Abstract
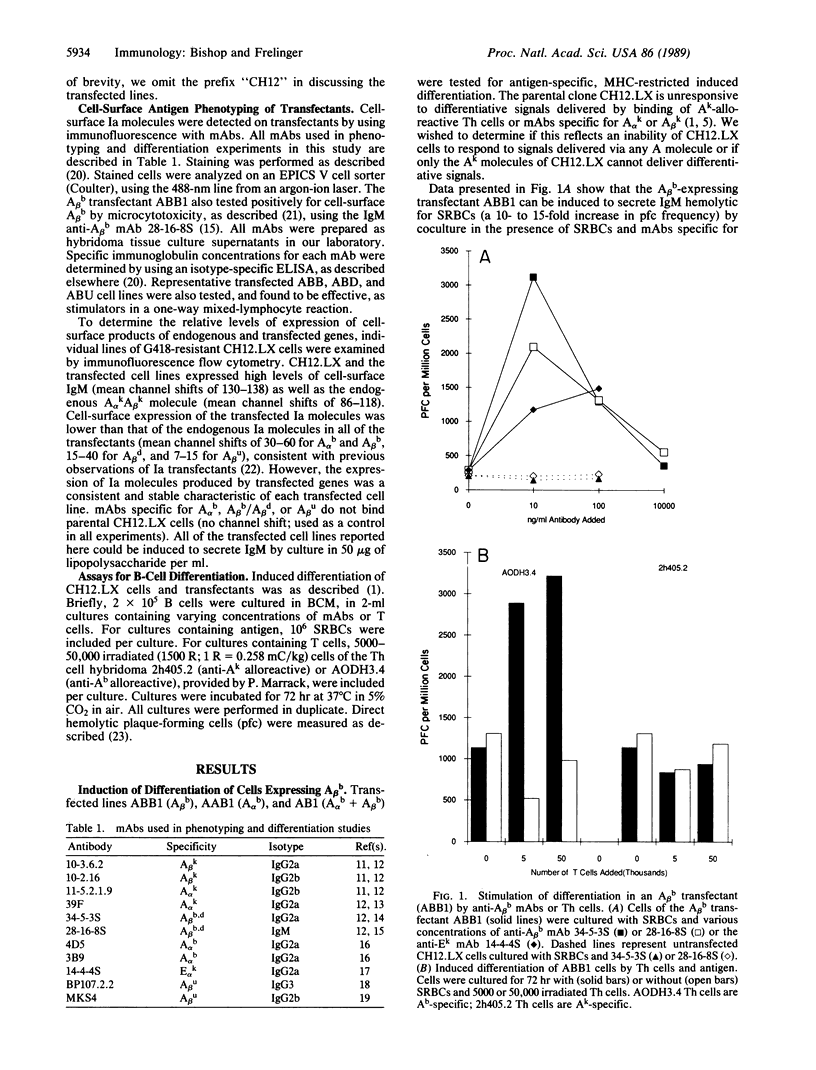
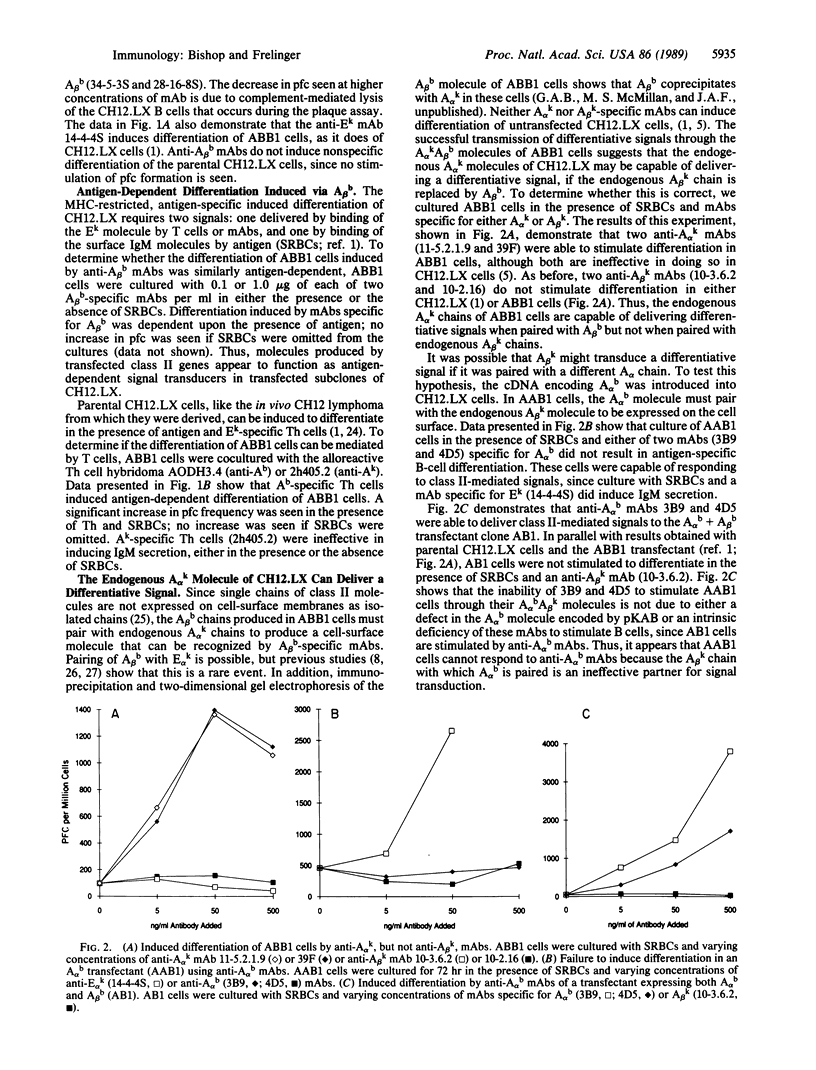
CH12.LX B cells are responsive to antigen-dependent differentiative signals transmitted through their surface Ek molecules. Although CH12.LX cells express surface Ak molecules with normal protein sequence, the Ak molecules do not deliver differentiative signal to these B cells. To determine whether introduction of a new A molecule into CH12.LX cells would correct this deficiency, CH12.LX cells were transfected with the genes encoding new Aalpha and/or Abeta molecules. It was found that transfected cells responded to antigen-specific signals delivered via the Ealphak Ebetak, Aalphab Abetab, or Aalphak Abetab molecule. However, the B cells did not respond to signals generated by the molecule Aalpha kAbetak, Aalphab Abetak, Aalphak Abetad, or Aalpha kAbetau. Comparison of these sequences suggests that two Abeta residues, His-47 and Trp-197, are important to the transmission of differentiative signals to B cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck B. N., Buerstedde J. M., Krco C. J., Nilson A. E., Chase C. G., McKean D. J. Characterization of cell lines expressing mutant I-Ab and I-Ak molecules allows the definition of distinct serologic epitopes on A alpha and A beta polypeptides. J Immunol. 1986 Apr 15;136(8):2953–2961. [PubMed] [Google Scholar]
- Bishop G. A., Haughton G. Induced differentiation of a transformed clone of Ly-1+ B cells by clonal T cells and antigen. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7410–7414. doi: 10.1073/pnas.83.19.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G. A., Haughton G. Role of the interleukin 2 receptor in differentiation of a clone of Ly-1+ B cells. J Immunol. 1987 May 15;138(10):3308–3313. [PubMed] [Google Scholar]
- Bishop G. A., McMillan M. S., Haughton G., Frelinger J. A. Signaling to a B-cell clone by Ek, but not Ak, does not reflect alteration of Ak genes. Immunogenetics. 1988;28(3):184–192. doi: 10.1007/BF00375858. [DOI] [PubMed] [Google Scholar]
- Braunstein N. S., Germain R. N. Allele-specific control of Ia molecule surface expression and conformation: implications for a general model of Ia structure-function relationships. Proc Natl Acad Sci U S A. 1987 May;84(9):2921–2925. doi: 10.1073/pnas.84.9.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C., Newell M. K., Justement L. B., McGuire J. C., Leach K. L., Chen Z. Z. Ia binding ligands and cAMP stimulate nuclear translocation of PKC in B lymphocytes. Nature. 1987 Jun 18;327(6123):629–632. doi: 10.1038/327629a0. [DOI] [PubMed] [Google Scholar]
- Choi E., McIntyre K., Germain R. N., Seidman J. G. Murine I-A beta chain polymorphism: nucleotide sequences of three allelic I-A beta genes. Science. 1983 Jul 15;221(4607):283–286. doi: 10.1126/science.6407114. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Estess P., Begovich A. B., Koo M., Jones P. P., McDevitt H. O. Sequence analysis and structure-function correlations of murine q, k, u, s, and f haplotype I-A beta cDNA clones. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3594–3598. doi: 10.1073/pnas.83.11.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger J. A., Neiderhuber J. E., David C. S., Shreffler D. C. Evidence for the expression of Ia (H-2-associated) antigens on thymus-derived lymphocytes. J Exp Med. 1974 Nov 1;140(5):1273–1284. doi: 10.1084/jem.140.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Malissen B. Analysis of the expression and function of class-II major histocompatibility complex-encoded molecules by DNA-mediated gene transfer. Annu Rev Immunol. 1986;4:281–315. doi: 10.1146/annurev.iy.04.040186.001433. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Quill H. Unexpected expression of a unique mixed-isotype class II MHC molecule by transfected L-cells. Nature. 1986 Mar 6;320(6057):72–75. doi: 10.1038/320072a0. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., McDevitt H. O. Variable synthesis and expression of E alpha and Ae (E beta) Ia polypeptide chains in mice of different H-2 haplotypes. Immunogenetics. 1981;12(3-4):321–337. doi: 10.1007/BF01561674. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landais D., Matthes H., Benoist C., Mathis D. A molecular basis for the Ia.2 and Ia.19 antigenic determinants. Proc Natl Acad Sci U S A. 1985 May;82(9):2930–2934. doi: 10.1073/pnas.82.9.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landias D., Beck B. N., Buerstedde J. M., Degraw S., Klein D., Koch N., Murphy D., Pierres M., Tada T., Yamamoto K. The assignment of chain specificities for anti-Ia monoclonal antibodies using L cell transfectants. J Immunol. 1986 Nov 1;137(9):3002–3005. [PubMed] [Google Scholar]
- LoCascio N. J., Arnold L. W., Corley R. B., Haughton G. Induced differentiation of a B cell lymphoma with known antigen specificity. J Mol Cell Immunol. 1984;1(3):177–190. [PubMed] [Google Scholar]
- Lotteau V., Teyton L., Burroughs D., Charron D. A novel HLA class II molecule (DR alpha-DQ beta) created by mismatched isotype pairing. Nature. 1987 Sep 24;329(6137):339–341. doi: 10.1038/329339a0. [DOI] [PubMed] [Google Scholar]
- Malissen B., Shastri N., Pierres M., Hood L. Cotransfer of the Ed alpha and Ad beta genes into L cells results in the surface expression of a functional mixed-isotype Ia molecule. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3958–3962. doi: 10.1073/pnas.83.11.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhuber J. E., Frelinger J. A., Dugan E., Coutinho A., Shreffler D. C. Effects of anti-Ia serum on mitogenic responses. I. Inhibition of the proliferative response to B cell mitogen, LPS, by specific anti-Ia sera. J Immunol. 1975 Dec;115(6):1672–1676. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Palacios R., Martinez-Maza O., Guy K. Monoclonal antibodies against HLA-DR antigens replace T helper cells in activation of B lymphocytes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3456–3460. doi: 10.1073/pnas.80.11.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres M., Devaux C., Dosseto M., Marchetto S. Clonal analysis of B- and T-cell responses to Ia antigens. I. Topology of epitope regions on I-Ak and I-Ek molecules analyzed with 35 monoclonal alloantibodies. Immunogenetics. 1981 Dec;14(6):481–495. doi: 10.1007/BF00350120. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Snow E. C., Noelle R. J. Thymus-dependent antigenic stimulation of hapten-specific B lymphocytes. Immunol Rev. 1987 Oct;99:173–192. doi: 10.1111/j.1600-065x.1987.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Symington F. W., Sprent J. A monoclonal antibody detecting an Ia specificity mapping in the I-A or I-E subregion. Immunogenetics. 1981;14(1-2):53–61. doi: 10.1007/BF00344299. [DOI] [PubMed] [Google Scholar]
- Widera G., Flavell R. A. The I region of the C57BL/10 mouse: characterization and physical linkage to H-2K of an SB beta-like class II pseudogene, psi A beta 3. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5500–5504. doi: 10.1073/pnas.82.16.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]


