Abstract
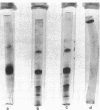
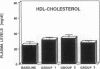
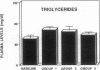
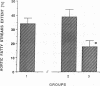
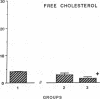
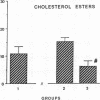
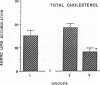
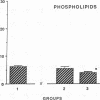
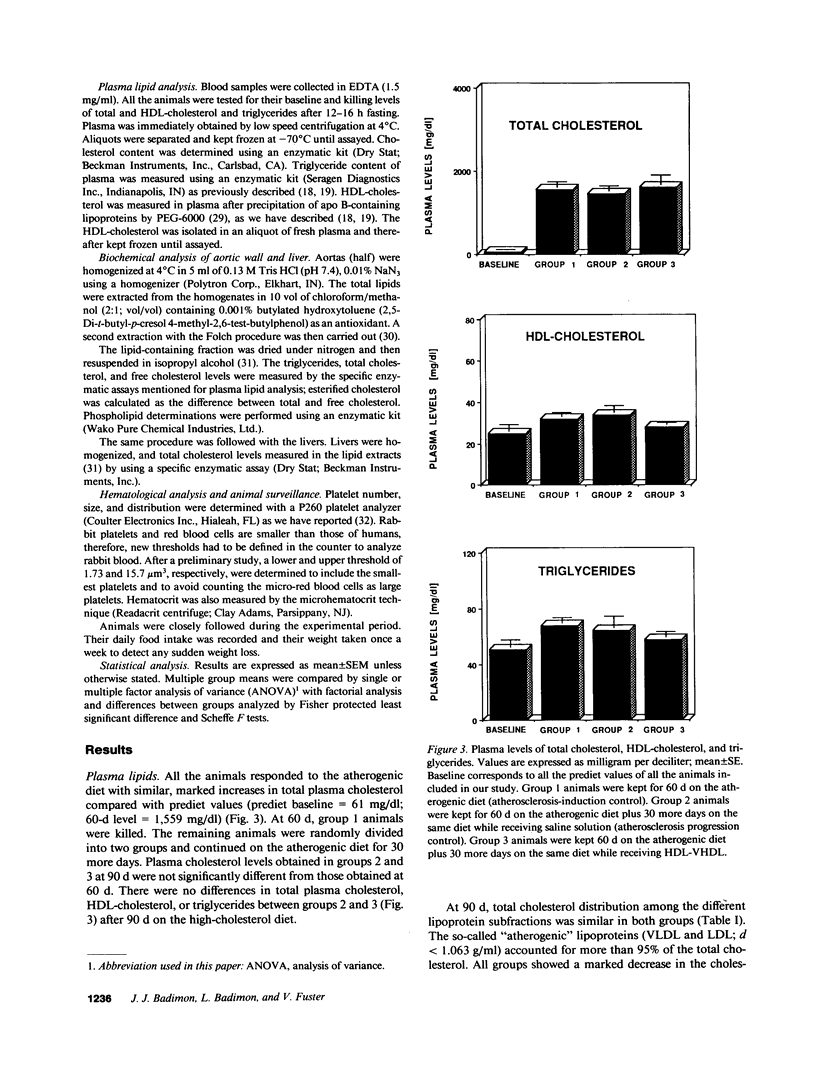
The effects of homologous plasma HDL and VHDL fractions on established atherosclerotic lesions were studied in cholesterol-fed rabbits. Atherosclerosis was induced by feeding the animals a 0.5% cholesterol-rich diet for 60 d (group 1). Another group of animals were maintained on the same diet for 90 d (group 2). A third group was also fed the same diet for 90 d but received 50 mg HDL-VHDL protein per wk (isolated from normolipemic rabbit plasma) during the last 30 d (group 3). Aortic atherosclerotic involvement at the completion of the study was 34 +/- 4% in group 1, 38.8 +/- 5% in group 2, and 17.8 +/- 4% in group 3 (P less than 0.005). Aortic lipid deposition was also significantly reduced in group 3 compared with group 1 (studied at only 60 d) and group 2. This is the first in vivo, prospective evidence of the antiatherogenic effect of HDL-VHDL against preexisting atherosclerosis. Our results showed that HDL plasma fractions were able to induce regression of established aortic fatty streaks and lipid deposits. Our results suggest that it may be possible not only to inhibit progression but even to reduce established atherosclerotic lesions by HDL administration.
Full text
PDF
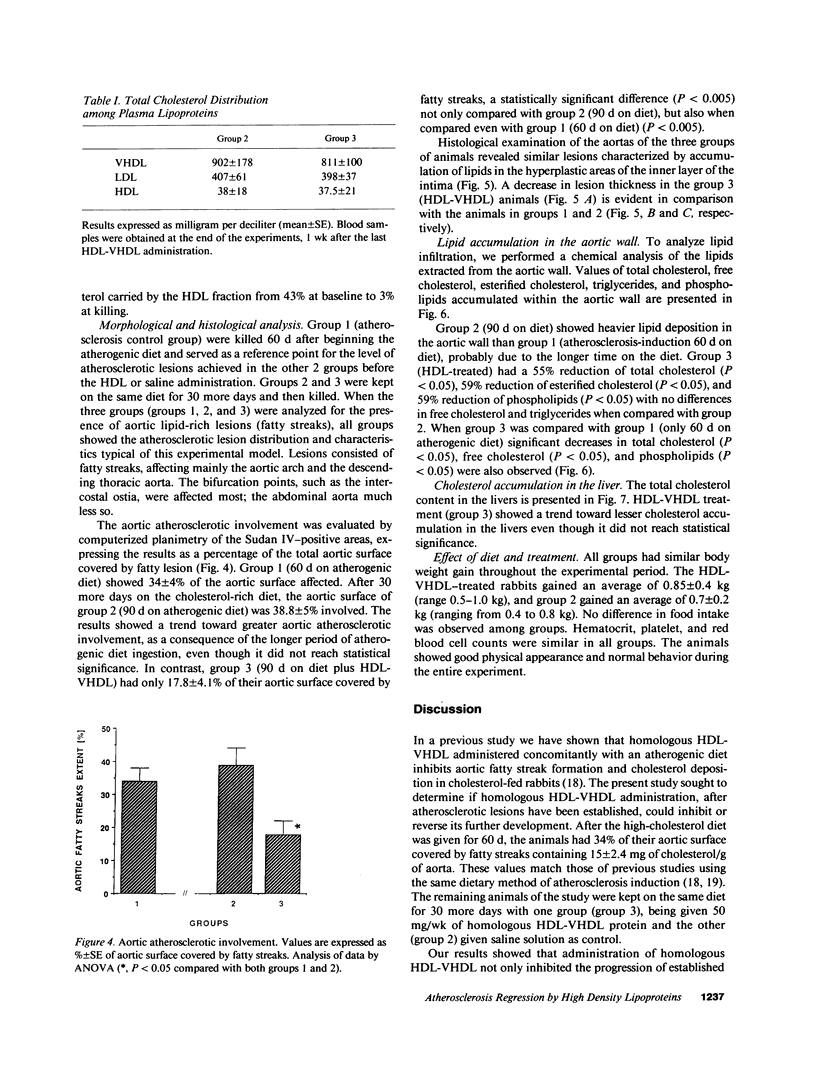

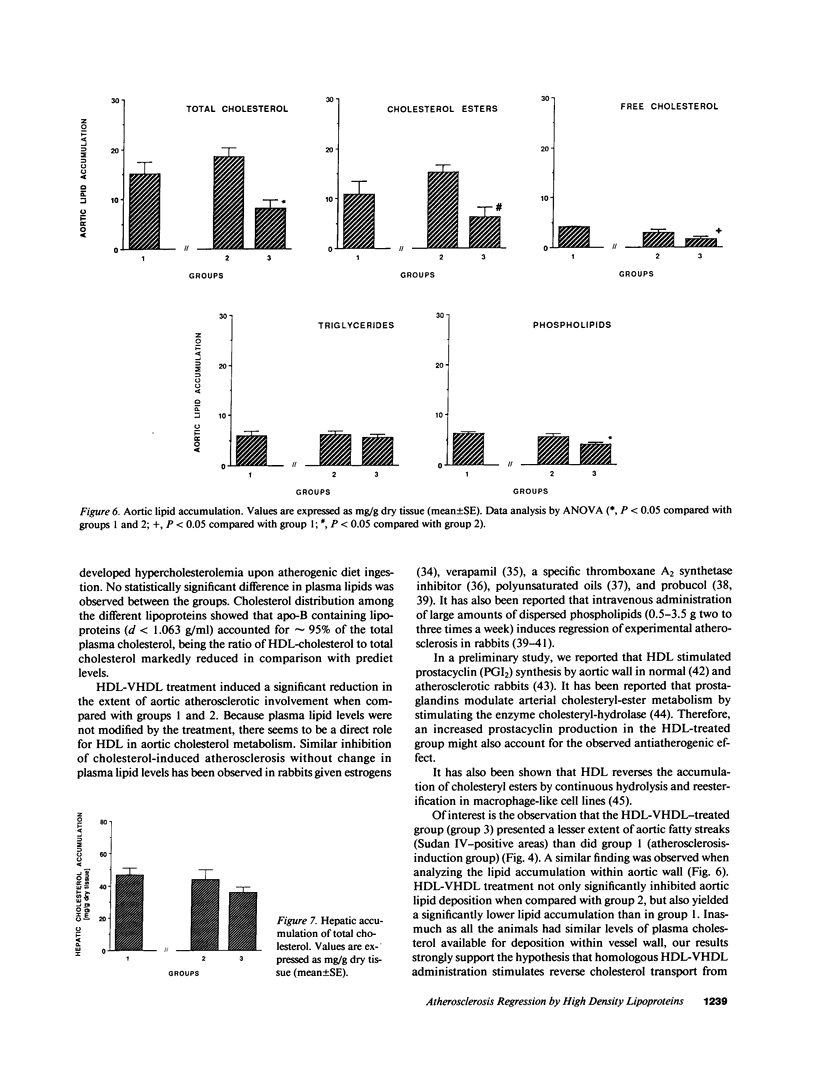


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arad Y., Badimon J. J., Badimon L., Hembree W. C., Ginsberg H. N. Dehydroepiandrosterone feeding prevents aortic fatty streak formation and cholesterol accumulation in cholesterol-fed rabbit. Arteriosclerosis. 1989 Mar-Apr;9(2):159–166. doi: 10.1161/01.atv.9.2.159. [DOI] [PubMed] [Google Scholar]
- BARR D. P., RUSS E. M., EDER H. A. Protein-lipid relationships in human plasma. II. In atherosclerosis and related conditions. Am J Med. 1951 Oct;11(4):480–493. doi: 10.1016/0002-9343(51)90183-0. [DOI] [PubMed] [Google Scholar]
- Badimon J. J., Badimon L., Galvez A., Dische R., Fuster V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab Invest. 1989 Mar;60(3):455–461. [PubMed] [Google Scholar]
- Bates S. R. Effect of HDL on the interaction of hyperlipemic LDL with monkey smooth muscle cells. Artery. 1980;7(4):303–315. [PubMed] [Google Scholar]
- Blankenhorn D. H., Nessim S. A., Johnson R. L., Sanmarco M. E., Azen S. P., Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987 Jun 19;257(23):3233–3240. [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FRIEDMAN M., BYERS S. O., ROSENMAN R. H. Resolution of aortic atherosclerotic infiltration in the rabbit by phosphatide infusion. Proc Soc Exp Biol Med. 1957 Jul;95(3):586–588. doi: 10.3181/00379727-95-23300. [DOI] [PubMed] [Google Scholar]
- Galvez A., Badimon L., Badimon J. J., Fuster V. Electrical aggregometry in whole blood from human, pig and rabbit. Thromb Haemost. 1986 Oct 21;56(2):128–132. [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Jr, Bangdiwala S., Tyroler H. A. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989 Jan;79(1):8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977 May;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- Gordon T., Kannel W. B., Castelli W. P., Dawber T. R. Lipoproteins, cardiovascular disease, and death. The Framingham study. Arch Intern Med. 1981 Aug;141(9):1128–1131. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN R. L., McGILL H. C., Jr, STRONG J. P., GEER J. C. Technics for studying atherosclerotic lesions. Lab Invest. 1958 Jan-Feb;7(1):42–47. [PubMed] [Google Scholar]
- Hajjar D. P. Prostaglandins modulate arterial cholesteryl ester metabolism. Enzyme. 1984;32(4):218–227. doi: 10.1159/000469481. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Robertson A. L., Jr, Chisolm G. M., 3rd LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979 Mar;32(3):213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Ho Y. K., Brown M. S., Goldstein J. L. Hydrolysis and excretion of cytoplasmic cholesteryl esters by macrophages: stimulation by high density lipoprotein and other agents. J Lipid Res. 1980 May;21(4):391–398. [PubMed] [Google Scholar]
- Hough J. L., Zilversmit D. B. Effect of 17 beta estradiol on aortic cholesterol content and metabolism in cholesterol-fed rabbits. Arteriosclerosis. 1986 Jan-Feb;6(1):57–63. doi: 10.1161/01.atv.6.1.57. [DOI] [PubMed] [Google Scholar]
- Izzo C., Grillo F., Murador E. Improved method for determination of high-density-lipoprotein cholesterol I. Isolation of high-density lipoproteins by use of polyethylene glycol 6000. Clin Chem. 1981 Mar;27(3):371–374. [PubMed] [Google Scholar]
- Jackson R. L., Gotto A. M., Stein O., Stein Y. A comparative study on the removal of cellular lipids from Landschütz ascites cells by human plasma apolipoproteins. J Biol Chem. 1975 Sep 25;250(18):7204–7209. [PubMed] [Google Scholar]
- Leth-Espensen P., Stender S., Ravn H., Kjeldsen K. Antiatherogenic effect of olive and corn oils in cholesterol-fed rabbits with the same plasma cholesterol levels. Arteriosclerosis. 1988 May-Jun;8(3):281–287. doi: 10.1161/01.atv.8.3.281. [DOI] [PubMed] [Google Scholar]
- Malinow M. R. Experimental models of atherosclerosis regression. Atherosclerosis. 1983 Aug;48(2):105–118. doi: 10.1016/0021-9150(83)90097-7. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miller N. E., Thelle D. S., Forde O. H., Mjos O. D. The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1977 May 7;1(8019):965–968. doi: 10.1016/s0140-6736(77)92274-7. [DOI] [PubMed] [Google Scholar]
- Morton R. E., Zilversmit D. B. Purification and characterization of lipid transfer protein(s) from human lipoprotein-deficient plasma. J Lipid Res. 1982 Sep;23(7):1058–1067. [PubMed] [Google Scholar]
- Oram J. F. Effects of high density lipoprotein subfractions on cholesterol homeostasis in human fibroblasts and arterial smooth muscle cells. Arteriosclerosis. 1983 Sep-Oct;3(5):420–432. doi: 10.1161/01.atv.3.5.420. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Johnson W. J., Rothblat G. H. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta. 1987 Jun 24;906(2):223–276. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- Reichl D., Miller N. E. The anatomy and physiology of reverse cholesterol transport. Clin Sci (Lond) 1986 Mar;70(3):221–231. doi: 10.1042/cs0700221. [DOI] [PubMed] [Google Scholar]
- Reichl D., Rudra D. N., Myant N. B., Pflug J. J. Further evidence for the role of high density lipoprotein in the removal of tissue cholesterol in vivo. Atherosclerosis. 1982 Jul;44(1):73–84. doi: 10.1016/0021-9150(82)90054-5. [DOI] [PubMed] [Google Scholar]
- Rhoads G. G., Gulbrandsen C. L., Kagan A. Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N Engl J Med. 1976 Feb 5;294(6):293–298. doi: 10.1056/NEJM197602052940601. [DOI] [PubMed] [Google Scholar]
- Rouleau J. L., Parmley W. W., Stevens J., Wikman-Coffelt J., Sievers R., Mahley R. W., Havel R. J. Verapamil suppresses atherosclerosis in cholesterol-fed rabbits. J Am Coll Cardiol. 1983 Jun;1(6):1453–1460. doi: 10.1016/s0735-1097(83)80049-7. [DOI] [PubMed] [Google Scholar]
- Skrinska V. A., Konieczkowski M., Gerrity R. G., Galang C. F., Rebec M. V. Suppression of foam cell lesions in hypercholesterolemic rabbits by inhibition of thromboxane A2 synthesis. Arteriosclerosis. 1988 Jul-Aug;8(4):359–367. doi: 10.1161/01.atv.8.4.359. [DOI] [PubMed] [Google Scholar]
- Son Y. S., Zilversmit D. B. Increased lipid transfer activities in hyperlipidemic rabbit plasma. Arteriosclerosis. 1986 May-Jun;6(3):345–351. [PubMed] [Google Scholar]
- St Clair R. W. Atherosclerosis regression in animal models: current concepts of cellular and biochemical mechanisms. Prog Cardiovasc Dis. 1983 Sep-Oct;26(2):109–132. doi: 10.1016/0033-0620(83)90026-9. [DOI] [PubMed] [Google Scholar]
- Stein O., Halperin G., Stein Y. Cholesteryl ester efflux from extracellular and cellular elements of the arterial wall. Model systems in culture with cholesteryl linoleyl ether. Arteriosclerosis. 1986 Jan-Feb;6(1):70–78. doi: 10.1161/01.atv.6.1.70. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. High density lipoproteins reduce the uptake of low density lipoproteins by human endothelial cells in culture. Biochim Biophys Acta. 1976 May 27;431(2):363–368. doi: 10.1016/0005-2760(76)90157-0. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y., Lefevre M., Roheim P. S. The role of apolipoprotein A-IV in reverse cholesterol transport studied with cultured cells and liposomes derived from an ether analog of phosphatidylcholine. Biochim Biophys Acta. 1986 Aug 14;878(1):7–13. doi: 10.1016/0005-2760(86)90337-1. [DOI] [PubMed] [Google Scholar]
- Stein O., Vanderhoek J., Stein Y. Cholesterol ester accumulation in cultured aortic smooth muscle cells. Induction of cholesterol ester retention by chloroquine and low density lipoprotein and its reversion by mixtures of high density apolipoprotein and sphingomyelin. Atherosclerosis. 1977 Apr;26(4):465–482. doi: 10.1016/0021-9150(77)90115-0. [DOI] [PubMed] [Google Scholar]
- Tall A. R., Krumholz S., Olivecrona T., Deckelbaum R. J. Plasma phospholipid transfer protein enhances transfer and exchange of phospholipids between very low density lipoproteins and high density lipoproteins during lipolysis. J Lipid Res. 1985 Jul;26(7):842–851. [PubMed] [Google Scholar]
- Via D. P., Plant A. L., Craig I. F., Gotto A. M., Jr, Smith L. C. Metabolism of normal and modified low-density lipoproteins by macrophage cell lines of murine and human origin. Biochim Biophys Acta. 1985 Mar 6;833(3):417–428. doi: 10.1016/0005-2760(85)90099-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams K. J., Werth V. P., Wolff J. A. Intravenously administered lecithin liposomes: a synthetic antiatherogenic lipid particle. Perspect Biol Med. 1984 Spring;27(3):417–431. doi: 10.1353/pbm.1984.0031. [DOI] [PubMed] [Google Scholar]
- Zilversmit D. B. Lipid transfer proteins. J Lipid Res. 1984 Dec 15;25(13):1563–1569. [PubMed] [Google Scholar]