Abstract
Anchorage to matrix is mediated for many cells not only by integrin-based focal adhesions but also by a parallel assembly of integral and peripheral membrane proteins known as the Dystroglycan Complex. Deficiencies in either dystrophin (mdx mice) or γ-sarcoglycan (γSG−/− mice) components of the Dystroglycan Complex lead to upregulation of numerous focal adhesion proteins, and the phosphoprotein paxillin proves to be among the most prominent. In mdx muscle, paxillin-Y31 and Y118 are both hyper-phosphorylated as are key sites in focal adhesion kinase (FAK) and the stretch-stimulatable pro-survival MAPK pathway, whereas γSG−/− muscle exhibits more erratic hyper-phosphorylation. In cultured myotubes, cell tension generated by myosin-II appears required for localization of paxillin to adhesions while vinculin appears more stably integrated. Over-expression of wild-type (WT) paxillin has no obvious effect on focal adhesion density or the physical strength of adhesion, but WT and a Y118F mutant promote contractile sarcomere formation whereas a Y31F mutant shows no effect, implicating Y31 in striation. Self-peeling of cells as well as Atomic Force Microscopy (AFM) probing of cells with or without myosin II inhibition indicate an increase in cell tension within paxillin-overexpressing cells. However, prednisolone, a first-line glucocorticoid for muscular dystrophies, decreases cell tension without affecting paxillin at adhesions, suggesting a non-linear relationship between paxillin and cell tension. Hypertension that results from upregulation of integrin adhesions is thus a natural and treatable outcome of dystroglycan complex down-regulation.
Keywords: adhesion, contractility, differentiation, paxillin, muscular dystrophy, hypertensive, glucocorticoid
INTRODUCTION
Tissue cells not only attach to but also pull on matrix as part of ‘tactile’ signaling mechanisms (Discher et al., 2005). Myosins invariably provide the pulling force in establishing a cytoskeletal tension, and cell anchorage generally occurs via the well-studied integrin-based focal adhesion system but also – in many cell types – via the dystroglycan complex (DGC). Identified first in myocytes (Campbell, 1995), the DGC is increasingly understood to be used by many cells (Campbell, 1995; Muschler et al., 2002) for anchorage to basal lamina. Integrin↔DGC signaling appears bidirectional (Yoshida et al., 1998), and yet the interplay with cell tension and contractility is unknown, as is any impact on cell differentiation.
The DGC linkage between the cytoskeleton and the extracellular matrix (ECM) is often perturbed or disrupted in muscular dystrophies (MD). Myoblasts are relatively unaffected because the DGC is expressed only in post-fusion, non-dividing myotubes, but tension-induced damage to the mature muscle membrane ultimately causes muscle weakness, massive degeneration, and premature death (Campbell, 1995; Straub and Campbell, 1997; Lim and Campbell, 1998; Cohn and Campbell, 2000). Importantly, in both dystrophin-deficient patients (Duchenne Muscular Dystrophy) as well as in dystrophin-deficient mdx mice, the contractile myotubes partially compensate for the lack of an intact DGC by up-regulating integrins, particularly α7β1 (Figure 1A, right sketch) (Hodges et al., 1997). An intermediate level of compensation occurs with deficiency of the DGC component γ-sarcoglycan, leading to what also appears to be a more apoptotic phenotype (Griffin et al., 2005). Intentional over-expression of α7β1 has proven protective (Yoshida et al., 1998; Allikian et al., 2004; Burkin et al., 2005), but whether this is strictly from stabilizing transmembrane force transmission or also from the recruitment of additional cytosolic proteins to the integrin complex has not yet been addressed.
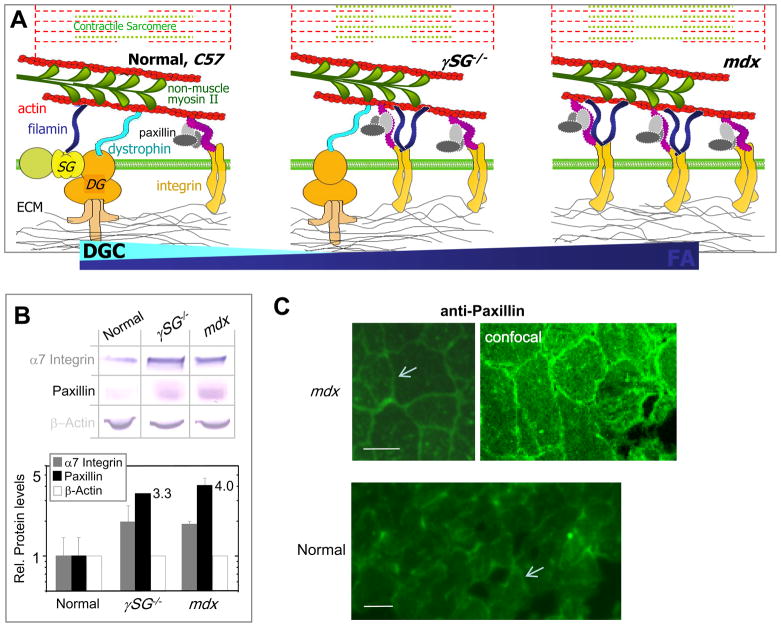
Figure 1. Upregulated adhesive complex components in muscular dystrophies.
(A) Sarcolemma scaffolding in normal (C57), γSG−/−, and mdx muscle cells illustrates the shift in cell attachment mediated by Dystroglycan Complex (DGC) to increasingly integrin-based Focal Adhesions (FA).
(B) Western blot analysis of C57, mdx and γSG−/− muscle lysates shows a two-fold upregulation of α7 integrins in dystrophic muscle together with 4-5 fold increases in paxillin (n=3). β-actin is used to normalize, and in so doing the integrin result appears consistent with past reports (Hodges et al., 1997).
(C) Muscle sections immunostained with monoclonal anti-paxillin plus secondary and imaged under identical conditions by conventional fluorescence microscopy and confocal (mdx). Secondary alone shows no labeling. Paxillin intensity in normal muscle appears dim relative to mdx, and only the latter showed sufficient intensity to be imaged by laser scanning confocal, suggesting paxillin localization to sarcolemma (arrows) as well as cytosol. Scalebars ~20 μm.
Talin, α-actinin, and perhaps filamin contribute scaffolding roles in integrin-based Focal Adhesions (FA), whereas other components such as paxillin, vinculin, and FAK diffuse in and out as part of a phospho-Tyrosine based signaling nexus (Panetti 2002; Shemesh et al., 2005; Zaidel-Bar et al., 2007; Pasapera et al., 2010). Essential for embryonic development (Furuta et al., 1995; Xu et al., 1998; Hagel et al., 2002; Charlesworth et al., 2006), FA-derived signals promote assembly of cytoskeletal tension structures such as stress fibers and also propagate cell survival signals into the MAPK pathway (Turner, 2000; Hagel et al., 2002; Brown and Turner, 2004) with activation of ERK (Fluck et al., 1999; Turner, 2000; Most et al., 2003; Schaeffer et al., 2003; Lunn and Rozengurt, 2004; Melendez et al., 2004; Mizukami et al., 2004; Subauste et al., 2004; Lin et al., 2005; Palfi et al., 2005; Vittal et al., 2005; Das et al., 2006; Peng et al., 2006; Wei et al., 2006) – which is already known to be enhanced in stretched mdx muscle (Kumar et al., 2004) and in γSG−/− muscle (Griffin et al., 2005). In maturing myotubes, FAs are the nucleation sites for myofibrillogenesis (McKenna et al., 1986; Sanger et al., 2002) during which extensive cytoskeletal remodeling ultimately replaces non-muscle myosin-II (NMM-II) mini-filaments with the contractile striations of skeletal muscle myosin-II (Figure 1A). Given the upregulation of integrins in muscular dystrophies, as well as the known enrichment of filamin at the sarcolemma of both mdx and γSG−/− mice (Thompson et al., 2000), we hypothesized that additional FA components would also be modulated and would influence downstream outputs ranging from cell tension and myofibrillogenesis to viability. Transcript profiles of mdx versus normal muscle indeed hint at increases in paxillin (+15%; see Table S1A) as well as other components, such as vinculin (+35%) and γ-actin, and the latter has recently been shown to be elevated at the protein level (Hanft et al., 2006). However, signaling and phenotype depend on the protein levels, post-translational modifications, and collective interactions with feedback loops in and between signaling networks. Here we demonstrate – as part of compensatory mechanisms within mouse dystrophic muscle – a major upregulation of paxillin and adhesive signaling that promotes general contractility, as confirmed by ectopic expression studies. We use micropatterned strips of collagen with finite length that standardize cell shape and permit novel studies of cell adhesion, and then we employ Atomic Force Microscopy to probe cell stiffness – comparing to cells relaxed by a myosin inhibitor – in order to determine an effective cell tension that relates to the adhesion-cytoskeleton state. Changes in signaling that are coupled to structure could suggest new interventions or better understanding of current therapeutic interventions, as illustrated here with initial data on the pro-relaxant activities of a major, clinical glucocorticoid.
RESULTS
Adhesive-contractile signaling is upregulated in muscular dystrophies
Tissue lysates from dystrophic mdx and γSG−/− muscle show paxillin to be upregulated compared to normal muscle in Western blots (Figure 1B). α7-integrin was confirmed to be up in both dystrophic cell types (Hodges et al., 1997), but paxillin overexpression appears even higher at about 4-fold in mdx and above 3-fold in γSG−/− muscle (+300% and +230%, respectively). Vinculin was also significantly elevated in mdx muscle (+84%; see Table S1B) although FAK was unchanged (not shown). Immunostaining of paxillin in normal and mdx muscle sections showed paxillin localization at the sarcolemma with perhaps more paxillin in mdx cells both at the membrane and throughout the cytosol (Figure 1C).
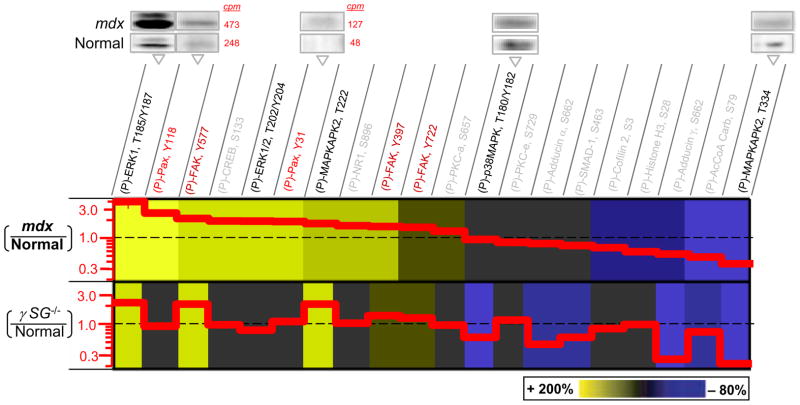
A number of (P)-Tyr sites on paxillin are known to be modified by cell stretch (Yano et al., 1996; Sokabe et al., 1997), shear stress (Cuvelier et al., 2005), and osmotic shock (Hirakawa et al., 2004). Screening of muscle tissue lysates for 20 phospho-sites within and beyond the paxillin-FAK-MAPK signaling nexus shows that relative levels of phosphorylation in mdx versus normal muscle vary from higher to lower (Figure 2), with quantitative changes made explicit in the step plots as red solid lines. Both mdx and γSG−/− dystrophic muscle types show significant hyperphosphorylation (beyond +25%) of (P)-ERK1/2, (P)-FAK, and (P)-MAPKAPK2T222. The greatest hyperactivation is found for (P)-ERK1, which is up 2.8-fold in resting mdx and 2.3-fold in γSG−/− muscle, while (P)-FAKY577 is up almost as high in both mdx and γSG−/− muscle. Some phosphoprotein levels such as (P)-PKC-α S657, -εS729 change little in both dystrophies whereas others are consistently and significantly decreased (below −25%) in both, especially (P)-MAPKAPK2T334 and (P)-Adducin-γS662. For paxillin, both (P)-paxillinY118 and (P)-paxillinY31 are increased more than 2-fold in mdx but unaffected in γSG−/− muscle. Additional signaling molecules such as CREB also show activation in mdx tissue (+1.9-fold) but remain unaltered in γSG−/− muscle.
Figure 2.
Hyperphosphorylated MAPKs, FAK, and Paxillin in muscular dystrophies. Phosphoprotein immunoblots of normal (C57) and dystrophic (mdx) muscle tissue were quantified (with radio-labeled secondary) and normalized for direct comparison to results of γSG−/− (Griffin et al., 2005). Representative blot images are shown with cpm’s indicated for phosphopaxillins to illustrate the methods. Greater phosphorylation of paxillin is found in mdx. Increased levels of phospho-paxillin (Fluck et al., 1999; Melendez et al., 2004; Subauste et al., 2004), ERK (Most et al., 2003; Schaeffer et al., 2003; Mizukami et al., 2004; Palfi et al., 2005; Vittal et al., 2005; Das et al., 2006; Wei et al., 2006), and FAK (Lunn and Rozengurt, 2004; Lin et al., 2005; Peng et al., 2006) are associated with either survival or apoptosis, but only γSG−/− cells have been reported to be apoptotic (Griffin et al., 2005). Representative error bars for phospho-ERK are <20% (S.D./Avg.; n=3).
Similarities and differences in relative activation of the paxillin-FAK-MAPK nexus were formally assessed with a simple cross-correlation analysis. Briefly, maximum activation among all proteins in the results of Fig. 2 is set to +1 (i.e. ERK, which is about 4-fold above Normal for mdx becomes +1), and the maximum deactivation is set to −1 (i.e. MAPKAPK2). The re-scaled result for each phosphoprotein in a set (eg. mdxERX = +1) is then multiplied by all of the others within a pairwise set (eg. mdxi x mdxj) and this is averaged over the set to obtain the cross-correlation coefficient, φ. If all proteins are up (or down) to the same extent relative to Normal cells, then they are correlated and φ = +1; anti-correlations (φ = −1) or null correlations (φ = 0) might also emerge. For mdx muscle, φ = +0.35 indicates a general hyperactivation for the selected set of signaling pathways; whereas for γSG−/− muscle, φ = +0.16 indicates less overall activation for the same set (Table 1). The difference occurs despite similar upregulation of α7-integrin (~2-fold per Figure 1C). The cross-correlation between γSG−/− and mdx is small (in absolute value) but negative, indicating a weak anti-correlation. This difference suggests different pathways are involved in the increased (P)-ERK1 in γSG−/− mice, while the smaller φ suggests a moderating effect of the residual DGC complex in signaling.
Table 1.
Cross-correlation within Pax-FAK-MAPK nexus
| Correlation Coefficient | mdx/Norm | γSG−/−/Norm |
|---|---|---|
| mdx/Norm | 0.35 | −0.039 |
| γSG−/−/Norm | −0.039 | 0.16 |
Since the paxillin-FAK-MAPK nexus is implicated in mechanoactivation within numerous cell types, and has the potential for long-term effects in contractile muscle, we assessed late-stage activation after a sustained muscle extension of 10% (Figure S2). In this imposed state of contractile tension, (P)-paxillin shows no sustained changes in either dystrophy whereas ERK and MAPKAP2 appear hyper-activated from 3- to 10-fold after 20 min in normal and dystrophic muscles. Hyper-activation is also seen for (P)-p38MAPK, which is not elevated significantly in unstimulated dystrophic muscle, and (P)-FAK is activated sustainably under mechanical stretch only in mdx muscle. Given the lack of acute paxillin response and the noted elevation of paxillin in dystrophy, we conducted model studies in C2C12 myocytes of paxillin distribution and dynamics as well as overexpression contributions to phenotype.
Paxillin localization to Focal Adhesions depends on Contractility
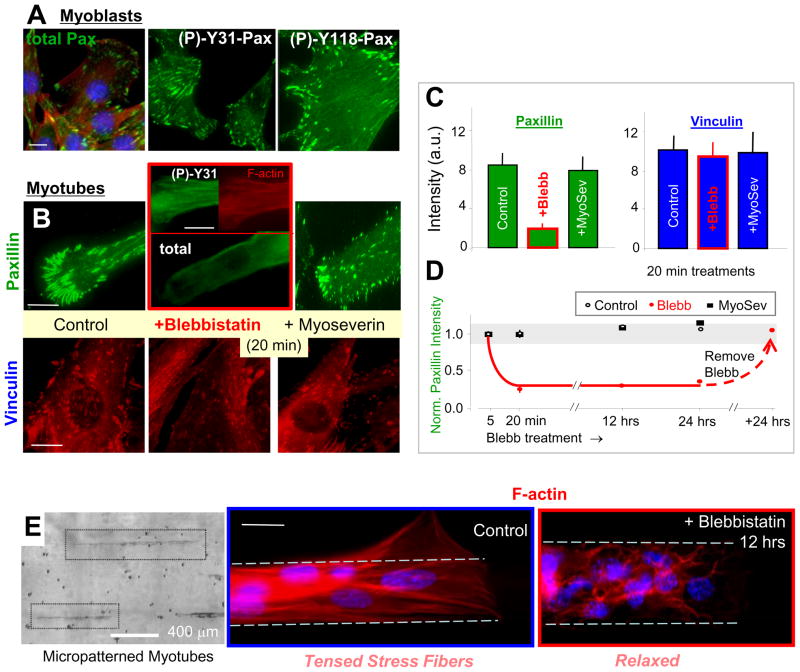
Immunostaining for paxillin and vinculin in myocytes cultured on collagen-coated glass (Figure 3A, B; Figure S3) demonstrates the typical localization to focal adhesions as well as cytosolic staining consistent with the muscle sections (Figure 1C). In culture, 6 days in differentiation medium causes myoblasts to stop dividing and fuse into multinucleated myotubes, but differences in FA density and intensity between myoblasts and definitively fused myotubes were generally not apparent here. Phospho-paxillin, specifically (P)-Y31 and (P)-Y118, preferentially localizes to FA, and treatment of cultured myotubes with blebbistatin, which inhibits non-muscle myosin as well as skeletal muscle myosin (Straight et al., 2003), shows that FA localization of paxillin – but not vinculin – is lost within 20 min (Figure 3B). Recent studies of fibroblasts show that a 60 min treatment with blebbistatin (at lower dose than here) leads to a partial loss of both paxillin and vinculin from adhesions (Pasapera et al., 2010), but the same studies also used FRAP (Fluorescence Recovery After Photobleaching) to show that GFP-paxillin is lost from adhesions more than 3-fold faster than GFP-vinculin. Quantitative intensity analysis here (Figure 3C, D) further shows that most (but not all) of paxillin is lost from FA in non-dividing myotubes, and while 5 min is not enough time, 20 min is sufficient for a saturating effect that is dynamically reversed after the drug is washed out. Formation of FA in non-muscle cells had long been known to depend on the contractile activity of non-muscle myosin, and, at least in T-lymphoblasts, paxillin also reportedly interacts in the cytosol with tubulin (Herreros et al., 2000). However, the putative tubulin depolymerizer drug myoseverin (Rosania et al., 2000), which also dedifferentiates myotubes back into mono-nucleated blasts (see below also), has no obvious effect on FA-localized paxillin or vinculin, consistent with the stated lack of obvious FA differences between myoblasts and myotubes. Nocadozole also has no effect (not shown).
Figure 3. Paxillin localization to focal adhesions is contractility dependent.
(A) Immunostaining of paxillin and its phosphorylated forms, (P)-Y31 and (P)-Y118, at focal adhesions and in the cytosol of myoblasts.
(B–C) Myotubes treated with blebbistatin, which inhibits non-muscle myosin-II and skeletal muscle myosin, show loss of paxillin but not vinculin from adhesions within 20 min. Note that the vinculin stained cells were within differentiated myotubes cultures and no distinctions with residual myoblasts were obvious. Myoseverin, which depolymerizes tubulin, has no such effect (total paxillin shown). Quantitative intensity analysis of 20 arbitrary myotubes (Avg. ± S.D.).
(D) Kinetics of paxillin loss and re-localization at focal adhesions after blebbistatin treatment and washout.
(E) Nascent myotubes attached to micropatterned collagen strips on glass exhibit stress fibers, whereas hours of blebbistatin treatment leads to relaxation of these structures into an anastamosing network of bundles. Scalebars ~10 μm.
Blebbistatin is expected to decrease cytoskeletal tension (contractility, or ‘prestress’) in both striated myotubes and also in the more stress-fiber filled cells that adhere to collagen-coated rigid glass; this is because blebbistatin inhibits both the non-muscle and skeletal muscle myosin-II isoforms. To test this effect, myotubes were grown on 20 μm wide strips of collagen-coated rigid glass that vary in length but help to standardize cell geometry (Figure 3E). Phalloidin-stained control cells show actin stress fibers aligned with the patterns, indicative of a tensed myotube. Blebbistatin treatment produces a more anastamosing network of relaxed actomyosin fibers. Functional assessment of the blebbistatin-relaxation of muscle tissue was performed by stretching excised muscles 10% and monitoring the tetanic force in a bath of blebbistatin (with slow permeation) (Figure S4; see Supplemental Methods). Within 45 min, the contractile force had indeed decreased (~60±15%), consistent with known effects of blebbistatin on skeletal muscle myosin (Linari M, 2004). Phospho-activation of ERK also proved to be about half that of untreated controls (Figure 2, S2). Stretch-activated phospho-signaling thus depends on contractility.
Paxillin overexpression promotes Myofibrillogenesis without affecting Adhesion
Given our original observation of dystrophic upregulation of paxillin, we overexpressed paxillin and phospho-mutants of paxillin in well-controlled cell cultures to study mobility and additional effects. C2C12 myoblasts transfected with GFP-paxillin (~30% transfection efficiency) overexpress total paxillin by well over fivefold at 24 hrs post-transfection by Western blot (not shown). FRAP studies of these cells show high paxillin mobility and full recovery of fluorescence within seconds (Figure S5), consistent with other recent reports for GFP-paxillin (von Wichert et al., 2003; Lele et al., 2006). Paxillin binding within FAs is suggested by the 3-fold slower recovery of paxillin fluorescence within bleached FAs compared to bleached cytosol (Figure S5). Blebbistatin treatment decreases cytosolic mobility, suggesting enhanced interactions in the cytosol when FA are inhibited. This highlights once again a relationship between paxillin and cell tension.
Culture results above for (P)-Y31 and (P)-Y118 in paxillin suggested no difference in FA localization and recent results (Pasapera et al., 2010) suggest no more than a ~10% difference in blebbistatin sensitivity, but these phospho-forms might still have differential roles in other adhesion-directed cell functions. Multinucleated myotubes initiate myofibrillogenesis at their adhesions as they exchange nonmuscle myosin II for skeletal muscle myosin II into a striated organization – a hallmark of striated muscle differentiation (Sanger et al., 2002). The degree of muscle myosin striation depended strongly on the ability of the overexpresed GFP-paxillin to be phosphorylated on Y31 (Figure 4A). In initial studies, we used micropatterned multi-layer cultures in which a first layer of cells is grown on collagen-strips for 1–2 days followed by plating of a second layer of myoblasts (Engler et al., 2004b; Griffin et al., 2004b); about 40% of upper control myotubes striate by day 6 in such cultures. In comparison, when Pax+ cells constitute the upper layer, nearly 100% of the green fluorescent Pax+-myotubes appeared striated; this was found for both chicken and mouse wild-type paxillin. We then investigated the effects of inhibiting phosphorylation at different sites on paxillin. While the Y118F mutant behaved similarily to WT, the Y31F mutant did not striate beyond WT and neither did the tetra-mutant Y31/40/118/181F (Figure 4B), which implicates phosphorylation of Y31 in contractility and striation. This result suggests that paxillin, specifically Y31 phospho-paxillin, and muscle tension are part of a feedback loop in which changes in one affects the other.
Figure 4. Paxillin overexpression promotes striation-differentiation and depends on Y31.
(A) Cell cultures immunostained for skeletal muscle myosin show contractile sarcomeric striations in nearly 100% of Pax+ myotubes (green). Most control cells show only stress fibers at day 6, with about 40% exhibiting striations. Similar results were found for chicken and mouse WT paxillin. Paxillin mutant Y118F gives a similar phenotype to WT, but mutant Y31F shows no effect.
(B) Quantitation for 30 cells per expt.; S.D. shown for n ≥ 3 transfections. Scalebars ~10 μm.
Myoseverin has also been shown to drastically affect adhesions and cytoskeleton of myotubes, causing muscle to de-differentiate into mononucleated myoblasts (Musa et al., 2003). Indeed, within 24 hrs and even within hours of drug addition, control myotubes treated with myoseverin began to fission and completely reverted to single cells within 48 hrs (Figure 5). Paxillin immunostaining also appears relatively diffuse by 24 hrs at which point 80% of cells are (non-apoptotic) monomyocytes. This process is reportedly reversible (Rosania et al., 2000), as washing out the drug allowed re-formation of myotubes, albeit roughened and imperfect myotubes. In Pax+ myotubes, structures are better protected from disassembly, and only 20% of Pax+ cells appear mononucleated. Assuming myoseverin’s reported action on microtubules, these results suggest that paxillin delays de-differentiation by maintaining microtubule structure – which we also observe (Figure 5). These de-differentiation results are consistent with a more committed contractile phenotype for Pax+ cells.
Figure 5.
Paxillin overexpression protects against de-differentiation. Phase contrast images of myotubes treated with DMSO solvent show no perturbation, but myoseverin treatment for 24 hrs (and as short as 6 hrs) leads to disassembly of control myotubes and microtubules. Pax+ protects myotubes against this de-differentiation and microtubules against depolymerization at 24 hrs. Scalebars ~20 μm.
The additional paxillin and contractile tension in Pax+ cells could in principle drive stronger adhesion, but direct measurements show that neither adhesion structure nor adhesiveness are affected. Vinculin immunostaining in day 6 myotubes attaching to the rigid ECM-coated glass indeed shows the same statistical distribution of FA area in Pax+ and GFP control myotubes (Figure S6A). The strength of adhesion of individual myotubes was determined by a micropipette aspiration method in which individual myotubes are controllably peeled into a large bore micropipette as the fluid shear stress pulls the cell in (Griffin et al., 2004a). The externally applied tension (not the cell-generated internal tension) can be estimated from the fluid mechanics and is visibly sufficient to break the cell adhesion bonds which limit the rate of peeling along the length of the myotube. Importantly, Pax+ and control cells yield the same cell detachment curve (Figure S6B). A minimum tension T0 (≈ 6 nN/μm) is always needed to initiate cell peeling, but in agreement with past theory and experiment (Griffin et al., 2004a) the peeling velocity then increases logarithmically with the externally applied tension. Paxillin over-expression thus does not alter – directly or indirectly – the adhesion strength of myotubes.
Hypertension of Paxillin-overexpressing C2C12 cells and primary mdx myotubes
Paxillin over-expression has no obvious effects on myoblasts (eg. FA density, spread area, stress fibers), which is consistent with past reports on a vascular smooth muscle cell line (Engler et al., 2004a), but Pax+ could still promote cytoskeletal contractility since myofibrillogenesis starts at the adhesions (Engler et al., 2004b; Griffin et al., 2004b). The contractile tension stresses the adhesions, but our cell peeling measurements reveal a minimum tension T0 that a cell would need to generate in order to detach itself. Given the high concentration of FA vinculin and paxillin at the ends of normal myotubes (Griffin et al., 2004a,b; Griffin et al., 2005) as well as the mdx myotubes shown here (Figure 6A-inset), these cells are attached more strongly at their ends, and so forced detachment relieves a barrier for self-peeling of these tensed cells (Figure 6A, B). One might consider the cell to be a rubberband that is stretched and glued to a benchtop most strongly at the ends of the rubberband but also lightly in the middle. The tension in the rubberband, like actomyosin contractility, is clear, and can drive some amount of slow self-peeling if bonds are weakened, but then the cell tension would relax and no further ‘self-peeling’ would occur – unless one forcibly pulled on the rubberband to quickly peel it as done in our micropipette aspirations above. Note that self-peeling is slow and does not involve micropipette aspiration or any externally imposed tension that drives more rapid peeling.
Figure 6. Hypertension of paxillin-overexpressing and mdx myotubes.
(A) Self-peeling is initiated by detaching the firmly attached end of the myotube at t = 0. Upper insets show cells on micropatterned strips with a typical distribution of FA proteins – here in mdx myotubes. Note the end-concentrated FA’s, which must be disrupted for the cells to peel under their own tension.
(B) Exponential relaxation of control, Pax+, and blebbistatin-treated myotubes for both single layer and cell-on-cell arrangements. The asymptotic relaxation amplitudes are used to obtain the relaxation attributable to myotube contractility (L - Lbleb). Pax+ myotubes are more contractile than controls: 40% moreso for the bottom layer and 60% moreso for the top layer of striated cells (8–10 cells each).
(C) Self-relaxation of 6-day old mdx myotubes (12 cells) suggests contractility between that of C57 and γSG−/− myotubes (Griffin et al., 2005). After subtraction of the blebbistatin results, mdx myotubes relax 16% versus 11% for C57 control cells and ~23% for γSG−/− myotubes.
(D) AFM probing of cell-on-cell arrangements yields the apparent myotube stiffness Eapp, which relates to the structural organization and also the intrinsic stress σ or contractility of the myotubes. Representative force-indentation curves are shown for control (CTL), Pax+ and blebbistatin treated myotubes.
(E) Normal myotubes have an elasticity of Eapp = 12 kPa, but Pax+ myotubes are significantly stiffer at 22 kPa. Since blebbistatin suppresses contractility, EBlebb = 3 kPa is subtracted from the two other cell systems to yield an intrinsic tension of 9 kPa for control myotubes and 19 kPa for Pax+ myotubes.
(F) EDL muscle was dissected from normal and mdx mice and also probed in buffer using AFM. mdx muscle have an Eapp of 18 kPa and appear 1.5 times stiffer than C57 muscle with a mean stiffness of 12 kPa. The relative frequency is normalized to 100 contacts on at least 10 myotubes.
As cells detach and self-peel, they relieve a fraction of their internal tension and exponentially approach a new equilibrium – similar to the blebbistatin-sensitive tetanic force relaxation at constant length (Figure S4). Indeed, blebbistatin-treated cells do not self-peel significantly when compared to controls, but Pax+ cells detach to a greater extent than controls. The amplitude of final relaxation (L – LBlebb) thus provides an indication of contractility or cell tension since blebbistatin treatment largely eliminates myosin-II based contractility (both non-muscle and skeletal muscle myosin II). Further estimations of cell tension from self-peeling are difficult although the initial cell tension should exceed the T0 (≈ 6 nN/μm) measured above. Nonetheless, the additional relaxation (and additional cell tension) in Pax+ cells is most prominent (+60%, Figure 6C) with the striated myotubes in the upper layer (per Figure 4’s skeletal muscle myosin II) compared to the lower layer of stress fiber rich cells (+40%). Paxillin overexpression in C2C12 cells thus drives striation (see Y31 requirement above) and thereby generates a more contractile phenotype.
Primary myotubes derived from mdx mouse myoblasts and grown in culture for one week are found to relax more than C57-derived control cells in the self-peeling measurements (Figure 6C). As shown here only for comparison, we reported similar results for γSG−/− myotubes (Griffin et al., 2005). The results thus suggest a hypertrophic or hypertensive phenotype for both dystrophic cell types here, although the γSG−/− derived myotubes seem to relax more and thus perhaps possess even greater hypertension. Indeed, a significant fraction of γSG−/− cells in culture – but not mdx cells – spontaneously relax and appear bulbous in the middle (Figure S7), suggesting that the needed balance between adhesion and contractility is shifted more to contractility.
Another physical measurement made on the various cell types is based on the general relation between cell elasticity and cell tension. In detailed studies by others of smooth muscle cells adhering to deformable gel substrates (Wang et al., 2002) (and expressing smooth muscle myosin-II), cell prestress σdeduced from traction stresses in the gels is found proportional to cell elasticity E as measured by a micro-bead method. Such results imply σ≅ a(E - Eo), in which Eo is the tension-free cell elasticity (positive) and a is a proportionality factor of order ~1–10. These two parameters (a, Eo) likely depend on cell-type and details of elasticity measurements. In order to estimate σ here with the skeletal myotubes we assume a ≈ 1 and that Eo = EBlebb. We measured cell elasticity using an atomic force microscope (AFM) in which the force required to indent a cell allows determination of E (Figure 6D) (Rotsch et al., 1999). Representative force-indentation curves for control (CTL), Pax+, and blebbistatin-treated cells indicate greater tension in CTL cells and Pax+ cells compared to the drug-relaxed cells (σ ≈ 0). Averaging such data over multiple locations and multiple myotubes we obtain a mean E = 12 kPa for the CTL myotubes, in agreement with past results (Collinsworth et al., 2002), but we obtain 22 kPa for the Pax+ myotube. We establish a ‘stress-free’ state of Eo = EBlebb ≈ 3–4 kPa for blebbistatin-treated myotube (Figure 6E, acute treatment of 1–2 hrs).
With a baseline Eo ≈ 3–4 kPa established, the various measures of cell elasticity yield an intrinsic cell tension σCTL ≈ 9 kPa for control cells and σPax+ ≈ 19 kPa for Pax+ myotubes. This +110% increase in apparent tension with paxillin overexpression is roughly consistent with the +60% increase in the apparent tension that drives self-peeling (Figure 6B). With primary cells likewise, elasticity measurements of fresh muscle excised from C57 controls and the paxillin-upregulated mdx mice (Figure 6F) show the latter is similarly hypertensive and also close to matching the relaxation differences (Figure 6C). A simple analysis of these biophysical results proves remarkably consistent with signaling changes: since the contractile prestress σ cannot exceed the critical peeling tension T0 without causing spontaneous cell detachment, one can estimate distinct ‘attachment length’ scales for the different cell types. For Pax+ myotubes, T0/σPax+ ≤ 0.3 μm, and for CTL myotubes, T0/σCTL ≤ 0.67 μm. The 2.3-fold smaller length scale for Pax+ phenotypes certainly reflects the higher contractility and also approximates the higher contractile signaling of Figure 2. Therefore, the various physical measurements here quantify in a very consistent way the hypertensive phenotype of paxillin over-expressing muscle cell lines as well as dystrophic muscle cells.
Prednisolone acts as a relaxant in culture
The glucocorticoid prednisolone (PDN) is currently the most widely used palliative treatment for muscular dystrophy. Mechanisms of PDN action are not well understood, and there are dose-limiting side effects (Lo Cascio et al., 1995), but PDN helps maintain muscle strength and muscle function for a few years (Fenichel et al., 1991). Anti-inflammatory effects might result from down-regulation of CAMs (cell adhesion molecules) on leukocytes and vascular cells (Wehling-Henricks et al., 2004), while further benefits possibly include enhanced myogenesis (Passaquin et al., 1993), stabilization of muscle fiber membranes (Jacobs et al., 1996), reduced necrosis (Takagi et al., 1998) or decrease in intracellular calcium levels (Vandebrouck et al., 1999). Gene expression profiling of PDN-treated mdx mice after 1 and 6 wks revealed over-expression of genes relating to metabolism and proteolysis as well as differential expression of genes relating to calcium metabolism (Fisher et al., 2005), which of course could impact contractility.
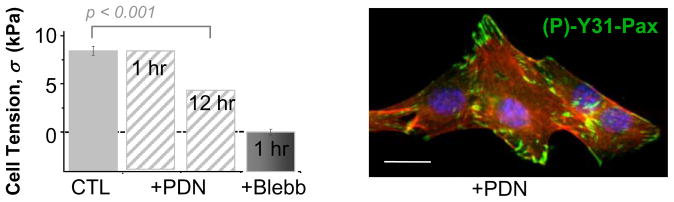
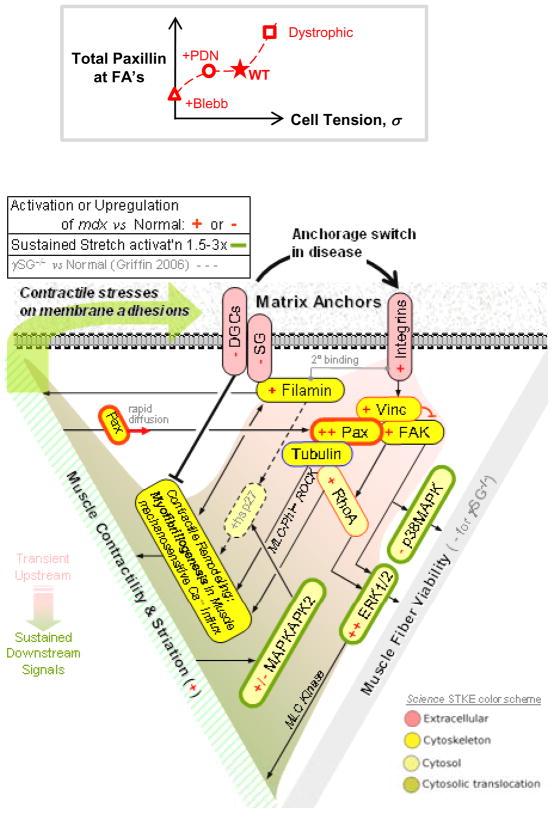
PDN effects on cultured myotubes are physically detectable by the methods here: AFM probing after a 12 hr treatment at the typical clinical dose (1 μM PDN) reveals statistically softer cells with Eapp ≈ 8 kPa corresponding to a cell tension of ~5 kPa (Figure 7). This is equivalent to relaxing ~50% of myosins when calibrated against full myosin inhibition by blebbistatin. However, a key difference in PDN activity is the fact that a 1 hr treatment has no significant relaxant effect whereas blebbistatin shows full effect well within 1 hr (also see Figure S4), which is more than sufficient time for inhibition of myosin-II’s ATPase. On the other hand, PDN had no clear effect on paxillin localization to adhesions or levels of (P)-Y31. All of the results collectively suggest a non-linear, likely multivariate, relationship between total paxillin in the FA’s and cell tension (Figure 8A, plot) with ~50% decreases in cell tension having no effect on paxillin whereas larger increases (~100% in Figure 8) produce an increase in cell contractility.
Figure 7. Prednisolone is a mild relaxant but Paxillin is unaffected.
Prednisolone (PDN, 1 μM) treatment for 12 hr, softens C2C12 myotubes to ~50% of that achieved with Blebbistatin. AFM measurements were converted to cell tensions per Figure 8D. Immunofluorescence and immunoblotting show that Y31-Paxillin is unaffected by PDN. Scalebar ~10 μm.
Figure 8. Non-linearities in paxillin vs tension and a Signaling Circuit for Anchorage-dependent Contractility and Viability.
(A) Non-linear relationship between total paxillin and cell tension, summarizing the various findings.
(B) The underlying Signaling circuit for anchorage dependent contractility and viability. Dystroglycan-related defects in the muscular dystrophies leads to upregulation of integrins and associated proteins. Upregulation of FA proteins such as paxillin, vinculin and FAK helps to preserve muscle integrity by maintaining a balance between adhesion and contractility. A role for MAPK proteins in sustained stretch-mediated mechanosignaling emerges as a common pathway in both dystrophies studied.
DISCUSSION
Shifting the cell attachment system from dystroglycan to focal adhesions might well be ‘mechanically’ neutral for the cells, but downstream remodeling and signaling derived from these different complexes appears significantly perturbed. Immunoblots and (P)-protein screens here (Figures 1B, 2) amplify and extend previously published mdx muscle transcript profiles (Table S1) which indicate at most a slight paxillin increase (+14%). Dystroglycan and γ-sarcoglycan are also well-known to be lacking at the protein level in mdx mice (Table S1) even though decreases in mRNA are similar in magnitude (about −15%). Paxillin is further shown here to signal and promote – in long term culture – myofibril formation and cytoskeletal tension which is synonymous with cell tension, contractility, and ‘prestress’ (Figure 4, 6). Surprisingly, increased paxillin does not lead to more abundant FA’s or stronger adhesion (Figure S6). In the short term, paxillin diffuses in and out of FA in seconds (Figures S4), and its localization closely couples to sustained activation of myosin-II (Figure 3) and perhaps also to ERK (Figure 2), ultimately promoting viability. It should be noted, however, that the phosphorylation states of the MAPK components appear more responsive than paxillin to 20 min of imposed tension (Figure S2), suggesting perhaps that phosphopaxillin contributes to long-term signaling – especially Y31 in the contractile differentiation (Figure 4) – rather than short-term signaling. Consistent with a hypertensive phenotype, the mdx transcriptome (Table S1) further indicates upregulation of (i) nonmuscle myosin-IIA as well as γ-actin, (ii) MAPK (ERK1/2), and of course (iii) integrins including α7. Such a profile provides a basis for hypertrophy that is usually attributed to injury-induced regeneration.
While (P)-Y31-paxillin seems to promote striation and contractility, non-muscle myosin IIs almost certainly contribute to cell tension and contractility as well as myofibril formation. RhoA is also found elevated in dystrophic muscle (not shown), but RhoA is not known to activate skeletal muscle myosin, and so it seems more likely that in the diseased muscle, skeletal muscle myosin is activated by elevated resting calcium (Law et al., 1994) as is typical of hypertensive states. Membrane stability/permeation as well as anchorage to basement membrane are expected to couple to contractility: an ‘A-C-V’ triangle of Anchorage-Contractility-Viability is illustrated in Figure 8. Using a signaling circuit appropriate to muscle, emphasis is first on the switch toward α7β1 integrins (Hodges et al., 1997; Allikian et al., 2004; Burkin et al., 2005) and integrin-based ECM signaling and also C-type filamin (Thompson et al., 2000) that occurs in the muscular dystrophies. Upregulation of the nexus-(Paxillin, Vinculin and FAK) drives cytoskeletal remodeling, which includes upregulation of γ-actin (Hanft et al., 2006) as well as vinculin and talin (Law et al., 1994) beyond what transcription profiles might suggest (Table S1). Increased filamins (Thompson et al., 2000) are interesting because they help sustain the tension in a cell and because they are mechanotransducers (Kasza et al., 2009). Hyperactivation of ERK1/2 and MAPKAPK2 in screens of both mdx and γSG−/− indicate downstream perturbations of common signaling pathways in muscular dystrophy. While ERK1/2 and MAPKAPK2 contribute to the contractile loop, ERK1/2 and FAK likely modulate the apoptosis/survival pathway. The differential regulation of FA proteins such as (P)-paxillin might also regulate the extent of adhesive viability response to prestress. As evidence, the hypertensive γSG−/− cells lack much (P)-paxillin and they also apoptose faster (Griffin et al., 2005).
Additional structures are coupled to signaling. During myofibrillogenesis, sarcomeric organization requires a stable microtubule network for mechanical support (Palazzo et al., 2004; Pizon et al., 2005). Since depletion of paxillin at FA inhibits force development without having any effect on myosin activity (Tang et al., 2002), paxillin might also be involved in stabilizing structural proteins like tubulin and thereby provide myofibrillar stability. In muscular dystrophies, microtubule depolymerization by LIMK1 and Rho GTPases is misregulated (Gorovoy et al., 2005; Hu et al., 2006). A major function of paxillin in vivo also seems to be to regulate the p190RhoGAP/p120-Ras-GAP regulatory pathway as well as tubulin polymerization (van Horck et al., 2001; Tsubouchi et al., 2002; Nishiyama et al., 2004); hence, it could be that rapid turnover of paxillin at FA ultimately provides adhesion stability. Although, we observe no significant changes in adhesion, our in vitro measurements on C2C12 cells were performed on rigid glass substrates that promote strong adhesions relative to soft matrices (Engler et al., 2004b). The inability to detect adhesion differences here suggests that adhesion responses may be saturated, and subtle changes in adhesion due to paxillin overexpression are completely over-ridden by the rigid substrate (Pelham, 1997).
Our biophysical measurements of self-peeling cells as well as our AFM measurements of cell tension provide novel evidence of a hypertensive disease state. Dystrophic cells with already weakened sarcolemma are more susceptible to injury from cell-generated forces, with hypertension driven apoptosis evident in γSG−/− cells. Blebbistatin treatment, which relaxes the cell tension, also downregulates ERK activation (Figure S4), thereby highlighting the tension-linked viability signaling in these cells. Elevated levels of spontaneous contractions in mdx mice can be brought down to normal levels in the presence of the muscle relaxant relaxin through upregulation of endogenous nitric oxide (Baccari et al., 2005). Here, the first-line gluococorticoid Prednisolone that is used to preserve muscle mass is also shown to relax muscle – perhaps through calcium regulation (Fisher et al., 2005) – and so part of its utility in treating dystrophic patients might lie in relaxing the unappreciated hypertensive phenotype of dystrophic cells. PDN also helps to show that the basic relationship between cell tension and total paxillin at adhesions is likely to be non-linear.
Material and Methods
Tissue lysates and Western Blot Analyses
TA muscles were isolated within 5 min of anesthetizing 8–10 week mdx, γSG−/−, and normal C57 mice, and the isolated muscles were either snap-frozen immediately in liquid nitrogen or else stretched by 10% for 20 min in Ca++ Ringer’s per (Griffin et al., 2005). Muscle lysates were made by grinding the tissues to powder, suspending in lysis buffer, and collecting the supernatant after centrifugation at 13,000 rpm at 4 °C. Lysates were stored at −70 °C, and western blotting was performed using the standard protocol with color developed by BNIP substrates. Monoclonal antibodies for paxillin were obtained for total protein (Research Diagnostics, NJ), while polyclonal antibodies were used for (P)-paxillin (BioSource). The phospho-protein screens of lysates were generated and quantitated as described elsewhere (Griffin et al., 2005).
Cell Cultures, Drug Treatments, and Transfections
In preparation for the experiments, coverslips or fully patterned slides were coated with ECM per (Griffin et al., 2004a) and seeded with roughly 104 cells. Primary myoblasts were harvested from 1-day-old mice. After harvesting, ~105 cells were plated onto the patterned glass coverslips. Cultures were maintained in DMEM supplemented with 22% horse serum (GIBCO), 8% embryo extract (US Biological, MA), 1% L-glutamine (GIBCO) and 1% penicillin/streptomycin at 10,000 units/ml and 10,000 μg/ml, respectively. Media was changed every other day. Skeletal muscle derived C2C12 mouse cells are maintained in 75 cm2 cell culture flasks (Corning, NY) in 10 mL DMEM (GIBCO Laboratories, Grand Island, NY) supplemented with 20 % fetal bovine serum (GIBCO), 0.5 % chick embryo extract (GIBCO), and 0.5 % penicillin/streptomycin (10,000 units/mL and 10,000 μg/mL, respectively) (GIBCO). Cells are passaged every 2–3 days by detaching with trypsin-EDTA (GIBCO). For differentiation, media is changed to differentiation media (DM) (DMEM supplemented with 10% horse serum (GIBCO) plus 1% penicillin/streptomycin), and replaced every other day. For cell-on-cell studies, a first layer of cells was established on the patterns, and one day later a second layer of C2C12 cells was plated and the media was switched within 24 hr to differentiation media (Griffin et al., 2004a).
Inhibition of myosin by 50 μM blebbistatin (Sigma) or 50 μM BTS (Sigma) used DMSO solvent controls in parallel. For studies with myoseverin (MS; Calbiochem), differentiated myotubes (14 days) were treated at 20 μM in DM. For studying the effect of prednisolone, one week old myotubes were incubated with 1 μM prednisolone for 12 hours before being probed with the AFM. Transfection with GFP-paxillin in C2C12 cells was performed using Lipofectamine-2000 following standard protocols.
Immunofluorescence, Microscopy, and FRAP
Tissue sections of the TA muscle from C57 and mdx mice were immunostained using paxillin antibody. Cultured cells were rinsed with phosphate buffered saline (PBS), fixed with 10% formaldehyde solution for 3 minutes, permeabilized with 0.5% Triton X100, and blocked in 5% BSA for 1 h at 37° C. Cells were incubated overnight at 4 °C in one of the following primary antibody solutions in PBS at a dilution of 1:100: mouse anti-myosin (Zymed), mouse anti-tubulin (BioSource), mouse anti-vinculin (Sigma). Secondary FITC anti-mouse IgG (Molecular Probes) was incubated at 1:300 for 1 h at 37 °C. Staining with secondary antibodies alone allowed for background detection. F-actin staining was perfomed with 60 μg/ml TRITC-phalloidin (Sigma), and cell nuclei were labeled with 1:100 Hoechst 33342 (Molecular Probes). Samples were mounted onto slides using Gel-Mount (Biomeda).
Microscopy was done with either a Nikon TE300 inverted microscope or Olympus IX71 microscope, using either a 40× (NA 0.45) or a 60× (NA 1.45) oil objective. Images were recorded under constant, calibrated settings with a Cascade CCD camera (Photometrics, Tucson, AZ), and fluorescence intensity was measured using ImageJ (NIH). For FRAP of GFP-paxillin, a nanosecond pulse laser (MicroPoint Laser, Photonic-Instruments, St. Charles, IL) exciting Coumarin-440 was done with a single pulse so as to limit bleaching of the mobile pool. Recovery curves, normalized to total signal to account for photobleaching, were computed for a circular area of maximum bleach; dbleach ~ 1μm.
Single cell peeling, Self-Relaxation, and AFM measures of Cell tension
Myotubes were forcibly peeled from the substrate by a micropipette peeling technique described elsewhere (Griffin et al., 2004a). Briefly, a large-bore micropipette was attached to a syringe pump and the flow rate was set to 10 μl/min. After one end of the cell was mechanically detached, the syringe pump was used to initiate a flow and cells were peeled from the substrate using a motorized stage. Because of the large size of the micropipette compared with the dimensions of the cell, the cell peeling velocity (Vpeel) is driven by the peeling tension (Tpeel), which is simply the integral of the shear stress over the length of cell inside the pipette.
For self-relaxation experiments per (Griffin et al., 2004b), briefly, one end of a patterned cell was mechanically detached with a glass micropipette incubated in BSA. As the cell relaxed, the stage was repositioned in order to image the cell.
For AFM measures of cell tension, samples were placed on the stage of an Asylum 1-D AFM (Asylum Research, Santa Barbara) and indented perpendicular to the myotube axis by a pyramid-tipped probe (Veeco) The spring constant was determined with the thermal calibration method and was within 10% of the nominal value. Force-indentation profiles were obtained for multiple myotubes (cultured or freshly isolated) at multiple locations with a total count of 100 measurements. Each profile was fit with a modified Hertz model for a cone (Rotsch et al., 1999) to determine their elastic modulus. Histograms of the determined elastic moduli and the intrinsic tension were plotted, and their mean magnitudes reported.
Supplementary Material
Acknowledgments
Support from the NIH, MDA, and NSF is very gratefully acknowledged. Mouse GFP-Paxillin was generously provided Dr. J.F. Mushinski (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allikian MJ, Hack AA, Mewborn S, Mayer U, McNally EM. Genetic compensation for sarcoglycan loss by integrin {alpha}7{beta}1 in muscle. J Cell Sci. 2004;117:3821–3830. doi: 10.1242/jcs.01234. [DOI] [PubMed] [Google Scholar]
- Baccari MC, Calamai F, Chiappini L, Vannucchi MG, Bani D. Relaxin restores altered ileal spontaneous contractions in dystrophic (mdx) mice. Ann N Y Acad Sci. 2005;1041:308–310. doi: 10.1196/annals.1282.047. [DOI] [PubMed] [Google Scholar]
- Bakay MZP, Chen J, Hoffman EP. A web-accessible complete transcriptome of normal human and DMD muscle. Neuromuscul Disord. 2002;Suppl 1:S125–41. doi: 10.1016/s0960-8966(02)00093-7. [DOI] [PubMed] [Google Scholar]
- Brown MC, Turner CE. Paxillin: Adapting to Change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. Transgenic Expression of {alpha}7{beta}1 Integrin Maintains Muscle Integrity, Increases Regenerative Capacity, Promotes Hypertrophy, and Reduces Cardiomyopathy in Dystrophic Mice. Am J Pathol. 2005;166:253–263. doi: 10.1016/s0002-9440(10)62249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Charlesworth P, Komiyama NH, Grant SG. Homozygous mutation of focal adhesion kinase in embryonic stem cell derived neurons: normal electrophysiological and morphological properties in vitro. BMC Neurosci. 2006;7:47–57. doi: 10.1186/1471-2202-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–1471. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Collinsworth AM, Zhang S, Kraus WE, Truskey GA. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Physiol Cell Physiol. 2002;283:C1219–1227. doi: 10.1152/ajpcell.00502.2001. [DOI] [PubMed] [Google Scholar]
- Cuvelier SL, Paul S, Shariat N, Colarusso P, Patel KD. Eosinophil adhesion under flow conditions activates mechanosensitive signaling pathways in human endothelial cells. J Exp Med. 2005;202:865–876. doi: 10.1084/jem.20041315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Tosaki A, Bagchi D, Maulik N, Das DK. Potentiation of a survival signal in the ischemic heart by resveratrol through p38 mitogen-activated protein kinase/mitogen- and stress-activated protein kinase 1/cAMP response element-binding protein signaling. J Pharmacol Exp Ther. 2006;317:980–988. doi: 10.1124/jpet.105.095133. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004a;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004b;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenichel GM, Florence JM, Pestronk A, Mendell JR, Moxley RT, 3rd, Griggs RC, Brooke MH, Miller JP, Robison J, King Wea. Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology. 1991;41:1874–1877. doi: 10.1212/wnl.41.12.1874. [DOI] [PubMed] [Google Scholar]
- Fisher I, Abraham D, Bouri K, Hoffman EP, Muntoni F, Morgan J. Prednisolone-induced changes in dystrophic skeletal muscle. Faseb J. 2005;19:834–836. doi: 10.1096/fj.04-2511fje. [DOI] [PubMed] [Google Scholar]
- Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW. Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol. 1999;277:152–162. doi: 10.1152/ajpcell.1999.277.1.C152. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- Gorovoy M, Niu J, Bernard O, Profirovic J, Minshall R, Neamu R, Voyno-Yasenetskaya T. LIM kinase 1 coordinates microtubule stability and actin polymerization in human endothelial cells. J Biol Chem. 2005;280:26533–26542. doi: 10.1074/jbc.M502921200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MA, Engler AJ, Barber TA, Healy KE, Sweeney HL, Discher DE. Patterning, Prestress, and Peeling Dynamics of Myocytes. Biophys J. 2004a;86:1209–1222. doi: 10.1016/S0006-3495(04)74195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MA, Feng H, Tewari M, Acosta P, Kawana M, Sweeney HL, Discher DE. {gamma}-Sarcoglycan deficiency increases cell contractility, apoptosis and MAPK pathway activation but does not affect adhesion. J Cell Sci. 2005;118:1405–1416. doi: 10.1242/jcs.01717. [DOI] [PubMed] [Google Scholar]
- Griffin MA, Sen S, Sweeney HL, Discher DE. Adhesion-contractile balance in myocyte differentiation. J Cell Sci. 2004b;117:5855–5863. doi: 10.1242/jcs.01496. [DOI] [PubMed] [Google Scholar]
- Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. The Adaptor Protein Paxillin Is Essential for Normal Development in the Mouse and Is a Critical Transducer of Fibronectin Signaling. Mol Cell Biol. 2002;22:901–915. doi: 10.1128/MCB.22.3.901-915.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft LM, Rybakova IN, Patel JR, Rafael-Fortney JA, Ervasti JM. Cytoplasmic gamma-actin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc Natl Acad Sci U S A. 2006;103:5385–5390. doi: 10.1073/pnas.0600980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros L, Rodriguez-Fernandez JL, Brown MC, Alonso-Lebrero JL, Cabanas C, Sanchez-Madrid F, Longo N, Turner CE, Sanchez-Mateos P. Paxillin Localizes to the Lymphocyte Microtubule Organizing Center and Associates with the Microtubule Cytoskeleton. J Biol Chem. 2000;275:26436–26440. doi: 10.1074/jbc.M003970200. [DOI] [PubMed] [Google Scholar]
- Hirakawa M, Oike M, Karashima Y, Ito Y. Sequential activation of RhoA and FAK/paxillin leads to ATP release and actin reorganization in human endothelium. J Physiol (Lond) 2004;558:479–488. doi: 10.1113/jphysiol.2004.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110:2873–2881. doi: 10.1242/jcs.110.22.2873. [DOI] [PubMed] [Google Scholar]
- Hu YL, Haga JH, Miao H, Wang Y, Li YS, Chien S. Roles of microfilaments and microtubules in paxillin dynamics. Biochem Biophys Res Commun. 2006;348:1463–1471. doi: 10.1016/j.bbrc.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Jacobs SC, Bootsma AL, Willems PW, Bar PR, Wokke JH. Prednisone can protect against exercise-induced muscle damage. J Neurol. 1996;243:410–416. doi: 10.1007/BF00869001. [DOI] [PubMed] [Google Scholar]
- Kasza KE, Nakamura F, Hu S, Kollmannsberger P, Bonakdar N, Fabry B, Stossel TP, Wang N, Weitz DA. Filamin A Is Essential for Active Cell Stiffening but not Passive Stiffening under External Force. Biobphys J. 2009;96:4326–4335. doi: 10.1016/j.bpj.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Khandelwal N, Malya R, Reid MB, Boriek AM. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. Faseb J. 2004;18:102–113. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- Law DJ, Allen DL, Tidball JG. Talin, vinculin and DRP (utrophin) concentrations are increased at mdx myotendinous junctions following onset of necrosis. J Cell Sci. 1994;107(Pt 6):1477–1483. doi: 10.1242/jcs.107.6.1477. [DOI] [PubMed] [Google Scholar]
- Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- Lim LE, Campbell KP. The sarcoglycan complex in limb-girdle muscular dystrophy. Curr Opin Neurol. 1998;11:443–452. doi: 10.1097/00019052-199810000-00006. [DOI] [PubMed] [Google Scholar]
- Lin CL, Zhang ZX, Xu YJ, Ni W, Chen SX. Focal adhesion kinase antisense oligodeoxynucleotides inhibit human pulmonary artery smooth muscle cells proliferation and promote human pulmonary artery smooth muscle cells apoptosis. Chin Med J (Engl) 2005;118(1):20–26. [PubMed] [Google Scholar]
- Linari MBR, Pellegrino MA, Reconditi M, Reggiani C, Lombardi The mechanism of the force response to stretch in human skinned muscle fibres with different myosin isoforms. J Physiol. 2004;554(Pt 2):335–352. doi: 10.1113/jphysiol.2003.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Cascio V, Kanis JA, Beneton MN, Bertoldo F, Adami S, Poggi G, Zanolin ME. Acute effects of deflazacort and prednisone on rates of mineralization and bone formation. Calcif Tissue Int. 1995;56:109–112. doi: 10.1007/BF00296340. [DOI] [PubMed] [Google Scholar]
- Lunn JA, Rozengurt E. Hyperosmotic Stress Induces Rapid Focal Adhesion Kinase Phosphorylation at Tyrosines 397 and 577: Role of Src family kinases and Rho family GTPases. J Biol Chem. 2004;279:45266–45278. doi: 10.1074/jbc.M314132200. [DOI] [PubMed] [Google Scholar]
- McKenna NM, Johnson CS, Wang YL. Formation and alignment of Z lines in living chick myotubes microinjected with rhodamine-labeled alpha-actinin. J Cell Biol. 1986;103:2163–2171. doi: 10.1083/jcb.103.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez J, Turner C, Avraham H, Steinberg SF, Schaefer E, Sussman MA. Cardiomyocyte apoptosis triggered by RAFTK/pyk2 via Src kinase is antagonized by paxillin. J Biol Chem. 2004;279:53516–53523. doi: 10.1074/jbc.M408475200. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Iwamatsu A, Aki T, Kimura M, Nakamura K, Nao T, Okusa T, Matsuzaki M, Yoshida K, Kobayashi S. ERK1/2 regulates intracellular ATP levels through alpha-enolase expression in cardiomyocytes exposed to ischemic hypoxia and reoxygenation. J Biol Chem. 2004;279:50120–50131. doi: 10.1074/jbc.M402299200. [DOI] [PubMed] [Google Scholar]
- Most P, Boerries M, Eicher C, Schweda C, Ehlermann P, Pleger ST, Loeffler E, Koch WJ, Katus HA, Schoenenberger CA, Remppis A. Extracellular S100A1 protein inhibits apoptosis in ventricular cardiomyocytes via activation of the extracellular signal-regulated protein kinase 1/2 (ERK1/2) J Biol Chem. 2003;278:48404–48412. doi: 10.1074/jbc.M308587200. [DOI] [PubMed] [Google Scholar]
- Musa H, Orton C, Morrison EE, Peckham M. Microtubule assembly in cultured myoblasts and myotubes following nocodazole induced microtubule depolymerisation. J Muscle Res Cell Motil. 2003;24:301–308. [PMC free article] [PubMed] [Google Scholar]
- Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- Nishiyama T, Kii I, Kudo A. Inactivation of Rho/ROCK signaling is crucial for the nuclear accumulation of FKHR and myoblast fusion. J Biol Chem. 2004;279:47311–47319. doi: 10.1074/jbc.M403546200. [DOI] [PubMed] [Google Scholar]
- Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- Palfi A, Toth A, Kulcsar G, Hanto K, Deres P, Bartha E, Halmosi R, Szabados E, Czopf L, Kalai T, Hideg K, Sumegi B, Toth K. The role of Akt and mitogen-activated protein kinase systems in the protective effect of poly(ADP-ribose) polymerase inhibition in Langendorff perfused and in isoproterenol-damaged rat hearts. J Pharmacol Exp Ther. 2005;315:273–282. doi: 10.1124/jpet.105.088336. [DOI] [PubMed] [Google Scholar]
- Panetti TS. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front Biosci review. 2002;7:143–150. doi: 10.2741/A771. [DOI] [PubMed] [Google Scholar]
- Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188:877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaquin AC, Metzinger L, Leger JJ, Warter JM, Poindron P. Prednisolone enhances myogenesis and dystrophin-related protein in skeletal muscle cell cultures from mdx mouse. J Neurosci Res. 1993;35:363–372. doi: 10.1002/jnr.490350403. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, Chen J, Chien K, Gu H, Guan JL. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V, Gerbal F, Diaz CC, Karsenti E. Microtubule-dependent transport and organization of sarcomeric myosin during skeletal muscle differentiation. Embo J. 2005;24:3781–3792. doi: 10.1038/sj.emboj.7600842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosania GR, Chang YT, Perez O, Sutherlin D, Dong H, Lockhart DJ, Schultz PG. Myoseverin, a microtubule-binding molecule with novel cellular effects. 2000;18:304. doi: 10.1038/73753. [DOI] [PubMed] [Google Scholar]
- Rotsch C, Jacobson K, Radmacher M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. PNAS. 1999;96:921–926. doi: 10.1073/pnas.96.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JW, Chowrashi P, Shaner NC, Spalthoff S, Wang J, Freeman NL, Sanger JM. Myofibrillogenesis in skeletal muscle cells. Clin Orthop Relat Res. 2002:S153–162. doi: 10.1097/00003086-200210001-00018. [DOI] [PubMed] [Google Scholar]
- Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL. Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochim Biophys Acta. 2003;1643:65–73. doi: 10.1016/j.bbamcr.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Shemesh T, Geiger B, Bershadsky AD, Kozlov MM. Focal adhesions as mechanosensors: a physical mechanism. Proc Natl Acad Sci U S A. 2005;102:12383–12388. doi: 10.1073/pnas.0500254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M, Naruse K, Sai S, Yamada T, Kawakami K, Inoue M, Murase K, Miyazu M. Mechanotransduction and intracellular signaling mechanisms of stretch-induced remodeling in endothelial cells. Heart Vessels. 1997;Suppl 12:191–193. [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting Temporal and Spatial Control of Cytokinesis with a Myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Straub V, Campbell KP. Muscular dystrophies and the dystrophin-glycoprotein complex. Curr Opin Neurol. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165:371–381. doi: 10.1083/jcb.200308011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Watanabe T, Kojima S, Endo Y. Effect of long-term administration of prednisolone on serum creatine kinase and muscle pathology of mdx mouse. Rinsho Shinkeigaku. 1998;38:724–728. [PubMed] [Google Scholar]
- Tang DD, Wu MF, Opazo Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol. 2002;542:501–513. doi: 10.1113/jphysiol.2002.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HGW, McNally EM, Watkins S, Kunkel LM. Filamin 2 (FLN2): A muscle-specific sarcoglycan interacting protein. J Cell Biol. 2000;148:115–126. doi: 10.1083/jcb.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi A, Sakakura J, Yagi R, Mazaki Y, Schaefer E, Yano H, Sabe H. Localized suppression of RhoA activity by Tyr31/118-phosphorylated paxillin in cell adhesion and migration. J Cell Biol. 2002;159:673–683. doi: 10.1083/jcb.200202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE. Paxillin interactions. J Cell Sci. 2000;113:4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- van Horck FP, Ahmadian MR, Haeusler LC, Moolenaar WH, Kranenburg O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J Biol Chem. 2001;276:4948–4956. doi: 10.1074/jbc.M003839200. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Imbert N, Duport G, Cognard C, Raymond G. The effect of methylprednisolone on intracellular calcium of normal and dystrophic human skeletal muscle cells. Neurosci Lett. 1999;269:110–114. doi: 10.1016/s0304-3940(99)00418-8. [DOI] [PubMed] [Google Scholar]
- Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. Embo J. 2003;22:5023–5035. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282:C606–616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- Wehling-Henricks M, Lee JJ, Tidball JG. Prednisolone decreases cellular adhesion molecules required for inflammatory cell infiltration in dystrophin-deficient skeletal muscle. Neuromuscul Disord. 2004;14:483–490. doi: 10.1016/j.nmd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Wei H, Campbell W, Vander Heide RS. Heat shock-induced cardioprotection activates cytoskeletal-based cell survival pathways. Am J Physiol Heart Circ Physiol. 2006;291:H638–647. doi: 10.1152/ajpheart.00144.2006. [DOI] [PubMed] [Google Scholar]
- Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- Yano Y, Geibel J, Sumpio BE. Tyrosine phosphorylation of pp125FAK and paxillin in aortic endothelial cells induced by mechanical strain. Am J Physiol. 1996;271:C635–649. doi: 10.1152/ajpcell.1996.271.2.C635. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Pan Y, Hanada H, Iwata Y, Shigekawa M. Bidirectional signaling between sarcoglycans and the integrin adhesion system in cultured L6 myocytes. J Biol Chem. 1998;273:1583–1590. doi: 10.1074/jbc.273.3.1583. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Milo R, Kam Z, Geiger B. A paxillin tyrosine phosphorylation switch regulates the assembly and form of cell-matrix adhesions. J Cell Sci. 2007;120:137–148. doi: 10.1242/jcs.03314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.