Abstract
Although caspase activation is generally thought to be necessary to induce apoptosis, recent evidence suggests that apoptosis can be activated in the setting of caspase inhibition. In this study, we tested the hypothesis that caspase-independent apoptotic pathways contribute to the development of heart failure in the absence of caspase activation. Acute cardiomyopathy was induced using a single dose of doxorubicin (Dox, 20 mg/kg) injected into male wild-type (WT) and transgenic (Tg) mice with a cardiac-specific expression of cytokine response modifier A (CrmA), a known caspase inhibitor. Early (6 day) survival was significantly better in CrmA Tg (81%) than WT (38%) mice. Twelve days after Dox injection, however, the mortality benefit had dissipated, and increased cardiac apoptosis was observed in both groups. There was, however, a significantly greater release of apoptosis-inducing factor (AIF) from mitochondria to cytosol in CrmA Tg compared with WT mice, which suggests that an enhancement of activation in caspase-independent apoptotic pathways had occurred. The administration of a poly(ADP-ribose) polymerase-1 inhibitor, 4-amino-1,8-naphthalimide (4-AN), to Dox-treated mice resulted in significantly improved cardiac function, a significant blockade of AIF released from mitochondria, and decreased cardiac apoptosis. There were also significantly improved survival in WT (18% without 4-AN vs. 89% with 4-AN) and CrmA Tg (13% without 4-AN vs. 93% with 4-AN) mice 12 days after Dox injection. In conclusion, these findings suggest that apoptosis can be induced in the heart lacking caspase activation via caspase-independent pathways and that enabling the inhibition of AIF activation may provide a significant cardiac benefit.
Keywords: apoptosis-inducing factor, poly(adenosine 5′-diphosphate-ribose) polymerase-1, doxorubicin, mortality, cardiomyopathy, cytokine response modifier A
apoptosis is a tightly regulated, cell deletion process known to play an important role in various cardiovascular diseases, such as myocardial infarction, reperfusion injury, and the development of heart failure (15, 19). Although the caspases, a family of cysteine proteases, have been thought of as the critical enzymes in the induction and execution of apoptosis, there is accumulating evidence to suggest that caspase-independent pathways play a significant role in cardiac cell death (3). The potential importance of caspase-independent pathways in the heart is highlighted by the fact that cardiomyocytes contain high levels of endogenous caspase inhibitors, which render them relatively resistant to caspase-dependent apoptosis (20). Although some studies have shown that caspase inhibition reduces the acute loss of myocardium in various animal models (17, 38), other studies indicate that caspase inhibition might not be able to completely inhibit apoptosis (7, 25).
Because of its potentially significant contribution to cell death in the heart, it is important to define the role of the caspase-independent pathway in cardiac apoptosis more fully. The best studied example of caspase-independent apoptosis involves apoptosis-inducing factor (AIF) (5, 8, 9, 28, 34). AIF is an NADH-oxidase produced as a 67-kDa protein containing an NH2-terminal mitochondrial localization signal sequence (28, 34). It is processed into a 57-kDa mature form by calpain in mitochondria, released into the cytosol in response to apoptotic stimulation, and is able to translocate into the nucleus and induce DNA fragmentation without caspase activation (20). This sequence of events is supported by evidence that AIF microinjected into cells can induce apoptotic changes, such as chromatin condensation, that cannot be blocked by caspase inhibition alone, as, for instance, by zVAD.fmk or the overexpression of Bcl-2 (5, 20, 28). Furthermore, AIF can trigger DNA fragmentation in apoptotic protease-activating factor-1 and caspase-9-deficient cells (5), an effect that is probably mediated through the direct activation of caspase-3 (16) by AIF. In the heart, AIF has been implicated in apoptosis induced by oxidative stress and heart failure (6, 32). Its mature form is released, together with cytochrome c, from mitochondria to the cytosol in ischemic neonatal cardiomyocytes characterized by caspase-independent DNA degradation (4). Several studies have shown that AIF is regulated by poly(ADP-ribose) polymerase-1 (PARP-1), a highly conserved 116-kDa nuclear enzyme, involved in DNA repair (40). PARP-1 facilitates both the release of AIF from mitochondria and AIF nuclear translocation without the mediation of Bcl-2 proteins and caspase activation (1, 13, 29, 40).
In the present study, we used α-myosin heavy chain (α-MHC) promotor to generate transgenic (Tg) mice with cardiac-specific expression of cytokine response modifier A (CrmA), a known inhibitor of caspases (10, 30, 41), to determine whether caspase-independent apoptosis is activated in the setting of caspase inhibition in vivo. Using acute doxorubicin (Dox)-induced cardiomyopathy with significant cardiac apoptosis (26, 31), we sought to define the contribution of caspase-independent apoptosis and to investigate its role in the development of heart failure by administering 4-amino-1,8-naphthalimide (4-AN), a potent (PARP-1) inhibitor.
MATERIALS AND METHODS
Generation of CrmA Tg mice.
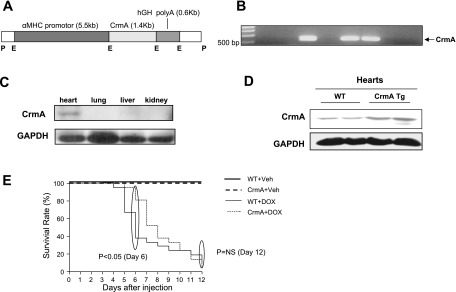
A schematic diagram of the construct used to generate Tg mice is shown in Fig. 1A. Cardiac-specific expression was achieved using α-MHC promoter. The genotypes of CrmA Tg mice were identified by polymerase chain reaction (PCR) with PCR primers as follows: forward MHC, 5′-CGGTGTAAAAGAGGCAGGGAAG-3′; and reverse MHC, 5′-ACTGACGAGATTGACGGTGGAG-3′. CrmA Tg mice were identified by the appearance of a 525-bp band (Fig. 1B). Western blot analysis for CrmA was performed with anti-CrmA antibody (BD Pharmingen, San Diego, CA). The investigations conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996), and were approved by the Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center.
Fig. 1.
Generation of cytokine response modifier A (CrmA) transgenic (Tg) mice and survival of doxorubicin (Dox)-induced cardiomyopathy in wild-type (WT) and CrmA Tg mice. A: schematic diagram of the construct used to generate Tg mice with cardiac-specific overexpression of CrmA. Cardiac-specific expression was achieved using α-myosin heavy chain (α-MHC) promoter. B: genotyping of CrmA Tg mice. CrmA Tg mice were identified by the appearance of a 525-bp band in tail genomic DNA. C: representative Western immunoblot analysis of different tissues from CrmA Tg mice. GAPDH was used as an internal control. D: representative Western immunoblot analysis of heart tissues from WT and CrmA Tg mice hearts. E: 12-day survival curves for WT and CrmA Tg mice after Dox (20 mg/kg) or vehicle (Veh) injection; n = 6 mice/group (Veh) and 21/group (Dox). The early term survival of WT/Dox mice was significantly less than CrmA/Dox mice (at day 6, P <0.05 by Wilcoxon's test), but the Dox groups did not differ at day 12. hGH, human growth hormone; P, Pvul; E, EcoR1; polyA, polyandenylation; NS, not significant.
Dox-induced cardiomyopathy and 4-AN treatment.
Twelve-week-old male CrmA Tg mice and wild-type (WT) littermates were treated with a single intraperitoneal injection of 20 mg/kg Dox (Bedford Labs, Bedford, OH), a dose shown to induce significant acute heart failure in 1 to 2 wk (18, 23). Control mice were treated intraperitoneally with an equal volume of vehicle, and all animals were observed daily for signs of heart failure. To determine the effect of PARP inhibition, WT and CrmA mice were treated daily with PARP-1 inhibitor 4-AN (3 mg·kg−1·day−1 ip; Biomol, Plymouth Meeting, PA) starting 1 day before the Dox injection.
Hemodynamic measurements.
Baseline cardiac function was analyzed using echocardiography. For cardiac functional analysis 10 days after Dox injection, which is just before the time point at which the most significant Dox-induced mortality is observed, we used the left ventricular (LV) pressure-volume loop measurement. This method uniquely provides measures of LV performance that are more specific to the heart and less affected by vascular loading conditions (27), which may be particularly important in our acute heart failure model. Pressure-volume parameters were measured under isoflurane (2%) inhalant anesthesia using a 1.4-Fr microtip pressure-volume catheter (Scisense, Ontario, Canada), inserted into the right common carotid artery and advanced into the left ventricle. Data were recorded using a PowerLab system (ADInstruments, Colorado Springs, CO). Beat-by-beat pressure-volume parameters including stroke work, cardiac output, preload, afterload, and contractility were measured and analyzed using CardioSoft Pro software (CardioSoft, Houston, TX).
Biochemical and histological analyses.
Western immunoblot analyses, the assays for caspase-3, -8, and -9, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and immunohistochemistry for AIF were preformed as described previously (11, 14, 32). More detailed descriptions of the analyses are outlined in the supplemental information that may be found posted with the online version of this article.
Statistical analyses.
All data are expressed as means ± SE. Statistical analyses between two groups were performed with unpaired Student t-tests. To test the significance of hemodynamic changes, we conducted two-way ANOVAs followed by Bonferroni post hoc analysis using GraphPad Prism 5.0 (San Diego, CA). Survival rates after Dox were analyzed with the Wilcoxon test or log-rank test. P values of <0.05 were considered significant.
RESULTS
Improvement in early, but not late, survival in CrmA Tg mice after Dox administration.
CrmA Tg mice showed cardiac-specific expression of CrmA as determined by protein expression (Fig. 1, C and D) but no morphological or echocardiographic differences from WT mice at baseline (supplemental Tables 1 and 2). Initially, to determine whether the cardiac-specific inhibition of caspase has an effect on mortality, Dox, which has been shown to induce acute cardiac dysfunction and death associated with the activation of cardiac apoptosis, was injected into WT and CrmA Tg mice. We compared four groups of mice: 1) WT + vehicle, 2) WT + Dox, 3) CrmA Tg + vehicle, and 4) CrmA Tg + Dox. As expected, there were no deaths associated with vehicle injection (Fig. 1E), but Dox caused a significant increase in mortality in both WT and CrmA Tg mice. The increase in mortality was not uniform, however. Six days after Dox injection, survival was significantly greater in CrmA Tg (81%) compared with WT (38%) mice (Fig. 1E). Furthermore, at 12 days, CrmA Tg mice no longer displayed a relative survival benefit (5% CrmA Tg vs. 10% WT, P = not significant).
We also measured cardiac function using the LV pressure-volume loop 5 and 10 days after Dox or control vehicle injection. Since changes in molecular and hemodynamic parameters precede changes in mortality, the hemodynamic and molecular analyses performed at 5 and 10 days correspond to the mortality findings at 6 days, where the peak mortality difference is found, and at 12 days. Although baseline cardiac function in untreated WT and CrmA Tg mice was similar (supplemental Table 2), a significant decrease in cardiac function was evident in WT, but not CrmA Tg, mice 5 days after Dox treatment (Table 1). However, after 10 days, there was no longer any difference between WT and CrmA Tg mice, and both groups showed a significant and similar decrease in cardiac function compared with controls (Table 2). This finding suggests that the early survival benefit in CrmA Tg mice noted at 6 days was associated with less severe cardiac dysfunction measured a day earlier. However, after 10 days, WT and CrmA Tg mice showed similar degrees of Dox-induced cardiac dysfunction and correspondingly similar mortality. The survival benefit associated with the cardiac-specific expression of the caspase inhibitor CrmA thus appears to be associated with the earlier, rather than the later, stages of Dox-induced cardiomyopathy.
Table 1.
LV hemodynamics 5 days after Dox injection in WT and CrmA Tg mice
| Vehicle | Dox | |
|---|---|---|
| LVEDP, mmHg | ||
| WT | 3.6 ± 0.8 | 5.0 ± 0.5 |
| CrmA | 3.7 ± 0.2 | 3.8 ± 0.4 |
| LVSP, mmHg | ||
| WT | 111.5 ± 2.6 | 94.3 ± 1.8*† |
| CrmA | 111.2 ± 2.1 | 101.8 ± 3.4 |
| dP/dtmax, mmHg/s | ||
| WT | 9,055 ± 234 | 6,258 ± 302*† |
| CrmA | 9,043 ± 339 | 8,815 ± 355 |
| dP/dtmin, mmHg/s | ||
| WT | 7,415 ± 467 | 5,152 ± 229*† |
| CrmA | 7,238 ± 564 | 7,159 ± 218 |
| SW, mmHg•μl | ||
| WT | 1,279 ± 63.0 | 849 ± 112.5*† |
| CrmA | 1,264 ± 81.9 | 1,164 ± 88.5 |
| CO, ml/min | ||
| WT | 5.1 ± 0.4 | 3.3 ± 0.4*† |
| CrmA | 5.3 ± 0.4 | 4.5 ± 0.3 |
Values are means ± SE; n = 5 to 6 mice. Dox, doxorubicin; LVEDP, left ventricular (LV) end-diastolic pressure; LVSP, LV systolic pressure, dP/dtmax and dP/dtmin, maximum and minimum first derivative of ventricular pressure with respect to time; SW, stroke work; CO, cardiac output; Tg, transgenic.
P < 0.05, Dox vs. vehicle;
P < 0.05, Dox wild-type (WT) vs. Dox cytokine response modifier A (CrmA).
Table 2.
LV hemodynamics 10 days after Dox injection in WT and CrmA Tg mice
| Vehicle | Dox | Dox + 4-AN | |
|---|---|---|---|
| LVEDP, mmHg | |||
| WT | 3.5 ± 1.1 | 7.1 ± 0.8* | 4.2 ± 0.5† |
| CrmA | 3.1 ± 0.3 | 7.1 ± 0.6* | 3.3 ± 0.5† |
| LVSP, mmHg | |||
| WT | 107.9 ± 2.2 | 91.24 ± 5.7* | 103.4 ± 3.8† |
| CrmA | 111.8 ± 3.2 | 82.3 ± 2.6* | 105.2 ± 6.6† |
| dP/dtmax, mmHg/s | |||
| WT | 8,693 ± 304 | 6,529 ± 715* | 8,102 ± 461† |
| CrmA | 8,896 ± 486 | 5,816 ± 1075* | 8,413 ± 265† |
| dP/dtmin, mmHg/s | |||
| WT | 7,282 ± 530 | 4,202 ± 480* | 6,914 ± 246† |
| CrmA | 6,945 ± 562 | 3,029 ± 548* | 6,026 ± 359† |
| CO, ml/min | |||
| WT | 5.0 ± 0.4 | 2.8 ± 0.2* | 4.8 ± 0.4† |
| CrmA | 5.2 ± 0.5 | 2.3 ± 0.1* | 4.4 ± 0.6† |
| τ, ms | |||
| WT | 12.4 ± 1.1 | 23.0 ± 4.0* | 13.6 ± 1.2† |
| CrmA | 11.5 ± 0.7 | 24.8 ± 2.3* | 14.0 ± 1.2† |
Values are means ± SE; n = 6 to 7 mice. τ, ventricular isovolumic relaxation time constant.
P < 0.05, Dox vs. vehicle;
P < 0.05, Dox vs. Dox + 4-amino-1,8-naphthalimide (4-AN).
Delayed activation of AIF in CrmA Tg mice after Dox injection.
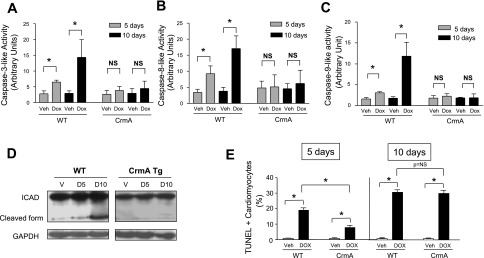
To determine the mechanisms that might contribute to the mortality differences in WT and CrmA Tg mice after Dox injection, we harvested hearts at 5 and 10 days for molecular, biochemical, and histological analyses. To confirm that CrmA overexpression resulted in the inhibition of caspase activation, we measured the activities of caspase-3, -8, and -9 in heart lysates from WT and CrmA Tg mice after Dox and vehicle injection. We found that Dox induced a significant activation of caspase-3, -8, and -9 activities in WT mice compared with vehicle at both 5 and 10 days (Fig. 2, A–C). In contrast, in CrmA Tg mouse hearts, caspase-3, -8, and -9 activities were nearly completely inhibited at 5 and 10 days after Dox injection. We also confirmed caspase inhibition in CrmA Tg mouse hearts by measuring the cleavage of the inhibitor of caspase-activated DNase (ICAD). We found significant ICAD cleavage in WT mouse hearts after Dox injection. In contrast, ICAD cleavage was completely attenuated both at 5 and 10 days after Dox injection in CrmA Tg mouse hearts (Fig. 2D).
Fig. 2.
Caspase inhibition in Dox-induced cardiomyopathy in WT and CrmA Tg mice. A–C: caspase-3-like (A), caspase-8-like (B), and caspase-9-like (C) activities in WT and CrmA Tg mice 5 and 10 days after Dox injection. Dox, 5 and 10 days after Dox injection. *P < 0.05; n = 5–7 mice/group. D: representative Western immunoblots of inhibitor of caspase-activated DNase (ICAD) of WT and CrmA Tg mice 5 (D5) and 10 (D10) days after Dox injection. V, Veh-injected control at 10 days. GAPDH is the internal loading control. E: terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining in WT and CrmA Tg mice 5 and 10 days after Dox injection. *P < 0.05; n = 5–7 mice/group.
To determine whether caspase inhibition in CrmA Tg mice inhibits cardiac apoptosis, we next assessed cardiac apoptosis using TUNEL staining. Dox significantly increased TUNEL-positive cardiomyocytes at both 5 and 10 days in both WT and CrmA Tg mice compared with vehicle-injected mice (Fig. 2E). However, there were significantly fewer TUNEL-positive cells in CrmA Tg than WT mice 5 days after Dox injection. This early protection against apoptosis in the CrmA Tg group, however, was no longer evident 10 days after Dox injection, suggesting that a significant increase in cardiac apoptosis occurred in response to Dox despite a complete inhibition of caspase (Fig. 2E).
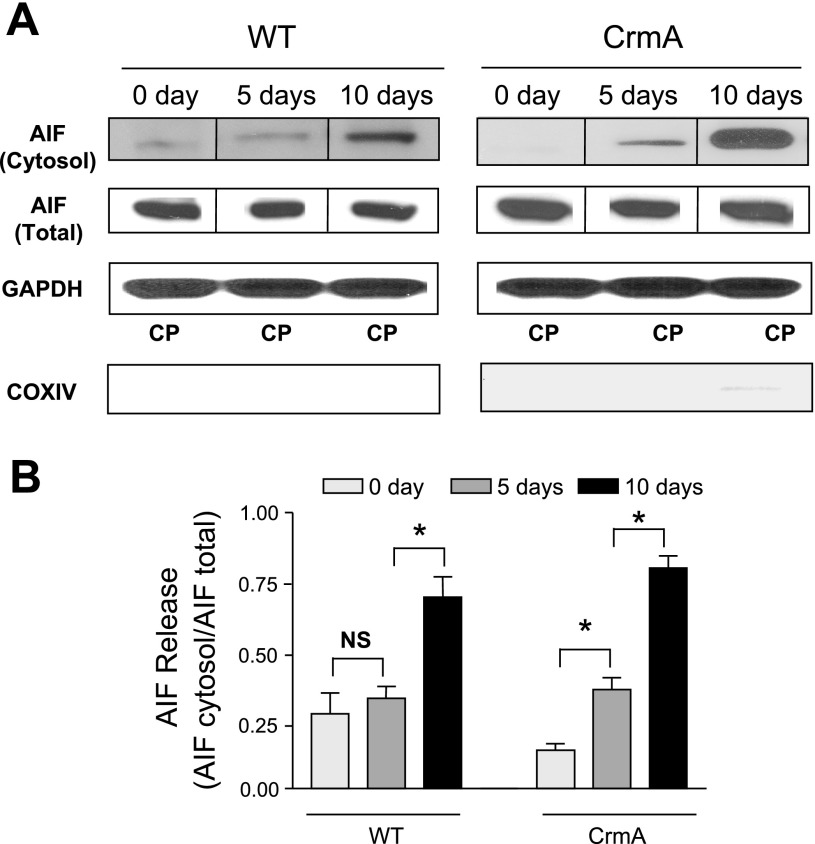
Significant cardiac apoptosis in the absence of caspase activation in CrmA Tg heart raises the possibility of increased activity in alternative, caspase-independent apoptotic pathways. We therefore examined the activation of AIF-induced apoptosis. AIF translocation from mitochondria is an early hallmark of AIF activation. WT mice showed no significant increase in the release of AIF from mitochondria to the cytosol 5 days after Dox injection (Fig. 3, A and B). In contrast, a significant release of AIF in CrmA Tg mice at 5 days suggested an early activation of AIF. Ten days after Dox injection, there was significant release of AIF in both WT (P < 0.05 vs. vehicle) and CrmA Tg (P < 0.05 vs. vehicle and 5-day Dox) mice but AIF release in CrmA Tg was significantly greater at 64% than in WT mice. This finding suggests that an activation of AIF may play a role in the late stage of Dox-induced cardiomyopathy and that this event may explain the loss of the early survival benefit conferred by caspase inhibition.
Fig. 3.
Apoptosis-inducing factor (AIF) release in Dox-induced cardiomyopathy in WT and CrmA Tg mice. A: representative Western immunoblots of AIF in the mitochondria-free cytosolic fraction and total AIF in WT and CrmA Tg mice 5 and 10 days after Dox injection. Individual representative images are identified by a line between images. The purity of the cytosolic proteins (CPs) was examined by immunoblots of (cytosolic specific) GAPDH and (mitochondrial specific) cytochrome-c oxidase subunit IV (COX IV). B: quantification of cytosolic protein content of AIF. *P < 0.05; n = 4 mice.
PARP-1 inhibition significantly improved mortality by inhibiting AIF-induced apoptosis.
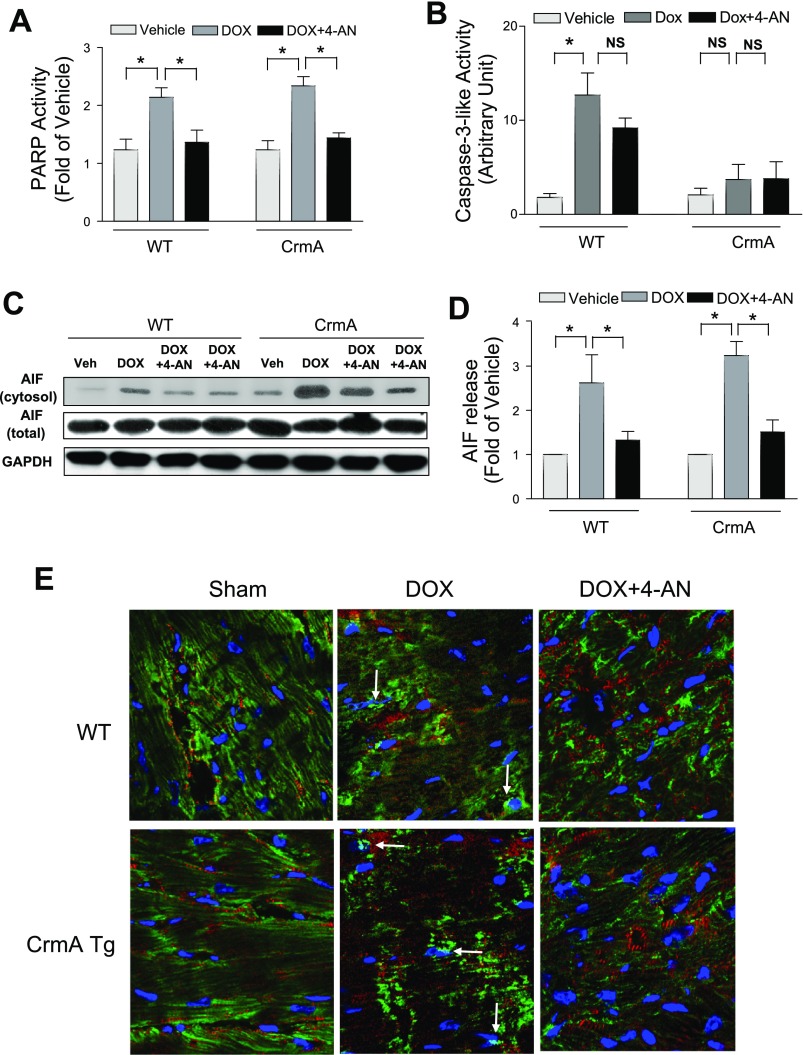
We next sought to determine whether we could improve the survival in Dox-induced cardiomyopathy by inhibiting AIF-induced apoptosis. PARP-1 has been shown to regulate AIF, including its translocation from the mitochondria to the nucleus (29, 40). To inhibit AIF, the mice were thus treated daily with a potent inhibitor of PARP-1 activation, 4-AN, starting 1 day before Dox injection using the following groups: 1) WT + Dox + vehicle, 2) WT + Dox + 4-AN, 3) CrmA Tg + Dox + vehicle, and 4) CrmA Tg + Dox + 4-AN. We found that Dox significantly increased PARP-1 activity in both WT and Tg mice 10 days after injection and, as expected, that 4-AN effectively reduced PARP-1 activation by Dox to the baseline level in both WT and CrmA Tg mice hearts (Fig. 4A). Caspase-3 activity was not significantly inhibited by 4-AN alone (Fig. 4B). To determine whether pretreatment with 4-AN attenuates AIF-induced apoptosis, we next examined AIF translocation from the mitochondria to the cytosol. We found that 4-AN significantly blocked Dox-induced AIF translocation in both WT and CrmA Tg mouse hearts (Fig. 4, C and D). We also observed significant AIF translocation to the nucleus after Dox injection in both WT and CrmA Tg mouse hearts, which were blocked by 4-AN (Fig. 4E). These findings suggest that PARP-1 inhibition effectively blocks Dox-induced PARP-1 activation as well as AIF translocation.
Fig. 4.
The effect of poly(ADP-ribose) polymerase (PARP) inhibition on AIF release 10 days after Dox injection with or without 4-amino-1,8-naphthalimide (4-AN). A: PARP activity assay in WT and CrmA Tg mice 10 days after Dox injection. *P < 0.05; n = 4 mice. B: caspase-3-like activities assay in WT and CrmA Tg mice 10 days after Dox injection. *P < 0.05; n = 4 mice. C: representative Western immunoblots of AIF in the mitochondria-free, cytosolic fraction of WT or CrmA heart after Veh, Dox, or Dox + 4-AN administration. GAPDH was used as an internal control. The 2 rightmost columns of blots represented the Dox + 4-AN conditions for the WT and CrmA groups. D: quantification of cytosolic protein content of AIF. *P < 0.05; n = 4 mice. E: AIF immunofluorescent staining of WT and CrmA Tg mice 5 days after Dox injection with or without 4-AN. Arrows indicate dual staining for AIF (green) and nuclei (blue), suggesting AIF translocation to the nuclei. AIF, green; nuclei, blue; α-actinin, red. Magnification = ×60.
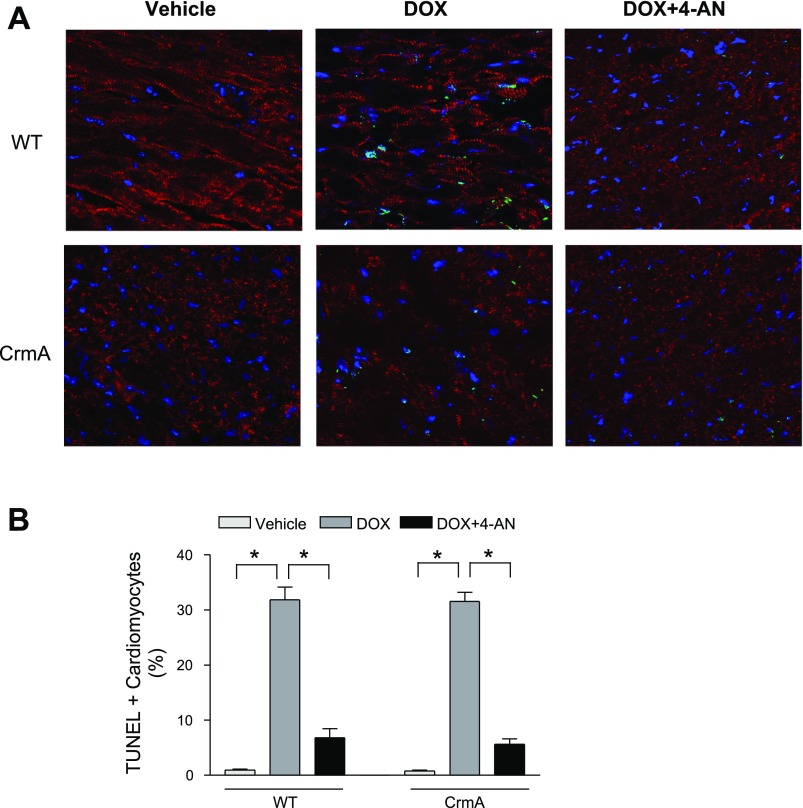
We next examined the question of whether inhibiting AIF translocation via PARP-1 inhibition affects the rate of Dox-induced cardiac apoptosis. Vehicle treatment was associated with very low levels of baseline cardiac apoptosis (<0.1%) in both WT and CrmA Tg mice hearts (Fig. 5, A and B). Ten days after Dox injection, the number of TUNEL-positive cardiomyocytes was significantly increased in both WT and CrmA Tg groups, but the increase was significantly smaller after 4-AN treatment in both WT (6.8 ± 1.6%) and CrmA Tg (5.6 ± 1.0%) compared with untreated hearts. Consistent with these results, we also found that treatment with 4-AN significantly mitigated the Dox-induced impairment of cardiac function in both groups (Table 1). PARP-1 inhibitor alone did not exert any significant effect on hemodynamic parameters in vehicle mice. These findings suggest that the attenuation of AIF activation by PARP inhibition significantly reduced cardiac apoptosis and improved cardiac function in Dox-induced cardiomyopathy.
Fig. 5.
TUNEL staining of WT and CrmA Tg mice 10 days after Dox injection with or without 4-AN. A: representative images of TUNEL staining in WT and CrmA Tg mice with or without 4-AN. TUNEL, green; nuclei, blue; α-actinin, red. Magnification = ×60. B: quantification of TUNEL staining. *P < 0.05; n = 6–8 mice.
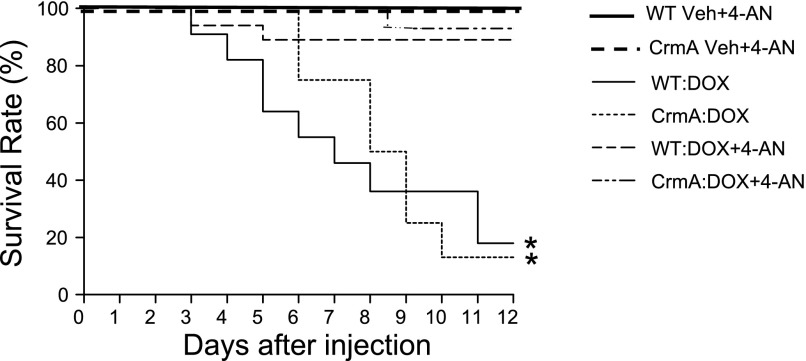
Finally, to determine whether the inhibition of AIF-induced apoptosis by 4-AN translates into improved survival in mice treated with Dox, we determined the survival rates for both WT and CrmA Tg mice after Dox injection with or without 4-AN. In this experiment, there were a 55% and 75% survival rate 6 days after Dox injection and an 18% and 13% survival rate 12 days after Dox injection in WT and CrmA Tg mice, respectively. We found that pretreatment with 4-AN significantly improved the survival in both WT (89% vs. 18%) and CrmA Tg (93% vs. 13%) mice compared with those without 4-AN 12 days after Dox injection (Fig. 6). Both WT and CrmA Tg mice showed a significant benefit, suggesting that the inhibition of AIF activation by 4-AN may plays an essential role in protecting against Dox-induced cardiac dysfunction.
Fig. 6.
Survival graph of WT and CrmA Tg mice with 4-AN (3 mg/kg) administration after Dox injection. Survival was monitored for up to 12 days. *P < 0.05; n = 6 mice/Veh groups and 10–18 mice/Dox groups.
DISCUSSION
In this study, we showed a significant activation of caspase-independent apoptosis in Dox-induced cardiomyopathy in Tg mice with cardiac-specific caspase inhibition via overexpression of CrmA. CrmA Tg mice showed an initial survival benefit associated with the inhibition of caspase activation in response to Dox-induced acute heart failure compared with WT littermates. However, there was a delayed but significant activation of AIF-induced, caspase-independent apoptosis, and the overall mortality rate was ultimately similar to WT mice. Treatment with 4-AN, a potent PARP inhibitor, significantly decreased AIF-induced apoptosis, improved cardiac function, and increased survival in both CrmA Tg and WT mice compared with those without 4-AN after Dox injection. We conclude that caspase-dependent apoptosis plays an important role in WT mice at 5 days, since there was both a significant increase in apoptosis and activation of caspases, compared with CrmA Tg mice, which showed no significant apoptosis at 5 days and no caspase activation. Nevertheless, caspase-independent apoptosis likely contributes significantly to Dox-induced cardiomyopathy even in WT mice, since treatment with 4-AN, a potent PARP inhibitor, significantly decreased AIF-induced apoptosis, improved cardiac dysfunction, and increased survival in both CrmA Tg and WT mice compared with those without 4-AN after Dox injection. These novel findings suggest the potentially significant role of caspase-independent apoptosis in the development of heart failure in this model.
Caspase inhibition has been shown to reduce the acute loss of myocardium in various animal models (17, 38). However, other studies indicate that caspase inhibition alone might not be sufficient to eliminate apoptosis completely (25). Several studies have shown that even in the presence of complete caspase inhibition, such as pharmacological caspase inhibition by zVAD.fmk, nuclear DNA fragmentation was present and significant tissue damage was still observed (12, 40). It thus appears that caspase-independent pathways, such as those mediated by PARP-1/AIF, may play an important role in the induction of apoptosis and contribute significantly to apoptotic cell death in the heart. In this study, we showed that caspase inhibition using CrmA successfully blocked caspase-3, -8, and -9 activation in CrmA Tg mice after Dox exposure, suggesting that this is an effective strategy for inhibiting caspase activation in a cardiac-specific manner in vivo. However, the initial improvement in survival was followed by a delayed but exaggerated increase in mortality, which negated the initial benefit of caspase inhibition by CrmA overexpression.
AIF and cytochrome c are important for cell viability when they are located in mitochondria, but when released from mitochondria, they both activate apoptotic programs. Our current findings suggest, however, that caspase-dependent and -independent apoptosis have different and distinct time courses. Although we observed an initial survival benefit after 6 days in response to acute heart failure induced by Dox in CrmA Tg mice compared with WT littermates, by 12 days after Dox injection the CrmA Tg survival rate had fallen to the WT level and the overall mortality rate was ultimately similar to that of WT mice. In addition, at 10 days after Dox injection, there was a significant release of AIF in both WT and CrmA Tg hearts without a change in total AIF. These findings also support the notion that in the setting of caspase inhibition, caspase-independent apoptosis, such as AIF-induced apoptosis, is activated. Indeed, numerous studies have reported that the mitochondrial release of AIF takes place downstream of cytochrome c release and is further enhanced in the setting of caspase inhibition (2, 21, 35).
PARP-1 is a highly conserved, 116-kDa nuclear enzyme involved in DNA repair (29, 40) that has been shown to facilitate both the release of AIF from mitochondria and AIF nuclear translocation (1, 13, 29, 40). After translocating to the nucleus, AIF mediates large-scale DNA fragmentations by enhancing the activity of endonuclasese G (21, 36). In the present studies, we demonstrated that PARP-1 inhibition significantly attenuates the Dox-induced AIF release from mitochondria in both WT and CrmA Tg mice. This result further supports the idea that PARP-1 activation is most likely the main mechanism of AIF activation and that AIF may be an essential downstream effector in the cell death program initiated by PARP-1. In fact, PARP-1/AIF activation may also play an important role in the induction of apoptosis in WT mice with Dox-induced heart failure. Since apoptosis is a highly orchestrated process involving multiple pathways, we cannot exclude the possibility that PARP-1 inhibition may be involved in caspase-dependent as well as caspase-independent pathways. Other investigators have shown that in a murine model of heart failure, PARP inhibition attenuates the development of hypertrophy and the mitochondrial-to-nuclear translocation of AIF. Molnar et al. (22) have demonstrated an activation of PARP-1 in the failing heart by showing an increased abundance of poly-ADP-ribosylated proteins (22). In a mouse model of heart failure induced by transverse aortic banding, the extent of the mitochondrial-to-nuclear translocation of AIF was reduced by the inhibition of PARP-1 activation, through the use of either isoindolinone-based PARP inhibitor (INO-1001) or PARP-1 genetic-deficient mice (37). The mechanism responsible for the PARP-1-dependent release of AIF from mitochondria remains to be identified, but it has been proposed that the cell death pathway initiated by PARP-1 activation may be mediated by AIF (40).
In the present study, we used a well-established mouse model of Dox-induced acute heart failure that shows a good correlation between the degree of myocardial apoptosis and the severity of Dox-induced heart failure (24). Dox-induced cardiomyopathy was chosen to test our hypothesis in an in vivo animal model of cardiac apoptosis during the progression of heart failure because apoptotic cell death is a key component in Dox-induced cardiotoxicity (26, 31). Although death associated with the administration of Dox in experimental animals may be multifactorial, cardiomyopathy is the most important cause of mortality after Dox injection (26, 39). Our results suggest that by inhibiting apoptosis, we may be able to significantly improve cardiac function and mortality in this model. Studies suggest that Dox induces caspase-independent as well as caspase-dependent apoptosis and showed that apoptosis is initiated via both caspase-3 activation and AIF nuclear translocation by mitochondrial damage after Dox exposure (33, 39). While further studies are needed to determine whether caspase-independent apoptosis plays a significant role in other models of cardiomyopathy, such as myocardial infarction, current studies suggest that the caspase-independent apoptotic route is also involved in the death of cardiomyocytes by Dox.
While the inhibition of apoptosis promises to be an important target for therapeutic intervention, better defining the significance of specific apoptotic mechanisms will be critical in understanding how the myocardium is lost during heart failure. Our findings provide further clarification of the role of caspase-independent apoptosis in the heart and contribute to a better understanding of the molecular mechanisms that are involved in AIF-induced cardiac apoptosis.
GRANTS
This study was supported in part by the grants from National Institutes of Health RO1-HL-091998 (to P. M. Kang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Alonso M, Tamasdan C, Miller DC, Newcomb EW. Flavopiridol induces apoptosis in glioma cell lines independent of retinoblastoma and p53 tumor suppressor pathway alterations by a caspase-independent pathway. Mol Cancer Ther 2: 139–150, 2003. [PubMed] [Google Scholar]
- 2. Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol 159: 923–929, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bae S, Yalamarti B, Kang PM. Role of caspase-independent apoptosis in cardiovascular diseases. Front Biosci 13: 2495–2503, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem 281: 22943–22952, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci 115: 4727–4734, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Chen M, Zsengeller Z, Xiao CY, Szabo C. Mitochondrial-to-nuclear translocation of apoptosis-inducing factor in cardiac myocytes during oxidant stress: potential role of poly(ADP-ribose) polymerase-1. Cardiovasc Res 63: 682–688, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Choudhury S, Bae S, Kumar SR, Ke Q, Yalamarti B, Choi JH, Kirshenbaum LA, Kang PM. Role of AIF in cardiac apoptosis in hypertrophic cardiomyocytes from Dahl salt-sensitive rats. Cardiovasc Res 85: 28–37, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23: 2785–2796, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol 158: 507–517, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gurevich RM, Regula KM, Kirshenbaum LA. Serpin protein CrmA suppresses hypoxia-mediated apoptosis of ventricular myocytes. Circulation 103: 1984–1991, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Han Y, Chen YS, Liu Z, Bodyak N, Rigor D, Bisping E, Pu WT, Kang PM. Overexpression of HAX-1 protects cardiac myocytes from apoptosis through caspase-9 inhibition. Circ Res 99: 415–423, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol Sci 25: 259–264, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Joseph B, Marchetti P, Formstecher P, Kroemer G, Lewensohn R, Zhivotovsky B. Mitochondrial dysfunction is an essential step for killing of non-small cell lung carcinomas resistant to conventional treatment. Oncogene 21: 65–77, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res 87: 118–125, 2000. [DOI] [PubMed] [Google Scholar]
- 15. Kang PM, Izumo S. Apoptosis in heart: basic mechanisms and implications in cardiovascular diseases. Trends Mol Med 9: 177–182, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311: 847–851, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laugwitz KL, Moretti A, Weig HJ, Gillitzer A, Pinkernell K, Ott T, Pragst I, Stadele C, Seyfarth M, Schomig A, Ungerer M. Blocking caspase-activated apoptosis improves contractility in failing myocardium. Hum Gene Ther 12: 2051–2063, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, Kanamori H, Khai NC, Maruyama R, Ogino A, Minatoguchi S, Fujiwara T, Fujiwara H. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation 113: 535–543, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Logue SE, Gustafsson AB, Samali A, Gottlieb RA. Ischemia/reperfusion injury at the intersection with cell death. J Mol Cell Cardiol 38: 21–33, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Lorenzo HK, Susin SA, Penninger J, Kroemer G. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ 6: 516–524, 1999. [DOI] [PubMed] [Google Scholar]
- 21. Modjtahedi N, Giordanetto F, Madeo F, Kroemer G. Apoptosis-inducing factor: vital and lethal. Trends Cell Biol 16: 264–272, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Molnar A, Toth A, Bagi Z, Papp Z, Edes I, Vaszily M, Galajda Z, Papp JG, Varro A, Szuts V, Lacza Z, Gero D, Szabo C. Activation of the poly(ADP-ribose) polymerase pathway in human heart failure. Mol Med 12: 143–152, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol 50: 528–536, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neilan TG, Blake SL, Ichinose F, Raher MJ, Buys ES, Jassal DS, Furutani E, Perez-Sanz TM, Graveline A, Janssens SP, Picard MH, Scherrer-Crosbie M, Bloch KD. Disruption of nitric oxide synthase 3 protects against the cardiac injury, dysfunction, and mortality induced by doxorubicin. Circulation 116: 506–514, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Okamura T, Miura T, Takemura G, Fujiwara H, Iwamoto H, Kawamura S, Kimura M, Ikeda Y, Iwatate M, Matsuzaki M. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res 45: 642–650, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther 300: 862–867, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Penninger JM, Kroemer G. Mitochondria, AIF and caspases–rivaling for cell death execution. Nat Cell Biol 5: 97–99, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Pieper AA, Brat DJ, Krug DK, Watkins CC, Gupta A, Blackshaw S, Verma A, Wang ZQ, Snyder SH. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc Natl Acad Sci USA 96: 3059–3064, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell 69: 597–604, 1992. [DOI] [PubMed] [Google Scholar]
- 31. Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 339: 900–905, 1998. [DOI] [PubMed] [Google Scholar]
- 32. Siu PM, Bae S, Bodyak N, Rigor DL, Kang PM. Response of caspase-independent apoptotic factors to high salt diet-induced heart failure. J Mol Cell Cardiol 42: 678–686, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest 117: 3730–3741, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446, 1999. [DOI] [PubMed] [Google Scholar]
- 35. Uren RT, Dewson G, Bonzon C, Lithgow T, Newmeyer DD, Kluck RM. Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J Biol Chem 280: 2266–2274, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Yang C, Chai J, Shi Y, Xue D. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science 298: 1587–1592, 2002. [DOI] [PubMed] [Google Scholar]
- 37. Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, Szabo C. Poly(ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther 312: 891–898, 2005. [DOI] [PubMed] [Google Scholar]
- 38. Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 97: 276–281, 1998. [DOI] [PubMed] [Google Scholar]
- 39. Youn HJ, Kim HS, Jeon MH, Lee JH, Seo YJ, Lee YJ, Lee JH. Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment. Mol Cell Biochem 270: 13–19, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297: 259–263, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem 272: 7797–7800, 1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.