Abstract
Deficiency of acyl CoA:cholesterol acyltransferase 2 (ACAT2) in mice results in a reduction in cholesterol ester synthesis in the small intestine and liver, which in turn limits intestinal cholesterol absorption, hepatic cholesterol gallstone formation, and the accumulation of cholesterol esters in the plasma lipoproteins. Here we examined the contribution of ACAT2-derived cholesterol esters to atherosclerosis by crossing ACAT2-deficient (ACAT2–/–) mice with apolipoprotein (apo) E-deficient (ApoE–/–) mice, an atherosclerosis-susceptible strain that has impaired apoE-mediated clearance of apoB-containing lipoproteins. ACAT2–/– ApoE–/– mice and ACAT2+/+ ApoE–/– (control) mice had similar elevations of plasma apoB and total plasma lipids; however, the lipid cores of the apoB-containing lipoproteins in ACAT2–/– ApoE–/– mice contained primarily triglycerides rather than cholesterol esters. At 30 wk of age, only the control mice had significant atherosclerosis, which was nearly absent in ACAT2–/– ApoE–/– mice. ACAT2 deficiency in the apoE-deficient background also led to a compensatory increase in the activity of lecithin/cholesterol acyltransferase, the major plasma cholesterol esterification enzyme, which increased high-density lipoprotein cholesterol esters. Our results demonstrate the crucial role of ACAT2-derived cholesterol esters in the development of atherosclerosis in mice and suggest that triglyceride-rich apoB-containing lipoproteins are not as atherogenic as those containing cholesterol esters. Our results also support the rationale of pharmacological inhibition of ACAT2 as a therapy for atherosclerosis.
Cholesterol exists in two major forms in vertebrates: as a free sterol and as a cholesterol ester in which the sterol moiety is covalently attached to a long-chain fatty acid. Free cholesterol is found mainly in cell membranes, where it plays important roles in modulating membrane fluidity and permeability. When the cholesterol content of membranes becomes excessive, cholesterol esters are synthesized. These neutral lipids are poorly soluble in the membrane. Therefore, they are either stored in cytosolic lipid droplets or secreted from cells as components of apolipoprotein (apo) B-containing lipoproteins.
The synthesis of cholesterol esters is catalyzed by esterification enzymes. These enzymes include lecithin:cholesterol acyltransferase (LCAT), which functions in the plasma [primarily on high-density lipoproteins (HDL)], and acyl CoA:cholesterol acyltransferase (ACAT) (1–3), which functions intracellularly. There are two known ACAT enzymes, which are products of different genes. ACAT1 is present in many tissues, with the highest expression levels in steroidogenic tissues, sebaceous glands, and macrophages (4–6). ACAT2 is present primarily in the liver and small intestine (7–9).
Gene knockout studies in mice have helped to define the in vivo functions of ACAT1 and ACAT2. ACAT1-deficient (ACAT1–/–) mice are healthy but lack cholesterol esters in the adrenal cortex and in macrophages (10). The lack of ACAT activity in macrophages facilitated studies to examine the contribution of ACAT1 to macrophage foam-cell formation and atherosclerosis. In hyperlipidemic mouse models, selective ACAT1 deficiency did not prevent atherosclerosis (11, 12) and, in one study (13), caused increased lesions, possibly as a result of toxicity from free cholesterol.
ACAT2 deficiency in mice led to a loss of cholesterol esterification activity in the small intestine and liver (14). When ACAT2-deficient (ACAT2–/–) mice consumed a low-fat low-cholesterol diet, there were no obvious phenotypic consequences from the loss of ACAT2. However, when these mice were fed a diet rich in fat and cholesterol, they were protected from diet-induced hypercholesterolemia and gallstone formation (14). This protection appeared to result from a reduced capacity for intestinal cholesterol absorption, which served to “shield” the mice from the effects of the diet.
ACAT2–/– mice also had a near-complete lack of cholesterol esters in the apoB-containing lipoproteins (14). Instead, these lipoprotein particles contained mostly triglycerides. This finding prompted the current study to determine the role of ACAT2-mediated cholesterol ester synthesis in atherosclerosis. We sought to introduce ACAT2 deficiency into a mouse model of atherosclerosis to generate two groups of mice in which plasma apoB-containing lipoproteins were similarly elevated but the lipid composition differed (i.e., one with mainly cholesterol esters and one with triglycerides in these particles). We therefore crossed ACAT2–/– mice with mice lacking apoE, an atherosclerosis-susceptible strain that has impaired clearance of apoB-containing lipoproteins (15, 16).
A second goal of these studies was to examine the role of triglycerides in atherogenesis. Cholesterol esters have long been recognized for their association with atherosclerosis (17). However, epidemiologic studies examining the role of plasma triglycerides in atherosclerosis have yielded conflicting results and have been difficult to interpret, because hypertriglyceridemia is frequently accompanied by low-HDL cholesterol levels and other potentially proatherogenic metabolic derangements (18, 19). To shed light on this aspect of atherogenesis, we studied ACAT2–/– mice in which the cholesterol esters in apoB-containing lipoproteins were “genetically replaced” with triglycerides.
Methods
Mice.
ACAT2–/– mice (14) (50% C57BL/6 and 50% 129Sv/Jae genetic background) were crossed with ApoE–/– mice (100% C57BL/6 background) to generate ACAT2–/– ApoE–/– and ACAT2+/+ ApoE–/– (control) mice. Mice used in studies were ≈87.5% C57BL/6 and 12.5% 129Sv/Jae genetic background. Mice were housed in a pathogen-free barrier facility (12-h/12-h light/dark cycle) and were fed chow (PicoLab Rodent Diet 20, Purina) for 27 weeks after weaning. All experiments were approved by the Committee on Animal Research of the University of California, San Francisco.
Plasma Lipid and Lipoprotein Analyses.
Colorimetric assays were used to measure plasma levels of total cholesterol (Spectrum kit, Abbott), free cholesterol (Wako, Neuss, Germany), and triglycerides (Triglycerides/GB kit, Boehringer Ingelheim). Plasma HDL cholesterol was determined after precipitation of apoB-containing lipoproteins with polyethylene glycol–glycine. All lipid assays included a plasma-based internal standard (Accutrol, Sigma). Lipids in lipoprotein fractions were analyzed after separation of pooled plasma samples (n = 3) by FPLC with a Pharmacia Superose-6 column (20). Fractions were assayed for total cholesterol, free cholesterol, and triglycerides (Triglycerides, Boehringer), and cholesterol ester levels were calculated by subtracting values of free cholesterol from total cholesterol.
For apoB immunoblotting, plasma was separated by 4% SDS/PAGE, transferred to a polyvinylidene difluoride (PVDF) membrane, and incubated with a polyclonal antibody that recognizes the amino terminus of apoB (21) (a gift from S. Young, Gladstone Institute of Cardiovascular Disease, San Francisco). For apoAI immunoblotting, plasma was separated by 12% SDS/PAGE, transferred to a PVDF membrane, and incubated with a polyclonal antibody that recognizes apoAI (a gift from K. Weisgraber, Gladstone Institute of Cardiovascular Disease). For all immunoblotting, binding was detected by enhanced chemiluminescence (Amersham Pharmacia).
Atherosclerotic Lesion Analyses.
On the basis of power calculation analyses, we initially enrolled 20 female mice per genotype in atherosclerosis studies. However, because the differences in atherosclerosis between genotypes were so large, the study was terminated early. Atherosclerosis results for 13 ACAT2+/+ ApoE–/– and 17 ACAT2–/– ApoE–/– mice are reported.
Female mice were killed at 30 weeks of age, after 27 weeks of chow feeding. Blood was collected by cardiac puncture. Tissues were fixed by perfusion with 3% paraformaldehyde in phosphate buffer (pH 7.3), and aortas were removed, opened longitudinally from the heart to the iliac bifurcation, and pinned out flat (22). Aortic images were captured with a Polaroid digital camera (DMC1) mounted on a Leica MZ6 stereo microscope and analyzed with photoshop 5.0.1 (Adobe Systems, Mountain View, CA) and fovea pro 1.0 (Reindeer Graphics, Asheville, NC). An image of each aorta was captured and divided into three regions (arch, thorax, and abdomen), from which both aortic surface and atherosclerotic lesion areas were quantified. Lesion areas were calculated as a percentage of total surface area (23). To complement the aortic surface area results, aortic root morphology was also examined in three ACAT2+/+ ApoE–/– and three ACAT2–/– ApoE–/– mice that had total aortic lesion areas representative of the means for each genotype. Aortic roots were fixed by transcardial perfusion with 3% paraformaldehyde in phosphate buffer (pH 7.3), embedded in OCT compound (Sakura Tissue-Tek, Torrance, CA), frozen, sectioned, and stained with Oil red O.
Immunoblotting of Hepatic Proteins.
For scavenger receptor class B, type I (SR-BI) immunoblotting, 125 μg of protein from liver homogenates was separated by 10% SDS/PAGE, transferred to a polyvinylidene difluoride membrane, and incubated with a polyclonal antibody that recognizes SR-BI (Novus Biologicals, Littleton, CO). Binding was detected by enhanced chemiluminescence (Amersham Pharmacia). The membrane was then stripped and reprobed with an antibody that recognizes low-density lipoprotein (LDL) receptor-related protein (a gift from J. Herz, University of Texas Southwestern, Dallas) to control for equal loading of protein per lane.
LCAT Activity.
LCAT activity in plasma was assayed as described (24). Briefly, recombinant HDL (rHDL) were synthesized from phosphatidylcholine:14C-cholesterol:apoAI in a molar ratio of 80:5:1. Cholesterol esterification was measured by adding rHDL (1.2 μg) to 500 μl of 1× Tris-borate-EDTA buffer containing 0.6% BSA, 2-mercaptoethanol, and 5 μl of mouse plasma as the LCAT source. Tubes were incubated at 37°C with shaking for 5 min, and the reaction was stopped by adding CHCl3:MeOH (1:2) containing 20 μg/ml free cholesterol and cholesterol ester. Lipids were extracted, resuspended in CHCl3:MeOH (2:1), and separated by TLC in hexane/ether/acetic acid (70:30:2, vol/vol/vol). The free cholesterol and cholesterol ester bands were scraped, and radioactivity was counted in a liquid scintillation counter. Fractional cholesterol esterification was calculated as dpm in cholesterol esters divided by dpm in cholesterol esters plus free cholesterol. Specific activity was calculated from the fractional esterification and the specific activity of the labeled substrate.
Cholesterol Ester Fatty Acid Composition.
Lipids were isolated from plasma by chloroform–methanol extraction (25) with cholesteryl tridecanoate added as an internal standard. Cholesterol esters were isolated by TLC (Silica Gel 60, EM Science), and the fatty acid moieties of the cholesterol esters were converted to fatty acid methyl esters for analysis by gas–liquid chromatography as described (26).
Statistical Methods.
Data are shown as mean ± SD unless stated otherwise. Means were compared with the Mann–Whitney rank–sum test. Regression analyses were performed with statview (SAS Institute, Cary, NC).
Results
Changes in Plasma Lipids and Lipoproteins in ACAT2–/– ApoE–/– Mice.
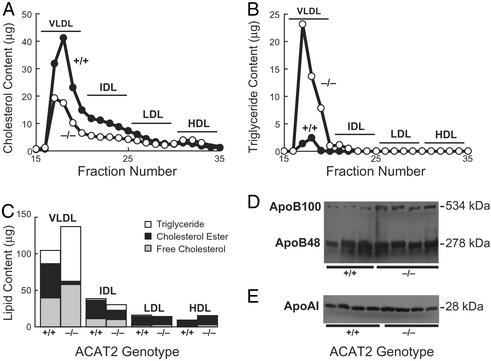
Introducing ACAT2 deficiency into ApoE–/– mice had marked effects on their plasma lipids (Table 1). Total plasma cholesterol levels were nearly 60% lower in ACAT2–/– ApoE–/– mice than in ACAT2+/+ ApoE–/– control mice. This reduction was due primarily to a >70% reduction in plasma cholesterol esters in the ACAT2–/– ApoE–/– mice. The reduction in plasma total cholesterol was due to lower cholesterol content in very low-density lipoproteins (VLDL) and the intermediate-density lipoproteins (Fig. 1A). Plasma-free cholesterol was also reduced by 18% in ACAT2–/– ApoE–/– mice.
Table 1.
Plasma lipid levels
| ACAT2+/+ApoE−/− | ACAT2−/−ApoE−/− | P | |
|---|---|---|---|
| Total cholesterol (TC) | 436.6 ± 91.7 | 179.7 ± 52.4 | <0.0001 |
| Cholesterol esters (CE) | 284.5 ± 71.3 | 78.8 ± 19.5 | 0.0005 |
| Free cholesterol (FC) | 130.4 ± 21.7 | 106.9 ± 18.6 | 0.028 |
| HDL cholesterol | 18.5 ± 5.7 | 29.8 ± 8.1 | 0.0019 |
| Triglyceride (TG) | 56.7 ± 26.7 | 108.3 ± 46.0 | 0.015 |
Plasma lipids (mg/dl) were measured in adult (20- to 26-week-old) female mice (ACAT2+/+ ApoE−/−: TC, n = 10; CE, n = 7; FC, n = 7, HDL, n = 10; TG, n = 9. ACAT2−/− ApoE−/−: TC, n = 20; CE, n = 11; FC, n = 12; HDL, n = 16; TG, n = 12). Data are shown as mean ± SD. P values were determined with the Mann–Whitney test.
Figure 1.
Plasma lipoproteins and apolipoproteins in chow-fed ACAT2+/+ ApoE–/– and ACAT2–/– ApoE–/– mice. Total cholesterol levels (A) and triglyceride levels (B) in plasma fractions isolated by FPLC are shown. Plasma samples from three 24- to 26-week-old female mice of each genotype were pooled for analysis. The experiment was repeated twice, and similar results were obtained, although some variability in triglyceride content was observed. (C) Plasma lipid composition in lipoproteins from female mice. Plasma samples from three mice of each genotype were pooled, and lipoproteins were separated by FPLC. Lipid contents in fractions were summed to determine the content of each of the major lipoprotein classes (VLDL, fractions 17–20; intermediate-density lipoproteins, fractions 21–25; LDL, fractions 26–30; HDL, fractions 31–35). Cholesterol ester values reflect the esterified cholesterol mass in the sample. (D) Plasma apoB levels. Plasma samples (0.5 μl) were subjected to SDS/PAGE and immunoblotting for apoB. Data are shown for three ACAT2+/+ ApoE–/– mice and four ACAT2–/– ApoE–/– mice. (E) Plasma apoAI levels. Plasma samples (1 μl) were subjected to SDS/PAGE and immunoblotting for apoAI. Data are shown for four mice of each genotype.
In contrast, plasma triglyceride levels were ≈90% higher in ACAT2–/– ApoE–/– mice than in controls. This increase was due to a higher triglyceride content in VLDL and intermediate-density lipoproteins (Fig. 1B). The total plasma lipid content in the lipoproteins was similar in ACAT2–/– ApoE–/– mice and controls, with the chief effect of ACAT2 deficiency being a marked reduction in the cholesterol ester content of the VLDL and intermediate-density lipoproteins and a corresponding increase in the triglyceride content in these lipoproteins (Fig. 1C).
Plasma apoB levels directly reflect the numbers of plasma lipoprotein particles (27) and correlate with atherosclerosis risk (28). Plasma levels of apoB48 were similarly elevated in ACAT2–/– ApoE–/– mice and controls (Fig. 1D), and plasma apoB100 levels appeared to be higher in the ACAT2–/– ApoE–/– mice. Thus, compared with ApoE–/– mice, which have impaired clearance of apoB48-containing lipoproteins (15, 16), the ACAT2–/– ApoE–/– mice had similar or higher levels of apoB-containing lipoproteins whose lipid composition differed from that in ApoE–/– mice.
In previous studies (14), ACAT2 deficiency did not affect plasma HDL cholesterol levels in mice fed chow or high-fat diets. However, in the setting of apoE deficiency, ACAT2 deficiency resulted in a 58% increase in plasma HDL cholesterol levels (Table 1 and Fig. 1C). The plasma levels of apoAI, the major apolipoprotein component of HDL, were similar in ACAT2–/– ApoE–/– mice and controls (Fig. 1E), indicating that, in HDL, the primary effect of ACAT2 deficiency was on the lipid content of the particles.
Prevention of Atherosclerosis in ACAT2–/– ApoE–/– Mice.
Atherosclerosis was nearly absent in ApoE–/– mice that lacked ACAT2 (Fig. 2). After 27 weeks on a chow diet, atherosclerotic lesions were present on the aortic surface in control mice (4.8 ± 2.9% of surface area) but not in ACAT2–/– ApoE–/– mice (0.1 ± 0.2%, P < 0.01) (Fig. 2A). This reduction in atherosclerosis in ACAT2–/– ApoE–/– mice was present in all regions of the aorta, with the most striking difference found in the proximal portion of the aortas (Figs. 2B and 3A). Examination of aortic root sections in a subset of mice confirmed that lesions were nearly absent in ACAT2–/– ApoE–/– mice (Fig. 3B). A similar prevention of atherosclerosis was observed in a separate study in which small numbers of mice were fed a Western-type diet for 9 weeks (not shown).
Figure 2.
Prevention of atherosclerosis in ACAT2–/– ApoE–/– mice. (A) Lesion areas as a percentage of total aortic surface in ACAT2+/+ ApoE–/– (n = 13) and ACAT2–/– ApoE–/– (n = 17) mice after 27 weeks of chow feeding. The horizontal bar indicates the mean. *, P < 0.001. (B) Lesion areas in the proximal (arch), middle (thorax), and distal (abdomen) thirds of aortas. *, P < 0.01. (C) Regression analysis of total aortic lesion areas and plasma lipid levels. Data are plotted for those mice in which both lipid levels and atherosclerosis levels were measured. Regression lines represent data from both genotypes.
Figure 3.
Representative images showing the extent of aortic atherosclerosis in chow-fed ACAT2+/+ ApoE–/– and ACAT2–/– ApoE–/– mice. (A) Pinned-out aortas showing surface lesions (arrows) in ACAT2+/+ ApoE–/– but not in ACAT2–/– ApoE–/– mice. (B) Cross sections of proximal aortic roots showing Oil-red-O-staining lesions (arrow points to example) in ACAT2+/+ ApoE–/– but not in ACAT2–/– ApoE–/– mice. (Bar = 500 μm.)
We examined the relationship of the extent of aortic atherosclerosis with plasma lipid levels for individual mice in the two study groups. Lesion area correlated best with total plasma cholesterol levels, although this positive correlation was attributable mainly to the correlation in control mice (Fig. 2C). In contrast, plasma triglyceride levels did not correlate with lesion area. As expected, there was a significant inverse correlation between plasma HDL cholesterol levels and lesion area.
Increased LCAT Activity in ACAT2–/– ApoE–/– Mice.
Because the increase in plasma HDL cholesterol in ACAT2–/– ApoE–/– mice was unexpected, we further investigated the mechanism for this finding. This increase was due mainly to an increase in cholesterol ester content (Fig. 1C and data not shown). Cholesterol esters in plasma HDL are cleared mainly by a selective uptake mechanism in hepatocytes that is mediated by SR-BI (29), and ApoE–/– mice have increased hepatic SR-BI levels (30). However, hepatic SR-BI levels were not different in ACAT2–/– ApoE–/– mice and controls (Fig. 4A), suggesting that impaired clearance did not account for the increase in HDL cholesterol esters in ACAT2–/– ApoE–/– mice. In contrast, activity levels of LCAT, the major enzyme responsible for synthesizing cholesterol esters in plasma, were significantly higher in ACAT2–/– ApoE–/– mice than in controls (Fig. 4B). Confirming this observation, the plasma cholesterol esters of ACAT2–/– ApoE–/– mice reflected increased LCAT activity (Fig. 4C), with greater proportional content of very long-chain polyunsaturated fatty acids, including C20:4ω6, C20:5ω3, and C22:6ω3, and lower proportions of long-chain saturated fatty acids, such as C16:0, C16:1, C17:0, and C18:1Δ9 (26). LCAT-derived cholesterol esters were present in apoB-containing lipoproteins and HDL (Fig. 1C).
Figure 4.
Analysis of determinants of HDL metabolism in ACAT2+/+ ApoE–/– and ACAT2–/– ApoE–/– mice. (A) Similar levels of SR-BI protein in livers from ACAT2+/+ ApoE–/– and ACAT2–/– ApoE–/– mice. Homogenized liver samples (125 μg of total protein) were subjected to SDS/PAGE and immunoblotting for SR-BI. Membranes were stripped and immunoblotting for the LDL receptor-related protein (LRP) was performed as a control for protein content. Data for four mice of each genotype are shown. (B) Increased LCAT activity in ACAT2–/– ApoE–/– mice. LCAT activity was determined in plasma samples from six mice of each genotype. *, P = 0.02. (C) Altered composition of plasma cholesterol esters in ACAT2–/– ApoE–/– mice. Cholesterol esters were isolated by TLC from plasma samples from six mice of each genotype. The fatty acid content of the cholesterol esters was analyzed by gas chromatography. Data for each fatty acid are expressed as a percentage (wt/wt) of the total cholesterol esters. *, P < 0.05.
Discussion
This study demonstrates that ACAT2-mediated cholesterol ester synthesis is crucial for atherogenesis in mice. The absence of ACAT2 in the small intestine and liver almost completely prevented atherosclerosis in apoE-deficient mice. Our findings implicate ACAT2 activity as a major determinant of susceptibility to atherosclerosis. The results also suggest that apoB-containing lipoproteins that are rich in triglycerides, as in ACAT2–/– ApoE–/– mice, are not as atherogenic as those containing cholesterol esters.
Previously, we showed that ACAT2 deficiency in the small intestine and liver of mice limits intestinal cholesterol absorption and cholesterol gallstone formation in response to diet and prevents the accumulation of cholesterol esters in the plasma lipoproteins (14). Now we show that the protective effects of ACAT2 deficiency on cholesterol-mediated disease extend to atherosclerosis. The protection from atherosclerosis we found in apoE-deficient mice lacking ACAT2 indicates that ACAT2 function is required for atherosclerosis to develop in this model. This conclusion agrees with the observation that hepatic ACAT activity levels correlate with atherosclerosis susceptibility in primates (31, 32). These findings suggest that conditions that increase intestinal and hepatic ACAT activity may promote atherosclerosis.
We suspect that the protective effect of ACAT2 deficiency on atherosclerosis relates primarily to the replacement of cholesterol esters with triglycerides in the cores of apoB-containing lipoproteins. The shift toward more polyunsaturated fatty acids in the cholesterol esters may also have contributed to atherosclerosis protection. Plasma apoB levels are highly correlated with atherosclerosis risk (28). However, the ACAT2–/– ApoE–/– mice did not develop atherosclerosis despite their high plasma levels of triglyceride-rich apoB-containing lipoproteins. This finding is of interest because of the controversy concerning plasma triglyceride levels and atherosclerosis. Elevated plasma triglycerides are considered a potential risk factor for atherosclerosis. However, hypertriglyceridemia is often accompanied by multiple potentially proatherogenic metabolic derangements, including low plasma HDL cholesterol levels, which makes the risk attributable to hypertriglyceridemia per se difficult to assess (reviewed in refs. 33 and 34). Our findings in this study strongly suggest that triglyceride-rich apoB-containing lipoproteins are not as atherogenic as those that are rich in cholesterol esters, although, strictly speaking, ACAT2–/– ApoE–/– mice are not a model of marked hypertriglyceridemia. Nevertheless, these data support the hypothesis that the inflammatory response in atherosclerosis occurs primarily in response to toxicity from cholesterol accumulation in lesions.
The increased plasma HDL cholesterol levels in ACAT2–/– ApoE–/– mice were unexpected because neither ACAT2- nor apoE-deficient mice have increases in HDL cholesterol levels. The increase in HDL cholesterol was primarily due to an increase in the cholesterol ester content; apoAI levels were not increased. The increase in HDL plasma cholesterol esters apparently results from an increase in LCAT activity, which may reflect a compensatory increase in response to an increased availability of cholesterol substrate on HDL particles in the absence of ACAT2 activity. This increase in LCAT activity and HDL cholesterol esters also may have contributed to the antiatherogenic effect of ACAT2 deficiency. However, we believe that this contribution is likely to have been small. The average increase in HDL cholesterol in ACAT2–/– ApoE–/– mice was ≈11 mg/dl, an increase that is unlikely to have caused atherosclerosis to decrease by ≈80%. Moreover, studies of transgenic mice overexpressing LCAT (with associated activity levels much higher than in ACAT2–/– ApoE–/– mice) have found either no difference or an increased susceptibility to atherosclerosis (35–37).
Our findings provide some insight into ongoing strategies to develop ACAT inhibitors to prevent or treat atherosclerosis. Because cholesterol esters play a prominent role in atherogenesis, numerous ACAT inhibitors have been developed in the past 20 years (reviewed in ref. 2). However, these agents were developed before the discovery of two different ACATs, and most of these compounds therefore act nonselectively. Recent studies in ACAT1–/– mice indicate that the selective loss of ACAT1 activity in the setting of hyperlipidemia does not prevent atherosclerosis and may cause detrimental effects due to free cholesterol deposition in tissues (11, 13). Conversely, the current study suggests that selective loss of ACAT2 activity in mice may be useful in preventing or treating atherosclerosis, in addition to preventing hypercholesterolemia and gallstones induced by dietary cholesterol (14). Taken together, our studies and those of others using nonselective inhibitors in animals (38–40) suggest that nonselective ACAT inhibitors or inhibitors that are more selective for ACAT2 may be the most desirable to study in humans.
Our findings highlight the role of ACAT2-mediated cholesterol ester synthesis in promoting atherosclerosis in mice. Genetic variability in ACAT2 gene expression may therefore contribute to susceptibility to atherosclerosis (32), as well as to diet-induced hypercholesterolemia and gallstones (14) in mice. Whether alterations in ACAT2 activity influence atherosclerosis susceptibility in humans is currently unclear. Important differences in lipoprotein metabolism and atherosclerosis development exist between mice and humans, and these differences limit the ability to extrapolate between species. For example, whereas ACAT2 is the major ACAT that functions in mouse liver, the contribution of ACAT2 (versus ACAT1) to hepatic cholesterol esterification activity in humans is uncertain. In addition, depletion of cholesterol esters in apoB-containing lipoproteins due to reduced ACAT2 activity is less likely to be as marked in humans, because humans (but not mice) have transfer of cholesterol esters from HDL to apoB-containing lipoproteins by cholesterol ester transfer protein. Nevertheless, our data provide further support for the rationale of inhibiting hepatic and intestinal ACAT activity as a pharmaceutical strategy to reduce atherosclerosis.
Acknowledgments
We thank R. Choi and Y. Terasawa for assistance in the early phases of these studies; R. Bituin for assistance in mouse husbandry; S. Ordway and G. Howard for editorial assistance; and H. Chen, K. Weisgraber, J. Parks, and S. Young for comments on the manuscript. This work was supported by National Institutes of Health Grants (NIH) (R01-HL57170 (to R.F.) and HL-49373 (to L.R.), an NIH postdoctoral fellowship training grant (to K.B.), a Sarnoff Fellowship (to E.W.), and the J. David Gladstone Institutes.
Abbreviations
- apo
apolipoprotein
- LCAT
lecithin:cholesterol acyltransferase
- HDL
high-density lipoprotein
- ACAT
acyl CoA:cholesterol acyltransferase
- ACAT1–/–
ACAT1-deficient
- ACAT2–/–
ACAT2-deficient
- SR-BI
scavenger receptor class B, type I
- LDL
low-density lipoprotein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Chang T Y, Chang C C Y, Cheng D. Annu Rev Biochem. 1997;66:613–638. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 2.Buhman K F, Accad M, Farese R V., Jr Biochim Biophys Acta. 2000;1529:142–154. doi: 10.1016/s1388-1981(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 3.Rudel L L, Lee R G, Cockman T L. Curr Opin Lipidol. 2001;12:121–127. doi: 10.1097/00041433-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Chang C C Y, Huh H Y, Cadigan K M, Chang T Y. J Biol Chem. 1993;268:20747–20755. [PubMed] [Google Scholar]
- 5.Uelmen P J, Oka K, Sullivan M, Chang C C Y, Chang T Y, Chan L. J Biol Chem. 1995;270:26192–26201. doi: 10.1074/jbc.270.44.26192. [DOI] [PubMed] [Google Scholar]
- 6.Meiner V, Tam C, Gunn M D, Dong L-M, Weisgraber K H, Novak S, Myers H M, Erickson S K, Farese R V., Jr J Lipid Res. 1997;38:1928–1933. [PubMed] [Google Scholar]
- 7.Cases S, Novak S, Zheng Y-W, Myers H M, Lear S R, Sande E, Welch C B, Lusis A J, Spencer T A, Krause B R, et al. J Biol Chem. 1998;273:26755–26764. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- 8.Anderson R A, Joyce C, Davis M, Reagan J W, Clark M, Shelness G S, Rudel L L. J Biol Chem. 1998;273:26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 9.Oelkers P, Behari A, Cromley D, Billheimer J T, Sturley S L. J Biol Chem. 1998;273:26765–26771. doi: 10.1074/jbc.273.41.26765. [DOI] [PubMed] [Google Scholar]
- 10.Meiner V L, Cases S, Myers H M, Sande E R, Bellosta S, Schambelan M, Pitas R E, McGuire J, Herz J, Farese R V., Jr Proc Natl Acad Sci USA. 1996;93:14041–14046. doi: 10.1073/pnas.93.24.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Accad M, Smith S J, Newland D L, Sanan D A, King L E, Jr, Linton M F, Fazio S, Farese R V., Jr J Clin Invest. 2000;105:711–719. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagyu H, Kitamine T, Osuga J-I, Tozawa R-I, Chen Z, Kaji Y, Oka T, Perrey S, Tamura Y, Ohashi K, et al. J Biol Chem. 2000;275:21324–21330. doi: 10.1074/jbc.M002541200. [DOI] [PubMed] [Google Scholar]
- 13.Fazio S, Major A S, Swift L L, Gleaves L A, Accad M, Linton M F, Farese R V., Jr J Clin Invest. 2001;107:163–171. doi: 10.1172/JCI10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhman K K, Accad M, Novak S, Choi R S, Wong J S, Hamilton R L, Turley S, Farese R V., Jr Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 15.Plump A S, Smith J D, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft J G, Rubin E M, Breslow J L. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 17.Windaus A. Hoppe-Seyler's Z Physiol Chem. 1910;67:174–176. [Google Scholar]
- 18.Grundy S M, Vega G L. Arch Intern Med. 1992;152:28–34. [PubMed] [Google Scholar]
- 19.Miller M. Clin Cardiol. 1999;22:II-1–6. doi: 10.1002/clc.4960221402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horie Y, Fazio S, Westerlund J R, Weisgraber K H, Rall S C., Jr J Biol Chem. 1992;267:1962–1968. [PubMed] [Google Scholar]
- 21.McCormick S P A, Ng J K, Véniant M, Borén J, Pierotti V, Flynn L M, Grass D S, Connolly A, Young S G. J Biol Chem. 1996;271:11963–11970. doi: 10.1074/jbc.271.20.11963. [DOI] [PubMed] [Google Scholar]
- 22.Palinski W, Tangirala R K, Miller E, Young S G, Witztum J L. Arterioscler Thromb Vasc Biol. 1995;15:1569–1576. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 23.Terasawa Y, Ladha Z, Leonard S W, Morrow J D, Newland D, Sanan D, Packer L, Traber M G, Farese R V., Jr Proc Natl Acad Sci USA. 2000;97:13830–13834. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks J S, Gebre A K, Furbee J W. Methods Mol Biol. 1999;109:123–131. doi: 10.1385/1-59259-581-2:123. [DOI] [PubMed] [Google Scholar]
- 25.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Furbee J W, Jr, Francone O, Parks J S. J Lipid Res. 2002;43:428–437. [PubMed] [Google Scholar]
- 27.Kane J P, Havel R J. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, Childs B, Kinzler K W, Vogelstein B, editors. Vol. 2. New York: McGraw–Hill; 2001. pp. 2717–2752. [Google Scholar]
- 28.Sniderman A, Shapiro S, Marpole D, Skinner B, Teng B, Kwiterovich P O., Jr Proc Natl Acad Sci USA. 1980;77:604–608. doi: 10.1073/pnas.77.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trigatti B, Rigotti A, Kreiger M. Curr Opin Lipidol. 2000;11:123–131. doi: 10.1097/00041433-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Arai T, Rinninger F, Varban L, Fairchild-Huntress V, Liang C-P, Chen W, Seo T, Deckelbaum R, Huszar D, Tall A R. Proc Natl Acad Sci USA. 1999;96:12050–12055. doi: 10.1073/pnas.96.21.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr T P, Parks J S, Rudel L L. Arterioscler Thromb. 1992;12:1274–1283. doi: 10.1161/01.atv.12.11.1274. [DOI] [PubMed] [Google Scholar]
- 32.Rudel L L, Davis M, Sawyer J, Shah R, Wallace J. J Biol Chem. 2002;277:31401–31406. doi: 10.1074/jbc.M204106200. [DOI] [PubMed] [Google Scholar]
- 33.Grundy S M. Am J Cardiol. 1999;83:25F–29F. doi: 10.1016/s0002-9149(99)00211-8. [DOI] [PubMed] [Google Scholar]
- 34.Rubins H B. J Cardiovasc Risk. 2000;7:339–345. doi: 10.1177/204748730000700507. [DOI] [PubMed] [Google Scholar]
- 35.Berard A M, Foger B, Remaley A, Shamburek R, Vaisman B L, Talley G, Paigen B, Hoyt R F, Jr, Marcovina S, Brewer H B, Jr, et al. Nat Med. 1997;3:744–749. doi: 10.1038/nm0797-744. [DOI] [PubMed] [Google Scholar]
- 36.Mehlum A, Muri M, Hagve T A, Solberg L A, Prydz H. Acta Pathol Microbiol Scand. 1997;105:861–868. doi: 10.1111/j.1699-0463.1997.tb05095.x. [DOI] [PubMed] [Google Scholar]
- 37.Furbee J W, Jr, Parks J S. Atherosclerosis. 2002;165:89–100. doi: 10.1016/s0021-9150(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 38.Bocan T M A, Krause B R, Rosebury W S, Mueller S B, Lu X, Dagle C, Major T, Lathia C, Lee H. Arterioscler Thromb Vasc Biol. 2000;20:70–79. doi: 10.1161/01.atv.20.1.70. [DOI] [PubMed] [Google Scholar]
- 39.Delsing D J M, Offerman E H, van Duyvenvoorde W, van der Boom H, de Wit E C M, Gijbels M J J, van der Laarse A, Jukema J W, Havekes L M, et al. Circulation. 2001;103:1778–1786. doi: 10.1161/01.cir.103.13.1778. [DOI] [PubMed] [Google Scholar]
- 40.Kusunoki J, Hansoty D K, Aragane K, Fallon J T, Badimon J J, Fisher E A. Circulation. 2001;103:2604–2609. doi: 10.1161/01.cir.103.21.2604. [DOI] [PubMed] [Google Scholar]