Abstract
Ion channels and lipid phosphatases adopt a transmembrane voltage sensor domain (VSD) that moves in response to physiological variations of the membrane potential to control their activities. However, the VSD movements and coupling to the channel or phosphatase activities may differ depending on various interactions between the VSD and its host molecules. BK-type voltage, Ca2+ and Mg2+ activated K+ channels contain the VSD and a large cytosolic domain (CTD) that binds Ca2+and Mg2+. VSD movements are coupled to BK channel opening with a unique allosteric mechanism and are modulated by Ca2+ and Mg2+ binding via the interactions among the channel pore, VSD and CTD. These properties are energetically advantageous for the pore to be controlled by multiple stimuli, revealing the adaptability of the VSD to its host molecules and showing the potential for intracellular signals to affect the VSD in order to modulate the function of its host molecules.

Jianmin Cui is the Spencer T. Olin Professor of Biomedical Engineering at Washington University in St Louis. He received a PhD in Physiology and Biophysics from State University of New York at Stony Brook and a post-doctoral training at Stanford University. He was an assistant professor of Biomedical Engineering at Case Western Reserve University before moving to St Louis. His research interests are on membrane permeation to ions, drugs and genes, including the molecular mechanisms of ion channel function and ultrasound-mediated drug/gene delivery. He is a recipient of the Established Investigator Award from the American Heart Association.
Introduction
BK channels are large conductance voltage and Ca2+ activated K+ channels encoded by the Slo1 gene (Atkinson et al. 1991). Similar to other K+ channels, BK channels contain a central ionic pore comprising transmembrane α helices S5 and S6 from four Slo1 subunits and the selectivity filter for K+ permeation (Atkinson et al. 1991; Adelman et al. 1992; Butler et al. 1993; Shen et al. 1994; Tseng-Crank et al. 1994; Doyle et al. 1998). The opening of BK channels repolarizes the membrane potential and reduces Ca2+ entry into the cell by closing voltage-dependent Ca2+ channels, which regulate various physiological processes including neurotransmitter release in synapses (Robitaille & Charlton, 1992; Raffaelli et al. 2004; Wang, 2008), contraction of smooth muscle cells in airway and blood vessels (Brayden & Nelson, 1992; Kotlikoff, 1993; Brenner et al. 2000), circadian pacemaker output in central nerve systems (Meredith et al. 2006; Pitts et al. 2006; Kent & Meridith, 2008) and electric tuning by hair cells in vertebrates (Art & Fettiplace, 1987; Fuchs et al. 1988; Hudspeth & Lewis, 1988). Each Slo1 subunit has a voltage sensor domain (VSD) formed by transmembrane segments S1–S4 and a large cytosolic domain (CTD) containing Ca2+ binding sites (Fig. 1). Voltage sensor movements in response to depolarization of the membrane potential and the binding of intracellular Ca2+ are separately coupled to the pore to activate the channel.
Figure 1. Voltage- and Ca2+-dependent activation of BK channels.
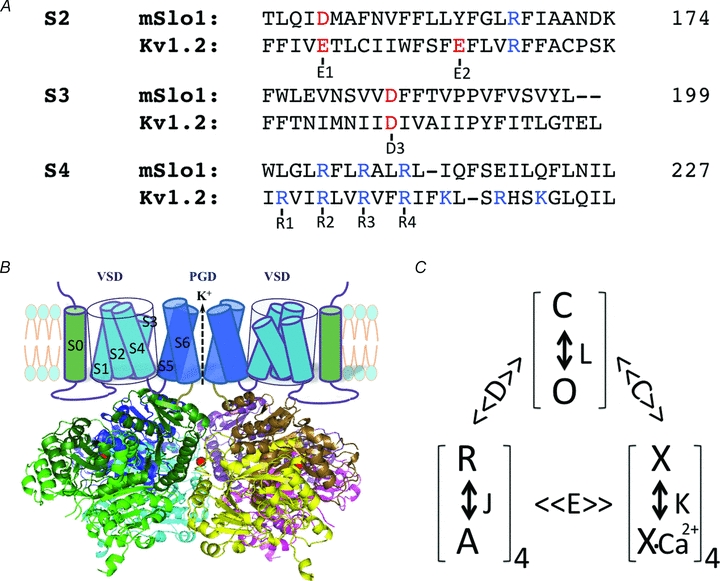
A, sequence alignment of S2–S4 in the voltage sensor domain of mSlo1 and KV1.2 channels, highlighting the conserved charged residues (red and blue) that are important in voltage-dependent activation of both BK and KV channels. The position of the last amino acid in each segment of mSlo1 is given (numbers). B, structure model of BK channels. The cartoon of the membrane-spanning domains only shows two subunits for clarity. The cytosolic domain is the tetrameric Slo1 structure (Yuan et al. 2010). The subunits are represented by different colours and the dark and light shades of each colour show the RCK1 and RCK2 structural domains within the same subunit (RCK: regulator for conductance of K+). A bound Ca2+ ion to the Ca2+ bowl in each subunit is shown as a red sphere. C, model of allosteric gating of BK channels by voltage and Ca2+. The channel can open and close (O and C) with an equilibrium constant L, which is modulated by the activation of four voltage sensors (R and A, with an equilibrium constant J) and the binding of four Ca2+ ions (a simplification from actual binding of eight Ca2+ ions, X and X-Ca2+, with a dissociation constant K) with the allosteric factor D and C, respectively. The voltage sensor activation and Ca2+ binding also affect each other with a factor E. The model was proposed by Horrigan & Aldrich (2002).
Similar molecular mechanisms may underlie the coupling between voltage sensor movements and pore opening in voltage gated K+ (KV) channels and BK channels (Cui et al. 2009). However, while in some KV channels, such as Shaker, the coupling between the voltage sensor and pore seems to be tight or even obligatory, i.e. the pore opens when and only when all the voltage sensors are activated, the coupling in BK channels is allosteric, i.e. the pore can open when the voltage sensor is either activated or at rest but the opening is favoured when the voltage sensor is activated. In addition, in BK channels the CTD is located close to the membrane-spanning voltage sensor and pore domains. This structural feature allows intracellular Mg2+ to bind closely to the cytosolic side of the voltage sensor and directly affect voltage sensor movements via an electrostatic interaction (Yang et al. 2007). This article will review the special properties of voltage sensing and the influence by Ca2+ and Mg2+ ions in BK channels.
Allosteric mechanisms of voltage- and Ca2+-dependent activation of BK channels
The VSDs of BK channels contain charged amino acid residues that are highly conserved among KV channels (Fig. 1A). The arginine residues at positions 207, 210 and 213 in the S4 segment (all residue numbers are based on the mbr5 sequence of mouse Slo1 (mSlo1) subunit, GenBank accession number, GI: 347143) (Butler et al. 1993) correspond to R2, R3 and R4 in KV channels (Gandhi & Isacoff, 2002), Asp153 in S2 corresponds to E1/D1 in Kv channels (Silverman et al. 2003; Wu et al. 2010), and Asp186 in S3 corresponds to D3 in KV channels (Fig. 1A). The S2 segment does not contain the conserved E2 residue of KV channels at the cytosolic side, but instead a positively charged Arg167 that is located four residues below the E2 position. Although the mutations of each of the three arginine residues in S4 alter voltage dependence of BK channel opening (Diaz et al. 1998; Cui & Aldrich, 2000), it was shown that only Arg213 contributes to voltage sensing (Ma et al. 2006). In addition, the charged residues Asp153 and Arg167 in S2 and Asp186 in S3 also contribute to voltage sensing. Neutralization of each of these residues in the four mSlo1 subunits reduced effective gating charge by 1.20, 0.92, 0.48 and 0.88, respectively, from 2.32 of the WT channel (Ma et al. 2006). The role of these charges is somewhat different from that of the equivalent charged residues in KV channels, where the arginine residues in S4 serve as the primary gating charges, while the negatively charged residues in S2 and S3 interact with S4 arginines to stabilize channel protein (Papazian et al. 1995; Tiwari-Woodruff et al. 1997; Long et al. 2005) and steer S4 movements during activation (Wu et al. 2010); only E2 in the S2 segment of the Shaker K+ channel had been shown to serve as a gating charge (Seoh et al. 1996). Since in BK channels the charged residues in S2, S3 and S4 contribute similarly to voltage sensing, the movements of the VSD in BK and KV channels may differ during activation. A model proposes that in BK channels the VSD undergoes a global conformation change that results in a repacking of all membrane-spanning segments in the VSD during activation, as opposed to a large movement of S4 alone in KV channels (Ma et al. 2006; Pantazis et al. 2010).
In BK channels, each Slo1 subunit contains an additional transmembrane segment S0 at the N-terminus of the VSD (Wallner et al. 1996) (Fig. 1B). In the BK channel structure, S0 is located at the periphery of VSD close to S2 (Liu et al. 2008; Wang & Sigworth, 2009). S0 is important for modulation of BK channels by auxiliary β subunits; it is also essential for the function of the Slo1 protein since the truncated Slo1 lacking S0 could not express any currents but the coexpression of the truncated Slo1 with a separate S0 segment restored channel function (Wallner et al. 1996). Mutations of S0 affect voltage-dependent activation of BK channels (Koval et al. 2007). Nevertheless, it is not clear how S0 contributes to the movements of the VSD during voltage dependent activation.
Voltage sensor activation in BK channels facilitates channel opening. In the absence of intracellular Ca2+, the channel can be activated by voltage; the macroscopic conductance and activation rate increase with depolarization (Meera et al. 1996; Cui et al. 1997). Likewise, the open probability of single BK channels increases with voltage (Nimigean & Magleby, 2000; Rothberg & Magleby, 2000; Talukder & Aldrich, 2000). Voltage sensor movements can be measured by gating currents (Stefani et al. 1997; Horrigan & Aldrich, 1999) and fluorescence changes from the fluorophore labelling the S3–S4 linker (Savalli et al. 2006) prior to channel opening. Mutations of charged amino acid residues in the VSD reduce the steepness of the voltage dependence of channel opening (Diaz et al. 1998; Cui & Aldrich, 2000; Ma et al. 2006). Finally, intracellular Mg2+ (Yang et al. 2007) and the auxiliary β subunits of BK channels (Bao & Cox, 2005; Orio & Latorre, 2005; Savalli et al. 2007; Yang et al. 2008b) modulate channel opening by altering voltage sensor function.
While it is clear that BK channels are activated by voltage, the channel can open even when the voltage sensors are not activated; conversely, the channel may remain closed when the voltage sensors are activated. For an ion channel that opens only after the voltage sensors are activated, the probability of channel opening (Po) depends on voltage with an exponential function at negative voltages, where the voltage sensors are at the resting state and the probability of their traverse to the activated state is low (Almers, 1978; Sigworth, 1994; Sigg & Bezanilla, 1997). For instance, the Shaker K+ channel shows such a Po–voltage relation at negative voltages where Po is as low as 10−7, suggesting the tight or even obligatory relation between voltage sensor activation and channel opening (Islas & Sigworth, 1999). However, for BK channels, the Po–V relation becomes flat as voltage decreases below 0 mV, deviating from the exponential relationship at Po≈ 10−5 and is much shallower at Po≤ 10−6 (Horrigan et al. 1999; Cui & Aldrich, 2000; Yang et al. 2010). This result indicates that BK channels can open spontaneously and independently of voltage sensor movements at negative voltages. Another piece of evidence for the non-obligatory relation between voltage sensor movements and channel opening is that the On-gating current elicited by a depolarizing voltage pulse from a negative voltage has a much faster time course than channel opening. The ionic current does not turn on until the On-gating current completes relaxation to 0 (Fig. 2B) (Horrigan & Aldrich, 1999), suggesting that the gating charges have moved to the activated state before the channels open. Thus, the voltage sensors can activate while the channel is closed and the activation of the voltage sensors facilitates channel opening. Based on these results and the voltage dependence of ionic and gating currents, an allosteric model was proposed for voltage-dependent activation of BK channels (Horrigan et al. 1999). In this model the voltage sensors can activate when the channel is either at the closed or open state but in open channels the activation of the voltage sensors is more favoured; reciprocally, the activation of the voltage sensors promotes channel opening (Fig. 1C).
Figure 2. Mg2+ modulation of the voltage sensor function in BK channels.
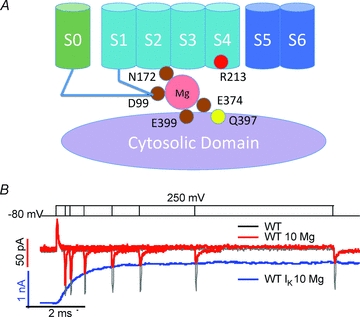
A, cartoon showing amino acid residues involved in Mg2+ binding and interaction with the voltage sensor domain. B, gating currents in the absence and presence of 10 mm Mg2+ (black and red traces) in response to various lengths of voltage pulses from −80 mV to +250 mV (top). The ionic current (blue trace) in response to a −80 to +250 mV voltage pulse in 10 mm Mg2+ is aligned with gating currents to show the correlation in time courses between channel opening and the decay in Off-gating current amplitude in 10 mm Mg2+. Adapted from Yang et al. (2007), ©2007 National Academy of Sciences of the USA.
The allosteric coupling between voltage sensor movements and channel opening in BK channels is particularly suitable for Ca2+-dependent activation. At voltages below – 150 mV, where the voltage sensors are at the resting state, an increase of intracellular Ca2+ concentration enhances Po by ∼104 (Horrigan & Aldrich, 2002; Sweet & Cox, 2008; Yang et al. 2010). Therefore, Ca2+ binding can open the channel without voltage sensor activation, which is energetically advantageous for Ca2+ to activate the channel, especially at negative voltages.
Two Ca2+ binding sites have been identified in the CTD of the Slo1 subunit by functional studies and mutagenesis. One site is located in the Ca2+ bowl that contains consecutive Asp residues at positions 895–901 (Moss et al. 1996a,b; Schreiber & Salkoff, 1997; Schreiber et al. 1999; Bian et al. 2001; Braun & Sy, 2001; Bao et al. 2004), and the other resides at Asp367 (Xia et al. 2002). Recently, an X-ray crystallographic structure of the CTD of Slo1 shows a Ca2+ ion binding to the Ca2+ bowl that is coordinated by the side chains from Asp898 and Asp900 and main chain carbonyl from Gln892 and Asp895 (Yuan et al. 2010), but the second Ca2+ binding site was not identified in the structure. Ca2+-dependent activation of BK channels can also be described by an allosteric model (McManus & Magleby, 1991; Cox et al. 1997), in which Ca2+ can bind to the channel in both the closed and open states but with a higher affinity when the channel is open, and therefore Ca2+ binding shifts the equilibrium between the closed and open states toward open (Fig. 1C). Because both voltage and Ca2+ promote BK channel opening and because both voltage sensor activation and Ca2+ binding are favoured when the channel is open, Ca2+ binding shifts voltage dependence of channel opening toward less positive voltages, while depolarization enhances the apparent affinity and cooperativity of Ca2+ binding (Cox et al. 1997; Cui et al. 1997). Thus, voltage sensor movements are modulated by Ca2+, and vice versa, through an allosteric connection via channel opening. In addition, Ca2+-dependent activation and voltage sensor movements also affect each other more directly (Horrigan & Aldrich, 2002; Sweet & Cox, 2008), possibly via the interaction between the VSD and CTD (see below).
Mg2+ activates BK channels through an electrostatic interaction with the VSD
In addition to the sites at the Ca2+ bowl and Asp367 that bind Ca2+ with affinities in the micromolar range, BK channels contain another site that binds both Ca2+ and Mg2+ with affinities in the millimolar range (Golowasch et al. 1986; Oberhauser et al. 1988; Shi & Cui, 2001; Zhang et al. 2001). Because this site binds metal ions at the physiological concentration of Mg2+, it is known as the Mg2+ binding site. This site is unique in that Mg2+ is coordinated by residues from both the CTD (Glu374 and Glu399) (Shi et al. 2002; Xia et al. 2002) and the VSD (Asp99 and Asn172) (Yang et al. 2008a) (Fig. 2A). It shows that the CTD in the BK channel is spatially very close to the VSD. Consistent with this result, it was shown that two Cys mutations that substitute for Asp99 in the VSD and Gln397 in the CTD can form a disulfide bond (Yang et al. 2008a) that is 2.9–4.6 Å in distance Cβ–Cβ of the two Cys residues (Hazes & Dijkstra, 1988). The close association between the VSD and CTD was also demonstrated in the electron cryomicroscopy structure of BK channels (Wang & Sigworth, 2009). Recently, it was shown that X-ray crystallographic structure of the Slo1 CTD fits well with the cytosolic face of the KV channel VSD (Yuan et al. 2010).
The Mg2+ ion bound to the BK channel is positioned close to the VSD, allowing it to affect the VSD through an electrostatic interaction. Evidence for the involvement of the VSD in Mg2+-dependent activation of BK channels was first shown in a study in which the mutation of Arg213 to Gln (R213Q) abolished Mg2+ sensitivity of BK channel activation (Hu et al. 2003). Since Arg213 is the only residue in S4 contributing to gating charge (Ma et al. 2006), this result suggested that Mg2+ might alter voltage sensor movements by an electrostatic interaction. The evidence supporting this idea was shown in a number of experiments (Yang et al. 2007). First, Mg2+-dependent activation depends on the ionic strength of the intracellular solution, and the effect of Mg2+ diminishes with increasing ionic strength, consistent with the idea that Mg2+ activates the channel with an electrostatic interaction. Second, a positive charge at residue 213 is necessary and sufficient for Mg2+-dependent activation. Neutralization of the charge of Arg213 with mutations R213Q or R213C abolishes Mg2+-dependent activation, while a covalent modification of R213C by a positively charged [2-(trimethylammonium)ethyl]methane thiosulfonate (MTSET+) restores Mg2+ sensitivity. This result suggests that Arg213 is the target of the electrostatic interaction with Mg2+. The third experiment supporting the electrostatic interaction is that positive charges added to Gln397 by either mutation or chemical modification can mimic the effect of Mg2+ to activate the channel. Gln397 is located close to the Mg2+ binding site, only two residues away from one of the Mg2+ coordinator Glu399 (Shi et al. 2002; Xia et al. 2002), and mutations of Gln397 to charged residues alter Mg2+ binding by electrostatic interactions (Yang et al. 2007). These results indicate that the electric field of the charges at 397 overlaps with that of the bound Mg2+ ion. Therefore, both charges can interact with Arg213 through the electric fields.
The electrostatic repulsion between Mg2+ and Arg213 in S4 slows down the return of the voltage sensor from the activated state to the resting state. This is supported by measuring the effect of Mg2+ on gating currents of BK channels (Yang et al. 2007). Interestingly, the effect of Mg2+ on gating currents depends on whether the channel is in the closed or open state. In BK channels, the voltage sensor is allosterically coupled to the activation gate and can activate in both the closed and open state (Horrigan & Aldrich, 1999). The On-gating current in response to a depolarization pulse decays rapidly before the onset of the ionic current (Fig. 2B), representing voltage sensor activation in the closed state. It was found that Mg2+ has little effect on the On-gating current but prolongs and reduces the amplitude of the Off-gating current upon membrane repolarization (Yang et al. 2007; Horrigan & Ma, 2008). The changes in the Off-gating current by Mg2+ are increasingly apparent with longer depolarization pulses and the time course matches with the onset of the ionic current elicited by the same voltage (Fig. 2B), indicating that the returning of the voltage sensor to the resting state as measured by the Off-gating current is slowed down by Mg2+ more when the channel is open than when the channel is closed. These results suggest that channel opening enhances the interaction between Mg2+ and S4, possibly by a conformational change that moves the bound Mg2+ closer to S4.
Summary
BK channels are activated by voltage and intracellular Ca2+ and Mg2+. The BK channel contains the transmembrane VSD as well as Ca2+ and Mg2+ binding sites in a large CTD. The voltage and metal sensors all control the opening of the same ionic pore in response to various physiological signals. While the architecture of the VSD in BK channels is similar to that of other voltage-dependent channels, the movements of the VSD during channel activation and the coupling between the VSD and the activation gate in BK channels are unique, and as such the charged residues in S2, S3 and S4 make similar contributions to voltage sensing and the VSD activation promotes channel opening through an allosteric mechanism. These properties are well suited to the function of BK channels to sense multiple stimuli and may be derived from the interactions of the VSD not only with the pore but also with the unique S0 segment and the CTD in BK channels. These interactions among different structural domains also mediate the modulation of VSD movements by Ca2+ via allosteric mechanisms and by Mg2+ via electrostatic interactions.
The movements of the VSD in response to depolarization can either open or close ion channels (Perozo et al. 1992; Mannikko et al. 2002) and can activate lipid phosphatases (Murata et al. 2005; Hossain et al. 2008), indicating that the VSD has the ability to adapt to various environments in its host protein molecules and functional requirements. The VSD may also be modulated by intracellular factors such as ligand binding, posttranslational modification, and the interactions with cytosolic domains, accessory proteins or subunits to alter the function of voltage-dependent ion channels (Perozo & Bezanilla, 1990; Jones et al. 1997; Terlau et al. 1997; Bao & Cox, 2005). The study of voltage-dependent activation of BK channels may provide insights into the principles of the VSD versatility and modulation that affect the function of VSD containing molecules.
Acknowledgments
Dr Roderick MacKinnon kindly provided coordinates for the BK channel gating ring structure in Fig. 1B. Allen Yang assisted in making Fig. 1B. Dr Urvi Lee and Mark Zaydman offered insightful comments on the manuscript. This work was supported by National Institutes of Health grants R01-HL70393 and R01-NS060706. J.C. is the Spencer T. Olin Professor of Biomedical Engineering.
References
- Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle ccochlea. J Physiol. 1987;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Bao L, Cox DH. Gating and ionic currents reveal how the BKCa channel's Ca2+sensitivity is enhanced by its β1 subunit. J Gen Physiol. 2005;126:393–412. doi: 10.1085/jgp.200509346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Kaldany C, Holmstrand EC, Cox DH. Mapping the BKCa channel's “Ca2+bowl”: side-chains essential for Ca2+ sensing. J Gen Physiol. 2004;123:475–489. doi: 10.1085/jgp.200409052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Favre I, Moczydlowski E. Ca2+-binding activity of a COOH-terminal fragment of the Drosophila BK channel involved in Ca2+-dependent activation. Proc Natl Acad Sci U S A. 2001;98:4776–4781. doi: 10.1073/pnas.081072398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AP, Sy L. Contribution of potential EF hand motifs to the calcium-dependent gating of a mouse brain large conductance, calcium-sensitive K+ channel. J Physiol. 2001;533:681–695. doi: 10.1111/j.1469-7793.2001.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Cox DH, Cui J, Aldrich RW. Allosteric gating of a large conductance Ca- activated K+channel. J Gen Physiol. 1997;110:257–281. doi: 10.1085/jgp.110.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Aldrich RW. Allosteric linkage between voltage and Ca2+-dependent activation of BK-type mslo1 K+channels. Biochemistry. 2000;39:15612–15619. doi: 10.1021/bi001509+. [DOI] [PubMed] [Google Scholar]
- Cui J, Cox DH, Aldrich RW. Intrinsic voltage dependence and Ca2+regulation of mslo large conductance Ca2+-activated K+channels. J Gen Physiol. 1997;109:647–673. doi: 10.1085/jgp.109.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66:852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz L, Meera P, Amigo J, Stefani E, Alvarez O, Toro L, Latorre R. Role of the S4 segment in a voltage-dependent calcium-sensitive potassium (hSlo) channel. J Biol Chem. 1998;273:32430–32436. doi: 10.1074/jbc.273.49.32430. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Nagai R, Evans MG. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci. 1988;8:2460–2467. doi: 10.1523/JNEUROSCI.08-07-02460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi CS, Isacoff EY. Molecular models of voltage sensing. J Gen Physiol. 2002;120:455–463. doi: 10.1085/jgp.20028678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J, Kirkwood A, Miller C. Allosteric effects of Mg2+on the gating of Ca2+-activated K+channels from mammalian skeletal muscle. J Exp Biol. 1986;124:5–13. doi: 10.1242/jeb.124.1.5. [DOI] [PubMed] [Google Scholar]
- Hazes B, Dijkstra BW. Model building of disulfide bonds in proteins with known three-dimensional structure. Protein Eng. 1988;2:119–125. doi: 10.1093/protein/2.2.119. [DOI] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca2+ J Gen Physiol. 1999;114:305–336. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Cui J, Aldrich RW. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca2+ J Gen Physiol. 1999;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Ma Z. Mg2+enhances voltage sensor/gate coupling in BK channels. J Gen Physiol. 2008;131:13–32. doi: 10.1085/jgp.200709877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MI, Iwasaki H, Okochi Y, Chahine M, Higashijima S, Nagayama K, Okamura Y. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J Biol Chem. 2008;283:18248–18259. doi: 10.1074/jbc.M706184200. [DOI] [PubMed] [Google Scholar]
- Hu L, Shi J, Ma Z, Krishnamoorthy G, Sieling F, Zhang G, Horrigan FT, Cui J. Participation of the S4 voltage sensor in the Mg2+-dependent activation of large conductance (BK) K+channels. Proc Natl Acad Sci U S A. 2003;100:10488–10493. doi: 10.1073/pnas.1834300100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ, Lewis RS. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas LD, Sigworth FJ. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J Gen Physiol. 1999;114:723–742. doi: 10.1085/jgp.114.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LP, Patil PG, Snutch TP, Yue DT. G-protein modulation of N-type calcium channel gating current in human embryonic kidney cells (HEK 293) J Physiol. 1997;498:601–610. doi: 10.1113/jphysiol.1997.sp021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent J, Meridith AL. BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus. PLoS One. 2008;3:e3884. doi: 10.1371/journal.pone.0003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlikoff MI. Potassium channels in airway smooth muscle: a tale of two channels. Pharmacol Ther. 1993;58:1–12. doi: 10.1016/0163-7258(93)90064-k. [DOI] [PubMed] [Google Scholar]
- Koval OM, Fan Y, Rothberg BS. A role for the S0 transmembrane segment in voltage-dependent gating of BK channels. J Gen Physiol. 2007;129:209–220. doi: 10.1085/jgp.200609662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zakharov SI, Yang L, Deng SX, Landry DW, Karlin A, Marx SO. Position and role of the BK channel α subunit S0 helix inferred from disulfide crosslinking. J Gen Physiol. 2008;131:537–548. doi: 10.1085/jgp.200809968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Campbell EB, MacKinnon R. Voltage sensor of KV1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- Ma Z, Lou XJ, Horrigan FT. Role of charged residues in the S1–S4 voltage sensor of BK channels. J Gen Physiol. 2006;127:309–328. doi: 10.1085/jgp.200509421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus OB, Magleby KL. Accounting for the Ca2+-dependent kinetics of single large-conductance Ca2+-activated K+ channels in rat skeletal muscle. J Physiol. 1991;443:739–777. doi: 10.1113/jphysiol.1991.sp018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannikko R, Elinder F, Larsson HP. Voltage-sensing mechanism is conserved among ion channels gated by opposite voltages. Nature. 2002;419:837–841. doi: 10.1038/nature01038. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between alpha (hslo) and β subunits (KV, Ca β) of maxi K channels. FEBS Lett. 1996;382:84–88. doi: 10.1016/0014-5793(96)00151-2. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss GW, Marshall J, Moczydlowski E. Hypothesis for a serine proteinase-like domain at the COOH terminus of Slowpoke calcium-activated potassium channels. J Gen Physiol. 1996a;108:473–484. doi: 10.1085/jgp.108.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss GW, Marshal J, Morabito M, Howe JR, Moczydlowski E. An evolutionarily conserved binding site for serine proteinase inhibitors in large conductance calcium-activated potassium channels. Biochemistry. 1996b;35:16024–16035. doi: 10.1021/bi961452k. [DOI] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Nimigean CM, Magleby KL. Functional coupling of the β1 subunit to the large conductance Ca2+-activated K+ channel in the absence of Ca2+. Increased Ca2+ sensitivity from a Ca2+-independent mechanism. J Gen Physiol. 2000;115:719–736. doi: 10.1085/jgp.115.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser A, Alvarez O, Latorre R. Activation by divalent cations of a Ca2+-activated K+channel from skeletal muscle membrane. J Gen Physiol. 1988;92:67–86. doi: 10.1085/jgp.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio P, Latorre R. Different effects of β1 and β2 subunits on BK channel activity. J Gen Physiol. 2005;125:395–411. doi: 10.1085/jgp.200409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis A, Gundenko V, Savalli N, Sigg D, Olcese R. Operation of the voltage sensor of a human voltage- and Ca2+-activated K+channel. Proc Natl Acad Sci U S A. 2010;107:4459–4464. doi: 10.1073/pnas.0911959107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian DM, Shao XM, Seoh SA, Mock AF, Huang Y, Wainstock DH. Electrostatic interacions of S4 voltage sensor in Shaker K+channel. Neuron. 1995;14:1293–1301. doi: 10.1016/0896-6273(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Perozo E, Bezanilla F. Phosphorylation affects voltage gating of the delayed rectifier K+channel by electrostatic interactions. Neuron. 1990;5:685–690. doi: 10.1016/0896-6273(90)90222-2. [DOI] [PubMed] [Google Scholar]
- Perozo E, Papazian DM, Stefani E, Bezanilla F. Gating currents in Shaker K+channels. Implications for activation and inactivation models. Biophys J. 1992;62:160–168. doi: 10.1016/S0006-3495(92)81802-7. discussion 169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+currents in suprachiasmatic nucleus neurons. Brain Res. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Gen Physiol. 2004;557:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg BS, Magleby KL. Voltage and Ca2+ activation of single large-conductance Ca2+-activated K+ channels described by a two-tiered allosteric gating mechanism. J Gen Physiol. 2000;116:75–99. doi: 10.1085/jgp.116.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalli N, Kondratiev A, de Quintana SB, Toro L, Olcese R. Modes of operation of the BKCa channel β2 subunit. J Gen Physiol. 2007;130:117–131. doi: 10.1085/jgp.200709803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savalli N, Kondratiev A, Toro L, Olcese R. Voltage-dependent conformational changes in human Ca2+- and voltage-activated K+ channel, revealed by voltage-clamp fluorometry. Proc Natl Acad Sci U S A. 2006;103:12619–12624. doi: 10.1073/pnas.0601176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J. 1997;73:1344–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M, Yuan A, Salkoff L. Transplantable sites confer calcium sensitivity to BK channels. Nat Neurosci. 1999;2:416–421. doi: 10.1038/8077. [DOI] [PubMed] [Google Scholar]
- Seoh SA, Sigg D, Papazian DM, Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Lagrutta A, Davies NW, Standen NB, Adelman JP, North RA. Tetraethylammonium block of Slowpoke calcium-activated potassium channels expressed in Xenopus oocytes: evidence for tetrameric channel formation. Pflugers Arch. 1994;426:440–445. doi: 10.1007/BF00388308. [DOI] [PubMed] [Google Scholar]
- Shi J, Cui J. Intracellular Mg2+ enhances the function of BK-type Ca2+-activated K+ channels. J Gen Physiol. 2001;118:589–606. doi: 10.1085/jgp.118.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Krishnamoorthy G, Yang Y, Hu L, Chaturvedi N, Harilal D, Qin J, Cui J. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 2002;418:876–880. doi: 10.1038/nature00941. [DOI] [PubMed] [Google Scholar]
- Sigg D, Bezanilla F. Total charge movement per channel. The relation between gating charge displacement and the voltage sensitivity of activation. J Gen Physiol. 1997;109:27–39. doi: 10.1085/jgp.109.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys. 1994;27:1–40. doi: 10.1017/s0033583500002894. [DOI] [PubMed] [Google Scholar]
- Silverman WR, Roux B, Papazian DM. Structural basis of two-stage voltage-dependent activation inK+channels. Proc Natl Acad Sci U S A. 2003;100:2935–2940. doi: 10.1073/pnas.0636603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E, Ottolia M, Noceti F, Olcese R, Wallner M, Latorre R, Toro L. Voltage-controlled gating in a large conductanceCa2+-sensitive K+channel (hslo) Proc Natl Acad Sci U S A. 1997;94:5427–5431. doi: 10.1073/pnas.94.10.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet TB, Cox DH. Measurements of the BKCa channel's high-affinityCa2+binding constants: effects of membrane voltage. J Gen Physiol. 2008;132:491–505. doi: 10.1085/jgp.200810094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder G, Aldrich RW. Complex voltage-dependent behavior of single unliganded calcium-sensitive potassium channels. Biophys J. 2000;78:761–772. doi: 10.1016/S0006-3495(00)76634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlau H, Heinemann SH, Stuhmer W, Pongs O, Ludwig J. Amino terminal-dependent gating of the potassium channel rag eag is compensated by a mutation in the S4 segment. J Gen Physiol. 1997;502:537–543. doi: 10.1111/j.1469-7793.1997.537bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff SK, Schulteis CT, Mock AF, Papazian DM. Electrostatic interactions between transmembrane segments meditate folding of Shaker K+channel subunits. Biophys J. 1997;72:1489–1500. doi: 10.1016/S0006-3495(97)78797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, Dichiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distant Ca2+- activated K+channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Determinant for β-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci U S A. 1996;93:14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sigworth FJ. Structure of BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 2009;461:292–295. doi: 10.1038/nature08291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW. Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol Neurobiol. 2008;38:153–166. doi: 10.1007/s12035-008-8039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Delaloye K, Zaydman MA, Nekouzadeh A, Rudy Y, Cui J. State-dependent electrostatic interactions of S4 arginines with E1 in S2 during KV7.1 activation. J Gen Physiol. 2010;135:595–606. doi: 10.1085/jgp.201010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- Yang H, Hu L, Shi J, Delaloye K, Horrigan FT, Cui J. Mg2+meditates interaction between the voltage sensor and cytosolic domain to activate BK channels. Proc Natl Acad Sci U S A. 2007;104:18270–18275. doi: 10.1073/pnas.0705873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Shi J, Zhan G, Yang J, Delaloye K, Cui J. Activation of Slo1 BK channels by Mg2+coordinated between the voltage sensor and RCK1 domains. Nat Struct Mol Biol. 2008a;15:1152–1159. doi: 10.1038/nsmb.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang G, Shi J, Lee US, Delaloye K, Cui J. Subunit-specific effect of the voltage sensor domain on Ca2+sensitivity of BK channels. Biophys J. 2008b;94:4678–4687. doi: 10.1529/biophysj.107.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Krishnamoorthy G, Saxena A, Zhang G, Shi J, Yang H, Delaloye K, Sept D, Cui J. An eplilepsy/dyskinesia-associated mutation enhances BK channel activation by potentiating Ca2+sensing. Neuron. 2010;66:871–883. doi: 10.1016/j.neuron.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Leonetti MD, Pico AR, Hsiung Y, Mackinnon R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 Å resolution. Science. 2010;329:182–186. doi: 10.1126/science.1190414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Solaro CR, Lingle CJ. Allosteric regulation of BK channel gating by Ca2+ and Mg2+ through a nonselective, low affinity divalent cation site. J Gen Physiol. 2001;118:607–636. doi: 10.1085/jgp.118.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]


