the pancreas such as pancreatitis and pancreatic cancer.
2. Functions of the Sonic Hedgehog Protein in the Bone Marrow and Their Therapeutic Implications.
Numerous external and internal stimuli are involved in the stringent control of BM-resident hematopoietic stem/progenitor cells and hematopoiesis in homeostatic conditions and after injury (Trowbridge et al., 2006; Kiuru et al., 2009; Merchant et al., 2010). In particular, it has been proposed that the activation of the Hh signaling pathway might induce the expansion of primitive BM-resident hematopoietic cells under homeostatic conditions and during acute regeneration (Trowbridge et al., 2006). Moreover, the SHH protein can cause changes in endosteal hematopoietic stem cell niche, including osteoblasts, and thereby alter early lymphoid differentiation (Kiuru et al., 2009). It has also been shown that SHH protein or oxysterols, certain naturally occurring oxygenated derivatives of cholesterol, including 20(S)-hydroxycholesterol, can induce the antiadipogenic and osteogenic effects on multipotent BM-stromal cells by activating the canonical Hh pathway through SMO signaling element (Kim et al., 2007a, 2010; Amantea et al., 2008). In fact, the osteogenic effect induced through the stimulation of the Hh cascade seems to be mediated, at least in part, via the enhanced expression of gene products associated with the Notch (HES-1, HEY-1, and HEY-2) and Wingless (Dkk-1) pathways (Amantea et al., 2008; Kim et al., 2010). Hence, the stimulation of the Hh cascade by the SHH protein or oxysterols in BM-resident stromal cells may constitute a potential approach to induce their osteogenic differentiation and bone-forming properties. This strategy could be used to treat diverse osteopenic disorders, such as the osteoporosis resulting from an increase of the adipocyte differentiation concomitant with a decrease of bone formation by osteoblasts occurring during chronological aging, which constitute the major causes of morbidity and mortality in elderly persons.
3. Functions of the Sonic Hedgehog Cascade in the Postnatal Developing and Adult Brain and Their Therapeutic Implications.
In postnatal development and adult brain, the SHH protein has been shown to provide critical roles in neurogenesis occurring in the subventricular zone and hippocampal dentate gyrus, the zones known as the niches of adult neural stem/progenitor cells in mice and human in homeostatic conditions and after brain injuries (Pola et al., 2001; Calcutt et al., 2003; Palma and Ruiz i Altaba, 2004; Palma et al., 2005; Sicklick et al., 2005, 2006; Alvarez-Medina et al., 2009; Bambakidis et al., 2009; Gulino et al., 2009; Sims et al., 2009; Wang et al., 2009a). More specifically, it has been shown that the SHH protein can display neuroprotective effects and cooperate with EGF to induce the proliferation of neural stem/progenitor cells in the subventricular zone and generate new olfactory interneurons in mice in vivo (Palma and Ruiz i Altaba, 2004; Palma et al., 2005). Furthermore, the SHH protein, which is synthesized and secreted by the Purkinje cells in the postnatal developing cerebellum and adult brain, can also promote, in a paracrine manner, the activation of the GLI-mediated canonical pathway and proliferation of GCPs (Wechsler-Reya and Scott, 1999; Charytoniuk et al., 2002a). It is noteworthy that reactive astrocytes in an injured cerebral cortex can also produce and secrete the SHH protein, which can stimulate Oligo2+ expressing progenitor cells (Amankulor et al., 2009). Hence, Oligo2+ GCPs can give rise to mature oligodendrocytes that contribute to re-myelination of injured axons. These observations support the therapeutic interest of stimulating the SHH pathway for treating diverse brain defects and injuries, neurodegenerative disorders, and cerebral cortical injuries such as multiple sclerosis (Bambakidis et al., 2009; Gulino et al., 2009; Zhang et al., 2009).
4. Functions of the Hedgehog Cascade in the Cardiovascular System and Their Therapeutic Implications.
Numerous accumulating lines of evidence have also indicated that the activation of Hh signaling cascade may promote the neovascularization process after severe ischemic injuries (Pola et al., 2001; Kusano et al., 2005; Asai et al., 2006; Lee et al., 2007; Lavine et al., 2008; Renault et al., 2009; Sims et al., 2009). More specifically, it has been shown that the SHH protein plays a critical role in coronary development and can promote the formation of coronary vessels in the embryonic and adult heart. Moreover, it has been shown that Hh signaling molecules are expressed in human peripheral monocytes, and the SHH protein induces the migration of monocytes in blood samples from control patients, but it does not induce a chemotactic effect on monocytes from diabetic patients with coronary artery disease (Dunaeva et al., 2010). The impaired response of diabetic patients to the SHH protein has been associated with a strong transcriptional up-regulation of the PTCH1 receptor, which can negatively regulate the SMO transducer activity. In addition, the SHH protein can also contribute to the neoangiogenesis process by promoting the proliferation, migration, and vascular endothelial growth factor production via the PI3K/Akt signaling pathway in BM-derived endothelial progenitor cells (EPCs), which might be recruited to injured tissues (Fu et al., 2006).
It is of therapeutic interest that the exogenous administration of the SHH protein or SHH gene transfer has been shown to induce angiogenesis and accelerate the repair of ischemic brain injury, acute and chronic myocardial ischemia, and skeletal muscle ischemia in animal models in vivo (Pola et al., 2001; Kusano et al., 2005). Moreover, a strategy consisting of topically applied SHH gene therapy also accelerated the cutaneous wound healing in a diabetic mouse model in vivo, in least in part, via the stimulation of dermal fibroblasts and indirectly by enhancing the recruitment of BM-derived EPCs at damaged skin, which in turn promoted microvasculature remodeling and wound repair (Asai et al., 2006). Hence, the stimulation of the Hh signaling pathway might represent a potential strategy to promote neoangiogenesis and arteriogenesis and thereby prevent diverse cardiovascular disorders such as ischemic injury and heart failure.
Despite great clinical interest to stimulate the Hh cascade to treat diverse human disorders, it is noteworthy that additional investigations are required to confirm the therapeutic benefit of this strategy versus its detrimental effect on normal adult stem/progenitor cells, including the potential induction of their malignant transformation and cancer development.
IV. Critical Functions of the Hedgehog Signaling Pathway in the Malignant Transformation of Cancer- and Metastasis-Initiating Cells and Their Progenies
The sustained activation of the Hh signaling pathway in tissue-resident adult stem/progenitor cells and their progenies has been proposed to represent a potential event that may contribute to their malignant transformation during cancer initiation and progression (Thayer et al., 2003; Cohen, 2003; Beachy et al., 2004; Karhadkar et al., 2004; Oniscu et al., 2004; Sanchez et al., 2004; Ohta et al., 2005; Datta and Datta, 2006; Mimeault et al., 2006, 2007a,b, 2008; Bian et al., 2007; Chen et al., 2007b; Clement et al., 2007; Ehtesham et al., 2007; Peacock et al., 2007; Stecca et al., 2007; Bhattacharya et al., 2008; Mimeault and Batra, 2008a; Schnidar et al., 2009). More specifically, inherited or somatic inactivating mutations in the PTCH1 or SUFU tumor suppressor gene leading to a loss-of-function and/or activating mutation in SMO oncogene that aberrantly activates Hh signal transduction may result in the development of diverse cancers (Fig. 6) (Dahmane et al., 1997; Reifenberger et al., 1998; Taylor et al., 2002; Berman et al., 2003; Beachy et al., 2004; Sheng et al., 2004; Douard et al., 2006). These cancer types include BCCs and common pediatric tumors such as medulloblastoma, a highly malignant tumor derived from cerebellar granule neuron progenitor cells, and rhabdomyosarcoma, a tumor that originates in the soft tissues of the body, including the skeletal muscles, tendons, and connective tissues (Dahmane et al., 1997; Reifenberger et al., 1998; Taylor et al., 2002; Berman et al., 2003; Ruiz i Altaba et al., 2004; Beachy et al., 2004). It has also been reported that the gene encoding HHIP, an endogenous Hh inhibitor, may be transcriptionally silenced by hypermethylation and chromatin remodeling in diverse cancers, such as gastrointestinal and hepatocellular carcinomas, and thereby contribute to the persistent activation of the Hh cascade (Taniguchi et al., 2007; Tada et al., 2008; Eichenmüller et al., 2009). In addition, an up-regulation of expression levels and/or activities of Hh signaling elements, including Hh ligands, SMO coreceptor, and GLI proteins often occurs during cancer initiation, and progression to locally invasive and metastatic disease stages. The overexpression of Hh signaling elements might result in the sustained growth and enhanced invasive properties of malignant cells in multiple myeloma, melanoma, glioma, gastrointestinal tract, pancreatic, hepatic, small-cell lung, prostate, mammary, and ovarian cancers (Thayer et al., 2003; Beachy et al., 2004; Karhadkar et al., 2004; Oniscu et al., 2004; Sanchez et al., 2004; Sheng et al., 2004; Ohta et al., 2005; Douard et al., 2006; Mimeault et al., 2006, 2007a,b; Bar et al., 2007; Bian et al., 2007; Chen et al., 2007b; Ehtesham et al., 2007; Peacock et al., 2007; Stecca et al., 2007; Bhattacharya et al., 2008; Liao et al., 2009; Mizuarai et al., 2009; Schnidar et al., 2009; Yang et al., 2010). More particularly, the reactivation of the Hh pathway and other developmental cascades, including EGFR and Wnt/β-catenin, in tissue-resident adult stem/progenitor cells during severe tissue injury, chronic inflammation, and intense stress along chronological aging might promote the cancer initiation and development (Beachy et al., 2004; Nielsen et al., 2008; Mimeault and Batra, 2009, 2010a,c; Strobel et al., 2010). Moreover, the up-regulation of Hh signaling components along the epithelial-mesenchymal transition process, might play a pivotal role in the proliferation, survival, invasion, and metastases of cancer stem/progenitor cells and their progenies at distant tissues (Mimeault and Batra, 2007, 2010a,c; Klarmann et al., 2009).
In support of the critical implication of the Hh signaling network in cancer initiation, it has been shown that PTCH knockout mice or SMOA1;Bmi(+/+) and SMOA1;Bmi(+/−) mice expressing SMO and BMI-1 spontaneously developed typical medulloblastoma arising from the expansion of the cerebellar granule neuron precursors (Michael et al., 2008; Yang et al., 2008; Ward et al., 2009). Furthermore, it has been shown that the activation of the Hh cascade by the SHH ligand may induce a transitory differentiation of prostate stem/progenitor cells into CD44+/p63(+/−) hyperplasia basal cells with a intermediate phenotype (CK8/14) (Chen et al., 2006a, 2007a). This early transforming event culminated toward tumorigenesis by giving rise to CD44, PTCH1, and GLI-expressing prostate cancer cells (Chen et al., 2006a, 2007a). In the same way, it has been reported that the Hh cascade is activated in human breast CD44+CD24−/lowLin− cancer stem cells, and the overexpression of GLI2 transcriptional activator in mammosphere-initiating cells resulted in the formation of ductal hyperplasia in a humanized nonobese diabetic-severe combined immunodeficient (NOD/SCID) mouse model in vivo (Liu et al., 2006; Tanaka et al., 2009). The overexpression of GLI1 in the mouse mammary gland also resulted in tumor development arising from the expansion of epithelial cells expressing the progenitor cell markers keratin 6 and BMI-1 (Fiaschi et al., 2009). Moreover, the activated Hh-GLI signaling pathway might regulate the expression levels of stemness genes, self-renewal ability, and survival of CD133+ glioma cancer stem cells and may contribute to sustained glioma growth and tumor cell survival in vivo (Clement et al., 2007).
In addition to the oncogenic effects induced through the activation of the Hh pathway in cancer cells, it has also been reported that Hh ligands can contribute to the pathogenesis of diverse human epithelial cancers, including pancreatic, colon, prostate, breast, and ovarian cancers by acting on the surrounding stromal cells and promoting the tumor neovascularization process (Yauch et al., 2008; Kasper et al., 2009; Olive et al., 2009; Shaw et al., 2009; Theunissen and de Sauvage, 2009; Nakamura et al., 2010; Walter et al., 2010). Moreover, different molecular cross-talk between the Hh cascade and other oncogenic signaling elements might cooperate for the tumor development and transition to invasive and metastatic disease stages.
A. Cross-Talks between the Hedgehog Cascade and Other Oncogenic Signaling Elements
A growing body of evidence has indicated that the aberrant activation of the Hh pathway combined with the occurrence of other oncogenic events, including the activating mutations in oncogenes such as K-RAS or inactivation of tumor suppressor gene products (p53, p16INK4A, and/or phosphatase and tensin homolog deleted chromosome 10), may cooperate in the malignant transformation of diverse epithelial cells and tumor development (Thayer et al., 2003; Hingorani et al., 2005; Pasca di Magliano et al., 2006; Carrière et al., 2007; Ji et al., 2007; Morton et al., 2007; Reinisch et al., 2007; Abe et al., 2008; Kasai et al., 2008; Frappart et al., 2009; Seto et al., 2009; Stecca and Ruiz I Altaba, 2009). For instance, it has been reported that the endogenous expression of mutated K-RAS (G12D) in a population of pancreatic exocrine progenitors characterized by the expression of nestin resulted in the formation of pancreatic intraepithelial neoplasias in a mouse model in vivo (Carrière et al., 2007). Moreover, the activation of the SHH signaling pathway cooperated with oncogenic K-RAS to promote pancreatic ductal adenocarcinoma development (Thayer et al., 2003; Pasca di Magliano et al., 2006; Ji et al., 2007; Kasai et al., 2008). More specifically, the malignant transformation of human pancreatic ductal epithelial cells induced by mutated K-RAS through the stimulation of RAF/extracellular-signal-regulated kinase kinase (MEK)/mitogen-activated protein kinase (MAPK) signaling elements was accompanied by an increase of the GLI transcriptional activity leading to enhanced GLI1 expression (Ji et al., 2007). It has also been reported that the oncogenic K-RAS-induced cell transformation in pancreatic epithelium may be mediated, at least in part, through an enhanced expression of SHH ligand (Thayer et al., 2003; Hingorani et al., 2005; Pasca di Magliano et al., 2006). In this regard, it is noteworthy that the oncogenic K-RAS has been observed to enhance the association of the SCL/TAL1 interrupting locus product, a cytoplasmic protein overexpressed in pancreatic ductal adenocarcinoma, with SUFU protein in pancreatic cancer cell lines (Kasai et al., 2008). Thus, the formation of the SCL/TAL1 interrupting locus-SUFU complexes may inhibit the repressive effect induced by SUFU protein on the GLI activity and result in an up-regulation of GLI target gene expression (Kasai et al., 2008). On the other hand, it has also been shown that the stimulation of the Hh signaling cascade may activate human double minute 2 and thereby increase the p53 degradation by ubiquitination and inhibit the p53-mediated tumor suppressive effect in human breast cancer cell lines (Abe et al., 2008).
In addition, the persistent activation of RTKs such as EGFR and platelet-derived growth factor receptor α as well as TGF-β/TGF-βR and Wnt/β-catenin also can cooperate with the canonical Hh-GLI pathway (Xie et al., 2001; Bigelow et al., 2005; Palma et al., 2005; Kasper et al., 2006; Maeda et al., 2006; Riobo et al., 2006; Riobó et al., 2006; Stecca et al., 2007; Nolan-Stevaux et al., 2009; Schnidar et al., 2009; Seto et al., 2009; Stecca and Ruiz, 2010). The integration of these signaling cascades may promote the acquisition of a more malignant behavior by cancer cells and the development of diverse aggressive cancers (Xie et al., 2001; Bigelow et al., 2005; Palma et al., 2005; Kasper et al., 2006b; Riobo et al., 2006a; Dennler et al., 2007, 2009; Stecca et al., 2007; Schnidar et al., 2009; Seto et al., 2009). The signaling cross-talk between Hh and other growth factor cascades may be mediated through different molecular mechanisms (Kasper et al., 2006b; Riobo et al., 2006a; Schnidar et al., 2009; Seto et al., 2009). In particular, an increase of GLI1 and GLI2 transcriptional expression may be induced through the activation of TGF-β/TGF-βR/Smads and Wnt/β-catenin during cancer progression (Dennler et al., 2007, 2009). Moreover, the stability and activities of the GLI1 and GLI2 transcriptional effectors of the Hh pathway may be modulated through the integration of distinct intracellular transduction signals induced through RTK activation. These transforming events consist of a sustained activation of RAS/RAF/MEK/extracellular signal-regulated kinase (ERK)/MAPK, PI3K/Akt/mammalian target of rapamycin (mTOR)/p70S6K2, and/or protein kinase C-δ (Kasper et al., 2006b; Riobo et al., 2006; Riobó et al., 2006; Stecca et al., 2007; Mizuarai et al., 2009; Schnidar et al., 2009; Seto et al., 2009). In fact, the stimulation of these distinct signaling elements can cooperate with GLI proteins in the regulation of specific target gene expression, including PTCH1 and GLI1, in a cancer cell type-dependent manner (Kasper et al., 2006b; Riobo et al., 2006a; Stecca et al., 2007; Schnidar et al., 2009; Seto et al., 2009). Numerous investigations have revealed that the overexpression of EGFR signaling elements frequently occurs in numerous aggressive and metastatic cancers, and can cooperate with the Hh pathway for the malignant transformation and survival of cancer cells.
B. Cross-Talks between the Hedgehog and Epidermal Growth Factor Receptor Signaling Cascades
The enhanced expression and/or activity of EGFR and its ligands, EGF, TGF-α, heparin-binding EGF, amphiregulin, and epiregulin has been associated with the development of diverse aggressive cancers, such as brain, skin, cervical, head and neck, renal, non–small-cell lung, liver, gastrointestinal, colorectal, pancreas, prostate, breast, and ovarian cancers and sarcomas (Ohsaki et al., 2000; Yarden and Sliwkowski, 2001; Di Lorenzo et al., 2002; Hernes et al., 2004; Hynes and Lane, 2005; Shepherd et al., 2005; Citri and Yarden, 2006; Shah et al., 2006; Cerciello et al., 2007; Mimeault and Batra, 2007, 2008a; Mimeault et al., 2008; Yonesaka et al., 2008; Schnidar et al., 2009; Seto et al., 2009). The EGFR signaling network can contribute to the tumor growth, invasiveness, and angiogenic process through autocrine and paracrine loops by activating diverse intracellular cascades. Among these signaling elements, there are MAPKs, PI3K/Akt, NF-κB, phospholipase Cγ, and the transcriptional repressor of E-cadherin expression, snail and twist (Fig. 6) (Yarden and Sliwkowski, 2001; Hynes and Lane, 2005; Angelucci et al., 2006; Citri and Yarden, 2006; Mimeault et al., 2006, 2008; Lo et al., 2007; Mimeault and Batra, 2007, 2008a).
Accumulating lines of evidence have indicated that different bidirectional cross-talk between Hh and EGFR signaling cascades may contribute to the malignant transformation of cancer cells. For instance, it has been shown that the stimulation of EGFR/RAS/RAF/MEK/ERK might lead to an activation of the GLI transcription factor and selective transcriptional modulation of GLI target gene expression in HaCaT keratinocytes and BCC, gastric, and pancreatic cancer cell lines (Bigelow et al., 2005; Kasper et al., 2006b; Schnidar et al., 2009; Seto et al., 2009). More specifically, the activation of the EGFR pathway resulted in the stimulation of transcription factors such as activator protein 1 family member JUN that cooperated with GLI1 and/or GLI2 activators for triggering selective expression of target gene products involved in the oncogenic transformation of BCC cell lines (Schnidar et al., 2009). Likewise, the stimulation of the Hh pathway can also modulate the expression and activities of EGFR signaling elements (Bigelow et al., 2005; Mimeault et al., 2006; Pasca di Magliano et al., 2006; Heo et al., 2007). For instance, it has been observed that the constitutive SHH expression was associated with an enhanced phosphorylation of EGFR in mouse embryonic stem cells and human HaCaT keratinocytes as well as an increase of collagen matrix invasion of HaCaT keratinocytes (Bigelow et al., 2005; Heo et al., 2007). It has also been reported that the EGFR signaling was activated in undifferentiated tumors formed in the GLI2-overexpressing Pdx-Cre;CLEG2 mouse model, and up-regulation of EGFR/Akt signaling contributed to the proliferation and survival of pancreatic cancer cells (Pasca di Magliano et al., 2006). The inhibition of the Hh cascade using cyclopamine was also accompanied by a down-regulation of EGFR expression level in prostate and pancreatic cancer cells (Mimeault et al., 2006; Hu et al., 2007).
Together, these observations have revealed that an up-regulation of Hh and RTKs, including EGFR signaling elements, during cancer progression and multiple cross-talks between these tumorigenic cascades can cooperate for the sustained growth, survival, and treatment resistance of cancer stem/progenitor cells and their progenies. Thus, the targeting of these tumorigenic signaling elements might constitute a potential therapeutic strategy of great clinical interest for overcoming treatment resistance and developing novel combination therapies against aggressive, metastatic, and recurrent cancers.
V. Targeted Therapies
The molecular targeting of different oncogenic products in cancer cells represents a potential strategy for improving the current therapeutic treatments by antihormonal therapies, radiotherapies, and chemotherapies against locally advanced, invasive, metastatic, and recurrent cancers. Because the resistance of cancer- and metastasis-initiating cells to current therapies can provide critical roles in tumor regrowth, metastases, and disease relapse, the molecular targeting of these immature cells endowed with high self-renewal and aberrant differentiation abilities constitutes a promising therapeutic strategy to prevent disease recurrence. The potential molecular targets include Hh/GLI, EGFR family members, Wnt/β-catenin, Notch, hyaluronan/CD44, TGF-β/TGF-βR, and stromal cell-derived factor-1/CXC chemokine receptor 4 signaling elements (Mimeault and Batra, 2007, 2008a,b, 2010a,b; Mimeault et al., 2008). Based on a growing body of experimental evidence indicating that the persistent activation of Hh and/or EGFR pathways represents a critical step in cancer progression to invasive and metastatic stages and disease recurrence, many efforts have been made to develop specific inhibitory agents targeting these tumorigenic cascades. We review the results from recent investigations supporting the clinical interest of targeting the Hh and/or EGFR cascades to eradicate the total tumor cell mass, improve current treatment, and develop new effective combination therapies against aggressive and recurrent cancers.
A. Targeting of the Canonical Hedgehog Tumorigenic Signaling Pathway
One of the therapeutic approaches to block the Hh signaling cascade is the use of a specific inhibitor of the SMO coreceptor. These chemical compounds include the natural plant-derived steroidal alkaloid cyclopamine and its derivatives, such as 3-keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl) cyclopamine (KAAD-cyclopamine), and semisynthetic d-homo-ring analogs IPI-269609 (Feldmann et al., 2008) and IPI-926 (Olive et al., 2009) as well as small synthetic molecules such as 2-chloro-N-[4-chloro-3-(2-pyridinyl)phenyl]-4-(methylsulfonyl)benzamide (GDC-0049; HhAntag691), a proline derivative Cur61414 (Rubin and de Sauvage, 2006), and N-(6-((2S,6R)-2,6-dimethylmorpholino)pyridin-3-yl)-2-methyl-4′-(trifluoromethoxy)biphenyl-3-carboxamide (NVP-LDE-225) (Table 1) (Williams et al., 2003; Romer et al., 2004; Mimeault et al., 2006; Riobo et al., 2006a; Rubin and de Sauvage, 2006; Bar et al., 2007; Chen et al., 2007b; Clement et al., 2007; Lauth et al., 2007; Peacock et al., 2007; Stecca et al., 2007; Feldmann et al., 2008; Büttner et al., 2009; Eichenmüller et al., 2009; Jimeno et al., 2009; Olive et al., 2009; Robarge et al., 2009; Tremblay et al., 2009; Mimeault and Batra, 2010b; Pan et al., 2010; Stanton and Peng, 2010; Yang et al., 2010). Moreover, small synthetic structural analogs of the second and third intracellular loops of the SMO proteins, such as small palmitoylated peptides as short as 10 residues, have also been designed (Remsberg et al., 2007). In addition, other therapeutic strategies include the use of anti-PTCH1 or SHH monoclonal antibody (mAb), endogenous Hh inhibitor, HHIP, small-molecule inhibitors of GLI1/GLI2 transcriptional activity, and silencing of GLI1 or GLI2 by siRNA or short hairpin RNAs (Table 1) (Karhadkar et al., 2004; Sanchez et al., 2004; Ohta et al., 2005; Rubin and de Sauvage, 2006; Bar et al., 2007; Clement et al., 2007; Ji et al., 2007, 2008; Kim et al., 2007b; Lauth et al., 2007; Nakamura et al., 2007; Peacock et al., 2007; Stecca et al., 2007; Thiyagarajan et al., 2007; Narita et al., 2008; Shaw and Prowse, 2008; Xu et al., 2008; Hyman et al., 2009; Jimeno et al., 2009; Tanaka et al., 2009). The targeting of GLI proteins in cancer cells is of particular interest for overcoming the development of resistance to the SMO inhibitors (Yauch et al., 2009) and Hh ligand-independent GLI activation.
TABLE 1.
Potential inhibitory agents of hedgehog and EGFR tumorigenic signaling elements
| Targeted Signaling Element | Name of Inhibitory Agent |
|---|---|
| Growth factor signaling element | |
| SHH ligand | Anti-SHH antibody, HHIP |
| SMO coreceptor | Cyclopamine, KAAD-cyclopamine, IPI-926, IPI-269609, GDC-0449, NVP-LDE-225 small palmitoylated peptides |
| GLI-1 or GLI-2 | siRNA, shRNA |
| EGFR (erbB1) | Anti-EGFR-antibody (mAb-C225, ICM-C225) |
| EGFR-TKI | Gefitinib, erlotinib, AG1478, EKB-569 |
| Wnt/β-catenin | Anti-Wnt antibody, WIF-1 |
| Notch | γ-Secretase inhibitor DAPT, Compound E, DBZ |
| VEGFR | Anti-VEGFR antibody (DC101) |
| VEGFR/EGFR | ZD6474 |
| Signal transduction element | |
| BMI-1 | Vorinostat, azacitidine decitabine |
| Bcl-2 | Antisense-Bcl-2 (oblimersen sodium), ABT-737 |
| Farnesyl transferase | FTI (BMS-214662, R115577) |
| RAF | Sorafenib, RAF265 |
| MEK | PD0325901, SL 327, U012 |
| PI3K/Akt | ZSTK474 |
| mTOR | Rapamycin, CCI-779 |
| NF-κB | IκBα inhibitor, sulfasalazine, bortezomib (PS-341), salinosporamide A (NPI-0052), parthenolide, DHMEQ |
| COX-2 | NS-396 (aspirine), etodolac, celecoxib, rofecoxib |
| ABC transporters | Cyclopamine, gefitinib, erlotinib |
ABT-737, 4-[4-[[2-(4-chlorophenyl)phenyl]methyl]piperazin-1-yl]-N-[4-[[(2R)-4-(dimethylamino)-1-phenylsulfanylbutan-2-yl]amino]-3-nitrophenyl]sulfonylbenzamide; BMS-214662, (3R)-3-benzyl-1-(1H-imidazol-5-ylmethyl)-4-thiophen-2-ylsulfonyl-3,5-dihydro-2H-1,4-benzodiazepine-7-carbonitrile; CCI-779, rapamycin 42-[3-hydroxy-2 (hydroxymethyl)-2-methylpropanoate]; Compound E, [(2S)-2-{[(3,5-difluorophenyl)-acetylamino}-N-[(3S)-1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-3-yl] propanamide]; DAPT, tert-butyl (2S)-2-[[(2S)-2-[[2-(3,5-difluorophenyl)acetyl]amino]propanoyl]amino]-2-phenylacetate; DBZ, (2S)-2-[2-(3,5-difluorophenyl)-acetylamino]-N-(5-methyl-6-oxo-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl)-propionamide; DHMEQ, dehydroxymethylepoxyquinomicin ; EKB-569, (E)-N-[4-(3-chloro-4-fluoroanilino)-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide; FTI, farnesyl transferase inhibitor; NS-396, 2-acetyloxybenzoic acid; PD0325901, N-[(2R)-2,3-dihydroxypropoxy]-3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzamide; RAF265, 1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-yl]pyridin-4-yl]oxy-N-[4-(trifluoromethyl)phenyl] benzimidazol-2-amine; R115577, 6-[(R)-amino-(4-chlorophenyl)-(3-methylimidazol-4-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2-one; salinosporamide A, (1S,2R,5R)-2-(2-chloroethyl)-5-[[(1S)-cyclohex-2-en-1-yl]-hydroxymethyl]-1-methyl-7-oxa-4-azabicyclo[3.2.0]heptane-3,6-dione; shRNA, short hairpin RNA; SL 327, α-[amino[(4-aminophenyl)thio]methylene]-2-(trifluoromethyl)benzeneacetonitrile; TKI, tyrosine kinase activity inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; ZD6474, N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl)methoxy]quinazolin-4-amine; ZSTK474, 2-(2-difluoromethylbenzimidazol-1-yl)-4,6-dimorpholino-1,3,5-triazine.
The data from numerous in vitro and in vivo studies have revealed that the blockade of the Hh pathway with these agent types results in an inhibition of growth and invasiveness, metastatic spread, and/or the apoptotic death of the cancer- and metastasis-initiating cells and their progenies, whereas the normal cells were insensitive to these cytotoxic effects (Berman et al., 2003; Williams et al., 2003; Beachy et al., 2004; Karhadkar et al., 2004; Kubo et al., 2004; Sheng et al., 2004; Romer and Curran, 2005; Douard et al., 2006; Mimeault et al., 2006; Rubin and de Sauvage, 2006; Clement et al., 2007; Ji et al., 2007; Lauth et al., 2007; Peacock et al., 2007; Remsberg et al., 2007; Stecca et al., 2007; Eichenmüller et al., 2009; Jimeno et al., 2009; Kobune et al., 2009; Liao et al., 2009; Robarge et al., 2009; Tanaka et al., 2009; Tremblay et al., 2009). It has been observed that the inhibition of the SMO signaling effector with cyclopamine inhibited tumor growth, invasion, and metastases of cancer cells with stem cell-like properties in human leukemia, multiple myeloma, and glioma as well as pancreatic, breast, and liver cancers (Liu et al., 2005b, 2006; Douard et al., 2006; Bar et al., 2007; Clement et al., 2007; Feldmann et al., 2007, 2008; Peacock et al., 2007; Stecca et al., 2007; Xu et al., 2008; Eichenmüller et al., 2009; Kobune et al., 2009; Tanaka et al., 2009). In particular, it has been observed that the systemic delivery of cyclopamine or a novel, orally bioavailable small-molecule SMO inhibitor, IPI-269609, prevented the metastatic spread of pancreatic cells and reduced the number of tumor cells expressing the stem cell-like marker aldehyde dehydrogenase in the animal model in vivo (Feldmann et al., 2007, 2008). Moreover, cyclopamine treatment or GLI-1 knockdown by siRNA was also effective at eradicating the clonogenic glioma cells expressing the stem cell-like markers in vitro and inhibiting intracranial growth of glioma stem cell-derived tumors in vivo (Bar et al., 2007; Clement et al., 2007; Xu et al., 2008).
In addition, recent investigations have revealed that the primary cilium might supply key functions for the activation of the SMO protein in certain types of cancer cells (Fig. 3) (Rosenbaum and Witman, 2002; Corbit et al., 2005; Haycraft et al., 2005; Rohatgi and Scott, 2007; Rohatgi et al., 2007; Han et al., 2008, 2009; Spassky et al., 2008; Bailey et al., 2009; Veland et al., 2009; Wong et al., 2009). Therefore, the interference with the IFT proteins and/or the inhibition of the translocation of SMO molecules to the ciliary structure may also represent an alternative therapeutic strategy. It has been shown that specific products of the sterol biosynthesis, including cholesterol and oxysterols, can provide critical roles for the SMO translocation to the primary cilium and activation of the Hh pathway in medulloblastoma cells, which are derived from the malignant transformation of cerebellar GCPs (Corcoran and Scott, 2006). It is noteworthy that the blockade of the sterol synthesis using specific inhibitors reduced the Hh pathway-mediated cell proliferation in medulloblastoma cells (Corcoran and Scott, 2006). In this regard, it has also been shown that SMO antagonists, including SANT-1 and SANT-2, can inhibit the translocation of the SMO molecule to the primary cilium (Wang et al., 2009b). Moreover, it has been reported that four Hh pathway inhibitors designated as HPIs that do not target SMO protein can act downstream of SUFU-modulated GLI activation by decreasing the extent of the SMO accumulation in the primary cilium induced in response to SHH ligand (Hyman et al., 2009). Therefore, the use of these Hh signaling inhibitors might constitute another potential therapeutic strategy to reduce the SMO accumulation in the ciliary structure and SMO-mediated Hh cascade activation in cancer cells.
B. Antitumoral Effects Induced by the Canonical Hedgehog Cascade Blockade in Tumor Stromal Cells
Accumulating evidence has revealed that the secreted SHH ligand can also contribute in a paracrine manner to tumor-stromal cell interactions by stimulating neighboring stromal cells, including fibroblasts, and enhancing the recruitment of BM-derived EPCs to tumor, thereby promoting tumor angiogenesis and disease progression (Bailey et al., 2007, 2009; Yamazaki et al., 2008; Yauch et al., 2008; Kasper et al., 2009; Olive et al., 2009; Shaw et al., 2009; Theunissen and de Sauvage, 2009; Nakamura et al., 2010; Walter et al., 2010). More specifically, the SHH protein has been shown to act through a paracrine mechanism by inducing the GLI1 expression in primary pancreatic cancer-associated stromal fibroblasts (CAF) established from human pancreatic adenocarcinoma that overexpress SMO coreceptor compared with normal pancreatic fibroblasts (Walter et al., 2010). It has also been noticed that the siRNA knockdown of SMO in primary CAFs was accompanied by a down-regulation of GLI1 expression level, suggesting that the targeting of the Hh pathway in CAFs might represent another therapeutic strategy to counteract the pancreatic cancer progression (Walter et al., 2010). In the same way, blockade of Hh signaling using the SMO inhibitor IPI-926 also induced antiangiogenic effects on a pancreatic cancer cell model in vivo (Olive et al., 2009). Moreover, the results from another recent study have revealed that the inhibition of the Hh cascade by using cyclopamine reduced the expression levels of GLI1 and GLI2 in stroma and inhibited the tumor neoangiogenic process and growth of pancreatic cancer cell-derived xenografts (Nakamura et al., 2010). This antiangiogenic effect of cyclopamine was mediated in part by an inhibition of the SHH release from pancreatic cancer cells and recruitment of BM-derived pro-angiogenic cells at primary pancreatic tumor (Nakamura et al., 2010). More specifically, it has been observed that a decrease of the angiotensin-1 and insulin-like growth factor-1 expression occurred in BM-derived proangiogenic cells after the cyclopamine treatment and resulted in a reduction of tumor neovascularization (Nakamura et al., 2010). In the same pathway, the GLI2 gene silencing by specific short hairpin RNAs also induced the apoptosis in a mouse BCC-like tumor cell line and markedly inhibited the tumor vascularization in an in vivo mouse tumor model (Ji et al., 2008).
C. Clinical Trials with the Canonical Hedgehog Cascade Inhibitors
Among the chemical compounds acting as the specific inhibitors of the canonical Hh pathway, only the SMO antagonists have been tested in humans. A preliminary study performed with the natural steroidal alkaloid cyclopamine, consisting of its topical application in a cream formulation to four patients with BCC, has revealed that the tumors rapidly regressed in all cases without adverse effects, and the normal skin and putative stem cells exposed to cyclopamine were preserved (Taş and Avci, 2004). Histological and immunohistochemical analyses have also indicated that the topical cyclopamine application resulted in an inhibition of the proliferation and induced the apoptotic death of tumor cells (Taş and Avci, 2004). The potential teratogenic effect of cyclopamine at high doses may limit its use. Other potent SMO inhibitors that have also reached the clinical trials include the orally active IPI-926 semisynthetic derivative of cyclopamine and different synthetic compounds such as GDC-0449, Cur61414, and NVP-LDE-225 (LoRusso et al., 2008; Molckovsky and Siu, 2008; Rudin et al., 2009; Von Hoff et al., 2009; Stanton and Peng, 2010).
The selective SMO antagonist GDC-0449 is among the more potent chemical compounds that has recently suspired great interest based on promising results from a phase I clinical trial. This small synthetic molecule was discovered through a high-throughput screening of a library of chemical compounds to analyze their potential inhibitory effect on the GLI1 expression by luciferase reporter gene assays followed by an optimization of the pharmacological properties of the most active SMO antagonists by medicinal chemistry (Robarge et al., 2009). The data from a phase I multicenter clinical trial consisting of the administration of orally active GDC-0449 in patients with advanced and metastatic cancers revealed that this pharmacological agent showed antitumoral activity and was well tolerated, with no grade 5 adverse events and dose-limiting toxicity, in a subset of cancer patients (LoRusso et al., 2008; Molckovsky and Siu, 2008; Von Hoff et al., 2009). More specifically, the results from this phase I clinical trial, obtained with 33 patients diagnosed with locally advanced or metastatic BCCs that were refractory to standard therapies, treated daily with oral GDC-0449 during a median time of 9.8 months, revealed that two patients showed a complete tumor response and 16 had a partial response to this treatment, whereas other patients had stable or progressive disease (Von Hoff et al., 2009). Moreover, a case report about the enrollment in a phase I clinical trial of a 26-year-old patient diagnosed with a systemic metastatic medulloblastoma associated with inactivating mutation in PTCH1 that was refractory to multiple prior clinical treatments indicated that rapid tumor regression and reduction of symptoms occurred in this patient after treatment with GDC-0449 (Rudin et al., 2009). Unfortunately, the development of resistance to GDC-0449 treatment associated with the occurrence of a mutation in the SMO protein impairing the binding of GDC-0449 to the SMO molecules led to tumor regrowth evident approximately 3 months after GDC-0449 treatment initiation at some sites (Rudin et al., 2009; Yauch et al., 2009). Consequently, this patient was removed from the clinical trial because of disease progression and died after approximately 2 months, despite a series of subsequent therapies (Rudin et al., 2009).
Hence, these data from a phase I clinical trial suggest that the GDC-0449 might induce the antitumoral effects in a subset of patients with locally advanced or metastatic BCCs or medulloblastomas characterized by a sustained activation of the Hh signaling cascade despite the intrinsic or acquired resistance to this treatment type, which might be prevalent in certain patients may counteract the efficacy of this therapeutic strategy. Phase I and II clinical trials are also ongoing to investigate long-term antitumoral activity and safety of GDC-0449, alone or in combination with current chemotherapeutic treatments, in patients diagnosed with a variety of cancers. These cancers include localized, metastatic, or recurrent BCC, medulloblastoma, glioblastoma multiforme, and small-cell lung, pancreatic, stomach, colorectal, breast, and ovarian cancers. Thus, the results from these additional clinical trials with GDC-0449 and other specific Hh inhibitors should confirm the therapeutic benefit and safety of inhibiting the Hh signaling pathway in treating patients with a wide range of solid tumors.
D. Targeting of the Noncanonical Hedgehog Tumorigenic Signaling Pathways
Emerging lines of experimental evidence have revealed that the activation of noncanonical Hh pathways may contribute in cooperation with the canonical Hh pathways mediated through Hh ligand/PTCH1/SMO/GLIs to the acquisition of more malignant phenotypes by cancer cells during disease progression (Lauth and Toftgård, 2007; Lauth et al., 2007; Jenkins, 2009; Stecca and Ruiz, 2010). More particularly, it has been reported that the oncogenic EWS-FLI1 fusion protein detected in the majority of Ewing's sarcomas, oncogenic K-RAS, and the activation of RTKs such as EGFR and platelet-derived growth factor receptor α as well as Wnt/β-catenin and the TGF-β1/TGF-βR system, can stimulate GLI expression and/or activities and Hh target gene expression during cancer development (Figs. 5 and 6) (Xie et al., 2001; Bigelow et al., 2005; Palma et al., 2005; Kasper et al., 2006; Maeda et al., 2006; Riobo et al., 2006; Riobó et al., 2006; Dennler et al., 2007, 2009; Ji et al., 2007; Stecca et al., 2007; Nolan-Stevaux et al., 2009; Schnidar et al., 2009; Seto et al., 2009; Stecca and Ruiz, 2010). Hence, these oncogenic signaling elements can cooperate with the canonical Hh ligand-induced signaling cascade in certain types of cancer cells. In fact, the bidirectional signaling cross-talk between these developmental pathways can cooperate to induce the epithelial-mesenchymal transition process in cancer cells and progression to invasive, metastatic, and recurrent cancers (Mimeault and Batra, 2007, 2010c; Yoo et al., 2008; Nolan-Stevaux et al., 2009; Stecca and Ruiz, 2010). Therefore, the targeting of these growth factor cascades and/or their intracellular signaling effectors, including GLI proteins, might constitute a promising therapeutic approach, alone or in combination with the specific inhibitor of canonical Hh pathway, to eradicate the cancer-initiating cells and their progenies (Dennler et al., 2007; Mimeault and Batra, 2007; Hyman et al., 2009). In support of this, it has been observed that the oncogenic K-RAS transformation of human pancreatic ductal epithelial cells, which resulted in an increase of GLI transcriptional activity, was inhibited by siRNA targeting K-RAS or a MEK-specific inhibitor, 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene (U0126) or 2′-amino-3′-methoxyflavone (PD98059) (Ji et al., 2007). In the same way, treatment of mouse pancreatic cell lines with siRNA constructs targeting K-RAS or GLI1 also inhibited expression of GLI1 and PTCH1 and induced the apoptotic death of these cancer cells in vitro (Nolan-Stevaux et al., 2009). Moreover, it has been observed that the TGF-β type I activin receptor-like kinase 5 (ALK5) inhibitor 4-(5-benzo(1,3)dioxol-5-yl-4-pyridin-2-yl-1H-imidazol-2-yl)benzamide (SB431542) completely abrogated the stimulatory effect induced by TGF-β on GLI2 expression and inhibited the growth of pancreatic cancer cells in vitro and in vivo (Dennler et al., 2007). It has also been shown that the small molecules termed 4-(2,4,5-tripyridin-4-ylthiophen-3-yl)pyridine (GANT58) and 2,2′-[[dihydro-2-(4-pyridinyl)-1,3(2H,4H)-pyrimidinediyl]bis (methylene)]bis[N,N-dimethyl]-benzenamine (GANT61), acting as inhibitors of GLI1- and GLI2-mediated Hh gene expression, inhibited the growth of the PANC-1 pancreatic and recurrent 22Rv1 prostate cancer cell lines in vitro and tumor growth of 22Rv1 xenografts in nude mice in vivo more potently than the SMO antagonist cyclopamine (Lauth et al., 2007). On the other hand, it has been reported that the activation of GSK3b, which may contribute to the inactivation of GLI proteins, might be inhibited through the stimulation of PI3K/Akt/mTOR/p70S6K2 intracellular signaling (Yuan et al., 2007; Mizuarai et al., 2009). In contrast, the silencing of p70S6K2 activated GSK3b, enhanced GLI degradation, and inhibited the viability of non–small-cell lung cancer cells (Yuan et al., 2007; Mizuarai et al., 2009). These data suggest that p70S6K2 might also represent a potential therapeutic target to inhibit Hh ligand-independent activation of the Hh cascade mediated through high GLI expression levels in certain types of cancer cells. In this regard, we reviewed in a more detailed manner the therapeutic strategies consisting of targeting EGFR, alone or in combination with a Hh inhibitor, for improving the current clinical cancer therapies.
E. Targeting of the Epidermal Growth Factor Receptor Tumorigenic Signaling Pathway
Numerous preclinical and clinical studies have indicated that the selective blockade of the EGFR signaling pathway may represent a potent strategy, alone or in combination with the other conventional treatments, to counteract cancer progression and prevent disease relapse (Yarden and Sliwkowski, 2001; Budiyanto et al., 2003; Chung and Saltz, 2005; Haruki et al., 2005; Hynes and Lane, 2005; Shelton et al., 2005; del Carmen et al., 2005; Angelucci et al., 2006; Citri and Yarden, 2006; Mimeault et al., 2006, 2010; Cerciello et al., 2007; Tortora et al., 2007; Shaw and Prowse, 2008; Griffero et al., 2009). Among the selective agents targeting the EGFR cascade are the anti-EGFR antibodies (mAb-C225 and ICM-C225, also designated cetuximab and erbitux, respectively), antisense oligonucleotide directed against EGFR or its ligands EGF and TGF-α, and the selective inhibitors of EGFR tyrosine kinase activity such as 4-(3′-chloroanilino)-6,7-dimethoxy-quinazoline (AG1478), N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine (gefitinib; Iressa; ZD1839) N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine (erlotinib; Tarceva; OSI-774) (Table 1) (Chung and Saltz, 2005; Haruki et al., 2005; Hynes and Lane, 2005; Shelton et al., 2005; Shepherd et al., 2005; del Carmen et al., 2005; Bonner et al., 2006; Citri and Yarden, 2006; Hanna et al., 2006; Khambata-Ford et al., 2007; Nogueira-Rodrigues et al., 2008; Shaw and Prowse, 2008; Yonesaka et al., 2008; Griffero et al., 2009; Montagut and Settleman, 2009). Alternatively, the molecular targeting of EGFR downstream signaling elements, including RAS/RAF/MEK, PI3K/Akt/mTOR, NF-κB, cyclooxygnase-2, and vascular endothelial growth factor/vascular endothelial growth factor receptor might represent another effective therapeutic approach (Table 1) (Chung and Saltz, 2005; Haruki et al., 2005; Hynes and Lane, 2005; Shelton et al., 2005; del Carmen et al., 2005; Citri and Yarden, 2006; Xu et al., 2008; Montagut and Settleman, 2009). Many in vitro and in vivo studies have revealed that these agents can induce an inhibition of the growth, invasiveness and apoptotic death of diverse cancer cell types and counteract the angiogenic process (Haruki et al., 2005; Hynes and Lane, 2005; Citri and Yarden, 2006; Mimeault et al., 2010). For instance, it has been reported that a specific inhibitor of EGFR tyrosine kinase activity, gefitinib or erlotinib, induced the antiproliferative and cytotoxic effects on EGFR+/CD133+ tumor-initiating cells from five patients with glioma blastomas (Griffero et al., 2009). Certain mAbs directed against EGFR (cetuximab) and EGFR tyrosine kinase activity inhibitors (gefitinib and erlotinib) have also reached clinical trials and are now used in the chemotherapeutic arsenals for treating diverse common solid tumors, including non–small-cell lung, breast, colorectal, head, and neck squamous cell and pancreatic cancers (Hynes and Lane, 2005; Shepherd et al., 2005; Bonner et al., 2006; Hanna et al., 2006; Khambata-Ford et al., 2007; Nogueira-Rodrigues et al., 2008; Yonesaka et al., 2008). The response of cancer patients to the blockade of the EGFR signaling cascade may be influenced by different factors, including the activating mutations in the EGFR and expression levels of EGFR ligands in cancer cells (Khambata-Ford et al., 2007; Yonesaka et al., 2008).
F. New Combination Therapies against Aggressive and Recurrent Cancers
In view of the fact that aggressive, metastatic, and recurrent cancers are typically characterized by activation of numerous oncogenic signaling elements, combination therapies may represent more effective treatments than monotherapies for improving the current therapeutic regimens and remedy the potential toxicity associated with the inclusion of high doses of individual drugs. In this regard, the molecular targeting of Hh and/or EGFR cascades has notably been shown to be a potential strategy for reversing the treatment resistance and improving the efficacy of the current antihormonal therapy, chemotherapy, and/or radiotherapy (Haruki et al., 2005; Hynes and Lane, 2005; del Carmen et al., 2005; Cerciello et al., 2007; Clement et al., 2007; Feldmann et al., 2007; Mimeault and Batra, 2007 2008a; Morton et al., 2007; Tortora et al., 2007; Mimeault et al., 2008, 2010; Shaw and Prowse, 2008; Jimeno et al., 2009; Kobune et al., 2009; Schnidar et al., 2009). For instance, the combined pharmacological inhibition of Hh/GLI and EGFR pathways by cyclopamine and gefitinib resulted in enhanced antiproliferative and apoptotic effects on prostate and pancreatic cell lines in vitro (Mimeault et al., 2006, 2010; Hu et al., 2007). Moreover, the combined use of cyclopamine and gefitinib also resulted in suppression of the growth of BCC cell lines derived from mice in vitro (Schnidar et al., 2009). We have also shown that a combination of gefitinib and/or cyclopamine with current chemotherapeutic drug docetaxel or mitoxantrone led to a more massive rate of apoptotic death on diverse metastatic prostate cancer cells, including side-population (SP) and non-SP cell fractions from invasive prostate cancer cells, relative to individual drugs or two-drug combination treatments (Mimeault et al., 2007a,b, 2010). Of particular interest is that the combination of cyclopamine with the current chemotherapeutic drug temozolomide also induced additive and synergistic anticarcinogenic effects on glioma stem cell culture cells in vitro (Clement et al., 2007). Likewise, a combination of cyclopamine and gemcitabine has been observed to inhibit tumor growth and metastatic spread and decrease the expression levels of stem cell-like markers, including aldehyde dehydrogenase detected by immunohistochemistry in a pancreatic cancer xenograft model in vivo (Feldmann et al., 2007; Jimeno et al., 2009). Moreover, the inhibition of Hh signaling with cyclopamine, HHIP, or anti-SHH mAb also induced apoptosis in CD34+ acute myeloid leukemic cells and significantly improved the cytotoxic effect induced by cytarabine on these leukemic cells (Kobune et al., 2009).
Of particular interest is that the Hh/GLI and EGF-EGFR system may contribute to the regulation of the expression and/or cellular localization of ABC transporters in certain cancer cells, including the SP cell fraction with stem cell-like properties (Fig. 6) (Chen et al., 2006; Meyer zu Schwabedissen et al., 2006; Sims-Mourtada et al., 2007; Mimeault et al., 2008; Zhang et al., 2009). Therefore, the inhibition of Hh and/or EGFR cascades may represent a potential strategy for reversing multidrug resistance phenotypes mediated through ABC multidrug efflux pumps. In support of this, cyclopamine has been observed to reduce the expression levels of ABCG2/breast cancer resistance and ABCB1/multidrug resistance protein-1/P-glycoprotein in the metastatic and tumorigenic PC3 prostate cancer cell line and to improve the cytotoxic effects induced by different chemotherapeutic drugs on diverse cancer cell lines (Sims-Mourtada et al., 2007). The SMO antagonist GDC-0449 (HhAntag691) was also effective in inhibiting the drug efflux pump activity of ABCG2 and multidrug resistance protein-1 (Zhang et al., 2009). Likewise, it has been shown that EGF can increase the expression of ABC transporters at the cell surface and in the SP fraction, whereas the blockade of the EGFR cascade can inhibit this effect (Takada et al., 2005; Chen et al., 2006b; Meyer zu Schwabedissen et al., 2006).
On the other hand, because the secreted ligands of Hh and EGFR cascades can act through a paracrine mode on surrounding tumor cells and stromal cells, the interference with their secretion and transport or SMO activity inhibition in host stromal cells could also constitute a potential adjuvant therapeutic strategy to counteract tumor development (Fig. 6). In this regard, it has been reported that blockade of the Hh pathway by using the SMO inhibitor IPI-926 improved tumor delivery and antitumoral efficacy of gemcitabine, at least in part, by disrupting the desmoplastic stroma in a pancreatic cancer cell model in vivo (Olive et al., 2009).
VI. Conclusions and Future Directions
Together, these recent studies have underlined the critical physiological roles played by the Hh signaling network during the developmental process and adult life in the maintenance of stem/progenitor cells and their progenies and complex molecular mechanisms involved in its regulation. Future studies, however, are necessary to more precisely delineate the signaling elements implicated in the positive and negative regulation of the transcriptional expression of Hh ligands and GLI proteins, SMO and GLI activation, and specific functions of the primary cilium in physiological and pathological conditions. In particular, it will be important to further determine the factors involved in the up-regulated expression of SHH and GLI activities and potential interactive cross-talk with other oncogenic pathways during cancer progression. The establishment of the precise molecular mechanisms of Hh signal transduction, including signaling elements involved in SMO translocation to the primary cilium and precise functions of IFT proteins in cellular responses induced in human normal and malignant cell types, is also of great interest. This additional work should help to develop novel potential pharmacological agents to modulate these cellular processes, and thereby counteract the activation of the Hh pathway and cancer development.
In addition, accumulating lines of experimental evidence have revealed that an aberrant activation of Hh/GLI and RTKs, such as the EGFR signaling cascade, frequently occur during cancer initiation and progression and these tumorigenic cascades may cooperate through multiple signaling cross-talks to the malignant transformation of cells, treatment resistance and disease relapse. Future studies are required to more precisely establish the molecular mechanisms and specific downstream signaling elements that may contribute to the cooperative or synergistic interactions of the Hh/GLIs and RTK signaling pathways, including EGFR, in cancer- and metastasis-initiating cells versus their differentiated progenies. Moreover, it is of great therapeutic interest to define drug resistance-associated molecules, including ABC transporters modulated through the inhibition of Hh and/or EGFR pathways, that could be targeted for reversing the chemoresistance of cancer- and metastasis-initiating cells. Hence, these additional investigations should lead to the identification of new potential targets to eradicate the total cancer cell mass and improve current therapies.
In view of the promising results from preclinical studies and a recent phase I clinical trial performed with specific SMO antagonists, and more particularly the successful tumor regression observed with GDC-0449 in certain patients with cancer, the targeting of the Hh cascade seems to represent a therapeutic strategy of great clinical interest. The data obtained with a broad spectrum of patients diagnosed with different cancer types treated with a Hh inhibitor over the long-term should confirm the therapeutic benefit and safety to selectively target the Hh cascade, alone or in combination with the current conventional therapies. In this regard, the establishment of accurate screening tests of diagnosis and molecular mechanisms associated with the intrinsic and acquired resistance of cancer patients to the treatment with a Hh or EGFR inhibitor or other therapeutic targets is also of great clinical interest. These additional studies should lead to an optimization of the choice of therapeutic regimens and personalized medicine as well as the development of novel effective multitarget strategies that could be used for improving the current cancer therapies against locally invasive and metastatic cancers, which are generally associated with a high rate of disease relapse and the death of cancer patients.
Acknowledgments.
This work was supported by the National Institutes of Health National Cancer Institute [Grants CA78590, CA111294, CA131944, CA133774, CA138791].
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.109.002329.
- aa
- amino acid(s)
- ABC
- ATP-binding cassette
- AG-1478
- 4-(3′-chloroanilino)-6,7-dimethoxy-quinazoline
- ALK5
- activin receptor-like kinase 5
- BCC
- basal cell carcinoma
- BM
- bone marrow
- BMI-1
- polycomb group protein-1
- CAF
- cancer-associated stromal fibroblast
- DHH
- Desert hedgehog
- EGF
- epidermal growth factor
- EGFR
- epidermal growth factor receptor
- EPC
- endothelial progenitor cell
- ERK
- extracellular signal-regulated kinase
- GANT58
- 4-(2,4,5-tripyridin-4-ylthiophen-3-yl)pyridine
- GANT61
- 2,2′-[[dihydro-2-(4-pyridinyl)-1,3(2H,4H)pyrimidinediyl]bis(methylene)]bis[N,N-dimethyl]-benzenamine
- GCP
- granule cell precursor
- GDC-0449
- 2-chloro-N-[4-chloro-3-(2-pyridinyl)phenyl]-4-(methylsulfonyl)benzamide
- GLI
- glioma-associated oncogene
- GLI3R
- glioma-associated oncogene 3 receptor
- GSK3b
- glycogen synthase kinase 3b
- Hh
- hedgehog
- Hhat
- Hh acyltransferase
- HHIP
- hedgehog-interacting protein
- IFT
- intraflagellar transport
- IHH
- Indian hedgehog
- KAAD-cyclopamine
- 3-keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl) cyclopamine
- mAb
- monoclonal antibody
- MAPK
- mitogen-activated protein kinase
- MEK
- extracellular-signal-regulated kinase kinase
- mTOR
- mammalian target of rapamycin
- NBCCS
- nevoid basal cell carcinoma syndrome
- NF-κB
- nuclear factor-κB
- NVP-LDE-225
- N-(6-((2S,6R)-2,6-dimethylmorpholino) pyridin-3-yl)-2-methyl-4′-(trifluoromethoxy)biphenyl-3-carboxamide
- PD032590
- N-[(2R)-2,3-dihydroxypropoxy]-3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzamide
- PD98059
- 2′-amino-3′-methoxyflavone
- PI3K
- phosphatidylinositol 3′ kinase
- PTCH1
- patched receptor 1
- RTK
- receptor tyrosine kinase
- SB431542
- 4-(5-benzo(1,3)dioxol-5-yl-4-pyridin-2-yl-1H-imidazol-2-yl)benzamide
- SHH
- sonic hedgehog
- siRNA
- small interference RNA
- SMO
- smoothened
- SP
- side population
- SUFU
- suppressor of fused
- TGF-β
- transforming growth factor-β
- U0126
- 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene
- Wnt
- Wingless ligand.
References
- Abe et al., 2008.Abe Y, Oda-Sato E, Tobiume K, Kawauchi K, Taya Y, Okamoto K, Oren M, Tanaka N. (2008) Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc Natl Acad Sci USA 105:4838–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn and Joyner, 2005.Ahn S, Joyner AL. (2005) In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature 437:894–897 [DOI] [PubMed] [Google Scholar]
- Alvarez-Medina et al., 2009.Alvarez-Medina R, Le Dreau G, Ros M, Martí E. (2009) Hedgehog activation is required upstream of Wnt signalling to control neural progenitor proliferation. Development 136:3301–3309 [DOI] [PubMed] [Google Scholar]
- Amankulor et al., 2009.Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. (2009) Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci 29:10299–10308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amantea et al., 2008.Amantea CM, Kim WK, Meliton V, Tetradis S, Parhami F. (2008) Oxysterol-induced osteogenic differentiation of marrow stromal cells is regulated by Dkk-1 inhibitable and PI3-kinase mediated signaling. J Cell Biochem 105:424–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci et al., 2006.Angelucci A, Gravina GL, Rucci N, Millimaggi D, Festuccia C, Muzi P, Teti A, Vicentini C, Bologna M. (2006) Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocr Relat Cancer 13:197–210 [DOI] [PubMed] [Google Scholar]
- Asai et al., 2006.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, Eaton E, Iwakura A, Tsutsumi Y, Hamada H, et al. (2006) Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation 113:2413–2424 [DOI] [PubMed] [Google Scholar]
- Bailey et al., 2009.Bailey JM, Mohr AM, Hollingsworth MA. (2009) Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene 28:3513–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey et al., 2007.Bailey JM, Singh PK, Hollingsworth MA. (2007) Cancer metastasis facilitated by developmental pathways: Sonic hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem 102:829–839 [DOI] [PubMed] [Google Scholar]
- Bak et al., 2003.Bak M, Hansen C, Tommerup N, Larsen LA. (2003) The Hedgehog signaling pathway—implications for drug targets in cancer and neurodegenerative disorders. Pharmacogenomics 4:411–429 [DOI] [PubMed] [Google Scholar]
- Bale, 2002.Bale AE. (2002) Hedgehog signaling and human disease. Annu Rev Genomics Hum Genet 3:47–65 [DOI] [PubMed] [Google Scholar]
- Bambakidis et al., 2009.Bambakidis NC, Horn EM, Nakaji P, Theodore N, Bless E, Dellovade T, Ma C, Wang X, Preul MC, Coons SW, et al. (2009) Endogenous stem cell proliferation induced by intravenous hedgehog agonist administration after contusion in the adult rat spinal cord. J Neurosurg Spine 10:171–176 [DOI] [PubMed] [Google Scholar]
- Bar et al., 2007.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, et al. (2007) Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells 25:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy et al., 2004.Beachy PA, Karhadkar SS, Berman DM. (2004) Tissue repair and stem cell renewal in carcinogenesis. Nature 432:324–331 [DOI] [PubMed] [Google Scholar]
- Berman et al., 2003.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, et al. (2003) Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425:846–851 [DOI] [PubMed] [Google Scholar]
- Bhardwaj et al., 2001.Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M. (2001) Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol 2:172–180 [DOI] [PubMed] [Google Scholar]
- Bhattacharya et al., 2008.Bhattacharya R, Kwon J, Ali B, Wang E, Patra S, Shridhar V, Mukherjee P. (2008) Role of hedgehog signaling in ovarian cancer. Clin Cancer Res 14:7659–7666 [DOI] [PubMed] [Google Scholar]
- Bian et al., 2007.Bian YH, Huang SH, Yang L, Ma XL, Xie JW, Zhang HW. (2007) Sonic hedgehog-Gli1 pathway in colorectal adenocarcinomas. World J Gastroenterol 13:1659–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow et al., 2005.Bigelow RL, Jen EY, Delehedde M, Chari NS, McDonnell TJ. (2005) Sonic hedgehog induces epidermal growth factor dependent matrix infiltration in HaCaT keratinocytes. J Invest Dermatol 124:457–465 [DOI] [PubMed] [Google Scholar]
- Bijlsma et al., 2006.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. (2006) Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol 4:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner et al., 2006.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578 [DOI] [PubMed] [Google Scholar]
- Bosanac et al., 2009.Bosanac I, Maun HR, Scales SJ, Wen X, Lingel A, Bazan JF, de Sauvage FJ, Hymowitz SG, Lazarus RA. (2009) The structure of SHH in complex with HHIP reveals a recognition role for the Shh pseudo active site in signaling. Nat Struct Mol Biol 16:691–697 [DOI] [PubMed] [Google Scholar]
- Budiyanto et al., 2003.Budiyanto A, Bito T, Kunisada M, Ashida M, Ichihashi M, Ueda M. (2003) Inhibition of the epidermal growth factor receptor suppresses telomerase activity in HSC-1 human cutaneous squamous cell carcinoma cells. J Invest Dermatol 121:1088–1094 [DOI] [PubMed] [Google Scholar]
- Buglino and Resh, 2008.Buglino JA, Resh MD. (2008) Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J Biol Chem 283:22076–22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner et al., 2009.Büttner A, Seifert K, Cottin T, Sarli V, Tzagkaroulaki L, Scholz S, Giannis A. (2009) Synthesis and biological evaluation of SANT-2 and analogues as inhibitors of the hedgehog signaling pathway. Bioorg Med Chem 17:4943–4954 [DOI] [PubMed] [Google Scholar]
- Calcutt et al., 2003.Calcutt NA, Allendoerfer KL, Mizisin AP, Middlemas A, Freshwater JD, Burgers M, Ranciato R, Delcroix JD, Taylor FR, Shapiro R, et al. (2003) Therapeutic efficacy of sonic hedgehog protein in experimental diabetic neuropathy. J Clin Invest 111:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter et al., 1998.Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, Rosenthal A, de Sauvage FJ. (1998) Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci USA 95:13630–13634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrière et al., 2007.Carrière C, Seeley ES, Goetze T, Longnecker DS, Korc M. (2007) The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci USA 104:4437–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta et al., 2006.Caserta TM, Kommagani R, Yuan Z, Robbins DJ, Mercer CA, Kadakia MP. (2006) p63 overexpression induces the expression of Sonic Hedgehog. Mol Cancer Res 4:759–768 [DOI] [PubMed] [Google Scholar]
- Cerciello et al., 2007.Cerciello F, Riesterer O, Sherweif M, Odermatt B, Ciernik IF. (2007) Is EGFR a moving target during radiotherapy of carcinoma of the uterine cervix? Gynecol Oncol 106:394–399 [DOI] [PubMed] [Google Scholar]
- Charytoniuk et al., 2002a.Charytoniuk D, Porcel B, Rodríguez Gomez J, Faure H, Ruat M, Traiffort E. (2002a) Sonic Hedgehog signalling in the developing and adult brain. J Physiol Paris 96:9–16 [DOI] [PubMed] [Google Scholar]
- Charytoniuk et al., 2002b.Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A, Ruat M. (2002b) Intrastriatal sonic hedgehog injection increases patched transcript levels in the adult rat subventricular zone. Eur J Neurosci 16:2351–2357 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2006a.Chen BY, Lin DP, Liu JY, Chang H, Huang PH, Chen YL, Chang HH. (2006a) A mouse prostate cancer model induced by Hedgehog overexpression. J Biomed Sci 13:373–384 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2007a.Chen BY, Liu JY, Chang HH, Chang CP, Lo WY, Kuo WH, Yang CR, Lin DP. (2007a) Hedgehog is involved in prostate basal cell hyperplasia formation and its progressing towards tumorigenesis. Biochem Biophys Res Commun 357:1084–1089 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2002.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. (2002) Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA 99:14071–14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2006b.Chen JS, Pardo FS, Wang-Rodriguez J, Chu TS, Lopez JP, Aguilera J, Altuna X, Weisman RA, Ongkeko WM. (2006b) EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope 116:401–406 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2007b.Chen X, Horiuchi A, Kikuchi N, Osada R, Yoshida J, Shiozawa T, Konishi I. (2007b) Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: it's [sic] inhibition leads to growth suppression and apoptosis. Cancer Sci 98:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang et al., 1996.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383:407–413 [DOI] [PubMed] [Google Scholar]
- Chuang and McMahon, 1999.Chuang PT, McMahon AP. (1999) Vertebrate hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature 397:617–621 [DOI] [PubMed] [Google Scholar]
- Chung and Saltz, 2005.Chung KY, Saltz LB. (2005) Antibody-based therapies for colorectal cancer. Oncologist 10:701–709 [DOI] [PubMed] [Google Scholar]
- Citri and Yarden, 2006.Citri A, Yarden Y. (2006) EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7:505–516 [DOI] [PubMed] [Google Scholar]
- Clement et al., 2007.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. (2007) HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17:165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, 2003.Cohen MM., Jr (2003) The hedgehog signaling network. Am J Med Genet A 123A:5–28 [DOI] [PubMed] [Google Scholar]
- Corbit et al., 2005.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. (2005) Vertebrate Smoothened functions at the primary cilium. Nature 437:1018–1021 [DOI] [PubMed] [Google Scholar]
- Corcoran and Scott, 2006.Corcoran RB, Scott MP. (2006) Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci USA 103:8408–8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui et al., 2010.Cui W, Wang LH, Wen YY, Song M, Li BL, Chen XL, Xu M, An SX, Zhao J, Lu YY, et al. (2010) Expression and regulation mechanisms of Sonic Hedgehog in breast cancer. Cancer Sci 101:927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane et al., 1997.Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. (1997) Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389:876–881 [DOI] [PubMed] [Google Scholar]
- Dai et al., 1999.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. (1999) Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem 274:8143–8152 [DOI] [PubMed] [Google Scholar]
- Datta and Datta, 2006.Datta S, Datta MW. (2006) Sonic Hedgehog signaling in advanced prostate cancer. Cell Mol Life Sci 63:435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies et al., 2000.Davies JP, Chen FW, Ioannou YA. (2000) Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science 290:2295–2298 [DOI] [PubMed] [Google Scholar]
- del Carmen et al., 2005.del Carmen MG, Rizvi I, Chang Y, Moor AC, Oliva E, Sherwood M, Pogue B, Hasan T.(2005) Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. J Natl Cancer Inst 97:1516–1524 [DOI] [PubMed] [Google Scholar]
- Dennler et al., 2007.Dennler S, André J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. (2007) Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res 67:6981–6986 [DOI] [PubMed] [Google Scholar]
- Dennler et al., 2009.Dennler S, André J, Verrecchia F, Mauviel A. (2009) Cloning of the human GLI2 promoter: transcriptional activation by transforming growth factor-beta via SMAD3/beta-catenin cooperation. J Biol Chem 284:31523–31531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker et al., 2009a.Dierker T, Dreier R, Migone M, Hamer S, Grobe K. (2009a) Heparan sulfate and transglutaminase activity are required for the formation of covalently cross-linked hedgehog oligomers. J Biol Chem 284:32562–32571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker et al., 2009b.Dierker T, Dreier R, Petersen A, Bordych C, Grobe K. (2009b) Heparan sulfate-modulated, metalloprotease-mediated sonic hedgehog release from producing cells. J Biol Chem 284:8013–8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo et al., 2002.Di Lorenzo G, Tortora G, D'Armiento FP, De Rosa G, Staibano S, Autorino R, D'Armiento M, De Laurentiis M, De Placido S, Catalano G, et al. (2002) Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin Cancer Res 8:3438–3444 [PubMed] [Google Scholar]
- Douard et al., 2006.Douard R, Moutereau S, Pernet P, Chimingqi M, Allory Y, Manivet P, Conti M, Vaubourdolle M, Cugnenc PH, Loric S. (2006) Sonic Hedgehog-dependent proliferation in a series of patients with colorectal cancer. Surgery 139:665–670 [DOI] [PubMed] [Google Scholar]
- Dunaeva et al., 2010.Dunaeva M, Voo S, van Oosterhoud C, Waltenberger J. (2010) Sonic hedgehog is a potent chemoattractant for human monocytes: diabetes mellitus inhibits Sonic hedgehog-induced monocyte chemotaxis. Basic Res Cardiol 105:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer et al., 2007.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. (2007) Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem 282:8959–8968 [DOI] [PubMed] [Google Scholar]
- Ehtesham et al., 2007.Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, Thompson RC, Cooper MK. (2007) Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene 26:5752–5761 [DOI] [PubMed] [Google Scholar]
- Eichberger et al., 2006.Eichberger T, Sander V, Schnidar H, Regl G, Kasper M, Schmid C, Plamberger S, Kaser A, Aberger F, Frischauf AM. (2006) Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics 87:616–632 [DOI] [PubMed] [Google Scholar]
- Eichenmüller et al., 2009.Eichenmüller M, Gruner I, Hagl B, Häberle B, Müller-Höcker J, von Schweinitz D, Kappler R. (2009) Blocking the hedgehog pathway inhibits hepatoblastoma gowth. Hepatology 49:482–490 [DOI] [PubMed] [Google Scholar]
- Feldmann et al., 2007.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, et al. (2007) Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res 67:2187–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann et al., 2008.Feldmann G, Fendrich V, McGovern K, Bedja D, Bisht S, Alvarez H, Koorstra JB, Habbe N, Karikari C, Mullendore M, et al. (2008) An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther 7:2725–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi et al., 2009.Fiaschi M, Rozell B, Bergström A, Toftgård R. (2009) Development of mammary tumors by conditional expression of GLI1. Cancer Res 69:4810–4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Kamenetsky et al., 2002.Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, et al. (2002) Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J Biol 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frappart et al., 2009.Frappart PO, Lee Y, Russell HR, Chalhoub N, Wang YD, Orii KE, Zhao J, Kondo N, Baker SJ, McKinnon PJ. (2009) Recurrent genomic alterations characterize medulloblastoma arising from DNA double-strand break repair deficiency. Proc Natl Acad Sci USA 106:1880–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu et al., 2006.Fu JR, Liu WL, Zhou JF, Sun HY, Xu HZ, Luo L, Zhang H, Zhou YF. (2006) Sonic hedgehog protein promotes bone marrow-derived endothelial progenitor cell proliferation, migration and VEGF production via PI 3-kinase/Akt signaling pathways. Acta Pharmacol Sin 27:685–693 [DOI] [PubMed] [Google Scholar]
- Fuse et al., 1999.Fuse N, Maiti T, Wang B, Porter JA, Hall TM, Leahy DJ, Beachy PA. (1999) Sonic hedgehog protein signals not as a hydrolytic enzyme but as an apparent ligand for patched. Proc Natl Acad Sci USA 96:10992–10999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano et al., 1999.Gaiano N, Kohtz JD, Turnbull DH, Fishell G. (1999) A method for rapid gain-of-function studies in the mouse embryonic nervous system. Nat Neurosci 2:812–819 [DOI] [PubMed] [Google Scholar]
- Gold et al., 2003.Gold DA, Baek SH, Schork NJ, Rose DW, Larsen DD, Sachs BD, Rosenfeld MG, Hamilton BA. (2003) RORalpha coordinates reciprocal signaling in cerebellar development through sonic hedgehog and calcium-dependent pathways. Neuron 40:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich et al., 1997.Goodrich LV, Milenković L, Higgins KM, Scott MP. (1997) Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277:1109–1113 [DOI] [PubMed] [Google Scholar]
- Griffero et al., 2009.Griffero F, Daga A, Marubbi D, Capra MC, Melotti A, Pattarozzi A, Gatti M, Bajetto A, Porcile C, Barbieri F, et al. (2009) Different response of human glioma tumor-initiating cells to EGFR kinase inhibitors. J Biol Chem 284:7138–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino et al., 2009.Gulino A, De Smaele E, Ferretti E. (2009) Glucocorticoids and neonatal brain injury: the hedgehog connection. J Clin Invest 119:243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn et al., 1996.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, et al. (1996) Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85:841–851 [DOI] [PubMed] [Google Scholar]
- Hahn et al., 1999.Hahn H, Wojnowski L, Miller G, Zimmer A. (1999) The patched signaling pathway in tumorigenesis and development: lessons from animal models. J Mol Med 77:459–468 [DOI] [PubMed] [Google Scholar]
- Hahn et al., 2000.Hahn H, Wojnowski L, Specht K, Kappler R, Calzada-Wack J, Potter D, Zimmer A, Müller U, Samson E, Quintanilla-Martinez L, et al. (2000) Patched target Igf2 is indispensable for the formation of medulloblastoma and rhabdomyosarcoma. J Biol Chem 275:28341–28344 [DOI] [PubMed] [Google Scholar]
- Han et al., 2009.Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. (2009) Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med 15:1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han et al., 2008.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. (2008) Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 11:277–284 [DOI] [PubMed] [Google Scholar]
- Hanna et al., 2006.Hanna N, Lilenbaum R, Ansari R, Lynch T, Govindan R, Jänne PA, Bonomi P. (2006) Phase II trial of cetuximab in ptients with previously treated non-small-cell lung cancer. J Clin Oncol 24:5253–5258 [DOI] [PubMed] [Google Scholar]
- Haruki et al., 2005.Haruki N, Kawaguchi KS, Eichenberger S, Massion PP, Olson S, Gonzalez A, Carbone DP, Dang TP. (2005) Dominant-negative Notch3 receptor inhibits mitogen-activated protein kinase pathway and the growth of human lung cancers. Cancer Res 65:3555–3561 [DOI] [PubMed] [Google Scholar]
- Haycraft et al., 2005.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. (2005) Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde et al., 2008.Hegde GV, Munger CM, Emanuel K, Joshi AD, Greiner TC, Weisenburger DD, Vose JM, Joshi SS. (2008) Targeting of sonic hedgehog-GLI signaling: a potential strategy to improve therapy for mantle cell lymphoma. Mol Cancer Ther 7:1450–1460 [DOI] [PubMed] [Google Scholar]
- Heo et al., 2007.Heo JS, Lee MY, Han HJ. (2007) Sonic hedgehog stimulates mouse embryonic stem cell proliferation by cooperation of Ca2+/protein kinase C and epidermal growth factor receptor as well as Gli1 activation. Stem Cells 25:3069–3080 [DOI] [PubMed] [Google Scholar]
- Hernes et al., 2004.Hernes E, Fosså SD, Berner A, Otnes B, Nesland JM. (2004) Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br J Cancer 90:449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani et al., 2005.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. (2005) Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7:469–483 [DOI] [PubMed] [Google Scholar]
- Hu et al., 2007.Hu WG, Liu T, Xiong JX, Wang CY. (2007) Blockade of sonic hedgehog signal pathway enhances antiproliferative effect of EGFR inhibitor in pancreatic cancer cells. Acta Pharmacol Sin 28:1224–1230 [DOI] [PubMed] [Google Scholar]
- Huangfu et al., 2003.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87 [DOI] [PubMed] [Google Scholar]
- Hyman et al., 2009.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, Sun M, Rack PG, Sinha S, Wu JJ, et al. (2009) Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci USA 106:14132–14137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes and Lane, 2005.Hynes NE, Lane HA. (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5:341–354 [DOI] [PubMed] [Google Scholar]
- Ikram et al., 2004.Ikram MS, Neill GW, Regl G, Eichberger T, Frischauf AM, Aberger F, Quinn A, Philpott M. (2004) GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol 122:1503–1509 [DOI] [PubMed] [Google Scholar]
- Ishibashi and McMahon, 2002.Ishibashi M, McMahon AP. (2002) A sonic hedgehog-dependent signaling relay regulates growth of diencephalic and mesencephalic primordia in the early mouse embryo. Development 129:4807–4819 [DOI] [PubMed] [Google Scholar]
- Jenkins, 2009.Jenkins D. (2009) Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal 21:1023–1034 [DOI] [PubMed] [Google Scholar]
- Ji et al., 2008.Ji J, Kump E, Wernli M, Erb P. (2008) Gene silencing of transcription factor Gli2 inhibits basal cell carcinoma like tumor growth in vivo. Int J Cancer 122:50–56 [DOI] [PubMed] [Google Scholar]
- Ji et al., 2007.Ji Z, Mei FC, Xie J, Cheng X. (2007) Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J Biol Chem 282:14048–14055 [DOI] [PubMed] [Google Scholar]
- Jia et al., 2009.Jia J, Kolterud A, Zeng H, Hoover A, Teglund S, Toftgård R, Liu A. (2009) Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Dev Biol 330:452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno et al., 2009.Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, García-García E, López-Ríos F, Matsui W, et al. (2009) A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther 8:310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al., 1996.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr., Scott MP. (1996) Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272:1668–1671 [DOI] [PubMed] [Google Scholar]
- Kalderon, 2000.Kalderon D. (2000) Transducing the hedgehog signal. Cell 103:371–374 [DOI] [PubMed] [Google Scholar]
- Karhadkar et al., 2004.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. (2004) Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 431:707–712 [DOI] [PubMed] [Google Scholar]
- Karpen et al., 2001.Karpen HE, Bukowski JT, Hughes T, Gratton JP, Sessa WC, Gailani MR. (2001) The sonic hedgehog receptor patched associates with caveolin-1 in cholesterol-rich microdomains of the plasma membrane. J Biol Chem 276:19503–19511 [DOI] [PubMed] [Google Scholar]
- Kasai et al., 2008.Kasai K, Inaguma S, Yoneyama A, Yoshikawa K, Ikeda H. (2008) SCL/TAL1 interrupting locus derepresses GLI1 from the negative control of suppressor-of-fused in pancreatic cancer cell. Cancer Res 68:7723–7729 [DOI] [PubMed] [Google Scholar]
- Kasper et al., 2009.Kasper M, Jaks V, Fiaschi M, Toftgård R. (2009) Hedgehog signalling in breast cancer. Carcinogenesis 30:903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper et al., 2006a.Kasper M, Regl G, Frischauf AM, Aberger F. (2006a) GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer 42:437–445 [DOI] [PubMed] [Google Scholar]
- Kasper et al., 2006b.Kasper M, Schnidar H, Neill GW, Hanneder M, Klingler S, Blaas L, Schmid C, Hauser-Kronberger C, Regl G, Philpott MP, et al. (2006b) Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol 26:6283–6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperczyk et al., 2009.Kasperczyk H, Baumann B, Debatin KM, Fulda S. (2009) Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J 23:21–33 [DOI] [PubMed] [Google Scholar]
- Katoh and Katoh, 2010.Katoh M, Katoh M. (2010) Integrative genomic analyses of CXCR4: transcriptional regulation of CXCR4 based on TGFbeta, Nodal, Activin signaling and POU5F1, FOXA2, FOXC2, FOXH1, SOX17, and GFI1 transcription factors. Int J Oncol 36:415–420 [DOI] [PubMed] [Google Scholar]
- Katoh and Katoh, 2006.Katoh Y, Katoh M. (2006) Hedgehog signaling pathway and gastrointestinal stem cell signaling network (review). Int J Mol Med 18:1019–1023 [PubMed] [Google Scholar]
- Kayed et al., 2003.Kayed H, Kleeff J, Keleg S, Büchler MW, Friess H. (2003) Distribution of Indian hedgehog and Its receptors patched and smoothened in human chronic pancreatitis. J Endocrinol 178:467–478 [DOI] [PubMed] [Google Scholar]
- Kayed et al., 2004.Kayed H, Kleeff J, Keleg S, Guo J, Ketterer K, Berberat PO, Giese N, Esposito I, Giese T, Büchler MW, et al. (2004) Indian hedgehog signaling pathway: expression and regulation in pancreatic cancer. Int J Cancer 110:668–676 [DOI] [PubMed] [Google Scholar]
- Khambata-Ford et al., 2007.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, et al. (2007) Expression of epiregulin and amphiregulin and K-Ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25:3230–3237 [DOI] [PubMed] [Google Scholar]
- Kim and Hebrok, 2001.Kim SK, Hebrok M. (2001) Intercellular signals regulating pancreas development and function. Genes Dev 15:111–127 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2007a.Kim WK, Meliton V, Amantea CM, Hahn TJ, Parhami F. (2007a) 20(S)-Hydroxycholesterol inhibits PPARgamma expression and adipogenic differentiation of bone marrow stromal cells through a hedgehog-dependent mechanism. J Bone Miner Res 22:1711–1719 [DOI] [PubMed] [Google Scholar]
- Kim et al., 2010.Kim WK, Meliton V, Tetradis S, Weinmaster G, Hahn TJ, Carlson M, Nelson SF, Parhami F. (2010) Osteogenic oxysterol, 20(S)-hydroxycholesterol, induces notch target gene expression in bone marrow stromal cells. J Bone Miner Res 25:782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2007b.Kim Y, Yoon JW, Xiao X, Dean NM, Monia BP, Marcusson EG. (2007b) Selective down-regulation of glioma-associated oncogene 2 inhibits the proliferation of hepatocellular crcinoma cells. Cancer Res 67:3583–3593 [DOI] [PubMed] [Google Scholar]
- Kinzler et al., 1987.Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O'Brien SJ, Wong AJ, Vogelstein B. (1987) Identification of an amplified, highly expressed gene in a human glioma. Science 236:70–73 [DOI] [PubMed] [Google Scholar]
- Kinzler and Vogelstein, 1990.Kinzler KW, Vogelstein B. (1990) The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol 10:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuru et al., 2009.Kiuru M, Hidaka C, Hubner RH, Solomon J, Krause A, Leopold PL, Crystal RG. (2009) Sonic hedgehog expands diaphyseal trabecular bone altering bone marrow niche and lymphocyte compartment. Mol Ther 17:1442–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarmann et al., 2009.Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, Mistree T, Thomas SB, Farrar WL. (2009) Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis 26:433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobune et al., 2009.Kobune M, Takimoto R, Murase K, Iyama S, Sato T, Kikuchi S, Kawano Y, Miyanishi K, Sato Y, Niitsu Y, et al. (2009) Drug resistance is dramatically restored by hedgehog inhibitors in CD34+ leukemic cells. Cancer Sci 100:948–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga et al., 2008.Koga K, Nakamura M, Nakashima H, Akiyoshi T, Kubo M, Sato N, Kuroki S, Nomura M, Tanaka M, Katano M. (2008) Novel link between estrogen receptor alpha and hedgehog pathway in breast cancer. Anticancer Res 28:731–740 [PubMed] [Google Scholar]
- Kogerman et al., 1999.Kogerman P, Grimm T, Kogerman L, Krause D, Undén AB, Sandstedt B, Toftgård R, Zaphiropoulos PG. (1999) Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol 1:312–319 [DOI] [PubMed] [Google Scholar]
- Kubo et al., 2004.Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, Kuroki S, Katano M. (2004) Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res 64:6071–6074 [DOI] [PubMed] [Google Scholar]
- Kusano et al., 2005.Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, et al. (2005) Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med 11:1197–1204 [DOI] [PubMed] [Google Scholar]
- Laner-Plamberger et al., 2009.Laner-Plamberger S, Kaser A, Paulischta M, Hauser-Kronberger C, Eichberger T, Frischauf AM. (2009) Cooperation between GLI and JUN enhances transcription of JUN and selected GLI target genes. Oncogene 28:1639–1651 [DOI] [PubMed] [Google Scholar]
- Lau et al., 2006.Lau J, Kawahira H, Hebrok M. (2006) Hedgehog signaling in pancreas development and disease. Cell Mol Life Sci 63:642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauth et al., 2007.Lauth M, Bergström A, Shimokawa T, Toftgård R. (2007) Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA 104:8455–8460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine et al., 2008.Lavine KJ, Kovacs A, Ornitz DM. (2008) Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J Clin Invest 118:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2007.Lee SW, Moskowitz MA, Sims JR. (2007) Sonic hedgehog inversely regulates the expression of angiopoietin-1 and angiopoietin-2 in fibroblasts. Int J Mol Med 19:445–451 [PubMed] [Google Scholar]
- Levy et al., 2007.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. (2007) Epidermal stem cells arise from the hair follicle after wounding. FASEB J 21:1358–1366 [DOI] [PubMed] [Google Scholar]
- Li et al., 2006.Li X, Deng W, Nail CD, Bailey SK, Kraus MH, Ruppert JM, Lobo-Ruppert SM. (2006) Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene 25:609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao et al., 2009.Liao X, Siu MK, Au CW, Chan QK, Chan HY, Wong ES, Ip PP, Ngan HY, Cheung AN. (2009) Aberrant activation of hedgehog signaling pathway contributes to endometrial carcinogenesis through beta-catenin. Mod Pathol 22:839–847 [DOI] [PubMed] [Google Scholar]
- Lin et al., 2007.Lin J, Feng L, Fukudome S, Hamajima Y, Huang T, Levine S. (2007) Cochlear stem cells/progenitors and degenerative hearing disorders. Curr Med Chem 14:2937–2943 [DOI] [PubMed] [Google Scholar]
- Litingtung et al., 2002.Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. (2002) Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418:979–983 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2005a.Liu A, Wang B, Niswander LA. (2005a) Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132:3103–3111 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2006.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. (2006) Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66:6063–6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2005b.Liu S, Dontu G, Wicha MS. (2005b) Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res 7:86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo et al., 2007.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. (2007) Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-rgulation of TWIST gene expression. Cancer Res 67:9066–9076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al., 2000.Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. (2000) Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 25:317–329 [DOI] [PubMed] [Google Scholar]
- LoRusso et al., 2008.LoRusso PM, Rudin CM, Borad MJ, Vernillet L, Darbonne WC, Mackey H, DiMartino JF, de Sauvage F, Low JA, Von Hoff DD. (2008) A first-in-human, first-in-class, phase 1 study of systemic hedgehog pathway antagonist, GDC-0449, in patients with advanced solid tumors (Abstract). J Clin Oncol 26 (Suppl):3516 [Google Scholar]
- Machold et al., 2003.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, et al. (2003) Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 39:937–950 [DOI] [PubMed] [Google Scholar]
- Maeda et al., 2006.Maeda O, Kondo M, Fujita T, Usami N, Fukui T, Shimokata K, Ando T, Goto H, Sekido Y. (2006) Enhancement of GLI1-transcriptional activity by beta-catenin in human cancer cells. Oncol Rep 16:91–96 [PubMed] [Google Scholar]
- Maity et al., 2005.Maity T, Fuse N, Beachy PA. (2005) Molecular mechanisms of Sonic hedgehog mutant effects in holoprosencephaly. Proc Natl Acad Sci USA 102:17026–17031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao et al., 2009.Mao H, Diehl AM, Li YX. (2009) Sonic hedgehog ligand partners with caveolin-1 for intracellular transport. Lab Invest 89:290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marigo et al., 1995.Marigo V, Roberts DJ, Lee SM, Tsukurov O, Levi T, Gastier JM, Epstein DJ, Gilbert DJ, Copeland NG, Seidman CE. (1995) Cloning, expression, and chromosomal location of SHH and IHH: two human homologues of the Drosophila segment polarity gene hedgehog. Genomics 28:44–51 [DOI] [PubMed] [Google Scholar]
- Matise and Joyner, 1999.Matise MP, Joyner AL. (1999) Gli genes in development and cancer. Oncogene 18:7852–7859 [DOI] [PubMed] [Google Scholar]
- McMahon et al., 2003.McMahon AP, Ingham PW, Tabin CJ. (2003) Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol 53:1–114 [DOI] [PubMed] [Google Scholar]
- Merchant et al., 2010.Merchant A, Joseph G, Wang Q, Brennan S, Matsui W. (2010) Gli1 regulates the proliferation and differentiation of HSCs and myeloid progenitors. Blood 115:2391–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen et al., 2006.Meyer zu Schwabedissen HE, Grube M, Dreisbach A, Jedlitschky G, Meissner K, Linnemann K, Fusch C, Ritter CA, Völker U, Kroemer HK. (2006) Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab Dispos 34:524–533 [DOI] [PubMed] [Google Scholar]
- Michael et al., 2008.Michael LE, Westerman BA, Ermilov AN, Wang A, Ferris J, Liu J, Blom M, Ellison DW, van Lohuizen M, Dlugosz AA. (2008) Bmi1 is required for Hedgehog pathway-driven medulloblastoma expansion. Neoplasia 10:1343–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault and Batra, 2007.Mimeault M, Batra SK. (2007) Interplay of distinct growth factors during epithelial mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol 18:1605–1619 [DOI] [PubMed] [Google Scholar]
- Mimeault and Batra, 2008a.Mimeault M, Batra SK. (2008a) Recent progress on normal and malignant pancreatic stem/progenitor cell research: therapeutic implications for the treatment of type 1 or 2 diabetes mellitus and aggressive pancreatic cancer. Gut 57:1456–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault and Batra, 2008b.Mimeault M, Batra SK. (2008b) Recent advances on the development of novel anti-cancer drugs targeting cancer stem/progenitor cells. Drug Dev Res 69:415–430 [Google Scholar]
- Mimeault and Batra, 2008c.Mimeault M, Batra SK. (2008c) Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev 4:27–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault and Batra, 2009.Mimeault M, Batra SK. (2009) Recent insights into the molecular mechanisms involved in aging and the malignant transformation of adult stem/progenitor cells and their therapeutic implications. Ageing Res Rev 8:94–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault and Batra, 2010a.Mimeault M, Batra SK. (2010a) Novel therapies against aggressive and recurrent epithelial cancers by molecular targeting tumor- and metastasis-initiating cells and their progenies. Anticancer Agents Med Chem 10:137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault and Batra, 2010b.Mimeault M, Batra SK. (2010b) New promising drug targets in cancer- and metastasis-initiating cells. Drug Discov Today 15:354–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault and Batra, 2010c.Mimeault M, Batra SK. (2010c) New advances on critical implications of tumor- and metastasis-initiating cells in cancer progression, treatment resistance and disease recurrence. Histol Histopathol 25:1057–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault et al., 2008.Mimeault M, Hauke R, Batra SK. (2008) Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin Pharmacol Ther 83:673–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault et al., 2010.Mimeault M, Johansson SL, Henichart JP, Depreux P, Batra SK. (2010) Cytotoxic effects induced by docetaxel, gefitinib, and cyclopamine on side population and nonside population cell fractions from human invasive prostate cancer cells. Mol Cancer Ther 9:617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault et al., 2007a.Mimeault M, Johansson SL, Vankatraman G, Moore E, Henichart JP, Depreux P, Lin MF, Batra SK. (2007a) Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther 6:967–978 [DOI] [PubMed] [Google Scholar]
- Mimeault et al., 2007b.Mimeault M, Mehta PP, Hauke R, Henichart JP, Depreux P, Lin MF, Batra SK. (2007b) Improvement of cytotoxic effects induced by mitoxantrone on hormone-refractory metastatic prostate cancer cells by co-targeting epidermal growth factor receptor and hedgehog signaling cascades. Growth Factors 25:400–416 [DOI] [PubMed] [Google Scholar]
- Mimeault et al., 2008.Mimeault M, Mehta PP, Hauke R, Batra SK. (2008) Functions of normal and malignant prostatic stem/progenitor cells in tissue regeneration and cancer progression and novel targeting therapies. Endocr Rev 29:234–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimeault et al., 2006.Mimeault M, Moore E, Moniaux N, Hénichart JP, Depreux P, Lin MF, Batra SK. (2006) Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells. Int J Cancer 118:1022–1031 [DOI] [PubMed] [Google Scholar]
- Ming et al., 1998.Ming JE, Roessler E, Muenke M. (1998) Human developmental disorders and the Sonic hedgehog pathway. Mol Med Today 4:343–349 [DOI] [PubMed] [Google Scholar]
- Mizuarai et al., 2009.Mizuarai S, Kawagishi A, Kotani H. (2009) Inhibition of p70S6K2 down-regulates Hedgehog/GLI pathway in non-small cell lung cancer cell lines. Mol Cancer 8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molckovsky and Siu, 2008.Molckovsky A, Siu LL. (2008) First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J Hematol Oncol 1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagut and Settleman, 2009.Montagut C, Settleman J. (2009) Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett 283:125–134 [DOI] [PubMed] [Google Scholar]
- Morton et al., 2007.Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M, Lewis BC. (2007) Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA 104:5103–5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura et al., 2010.Nakamura K, Sasajima J, Mizukami Y, Sugiyama Y, Yamazaki M, Fujii R, Kawamoto T, Koizumi K, Sato K, Fujiya M, et al. (2010) Hedgehog promotes neovascularization in pancreatic cancers by regulating Ang-1 and IGF-1 expression in bone-marrow derived pro-angiogenic Cells. PLoS ONE 5:e8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura et al., 2007.Nakamura M, Kubo M, Yanai K, Mikami Y, Ikebe M, Nagai S, Yamaguchi K, Tanaka M, Katano M. (2007) Anti-patched-1 antibodies suppress hedgehog signaling pathway and pancreatic cancer proliferation. Anticancer Res 27:3743–3747 [PubMed] [Google Scholar]
- Nakashima et al., 2006.Nakashima H, Nakamura M, Yamaguchi H, Yamanaka N, Akiyoshi T, Koga K, Yamaguchi K, Tsuneyoshi M, Tanaka M, Katano M. (2006) Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res 66:7041–7049 [DOI] [PubMed] [Google Scholar]
- Narita et al., 2008.Narita S, So A, Ettinger S, Hayashi N, Muramaki M, Fazli L, Kim Y, Gleave ME. (2008) GLI2 knockdown using an antisense oligonucleotide induces apoptosis and chemosensitizes cells to paclitaxel in androgen-independent prostate cancer. Clin Cancer Res 14:5769–5777 [DOI] [PubMed] [Google Scholar]
- Nery et al., 2001.Nery S, Wichterle H, Fishell G. (2001) Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 128:527–540 [DOI] [PubMed] [Google Scholar]
- Nielsen et al., 2004.Nielsen CM, Williams J, van den Brink GR, Lauwers GY, Roberts DJ. (2004) Hh pathway expression in human gut tissues and in inflammatory gut diseases. Lab Invest 84:1631–1642 [DOI] [PubMed] [Google Scholar]
- Nielsen et al., 2008.Nielsen SK, Møllgård K, Clement CA, Veland IR, Awan A, Yoder BK, Novak I, Christensen ST. (2008) Characterization of primary cilia and Hedgehog signaling during development of the human pancreas and in human pancreatic duct cancer cell lines. Dev Dyn 237:2039–2052 [DOI] [PubMed] [Google Scholar]
- Nogueira-Rodrigues et al., 2008.Nogueira-Rodrigues A, do Carmo CC, Viegas C, Erlich F, Camisão C, Fontão K, Lima R, Herchenhorn D, Martins RG, Moralez GM, et al. (2008) Phase I trial of erlotinib combined with cisplatin and radiotherapy for patients with locally advanced cervical squamous cell cancer. Clin Cancer Res 14:6324–6329 [DOI] [PubMed] [Google Scholar]
- Nolan-Stevaux et al., 2009.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernández-Zapico ME, Hanahan D. (2009) GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev 23:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard and Wieschaus, 1980.Nüsslein-Volhard C, Wieschaus E. (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801 [DOI] [PubMed] [Google Scholar]
- Odent et al., 1999.Odent S, Atti-Bitach T, Blayau M, Mathieu M, Aug J, Delezo de AL, Gall JY, Le Marec B, Munnich A, David V, et al. (1999) Expression of the sonic hedgehog (SHH) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum Mol Genet 8:1683–1689 [DOI] [PubMed] [Google Scholar]
- Ohsaki et al., 2000.Ohsaki Y, Tanno S, Fujita Y, Toyoshima E, Fujiuchi S, Nishigaki Y, Ishida S, Nagase A, Miyokawa N, Hirata S, et al. (2000) Epidermal growth factor receptor expression correlates with poor prognosis in non-small cell lung cancer patients with p53 overexpression. Oncol Rep 7:603–607 [DOI] [PubMed] [Google Scholar]
- Ohta et al., 2005.Ohta M, Tateishi K, Kanai F, Watabe H, Kondo S, Guleng B, Tanaka Y, Asaoka Y, Jazag A, Imamura J, et al. (2005) p53-independent negative regulation of p21/cyclin-dependent kinase-interacting protein 1 by the sonic hedgehog-glioma-associated oncogene 1 pathway in gastric carcinoma cells. Cancer Res 65:10822–10829 [DOI] [PubMed] [Google Scholar]
- Olive et al., 2009.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324:1457–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oniscu et al., 2004.Oniscu A, James RM, Morris RG, Bader S, Malcomson RD, Harrison DJ. (2004) Expression of Sonic hedgehog pathway genes is altered in colonic neoplasia. J Pathol 203:909–917 [DOI] [PubMed] [Google Scholar]
- Paladini et al., 2005.Paladini RD, Saleh J, Qian C, Xu GX, Rubin LL. (2005) Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol 125:638–646 [DOI] [PubMed] [Google Scholar]
- Palma and Ruiz i Altaba, 2004.Palma V, Ruiz i Altaba A. (2004) Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development 131:337–345 [DOI] [PubMed] [Google Scholar]
- Palma et al., 2005.Palma V, Lim DA, Dahmane N, Sánchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. (2005) Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132:335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al., 2010.Pan S, Wu X, Jiang J, Gao W, Wan Y, Cheng D, Han D, Liu J, Englund NP, Wang Y, et al. (2010) Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med Chem Lett 1:130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al., 2009.Park HJ, Carr JR, Wang Z, Nogueira V, Hay N, Tyner AL, Lau LF, Costa RH, Raychaudhuri P. (2009) FoxM1, a critical regulator of oxidative stress during opncogenesis. EMBO J 28:2908–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al., 2000.Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. (2000) Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127:1593–1605 [DOI] [PubMed] [Google Scholar]
- Parkin and Ingham, 2008.Parkin CA, Ingham PW. (2008) The adventures of sonic hedgehog in development and repair. I. Hedgehog signaling in gastrointestinal development and disease. Am J Physiol Gastrointest Liver Physiol 294:G363–G367 [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano et al., 2006.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. (2006) Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev 20:3161–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathi et al., 2001.Pathi S, Pagan-Westphal S, Baker DP, Garber EA, Rayhorn P, Bumcrot D, Tabin CJ, Blake Pepinsky R, Williams KP. (2001) Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech Dev 106:107–117 [DOI] [PubMed] [Google Scholar]
- Peacock et al., 2007.Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, et al. (2007) Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci USA 104:4048–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola et al., 2001.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, et al. (2001) The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7:706–711 [DOI] [PubMed] [Google Scholar]
- Porter et al., 1995.Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, Beachy PA. (1995) The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature 374:363–366 [DOI] [PubMed] [Google Scholar]
- Raffel et al., 1997.Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, James CD. (1997) Sporadic medulloblastomas contain PTCH mutations. Cancer Res 57:842–845 [PubMed] [Google Scholar]
- Rahnama et al., 2006.Rahnama F, Shimokawa T, Lauth M, Finta C, Kogerman P, Teglund S, Toftgård R, Zaphiropoulos PG. (2006) Inhibition of GLI1 gene activation by Patched1. Biochem J 394:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallu et al., 2002.Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. (2002) Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development 129:4963–4974 [DOI] [PubMed] [Google Scholar]
- Regl et al., 2002.Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM, Aberger F. (2002) Human GLI2 and GLI1 are part of a positive feedback mechanism in basal cell carcinoma. Oncogene 21:5529–5539 [DOI] [PubMed] [Google Scholar]
- Reifenberger et al., 1998.Reifenberger J, Wolter M, Weber RG, Megahed M, Ruzicka T, Lichter P, Reifenberger G. (1998) Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res 58:1798–1803 [PubMed] [Google Scholar]
- Reinisch et al., 2007.Reinisch CM, Uthman A, Erovic BM, Pammer J. (2007) Expression of BMI-1 in normal skin and inflammatory and neoplastic skin lesions. J Cutan Pathol 34:174–180 [DOI] [PubMed] [Google Scholar]
- Remsberg et al., 2007.Remsberg JR, Lou H, Tarasov SG, Dean M, Tarasova NI. (2007) Structural analogues of smoothened intracellular loops as potent inhibitors of hedgehog pathway and cancer cell growth. J Med Chem 50:4534–4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault et al., 2009.Renault MA, Roncalli J, Tongers J, Misener S, Thorne T, Jujo K, Ito A, Clarke T, Fung C, Millay M, et al. (2009) The Hedgehog transcription factor Gli3 modulates angiogenesis. Circ Res 105:818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo et al., 2006.Riobo NA, Haines GM, Emerson CP., Jr (2006) Protein kinase C-delta and mitogen-activated protein/extracellular signal-regulated kinase-1 control GLI activation in hedgehog signaling. Cancer Res 66:839–845 [DOI] [PubMed] [Google Scholar]
- Riobó et al., 2006.Riobó NA, Lu K, Ai X, Haines GM, Emerson CP., Jr (2006) Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA 103:4505–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié et al., 2009.Rittié L, Stoll SW, Kang S, Voorhees JJ, Fisher GJ. (2009) Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell 8:738–751 [DOI] [PubMed] [Google Scholar]
- Robarge et al., 2009.Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, Gould SE, Guichert O, Gunzner JL, Halladay J, Jia W, Khojasteh C, Koehler MF, Kotkow K, La H, Lalonde RL, Lau K, Lee L, Marshall D, Marsters JC, Jr., Murray LJ, Qian C, Rubin LL, Salphati L, Stanley MS, Stibbard JH, Sutherlin DP, Ubhayaker S, Wang S, Wong S, Xie M. (2009) GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett 19:5576–5581 [DOI] [PubMed] [Google Scholar]
- Rohatgi et al., 2007.Rohatgi R, Milenkovic L, Scott MP. (2007) Patched1 regulates hedgehog signaling at the primary cilium. Science 317:372–376 [DOI] [PubMed] [Google Scholar]
- Rohatgi and Scott, 2007.Rohatgi R, Scott MP. (2007) Patching the gaps in Hedgehog signalling. Nat Cell Biol 9:1005–1009 [DOI] [PubMed] [Google Scholar]
- Romer and Curran, 2005.Romer J, Curran T. (2005) Targeting medulloblastoma: small-molecule inhibitors of the Sonic Hedgehog pathway as potential cancer therapeutics. Cancer Res 65:4975–4978 [DOI] [PubMed] [Google Scholar]
- Romer et al., 2004.Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, et al. (2004) Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−) P53(−/−) mice. Cancer Cell 6:229–240 [DOI] [PubMed] [Google Scholar]
- Rosenbaum and Witman, 2002.Rosenbaum JL, Witman GB. (2002) Intraflagellar transport. Nat Rev Mol Cell Biol 3:813–825 [DOI] [PubMed] [Google Scholar]
- Rubin and de Sauvage, 2006.Rubin LL, de Sauvage FJ. (2006) Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov 5:1026–1033 [DOI] [PubMed] [Google Scholar]
- Rudin et al., 2009.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, et al. (2009) Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 361:1173–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba et al., 2007.Ruiz i Altaba A, Mas C, Stecca B. (2007) The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol 17:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba et al., 2004.Ruiz i Altaba A, Stecca B, Sánchez P. (2004) Hedgehog–Gli signaling in brain tumors: stem cells and paradevelopmental programs in cancer. Cancer Lett 204:145–157 [DOI] [PubMed] [Google Scholar]
- Ruppert et al., 1990.Ruppert JM, Vogelstein B, Arheden K, Kinzler KW. (1990) GLI3 encodes a 190-kilodalton protein with multiple regions of GLI similarity. Mol Cell Biol 10:5408–5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez et al., 2004.Sanchez P, Hernández AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. (2004) Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA 101:12561–12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki et al., 1999.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. (1999) Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126:3915–3924 [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich et al., 2006.Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, Scheidereit C. (2006) NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development 133:1045–1057 [DOI] [PubMed] [Google Scholar]
- Schnidar et al., 2009.Schnidar H, Eberl M, Klingler S, Mangelberger D, Kasper M, Hauser-Kronberger C, Regl G, Kroismayr R, Moriggl R, Sibilia M, et al. (2009) Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res 69:1284–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto et al., 2009.Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, Mohri D, Tanaka Y, Ijichi H, Tateishi K, et al. (2009) Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Mol Carcinog 48:703–712 [DOI] [PubMed] [Google Scholar]
- Shah et al., 2006.Shah RB, Ghosh D, Elder JT. (2006) Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate 66:1437–1444 [DOI] [PubMed] [Google Scholar]
- Shaw et al., 2009.Shaw A, Gipp J, Bushman W. (2009) The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene 28:4480–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw and Prowse, 2008.Shaw G, Prowse DM. (2008) Inhibition of androgen-independent prostate cancer cell growth is enhanced by combination therapy targeting Hedgehog and ErbB signalling. Cancer Cell Int 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton et al., 2005.Shelton JG, Steelman LS, Abrams SL, Bertrand FE, Franklin RA, McMahon M, McCubrey JA. (2005) The epidermal growth factor receptor gene family as a target for therapeutic intervention in numerous cancers: what's genetics got to do with it? Expert Opin Ther Targets 9:1009–1030 [DOI] [PubMed] [Google Scholar]
- Sheng et al., 2004.Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. (2004) Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd et al., 2005.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, et al. (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132 [DOI] [PubMed] [Google Scholar]
- Shi et al., 2008.Shi Y, Sun G, Zhao C, Stewart R. (2008) Neural stem cell self-renewal. Crit Rev Oncol Hematol 65:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicklick et al., 2005.Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang J, Zdanowicz M, Camp T, Torbenson MS, et al. (2005) Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest 85:1368–1380 [DOI] [PubMed] [Google Scholar]
- Sicklick et al., 2006.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, Fair JH, Ludlow JW, McClelland RE, et al. (2006) Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol 290:G859–G870 [DOI] [PubMed] [Google Scholar]
- Sims et al., 2009.Sims JR, Lee SW, Topalkara K, Qiu J, Xu J, Zhou Z, Moskowitz MA. (2009) Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke 40:3618–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Mourtada et al., 2007.Sims-Mourtada J, Izzo JG, Ajani J, Chao KS. (2007) Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene 26:5674–5679 [DOI] [PubMed] [Google Scholar]
- Spassky et al., 2008.Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. (2008) Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol 317:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer et al., 2009.Spicer LJ, Sudo S, Aad PY, Wang LS, Chun SY, Ben-Shlomo I, Klein C, Hsueh AJ. (2009) The hedgehog-patched signaling pathway and function in the mammalian ovary: a novel role for hedgehog proteins in stimulating proliferation and steroidogenesis of theca cells. Reproduction 138:329–339 [DOI] [PubMed] [Google Scholar]
- Stanton and Peng, 2010.Stanton BZ, Peng LF. (2010) Small-molecule modulators of the Sonic Hedgehog signaling pathway. Mol Biosyst 6:44–54 [DOI] [PubMed] [Google Scholar]
- Stecca and Ruiz i Altaba, 2009.Stecca B, Ruiz i Altaba A. (2009) A GLI1–p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J 28:663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecca et al., 2007.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz I Altaba A. (2007) Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA 104:5895–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecca and Ruiz I Altaba, 2010.Stecca B, Ruiz I Altaba A. (2010) Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG Signals. J Mol Cell Biol 2:84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepan et al., 2005.Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. (2005) Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem 280:15700–15708 [DOI] [PubMed] [Google Scholar]
- Stone et al., 1996.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et al. (1996) The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384:129–134 [DOI] [PubMed] [Google Scholar]
- Strobel et al., 2010.Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, Fernández-Del Castillo C, Warshaw AL, Thayer SP. (2010) Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 138:1166–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt et al., 2001.Strutt H, Thomas C, Nakano Y, Stark D, Neave B, Taylor AM, Ingham PW. (2001) Mutations in the sterol-sensing domain of Patched suggest a role for vesicular trafficking in Smoothened regulation. Curr Biol 11:608–613 [DOI] [PubMed] [Google Scholar]
- Tada et al., 2008.Tada M, Kanai F, Tanaka Y, Tateishi K, Ohta M, Asaoka Y, Seto M, Muroyama R, Fukai K, Imazeki F, et al. (2008) Down-regulation of hedgehog-interacting protein through genetic and epigenetic alterations in human hepatocellular carcinoma. Clin Cancer Res 14:3768–3776 [DOI] [PubMed] [Google Scholar]
- Taipale et al., 2002.Taipale J, Cooper MK, Maiti T, Beachy PA. (2002) Patched acts catalytically to suppress the activity of Smoothened. Nature 418:892–897 [DOI] [PubMed] [Google Scholar]
- Takada et al., 2005.Takada T, Suzuki H, Gotoh Y, Sugiyama Y. (2005) Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos 33:905–909 [DOI] [PubMed] [Google Scholar]
- Tanaka et al., 2009.Tanaka H, Nakamura M, Kameda C, Kubo M, Sato N, Kuroki S, Tanaka M, Katano M. (2009) The Hedgehog signaling pathway plays an essential role in maintaining the CD44+CD24−/low subpopulation and the side population of breast cancer cells. Anticancer Res 29:2147–2157 [PubMed] [Google Scholar]
- Taniguchi et al., 2007.Taniguchi H, Yamamoto H, Akutsu N, Nosho K, Adachi Y, Imai K, Shinomura Y. (2007) Transcriptional silencing of hedgehog-interacting protein by CpG hypermethylation and chromatic structure in human gastrointestinal cancer. J Pathol 213:131–139 [DOI] [PubMed] [Google Scholar]
- Taş and Avci, 2004.Taş S, Avci O. (2004) Rapid clearance of psoriatic skin lesions induced by topical cyclopamine. A preliminary proof of concept study. Dermatology 209:126–131 [DOI] [PubMed] [Google Scholar]
- Taylor et al., 2002.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, et al. (2002) Mutations in SUFU predispose to medulloblastoma. Nat Genet 31:306–310 [DOI] [PubMed] [Google Scholar]
- Thayer et al., 2003.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, et al. (2003) Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425:851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen and de Sauvage, 2009.Theunissen JW, de Sauvage FJ. (2009) Paracrine Hedgehog signaling in cancer. Cancer Res 69:6007–6010 [DOI] [PubMed] [Google Scholar]
- Thiyagarajan et al., 2007.Thiyagarajan S, Bhatia N, Reagan-Shaw S, Cozma D, Thomas-Tikhonenko A, Ahmad N, Spiegelman VS. (2007) Role of GLI2 transcription factor in growth and tumorigenicity of prostate cells. Cancer Res 67:10642–10646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas et al., 2000.Thomas MK, Rastalsky N, Lee JH, Habener JF. (2000) Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes 49:2039–2047 [DOI] [PubMed] [Google Scholar]
- Tokhunts et al., 2010.Tokhunts R, Singh S, Chu T, D'Angelo G, Baubet V, Goetz JA, Huang Z, Yuan Z, Ascano M, Zavros Y, et al. (2010) The full-length unprocessed hedgehog protein is an active signaling molecule. J Biol Chem 285:2562–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora et al., 2007.Tortora G, Gelardi T, Ciardiello F, Bianco R. (2007) The rationale for the combination of selective EGFR inhibitors with cytotoxic drugs and radiotherapy. Int J Biol Markers 22:S47–S52 [PubMed] [Google Scholar]
- Trowbridge et al., 2006.Trowbridge JJ, Scott MP, Bhatia M. (2006) Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci USA 103:14134–14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay et al., 2009.Tremblay MR, Lescarbeau A, Grogan MJ, Tan E, Lin G, Austad BC, Yu LC, Behnke ML, Nair SJ, Hagel M, et al. (2009) Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926). J Med Chem 52:4400–4418 [DOI] [PubMed] [Google Scholar]
- Tsanev et al., 2009.Tsanev R, Tiigimägi P, Michelson P, Metsis M, Østerlund T, Kogerman P. (2009) Identification of the gene transcription repressor domain of Gli3. FEBS Lett 583:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant and Monard, 2009.Vaillant C, Monard D. (2009) SHH pathway and cerebellar development. Cerebellum 8:291–301 [DOI] [PubMed] [Google Scholar]
- Varjosalo et al., 2006.Varjosalo M, Li SP, Taipale J. (2006) Divergence of hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell 10:177–186 [DOI] [PubMed] [Google Scholar]
- Varjosalo and Taipale, 2008.Varjosalo M, Taipale J. (2008) Hedgehog: functions and mechanisms. Genes Dev 22:2454–2472 [DOI] [PubMed] [Google Scholar]
- Veland et al., 2009.Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. (2009) Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol 111:p39–p53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff et al., 2009.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, et al. (2009) Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 361:1164–1172 [DOI] [PubMed] [Google Scholar]
- Vyas et al., 2008.Vyas N, Goswami D, Manonmani A, Sharma P, Ranganath HA, VijayRaghavan K, Shashidhara LS, Sowdhamini R, Mayor S. (2008) Nanoscale organization of hedgehog is essential for long-range signaling. Cell 133:1214–1227 [DOI] [PubMed] [Google Scholar]
- Walter et al., 2010.Walter K, Omura N, Hong SM, Griffith M, Vincent A, Borges M, Goggins M. (2010) Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res 16:1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2000.Wang B, Fallon JF, Beachy PA. (2000) Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100:423–434 [DOI] [PubMed] [Google Scholar]
- Wang and Li, 2006.Wang B, Li Y. (2006) Evidence for the direct involvement of {Beta}TrCP in Gli3 protein processing. Proc Natl Acad Sci USA 103:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2009a.Wang X, Mao X, Xie L, Greenberg DA, Jin K. (2009a) Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J Cereb Blood Flow Metab 29:1644–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2009b.Wang Y, Zhou Z, Walsh CT, McMahon AP. (2009b) Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc Natl Acad Sci USA 106:2623–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward et al., 2009.Ward RJ, Lee L, Graham K, Satkunendran T, Yoshikawa K, Ling E, Harper L, Austin R, Nieuwenhuis E, Clarke ID, et al. (2009) Multipotent CD15+ cancer stem cells in patched-1-deficient mouse medulloblastoma. Cancer Res 69:4682–4690 [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya and Scott, 1999.Wechsler-Reya RJ, Scott MP. (1999) Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22:103–114 [DOI] [PubMed] [Google Scholar]
- Williams et al., 2003.Williams JA, Guicherit OM, Zaharian BI, Xu Y, Chai L, Wichterle H, Kon C, Gatchalian C, Porter JA, Rubin LL, et al. (2003) Identification of a small molecule inhibitor of the hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci USA 100:4616–4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong et al., 2009.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr., Dlugosz AA, Reiter JF. (2009) Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 15:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie et al., 2001.Xie J, Aszterbaum M, Zhang X, Bonifas JM, Zachary C, Epstein E, McCormick F. (2001) A role of PDGFRalpha in basal cell carcinoma proliferation. Proc Natl Acad Sci USA 98:9255–9259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al., 2008.Xu Q, Yuan X, Liu G, Black KL, Yu JS. (2008) Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells 26:3018–3026 [DOI] [PubMed] [Google Scholar]
- Yakushiji et al., 2007.Yakushiji N, Suzuki M, Satoh A, Sagai T, Shiroishi T, Kobayashi H, Sasaki H, Ide H, Tamura K. (2007) Correlation between Shh expression and DNA methylation status of the limb-specific Shh enhancer region during limb regeneration in amphibians. Dev Biol 312:171–182 [DOI] [PubMed] [Google Scholar]
- Yamazaki et al., 2008.Yamazaki M, Nakamura K, Mizukami Y, Ii M, Sasajima J, Sugiyama Y, Nishikawa T, Nakano Y, Yanagawa N, Sato K, et al. (2008) Sonic hedgehog derived from human pancreatic cancer cells augments angiogenic function of endothelial progenitor cells. Cancer Sci 99:1131–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al., 2010.Yang Y, Tian X, Xie X, Zhuang Y, Wu W, Wang W. (2010) Expression and regulation of hedgehog signaling pathway in pancreatic cancer. Langenbecks Arch Surg 395:515–525 [DOI] [PubMed] [Google Scholar]
- Yang et al., 2008.Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schüller U, Machold R, Fishell G, Rowitch DH, et al. (2008) Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell 14:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden and Sliwkowski, 2001.Yarden Y, Sliwkowski MX. (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137 [DOI] [PubMed] [Google Scholar]
- Yauch et al., 2009.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, et al. (2009) Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science 326:572–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch et al., 2008.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al. (2008) A paracrine requirement for hedgehog signalling in cancer. Nature 455:406–410 [DOI] [PubMed] [Google Scholar]
- Yonesaka et al., 2008.Yonesaka K, Zejnullahu K, Lindeman N, Homes AJ, Jackman DM, Zhao F, Rogers AM, Johnson BE, Jänne PA. (2008) Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res 14:6963–6973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo et al., 2008.Yoo YA, Kang MH, Kim JS, Oh SC. (2008) Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis 29:480–490 [DOI] [PubMed] [Google Scholar]
- Yuan et al., 2007.Yuan Z, Goetz JA, Singh S, Ogden SK, Petty WJ, Black CC, Memoli VA, Dmitrovsky E, Robbins DJ. (2007) Frequent requirement of hedgehog signaling in non-small cell lung carcinoma. Oncogene 26:1046–1055 [DOI] [PubMed] [Google Scholar]
- Yue et al., 2009.Yue S, Chen Y, Cheng SY. (2009) Hedgehog signaling promotes the degradation of tumor suppressor Sufu through the ubiquitin-proteasome pathway. Oncogene 28:492–499 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2009.Zhang Y, Laterra J, Pomper MG. (2009) Hedgehog pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP and ABCB1/Pgp. Neoplasia 11:96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al., 2006.Zhou JX, Jia LW, Liu WM, Miao CL, Liu S, Cao YJ, Duan EK. (2006) Role of sonic hedgehog in maintaining a pool of proliferating stem cells in the human fetal epidermis. Hum Reprod 21:1698–1704 [DOI] [PubMed] [Google Scholar]
- Zurawel et al., 2000.Zurawel RH, Allen C, Chiappa S, Cato W, Biegel J, Cogen P, de Sauvage F, Raffel C. (2000) Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes Cancer 27:44–51 [DOI] [PubMed] [Google Scholar]