Abstract
It has been hypothesized that individuals who have higher demands for spatially based behaviours should show increases in hippocampal attributes. Some avian species have been shown to use a spatially based representation of their environment during migration. Further, differences in hippocampal attributes have been shown between migratory and non-migratory subspecies as well as between individuals with and without migratory experience (juveniles versus adults). We tested whether migratory behaviour might also be associated with increased hippocampal neurogenesis, and whether potential differences track previously reported differences in hippocampal attributes between a migratory (Zonotrichia leucophrys gambelii) and non-migratory subspecies (Z. l. nuttalli) of white-crowned sparrows. We found that non-migratory adults had relatively fewer numbers of immature hippocampal neurons than adult migratory birds, while adult non-migrants had a lower density of new hippocampal neurons than adult and juvenile migratory birds and juvenile non-migratory birds. Our results suggest that neurogenesis decreases with age, as juveniles, regardless of migratory status, exhibit similar and higher levels of neurogenesis than non-migratory adults. However, our results also suggest that adult migrants may either seasonally increase or maintain neurogenesis levels comparable to those found in juveniles. Our results thus suggest that migratory behaviour in adults is associated with maintained or increased neurogenesis and the differential production of new neurons may be the mechanism underpinning changes in the hippocampal architecture between adult migratory and non-migratory birds.
Keywords: neurogenesis, migration, white-crowned sparrows, doublecortin, spatial processing
1. Introduction
Differential demands on learning and memory have been shown to affect brain structures differently. This has been well-documented in the hippocampus, the area of the brain thought to be involved in spatial processing. Animals that have a greater reliance on spatial learning and memory tend to have larger hippocampi, more hippocampal neurons, and increased neurogenesis [1–6]. It has been shown that adults of some avian species use a learned representation of their spatial environment to orient during navigation and this representation appears to involve the hippocampus (e.g. [7]). For example, some studies have suggested that long-distance adult migratory birds use a learned spatial representation of their environment rather than a purely innate mechanism to navigate during migration (e.g. [8,9]). Consistent with these findings, migratory birds have also been shown to have better long-term memory [10], better spatial memory [11,12], and increased hippocampal volume and number of neurons than non-migratory birds [11–13]. This is presumed to be due to the need to learn and remember the spatial information associated with the migratory route and stopover sites (e.g. [10]). Thus, it is likely that there is a relationship between migratory behaviour, the need to learn and remember the spatial environment during migration, and the architecture of the hippocampus.
However, the mechanism underlying the maintenance of increased hippocampal volume and neuron numbers in migratory birds is unresolved. One possibility is that the increase in hippocampal volume and number of neurons may be a result of the addition of new neurons. It is now accepted that hippocampal neurons are continually produced throughout life, although the rate of production declines with age (e.g. [14–18]). These new neurons appear to structurally integrate into the hippocampal neural network, possess characteristics of functional neurons, and appear to be important for spatial discrimination [19–22]. Further, the use of spatial memory and learning has been shown to affect hippocampal neurogenesis in mammals and birds [6,23,24]. Consequently, a greater production of new hippocampal neurons owing to increased demands on learning and memory during migration may underlie differences in hippocampal attributes between migratory and non-migratory birds.
Because some migratory birds have been suggested to use spatial memory and learning to a large extent during migration (e.g. [10]), our study addresses whether increased hippocampal neurogenesis is associated with migration and if the addition of new neurons into the hippocampus tracks the increase in hippocampal attributes previously shown in migratory birds (e.g. [11–13]). We tested whether adult migratory Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii) exhibit increased neurogenesis compared with adult individuals of a closely related non-migratory subspecies, Nuttall's white-crowned sparrows (Z. l. nuttalli). The Gambel's subspecies of white-crowned sparrow is a long-distance migrant that uses multiple stopover sites [25], and thus, may be particularly dependent upon spatial memory during migration. We predicted that adult migrants should show increased levels of neurogenesis during migration compared with adult non-migratory birds during the same time period, possibly owing to increased demands on spatial processing.
Further, we also address whether these potential differences in neurogenesis between migratory and non-migratory birds could be related to experience with the migratory route and/or development by comparing adults and juveniles of both the migratory and non-migratory subspecies. Previous work indicates that migratory juvenile Gambel's white-crowned sparrows use an innate directional programme during their first migration, whereas adults seem to use experience ([9]; see also [8]). Because of this, we also predicted that migratory adults should show increased neurogenesis compared with migratory juveniles. Non-migratory juveniles and non-migratory adults should exhibit similar levels of neurogenesis. An alternative prediction, since neurogenesis has been shown to be very high during ontogeny and decline with age (e.g. [14–18]), is that juveniles of both subspecies will have greater levels of neurogenesis than their respective adult counterparts. However, migratory adults may counteract the downregulation of neurogenesis occurring during ageing with upregulation owing to spatial processing during migration. If so, we would expect to see the number of new neurons in adult migrants more similar to juveniles than to adult non-migrants.
To visualize neurogenesis, we used the marker doublecortin. Doublecortin only labels proteins specifically expressed in immature neurons that were produced within the past approximately 25 days [26–28] and thus reflects hippocampal neurogenesis specifically during the migration period in Gambel's white-crowned sparrows (approx. 29 days; [29]). Because doublecortin is an endogenous marker, it appears to be a viable alternative to the traditional BrdU method for detecting and quantifying neurogenesis [28,30–32]. Using exogenous markers such as BrdU would be extremely difficult in migratory birds as the same individuals would need to be captured twice, prior to and after the migration.
2. Material and methods
The two subspecies used in this study, Z. l. gambelii and Z. l. nuttalli, differ greatly in their migration habits. Zonotrichia leucophrys gambelii migrate yearly from their breeding grounds in Alaska and Canada to their wintering grounds in California [25]. Conversely, adult and juvenile non-migratory Z. l. nuttalli are sedentary and are found year-round on the coast of California [25].
All subjects were collected as part of an earlier study [12]. Briefly, 14 migratory Z. l. gambelii were captured on 8 October 2004 during migration near Davis, CA, USA. Individuals were identified as adults (n = 7) or juveniles (n = 7) based on plumage colour. Because they were caught during fall migration, adult migratory subjects had completed at least 1.5 full migrations whereas juveniles had completed only one-half of a full migration. Thus, at the time of capture, juveniles had not probably engaged a learned, spatially based navigational system and were considered inexperienced. All migratory individuals were sacrificed on 9 October 2004. Seven adult and seven juvenile non-migratory Z. l. nuttalli were captured on 12 October 2004 near the coast in Sonoma County and sacrificed on 14 October 2004.
All birds were perfused and their brains were removed, processed and sectioned as previously described [12]. Briefly, birds were anaesthetized with a lethal overdose of Nembutal and transcardially perfused with 4 per cent paraformaldehyde in 0.1 M phosphate buffer. Brains were extracted and post-fixed in 4 per cent paraformaldehyde for one week before cryoprotection. Brains were cryoprotected in 30 per cent sucrose and flash-frozen on dry ice. Brains were stored at −70°C until sectioning. Brains were sectioned on a cryostat at −20°C in the coronal plane every 40 µm and every 12th section was subjected to immunohistochemistry.
Sections were processed for doublecortin, an endogenous marker of immature neurons [6,28,30–33]. In both birds and mammals, only new neurons express doublecortin (e.g. [26–28]), which eliminates the need for BrdU injections and double labelling to establish neuronal identity. Changes in neurogenesis measured with doublecortin in response to behavioural experiences known to affect neurogenesis mirror changes measured with BrdU labelling (32). Unlike BrdU, doublecortin shows only transient expression, which lasts approximately 25 days in passerine birds [28]. Therefore, neurogenesis measured by using doublecortin only labels neurons that were produced within 25 days of sacrifice. Doublecortin is well suited to answer questions regarding the birth of new neurons during migration of Z. l. gambelii, as this subspecies has been noted to have a fairly long temporal migration (approx. 29 days; [29]). Since doublecortin only labels new neurons born within the past 25 days, our data were collected on neurons that were born during migration only.
To visualize doublecortin, sections were washed in Tris-buffered saline (TBS), incubated in 30 per cent hydrogen peroxide plus TBS (1 : 50) at room temperature for 30 min, washed in TBS, incubated in blocking buffer (normal horse serum (1∶33.3), TX-100 (1∶39.8) and TBS) at room temperature for 30 min, and then incubated in anti-doublecortin antibody plus blocking buffer (1 : 200; Santa Cruz Biotechnology, Santa Cruz, CA, USA; SC-8066) overnight (approx. 18 h) at 4°C. The following day, sections were washed in TBS, incubated in biotinylated horse anti-goat antibody in blocking buffer (1∶200; Vector Laboratories, Burlingame, CA, USA; BA-9500) at room temperature for 2 h, washed in TBS, incubated in ABC Elite kit (Vector Laboratories; PK-6100) at room temperature for 1 h, washed in TBS, reacted with DAB + nickle kit (Vector Laboratories; SK-4100) at room temperature for 2 min, washed in TBS, and mounted on slides. Slides were dried, lightly Nissl-stained, and covered with a coverslip.
We also performed a negative control to account for non-specific binding of the secondary antibody. To do so, we used the same protocol as above, but replaced the anti-doublecortin antibody with TBS during the overnight incubation. We found that the elimination of the primary antibody suppressed staining, indicating that our protocol specifically stained cells that expressed doublecortin, rather than staining non-specifically.
The lateral hippocampal formation boundary was determined by the change in density of Nissl-stained cells at the boundary, as per [1] and as in our previous study [6]. Doublecortin-labelled cells within the hippocampus were easily identified, as they stained darkly compared with surrounding Nissl-stained cells. They were also morphologically similar to doublecortin-labelled cells described by Boseret et al. [34]—round multipolar cells and fusiform cells, which are typical morphotypes of differentiating neurons and migrating neurons, respectively. From a previous study with these individuals, we had used stereological measurements to estimate hippocampal volume using the Cavalieri method (StereoInvestigator software, Microbrightfield, Inc., Colchester, VT, USA; microscope, Leica M4000B, Bannockburn, IL, USA; values reported in and taken from Pravosudov et al. [12]).
The number of doublecortin-positive cells was high, preventing exhaustive counts throughout the entire hippocampal formation. Consequently, we used the optical fractionator method [35] to estimate the number of doublecortin-positive cells, similar to previous studies [6,31,32]. Doublecortin neuron counts were performed with an optimal grid size of 130 µm, counting frame of 70 × 70 µm, and dissector height of 5 µm. The left and right hemispheres were both measured for neurons expressing doublecortin and then summed to produce the given values. There were no significant differences between left and right hemispheres of the hippocampus in terms of the number of neurons expressing doublecortin (paired t-test: t27 =− 1.67, p = 0.105).
To ascertain if the effect of our groups was global (i.e. occurring outside of the hippocampus), we also estimated the number of doublecortin-positive cells and the density of doublecortin-positive cells in another brain region, the hyperpallium apicale (HA; formerly the hyperstriatum accessorium), which is laterally adjacent to the hippocampus. We followed the protocol of Barnea & Nottebohm [36] and LaDage et al. [6], measuring the number of cells in the HA, up to a distance of 3 mm from the midline. Again, the doublecortin-positive cells were estimated in the left and right hemispheres of the HA and summed. There were significant differences between the right and left hemispheres of the HA in number and density of doublecortin-positive cells (paired t-test, number: t27 =− 2.907, p = 0.007; density: t27 =− 3.549, p = 0.001).
Differences among treatments in the number of neurons expressing doublecortin were determined by general linear model (GLM), followed by two-tailed Newman–Keuls pairwise comparisons when appropriate. The density of neurons expressing doublecortin was calculated by dividing the number of neurons expressing doublecortin by the total volume of the hippocampus (data collected on hippocampal volume from Pravosudov et al. [12]). Differences among groups in the density of neurons expressing doublecortin were also determined by GLM, followed by Newman–Keuls pairwise comparisons when appropriate. We considered all results to be statistically significant if p < 0.050.
3. Results
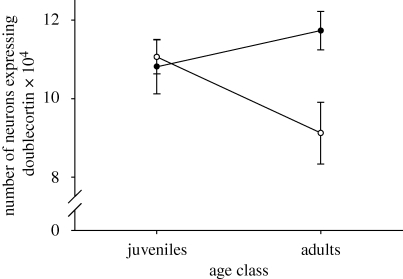
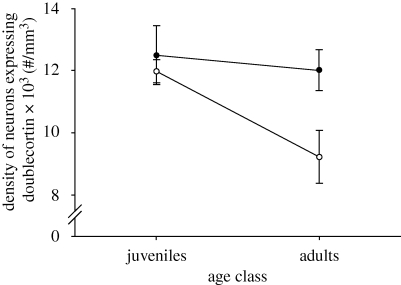
There was a significant subspecies×age class interaction in the number of neurons expressing doublecortin in the hippocampal formation (F1,24 = 5.375, p = 0.029). Adult non-migratory sparrows had fewer neurons expressing doublecortin than adult migratory sparrows (p = 0.030) and showed a trend towards decreased number of neurons expressing doublecortin when compared with juvenile non-migrants (p = 0.064) and to juvenile migratory birds (p = 0.087). All other comparisons were non-significant (adult versus juvenile migratory, p = 0.552; migratory versus non-migratory juveniles, p = 0.780; adult migratory versus juvenile non-migratory, p = 0.450; figure 1). There was also an effect of subspecies and of age class on the density of neurons expressing doublecortin (subspecies: F1,24 = 5.004, p = 0.035; age class: F1,24 = 4.797, p = 0.038) but no statistically significant effect of the interaction (F1,24 = 2.372, p = 0.137). Adult non-migratory birds had lower densities of neurons expressing doublecortin than adult migratory birds (p = 0.035), juvenile migratory birds (p = 0.022) and juvenile non-migratory birds (p = 0.015). All other comparisons were non-significant (adult versus juvenile migratory, p = 0.650; migratory versus non-migratory juveniles, p = 0.875; adult migratory versus juvenile non-migratory, p = 0.974; figure 2).
Figure 1.
Total number of hippocampal neurons expressing doublecortin (mean ± s.e.m.) in adult and juvenile migratory (Z. l. gambelii, filled circle) and non-migratory (Z. l. nuttalli, open circle) sparrows. n = 7 per group.
Figure 2.
Density of hippocampal neurons (no. per mm3) expressing doublecortin (mean ± s.e.m.) in adult and juvenile migratory (Z. l. gambelii, filled circle) and non-migratory (Z. l. nuttalli, open circle) sparrows. n = 7 per group.
We also found that the number and density of doublecortin-positive cells in the HA was not affected by subspecies (number: F1,24 = 0.047, p = 0.831; density: F1,24 = 0.346, p = 0.562) and there was no significant interaction between subspecies and age class (number: F1,24 = 0.298, p = 0.590; density: F1,24 = 0.002, p = 0.966). However, there was a significant effect of age class on the number and density of doublecortin-positive cells in the HA (number: F1,24 = 22.506, p < 0.001; density: F1,24 = 39.879, p < 0.001; migratory adults, number: 22.844 × 103 ± 1.604, density: 10.796 × 103 ± 0.749 (no. per mm3); migratory juveniles, number: 33.571 × 103 ± 3.779, density: 16.042 × 103 ± 1.160 (no. per mm3); non-migratory adults, number: 22.002 × 103 ± 1.389, density: 10.268 × 103 ± 0.714 (no. per mm3); non-migratory juveniles, number: 35.518 × 103 ± 2.708, density: 15.586 × 103 ± 0.618 (no. per mm3)).
4. Discussion
We found that migratory adult white-crowned sparrows had relatively more and a higher density of new hippocampal neurons than adult non-migratory white-crowned sparrows. This difference does not appear to be global, as the number and density of doublecortin-positive cells in an adjacent region (HA) did not differ among subspecies. This is consistent with the results of Pravosudov et al. [12] in that, in these same individuals, migratory sparrows had more total hippocampal neurons than non-migratory sparrows but with comparable absolute hippocampal volumes. Thus, taken together, adult migratory sparrows had a higher density of both new and total number of neurons in the hippocampus compared with adult non-migrants. Similarly, Cristol et al. [11] found that migratory juncos had a higher density of hippocampal neurons than non-migrants, although they did not address the age of these neurons. Previous studies have not documented the mechanism underpinning these differences, but our results suggest that the production of new hippocampal neurons may explain the difference in the number of hippocampal neurons previously found between migratory and non-migratory white-crowned sparrows [12]. To our knowledge, this is the first study to document a relationship between migratory behaviour and hippocampal neurogenesis.
Hippocampal neurogenesis has been suggested to be regulated by spatial processing, in that individuals having higher demands for spatially dependent behaviours show an increase in the number of new neurons in the hippocampus [6,33,37–39]. Many species of migratory birds, including white-crowned sparrows, exhibit behaviours that are consistent with increased demands on spatial learning and memory, at least during migration. For example, migratory individuals have been shown to return to the same wintering grounds and stopover sites each year (e.g. [40–43]), and orientation towards these areas have been suggested to be learned. In addition, migratory birds perform better on spatial memory tasks [11,12] and have a better long-term memory [10] than non-migratory birds.
Although migratory and non-migratory adults may possess different numbers of new hippocampal neurons, this effect may have been due to experience with spatially based learning and memory during migration, rather than a product of inherent subspecies differences. It has been shown that first-time migrant white-crowed sparrows, i.e. the juveniles in our study, use an innate orientation programme during their first migration rather than an adult-typical, learned form of navigation ([9]; see also [44]). Further, in some birds, acquisition of a spatial navigation system appears to occur during development [45]. Thus, assuming juvenile migratory sparrows were not using spatially based memory during the first half of their first migration, and if the differences we found between migratory and non-migratory sparrows were a function of previous experience with migration, we expected that juvenile migrants would have fewer numbers of new neurons than migratory adults. We found, however, that migratory adults and juveniles had similar numbers and densities of new hippocampal neurons. Although we may conclude that these results indicated intrinsic differences in neurogenesis between subspecies, our data comparing adult and juvenile non-migrants suggest an alternative explanation.
Our data also show that non-migratory juveniles have more and a higher density of new neurons than non-migratory adults, and the number and density of new neurons are similar to those found in migratory juveniles and adults. This may not be surprising, as previous studies have shown that neurogenesis is very high during development and declines with age (e.g. [14–18]). Thus, juveniles of both subspecies may show similar levels of neurogenesis owing to brain development in general, rather than neurogenesis regulated by spatial learning and memory per se. This may be supported by our data showing that neurogenesis in the HA were different between the age classes but not between subspecies, thus suggesting the effect of age on neurogenesis may not be specific to particular brain regions. It may be, then, that hippocampal neurogenesis is downregulated in non-migratory adult birds while, in migratory adults, neurogenesis is stable or upregulated during migration. An upregulation pattern in neurogenesis has been shown in studies of ageing mice, in that individuals housed in enriched environments showed an upregulation of neurogenesis compared with littermates housed in standard conditions (e.g. [46–48]). Although it would be interesting, unfortunately, with our data, we cannot decipher if migratory adults show variability in new neuron numbers based on periods of migration or if neurogenesis is stable between development and adulthood. Because of this, it may be that migratory birds have been selected for higher neurogenesis in general, suggesting that neurogenesis is not necessarily related to the migratory period itself. To determine such, migratory birds would have to be sampled throughout the year to determine neurogenesis rates outside of the migration period.
There are also other possible interpretations of our results. First, motor stimulation has been shown to have dramatic effects on neurogenesis. Mice that engage in voluntary physical activity exhibit increased neurogenesis compared with conspecifics denied such an opportunity (e.g. [49,50]). Migratory behaviour probably entails a large increase in motor stimulation compared with birds that do not migrate, which may potentially have caused the differences found between adult migratory and non-migratory sparrow subspecies in this study. However, juvenile migrants probably engaged in similar amounts of motor activity as adults during migration (at least relative to non-migrants), but did not exhibit more or more densely packed neurons compared with juvenile non-migrants. Thus, if motor stimulation can explain our results, it appears that it either differentially affects neurogenesis in adult and juvenile sparrows, or there is a ceiling to the additive effects (e.g. motor stimulation, development, spatial processing) that contribute to increased neurogenesis.
Also, it may be that non-migratory adults reflect the mean value of neurogenesis in both subspecies. Some aspect of migratory behaviour may select against individuals that exhibit lower neurogenesis, whereby only individuals with increased neurogenesis complete the migratory route. Thus, our data may reflect only a selected subset of all migratory individuals. To ascertain such, data on neurogenesis in individuals before migration would need to be gathered.
In conclusion, our study suggests that the population of new hippocampal neurons was significantly affected by migratory status. Non-migratory adults had less and a lower density of new hippocampal neurons than migratory adults. Further, neurogenesis was similar between adult and juvenile migrants, while adult non-migrants showed lower levels of neurogenesis compared with juvenile non-migrants. Further work is needed to investigate whether alternative factors such as exercise and stress could contribute to the patterns we found in neurogenesis between adults and juveniles of both subspecies. Also, investigation into whether adult migrants show variable numbers of new neurons, depending upon the time of year, and whether the adult population before migration shows a pattern consistent with this study would contribute to a fuller picture of neurogenesis patterns in this species. Similarly, it would be interesting to know, if migratory adults do indeed initiate increases in hippocampal neurogenesis during migration, what initiates the increase in neurogenesis, if it is stable throughout the migratory route, and whether neurogenesis rates are stable on the wintering grounds.
Acknowledgements
All procedures were approved by the Institutional Animal Care and Use Committee at the University of California Davis (10804).
We thank Marty Morton for help collecting birds and Alicja Omanska for her assistance. Birds were collected under California State and Federal Scientific Collecting Permit (801064-04). This research was supported by grants from the National Institutes of Health (MH079892 and MH076797) and the National Science Foundation (IOB-0615021) to V.P. Comments by three anonymous reviewers greatly improved the manuscript.
References
- 1.Krebs J. R., Sherry D. F., Healy S. D., Perry V. H., Vaccarino A. L.1989Hippocampal specialization of food-storing birds. Proc. Natl Acad. Sci. USA 86, 1388–1392 10.1073/pnas.86.4.1388 (doi:10.1073/pnas.86.4.1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherry D. F., Vaccarino A. L., Buckenham K., Herz R. S.1989The hippocampal complex of food storing birds. Brain Behav. Evol. 34, 308–317 10.1159/000116516 (doi:10.1159/000116516) [DOI] [PubMed] [Google Scholar]
- 3.Jacobs L. F., Gaulin S. J. C., Sherry D. F., Hoffman G. E.1990Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proc. Natl Acad. Sci. USA 87, 6349–6352 10.1073/pnas.87.16.6349 (doi:10.1073/pnas.87.16.6349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healy S. D., Krebs J. R.1996Food storing and the hippocampus in Paridae. Brain Behav. Evol. 47, 195–199 10.1159/000113239 (doi:10.1159/000113239) [DOI] [PubMed] [Google Scholar]
- 5.Roth T. C., II, Pravosudov V. V.2009Hippocampal volumes and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc. R. Soc. B 276, 401–405 10.1098/rspb.2008.1184 (doi:10.1098/rspb.2008.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaDage L. D., Roth T. C., II, Fox R. A., Pravosudov V. V.2010Ecologically-relevant spatial memory use modulates hippocampal neurogenesis. Proc. R. Soc. B 277, 1071–1079 10.1098/rspb.2009.1769 (doi:10.1098/rspb.2009.1769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bingman V. P., Able K. P.2002Maps in birds: representational mechanisms and neural bases. Curr. Opin. Neurobiol. 12, 745–750 10.1016/S0959-4388(02)00375-6 (doi:10.1016/S0959-4388(02)00375-6) [DOI] [PubMed] [Google Scholar]
- 8.Perdeck A. C.1967Orientation of Starlings after displacement to Spain. Ardea 46, 1–37 [Google Scholar]
- 9.Thorup K., Bisson I.-A., Bowlin M. S., Holland R. A., Wingfield J. C., Ramenofsky M., Wikelski M.2007Evidence for a navigational map stretching across the continental US in a migratory songbird. Proc. Natl Acad. Sci. USA 104, 18 115–18 119 10.1073/pnas.0704734104 (doi:10.1073/pnas.0704734104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mettke-Hoffmann C., Gwinner E.2003Long-term memory for a life on the move. Proc. Natl Acad. Sci. USA 100, 5863–5866 10.1073/pnas.1037505100 (doi:10.1073/pnas.1037505100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristol D. A., Reynolds E. B., LeClerc J. E., Donner A. H., Farabaugh C. S., Ziegenfus C. W. S.2003Migratory dark-eyed juncos, Junco hyemalis, have better spatial memory and denser hippocampal neurons than nonmigratory conspecifics. Anim. Behav. 66, 317–328 10.1006/anbe.2003.2194 (doi:10.1006/anbe.2003.2194) [DOI] [Google Scholar]
- 12.Pravosudov V. V., Kitaysky A. S., Omanska A.2006The relationship between migratory behavior, memory and the hippocampus: an intraspecific comparison. Proc. R. Soc. B 273, 2641–2649 10.1098/rspb.2006.3624 (doi:10.1098/rspb.2006.3624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healy S. D., Gwinner E., Krebs J. R.1996Hippocampal volume in migratory and non-migratory warblers: effects of age and experience. Behav. Brain Res. 81, 61–68 10.1016/S0166-4328(96)00044-7 (doi:10.1016/S0166-4328(96)00044-7) [DOI] [PubMed] [Google Scholar]
- 14.Barnea A., Nottebohm F.1996Recruitment and replacement of hippocampal neurons in young and adult chickadees: an addition to the theory of hippocampal learning. Proc. Natl Acad. Sci. USA 93, 714–718 10.1073/pnas.93.2.714 (doi:10.1073/pnas.93.2.714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn H. G., Dickinson-Anson H., Gage F. H.1996Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16, 2027–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling C., Zuo M., Alvarez-Buylla A., Cheng F.1997Neurogenesis in juvenile and adult ring doves. J. Comp. Neurol. 379, 300–312 (doi:10.1002/(SICI)1096-9861(19970310)379:2<300::AID-CNE10>3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- 17.Heine V. M., Maslam S., Joëls M., Lucassen P. J.2004Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus–pituitary–adrenal axis activation. Neurobiol. Aging 25, 361–375 10.1016/S0197-4580(03)00090-3 (doi:10.1016/S0197-4580(03)00090-3) [DOI] [PubMed] [Google Scholar]
- 18.McDonald H. Y., Wojtowicz J. M.2005Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci. Lett. 385, 70–75 10.1016/j.neulet.2005.05.022 (doi:10.1016/j.neulet.2005.05.022) [DOI] [PubMed] [Google Scholar]
- 19.Markakis E. A., Gage F. H.1999Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 406, 449–460 (doi:10.1002/(SICI)1096-9861(19990419)406:4<449::AID-CNE3>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 20.van Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D., Gage F. H.2002Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 10.1038/4151030a (doi:10.1038/4151030a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempermann G., Wiskott L., Gage F. H.2004Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 14, 186–191 10.1016/j.conb.2004.03.001 (doi:10.1016/j.conb.2004.03.001) [DOI] [PubMed] [Google Scholar]
- 22.Clelland C. D., et al. 2009A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 10.1126/science.1173215 (doi:10.1126/science.1173215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenough W. T., Cohen N. J., Juraska J. M.1999New neurons in old brains: learning to survive? Nat. Neurosci. 2, 203–205 10.1038/6300 (doi:10.1038/6300) [DOI] [PubMed] [Google Scholar]
- 24.Leuner B., Gould E., Shors T. J.2006Is there a link between adult neurogenesis and learning? Hippocampus 16, 216–224 10.1002/hipo.20153 (doi:10.1002/hipo.20153) [DOI] [PubMed] [Google Scholar]
- 25.Chilton G., Baker M. C., Barrentine C. D., Cunningham M. A.1995White-crowned sparrow (Zonotrichia leucophrys). In The birds of North America, no 183 (eds Poole A., Gill F.), Washington, DC: The Academy of Natural Sciences and: The American Ornithologists' Union [Google Scholar]
- 26.Francis F., et al. 1999Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23, 247–256 10.1016/S0896-6273(00)80777-1 (doi:10.1016/S0896-6273(00)80777-1) [DOI] [PubMed] [Google Scholar]
- 27.Gleeson J. G., Lin P. T., Flanagan L. A., Walsh C. A.1999Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23, 257–271 10.1016/S0896-6273(00)80778-3 (doi:10.1016/S0896-6273(00)80778-3) [DOI] [PubMed] [Google Scholar]
- 28.Balthazart J., Boseret G., Konkle A. T. M., Hurley L. L., Ball G. F.2008Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur. J. Neurosci. 27, 801–817 10.1111/j.1460-9568.2008.06059.x (doi:10.1111/j.1460-9568.2008.06059.x) [DOI] [PubMed] [Google Scholar]
- 29.King J. R., Barker S., Farner D. S.1963A comparison of energy reserves during autumnal and vernal migratory periods in the white-crowned sparrow Zonotrichia leucophrys gambelii. Ecology 44, 513–521 [Google Scholar]
- 30.Brown J. P., Couillard-Després S., Cooper-Kuhn C. M., Winkler J., Aigner L., Kuhn H. G.2003Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10 10.1002/cne.10874 (doi:10.1002/cne.10874) [DOI] [PubMed] [Google Scholar]
- 31.Rao M. S., Shetty A. K.2004Efficacy of doublecortin as a marker to analyze the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 19, 234–246 10.1111/j.0953-816X.2003.03123.x (doi:10.1111/j.0953-816X.2003.03123.x) [DOI] [PubMed] [Google Scholar]
- 32.Couillard-Despres S., et al. 2005Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 21, 1–14 10.1111/j.1460-9568.2004.03813.x (doi:10.1111/j.1460-9568.2004.03813.x) [DOI] [PubMed] [Google Scholar]
- 33.Hairston I. S., Little M. T. M., Scanlon M. D., Barakat M. T., Palmer T. D., Sapolsky R. M., Heller H. C.2005Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J. Neurophysiol. 94, 4224–4233 10.1152/jn.00218.2005 (doi:10.1152/jn.00218.2005) [DOI] [PubMed] [Google Scholar]
- 34.Boseret G., Ball G. F., Balthazart J.2007The microtubule-associate protein doublecortin is broadly expressed in the telencephalon of adult canaries. J. Chem. Neuoranat. 33, 140–154 10.1016/j.jchemneu.2007.02.002 (doi:10.1016/j.jchemneu.2007.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West M. J., Ostergaard K., Andreassen O. A., Finsen B.1996Estimation of the number of somatostatin neurons in the striatum: an in situ hybridization study using the optical fractionator method. J. Comp. Neuorol. 370, 11–22 (doi:10.1002/(SICI)1096-9861(19960617)370:1<11::AID-CNE2>3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- 36.Barnea A., Nottebohm F.1994Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc. Natl Acad. Sci. USA 91, 11 217–11 221 10.1073/pnas.91.23.11217 (doi:10.1073/pnas.91.23.11217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J.1999Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265 10.1038/6365 (doi:10.1038/6365) [DOI] [PubMed] [Google Scholar]
- 38.Ambrogini P., Cuppini R., Cuppini C., Ciaroni S., Cecchini T., Ferri P., Sartini S., Del Grande P.2000Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci. Lett. 286, 21–24 10.1016/S0304-3940(00)01074-0 (doi:10.1016/S0304-3940(00)01074-0) [DOI] [PubMed] [Google Scholar]
- 39.Döbrössy M. D., Drapeau E., Aurousseau C., Le Moal M., Piazza P. V., Abrous D. N.2003Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry 8, 974–982 10.1038/sj.mp.4001419 (doi:10.1038/sj.mp.4001419) [DOI] [PubMed] [Google Scholar]
- 40.Winker K., Warner D. W., Weisbrod A. R.1991Unprecedented stopover site fidelity in a Tennessee warbler. Wilson Bull. 103, 512–514 [Google Scholar]
- 41.Cantos F. J., Tellería J. L.1994Stopover site fidelity of four migrant warblers in the Iberian Peninsula. J. Avian Biol. 25, 131–134 10.2307/3677031 (doi:10.2307/3677031) [DOI] [Google Scholar]
- 42.Cuadrado M., Senar J. C., Copete J. L.1995Do all blackcaps Sylvia atricapilla show winter site fidelity? Ibis 137, 70–75 10.1111/j.1474-919X.1995.tb03221.x (doi:10.1111/j.1474-919X.1995.tb03221.x) [DOI] [Google Scholar]
- 43.Rimmer C. C., Darmstadt C. H.1996Non-breeding site fidelity in northern shrikes. J. Field Ornithol. 67, 360–366 [Google Scholar]
- 44.Sutherland W. J.1988The heritability of migration. Nature 334, 471–472 10.1038/334471a0 (doi:10.1038/334471a0) [DOI] [Google Scholar]
- 45.Gagliardo A., Ioalé P., Odetti F., Bingman V. P.2001The ontogeny of the homing pigeon navigational map: evidence for a sensitive learning period. Proc. R. Soc. Lond. B 268, 197–202 10.1098/rspb.2000.1350 (doi:10.1098/rspb.2000.1350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kempermann G., Kuhn H. G., Gage F. H.1998Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 18, 3206–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kempermann G., Gast D., Gage F. H.2002Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 52, 135–143 10.1002/ana.10262 (doi:10.1002/ana.10262) [DOI] [PubMed] [Google Scholar]
- 48.van Praag H., Shubert T., Zhao C., Gage F. H.2005Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685 10.1523/JNEUROSCI.1731-05.2005 (doi:10.1523/JNEUROSCI.1731-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Praag H., Kempermann G., Gage F. H.1999aRunning increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 2, 266–270 10.1038/6368 (doi:10.1038/6368) [DOI] [PubMed] [Google Scholar]
- 50.van Praag H., Christie B. R., Sejnowski T. J., Gage F. H.1999bRunning enhances neurogenesis, learning and long-term potentiation in mice. Proc. Natl Acad. Sci. USA 96, 13 427–13 431 10.1073/pnas.96.23.13427 (doi:10.1073/pnas.96.23.13427) [DOI] [PMC free article] [PubMed] [Google Scholar]