Abstract
Axonal degeneration causes morbidity in many neurological conditions, including stroke, neurotrauma and multiple sclerosis. Given the limited ability of CNS neurons to regenerate, combined with the observation that axonal damage causes clinical disability, has spurred efforts to investigate the mechanisms of axonal degeneration. Ca influx from outside the axon is a key mediator of injury. More recently substantial pools of intra-axonal Ca sequestered in “axoplasmic reticulum” have been reported. These Ca stores are under control of multimolecular “nanocomplexes” located along the internode under the myelin. Overactivation of these complexes during disease may lead to lethal release of Ca from intra-axonal stores. Rich receptor pharmacology offers tantalizing therapeutic options targeting these nanocomplexes in the many diseases where axonal degeneration is prominent.
Keywords: white matter injury, axonal degeneration, axon, glutamate receptor, Ca stores, myelin
Introduction
Myelinated axons, critical for reliable transmission of signal within the central and peripheral nervous systems, exhibit remarkably distincitve specialized regions. At the nodes of Ranvier, high densities of sodium (Na) channels conduct inward depolarizing currents, whereas internodal/juxtaparanodal potassium (K) channels maintain electrical stability and polarization. Myelin, produced and maintained by Schwann cells in the PNS (peripheral nervous system) and oligodendrocytes in the central nervous system (CNS), insulates 99% of the surface of axons, providing a low-capacitance, high-resistance protective covering. Together, this unique and highly specialized structure supports rapid and efficient saltatory impulse propagation [1]. However, the need for a continuous energy supply to maintain appropriate ion concentrations to support uninterrupted action potential generation places central fibers in a potentially vulnerable state. Thus, when energy demand exceeds supply, ionic dysregulation within the axon occurs causing an influx in Na and Ca and an efflux of K. Under injury conditions, such as anoxia/ischemia or trauma, conduction failure ensues as ion gradients cannot be re-established for proper action potential generation. Given the key importance of axons in the nervous system, it is not surprising that damage to myelinated fibers results in clinical disability, which is often severe. This article will review current knowledge of the molecular mechanisms of axonal degeneration, with specific emphasis on the axon cylinder per se, and the recently discovered internodal nanocomplexes containing glutamate receptors and other signalling molecules whose arrangement resembles the post-synaptic membrane of conventional synapses. While much of the insight was gained using anoxic and ischemic models, given the mechanistic overlap in other injury paradigms [2], the basic signaling pathways culminating in axonal damage are likely relevant to other disease mechanisms such as trauma and inflammatory/immune attack. The reader is also referred to additional complementary reviews [3-8].
Anoxia/ischemia causes conduction failure in CNS white matter
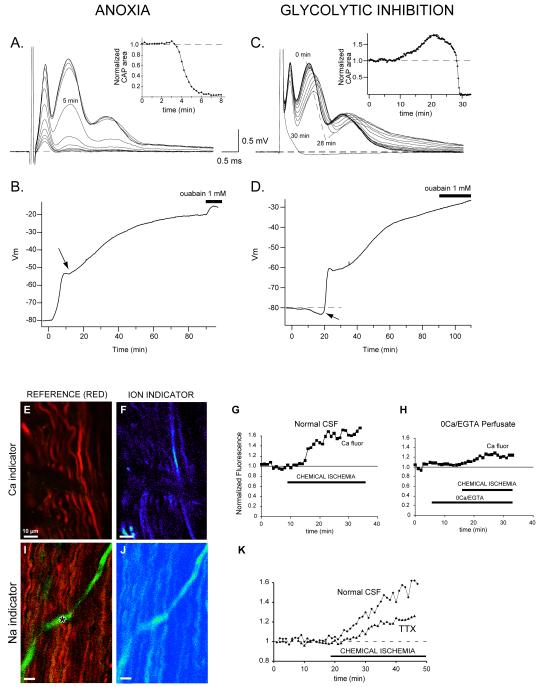
The normal function of CNS white matter depends on a continuous supply of oxygen and glucose because CNS energy reserves are extremely limited. Not surprisingly, disruptions of energy supply due to anoxia/ischemia cause loss of ionic homeostasis, depolarization and rapid conduction failure of central fibers. Ex vivo preparations of live central myelinated fibers (e.g., optic nerve and spinal dorsal columns) continue to be useful models to study axo-glial injury caused by a variety of insults. As shown in Figure 1A-D, conduction along a central white matter tract is inhibited within minutes of anoxia, in parallel with a strong depolarization of resting membrane potential [9-11]. Similarly, chemical ischemia (e.g., using the glycolytic inhibitor iodoacetate) also causes a marked depolarization and failure of electrogenesis [12, 13]. Collectively, these examples emphasize the absolute reliance of white matter on oxygen and glucose for continued function.
Figure 1.
Effect of anoxia or glycolytic block on optic nerve propagated compound action potentials (CAP) or resting membrane potentials (Vm). A: representative tracings show a rapid loss of the compound action potential recorded from rat optic nerve in vitro. Traces are displayed at 1 min intervals with anoxia beginning at time zero. Inset shows rate of decline of the area under the CAP. B: graph of rat optic nerve compound Vm recorded in a grease gap chamber. Chemical anoxia (2 mM NaCN) induces a rapid membrane depolarization that coincides with the loss of excitability as shown in panel A. After 10–15 min of anoxia there is an abrupt change in the rate of depolarization (arrow), which is never seen with ouabain application alone. This might reflect intrinsic mechanisms designed to limit a potentially deleterious membrane depolarization. Subsequent inhibition of Na+-K+-ATPase with ouabain after 90 min of anoxia induces an additional small depolarization indicating a minor contribution of glycolysis to Na+ and K+ pumping. C: in contrast to anoxia, the effects of glycolytic inhibition (1 mM iodoacetate) are delayed 10–20 min. CAP tracings are shown at intervals of 2 min. The initial manifestation is a rise in the CAP area that can exceed control area by 50% or more, as shown in the inset. A delay in peak latencies also occurs (compare tracings from time zero and 28 min); taken together, these features are characteristic of a transient initial hyperpolarization (see panel D). Excitability then fails very abruptly. D: Vm during glycolytic inhibition illustrates the delayed effects, with the initial change being a transient hyperpolarization (arrow) which closely coincides with the increase in CAP area illustrated in panel C. This is then followed by a rapid and massive depolarization corresponding to the sudden loss of excitability (panel C). Application of ouabain after 90 min of glycolytic block has no effect, indicating complete Na+-K+-ATPase failure under these conditions (compare with panel B). In parallel with electrophysiological failure, there is accumulation of abnormal levels of Ca (E-H) and Na (I-K) ion in ischemic axons, outlined by a red reference dye in E and I (the asterisk denotes a dye-filled capillary). The Ca and Na fluorescent indicators are shown in pseudocolor (F, J). Axonal Ca increases even in the absence of extracellular Ca (H). The Na rise is significantly, but incompletely, inhibited by blocking voltage-gated Na channels with tetrodotoxin (TTX) (K). Modified from refs [15, 17] with permission.
In the absence of an adequate energy supply, axoplasmic K falls while Na and Ca rise (Figure 1E-K), the latter from a nominal baseline of ~100 nM of free ion to tens of micromolar [15]. Although extracellular Ca accumulation in axons via reversal of the Na-Ca exchanger has been implicated in irreversible axonal damage [16], ischemic axons also suffer a rise in intra-axonal free [Ca] even in Ca-free perfusate (see below). In addition, some white matter tracts contain enough intracellular Ca to severely damage axons without any influx of this ion from the extracellular space [17]. Once intra-axonal Ca rises to toxic levels, irreversible axon damage occurs likely mediated by Ca-dependent proteases (e.g., calpains), activation of Ca-dependent phospholipases, disruption of mitochondrial oxidative phosphorylation (either by a potential mitochondrial Ca deficit induced by high cytosolic Na and export of free Ca from the matrix by mitochondrial Na-Ca exchange, or by mitochondrial Ca overload which occurs especially during the ‘reperfusion’” phase, with Ca excessively driven into the matrix via the Ca uniporter), and formation of free radicals [13, 18]. Furthermore, an increase in axoplasmic Ca has also been demonstrated in a wide range of experimental nerve injury models that produce acute or chronic axon degeneration, suggesting that intra-axonal Ca loading is an important pathologic event in many models of axon damage, for review see [19].
Taken together, an initial mechanism of central axon injury was proposed involving loss of ionic homeostasis through Na-K ATPase failure, Na entry through persistent Na channels [20, 21] and K efflux, causing reversal of the Na-Ca exchanger and the resultant accumulation of intra-axonal Ca. Importantly, the removal of Ca or Na, or applying antagonists of voltage-gated Na channels or Na-Ca exchange inhibitors, protected both rat optic nerve and spinal white matter tracts, and improved functional recovery [16, 22-26]. However, more severe insults such as ischemia (as opposed to anoxia alone) seem to recruit additional Ca-dependent injury mechanisms. Therefore, deciphering all the routes of intra-axonal Ca loading is of fundamental importance as it is likely to initiate irreversible damage to axon structure and function.
Ca release from intra-axonal stores
Previous work has shown that the levels of axoplasmic Ca increase in response to in vitro ischemia in myelinated axons of both rat optic nerve and spinal dorsal columns in the absence of extracellular Ca, suggesting that intracellular Ca stores might play an important role in axonal degeneration (Figure 1H) [15, 17]. Potentially substantial sources of intra-axonal Ca include mitochondria [27, 28], endoplasmic reticulum (ER) [29-31] [which can release Ca through ryanodine receptors, Ins(1,4,5,)P3 receptors, nicotinic acid adenine dinucleotide phosphate (NAADP)-sensitive receptors and sphingolipid Ca release-mediating protein of the ER (SCaMPER)], and cytosolic Ca-binding proteins [32]. As discussed in more detail below, recent work supports the notion that many of the above sources of intracellular Ca come into play in more severe injuries. Specifically, in addition to influx of extracellular Ca across the axolemma, intra-axonal calcium overload occurs through ryanodine-receptor-mediated release from ER in ischemic dorsal column axons [17], release via Ins(1,4,5,)P3 receptors in response to Ins(1,4,5,)P3 generation by phospholipase C [15, 33], and release from mitochondria [15]. Importantly, these studies unveil the complexity of axonal injury mechanisms and offer additional therapeutic targets to preserve axon structure and function following an insult.
‘Excitation-contraction coupling’-like Ca release: role of L-type Ca channels and ryanodine receptors
In skeletal muscle, depolarization sensed by L-type Ca channels is used to activate ryanodine receptors to release Ca from the sarcoplasmic reticulum, in turn promoting contraction. Interestingly, the application of the L-type Ca channel blocker nimodipine to ischemic dorsal columns maintained in zero Ca perfusate (the latter by itself failing to protect the tissue from injury) showed remarkable functional recovery [17]. Intriguingly, a similar ‘excitation-contraction coupling’ mechanism found in muscle cells might also operate in ischemic spinal axons, given that the protective action of nimodipine could not occur through inhibition of Ca influx through the channel, as all Ca was already removed from the extracellular space. It is important to note that dihydropyridines such as nimodipine block Ca channels by interfering with the voltage sensor of the channel [34], not by blocking its pore. Moreover, blocking ryanodine receptors directly using ryanodine or depleting ER Ca stores by pre-application of the ER Ca-ATPase inhibitor, thapsigargin, were also very protective in the same paradigm. Taken together, these data suggest that the pathological intra-axonal Ca rise following more severe insults occurs in part through axonal ER release, triggered by L-type Ca channel activation of ryanodine receptors.
To further substantiate these findings, changes in axoplasmic Ca levels were measured using fluorescent dyes and confocal microscopy (Figure 1E-K). These studies revealed that free axoplasmic Ca rises substantially during ischemia even in zero Ca perfusate, and this Ca increase can be greatly reduced by ryanodine [15, 17] or nimodipine [35]. Ultrastructural studies demonstrate the existence of subaxolemmal cisternae [17, 36] and triple-label immunofluorescence and co-immunoprecipitation confirm a spatial and physical association between voltage-gated Ca channels and ryanodine receptors near the axolemma of central myelinated axons [17]. Interestingly, the release of Ca from intra-axonal stores was highly dependent on Na influx, similar to the dependence of extracellular Ca entry on Na and reversal of Na/Ca exchange [15]. However, as extracellular Ca was removed in these studies, the axolemmal Na/Ca exchanger is an unlikely mediator of intra-axonal Ca accumulation; instead, these observations suggest a potentially direct, yet unknown, role for Na in mediating ER Ca release. Notably, functional recovery, while greatly improved by interference with this ‘excitation-contraction coupling’-like Ca release mechanism, is still incomplete in dorsal column axons following ischemia[dhh1], suggesting that other Ca sources may play a role, or perhaps indicating that not all injury pathways depend solely on Ca overload.
In addition to L-type Ca channels, additional voltage-gated Ca channels also play a role in CNS white matter injury. For example, Fern and colleagues showed that blockers of N-type Ca channels are protective against in vitro anoxia [37]. More recently, P/Q-type Ca channels were shown to be present on immature pre-myelinated axons [38]; whether these persist into adulthood and contribute to axonal injury is plausible, but currently unproven. xx[dhh2]
In summary, the role of voltage-gated Ca channels in axonal injury is supported by several studies that have shown remarkable protection using pharmacological inhibition. In addition to a direct protective effect of blocking voltage-gated Ca channel mediated Ca entry, interfering with the L-type Ca channel voltage sensor may prevent coupling to axonal ryanodine receptors and subsequent damaging Ca release from axonal Ca stores.
Ins(1,4,5,)P3 receptors as a source of intra-axonal Ca
The inositol trisphosphate Ins(1,4,5,)P3) receptor also mobilizes Ca from ER stores when bound by its ligand Ins(1,4,5,)P3 and contributes to intraaxonal Ca release in ischemic axons [15, 33]. To disrupt Ins(1,4,5,)P3 signaling, three reagents were used to probe mechanisms: U73122, an irreversible antagonist of phospholipase C (PLC), an enzyme that liberates Ins(1,4,5,)P3 from the plasma membrane phospholipid, PtdIns(4,5)P2 [39]; neomycin, which complexes phosphatidylinositol lipids to render them unavailable as phospholipase C substrates; and heparin, which inhibits Ins(1,4,5,)P3-induced Ca release by binding to Ins(1,4,5,)P3-binding sites. Each of the three antagonists significantly reduced the peak axonal Ca response compared with antagonist-free controls, suggesting that a substantial component of ischemia-induced intra-axonal Ca release is mediated by the Ins(1,4,5,)P3 signaling pathway [15].
To further investigate the mechanism of Ins(1,4,5,)P3-mediated intra-axonal calcium release, the Group I metabotropic glutamate receptor (mGluR-I) antagonist 1-aminoindan-1,5-dicarboxylic acid (AIDA) was added to zero-Ca-treated dorsal column slices, thus interfering with putative G-protein-coupled metabotropic receptor activation of PLC, which should in turn reduce Ins(1,4,5,)P3 generation [40]. Indeed, AIDA treatment alone was protective against a severe one hour ischemic insult [33]. Based on these studies, it is probable that mGluR-I receptors activate PLC, leading to Ins(1,4,5,)P3 production and Ca release, which then contributes significantly to central myelinated fiber injury.
Mitochondria as a source of intra-axonal Ca
Mitochondria also store and release intracellular Ca [41]. Under normal physiological conditions, Ca efflux from mitochondria is mediated primarily by the mitochondrial Na/Ca exchanger [42]. Furthermore, under certain conditions, both the Ca uniporter and the permeability transition pore (MPTP) can also release Ca [43]. To investigate the role of mitochondria in mediating Ca release during ischemia of optic nerves in vitro, the nerves were treated with three inhibitors of mitochondrial Ca release; CGP37157, a blocker of mitochondrial Na/Ca exchange; Ru360, an inhibitor of the mitochondrial Ca uniporter; and cyclosporin A, a MPTP blocker [15]. Treatment with CGP37157 reduced intra-axonal Ca levels greatly, but neither Ru360 nor cyclosporin A had this effect, suggesting that the majority of mitochondrial Ca release during ischemia is mediated by the mitochondrial Na/Ca exchanger [15], likely driven to export Ca from the mitochondrial matrix by substantial intra-axonal Na accumulation.
Based on the above studies, and because several sources of intra-axonal Ca release contribute to overall axonal Ca load, combinational treatments were applied to target both Ins(1,4,5,)P3 receptors and mitochondrial Na/Ca-exchanger-mediated Ca release. These combination strategies reduced the intra-axonal Ca rise much more effectively than any single treatment alone, suggesting that these pathways work independently and are major contributors to intracellular Ca release during ischemia. Furthermore, the greatly restrained Ca rise was paralleled by an improved electrophysiological recovery, underscoring a direct relationship between axonal Ca accumulation and functional injury [15].
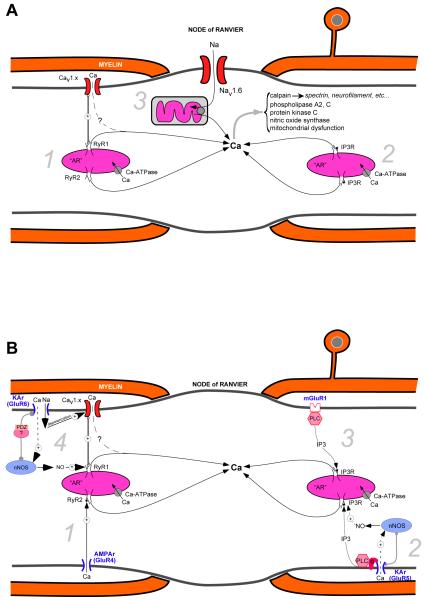
Collectively, these results indicate that, in some central white matter tracts, as the severity of injury increases, additional Ca-dependent injury mechanisms are recruited, involving release from intracellular pools. These experiments raise the strong possibility that controlling only the extracellular Ca influx is necessary but not sufficient to protect this tissue against more severe insults, with additional control of Ca release from intracellular stores being required for optimal protection. A summary diagram of intracellular Ca stores contributing to axonal damage is shown in Figure 2A.
Figure 2.
Sources of intra-axonal Ca release. A. Central myelinated axons possess formidable intracellular stores of Ca containing sufficient quantities to cause significant damage to the fiber. The Ca pools are stored in the axoplasmic reticulum (AR), the axonal analog of the endoplasmic reticulum (ER) in other cells, and mitochondria. The main intracellular Ca release channels are ryanodine receptors [1] (normally activated by Ca or by depolarization sensed by axolemmal voltage-sensitive Ca channels) and channels stimulated by inositol trisphosphate Ins(1,4,5)P3 [2]. Na loading of the axon through nodal Nav1.6 promotes Ca release from mitochondria by reverse mitochondrial Na-Ca exchange [3]. B. Proposed relationship between axonal glutamate receptors and intra-axonal Ca stores. The intracellular Ca release channels are under complex control of neurotransmitter receptors (mainly glutamate), expressed in clusters, together with related signaling molecules, along the internodal axolemma under the myelin sheath. GluR4 AMPA receptors permeate small amounts of Ca that, in turn, release Ca from the AR via ‘cardiac-type’ Ca-induced Ca release [1]. By contrast, axonal Ca increases from activation of GluR5 kainate receptors occur mainly via a G-protein-coupled, phospholipase C (‘PLC’)-dependent synthesis of IP3, which in turn activates Ins(1,4,5)P3 receptors on the AR [2]; this latter mechanism is partially dependent on NO, which is synthesized by neuronal nNOS, itself activated by small amounts of Ca entry via the GluR5 receptor; the locally produced NO may then further upregulate Ins(1,4,5)P3 receptor activity. Group I metabotropic glutamate receptors also activate Ins(1,4,5)P3-dependent Ca stores via PLC [3]. Stimulation of GluR6 kainate receptors induces a local depolarization and a small amount of Ca entry. The depolarization activates L-type Ca channels (Cav), whereas the kainate-receptor-mediated Ca2+ influx stimulates nNOS, which is scaffolded in the vicinity of the receptor. A PDZ-containing adaptor protein likely plays a role in organizing at least some of the signaling molecules into a functional ‘nanocomplex’ [4]. As with GluR5 receptors, locally generated NO might upregulate the activity of ryanodine receptors, which are activated by the depolarization-induced conformational change of the Ca channel, leading to release of Ca from the AR. The physiological roles of these nanocomplexes are unknown. However, overactivation of these signaling molecules by pathological levels of glutamate, NO and depolarization could be a major determinant of axonal degeneration in a variety of disorders. Mitochondria are omitted from panel B for clarity. xx[dhh5]
Glutamate receptors and axonal injury
Glutamate excitotoxicity has been well described as an important contributor to grey matter and, more recently, white matter degeneration. Indeed, with regards to the latter, antagonists of ionotropic glutamate receptors (i.e. AMPA/kainate receptors and, more recently, NMDA receptors) are protective in several models of white matter injury [33, 44-47]. By contrast, applying agonists of AMPA/kainate receptors or increasing extracellular glutamate levels by blocking glutamate transport promotes axonal injury [48-51]. Although the precise mechanisms of glutamate-mediated white matter injury are still being elucidated, excitotoxic glial injury is supported by the observations that these cells express Ca-permeable glutamate receptors, and antagonists of these receptors prevent glial cell death (for review, see [52]). In addition to glia and myelin [53] as targets of glutamate and purinergic receptor [8,14] excitotoxicity, more recent work (discussed in more detail below) reveals that functional AMPA/kainate receptors are present on central axons in discrete signaling ‘nanocomplexes’ that exert additional control over the intra-axonal Ca stores described above [54, 55].
To see whether myelinated axons express functional glutamate receptors that are capable of mediating intra-axonal Ca release, rat dorsal column axons were loaded with a Ca-sensitive indicator, then exposed to glutamate receptor agonists, and intra-axonal Ca changes were measured using confocal microscopy. Specifically, GluR5-containing kainate receptors were activated using the selective agonist (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA), and AMPA receptors were activated using AMPA. Following agonist application, robust increases in axoplasmic Ca were detected and could be blocked using the combined AMPA/kainate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzoquinoxaline-2,3-dione (NBQX). Curiously, the AMPA-mediated Ca flux was greatly reduced both in conditions of zero extracellular Ca (without additional antagonists) or following ryanodine inhibition of ryanodine receptors (in 2 mM bath Ca), suggesting that AMPA-induced Ca loading in axons is mainly mediated by ryanodine-sensitive Ca stores rather than influx across the axolemma. The extracellular Ca dependence further indicates that a cardiac-type Ca-induced Ca release mechanism is triggered by small amounts of Ca admitted through AMPA receptors. By contrast, the GluR5-mediated intra-axonal Ca response was reduced but not abolished in zero external Ca conditions, suggesting that the GluR5 effect was mediated in part by Ca release from intracellular stores, largely independently of extracellular Ca influx [55].
To further investigate the source of intra-axonal Ca mediated by GluR5 activation, dorsal columns were treated with pertussis toxin to block any putative kainate-receptor-coupled G protein signaling [56], or treated with inhibitors of phospholipase C or blockers of Ins(1,4,5,)P3 receptors to disrupt Ins(1,4,5,)P3-mediated Ca release. Blocking this pathway prevented the majority of intra-axonal Ca increases, revealing an important link between GluR5-containing kainate receptors, noncanonical signaling through G-proteins, activation of phospholipase C and release of Ca from axoplasmic Ins(1,4,5,)P3-dependent Ca stores. However, inhibition of L-type Ca channel activation did not prevent the axoplasmic Ca increases, signifying that L-type Ca channels do not play a significant role in AMPA/GluR5-mediated axoplasmic Ca accumulation [55], in stark contrast to the effect of GluR6 activation (see below).
In addition to the GluR5 studies, parallel sets of experiments were designed to examine the role of GluR6-containing kainate receptors in causing axoplasmic Ca deregulation. Activation of kainate receptors using kainate or SYM2081 (a GluR6 kainate receptor agonist) at concentrations that significantly reduce compound action potentials, cause an increase in intra-axonal Ca. Importantly, application of 3-(hydroxyamino)-6-nitro-6,7,8,9-tetrahydrobenzo[g]indol-2-one (NS-102), an antagonist of GluR6-containing kainate receptors, strongly reduced the Ca response induced by kainate, whereas AMPA receptor antagonists did not [54]. Because removal of extracellular Ca did not completely prevent intra-axonal Ca rise, axonal Ca stores were targeted to tease out the mechanism of GluR6-mediated intra-axonal Ca accumulation. In agreement with previous studies that showed that ischemia-induced depolarization of dorsal column axons releases Ca from ryanodine-dependent axonal stores [17], the GluR6-mediated axonal Ca rise was derived from ryanodine-sensitive Ca stores [54]. In addition, and in contrast to GluR5 kainate and AMPA receptor activation, inhibiting L-type Ca channels by nimodipine or nifedipine strongly inhibited the intra-axonal Ca increase mediated by GluR6 kainate receptors, and improved functional recovery [54]. Furthermore, replacing Na with the impermeable cation NMDG, but not the kainate receptor-permeable Li ion, almost completely blocked the GluR6-mediated Ca rise, supporting the notion that ryanodine-sensitive release of intra-axonal Ca is also depolarization dependent [54].
Taken together, the results from these glutamate receptor agonist studies suggest that, although GluR5 kainate-receptor-mediated intra-axonal Ca responses show some dependence on extracellular Ca, the majority of GluR5-dependent axonal Ca increase occurs via G proteins, activation of PLC, and release of Ca from intra-axonal ins(1,4,5)P3P3-dependent Ca stores. By contrast, GluR6 receptor-mediated intra-axonal Ca responses involve depolarization-dependent Ca release from ryanodine-sensitive Ca stores, involving L-type voltage-gated Ca channels that function as voltage sensors, rather than Ca-permeable pores. Figure 2B illustrates the mechanisms of glutamate receptor-mediated axonal Ca deregulation, and the intimate relationship between these receptors and intra-axonal Ca stores.
Signaling ‘nanocomplexes’ on myelinated axons: potential effectors of direct axonal injury
Although the above experiments clearly show that AMPA/kainate receptors are involved in the mobilization of intra-axonal Ca, they do not provide direct evidence that these receptors are axonal. Indeed, previous reports suggested that the protective effects of AMPA/kainate antagonists in white matter injury are due to protection of oligodendrocytes, with subsequent indirect effects on axonal sparing [47, 57]. To address whether functional glutamate receptors are expressed on the axolemma, experiments were designed to selectively load axons with various reagents to disrupt the proposed axonal GluR6-containing receptor signalling mechanism. Towards this end, axons were loaded with nitric oxide (NO) scavengers, and intra-axonal Ca rises were measured following kainate application. In contrast to bath-applied NO scavengers, axonal loading of the NO scavenger myoglobin or hydroxocobalamin potently reduced GluR6-containing glutamate receptor-mediated axonal Ca release [54]. In addition, inhibiting intra-axonal NO synthase (NOS) was also highly effective [54]. This role of NO in mobilizing intra-axonal Ca may reflect the known deleterious effects of this messenger on axonal function. Smith and colleagues [71] have conducted detailed studies on the actions of this gas on myelinated axonal function, and have found that concentrations that are thought to be present in inflammatory lesions for instance, induce significant irreversible conduction failure, that is dependent on the ongoing level of electrical activity in the fibers.
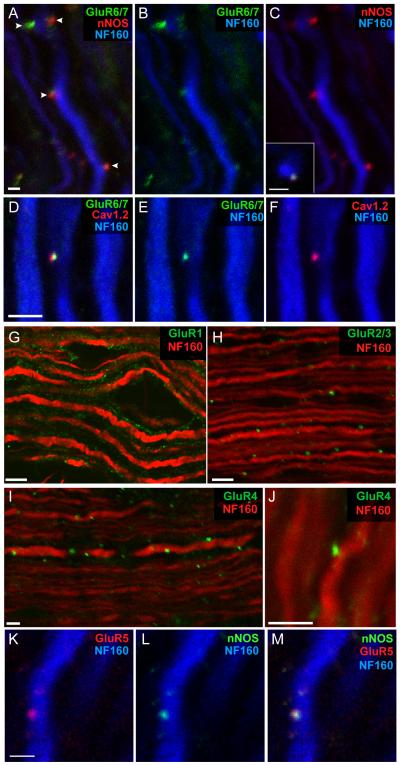
The above results suggest a close association of glutamate receptors on the axolemma and coupling with discrete signaling complexes that might provide a mechanistic link between axonal glutamate receptor activation and Ca release from intra-axonal Ca stores, with NO as an important positive modulator of this coupling. Such a role for NO is potentially very important in the context of neuroinflammation, where substantial amounts of this gas are generated by inflammatory cells. To tease out the presumed arrangement of these axonal ‘nanocomplexes’, immunohistochemistry, immunoelectron microscopy and immunoprecipitation assays were performed to show a spatial and physical interaction of these molecules within axons. As shown in Figure 3, punctate staining for GluR6/7, neuronal NOS (nNOS) and L-type Ca channels is localized to the periphery of neurofilament-positive axons. In addition,immunoelectron microscopy localized GluR6/7 to the axolemma and to clusters beneath the axolemma, within the axon itself [54]. Furthermore, immunoprecipitation confirmed a physical association between GluR6/7 and nNOS. Finally, a small peptide designed to disrupt GluR6 adaptor protein interactions mediated by PDZ domains was selectively loaded into axons (confirmed by a fluorescent tag linked to the peptide). Importantly, when axons were loaded with this peptide, GluR6-mediated intra-axonal Ca rises were blocked, indicating that the PDZ-binding motif on the C-terminus of GluR6 might position/cluster these receptors in close association with nNOS and other axonal signaling molecules that lead to intra-axonal Ca release [54]. Perhaps not surprisingly, very similar arrangements of receptors, adaptor proteins and effector molecules are found at interneuronal synapses [58-60]. Figure 3 illustrates that AMPA and GluR5 kainate receptors also appear in distinct internodal nanocomplexes, colocalized with relevant signalling proteins.
Figure 3.
The internodal axolemma of myelinated CNS fibers expresses a rich variety of glutamate receptors and related signaling molecules. (A–C) Triple-immunolabeled spinal axons exhibit punctate regions of co-localized GluR6/7 and nNOS clusters (arrowheads) at the surface of neurofilament-stained axon cylinders. Inset: transverse view of a surface cluster in another fiber. (D–F) GluR6/7 clusters also co-localize with Cav1.2 L-type Ca2+ channels. AMPA receptor subunits are also expressed on myelinated axons: (G) GluR1 staining was patchy but is seen mainly in the myelin sheath. (H–J) By contrast, GluR2/3 (mainly GluR3 given the absence of GluR2 in myelinated axons) and GluR4 staining was punctate, and was localized at the surface of neurofilament-positive axonal profiles. (K–M) Likewise, GluR5 kainate receptor subunits were also expressed on the surfaces of axons in punctate areas, co-localized with nNOS. Scale bars: A–F, K–M, 2 μm; G–J 5 μm. Modified from refs. [54, 55] with permission. xx[dhh6]
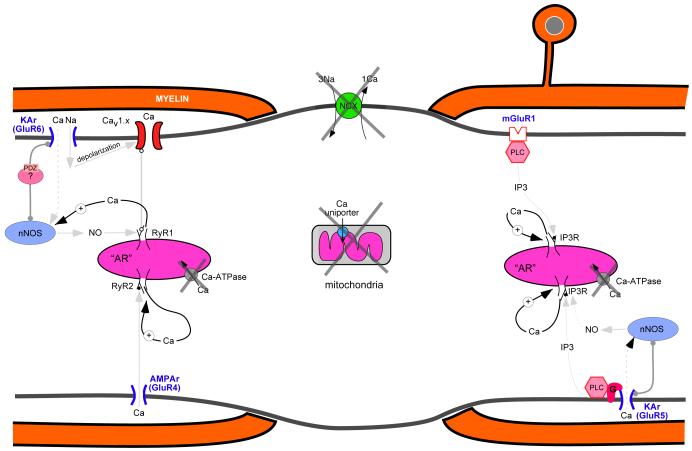
Interestingly, these studies and others [51], suggest that glutamate might directly activate internodal axonal glutamate receptors, promoting the release of substantial amounts of Ca from axoplasmic Ca stores. This proposed feed-forward mechanism [54] would involve activation of GluR6-containing kainate receptors (presumably through glutamate release from glia and inflammatory cells and/or reversal of axonal glutamate transporters under injurious conditions), subsequent depolarization of the internodal axolemma and a small localized rise in intracellular Ca through Ca-permeable kainate receptors. In turn, this local Ca response could promote NO synthesis by nNOS and modulation of L-type Ca-channel-mediated opening of ryanodine receptors on the subaxolemmal ER and the subsequent release of Ca. In parallel, activation of GluR5 receptors would lead to a mainly G-protein-coupled, phospholipase-C-dependent production of Ins(1,4,5,)P3 which would activate Ins(1,4,5,)P3 receptors on subaxolemmal ER and release Ca. Therefore, two divergent pathways are potentially activated through glutamate release to cause irreversible Ca-mediated axonal damage. Even worse, given the strong Ca dependence of many of the signalling molecules (nNOS, phospholipase C as well as the ryanodine and Ins(1,4,5,)P3 channels themselves), there is the potential for a catastrophic ‘runaway’ Ca release from internal pools (Figure 4). Importantly, as more is learned about these proposed axonal signaling nanocomplexes in normal axonal physiology and disease (Box 1), additional targets for therapeutic strategies should become available for the prevention of axonal degeneration (Box 2).
Figure 4.
Potential positive feedback of internal Ca release leading to a runaway emptying of intra-axonal stores. Under normal conditions, Ca homeostatic mechanisms (membrane Na-Ca exchanger [NCX], axoplasmic reticulum Ca-ATPase, Ca-binding proteins, mitochondria) maintain tight control over cellular Ca fluctuations. During pathological states, however, failure of Ca homeostasis (large Xs depict failure of the major Ca homeostatic systems) not only leads to excessive accumulation of released Ca, but this released Ca may in turn promote accelerated and unrestrained additional release of this ion by virtue of the Ca-dependence of nNOS, phospholipase C, and intracellular ryanodine and IP3 Ca channels. This may ultimately cause an irreversible and catastrophic accumulation of axonal Ca levels, causing structural and functional demise of the axon.
Box 1. Outstanding questions.
Important questions that remain unanswered are (1) are intra-axonal Ca release mechanisms important in axonal degeneration in in vivo models. (2) If so what pathways will be more effective to target when several converge. (3) What are the normal physiological roles of these signalling nanocomplexes within the internodal region of the axon, an area thought to be of little functional importance. (4) During disease progression such as following demyelination, do axons become more vulnerable due to exposed nanocomplexes harboring glutamate and likely other transmitter receptors
Most of the work to date has been completed using in vitro models of excised spinal and nerve tissue. Although these models are useful to study the mechanisms of axonal degeneration by allowing the internal and external axon environment to be manipulated, they are limited to acute studies due to tissue viability. As axon degeneration is likely to occur over days to months, in vivo models in combination with advanced optical imaging techniques will allow these dynamic events to be followed as they are occurring over time. In addition, such techniques will likely provide valuable information regarding what Ca release pathways are activated following axonal degeneration in vivo with the goal to uncover specific targets for therapeutic intervention. Indeed, whether intraaxonal Ca release and/or extracellular Ca contribute to axon degeneration in vivo is currently unknown, though both sources are strongly suspected based on detailed in vitro studies from a number of labs.
Box 2. Therapeutic implications.
Axonal degeneration and subsequent axonal loss are well characterized following stroke, spinal cord injury, and traumatic brain injury. Of particular importance will be to prevent secondary degeneration of axons that have survived the initial insult but later succumb to excitotoxic or inflammatory attack, in order to prevent further neurological decline. It is anticipated that many of the Ca deregulation pathways described previously play a role following trauma and potential therapeutic targets include glutamate receptors on axons and preventing extracellular and intracellular Ca store-mediated pathological intra-axonal Ca accumulation. Based on detailed studies, the most attractive potential targets may include voltage-gated Na and Ca channels, ionotropic and metabotropic glutamate receptors, plasmalemmal and mitochondrial Na-Ca exchangers, and even intracellular Ca release channels such as ryanodine and Ins(1,4,5,)P3 receptors (see Figure 2).
Although the majority of studies described in this manuscript use models of hypoxia/ischemia to investigate the mechanisms of axonal degeneration, similar mechanisms may contribute to secondary loss of axons following spinal cord injury and traumatic brain injury. Less intuitive is the notion that may contribute to axonal loss in such divergent diseases such as Alzheimer’s Disease (AD) and multiple sclerosis. With regards to the former, careful microscopic analyses of postmortem AD brains show significant white matter atrophy that precedes cortical atrophy (see Table 1). The implications from these studies in AD is that axon loss may precede somatic degeneration and highlight preventing axonal degeneration as an important therapeutic goal. Axon loss in multiple sclerosis is a prominent feature and may underlie the irreversible neurological decline associated with this disease. In particular, demyelination associated with the disease may unveil glutamate (and likely other) receptors on the axolemma and thereby making axons vulnerable to excitotoxic/inflammatory attack.
Concluding remarks
Studies of white matter injury mechanisms over the past two decades have provided insight into how anoxia/ischemia leads to axonal conduction failure. Importantly, antagonists of voltage-gated Na channels or Na-Ca exchanger inhibitors strongly protect central white matter tracts. However, more severe insults such as ischemia (as opposed to anoxia alone) seem to recruit additional Ca-dependent injury mechanisms, including the release of Ca from intra-axonal stores, that appear resistant to the above therapeutic strategies. This suggests that, to protect axons completely from Ca-mediated injury, blocking multiple routes of intracellular Ca accumulation will be necessary. Axonal mitochondria and the axoplasmic reticulum emerge as key intra-axonal Ca sources and unveil additional potential therapeutic targets (e.g. ryanodine and Ins(1,4,5)P3 receptors, and the mitochondrial Na-Ca exchanger)..
Glutamate-dependent excitotoxicity not only plays an important role in grey matter injury but is also likely to play a key role in white matter degeneration as well. With this in mind, pioneering studies investigating white matter injury mechanisms have revealed that different domains of the oligodendrocyte (i.e., soma, processes and myelin sheath) [53, 65, 66] express functional glutamate receptors that, when activated, allow pathological Ca accumulation [47, 53, 67]. Now adding to the list of glutamate-vulnerable white matter elements is the axon itself. [dhh3]Axonal AMPA, kainate and likely metabotropic glutamate receptors couple with discrete signaling nanocomplexes within the axon that, when over-stimulated, can promote pathological intra-axonal Ca accumulation. While glutamatergic signaling has been the focus, it appears likely that other transmitter systems may also be present and functional along the internode of mature myelinated fibers [14, 68, 69]. Although further studies will be necessary to determine a physiological role for these nanocomplexes located within the internodal region of the axon that was thought to be relatively passive, it is tempting to speculate that these networks provide a signalling conduit between the parent myelinating cell, myelin sheath and axon. Importantly, overstimulation of these signaling nanocomplexes could lead to focal Ca release, induce focal swellings from local cytoarchitectural failure, and eventual axonal transection as described previously (see discussion) [54]. Interestingly, multiple sclerosis and other diseases of CNS white matter induce very focal axonal swellings, which then proceed to complete transection [63]. One could speculate that these focal swellings are due to focal Ca release and structural damage to the fiber, mediated by the aforementioned nanocomplexes.
The past decade has seen major advances in our understanding of the makeup of various signalling molecules and complexes expressed on axons, myelinating cells and the myelin sheath they produce. Consequently, it is now clear that, to maximize white matter sparing and functional outcome, therapeutic strategies will need to be directed at preserving the oligodendrocyte–myelin–axon unit as a whole, as targeting any single component will likely be insufficient to optimally preserve function. xx[dhh4] Therefore, the next steps in preserving axon function will likely necessitate a thorough investigation into these fascinating axonal signalling complexes to determine the role of receptor activation in normal axon physiology and following disease.
Table 1. Examples of neurological disorders with significant primary axon damage.
| Disease | Comments | references |
|---|---|---|
| Alzheimer’s disease | White matter changes included partial loss of axons. Preclinical white matter atrophy precedes cortical atrophy, suggesting that axonal damage may be an independent factor in disease progression/disability. |
[61,62] |
| Multiple sclerosis | Substantial axon loss early in the progression of disease. Transected axons underlie irreversible neurological decline. |
[3, 63, 64] |
| Spinal cord injury | Axon dysfunction/loss is the main cause of morbidity. |
[7, 23, 25, 46] |
| Traumatic brain injury | Diffuse axonal injury (“DAI”) is thought to be the main pathological substrate, occurring mainly in a delayed manner secondary to biochemical disturbances rather than primary mechanical axotomy. |
[2, 6] |
| Stroke | The commonest disabling neurological disorder; almost all strokes affect gray matter and connecting myelinated fibers in the brain. |
[45, 47, 60] |
| Inherited leukodystrophies | A large variety of inborn errors of metabolism result in dysmyelination and axonal degeneration at any age. |
[51] |
| Periventricular white matter injury |
A common disorder of premature infants resulting in subcortical white matter damage, and cerebral palsy clinically. The immature oligodendrocyte is thought to be a major target. |
[70] |
Acknowledgements
Work in the Stys laboratory has been supported by Alberta Heritage Foundation for Medical Research, Canadian Institutes for Health Research, Canada Research Chairs, Dr. Frank Leblanc Chair for Spinal Cord Research, MS Society of Canada, National MS Society, Heart and Stroke Foundation of Ontario and NIH.
Glossary
- Anoxia
absence of oxygen supply to an organ or tissue
- Chemical ischemia
the use of a chemical to mimic the effects of ischemia
- Hypoxia
low oxygen supply to an organ or tissue
- Ischemia
Inadequate blood supply (circulation) to an organ or tissue
- PDZ-domain
a protein-protein interaction domain
- PDZ-binding motif
the sequence of amino acids that allow PDZ-containing proteins to bind. For example, glutamate receptor COOH-terminus residues provide a direct binding site for PDZ domain-containing proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: the authors declare no conflicts.
References
- 1.Waxman SG, Kocsis JD, Stys PK. The Axon : structure, function and pathophysiology. Oxford University Press; New York; Oxford: 1995. p. xv.p. 692. [2] of col. plates. [Google Scholar]
- 2.Wolf JA, et al. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21(6):1923–30. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8(3):280–91. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- 4.Stys PK. General mechanisms of axonal damage and its prevention. J Neurol Sci. 2005;233(1-2):3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins A, Scolding N. Protecting axons in multiple sclerosis. Mult Scler. 2008;14(8):1013–25. doi: 10.1177/1352458508091370. [DOI] [PubMed] [Google Scholar]
- 6.Buki A, Povlishock JT. All roads lead to disconnection?--Traumatic axonal injury revisited. Acta Neurochir (Wien) 2006;148(2):181–93. doi: 10.1007/s00701-005-0674-4. discussion 193-4. [DOI] [PubMed] [Google Scholar]
- 7.Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21(6):754–74. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 8.Matute C. Calcium dyshomeostasis in white matter pathology. Cell Calcium. 2009 doi: 10.1016/j.ceca.2009.12.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Stys PK, et al. Role of extracellular calcium in anoxic injury of mammalian central white matter. Proc Natl Acad Sci U S A. 1990;87(11):4212–6. doi: 10.1073/pnas.87.11.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leppanen L, Stys PK. Ion transport and membrane potential in CNS myelinated axons. II. Effects of metabolic inhibition. J Neurophysiol. 1997;78(4):2095–107. doi: 10.1152/jn.1997.78.4.2095. [DOI] [PubMed] [Google Scholar]
- 11.Fern R, et al. Axon conduction and survival in CNS white matter during energy deprivation: a developmental study. J Neurophysiol. 1998;79(1):95–105. doi: 10.1152/jn.1998.79.1.95. [DOI] [PubMed] [Google Scholar]
- 12.Stys PK, Hubatsch DA, Leppanen LL. Effects of K+ channel blockers on the anoxic response of CNS myelinated axons. Neuroreport. 1998;9(3):447–53. doi: 10.1097/00001756-199802160-00017. [DOI] [PubMed] [Google Scholar]
- 13.Stys PK. Anoxic and ischemic injury of myelinated axons in CNS white matter: from mechanistic concepts to therapeutics. J Cereb Blood Flow Metab. 1998;18(1):2–25. doi: 10.1097/00004647-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Matute C, et al. P2X(7) receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J Neurosci. 2007;210:693–702. doi: 10.1523/JNEUROSCI.0579-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolaeva MA, Mukherjee B, Stys PK. Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J Neurosci. 2005;25(43):9960–7. doi: 10.1523/JNEUROSCI.2003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci. 1992;12(2):430–9. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouardouz M, et al. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron. 2003;40(1):53–63. doi: 10.1016/j.neuron.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Young W. Role of calcium in central nervous system injuries. J Neurotrauma. 1992;9(Suppl 1):S9–25. [PubMed] [Google Scholar]
- 19.LoPachin RM, Lehning EJ. Mechanism of calcium entry during axon injury and degeneration. Toxicol Appl Pharmacol. 1997;143(2):233–44. doi: 10.1006/taap.1997.8106. [DOI] [PubMed] [Google Scholar]
- 20.Stys PK, et al. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci U S A. 1993;90(15):6976–80. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor CP. Na+ currents that fail to inactivate. Trends Neurosci. 1993;16(11):455–60. doi: 10.1016/0166-2236(93)90077-y. [DOI] [PubMed] [Google Scholar]
- 22.Waxman SG, et al. Protection of the axonal cytoskeleton in anoxic optic nerve by decreased extracellular calcium. Brain Res. 1993;614(1-2):137–45. doi: 10.1016/0006-8993(93)91027-p. [DOI] [PubMed] [Google Scholar]
- 23.Imaizumi T, Kocsis JD, Waxman SG. Anoxic injury in the rat spinal cord: pharmacological evidence for multiple steps in Ca(2+)-dependent injury of the dorsal columns. J Neurotrauma. 1997;14(5):299–311. doi: 10.1089/neu.1997.14.299. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Jiang Q, Stys PK. Important role of reverse Na(+)-Ca(2+) exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84(2):1116–9. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na(+)-K(+)-ATPase, the Na(+)-H+ exchanger, and the Na(+)-Ca2+ exchanger. J Neurosci. 1996;16(2):545–52. doi: 10.1523/JNEUROSCI.16-02-00545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehlings MG, Agrawal S. Role of sodium in the pathophysiology of secondary spinal cord injury. Spine (Phila Pa 1976) 1995;20(20):2187–91. doi: 10.1097/00007632-199510001-00002. [DOI] [PubMed] [Google Scholar]
- 27.Thayer SA, Miller RJ. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholls DG, et al. Interactions between mitochondrial bioenergetics and cytoplasmic calcium in cultured cerebellar granule cells. Cell Calcium. 2003;34(4-5):407–24. doi: 10.1016/s0143-4160(03)00144-1. [DOI] [PubMed] [Google Scholar]
- 29.Pozzan T, et al. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 30.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 31.Petersen OH, Verkhratsky A. Endoplasmic reticulum calcium tunnels integrate signalling in polarised cells. Cell Calcium. 2007;42(4-5):373–8. doi: 10.1016/j.ceca.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1(4):241–58. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- 33.Ouardouz M, et al. Complex interplay between glutamate receptors and intracellular Ca2+ stores during ischaemia in rat spinal cord white matter. J Physiol. 2006;577(Pt 1):191–204. doi: 10.1113/jphysiol.2006.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325(6106):717–20. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- 35.Ouardouz M, et al. Protection of ischemic rat spinal cord white matter: Dual action of KB-R7943 on Na+/Ca2+ exchange and L-type Ca2+ channels. Neuropharmacology. 2005;48(4):566–75. doi: 10.1016/j.neuropharm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Metuzals J, et al. Organization of the cortical endoplasmic reticulum in the squid giant axon. J Neurocytol. 1997;26(8):529–39. doi: 10.1023/a:1015482407202. [DOI] [PubMed] [Google Scholar]
- 37.Fern R, Ransom BR, Waxman SG. Voltage-gated calcium channels in CNS white matter: role in anoxic injury. J Neurophysiol. 1995;74(1):369–77. doi: 10.1152/jn.1995.74.1.369. [DOI] [PubMed] [Google Scholar]
- 38.Alix JJ, Dolphin AC, Fern R. Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation. J Physiol. 2008;586(Pt 17):4069–89. doi: 10.1113/jphysiol.2008.155077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin W, et al. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994;642(1-2):237–43. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- 40.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34(1):1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 41.Parekh AB. Mitochondrial regulation of store-operated CRAC channels. Cell Calcium. 2008;44(1):6–13. doi: 10.1016/j.ceca.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Castaldo P, et al. Role of the mitochondrial sodium/calcium exchanger in neuronal physiology and in the pathogenesis of neurological diseases. Prog Neurobiol. 2009;87(1):58–79. doi: 10.1016/j.pneurobio.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 44.Wrathall JR, Choiniere D, Teng YD. Dose-dependent reduction of tissue loss and functional impairment after spinal cord trauma with the AMPA/kainate antagonist NBQX. J Neurosci. 1994;14(11 Pt 1):6598–607. doi: 10.1523/JNEUROSCI.14-11-06598.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakiri Y, et al. Testing NMDA receptor block as a therapeutic strategy for reducing ischaemic damage to CNS white matter. Glia. 2008;56(2):233–40. doi: 10.1002/glia.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci. 1997;17(3):1055–63. doi: 10.1523/JNEUROSCI.17-03-01055.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tekkok SB, Goldberg MP. Ampa/kainate receptor activation mediates hypoxic oligodendrocyte death and axonal injury in cerebral white matter. J Neurosci. 2001;21(12):4237–48. doi: 10.1523/JNEUROSCI.21-12-04237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20(3):1190–8. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Domercq M, et al. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia. 2005;52(1):36–46. doi: 10.1002/glia.20221. [DOI] [PubMed] [Google Scholar]
- 50.Matute C. Characteristics of acute and chronic kainate excitotoxic damage to the optic nerve. Proc Natl Acad Sci U S A. 1998;95(17):10229–34. doi: 10.1073/pnas.95.17.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitt D, et al. Dysmyelinated axons in shiverer mice are highly vulnerable to alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor-mediated toxicity. Brain Res. 1309:146–54. doi: 10.1016/j.brainres.2009.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matute C, et al. Excitotoxic damage to white matter. J Anat. 2007;210(6):693–702. doi: 10.1111/j.1469-7580.2007.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Micu I, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439(7079):988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 54.Ouardouz M, et al. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann Neurol. 2009;65(2):151–9. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ouardouz M, et al. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann Neurol. 2009;65(2):160–6. doi: 10.1002/ana.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rozas JL, Paternain AV, Lerma J. Noncanonical signaling by ionotropic kainate receptors. Neuron. 2003;39(3):543–53. doi: 10.1016/s0896-6273(03)00436-7. [DOI] [PubMed] [Google Scholar]
- 57.Matute C, et al. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24(4):224–30. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- 58.Sattler R, et al. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284(5421):1845–8. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 59.Liu XM, et al. Neuroprotection of Tat-GluR6-9c against neuronal death induced by kainate in rat hippocampus via nuclear and non-nuclear pathways. J Biol Chem. 2006;281(25):17432–45. doi: 10.1074/jbc.M513490200. [DOI] [PubMed] [Google Scholar]
- 60.Aarts M, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298(5594):846–50. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 61.de la Monte SM. Quantitation of cerebral atrophy in preclinical and end-stage Alzheimer’s disease. Ann Neurol. 1989;25(5):450–9. doi: 10.1002/ana.410250506. [DOI] [PubMed] [Google Scholar]
- 62.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19(3):253–62. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 63.Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338(5):278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 64.Ganter P, Prince C, Esiri MM. Spinal cord axonal loss in multiple sclerosis: a post-mortem study. Neuropathol Appl Neurobiol. 1999;25(6):459–67. doi: 10.1046/j.1365-2990.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 65.Karadottir R, et al. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438(7071):1162–6. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438(7071):1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 67.Matute C, et al. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A. 1997;94(16):8830–5. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikolaeva MA, et al. Effects of the noradrenergic system in rat white matter exposed to oxygen-glucose deprivation in vitro. J Neurosci. 2009;29(6):1796–804. doi: 10.1523/JNEUROSCI.5729-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang CL, et al. Nicotinic acetylcholine receptors in mouse and rat optic nerves. J Neurophysiol. 2004;91(2):1025–35. doi: 10.1152/jn.00769.2003. [DOI] [PubMed] [Google Scholar]
- 70.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–3. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 71.Smith KJ, Kapoor R, Hall SM, Davies M. Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol. 2001;49:470–476. [PubMed] [Google Scholar]