Abstract
During the first wave of spermatogenesis, and in response to ionizing radiation, elevated mutant frequencies are reduced to a low level by unidentified mechanisms. Apoptosis is occurring in the same time frame that the mutant frequency declines. We examined the role of apoptosis in regulating mutant frequency during spermatogenesis. Apoptosis and mutant frequencies were determined in spermatogenic cells obtained from Bax-null or Trp53-null mice. The results showed that spermatogenic lineage apoptosis was markedly decreased in Bax-null mice and was accompanied by a significantly increased spontaneous mutant frequency in seminiferous tubule cells compared to that of wild-type mice. Apoptosis profiles in the seminiferous tubules for Trp53-null were similar to control mice. Spontaneous mutant frequencies in pachytene spermatocytes and in round spermatids from Trp53-null mice were not significantly different from those of wild-type mice. However, epididymal spermatozoa from Trp53-null mice displayed a greater spontaneous mutant frequency compared to that from wild-type mice. A greater proportion of spontaneous transversions and a greater proportion of insertions/deletions 15 days after ionizing radiation were observed in Trp53-null mice compared to wild-type mice. Base excision repair activity in mixed germ cell nuclear extracts prepared from Trp53-null mice was significantly lower than that for wild-type controls. These data indicate that BAX-mediated apoptosis plays a significant role in regulating spontaneous mutagenesis in seminiferous tubule cells obtained from neonatal mice, whereas tumor suppressor TRP53 plays a significant role in regulating spontaneous mutagenesis between postmeiotic round spermatid and epididymal spermatozoon stages of spermiogenesis.
Keywords: apoptosis, gamete biology, ionizing radiation, mutant frequency, spermatogenesis
The frequency of mutation in spermatogenic cells is altered in a cell type-specific manner in mice deficient in the proapoptotic protein BAX or tumor suppressor protein P53.
INTRODUCTION
Spontaneous mutant frequency in spermatogenic cells is lower than that in somatic tissues in a Lac I mouse model, official symbol Tg(TacLIZa)A1Jsh [1, 2]. However, the mutant frequency changes during spermatogenesis [1]. Primitive type A (collected from 6-day-old mice) and type A spermatogonia (collected from 8-day-old mice) have the highest spontaneous mutant frequencies, followed by type B spermatogonia, preleptotene spermatocytes, leptotene and zygotene spermatocytes, pachytene spermatocytes, round spermatids, and epididymal spermatozoa, all of which display a similarly low mutant frequency [1]. Ionizing radiation (IR) induces an increased mutant frequency that also declines as cells progress through spermatogenesis [3]. These data clearly show that one or more mechanisms reduce mutation load during spermatogenesis.
Repair of DNA plays a large role in regulating mutant frequency [4–6]. In particular, the base excision repair (BER) pathway plays a major role in regulating mutant frequency in the rodent male germline [7, 8]. It is unlikely, however, that DNA repair can mediate a decline in mutant frequency for fixed mutations. Apoptosis is another mechanism that may function in male germ cells to mediate a decline in mutant frequency during spermatogenesis by removing cells with a high mutant frequency [1, 3]. However, little is known about the quantitative effects of apoptosis on mutant frequency, particularly in the germline.
Apoptosis occurs extensively in the first wave of spermatogenesis in rodents and is critical for the elimination of abnormal germ cells. Up to 75% of the original early spermatogonia are lost and will not develop to the spermatocyte stage [9]. Later, in the mature mouse, germ cell apoptosis is observed primarily among spermatogonia and spermatocytes [10]. Apoptosis is a complex process comprised of two main pathways (intrinsic and extrinsic), each of which is regulated at multiple levels. The apoptosis regulator BCL-2 family is a major regulator of the intrinsic pathway [11], which is essential for normal balance of male germ cell survival or death. Some members of this family promote cell survival (e.g., BCL2, BCL2L1, and BCL2L2), whereas others antagonize it (e.g., BAX, BAK1, and BCL2L11, also known as BIM) [12]. Pro-apoptotic BAX appears to be essential for progression through the first wave of spermatogenesis [13]. BAX protein is abundantly expressed in mouse testis between 1 and 3 wk after birth [14]. In adult mice, BAX is expressed at low levels in male germ cells and is restricted to spermatogonia [14, 15]. Bax-null male mice are infertile as a result of disordered seminiferous tubules with excessive numbers of spermatogonia and preleptotene spermatocytes, consistent with failed apoptosis during the first wave of spermatogenesis [16, 17]. These findings indicate the importance of BAX-mediated apoptosis during spermatogenesis.
The intrinsic pathway of apoptosis is also regulated by the tumor suppressor TP53 protein. Upon activation by DNA damage or other signaling pathways, TP53 protein promotes the expression of a number of genes that are involved in apoptosis [18–20]. Most of the effects of tumor suppressor TP53 are ascribed to its function as a transcription factor. Tumor suppressor TP53 is also reported to directly activate components of the apoptotic machinery by regulating the activity of BCL2 family members (e.g., BAX [21]) or inducing FAS to bind with FADD [22]. Loss of TP53 confers a survival advantage after exposure to a variety of DNA-damaging agents [23], which is consistent with the report that mice deficient for tumor suppressor TRP53 are susceptible to spontaneous tumors [24]. Tumor suppressor TRP53 is reported to play an important role in apoptosis during normal spermatogenesis and during responses to DNA damage [25–27]. These findings demonstrate a role of tumor suppressor gene TP53 in modulating apoptosis and spermatogenesis.
To address the hypothesis that cell death may play a role in regulating mutant frequency during spermatogenesis, Bax-null or Trp53-null mice were used. Apoptosis was examined in testes from 1) prepubertal Bax-null mice, 2) young adult Trp53-null mice, and 3) young adult Trp53-null mice after IR treatment. Germline mutant frequencies were measured by crossing each line with Lac I transgenic mice (Tg(TacLIZa)A1Jsh). Because tumor suppressor TP53 has been implicated in BER [28, 29], this DNA repair activity was measured in spermatogenic cells obtained from Trp53-null mice.
MATERIALS AND METHODS
Animals
Six pairs of C57BL/6 mice heterozygous for the Trp53 gene (Trp53−/+) and homozygous for the Lac I transgene (Lac I+/+) gene were purchased from Taconic. Double-homozygous male mice (Trp53−/−/Lac I+/+) were generated at the University of Texas Health Science Center at San Antonio animal facility. Wild-type male and female mice (C57BL/6) homozygous for the Lac I gene (Trp53+/+/Lac I+/+) were obtained from Taconic or from in-house breeding regimens. All the animals used in the present experiments carried a Lac I gene; thus, we named the mice based on the status of the Trp53 gene—namely, Trp53 null (Trp53−/−) or Trp53 wild type (Trp53+/+). Female Bax−/− mice were crossed with male Lac I+/+ mice to generate double-heterozygous progeny that were then intercrossed to obtain Bax−/− mice carrying a Lac I gene. All animal procedures were approved by the Institutional Animal Care and Use Committee. The animal facility is Association for Assessment and Accreditation of Laboratory Animal Care accredited.
IR Treatment
Five male mice each of the Trp53−/− and wild-type genotypes were placed in an acrylic holding rack for exposure to IR in a GammaCell 40 (Atomic Energy of Canada Limited) to identify an appropriate radiation dose. The specific activity of the 137Cs was 1.2 Gy/min. Four-month-old male mice were subjected to whole-body exposure to 1.2 or 2.4 Gy of IR. The mice were killed at 15 or 49 days after irradiation, and the testes were then removed and fixed in formalin for histological examination. A dose of 1.2 Gy of IR was found to be below the threshold of morphological disruption of the seminiferous tubules and, therefore, was chosen for all subsequent experiments. In contrast, 2.4 Gy of IR was associated with disruption of spermatogenesis in mice of both genotypes.
Thirty male Trp53-null and 20 male wild-type mice were whole-body irradiated with 1.2 Gy of gamma-radiation at the age of 4 mo. The sham-treated control mice (10 male Trp53−/− and 5 male wild-type mice) were placed into the exposing chamber, but the chamber was not lowered to the radiation source for exposure. The control mice were mock-exposed for the same period of time as the IR-treated mice. The mice were killed at 15 or 49 days after irradiation, and the testes were then removed for mutant frequency analyses. Cells collected 15 days after irradiation are descendants of irradiated spermatogonial progenitors, whereas cells collected 49 days after irradiation are the descendants of irradiated spermatogonial stem cells. These time points were included in an effort to distinguish between the potentially different effects on stem cells compared to nonstem cells.
Preparation of Spermatogenic Cells
Mice were anesthetized with Isoflurane (Abbott Laboratories) and then euthanized by cervical dislocation. Ten Trp53-null mice were used at each time point (i.e., 0, 15, and 49 days after 1.2 Gy of IR), whereas five, seven, and eight wild-type mice were used at 0, 15, and 49 days after IR. Pachytene spermatocytes and round spermatids were prepared by using a Sta Put gradient system as described previously [30]. Briefly, testes were removed from mice and decapsulated. The testes were then placed in EKRB medium (120.1 mM NaCl, 4.8 mM KCl, 25.2 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgSO4·7H2O, and 1.3 mM CaCl2, supplemented with 11.1 mM glucose, 2 mM glutamine, 1× essential amino acids, 1× nonessential amino acids, 100 μg/ml streptomycin, and 100 U/ml penicillin; Gibco BRL). Collagenase (Sigma-Aldrich) was added to the buffer to a final concentration of 0.5 mg/ml. The flask was shaken for 15 min at 32°C. The tubules were allowed to settle to the bottom of the flask, and the supernatant was discarded. After two washes in EKRB media, the tubules were digested with 0.25 mg/ml of trypsin and 1 μg/ml of DNase for 15 min with shaking at 32°C. The cells were filtered through a 70-μm cell strainer, the filtrate centrifuged at 500 × g for 10 min, and the cells resuspended in EKRB medium containing 0.5% (w/v) bovine serum albumin (BSA). The cell suspension was then loaded on a 2–4% BSA gradient (Sta Put). The cell fractions were collected, and the cell populations were examined under the microscope. The purity of pachytene spermatocytes was greater than 90%, whereas the purity of round spermatids was greater than 94%.
The seminiferous tubule cells (defined as all the cell types within the seminiferous tubules) from 10-day-old mice consisted of approximately 50% germ cells (type A spermatogonia, type B spermatogonia, preleptotene spermatocytes, and leptotene spermatocytes) and 50% Sertoli cells [30]. Because of the difficulty in obtaining sufficient numbers of 10-day-old male Bax−/− mice, we were unable to perform enrichments of defined spermatogenic cell types. Thus, seminiferous tubule cells used in the mutagenesis assay were prepared similarly to cells from Trp53-null mice, but without the Sta Put gradient separation procedure. Seven 10-day-old male Bax−/− mice and twelve 10-day-old wild-type mice were used.
Epididymal spermatozoa were prepared as follows: Epididymides were minced with a scalpel in 1× PBS and then incubated at 33°C for 30 min. The resulting mixture was filtered through a 70-μm cell strainer in a 50-ml conical tube. Cells were collected by centrifuging at 1000 × g for 10 min, then snap-frozen in liquid nitrogen and stored at −80°C until use.
Mutagenesis Assay
High-molecular-weight genomic DNA was prepared using the RecoverEase DNA isolation kit according to the manufacturer's recommendations (Stratagene). Lambda phage shuttle vectors harboring the bacterial Lac I gene were recovered from high-molecular-weight genomic DNA samples using Stratagene's Transpack in vitro packaging extracts. Packaged phage were mixed with Escherichia coli SCS-8 cells and added to top agarose containing 5-bromo-4-chloro-3-indoyol-betagalactopyranoside and plated on NZY agar. After incubation overnight at 37°C, recovered plaque-forming units (pfus) were counted. Blue mutant plaques were visually identified, cored, and replated at low density under the same incubation conditions to confirm the mutant. Mutant frequency was determined by dividing the number of confirmed mutant plaques by the total number of pfus recovered.
DNA Sequence Analysis
All mutants obtained from Trp53−/− mice were sequenced by the DNA Sequencing Facility, Center for Biomedical Research, University of Victoria, Canada. For unknown reasons, one mutant from a Trp53−/− mouse and three mutants from wild-type mice could not be sequenced after multiple attempts. Very few mutants were recovered from untreated wild-type mice because of the low spontaneous mutant frequency in the male germline. Therefore, historical data were combined with the data in the present study for comparison. No significant differences were found in mutation spectra derived from the current data and the historical data [31]. Mice used in historical studies and in the present study had the same genetic background (C57BL/6) and similar age. In all, 24 of 30 mutants from Bax-null mice and 31 of 48 mutants from wild-type mice were sequenced.
Nuclear Extract Preparation and BER Assay
Nuclear extracts were prepared from mixed germ cells (i.e., spermatogenic cells in adult mice relatively free of somatic cells) obtained from 4-mo-old Trp53−/− mice and wild-type mice as described elsewhere [32, 33]. Mixed germ cells were prepared using the same procedure as described earlier for seminiferous tubule cells. For adult mice, this procedure yields predominantly germ cells (approximately 3% Sertoli cells) [30].
The BER assay was performed as previously described [34, 35]. Briefly, 3 pmol of BER substrate (i.e., a 51-mer oligonucleotide containing a single G:U mismatch and a 5′ fluorescein label on the U-containing strand; Integrated DNA technologies) were added to the nuclear extracts, then incubated at 37°C for 10 min in reaction buffer (100 mM Tris-HCl [pH 7.5]; 0.1 mM ethylenediaminetetra-acetic acid [EDTA]; 5 mM MgCl2; 1 mM dithiothreitol; 2 mM ATP; 0.5 mM NAD; 20 μM each of dATP, dGTP, and dTTP; 5 mM ditrisphosphocreatine; 10 U of creatine phosphokinase; 20 nM unlabeled dCTP; and 20 μCi of α-[33P]dCTP [3000 Ci/mmol]). Reactions were stopped by placing the tubes on ice and adding 4.5 μl of stop solution (50 mM EDTA, 0.3 M NaCl, and 80% formamide). Samples were subjected to PAGE. The bands of interest were quantified using a Personal Molecular Imager (Molecular Imager FX; Bio-Rad).
Determination of Apoptosis
Testes from five 10-day-old Bax−/− and six wild-type controls were collected and fixed in 10% formalin solution. Tissues were paraffin embedded, and TUNEL assays [36] were performed on testis sections (thickness, 4 μm) by the San Antonio Cancer Institute Pathology Core Shared Resource. The sections were then examined under the microscope. The apoptotic prevalence was calculated by the number of seminiferous tubules with at least one TUNEL-positive cell divided by total number of tubules counted. Fifteen days after IR, three adult Trp53-null and three wild-type mice were subjected to TUNEL assays using the same procedure. For wild-type mice, 651 tubules (mean number of tubules examined per mouse ± SD, 217 ± 22) were assayed, and 137 tubules (46 ± 9) were found with at least one TUNEL-positive cell per tubule. For Trp53-null mice, 445 tubules (148 ± 134) were assayed, and 97 tubules (32 ± 28) were observed with at least one TUNEL-positive cell per tubule.
Statistical Analyses
Mutant frequency data were analyzed by a Poisson regression model, with parameter estimates obtained by the method of maximum likelihood [37]. Statistical tests of differences used the likelihood ratio test. Comparisons among groups were Bonferroni adjusted. Mutation spectra from germ cells were compared for differences among radiation exposure, cell type, and days after IR exposure using a chi-square test. If small frequencies were encountered, an exact test was carried out. Fractions of tubules containing one or more apoptotic cells were analyzed using ANOVA for repeated measurements. All computations were carried out using SAS software (Version 9.1; SAS Institute).
RESULTS
Decreased Apoptosis and Increased Mutant Frequency in Bax-Null Seminiferous Tubule Cells
The spontaneous mutant frequency for seminiferous tubule cells obtained from 10-day-old Bax-null mice was significantly higher than that in wild-type mice of the same age (mean ± SEM, 3.03 ± 0.44 × 10−5 vs. 1.78 ± 0.33 × 10−5; P = 0.0212) (Table 1). The prevalence of apoptosis was significantly lower in Bax-null mice (11.1% ± 3.8% of tubules with apoptotic cells obtained in six mice) compared to wild-type mice (28.2% ± 4.6 % of tubules with apoptotic cells obtained in five mice; P < 0.05) (Fig. 1).
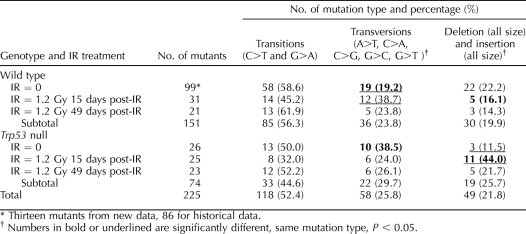
TABLE 1.
Mutant frequency in seminiferous tubule cells from Bax-null and wild-type mice.*
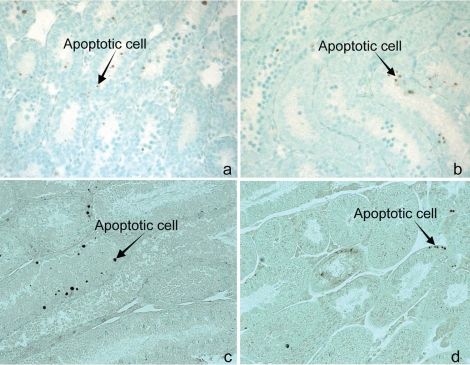
FIG. 1.
Representative TUNEL pictures from wild-type (a) and Bax-null (b) testis (10 days old) and from wild-type (c) and Trp53-null (d) testis (4 months old). Brown cells are apoptotic cells. Arrows point to selected examples. Original magnification ×160 (a and b) and ×200 (c and d).
Similar Spontaneous Mutation Spectra for Bax-Null and Wild-Type Seminiferous Tubule Cells
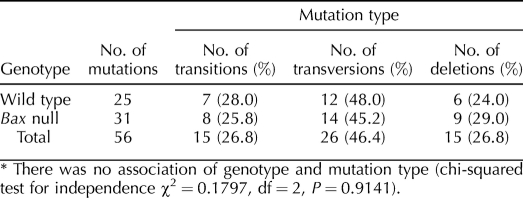
Analysis of mutation spectra for seminiferous tubule cells isolated from Bax-null mice or wild-type mice revealed no difference in the proportion of transitions, transversions, or deletions (Table 2). Mutants obtained from Bax-null mice displayed 25.8% transitions, 45.2% transversions, and 29.0% deletions as compared to 28.0%, 48.0%, and 24.0%, respectively, for wild-type seminiferous tubule cells.
TABLE 2.
Mutational spectra in seminiferous tubule cells from Bax-null and wild-type mice.*
Apoptosis Following IR in Spermatogenic Cells from Trp53-Null Mice and Wild-Type Mice
Our previous studies with IR-mediated mutagenesis in spermatogenic cells indicated that mechanisms exist to eliminate cells with elevated mutant frequencies during spermatogenesis [1, 3]. In several cell types, the apoptotic response to IR is dependent on the tumor suppressor gene TP53. To determine whether IR-induced apoptosis could occur in the spermatogenic cells in the absence of tumor suppressor gene Trp53, Trp53-null and wild-type mice were irradiated with a single dose of gamma-radiation, then apoptotic activity was measured by TUNEL in the whole testes 15 days later. Interestingly, apoptosis was observed in testes in both genotypes. Approximately 22% of seminiferous tubules in the Trp53-null mice were observed to have at least one apoptotic cell, which was similar to the level observed in the wild-type mice (21%; P = 0.6865) (Fig. 1).
Cell Type-Specific Spontaneous Mutant Frequency in Trp53-Null Mice
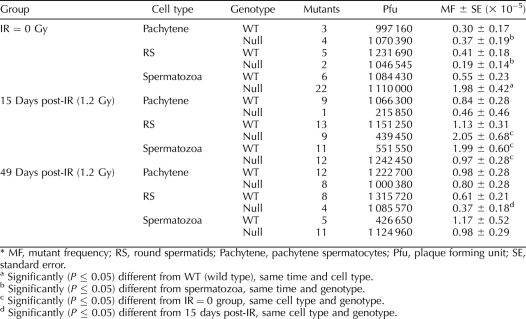
Pachytene spermatocytes, round spermatids, and epididymal spermatozoa were collected from Trp53-null and wild-type mice to analyze spontaneous mutant frequencies (Table 3). As shown in Figure 2, a significantly increased spontaneous mutant frequency was observed in epididymal spermatozoa obtained from the Trp53-null mice in comparison to the wild-type mice (P < 0.01). In contrast, the spontaneous mutant frequencies for pachytene spermatocytes and round spermatids were similar for Trp53-null mice and wild-type mice (Fig. 2).
TABLE 3.
Mutant frequency in spermatogenic cells in wild-type mice and Trp53-null mice.
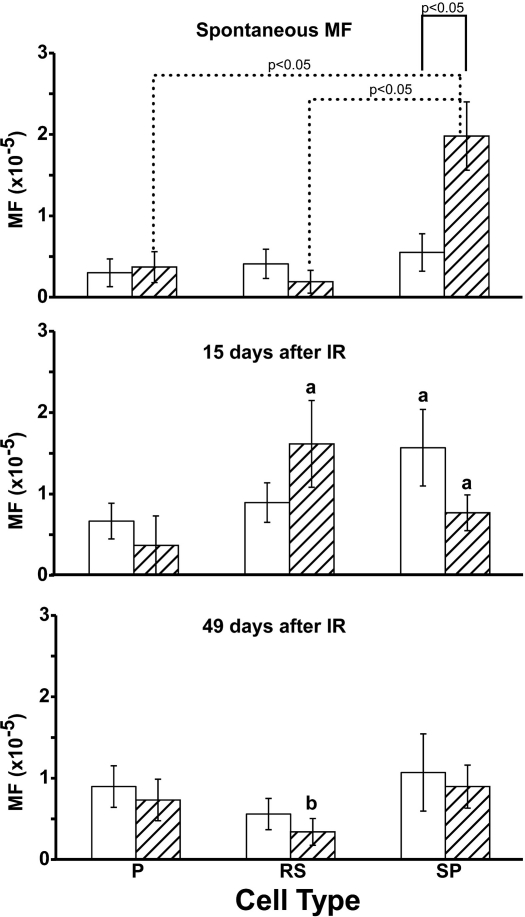
FIG. 2.
Mutant frequencies (MF) in different spermatogenic cell types from Trp53-null mice (hatched bars) and wild-type mice (white bars). P, pachytene spermatocytes; RS, round spermatids; SP, spermatozoa. Error bars represent the standard error. The letter a indicates significant (P ≤ 0.05) difference from spontaneous mutation, same cell type and genotype; the letter b indicates significant (P ≤ 0.05) difference from 15 days post-IR, same cell type and genotype.
Mutagenic Response to IR in Trp53-Null Spermatogenic Cells
Mutant frequencies in pachytene spermatocytes, round spermatids, or epididymal spermatozoa were similar in Trp53-null mice and wild-type mice at 15 and 49 days after IR treatment. Mutant frequency in round spermatids from Trp53-null mice, isolated 15 days after IR, was significantly higher than that measured in round spermatids isolated 49 days after IR (P < 0.05) (Fig. 2). Mutant frequency in epididymal spermatozoa from the wild-type mice determined 15 days after IR treatment was significantly increased compared to that in the mice not treated with IR (P < 0.05) (Fig. 2).
Spontaneous and IR-Induced Mutational Spectra in Trp53-Null Mice and Wild-Type Mice
The number of recovered spontaneous mutants was low in enriched spermatogenic cell types (i.e., pachytene spermatocytes, round spermatids, and spermatozoa). We therefore combined the data from those cell types with the same genotype and same treatment group to increase the power of the statistical analysis (Table 4). The mutation spectra in the Lac I gene revealed that transition mutations (C to T and G to A) were the most abundant mutation detected in spermatogenic cells regardless of genotype and IR treatment, except that deletion and insertion mutations were the most abundant mutations in Trp53-null germ cells 15 days after IR treatment (Table 4).
TABLE 4.
Mutation spectra in combined spermatogenic cell types in Trp53-null and wild-type mice.
A comparison of mutation spectra between Trp53-null and wild-type mice showed that the fraction of transversions in wild-type (IR = 0) was significantly lower than that in Trp53-null (IR = 0) mice (19.2% vs. 38.5%; P = 0.0383) (Table 4). Further analysis did not reveal significant differences among specific transversions (i.e., A to T, C to A, C to G, G to C, or G to T) between the two groups. After IR treatment, the proportion of transversion mutations was similar among mutants recovered from wild-type and Trp53-null germ cells. In contrast, the proportion of deletions and insertions was significantly greater among mutants recovered from Trp53-null germ cells 15 days after IR treatment as compared to wild-type cell (44.0% vs. 16.1%; P = 0.0217) (Table 4). GC to CG transversions occurred more frequently among mutants recovered from wild-type germ cells, with and without IR treatment as compared to Trp53-null mice (76.7% vs. 25%; P = 0.0007).
In wild-type mice, IR treatment markedly increased the fraction of transversions from 19.2% in IR = 0 groups to 38.7% at 15 days after IR treatment (P = 0.0293) (Table 4). However, the fraction of transversions dropped to 23.8% at 49 days after IR treatment (P > 0.05). In Trp53-null mice, IR treatment increased the fraction of deletions and insertions from 11.5% in IR = 0 groups to 44% at 15 days after IR treatment (P = 0.0100); however, the proportion of deletion/insertion mutations then decreased to 21.7% at 49 days after IR treatment (P > 0.05) (Table 4).
BER Activity in Mixed Germ Cells for Trp53-Null Mice
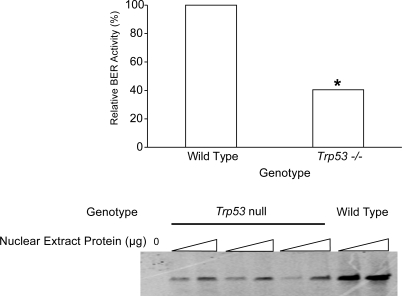
The BER activities were measured in nuclear extracts prepared from mixed germ cells obtained from Trp53-null mice and wild-type mice. BER activity in mixed germ cells from Trp53-null mice was approximately 40% (P = 0.0013; 95% confidence interval, 24.9–65.9%) of the levels in wild-type mice (Fig. 3).
FIG. 3.
BER activity in Trp53-null and wild-type spermatogenic cells. Top) Quantitative data obtained from three independent experiments. An asterisk indicates a significant (P < 0.05) difference compared to the wild type. Bottom) representative image of BER assay (Trp53-null sample was triplicate). The bands represent completely repaired oligonucleotide.
DISCUSSION
Spermatogenesis is a well-organized and finely regulated differentiation process in which spermatogonia proliferate and differentiate into haploid spermatozoa that carry half of the DNA required for the development of offspring. For healthy reproduction, it is essential to maintain germline genetic integrity. In previous studies [1, 3], we found that mutant frequency declines during the first wave of spermatogenesis and after IR (following an initial increase in mutant frequency). These observations suggest that spermatogenic cells possess one or more mechanisms to eliminate cells with mutations, thereby leaving nonmutated cells to complete spermatogenesis. Apoptosis plays a significant role in ensuring cellular homeostasis during spermatogenesis [13, 26]. Intercellular bridges exist between differentiating spermatogenic cells, contributing to the synchronous development of germ cells derived from a single spermatogonium [38]. All daughter cells from a common spermatogonium can be selectively eliminated via apoptosis, whereas unrelated colonies are preserved [39]. The intercellular bridges allow macromolecules critical to the differentiation process to be shared among the daughter cells [40–42], which may share apoptotic signals within the colony. Apoptosis in male germ cells can also be initiated in the presence of excessive DNA damage [43]. Thus, apoptosis appears to be a possible mechanism for removing mutant cells.
Apoptosis consists of at least two pathways (i.e., intrinsic and extrinsic pathways) [44]. A common class of proteins, caspases, is the executor in both pathways. Each pathway is regulated at multiple levels by various proteins. BAX is a pro-apoptosis protein in the intrinsic pathway and resides mainly in the cytosol of healthy cells as a soluble monomer [45–47]. In response to apoptotic signals, BAX undergoes conformational changes, translocates to the mitochondrial outer membrane, and forms large oligomeric complexes [46, 48, 49], which facilitate the release of cytochrome c from the mitochondrial intermembrane space into the cytosol [50, 51] as part of the cascade of events that comprise apoptosis. In the present study, the prevalence of apoptosis in testes of Bax-null mice was significantly decreased, which is consistent with its proapoptotic role in the germline. Accompanying this decrease in the prevalence of apoptosis, a significantly increased mutant frequency was observed for seminiferous tubule cells obtained from neonatal Bax-null mice. The cells were collected from 10-day-old mice, at a time when type A and type B spermatogonia are the predominant germ cell types in the testis [30]. These findings suggest that BAX-mediated apoptosis affects mutant frequency in premeiotic spermatogenic cells of neonatal mice. Bax expression is restricted to spermatogonia in adult mice [14, 15]. Thus, BAX-involved apoptosis may prevent spermatogonia with a high spontaneous mutation load from progressing through spermatogenesis and, thereby, reducing germline mutant frequency.
The seminiferous tubule cell preparations from 10-day-old mice used in the present study were comprised mainly of germ cells and Sertoli cells, with germ cells representing approximately 50% of the cells in the preparations. A lower mutant frequency was detected for the seminiferous tubule cell preparations from wild-type mice in the present study, similar to the level of mutant frequency determined by using enriched preparations of defined spermatogenic cell types [1] and consistent with the interpretation that the lower mutant frequency for wild-type mice resulted from the germ cells in the seminiferous tubule cell preparations. Thus, Bax appears to play an important role in regulating mutant frequency in the germline.
In contrast to the profile of apoptosis, mutation spectra were similar for Bax-null cells as compared to wild-type cells. Bax-null and wild-type seminiferous tubule cells exhibited a high proportion of deletion/insertion mutations compared to cells that had progressed further through spermatogenesis. These findings suggest that mutation spectra in the Lac I gene may be dependent on cell type. The greater mutant frequency but similar mutation spectrum in Bax-null spermatogonial cells may reflect the failure to remove mutated spermatogonia that would die when a BAX-dependent apoptosis pathway functions normally. One would expect to find a greater prevalence of deletions/insertions among spermatocytes in Bax-null mice. Unfortunately, spermatogenesis is severely compromised in these mice, and we were unable to directly assess mutations in spermatocytes from Bax-null mice. Epididymal spermatozoa were the only cell type examined from Trp53-null mice that displayed a significantly increased spontaneous mutant frequency compared to the wild-type mice, indicating that tumor suppressor gene Trp53 influences mutant frequency among postmeiotic cells. Apoptosis still occurs in spermatozoa [52]. However, TRP53 expression was only detected in pachytene spermatocytes in wild-type adult mouse testis using immunohistochemistry staining [25], which implies that Trp53 may not be involved directly in the process of apoptosis in spermatozoa. Evidence of apoptosis, such as loss of the integrity of the mitochondrial membrane potential and activated caspase 3, has been found in spermatozoa [25, 53]. Caspase 3 in particular is a downstream effecter of TP53 [54]. Loss of Trp53 functions in Trp53-null mice may decrease the activation of caspase 3, resulting in decreased apoptosis and higher mutant frequency in spermatozoa. The observation of increased mutant frequency only in epididymal spermatozoa indicates that tumor suppressor TRP53-regulated mutant frequency may be cell-type specific among the spermatogenic cells. Interestingly, differences in mutant frequency were not detected in sperm from Trp53-null mice after IR treatment, which indicated that postmeiotic spermatogenic cells from Trp53-null mice had a response to IR similar to that of wild-type mice. In contrast to spontaneous mutant frequency in spermatozoa, mutant frequencies for other spermatogenic cells examined from Trp53-null mice were similar to those for the wild-type mice, whether or not they were exposed to IR. This was also reported for somatic tissues from Trp53-null mice [23, 55].
Previous studies have demonstrated a difference in the prevalence of apoptosis within 24 h of IR between wild-type mice and Trp53-null mice [56]. Thus, we hypothesized that the Trp53-null mice would display a different mutagenic response to IR than wild-type mice. Pachytene spermatocytes were descendants from irradiated progenitor spermatogonia, round spermatids from primary spermatocytes, and epidydimal spermatozoa from spermatids 15 days after IR according to the literatures [30, 57]. Therefore, cells were isolated 15 days after IR were descended from irradiated progenitors and meiotic cells, whereas those isolated 49 days after IR were descended from irradiated stem cells [30, 57]. IR produces its maximum genetic damage in germ cell stages ranging from midpachytene spermatocytes through early spermatids, as determined by dominant lethal and specific locus assays [58]. Our results are consistent with the previous findings. Importantly, tumor suppressor TRP53 is not required to restore a low mutant frequency among spermatogenic cells descended from irradiated spermatogenic stem cells.
Mutation spectra can be distinctive after exposure to different genotoxic agents as well as for deficiencies in specific DNA repair activities. For example, cells deficient in OGG1, a DNA glycosylase that removes 8-oxode-oxy guanine from DNA, show a greater prevalence of GC to TA transversion mutations because of the decreased repair of oxidized guanine [59]. Mutation spectra can be affected even when mutant frequencies are not [60]. Markedly increased spontaneous mutant frequency was observed in epididymal spermatozoa from Trp53-null mice. However, the mutation spectra obtained for spermatozoa did not show a significant difference between Trp53-null and wild-type mice (data not shown). Combined mutation spectra data from pachytene spermatocytes, round spermatids, and epididymal spermatozoa revealed that the prevalence of transversions in non-IR-treated Trp53-null mice was greater than that in wild-type mice even though mutant frequencies were similar. Mutation spectrum is altered in spermatogenic cells from Trp53-null mice after IR treatment. Tumor suppressor TP53 is involved in the DNA damage response to IR and in the response to signal cell death. The increased prevalence of deletion/insertion mutations at 15 days after IR in Trp53-null mice further suggests that the response to damage is altered in the absence of tumor suppressor TRP53 or that tumor suppressor TRP53 normally functions to remove cells with damage that is likely to lead to insertion/deletion mutations. The prevalence of deletions among meiotic and postmeiotic spermatogenic cells is low relative to that detected in premeiotic spermatogonia. Trp53-null mice have an unusually high prevalence of deletion/insertion mutations 15 days after IR. Together, these data suggest that cells carrying damage that leads to deletions/insertions may be preferentially removed by cell death responses when the Trp53 gene is intact.
From a biological perspective, it is intriguing that the mutant frequency was affected in postmeiotic cells from Trp53-null mice. DNA replication provides an opportunity to create mutations from unrepaired DNA damage or through misincorporation by a replicative DNA polymerase. However, DNA replication is completed by primary spermatocytes. We have previously reported that the spontaneous mutant frequency increases significantly between pachytene spermatocytes (the last cell type of primary spermatocytes) and epididymal spermatozoa obtained from old wild-type mice [1]. It is unclear how mutations would become fixed in postreplicative cells unless it occurs through unscheduled DNA synthesis. One possibility is that low levels of tumor suppressor TRP53-dependent DNA repair do occur after the round spermatid stage of spermatogenesis and that in the absence of tumor suppressor TRP53, the normally low level of repair activity is compromised. Several DNA repair proteins interact with tumor suppressor TP53, including those involved in nucleotide excision repair, BER, mismatch repair, nonhomologous end-joining, and homologous recombination [61]. Thus, deficiencies in tumor suppressor TRP53 may lead to differences in postreplicative repair in germ cells, thereby impacting spectrum and frequency in response to spontaneous damage and affecting spectrum after IR.
Ionizing radiation is known to induce apoptosis in mouse testis [62]. The prevalence of apoptosis was similar among seminiferous tubules from Trp53-null mice and wild-type mice at 15 days after IR. However, we cannot exclude the possibility of a difference in prevalence of apoptosis between Trp53-null mice and wild-type mice at time points earlier than 15 days after IR. Hasegawa et al. [56] reported increased apoptosis that peaked 12 to 24 h after irradiation in spermatogonia from wild-type mice, but not from Trp53-null mice, and which then returned to the baseline levels at 3 days after irradiation with 0.5 Gy. The baseline apoptosis activity in Trp53-null mice in the present study could indicate that Trp53-independent apoptosis pathways function in spermatogenic cells. Other proteins, such as TRP63 and TRP73, can activate the promoters of several tumor suppressor Trp53-responsive genes implicated in apoptosis, including the Bax gene [63–65]. Therefore, apoptosis can still occur without tumor suppressor Trp53. At present, the extent to which these pathways mediate selective apoptosis in spermatogenic cells with or without IR is unclear.
Multiple DNA repair pathways function in male germline cells, including the BER pathway that elicits responses to single base DNA damage and abasic sites [66–68]. BER activity varies among mammalian tissues, with the highest level found in spermatogenic cells [32, 34, 69, 70]. Consistent with high levels of BER activity, spontaneous mutant frequency in spermatogenic cells is markedly lower than that in somatic tissues [1, 2]. Extracts prepared from a cell line that overexpressed wild-type tumor suppressor Trp53 displayed enhanced BER activity [28], whereas removal of tumor suppressor TRP53 protein from those cell line extracts significantly reduced BER activity [28, 29]. In the present study, a 60% decrease in BER activity was detected in mixed germ cells from Trp53-null mice as compared to levels in wild-type mixed germ cells. Together, these data demonstrate that tumor suppressor TRP53 is important for BER activity in somatic and germ cells. The data presented herein indicate that absence of tumor suppressor TRP53 likely affects the DNA repair response to spontaneous and induced DNA damage.
Theoretically, the levels of DNA damage should increase in cells with reduced DNA repair capacity caused by tumor suppressor TP53 deficiency. Because DNA damage is more likely to be mutagenic in an environment of reduced DNA repair, one would expect to see elevated mutagenesis. Indeed, the mutant frequency was elevated in epididymal spermatozoa obtained from Trp53-null mice. However, an elevated mutant frequency was not detected in spermatogenic cells from Trp53-null mice after IR as compared to wild-type germ cells. A possible explanation is that germ cells with more abundant DNA lesions and/or mutations are selectively eliminated via phagocytosis by Sertoli cells. Sertoli cells interact extensively with germ cells and play indispensable roles during spermatogenesis, because they form a blood-testis barrier, provide nutrients to germ cells, and phagocytically remove apoptotic spermatogenic cells [71–74]. It is also possible that other DNA repair pathways are utilized to remove the DNA damage when BER activity is reduced. Previously, recombination repair was found to be elevated following IR in mismatch repair-deficient mice [5]. This backup scenario seems unlikely, because studies utilizing mice heterozygous for BER genes reveal an elevated spontaneous mutation frequency in spermatogenic cells [7, 8], thereby suggesting other repair pathways do not compensate adequately for diminished BER. It is also possible that apoptosis is mediated by pathways other than those dependent on tumor suppressor TRP53, thereby eliminating the cells that would result in a greater mutant frequency.
In summary, evidence has been provided that apoptosis plays a measurable and important role in regulating mutant frequency in seminiferous tubule cells using Bax-null mice. Tumor suppressor TRP53 has been shown to play an important role in regulating mutant frequency during postmeiotic stages of spermiogenesis. Elimination of germ cells with a greater mutation load appears as a promising mechanism to maintain a low mutant frequency in spermatogenic cells that in turn increases the likelihood of healthy offspring.
Footnotes
Supported by grant AG21163 from the National Institute on Aging of the National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not necessarily reflect the view of the NIH or the Veteran's Health Care System.
REFERENCES
- Walter CA, Intano GW, McCarrey JR, McMahan CA, Walter RB.Mutation frequency declines during spermatogenesis in young mice but increases in old mice. Proc Natl Acad Sci U S A 1998; 95: 10015–10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SW, Provost GS, Fieck A, Kretz PL, Bullock WO, Putman DL, Sorge JA, Short JM.Analysis of spontaneous and induced mutations in transgenic mice using a lambda ZAP/lacI shuttle vector. Environ Mol Mutagen 1991; 18: 316–321. [DOI] [PubMed] [Google Scholar]
- Xu G, Intano GW, McCarrey JR, Walter RB, McMahan CA, Walter CA.Recovery of a low mutant frequency after ionizing radiation-induced mutagenesis during spermatogenesis. Mutat Res 2008; 654: 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehata H, Yanase F, Mori T, Nikaido O, Tanaka K, Ono T.Mutation spectrum in UVB-exposed skin epidermis of Xpa-knockout mice: frequent recovery of triplet mutations. Environ Mol Mutagen 2007; 48: 1–13. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ponomareva ON, Lasarev M, Turker MS.High frequency induction of mitotic recombination by ionizing radiation in Mlh1 null mouse cells. Mutat Res 2006; 594: 189–198. [DOI] [PubMed] [Google Scholar]
- Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE.Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A 1999; 96: 13300–13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D, Herbert DC, McMahan CA, Rotrekl V, Sobol RW, Wilson SH, Walter CA.Mutagenesis is elevated in male germ cells obtained from DNA polymerase-beta heterozygous mice. Biol Reprod 2008; 79: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huamani J, McMahan CA, Herbert DC, Reddick R, McCarrey JR, MacInnes MI, Chen DJ, Walter CA.Spontaneous mutagenesis is enhanced in Apex heterozygous mice. Mol Cell Biol 2004; 24: 8145–8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins C, Oakberg EF.Morphological and quantitative analysis of spermatogonia in mouse testes using whole mounted seminiferous tubules. I. The normal testes. Anat Rec 1978; 192: 519–528. [DOI] [PubMed] [Google Scholar]
- Blanco-Rodriguez J, Martinez-Garcia C, Porras A.Correlation between DNA synthesis in the second, third and fourth generations of spermatogonia and the occurrence of apoptosis in both spermatogonia and spermatocytes. Reproduction 2003; 126: 661–668. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y.Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol 2003; 195: 158–167. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S.The Bcl-2 protein family: arbiters of cell survival. Science 1998; 281: 1322–1326. [DOI] [PubMed] [Google Scholar]
- Print CG, Loveland KL.Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays 2000; 22: 423–430. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P.An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J 1997; 16: 2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Shabaik A, Miyashita T, Wang HG, Reed JC.Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 1994; 145: 1323–1336. [PMC free article] [PubMed] [Google Scholar]
- Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ.Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 1995; 270: 96–99. [DOI] [PubMed] [Google Scholar]
- Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM.Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Reprod 2002; 66: 950–958. [DOI] [PubMed] [Google Scholar]
- Owen-Schaub LB, Zhang W, Cusack JC, Angelo LS, Santee SM, Fujiwara T, Roth JA, Deisseroth AB, Zhang WW, Kruzel E, Radinsky R.Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol 1995; 15: 3032–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Reed JC.Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 1995; 80: 293–299. [DOI] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N.Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000; 288: 1053–1058. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR.Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004; 303: 1010–1014. [DOI] [PubMed] [Google Scholar]
- Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P.Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 1998; 282: 290–293. [DOI] [PubMed] [Google Scholar]
- Sands AT, Suraokar MB, Sanchez A, Marth JE, Donehower LA, Bradley A.p53 deficiency does not affect the accumulation of point mutations in a transgene target. Proc Natl Acad Sci U S A 1995; 92: 8517–8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A.Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumors. Nature 1992; 356: 215–221. [DOI] [PubMed] [Google Scholar]
- Beumer TL, Roepers-Gajadien HL, Gademan IS, van Buul PP, Gil-Gomez G, Rutgers DH, de Rooij DG.The role of the tumor suppressor p53 in spermatogenesis. Cell Death Differ 1998; 5: 669–677. [DOI] [PubMed] [Google Scholar]
- Baum JS, St George JP, McCall K.Programmed cell death in the germline. Semin Cell Dev Biol 2005; 16: 245–259. [DOI] [PubMed] [Google Scholar]
- Schwartz D, Goldfinger N, Kam Z, Rotter V.p53 Controls low DNA damage-dependent premeiotic checkpoint and facilitates DNA repair during spermatogenesis. Cell Growth Differ 1999; 10: 665–675. [PubMed] [Google Scholar]
- Offer H, Wolkowicz R, Matas D, Blumenstein S, Livneh Z, Rotter V.Direct involvement of p53 in the base excision repair pathway of the DNA repair machinery. FEBS Lett 1999; 450: 197–204. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ahn J, Wilson SH, Prives C.A role for p53 in base excision repair. EMBO J 2001; 20: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR.Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol 1993; 225: 84–113. [DOI] [PubMed] [Google Scholar]
- Walter CA, Intano GW, McMahan CA, Kelner K, McCarrey JR, Walter RB.Mutation spectral changes in spermatogenic cells obtained from old mice. DNA Repair (Amst) 2004; 3: 495–504. [DOI] [PubMed] [Google Scholar]
- Intano GW, McMahan CA, Walter RB, McCarrey JR, Walter CA.Mixed spermatogenic germ cell nuclear extracts exhibit high base excision repair activity. Nucleic Acids Res 2001; 29: 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, Beard WA, Wilson SH.Studies of gapped DNA substrate binding by mammalian DNA polymerase beta. Dependence on 5′-phosphate group. J Biol Chem 1994; 269: 18096–18101. [PubMed] [Google Scholar]
- Intano GW, McMahan CA, McCarrey JR, Walter RB, McKenna AE, Matsumoto Y, MacInnes MA, Chen DJ, Walter CA.Base excision repair is limited by different proteins in male germ cell nuclear extracts prepared from young and old mice. Mol Cell Biol 2002; 22: 2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal RK, Prasad R, Wilson SH.DNA polymerase beta conducts the gap-filling step in uracil-initiated base excision repair in a bovine testis nuclear extract. J Biol Chem 1995; 270: 949–957. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA.Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992; 119: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA.Generalized Statistical Models. New York:Chapman and Hall;1990. [Google Scholar]
- Weber JE, Russell LD.A study of intercellular bridges during spermatogenesis in the rat. Am J Anat 1987; 180: 1–24. [DOI] [PubMed] [Google Scholar]
- Tres LL, Kierszenbaum AL.Cell death patterns of the rat spermatogonial cell progeny induced by Sertoli cell geometric changes and Fas (CD95) agonist. Dev Dyn 1999; 214: 361–371. [DOI] [PubMed] [Google Scholar]
- Braun RE, Behringer RR, Peschon JJ, Brinster RL, Palmiter RD.Genetically haploid spermatids are phenotypically diploid. Nature 1989; 337: 373–376. [DOI] [PubMed] [Google Scholar]
- Caldwell KA, Handel MA.Protamine transcript sharing among postmeiotic spermatids. Proc Natl Acad Sci U S A 1991; 88: 2407–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales CR, Wu XQ, Hecht NB.The DNA/RNA-binding protein, TB-RBP, moves from the nucleus to the cytoplasm and through intercellular bridges in male germ cells. Dev Biol 1998; 201: 113–123. [DOI] [PubMed] [Google Scholar]
- Olsen AK, Lindeman B, Wiger R, Duale N, Brunborg G.How do male germ cells handle DNA damage? Toxicol Appl Pharmacol 2005; 207: 521–531. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Lue Y, Diaz-Romero M, Yen PH, Wang C, Swerdloff RS.Deciphering the pathways of germ cell apoptosis in the testis. J Steroid Biochem Mol Biol 2003; 85: 175–182. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ.Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem 1998; 273: 10777–10783. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Sanchez B, Martinou JC.Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem 2001; 276: 11615–11623. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ.Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 1997; 139: 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ.Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A 1997; 94: 3668–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P.Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem 2001; 276: 18361–18374. [DOI] [PubMed] [Google Scholar]
- Adams JM.Ways of dying: multiple pathways to apoptosis. Genes Dev 2003; 17: 2481–2495. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G.The pathophysiology of mitochondrial cell death. Science 2004; 305: 626–629. [DOI] [PubMed] [Google Scholar]
- Aziz N, Said T, Paasch U, Agarwal A.The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum Reprod 2007; 22: 1413–1419. [DOI] [PubMed] [Google Scholar]
- Paasch U, Grunewald S, Dathe S, Glander HJ.Mitochondria of human spermatozoa are preferentially susceptible to apoptosis. Ann N Y Acad Sci 2004; 1030: 403–409. [DOI] [PubMed] [Google Scholar]
- Harris SL, Levine AJ.The p53 pathway: positive and negative feedback loops. Oncogene 2005; 24: 2899–2908. [DOI] [PubMed] [Google Scholar]
- Buettner VL, Nishino H, Haavik J, Knoll A, Hill K, Sommer SS.Spontaneous mutation frequencies and spectra in p53 (+/+) and p53 (−/−) mice: a test of the ‘guardian of the genome' hypothesis in the Big Blue transgenic mouse mutation detection system. Mutat Res 1997; 379: 13–20. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Zhang Y, Niibe H, Terry NH, Meistrich ML.Resistance of differentiating spermatogonia to radiation-induced apoptosis and loss in p53-deficient mice. Radiat Res 1998; 149: 263–270. [PubMed] [Google Scholar]
- Oakberg EF.Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat 1956; 99: 507–516. [DOI] [PubMed] [Google Scholar]
- Russell WL, Bangham JW, Russell LB.Differential response of mouse male germ-cell stages to radiation-induced specific-locus and dominant mutations. Genetics 1998; 148: 1567–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Yang H, Cunanan C, Okamoto K, Shibata D, Pan J, Barnes DE, Lindahl T, McIlhatton M, Fishel R, Miller JH.Deficiencies in mouse Myh and Ogg1 result in tumor predisposition and G to T mutations in codon 12 of the K-ras oncogene in lung tumors. Cancer Res 2004; 64: 3096–3102. [DOI] [PubMed] [Google Scholar]
- Zhou ZQ, Manguino D, Kewitt K, Intano GW, McMahan CA, Herbert DC, Hanes M, Reddick R, Ikeno Y, Walter CA.Spontaneous hepatocellular carcinoma is reduced in transgenic mice overexpressing human O6-methylguanine-DNA methyltransferase. Proc Natl Acad Sci U S A 2001; 98: 12566–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Harris CC.p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol 2005; 6: 44–55. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Wilson G, Russell LD, Meistrich ML.Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiat Res 1997; 147: 457–467. [PubMed] [Google Scholar]
- Jost CA, Marin MC, Kaelin WG., Jrp73 Is a human p53-related protein that can induce apoptosis. Nature 1997; 389: 191–194. [DOI] [PubMed] [Google Scholar]
- Lee CW, La Thangue NB.Promoter specificity and stability control of the p53-related protein p73. Oncogene 1999; 18: 4171–4181. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F.p63, A p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 1998; 2: 305–316. [DOI] [PubMed] [Google Scholar]
- Wood RD.DNA repair in eukaryotes. Annu Rev Biochem 1996; 65: 135–167. [DOI] [PubMed] [Google Scholar]
- Lindahl T, Wood RD.Quality control by DNA repair. Science 1999; 286: 1897–1905. [DOI] [PubMed] [Google Scholar]
- Nilsen H, Krokan HE.Base excision repair in a network of defense and tolerance. Carcinogenesis 2001; 22: 987–998. [DOI] [PubMed] [Google Scholar]
- Olsen AK, Bjortuft H, Wiger R, Holme J, Seeberg E, Bjoras M, Brunborg G.Highly efficient base excision repair (BER) in human and rat male germ cells. Nucleic Acids Res 2001; 29: 1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabelof DC, Raffoul JJ, Yanamadala S, Ganir C, Guo Z, Heydari AR.Attenuation of DNA polymerase beta-dependent base excision repair and increased DMS-induced mutagenicity in aged mice. Mutat Res 2002; 500: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WW, Musto NA, Mather JP, Bardin CW.Sertoli cells secrete both testis-specific and serum proteins. Proc Natl Acad Sci U S A 1981; 78: 7565–7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M, Fawcett DW.The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970; 3: 308–326. [DOI] [PubMed] [Google Scholar]
- Griswold MD.Interactions between germ cells and Sertoli cells in the testis. Biol Reprod 1995; 52: 211–216. [DOI] [PubMed] [Google Scholar]
- Fujisawa M.Cell-to-cell cross talk in the testis. Urol Res 2001; 29: 144–151. [DOI] [PubMed] [Google Scholar]