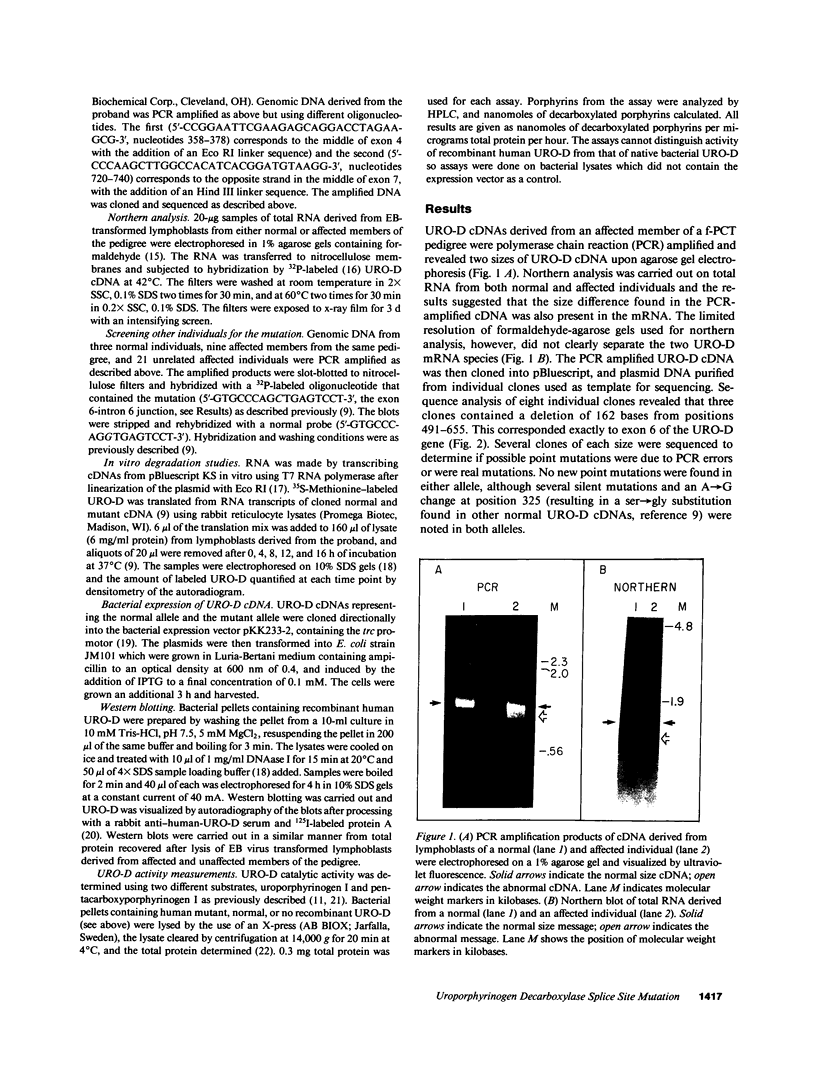
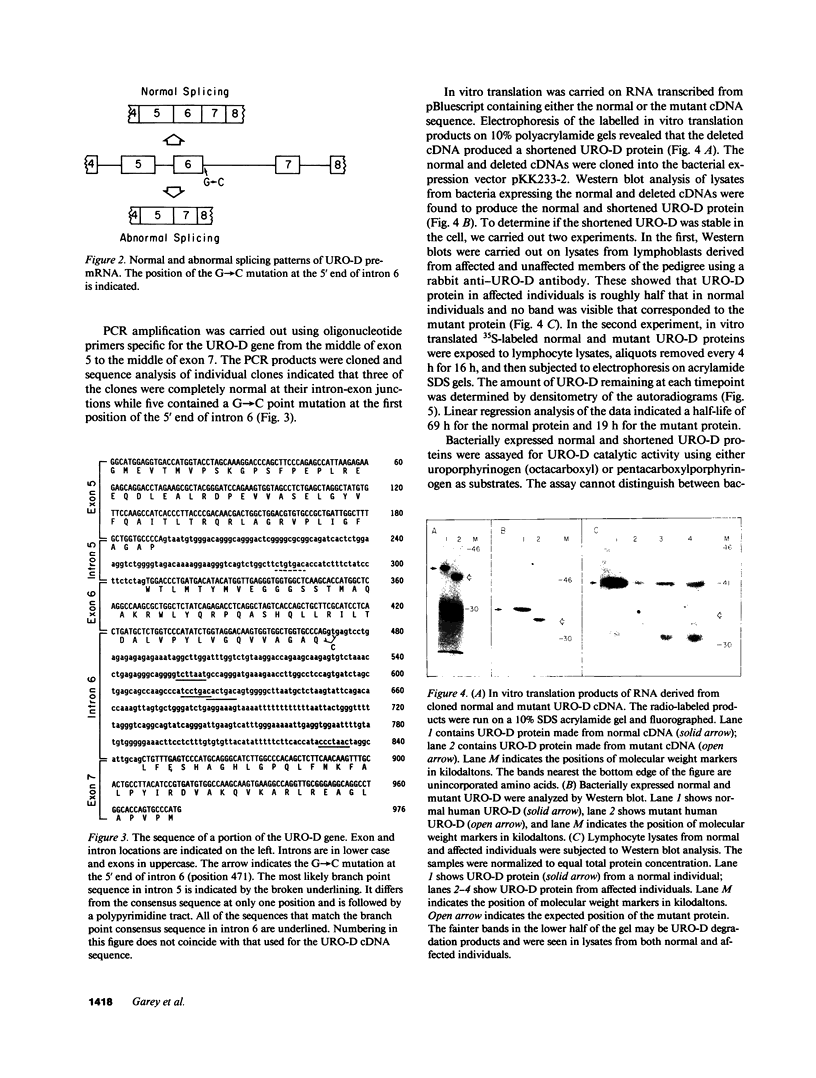
Abstract
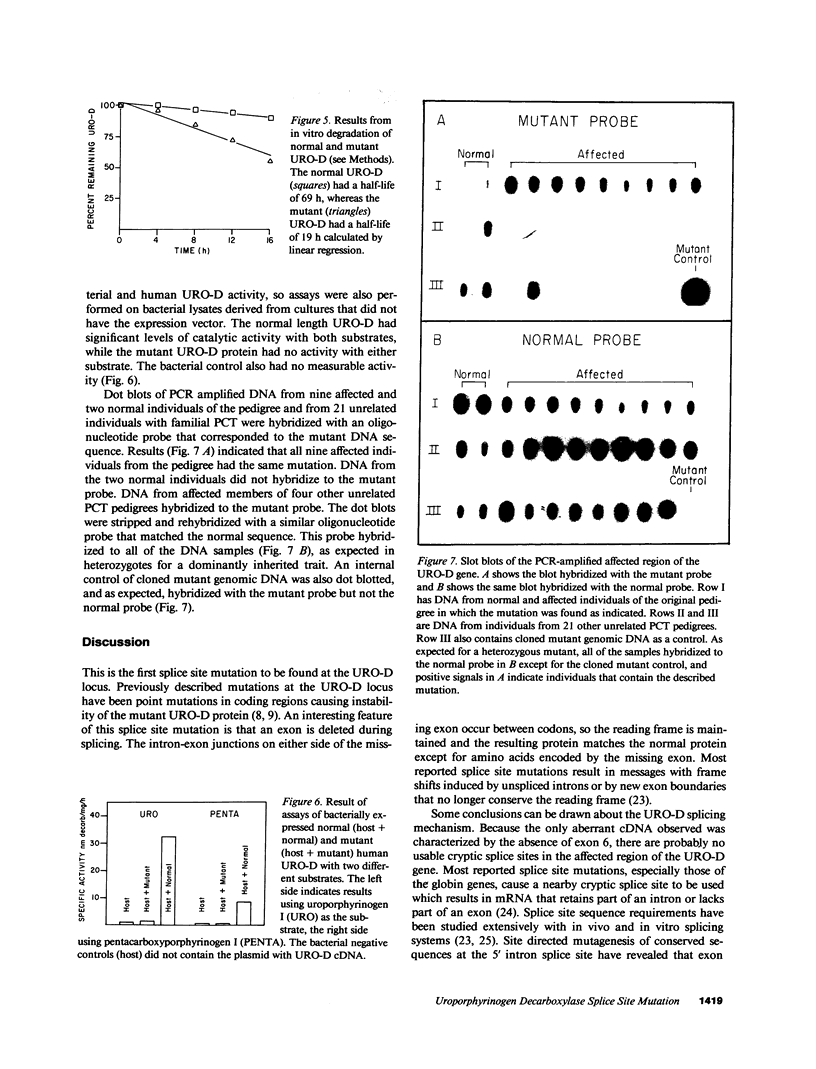
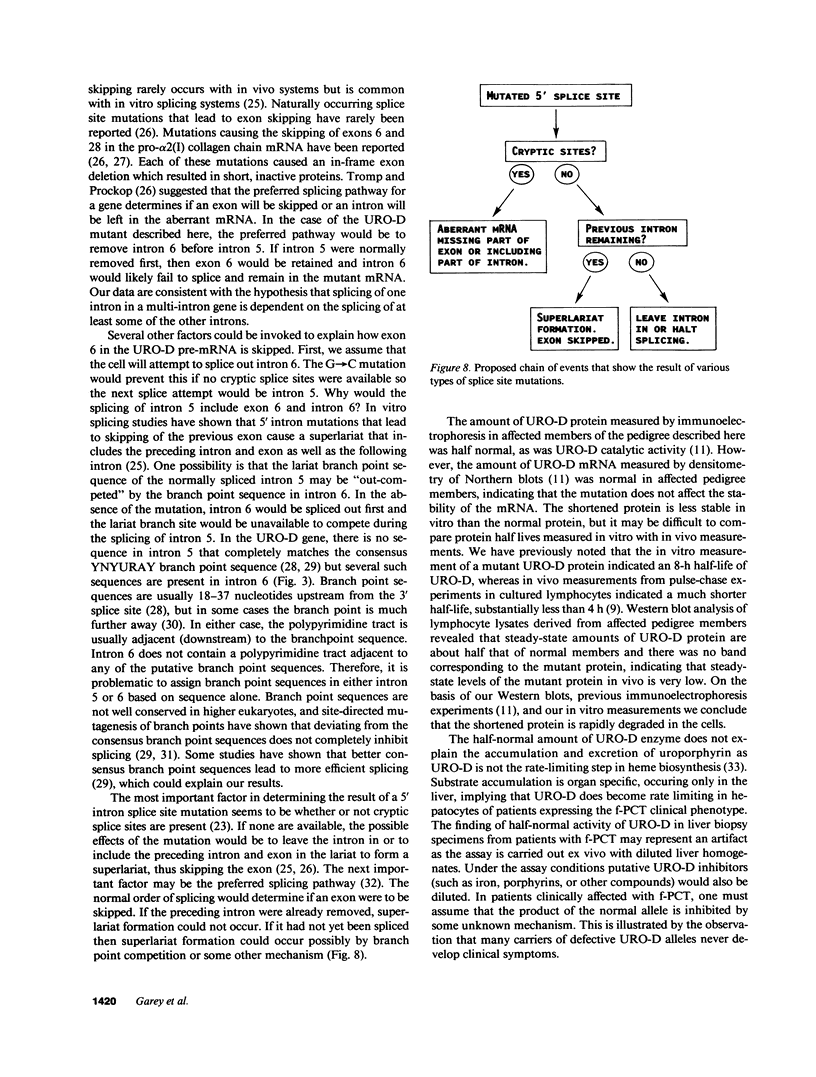
Uroporphyrinogen decarboxylase (URO-D) is a cytosolic heme-biosynthetic enzyme that converts uroporphyrinogen to coproporphyrinogen. Defects at the uroporphyrinogen decarboxylase locus cause the human genetic disease familial porphyria cutanea tarda. A splice site mutation has been found in a pedigree with familial porphyria cutanea tarda that causes exon 6 to be deleted from the mRNA. The intron/exon junctions on either side of exon 6 fall between codons, so the resulting protein is shorter than the normal protein, missing only the amino acids coded by exon 6. The shortened protein lacks catalytic activity, is rapidly degraded when exposed to human lymphocyte lysates, and is not detectable by Western blot analysis in lymphocyte lysates derived from affected individuals. The mutation was detected in five of 22 unrelated familial porphyria cutanea tarda pedigrees tested, so it appears to be common. This is the first splice site mutation to be found at the URO-D locus, and the first mutation that causes familial porphyria cutanea tarda to be found in more than one pedigree.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Barnard G. F., Akhtar M. Stereochemical and mechanistic studies on the decarboxylation of uroporphyrinogen III in haem biosynthesis. J Chem Soc Perkin 1. 1979;10:2354–2360. doi: 10.1039/p19790002354. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Desnick R. J. Assays of the heme biosynthetic enzymes. Preface. Enzyme. 1982;28(2-3):91–93. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Tovey J. A., Sheppard D. M. Purification of uroporphyrinogen decarboxylase from human erythrocytes. Immunochemical evidence for a single protein with decarboxylase activity in human erythrocytes and liver. Biochem J. 1983 Oct 1;215(1):45–55. doi: 10.1042/bj2150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garey J. R., Hansen J. L., Harrison L. M., Kennedy J. B., Kushner J. P. A point mutation in the coding region of uroporphyrinogen decarboxylase associated with familial porphyria cutanea tarda. Blood. 1989 Mar;73(4):892–895. [PubMed] [Google Scholar]
- Hansen J. L., Pryor M. A., Kennedy J. B., Kushner J. P. Steady-state levels of uroporphyrinogen decarboxylase mRNA in lymphoblastoid cell lines from patients with familial porphyria cutanea tarda and their relatives. Am J Hum Genet. 1988 Jun;42(6):847–853. [PMC free article] [PubMed] [Google Scholar]
- Helfman D. M., Ricci W. M. Branch point selection in alternative splicing of tropomyosin pre-mRNAs. Nucleic Acids Res. 1989 Jul 25;17(14):5633–5650. doi: 10.1093/nar/17.14.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. H., Sancovich H. A., Ferramola A. M., Evans N., Games D. E., Matlin S. A., Elder G. H., Smith S. G. Macrocyclic intermediates in the biosynthesis of porphyrins. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):191–206. doi: 10.1098/rstb.1976.0009. [DOI] [PubMed] [Google Scholar]
- Kawanishi S., Seki Y., Sano S. Uroporphyrinogen decarboxylase. Purification, properties, and inhibition by polychlorinated biphenyl isomers. J Biol Chem. 1983 Apr 10;258(7):4285–4292. [PubMed] [Google Scholar]
- Kushner J. P., Barbuto A. J., Lee G. R. An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity. J Clin Invest. 1976 Nov;58(5):1089–1097. doi: 10.1172/JCI108560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang K. M., Spritz R. A. In vitro splicing pathways of pre-mRNAs containing multiple intervening sequences? Mol Cell Biol. 1987 Oct;7(10):3428–3437. doi: 10.1128/mcb.7.10.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. The role of the mammalian branchpoint sequence in pre-mRNA splicing. Genes Dev. 1988 Oct;2(10):1268–1276. doi: 10.1101/gad.2.10.1268. [DOI] [PubMed] [Google Scholar]
- Romana M., Dubart A., Beaupain D., Chabret C., Goossens M., Romeo P. H. Structure of the gene for human uroporphyrinogen decarboxylase. Nucleic Acids Res. 1987 Sep 25;15(18):7343–7356. doi: 10.1093/nar/15.18.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roméo P. H., Raich N., Dubart A., Beaupain D., Pryor M., Kushner J., Cohen-Solal M., Goossens M. Molecular cloning and nucleotide sequence of a complete human uroporphyrinogen decarboxylase cDNA. J Biol Chem. 1986 Jul 25;261(21):9825–9831. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Investigations of rat liver uroporphyrinogen decarboxylase. Comparisons of porphyrinogens I and III as substrates and the inhibition by porphyrins. Biochem J. 1981 Apr 1;195(1):241–250. doi: 10.1042/bj1950241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Porro E. B., Patton J. G., Nadal-Ginard B. Scanning from an independently specified branch point defines the 3' splice site of mammalian introns. Nature. 1989 Nov 16;342(6247):243–247. doi: 10.1038/342243a0. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Straka J. G., Kushner J. P., Pryor M. A. Uroporphyrinogen decarboxylase. A method for measuring enzyme activity. Enzyme. 1982;28(2-3):170–185. [PubMed] [Google Scholar]
- Straka J. G., Kushner J. P. Purification and characterization of bovine hepatic uroporphyrinogen decarboxylase. Biochemistry. 1983 Sep 27;22(20):4664–4672. doi: 10.1021/bi00289a009. [DOI] [PubMed] [Google Scholar]
- Straus D., Gilbert W. Chicken triosephosphate isomerase complements an Escherichia coli deficiency. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2014–2018. doi: 10.1073/pnas.82.7.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toback A. C., Sassa S., Poh-Fitzpatrick M. B., Schechter J., Zaider E., Harber L. C., Kappas A. Hepatoerythropoietic porphyria: clinical, biochemical, and enzymatic studies in a three-generation family lineage. N Engl J Med. 1987 Mar 12;316(11):645–650. doi: 10.1056/NEJM198703123161101. [DOI] [PubMed] [Google Scholar]
- Tromp G., Prockop D. J. Single base mutation in the pro alpha 2(I) collagen gene that causes efficient splicing of RNA from exon 27 to exon 29 and synthesis of a shortened but in-frame pro alpha 2(I) chain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5254–5258. doi: 10.1073/pnas.85.14.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D., Bernard M., Combates N., Wirtz M. K., Hollister D. W., Steinmann B., Ramirez F. Identification of a mutation that causes exon skipping during collagen pre-mRNA splicing in an Ehlers-Danlos syndrome variant. J Biol Chem. 1988 Jun 25;263(18):8561–8564. [PubMed] [Google Scholar]
- Zhuang Y. A., Goldstein A. M., Weiner A. M. UACUAAC is the preferred branch site for mammalian mRNA splicing. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2752–2756. doi: 10.1073/pnas.86.8.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H., Grandchamp B., Beaumont C., Picat C., Nordmann Y. Uroporphyrinogen decarboxylase structural mutant (Gly281----Glu) in a case of porphyria. Science. 1986 Nov 7;234(4777):732–734. doi: 10.1126/science.3775362. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Hansen J., Picat C., Grandchamp B., Kushner J., Roberts A., Elder G., Nordmann Y. Prevalence of the 281 (Gly----Glu) mutation in hepatoerythropoietic porphyria and porphyria cutanea tarda. Hum Genet. 1988 Jan;78(1):101–102. doi: 10.1007/BF00291248. [DOI] [PubMed] [Google Scholar]
- de Verneuil H., Sassa S., Kappas A. Purification and properties of uroporphyrinogen decarboxylase from human erythrocytes. A single enzyme catalyzing the four sequential decarboxylations of uroporphyrinogens I and III. J Biol Chem. 1983 Feb 25;258(4):2454–2460. [PubMed] [Google Scholar]