Abstract
The Notch cell-cell signaling pathway is used extensively in cell fate specification during metazoan development. In many cell lineages, the conditional role of Notch signaling is integrated with the autonomous action of the Numb protein, a Notch pathway antagonist. During Drosophila sensory bristle development, precursor cells segregate Numb asymmetrically to one of their progeny cells, rendering it unresponsive to reciprocal Notch signaling between the two daughters. This ensures that one daughter adopts a Notch-independent, and the other a Notch-dependent, cell fate. In a genome-wide survey for potential Notch pathway targets, the second intron of the numb gene was found to contain a statistically significant cluster of binding sites for Suppressor of Hairless, the transducing transcription factor for the pathway. We show that this region contains a Notch-responsive cis-regulatory module that directs numb transcription in the pIIa and pIIIb cells of the bristle lineage. These are the two precursor cells that do not inherit Numb, yet must make Numb to segregate to one daughter during their own division. Our findings reveal a new mechanism by which conditional and autonomous modes of fate specification are integrated within cell lineages.
Keywords: Asymmetric cell division, Notch signaling, numb, Suppressor of Hairless, Default repression, Cis-regulatory module, Drosophila
INTRODUCTION
Metazoan development relies on two broad categories of mechanisms for specifying cell fate: autonomous, in which fate is determined by factors inherited by, or expressed in, the cell itself; and conditional, in which fate is determined by external factors, particularly cell-cell signaling. The cell lineage that gives rise to Drosophila adult mechanosensory organs (Fig. 1) is well known for its elegant combination of these two modes of fate specification (Hartenstein and Posakony, 1990; Posakony, 1994; Rhyu et al., 1994; Frise et al., 1996; Guo et al., 1996). At each of several precursor cell divisions in this lineage, the two daughter cells signal to each other via the Notch pathway. The fate of one daughter is specified by this signal. The other daughter inherits the Notch pathway antagonist Numb, asymmetrically segregated from the precursor cell. This renders the second daughter immune to the reciprocal Notch signal, ensuring that it adopts the alternative, Notch-independent, cell fate. The fly sensory organ lineage thus embodies a universal strategy for generating cell fate asymmetry during development.
Fig. 1.
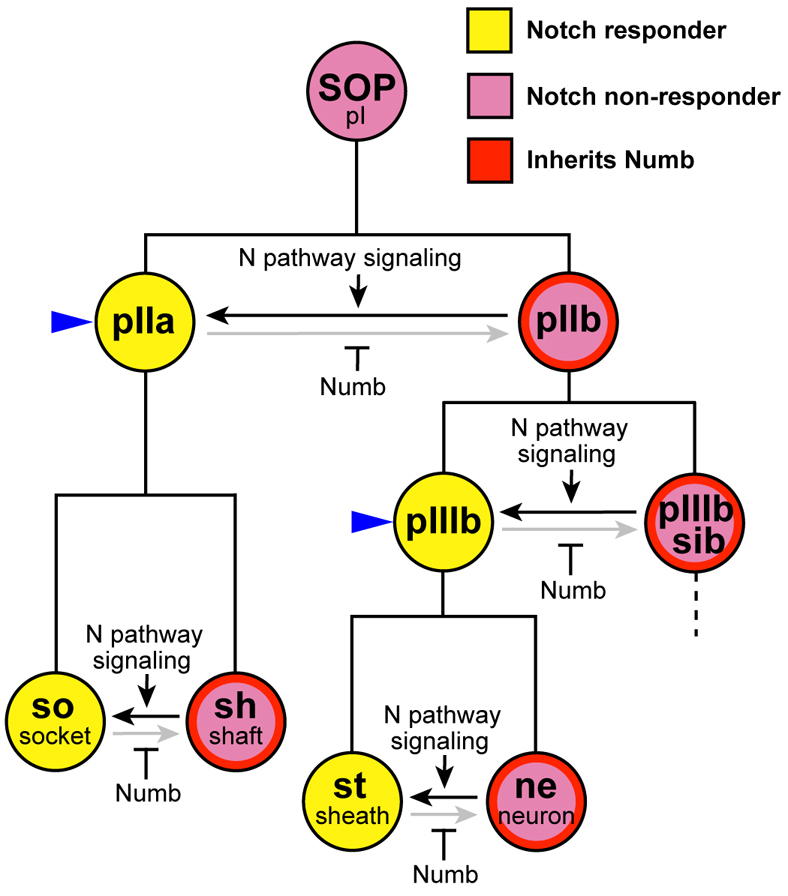
Cell fate specification in the Drosophila mechanosensory bristle lineage. The adult mechanosensory bristle lineage in Drosophila originates from a single sensory organ precursor cell (SOP or pI) and consists of a series of asymmetric cell divisions in which one daughter (yellow) adopts a Notch-dependent, and the other daughter (purple) a Notch-independent, cell fate. These outcomes are the result of the repeated use of a combination of conditional and autonomous cell fate specification mechanisms. At each division, the two daughter cells signal to each other via the Notch pathway (horizontal arrows), and one daughter is rendered immune to this signal by its inheritance of the Numb protein (red), segregated asymmetrically in the dividing precursor cell. The Notch-dependent precursor cells in the lineage, pIIa and pIIIb, are indicated with blue arrowheads.
In this lineage, the fates of two of the precursor cells (pIIa and pIIIb) are specified by Notch signaling (Fig. 1, blue arrowheads). It is essential, therefore, that these two cells do not inherit substantial amounts of Numb from their respective mother cells. However, each must make Numb to distribute to its own Notch-independent daughter cell. The solution to this regulatory problem has been a lingering question (Rhyu et al., 1994).
A previous report from our laboratory described the application of a computational method called SCORE to identify statistically significant clusters of transcription factor binding sites in the genome (Rebeiz et al., 2002). We used this method in an attempt to identify targets of Suppressor of Hairless [Su(H)], the transducing transcription factor for the Notch pathway in Drosophila. Besides recovering multiple genes already known to be activated directly by Su(H), the SCORE technique identified as potential targets several other core members of the Notch pathway, including Delta (which encodes a ligand for the Notch receptor), neuralized (which encodes an E3 ubiquitin ligase essential for promoting endocytosis and activation of the Delta protein) and numb. Here, we describe our identification and analysis of an intronic cis-regulatory module that is responsible for the transcriptional activation of numb in bristle precursor cells in response to Notch signaling. Our findings illuminate a previously unrecognized regulatory linkage that further intertwines the conditional and autonomous modes of cell fate specification.
MATERIALS AND METHODS
Fly stocks
w1118 is a spontaneous partial deletion of the white locus that eliminates gene function (Tweedie et al., 2009). sca-GAL4 and pnr-GAL4 are P-element `enhancer trap' insertions into the scabrous and pannier loci, respectively, driving expression of GAL4 (Hinz et al., 1994; Calleja et al., 1996). Nts1 is a temperature-sensitive point mutation of Notch induced by ethyl methanesulfonate (EMS) mutagenesis (Shellenbarger and Mohler, 1975); N81k1 is an X-ray-induced deficiency lacking DNA from chromosomal region 3C5-3C10, which overlaps the Notch locus (3C7-3C9) (Grimwade et al., 1985). UAS-numb flies carry a numb coding region transgene (Wang et al., 1997) that can be misexpressed using the GAL4-UAS system (Brand and Perrimon, 1993). numb1 is a strong hypomorphic mutation caused by a P-element transposon insertion at 30B (Uemura et al., 1989); numb2 is a diepoxybutane (DEB)-induced amorphic or strong hypomorphic allele first described by Frise et al. (Frise et al., 1996); numb796 is a strong loss-of-function allele induced by EMS mutagenesis (Buescher et al., 1998). Df(2L)30A-C is an X-ray-induced deficiency lacking DNA from chromosomal region 30A3-C5, which overlaps the numb locus (30B3-B5) (Uemura et al., 1989; Tweedie et al., 2009).
Reporter gene constructs
Fragments tested in reporter constructs were amplified by PCR on genomic DNA templates; primer sequences are supplied in Table S1 in the supplementary material. Binding site mutants were created by overlap-extension PCR (Ho et al., 1989); see Table S1 in the supplementary material for mutagenesis primer sequences. The CD2 fragment and all mutant variants thereof were PCR cloned from the Celera (Alameda, CA, USA) sequencing strain and are fully compliant with the Celera genome sequence (Adams et al., 2000). Fragments were inserted into the KpnI and BglII restriction sites of the GFP reporter vector pH-Stinger (Barolo et al., 2000).
Germline transformation
P element-mediated germline transformation was performed as previously described (Rubin and Spradling, 1982) using w1118 as the recipient strain.
Generation of flies bearing an inducible Su(H) short-hairpin RNAi construct
A GAL4-inducible RNAi construct for reducing Su(H) activity was designed according to Haley et al. (Haley et al., 2008). This transgene expresses a 71-nucleotide stem-loop sequence within a pre-miR-1 scaffold that leads to the production of 21-nucleotide silencing RNAs; these recognize a complementary sequence within the second exon of Su(H). Two synthetic oligonucleotides (IDT, Coralville, IA, USA; see Table S1 in the supplementary material) were annealed in 1× TE (Tris-EDTA) buffer and cloned into the pUAST-based vector pNE3 (Ben Haley, UC Berkeley, CA, USA). The final product was confirmed by sequencing (GENEWIZ, South Plainfield, NJ, USA) and inserted into the germline of w1118 embryos. Five independent insertion lines were obtained; when tested using pnr-GAL4 as the driver, all five lines gave the same `balding' phenotype shown in Fig. S4 in the supplementary material.
Reduction of Notch pathway activity
Su(H) short-hairpin (sh) RNAi expression
UAS-Su(H)shRNA flies were crossed to CD2-GFP flies, and the progeny crossed to pnr-GAL4/TM6c flies. Tubby (Tb)+ pupae at 0-3 hours after puparium formation (APF) were collected from this latter cross and aged at 25°C for 13-15 hours before dissection and staining. Tissue genotypes were distinguished by the cellular phenotype at scutellar macrochaete positions (which are transformed when both driver and responder are present), and by the detection of GFP expression.
Notch loss of function
Males of the genotype Nts1/Y; CD2-GFP/+ were crossed to wa N81k1/FM7, Kr-GAL4>UAS-GFP females at 18°C. Pupae at 0-4 hours APF were collected, aged at 18°C for an additional 24 hours and separated on the basis of Kr>GFP expression under an epifluorescence dissection microscope. Immediately prior to dissection, pupae were exposed to a temperature of 37°C for 2 hours. Presence of the CD2-GFP chromosome was detected by GFP expression at macrochaete positions.
numb misexpression experiments
Flies of the genotype y w; sca-GAL4/Cyo were crossed to flies of the genotype w1118; numbCD2-GFP; UAS-numb/TM6B. Progeny lacking either the sca-GAL4 driver or the UAS-numb responder were used as controls.
numb rescue constructs
Cloning of a 19.5 kb numb genomic DNA rescue construct was carried out in three steps. First, a 538 bp fragment upstream of the proximal promoter was PCR amplified (primers: forward 5′-ttgcggccgcaaGGTAAATCTGAAGGCGAAGCCATG-3′, reverse 5′-gcggccgcgaccgccgggtcGTGTCCCCGGGATAAGGTTGCAGATGCAA-3′; uppercase, genomic sequence; lowercase, bases added for cloning purposes) and cloned into pCR2.1-TOPO. Both primers contain synthetic NotI sites at their 5′ ends (bold). Internal to the 3′ NotI site, a synthetic DrdI and an endogenous SmaI site (both underlined) were used to clone a 19.5 kb SmaI/DrdI fragment containing the entire numb proximal promoter and transcription unit (see Fig. 2A), digested from BAC clone RP98-6D23 (Hoskins et al., 2000) into the pCR2.1-TOPO clone containing the above-mentioned 538 bp fragment. The 19.5 kb fragment was then cloned into CaSpeR(NotI) (Malicki et al., 1993) using the flanking NotI sites. In clones used for both wild-type and CD2 deletion rescue constructs, the DrdI/SmaI fragment insertion occurred in the reverse orientation, yielding a fully functional rescue transgene, with a small portion of 5′ noncoding sequence inserted downstream of the gene, which should have no effect on the outcome of our experiments.
Fig. 2.
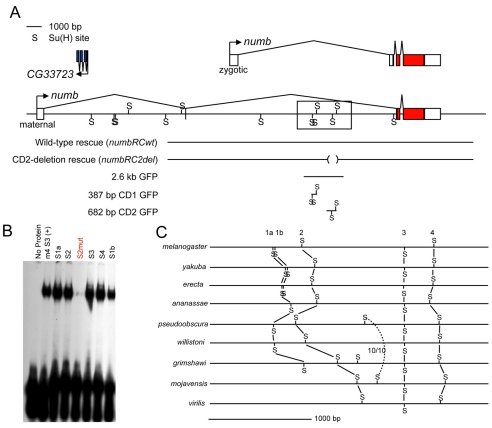
A cluster of conserved Su(H) binding sites in the Drosophila numb gene. (A) Diagram of the numb locus and genomic DNA fragments used in this study. The diagram is drawn to scale, and shows the position of high-affinity Su(H) binding motifs (S). The upper transcript isoform is zygotically expressed; the lower isoform is maternally expressed (Uemura et al., 1989). Boxed area marks the cluster of five predicted Su(H) binding sites found by SCORE analysis (Rebeiz et al., 2002). Genomic DNA fragments used for numb mutant rescue experiments (numbRC) are indicated, as are fragments tested for enhancer activity in GFP reporter transgenes. Red boxes indicate numb coding sequences. (B) Electrophoretic mobility shift assay (EMSA) analysis of predicted Su(H) binding sites in the boxed region shown in A. Sites are numbered as in C. All five sites are efficiently bound in vitro by a purified GST-Su(H) fusion protein; the binding of a positive control Su(H) site [m4 S3 (+); S3 from the E(spl)m4 gene (Bailey and Posakony, 1995)] is shown for comparison. A single-base substitution in numb site S2 (S2mut, red) almost completely abolishes binding. (C) Patterns of evolutionary conservation of Su(H) binding sites in the identified cluster. Solid lines indicate site orthology, based on the presence of a conserved site motif in a comparable location and on shared sequences adjacent to individual sites. Dotted line connects a pair of site occurrences (10/10 match) in D. pseudoobscura and D. mojavensis only. Data for D. simulans, D. sechellia and D. persimilis are omitted for clarity; all five sites are conserved in all three of these species, except site 1b, which is absent in D. persimilis. See Fig. S1 in the supplementary material for sequence alignments. Diagrams in A and C were generated using the GenePalette software tool (Rebeiz and Posakony, 2004).
To delete the CD2 enhancer, overlap-extension PCR (Ho et al., 1989) was used to generate a 1.6 kb fragment that overlapped endogenous PmeI and SacI sites (external primers: 5′-GAATCCACAAGTATTCGCCAGATG-3′, 5′-TGAACTGTATCTGTGTGCTCGGAG-3′; internal primers: 5′-TTTGCAGTATTTAAAATAGTAATTAATAGATGGTAAAAACTTTAAAACTT-3′, 5′-AAGTTTTAAAGTTTTTACCATCTATTAATTACTATTTTAAATACTGCAAA-3′). This 1.6 kb fragment was cloned via PmeI/SacI sites into a 5.3 kb PmeI/SacII fragment contained in pH-Stinger (Barolo et al., 2000). Next, the 5.3 kb PmeI/SacII fragment harboring a deletion of the CD2 enhancer was cloned into the wild-type numb rescue fragment contained in pCR2.1-TOPO, and subsequently inserted into CaSpeR(NotI). Transgenic fly lines carrying either the wild-type (numbRCwt) or CD2 deletion (numbRC2del) rescue constructs were generated by Genetic Services (Cambridge, MA, USA).
numb rescue experiments
Flies of the genotype w; Df(2L)30A-C/CyO or w; numb1/CyO were crossed to flies of the genotype w; numb796/CyO; numbRCX, where X refers to either the wild-type (wt) or CD2 enhancer deletion (2del) rescue construct variant. Curly+ progeny were collected and scored for the `double socket' phenotype at macrochaete positions along the dorsal head and thorax, in postorbital bristle rows and at bristle positions around the circumference of the T1 femur. Three independent insertion lines were analyzed for each rescue construct variant; the statistical significance of phenotypic differences between the variant groups was evaluated by the Mann-Whitney U test.
To evaluate pIIIb daughter cell fates, the same cross was performed except that CyO, Kr-GAL4 and UAS-GFP balancers were substituted and rescue construct lines wt-A and 2del-B were used. Pupae lacking Kr>GFP expression were dissected and stained at 24-30 hours APF. For each genotype, confocal images of the microchaete field from five nota were collected. Only positions for which a Su(H)-positive cell(s) could be easily matched with a Pros/Pros or Pros/Elav pair were marked and counted.
Transgene rescue of the numb2 mutant phenotype in mosaic clones
To assay phenotypic rescue in numb2 mutant clones, females of the genotype y w Ubx-FLP; FRT40A were crossed to males of the genotype w/Y; y+ numb2 ck FRT40A/CyO; numbRCX/Sb, where X is either `wt' or `2del'. Flies not carrying the CyO balancer were sorted for presence of the rescue construct (numbRC). Because of the dark eye color provided by the Ubx-FLP transgene, Sb was used to identify flies that did not receive the rescue construct chromosome, and annotated as `no RC'; Sb+ flies were assumed to contain a copy of the rescue construct. Forty macrochaete bristle positions on the dorsal head and thorax per fly were assayed for the ck mutant phenotype, indicating numb2 mutant territories, and the bristle phenotype in these territories was scored as shown in Table S2 in the supplementary material.
Immunohistochemistry
Staining of pupal nota was performed on animals incubated at 25°C and dissected at timed stages measured in hours APF. Timed pupae were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA, USA) with 0.1% Triton X-100 (Sigma) in PBS. For experiments using the anti-Hamlet antibody, the Triton X-100 concentration in the fixative solution was raised to 0.3% to increase tissue permeability. Before both primary and secondary antibody incubations, samples were blocked for 1 hour in a 1:10 solution of western blocking reagent (Roche) in PBS with 0.1% Triton X-100. Stained tissues were imaged on a Leica confocal microscope.
Primary antibodies used in this study were: mouse-anti-Cut [1:100; Developmental Studies Hybridoma Bank (DSHB)] (Blochlinger et al., 1990), mouse anti-Prospero (1:10; DSHB) (Spana and Doe, 1995), guinea pig anti-Hamlet (1:1000) (Moore et al., 2002), guinea pig anti-Senseless (1:2000) (Nolo et al., 2000), guinea pig anti-Numb (1:2000) (O'Connor-Giles and Skeath, 2003), rabbit anti-Su(H) (1:1000; Santa Cruz Biotechnology), rabbit anti-CG3227 (1:2000), rabbit anti-GFP (1:500; Invitrogen) and rat anti-Elav (1:200; DSHB). Secondary antibodies used (all from Invitrogen) were: Alexa 555-conjugated goat anti-mouse (1:1000), Alexa 647-conjugated goat anti-mouse (1:400), Alexa 555-conjugated goat anti-guinea pig (1:400), Alexa 647-conjugated goat anti-guinea pig (1:1000), Alexa 647-conjugated goat anti-rabbit (1:1000), Alexa 647-conjugated goat anti-rat (1:1000) and Alexa 488-conjugated chicken anti-rabbit (1:1000). Hoechst stain (1:1000; Invitrogen) was used as a DNA marker.
In situ hybridization
A 1.8 kb PCR-cloned genomic DNA fragment overlapping the last exon of numb was cloned into EcoRI and NotI sites of pBluescript SK I and used to transcribe a digoxygenin-labeled riboprobe (see Table S1 in the supplementary material for PCR primer sequences). In situ hybridization was performed as previously described (Reeves and Posakony, 2005) with the modification that instead of the normal 1:1500 dilution of 10 mg/ml proteinase K, a 1:25,000 dilution was used to protect protein epitopes for detection of the Cut protein by antibody stain (above) after the in situ hybridization step.
Mobility shift assays
Electrophoretic mobility shift assays (EMSAs) using purified GST-Su(H) were performed as described previously (Bailey and Posakony, 1995). Oligonucleotide probe sequences are listed in Table S1 in the supplementary material.
Scanning electron microscopy (SEM)
Adult flies were collected for SEM and prepared as described previously (Miller et al., 2009).
RESULTS
Experimental verification and evolutionary conservation of computationally identified Su(H) binding sites in numb
To obtain a preliminary assessment of the functionality of the putative Su(H) binding site cluster identified in Drosophila melanogaster numb by the SCORE computational method (Fig. 2A) (Rebeiz et al., 2002), we carried out two additional analyses: an electrophoretic mobility shift assay (EMSA) and phylogenetic footprinting (Fig. 2B,C).
The SCORE survey of the genome used a stringent definition of the DNA-binding specificity of Su(H), requiring a sequence that matches the motif YGTGDGAA (TGTGTGAA omitted). From previous studies of the interactions of Su(H) with its target genes, such sites are predicted to be bound by Su(H) protein with high affinity (Tun et al., 1994; Bailey and Posakony, 1995; Nellesen et al., 1999). Indeed, we found by EMSA that Su(H) efficiently bound all five predicted sites in the numb cluster in a sequence-specific manner (Fig. 2B).
Sequence comparisons revealed that, of the five Su(H) sites in the cluster (designated 1a, 1b, 2, 3 and 4), three (sites 2, 3 and 4) are precisely conserved in all 12 Drosophila species with sequenced genomes, with the exception of a single nucleotide change (CGTGGGAA to CGTGAGAA) in site 3 of D. virilis, which remains compatible with high-affinity binding by Su(H) (Fig. 2C; see Fig. S1 in the supplementary material for sequence alignments). Site 1a is exactly conserved in 10 of the 12 species, being absent only in the sister pair D. virilis and D. mojavensis. Finally, the fifth site (1b) shows conservation only among members of the melanogaster subgroup. This site lies only eight bp away from site 1a, a distance that is likely to preclude simultaneous occupancy of both sites. We have previously observed similar evolutionary instability of adjacent Su(H) sites (Castro et al., 2005). Overall, the phylogenetic footprinting analysis indicated that four of the five sites in the numb cluster have withstood selection pressure for 40-60 million years, implying a functional role for these motifs.
Enhancer activity of the Su(H) binding site cluster region in numb
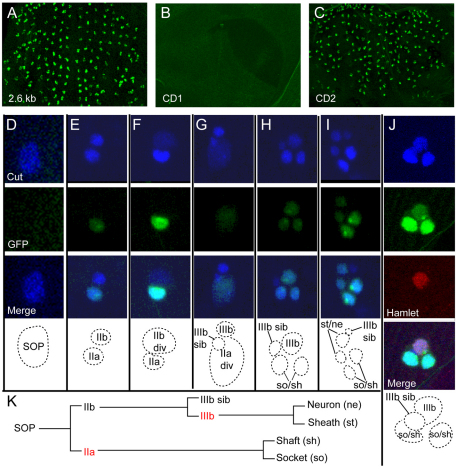
To test directly whether the Su(H) binding site cluster in the numb intron identifies one or more functional cis-regulatory modules, we incorporated into a GFP reporter transgene a 2.6 kb genomic DNA fragment bearing all five of the Su(H) sites identified in silico (see Fig. 2A). In multiple independent transgenic lines carrying this construct, we observed GFP expression in the embryonic CNS and PNS (data not shown); in the CNS, optic lobes, retinal field and Johnston's organ primordium of late third-instar larvae (data not shown); and in pupal-stage precursor cells for the microchaete bristles of the notum (Fig. 3A). We next tested the activity of two smaller fragments from this 2.6 kb region: CD1, bearing sites 1a, 1b and 2; and CD2, containing sites 3 and 4 (see Fig. 2A). The 387 bp CD1 fragment directed expression in the embryonic CNS (see Fig. S2 in the supplementary material), but displayed no activity in the microchaete field (Fig. 3B). However, the CD2 fragment, spanning 682 bp and containing two conserved Su(H) binding sites, directed strong GFP expression in developing microchaetes (Fig. 3C). Like the 2.6 kb fragment, it was also active in the embryonic CNS (see Fig. S2 in the supplementary material), the retinal field, the larval brain and the Johnston's organ primordium of the eye-antenna disc (data not shown). We conclude that the SCORE method has indeed identified regions of the numb gene that display transcriptional regulatory activity in vivo.
Fig. 3.
The numb CD2 enhancer is active in the pIIa and pIIIb cells of the mechanosensory bristle lineage in Drosophila. (A-C) Thoracic microchaete field at 24 hours after puparium formation (APF), after all cell divisions in the bristle lineage have been completed. GFP reporter gene activity is shown in green. (A) A 2.6 kb genomic DNA fragment containing the Su(H) binding site cluster (Fig. 2A) displays enhancer activity in the bristle lineage. (B) CD1, a 387 bp subfragment containing three Su(H) sites (Fig. 2A), fails to direct reporter gene expression in the bristle lineage. (C) CD2, a 682 bp subfragment containing two Su(H) sites (see Fig. 2A), recapitulates the activity of the parent 2.6 kb fragment. (D-J) Temporal analysis of CD2 reporter gene activity (GFP, green) in the bristle lineage (see K). Anti-Cut antibody (blue) labels all cells of the lineage. Schematic diagrams show the inferred identity of each cell (dotted lines). (D) No reporter gene expression is evident at the one-cell (SOP) stage (D). Following division of the SOP, GFP is expressed in the posterior pIIa cell (E,F), but is absent from the anterior pIIb cell, even as it nears division (div) (F). As the pIIa cell divides (G), GFP is distributed to its daughters, the presumptive socket (so) and shaft (sh) cell pair (H). GFP then accumulates in one of the anterior cells, presumably pIIIb, based on its larger size relative to the pIIIb sib cell (H). When pIIIb divides, GFP is distributed to its progeny, the presumptive neuron (ne) and sheath (st) cells (I). Anti-Hamlet labeling of a four-cell microchaete position, demonstrating that the anterior GFP-positive cell at this stage is pIIIb, which specifically expresses Hamlet (J, red). (K) Schematic of the cell lineage of notum microchaetes. The pIIa and pIIIb cells are highlighted in red. See also Fig. S2 in the supplementary material.
Cell-type specificity of the CD2 cis-regulatory module during bristle development
To define the cell-type specificity of the numb CD2 enhancer activity in the bristle lineage (Fig. 3K), we performed a detailed analysis of GFP accumulation from the reporter transgene in pupal nota at 16-18 hours APF. We used a monoclonal antibody against the Cut protein to fluorescently label the nuclei of all cells in developing microchaetes (Blochlinger et al., 1993).
No GFP was detected in the sense organ precursor (SOP), even when its division was imminent (Fig. 3D). At the two-cell stage of the lineage, however, many developing bristle organs expressed nuclear GFP in the posterior daughter of the SOP, the pIIa cell (Fig. 3E). Other two-cell positions displayed no GFP, consistent with a lag between the birth of the pIIa cell and the appearance of detectable GFP fluorescence. Because we never observed GFP in the anterior cell of a two-cell pair (pIIb), we conclude that reporter gene transcription is first stimulated in pIIa and not in the SOP. Fig. 3F shows a later-stage two-cell position, in which the pIIb cell was about to divide. Here, the intensity of GFP in pIIa was increased in comparison to the early two-cell position shown in Fig. 3E. By contrast, even as pIIb was dividing, no GFP was detectable in this cell (Fig. 3F). Fig. 3G shows a three-cell position, in which pIIb had already divided to yield its pIIIb and pIIIb sib daughters, and the posterior, GFP-positive pIIa cell was about to divide, as shown by diffuse fluorescence of both GFP and the anti-Cut antibody. In the four-cell position shown in Fig. 3H, the pIIa cell had divided, and both of its progeny, the presumptive socket and shaft cells, exhibited what is likely to be inherited GFP fluorescence, though we cannot rule out the possibility that the CD2 enhancer continued to be active in one or both of these cells. Also at the four-cell stage, an anterior Cut-positive cell had also begun accumulating GFP (Fig. 3H). Based on its anterior location, size and apical disposition, this cell is putatively pIIIb. The transcription factor Hamlet (Ham) is first expressed in the microchaete lineage in pIIIb, and has been shown to be a crucial regulator of the fates of its progeny (Moore et al., 2004). Staining pupal nota at 17 hours APF with anti-Ham antibody confirmed that the newly GFP-positive cell was indeed pIIIb (Fig. 3J). Finally, once pIIIb divides, GFP is inherited by its progeny, the presumptive sheath cell and neuron (though, again, we cannot exclude continued enhancer activity in these cells). Thus, when the microchaete lineage divisions had been completed, a total of four GFP-positive cells were observed (Fig. 3I), representing the initial activation of the numb CD2 enhancer in the pIIa and pIIIb cells, and (at least) the perdurance of GFP in their postmitotic progeny. It is also clear from our analysis that the pIIIb sib cell and its precursors within the lineage (pIIb, SOP) do not activate the CD2 enhancer, as no GFP was ever observed in these cells. The cells that did activate novel transcription directed by the enhancer (pIIa and pIIIb) were Notch-responsive cells, consistent with the presence of two conserved Su(H) binding sites in the CD2 fragment.
numb transcript accumulation in Notch-responsive cells of the bristle lineage
The foregoing analysis of the activity of the CD2 enhancer fragment suggests that numb might be subject to selective transcriptional activation in the Notch-responsive precursor cells of the bristle lineage. To investigate this question, we carried out in situ hybridization assays on pupal nota at 16 hours APF, when most microchaete organs were at the two- to three-cell stage of bristle development (Fig. 4). We found that, at this time, evenly spaced cells or cell clusters specifically accumulated elevated levels of numb transcript (Fig. 4A; see Fig. S3 in the supplementary material). The spacing of these strongly expressing cells was reminiscent of the microchaete pattern in the notum. numb transcript was also observed at low levels across the epidermal field (Fig. 4A; see Fig. S3 in the supplementary material).
Fig. 4.
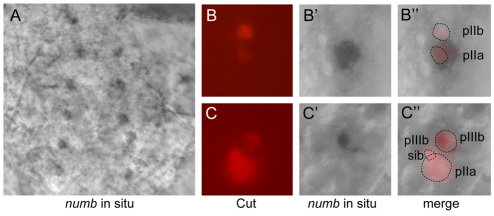
numb transcript accumulates at higher levels in specific cells of the bristle lineage in Drosophila. (A) At 16 hours after puparium formation (APF), elevated levels of transcript from the endogenous numb gene accumulate at discrete positions within the microchaete field of the developing notum, against a background of low-level ubiquitous expression. Anterior is at the top; image is centered on the notum midline and shows two symmetrical microchaete rows. See Fig. S3 in the supplementary material for a lower-magnification view of the full notum. (B-B′) numb transcript (B′,B′) accumulates specifically in the posterior pIIa cell, identified by anti-Cut staining of a developing microchaete at the two-cell stage (red in B and B′; see Fig. 3K for a lineage diagram). (C-C′) numb transcript accumulation (C′,C′) in the pIIIb cell, identified by anti-Cut staining of a developing microchaete at the three-cell stage (red in C and C′). The anterior pIIb cell has divided into the pIIIb and pIIIb sib cells. The larger pIIIb cell accumulates numb transcript, whereas the smaller, apical pIIIb sib cell does not (C′,C′).
To assess whether the strongly expressing cells belong to the microchaete lineage, the in situ-hybridized tissue was also labeled fluorescently with anti-Cut antibody. Cut-positive SOP positions showed only the low epidermal levels of numb transcript accumulation. However, a number of two-cell positions displayed strong numb transcript signals in the more posterior Cut-labeled cell, which is pIIa (Fig. 4B-B′). In a subset of three-cell positions, a small apical anterior Cut-positive nucleus was seen to be surrounded by cytoplasmic numb transcript (Fig. 4C-C′). Based on the size and location of this cell, we interpret it to be pIIIb. Therefore, strong transcript accumulation from the endogenous numb gene was observed in the pIIa cell and probably the pIIIb cell, but not in the SOP or pIIb cells. Our expression analysis thus shows that elevated numb transcript levels appear in the bristle lineage in a pattern consistent with the activity of the CD2 enhancer fragment, i.e. in those precursor cells with Notch-specified fates.
Default repression by Su(H) is required for the normal pattern of numb CD2 enhancer activity
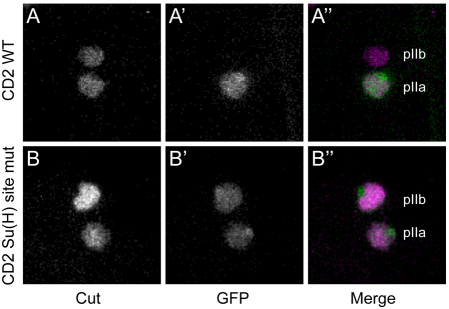
The presence of two highly conserved Su(H) binding sites within the numb CD2 enhancer fragment suggests that the specific activation of the enhancer in response to Notch signaling in pIIa and pIIIb is dependent on Su(H). Introduction of single-base mutations (YGTGDGAA to YGTGDCAA) into both of the Su(H) binding sites resulted in the appearance of robust ectopic reporter gene expression in pIIb, without severely affecting its expression in pIIa (Fig. 5). This result suggests that the transcriptional activation function of Su(H) does not provide the principal contribution to the activity of the enhancer in pIIa; instead, expression is evidently driven largely by other activators bound to the module. Significantly, however, the experiment also reveals that Su(H) does indeed act as a `default repressor' of the CD2 enhancer (Barolo and Posakony, 2002). This means that, immediately following the SOP division, Su(H) in its repressive mode would act to prevent CD2 activation in both pIIa and pIIb. The repressive state would persist in pIIb, but would be relieved in pIIa by the Notch signaling event that specifies the pIIa fate, thus permitting the enhancer to drive numb transcription specifically in the latter cell.
Fig. 5.
Mutation of the Su(H) binding sites in the numb CD2 enhancer yields ectopic activity in the Notch-independent pIIb cell. (A-B′) Developing Drosophila microchaetes at the two-cell stage, marked by Cut immunoreactivity (A,B; magenta in merge panels A′ and B′). The posterior pIIa cell and anterior pIIb cell are indicated. (A′,A′) The wild-type numb CD2 enhancer-reporter transgene (GFP; green in A′) is active only in Notch-dependent pIIa. (B′,B′) numb CD2 reporter gene bearing single-base mutations in its two Su(H) binding sites (GFP; green in B′) is active in both pIIa and pIIb.
Activation of the numb CD2 enhancer module depends on relief of Su(H)-mediated repression by Notch signaling
The data presented thus far are consistent with the model that numb transcription is stimulated by the CD2 enhancer in response to the asymmetric Notch signaling events that specify the fates of the pIIa and pIIIb precursor cells. We sought to test directly whether Notch signaling is necessary for activation of the CD2 enhancer module.
We first wanted to show that Su(H), as the transducing transcription factor for the Notch pathway, is required in trans for the cell type-specific activity of the enhancer, as implied by the Su(H)-binding-site-mutation experiment just described (Fig. 5). We used a pannier (pnr)-GAL4 driver to express a Su(H) short-hairpin RNAi construct in the central region of the pupal notum; the flanking regions served as the control tissue (Fig. 6A). This treatment resulted in a complete `balding' phenotype in the adult thorax (see Fig. S4 in the supplementary material) mimicking that observed in Su(H)-mutant mosaic territories (Schweisguth, 1995), indicating its effectiveness in reducing Su(H) gene activity.
Fig. 6.
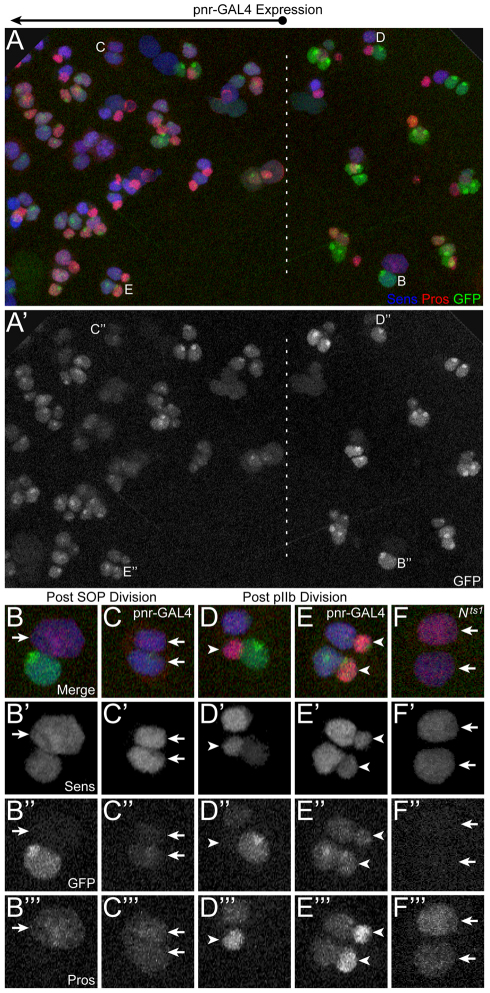
Response of the numb CD2 enhancer to perturbations in Notch signaling. (A-E′′) CD2-GFP reporter gene expression (green) in the genotype w; CD2-GFP/+; pnr-GAL4/UAS-Su(H)shRNA. (A,A') Wide-field view of pupal notum, with approximate boundary between GAL4-expressing and non-expressing territories denoted by a dashed line, as indicated above panel A. Positions enlarged in subsequent panels are labeled. (B-C′′) GFP expression in progeny cells following the SOP division in either the GAL4-non-expressing (B-B′′) or GAL4-expressing (C-C′′) territory. (D-E′′) GFP expression in progeny cells following the pIIb division in either the GAL4-non-expressing (D-D′′) or GAL4-expressing (E-E′′) territory. (F-F′′) Lack of GFP expression at the two-cell stage in a Nts1/N81k1 background following temperature shift. Cells are marked by anti-Sens (blue in A-F) and anti-Pros (red in A-F) immunoreactivity. GFP is also absent from four-cell Pros-positive positions (data not shown). Arrows in B-C′′ and F-F′′ indicate cells adopting the pIIb fate. Arrowheads in D-E′′ indicate cells adopting the pIIIb sib fate.
At the two-cell stage, Su(H) RNAi caused both of the progeny of the SOP to adopt the pIIb fate, owing to a failure of Notch signal transduction in the pIIa cell (Fig. 6B,C). Nevertheless, as expected from the result presented in Fig. 5, both of these cells expressed GFP from the CD2 enhancer-reporter transgene (Fig. 6C′), as Su(H) was no longer able to repress the enhancer in either cell. This experiment also suggests that Su(H) does normally make some contribution to the activation of the enhancer in pIIa, as the Su(H) RNAi treatment appeared to reduce the level of GFP accumulation in this cell relative to that observed in the wild-type territory (compare Fig. 6B′ and 6C′). Later, following the division of pIIb [which yields three cells in wild-type territory and four cells in the Su(H) RNAi domain], GFP from the CD2 reporter appeared only in pIIa and pIIIb at wild-type positions, but was expressed in all four cells (two `pIIIb' and two pIIIb sib cells) where Su(H) RNAi was active (Fig. 6D,E).
We directly investigated the role of Notch signaling in activating the CD2 enhancer by examining expression of the reporter gene in notum tissue of female pupae bearing the temperature-sensitive allele Nts1 in trans to a Notch null allele (Nts1/N81k1). Exposure of these animals to 37°C for two hours at the appropriate time caused a failure of the Notch signaling event that specifies the pIIa cell, yielding two-cell positions in which both daughters of the SOP have adopted the pIIb fate (Fig. 6F) (Hartenstein and Posakony, 1990; Posakony, 1994). Here, in contrast to what was observed when Su(H) activity was reduced (Fig. 6C′), neither cell expressed the CD2 reporter (Fig. 6F′). Combining the results presented in Figs 5 and 6, we conclude that Notch signaling is indeed required to activate the CD2 enhancer specifically in pIIa, and that it does this, in part, by relieving Su(H)-mediated repression. We confirmed the role of Notch signaling in CD2 enhancer activation in a separate experiment in which overexpression of Numb was used to abrogate Notch signaling activity throughout the sensory organ lineage (Reddy and Rodrigues, 1999a) (see Figs S5 and S6 in the supplementary material).
The numb CD2 enhancer is required for proper specification of the shaft and neuron cell fates
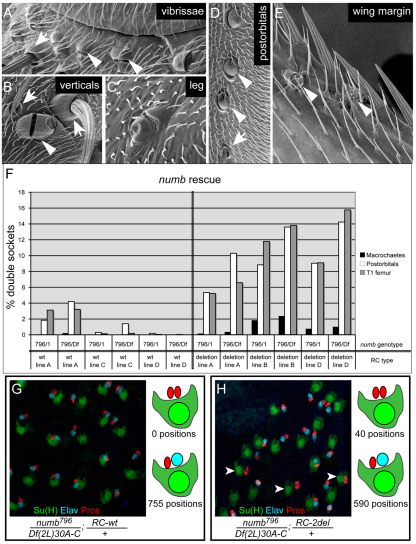
The specific activation of the numb CD2 enhancer in the pIIa and pIIIb precursor cells of the bristle lineage suggested to us that it might have a role in specifying the fates of their Numb-inheriting progeny cells, the shaft cell and neuron, respectively. We tested this prediction by carrying out rescue experiments using genomic DNA transgenes containing the numb locus. We found that a 20 kb fragment that includes the proximal (zygotic) promoter and entire coding region, along with substantial amounts of 5′ and 3′ flanking sequence (Fig. 2A), efficiently rescued the lethality associated with two different numb loss-of-function genotypes (Tweedie et al., 2009). Moreover, adult mechanosensory organ development was almost completely normal in numb mutant flies bearing the wild-type rescue fragment (Fig. 7F; see Table S2 in the supplementary material). By contrast, mutant flies bearing a version of this construct in which the numb CD2 enhancer had been deleted (Fig. 2A) displayed a widespread `double socket' phenotype, in which the shaft cell was transformed into a second socket cell (Fig. 7A-F; see Table S2 in the supplementary material). Similarly, pupal-stage nota of CD2 enhancer-deleted animals showed frequent transformation of the sensory neuron into a second sheath cell (Fig. 7G,H). These results demonstrate that the function of the CD2 enhancer module is required for the normal specification of the numb-dependent, Notch-independent shaft and neuron cell fates.
Fig. 7.
Deletion of the numb CD2 enhancer causes shaft-to-socket and neuron-to-sheath cell fate transformations. (A-E) Scanning electron micrograph images of numb796/Df(2L)30A-C; numbRC2del/+ flies, showing the appearance of a `double socket' phenotype at multiple bristle positions across the body. (A) Head vibrissae; anterior is to the left. (B) Inner vertical (left)/outer vertical (right) pair. (C) Bristle position on the T1 femur. (D) Postorbital positions on the posterior head. (E) Bristles on the anterior wing margin also show the double socket phenotype. In A, B, D and E, arrowheads point to bristle positions showing a double socket phenotype; arrows indicate positions at which the shaft fate has been properly specified. (F) Quantification of bristle phenotypes observed when either the numbRCwt (left half of the graph) or the numbRC2del (right half of the graph) rescue transgene is present in either a numb796/numb1 (`796/1') or a numb796/Df(2L)30A-C (`796/Df') background. Displayed are the results for three independent insertions of numbRCwt (lines A, C and D) and numbRC2del (lines A, B and D). Bristles positions scored were head and thorax dorsal macrochaetes, postorbital bristles and T1 femur positions, as indicated. numbRC2del results are highly significantly different from the numbRCwt results by the Mann-Whitney U test for both numb1 and Df(2L)30A-C (P<0.001). See also Table S2 in the supplementary material. (G,H) Illustration and quantification of the effect of deleting the CD2 enhancer on the fates of the progeny of pIIIb. Microchaete positions in pupal nota at 24-30 hours APF are marked with anti-Su(H) (green; socket cell), anti-Elav (blue; neuron) and anti-Pros (red; sheath cell) antibodies. In numb796/Df(2L)30A-C animals bearing one copy of the wild-type numb rescue construct (RC-wt), no `double sheath' positions are observed (G), whereas those carrying one copy of the CD2 deletion construct (RC-2del) show a significant prevalence of this phenotype, reflecting a failure to specify the numb-dependent neuron fate (H).
DISCUSSION
Successful computational identification of cis-regulatory modules based on binding site clustering
The transcriptional regulation of the numb gene has not previously received much attention because most experimental efforts have been focused on Numb protein localization, asymmetric segregation and function as a Notch pathway inhibitor. The motivation for the present study originated in a computational search of the fly genome for new Notch pathway target genes based on statistically significant clustering of Su(H) binding sites (Rebeiz et al., 2002). Although it has been suggested that homotypic site clustering is not a general property of cis-regulatory modules in Drosophila, and therefore that this parameter is of limited utility in computational prediction of enhancers (Li et al., 2007), the data that we have presented here and in other reports (Bailey and Posakony, 1995; Nellesen et al., 1999; Lai et al., 2000; Rebeiz et al., 2002; Castro et al., 2005) indicate that this approach can be quite effective in the case of Su(H) and other transcription factors. One beneficial feature of our SCORE method (Rebeiz et al., 2002) is the use of a largely unbiased window size (100-5000 bp) for the identification of statistically significant binding site clusters. This wide range allows the detection of local maxima that do not necessarily conform to the size expected for a canonical cis-regulatory module. Judging from the present study, the unbiased window-size approach might permit functional enhancer elements to be detected owing to the proximity of multiple enhancers with similar binding inputs. In any case, the SCORE technique successfully identified a functional cis-regulatory module within the ∼50 kb of non-coding DNA within and surrounding numb.
Role of the Notch-activated numb CD2 enhancer in the specification of the shaft and neuron cell fates
We have shown here that a 20 kb genomic DNA fragment is capable of nearly complete phenotypic rescue of two different numb loss-of-function genotypes, and that deletion of the numb CD2 enhancer from this fragment results in widespread `double socket' and `double sheath' phenotypes, reflecting a failure to specify the numb-dependent shaft and neuron cell fates. Thus, transcriptional activation of numb in the pIIa and pIIIb precursor cells, in response to the Notch signaling events that specify their respective fates, plays an important role in the proper specification of the Notch-independent progeny cell fate.
Given the high proportion of sensory organs in which the shaft and neuron cell fates are correctly specified in the absence of the CD2 enhancer, it seems clear that CD2 is not the only source of Numb for pIIa and pIIIb. We confirmed this inference directly by detecting Numb crescents in dividing pIIa cells in tissue lacking CD2 function, having first demonstrated that the numb796 allele is protein-null (see Fig. S7 in the supplementary material).
What might be the source of this additional Numb protein? It is, of course, possible that numb is served by a second enhancer module that also contributes to the transcriptional activation of the gene in pIIa and pIIIb in response to Notch signaling; there is substantial precedent for such `shadow' or `secondary' enhancers in insects (Hong et al., 2008; Frankel et al., 2010). However, it is very likely that the basal level of Numb protein that is detected in all cells in the epidermis (Rhyu et al., 1994) also accumulates in developing sensory organ cells, including pIIa and pIIIb, independently of the CD2 enhancer. This protein would presumably be segregated by the two precursor cells to their shaft and neuron daughter cells, respectively, and might suffice, in most cases, to inhibit Notch signaling in those cells.
What, then, would generate the need for the numb CD2 enhancer activity? Integrating all of our findings, we currently favor the following evolutionary scenario. Among the cells in the bristle lineage, the pIIa and pIIIb precursors face a unique challenge: because their own fates are specified by Notch signaling, it is crucial that they do not inherit Numb, yet each must make sufficient Numb to distribute asymmetrically to one of their progeny cells (Rhyu et al., 1994). In an ancestral sensory organ lineage, the ubiquitous basal level of Numb accumulation might have been adequate to supply the needs of pIIa and pIIIb. But, perhaps as the execution of the lineage became faster in some rapidly developing insects [the time from birth to division for pIIa and pIIIb is only 3-4 hours in Drosophila (Hartenstein and Posakony, 1989; Reddy and Rodrigues, 1999b)], Numb accumulation in these cells failed to meet the required threshold, resulting in unacceptably high failure rates in shaft cell and neuron specification. The emergence of the CD2 enhancer would then have offered the selective advantage of supplementing the basal Numb specifically in these two Notch-dependent precursor cells, without elevating the global activity of the gene. In this scenario, CD2 represents an evolutionary adaptation for ensuring the fidelity of two cell fate decisions during mechanosensory organ development.
Integration of conditional and autonomous modes of cell fate specification
The Drosophila external sensory organ lineage has stood for many years as an elegant example of the integration of conditional and autonomous mechanisms of cell fate specification (Posakony, 1994). The repeated use of a combination of bi-directional Notch signaling between sister cells and asymmetric segregation of the Notch pathway antagonist Numb is a highly effective strategy for ensuring the proper specification of cell fates in a succession of asymmetric cell divisions. This is particularly so because the orientation of the mitotic spindles and the segregation of Numb are tied to the planar polarity system (Bellaiche et al., 2001), such that the appropriate fate is assigned to the appropriate daughter with extremely high fidelity. The results reported here bring this Notch-Numb partnership full circle by demonstrating that a reciprocal regulatory linkage also exists: Notch signaling regulates numb (Fig. 8).
Fig. 8.
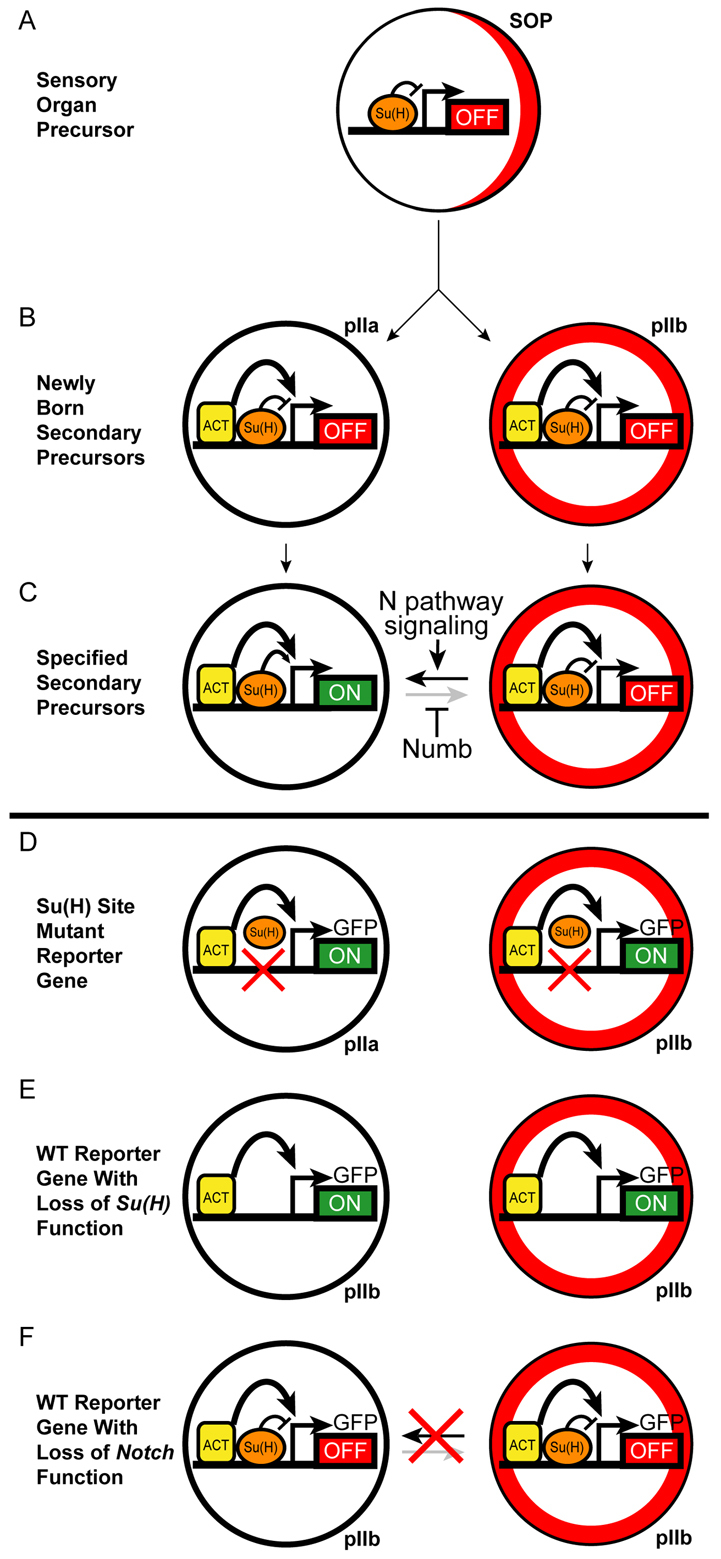
Model for the Notch-stimulated activation of numb transcription in the pIIa precursor cell. (A) As the Notch-independent SOP prepares to divide, it segregates Numb protein (red) in a crescent to its anterior side (right). Su(H) binds the CD2 enhancer module and represses it (OFF). (B) Immediately after the division of the SOP, pre-loaded transcriptional activator proteins (ACT) are bound to the CD2 enhancer in both daughter cells. Su(H) in its repressor mode keeps the enhancer OFF in both cells. The anterior daughter, the presumptive pIIb, has inherited Numb from the SOP (red ring). (C) Bi-directional Notch (N) signaling between the two daughter cells then specifies the pIIa fate (black horizontal arrow) and relieves `default repression' of the CD2 enhancer by Su(H) in this cell, permitting the bound ACT proteins to activate numb transcription (ON) in partnership with activated Su(H). Inherited Numb protein in pIIb renders this cell unresponsive to the reciprocal Notch signal (gray horizontal arrow), and Su(H) continues to repress the enhancer. (D-F) Interpretation of experiments with the GFP reporter gene (see Figs 5, 6). (D) Mutation of the two binding sites in the CD2 module prevents repression by Su(H), leaving ACT-stimulated reporter activity in pIIa and permitting ectopic activation of the reporter in pIIb. (E) Loss of Su(H) gene function causes cell-autonomous failure of pIIa specification (so that both sisters adopt the pIIb fate) and activation of the wild-type CD2 reporter gene in both cells [due to loss of Su(H)-mediated repression]. (F) Loss of Notch function likewise causes cell-autonomous failure of pIIa specification, but the wild-type CD2 reporter remains off in both cells owing to continued repression by Su(H).
We have shown that, although Notch signaling is essential to the activation of the numb bristle enhancer, the transcriptional activation function of Su(H) is not strictly required for enhancer activity. Accordingly, we suggest that Notch signaling acts here in large part as a trigger, relieving Su(H)-mediated `default repression' and permitting other activators bound to the enhancer to drive numb transcription (Fig. 8). Some or all of these activators are likely to be expressed in both pIIa and pIIb, as implied by the nearly equivalent level of reporter gene activity observed in the two cells when the Su(H) binding sites of the enhancer are mutated. We further suggest that this regulatory strategy is relevant to the question of timing raised earlier. Having Notch signaling act as a trigger for the action of a pre-assembled complex of other activators might help to ensure that the transcriptional response is very rapid, allowing sufficient numb mRNA to be accumulated and translated in pIIa and pIIIb before they divide.
Supplementary Material
Acknowledgments
We are grateful to Feng Liu for constructing and providing the UAS-Su(H)shRNA lines and to Ben Haley for providing the pNE3 vector. We thank Tammie Stone for performing the EMSAs. Adrian Moore and the laboratory of Yuh Nung Jan kindly provided anti-Hamlet antibody and fly stocks, respectively. The SEM images were collected at San Diego State University with generous help from Steve Barlow. S.W.M. received support from NIH predoctoral training grant GM07240. This work was supported by NIH grant GM046993 to J.W.P. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.050161/-/DC1
References
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., Amanatides P. G., Scherer S. E., Li P. W., Hoskins R. A., Galle R. F., et al. (2000). The genome sequence of Drosophila melanogaster. Science 287, 2185-2196 [DOI] [PubMed] [Google Scholar]
- Bailey A. M., Posakony J. W. (1995). Suppressor of Hairless directly activates transcription of Enhancer of split Complex genes in response to Notch receptor activity. Genes Dev. 9, 2609-2622 [DOI] [PubMed] [Google Scholar]
- Barolo S., Posakony J. W. (2002). Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 16, 1167-1181 [DOI] [PubMed] [Google Scholar]
- Barolo S., Carver L. A., Posakony J. W. (2000). GFP and β-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. BioTechniques 29, 726-732 [DOI] [PubMed] [Google Scholar]
- Bellaiche Y., Gho M., Kaltschmidt J., Brand A., Schweisguth F. (2001). Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat. Cell Biol. 3, 50-57 [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Bodmer R., Jan L. Y., Jan Y. N. (1990). Patterns of expression of Cut, a protein required for external sensory organ development, in wild-type and cut mutant Drosophila embryos. Genes Dev. 4, 1322-1331 [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Jan L. Y., Jan Y. N. (1993). Postembryonic patterns of expression of cut, a locus regulating sensory organ identity in Drosophila. Development 117, 441-450 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415 [DOI] [PubMed] [Google Scholar]
- Buescher M., Yeo S., Udolph G., Zavortink M., Yang X., Tear G., Chia W. (1998). Binary sibling neuronal cell fate decisions in the Drosophila embryonic central nervous system are nonstochastic and require inscuteable-mediated asymmetry of ganglion mother cells. Genes Dev. 12, 1858-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja M., Moreno E., Pelaz S., Morata G. (1996). Visualization of gene expression in living adult Drosophila. Science 274, 252-255 [DOI] [PubMed] [Google Scholar]
- Castro B., Barolo S., Bailey A. M., Posakony J. W. (2005). Lateral inhibition in proneural clusters: Cis-regulatory logic and default repression by Suppressor of Hairless. Development 132, 3333-3344 [DOI] [PubMed] [Google Scholar]
- Frankel N., Davis G., Vargas D., Wang S., Payre F., Stern D. (2010). Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 466, 490-493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frise E., Knoblich J. A., Younger-Shepherd S., Jan L. Y., Jan Y. N. (1996). The Drosophila Numb protein inhibits signaling of the Notch receptor during cell-cell interaction in sensory organ lineage. Proc. Natl. Acad. Sci. USA 93, 11925-11932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwade B. G., Muskavitch M. A., Welshons W. J., Yedvobnick B., Artavanis-Tsakonas S. (1985). The molecular genetics of the Notch locus in Drosophila melanogaster. Dev. Biol. 107, 503-519 [DOI] [PubMed] [Google Scholar]
- Guo M., Jan L. Y., Jan Y. N. (1996). Control of daughter cell fates during asymmetric division: Interaction of Numb and Notch. Neuron 17, 27-41 [DOI] [PubMed] [Google Scholar]
- Haley B., Hendrix D., Trang V., Levine M. (2008). A simplified miRNA-based gene silencing method for Drosophila melanogaster. Dev. Biol. 321, 482-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W. (1989). Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 107, 389-405 [DOI] [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W. (1990). A dual function of the Notch gene in Drosophila sensillum development. Dev. Biol. 142, 13-30 [DOI] [PubMed] [Google Scholar]
- Hinz U., Giebel B., Campos-Ortega J. A. (1994). The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell 76, 77-87 [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51-59 [DOI] [PubMed] [Google Scholar]
- Hong J., Hendrix D., Levine M. (2008). Shadow enhancers as a source of evolutionary novelty. Science 321, 1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins R., Nelson C., Berman B., Laverty T., George R., Ciesiolka L., Naeemuddin M., Arenson A., Durbin J., David R., et al. (2000). A BAC-based physical map of the major autosomes of Drosophila melanogaster. Science 287, 2271-2274 [DOI] [PubMed] [Google Scholar]
- Lai E. C., Bodner R., Posakony J. W. (2000). The Enhancer of split Complex of Drosophila includes four Notch-regulated members of the Bearded gene family. Development 127, 3441-3455 [DOI] [PubMed] [Google Scholar]
- Li L., Zhu Q., He X., Sinha S., Halfon M. (2007). Large-scale analysis of transcriptional cis-regulatory modules reveals both common features and distinct subclasses. Genome Biol. 8, R101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki J., Bogarad L., Martin M., Ruddle F., McGinnis W. (1993). Functional analysis of the mouse homeobox gene HoxB9 in Drosophila development. Mech. Dev. 42, 139-150 [DOI] [PubMed] [Google Scholar]
- Miller S., Avidor-Reiss T., Polyanovsky A., Posakony J. (2009). Complex interplay of three transcription factors in controlling the tormogen differentiation program of Drosophila mechanoreceptors. Dev. Biol. 329, 386-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. W., Jan L. Y., Jan Y. N. (2002). hamlet, a binary genetic switch between single- and multiple-dendrite neuron morphology. Science 297, 1355-1358 [DOI] [PubMed] [Google Scholar]
- Moore A., Roegiers F., Jan L., Jan Y. (2004). Conversion of neurons and glia to external-cell fates in the external sensory organs of Drosophila hamlet mutants by a cousin-cousin cell-type respecification. Genes Dev. 18, 623-628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellesen D. T., Lai E. C., Posakony J. W. (1999). Discrete enhancer elements mediate selective responsiveness of Enhancer of split Complex genes to common transcriptional activators. Dev. Biol. 213, 33-53 [DOI] [PubMed] [Google Scholar]
- Nolo R., Abbott L. A., Bellen H. J. (2000). Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349-362 [DOI] [PubMed] [Google Scholar]
- O'Connor-Giles K. M., Skeath J. (2003). Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev. Cell 5, 231-243 [DOI] [PubMed] [Google Scholar]
- Posakony J. W. (1994). Nature versus nurture: asymmetric cell divisions in Drosophila bristle development. Cell 76, 415-418 [DOI] [PubMed] [Google Scholar]
- Rebeiz M., Posakony J. W. (2004). GenePalette: A universal software tool for genome sequence visualization and analysis. Dev. Biol. 271, 431-438 [DOI] [PubMed] [Google Scholar]
- Rebeiz M., Reeves N. L., Posakony J. W. (2002). SCORE: A computational approach to the identification of cis-regulatory modules and target genes in whole-genome sequence data. Proc. Natl. Acad. Sci. USA 99, 9888-9893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G. V., Rodrigues V. (1999a). Sibling cell fate in the Drosophila adult external sense organ lineage is specified by Prospero function, which is regulated by Numb and Notch. Development 126, 2083-2092 [DOI] [PubMed] [Google Scholar]
- Reddy G. V., Rodrigues V. (1999b). A glial cell arises from an additional division within the mechanosensory lineage during development of the microchaete on the Drosophila notum. Development 126, 4617-4622 [DOI] [PubMed] [Google Scholar]
- Reeves N., Posakony J. W. (2005). Genetic programs activated by proneural proteins in the developing Drosophila PNS. Dev. Cell 8, 413-425 [DOI] [PubMed] [Google Scholar]
- Rhyu M. S., Jan L. Y., Jan Y. N. (1994). Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76, 477-491 [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. (1982). Genetic transformation of Drosophila with transposable element vectors. Science 218, 348-353 [DOI] [PubMed] [Google Scholar]
- Schweisguth F. (1995). Suppressor of Hairless is required for signal reception during lateral inhibition in the Drosophila pupal notum. Development 121, 1875-1884 [DOI] [PubMed] [Google Scholar]
- Shellenbarger D. L., Mohler J. D. (1975). Temperature-sensitive mutations of the Notch locus in Drosophila melanogaster. Genetics 81, 143-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spana E. P., Doe C. Q. (1995). The prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development 121, 3187-3195 [DOI] [PubMed] [Google Scholar]
- Tun T., Hamaguchi Y., Matsunami N., Furukawa T., Honjo T., Kawaichi M. (1994). Recognition sequence of a highly conserved DNA binding protein RBP-Jk. Nucleic Acids Res. 22, 965-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie S., Ashburner M., Falls K., Leyland P., Mcquilton P., Marygold S., Millburn G., Osumi-Sutherland D., Schroeder A., Seal R., et al. (2009). FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 37, D555-D559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Shepherd S., Ackerman L., Jan L. Y., Jan Y. N. (1989). numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 58, 349-360 [DOI] [PubMed] [Google Scholar]
- Wang S., Younger-Shepherd S., Jan L. Y., Jan Y. N. (1997). Only a subset of the binary cell fate decisions mediated by Numb/Notch signaling in Drosophila sensory organ lineage requires Suppressor of Hairless. Development 124, 4435-4446 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.