Abstract
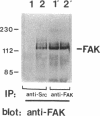
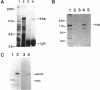
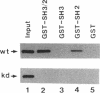
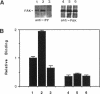
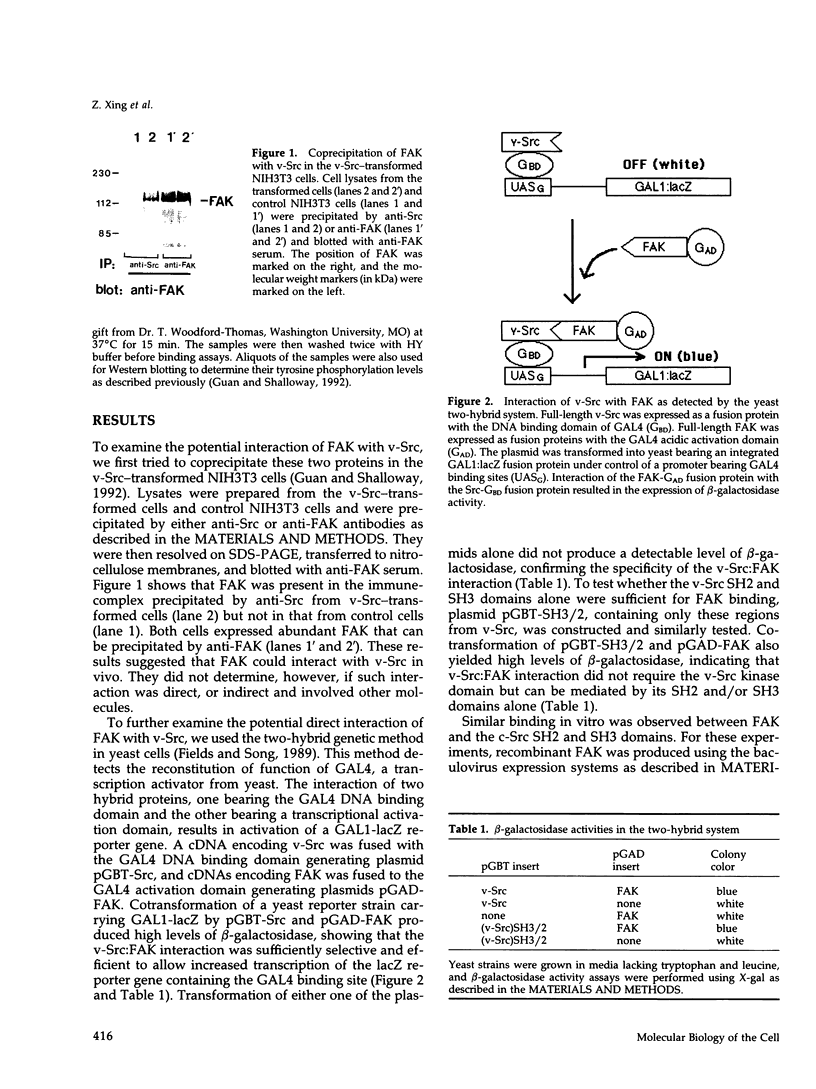
The recently described focal adhesion kinase (FAK) has been implicated in signal transduction pathways initiated by cell adhesion receptor integrins and by neuropeptide growth factors. To examine the mechanisms by which FAK relays signals from the membrane to the cell interior, we carried out a series of experiments to detect potential FAK interactions with proteins containing Src homology 2 (SH2) domains that are important intracellular signaling molecules. Using v-Src-transformed NIH3T3 cells, we showed that FAK was present in the immune-complex precipitated by anti-Src antibody, suggesting potential interaction of FAK with v-Src in vivo. We also showed potentially direct interaction of FAK with v-Src in vivo using the yeast two-hybrid system. Using recombinant FAK expressed in insect cells and bacterial fusion proteins containing Src SH2 domains, we showed direct binding of FAK to the Src SH2 domain but not to the SH3 domain in vitro. A kinase-defective mutant of FAK, which is not autophosphorylated, did not interact with the Src SH2 domain under the same conditions, suggesting the involvement of the FAK autophosphorylation sites. Treatment of FAK with a protein-tyrosine phosphatase decreased its binding to the Src SH2 domain, whereas autophosphorylation in vitro increased its binding. These results confirm the importance of FAK autophosphorylation sites in its interaction with SH2 domain-containing proteins. Taken together, these results suggest that FAK may mediate signal transduction events initiated on the cell surface by kinase activation and autophosphorylation that result in its binding to other key intracellular signaling molecules.
Full text
PDF


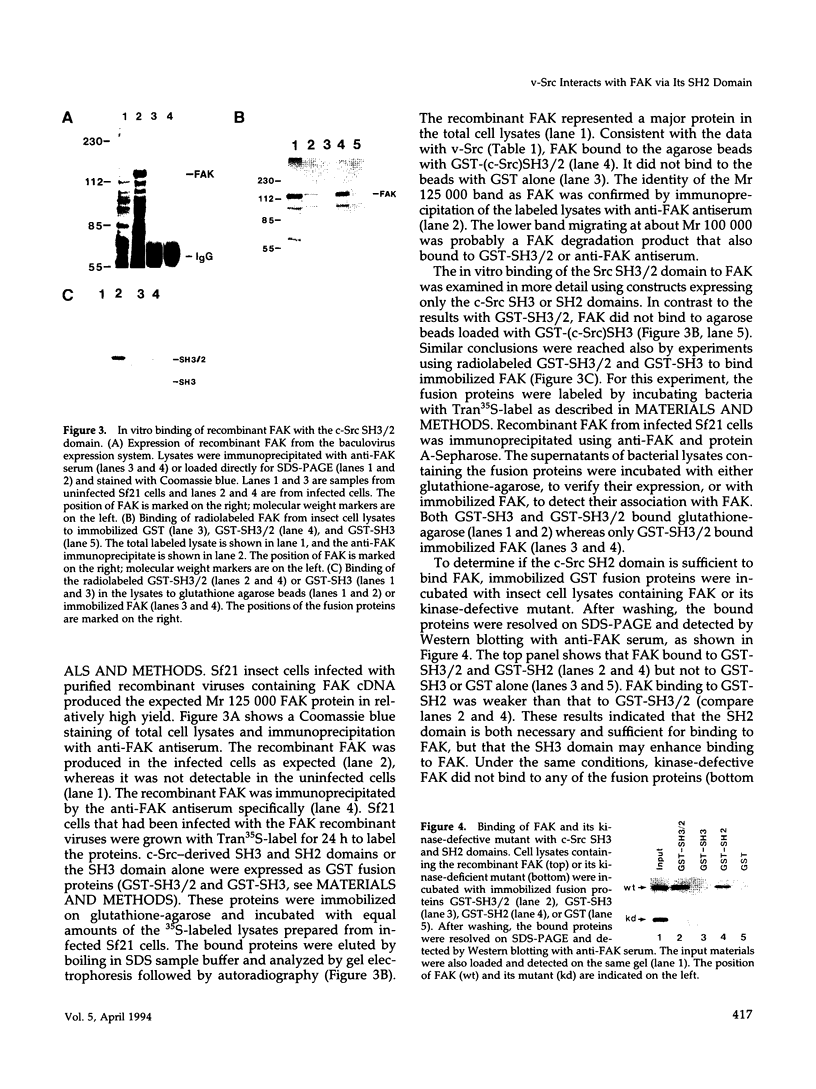


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André E., Becker-André M. Expression of an N-terminally truncated form of human focal adhesion kinase in brain. Biochem Biophys Res Commun. 1993 Jan 15;190(1):140–147. doi: 10.1006/bbrc.1993.1022. [DOI] [PubMed] [Google Scholar]
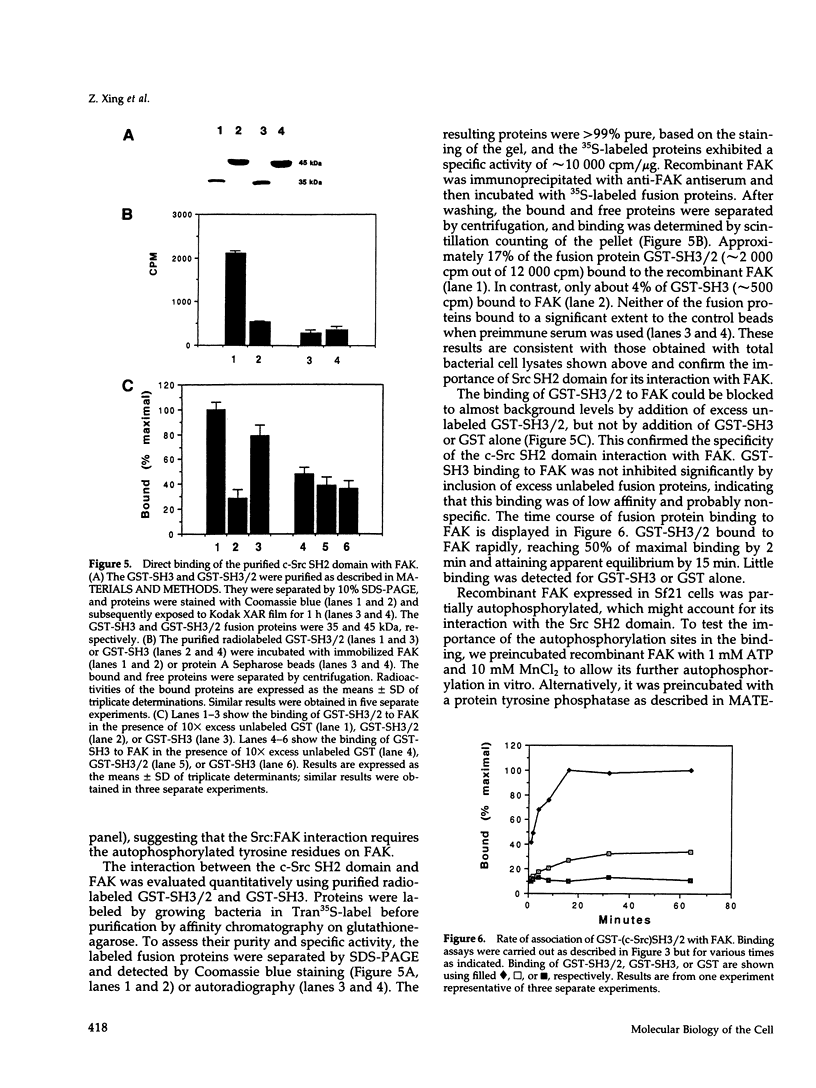
- Bibbins K. B., Boeuf H., Varmus H. E. Binding of the Src SH2 domain to phosphopeptides is determined by residues in both the SH2 domain and the phosphopeptides. Mol Cell Biol. 1993 Dec;13(12):7278–7287. doi: 10.1128/mcb.13.12.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockholt S. M., Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J Biol Chem. 1993 Jul 15;268(20):14565–14567. [PubMed] [Google Scholar]
- Brugge J. S., Jarosik G., Andersen J., Queral-Lustig A., Fedor-Chaiken M., Broach J. R. Expression of Rous sarcoma virus transforming protein pp60v-src in Saccharomyces cerevisiae cells. Mol Cell Biol. 1987 Jun;7(6):2180–2187. doi: 10.1128/mcb.7.6.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
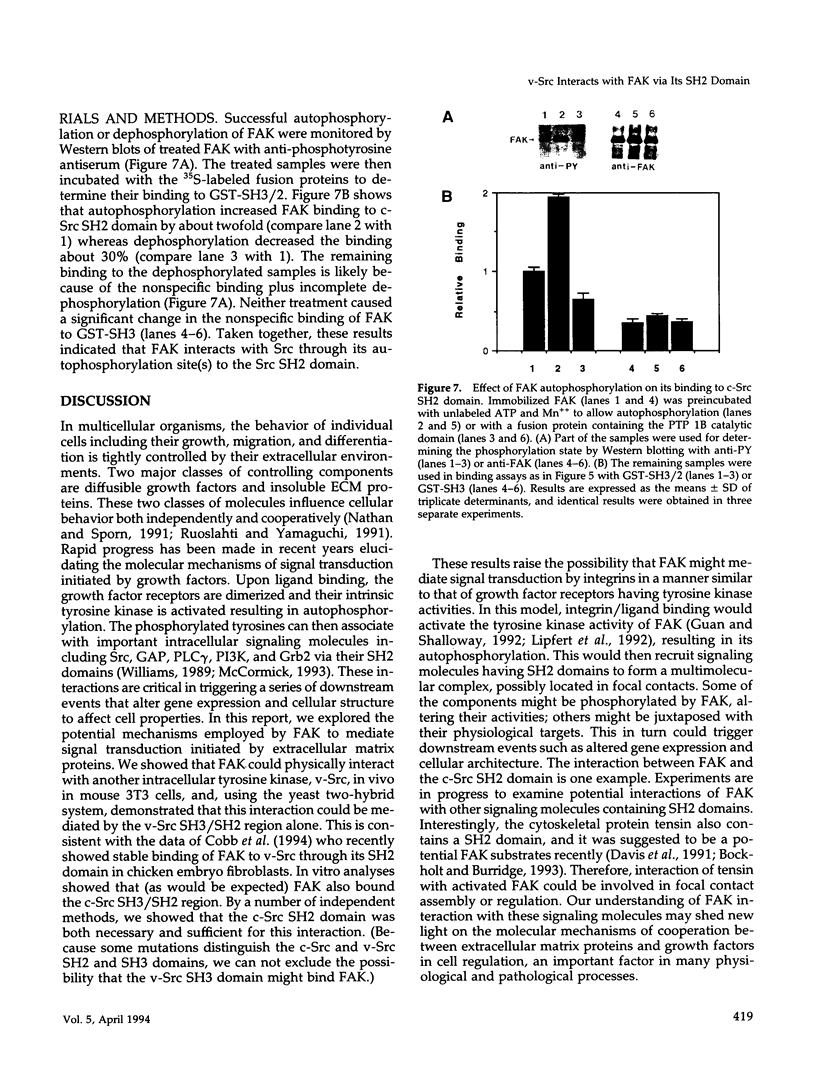
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Brugge J. S. Redistribution of activated pp60c-src to integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol Cell Biol. 1993 Mar;13(3):1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb B. S., Schaller M. D., Leu T. H., Parsons J. T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994 Jan;14(1):147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Davis S., Lu M. L., Lo S. H., Lin S., Butler J. A., Druker B. J., Roberts T. M., An Q., Chen L. B. Presence of an SH2 domain in the actin-binding protein tensin. Science. 1991 May 3;252(5006):712–715. doi: 10.1126/science.1708917. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J. D., Schaller M. D., Parsons J. T. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993 Nov;123(4):993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Coussens P. M., Danko A. V., Shalloway D. Overexpressed pp60c-src can induce focus formation without complete transformation of NIH 3T3 cells. Mol Cell Biol. 1985 May;5(5):1073–1083. doi: 10.1128/mcb.5.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Morrison D. K., Wong G., McCormick F., Williams L. T. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990 Apr 6;61(1):125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989 Sep 22;58(6):1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Song X. H. Bradykinin and bombesin rapidly stimulate tyrosine phosphorylation of a 120-kDa group of proteins in Swiss 3T3 cells. J Biol Chem. 1991 Apr 25;266(12):7746–7749. [PubMed] [Google Scholar]
- Lipfert L., Haimovich B., Schaller M. D., Cobb B. S., Parsons J. T., Brugge J. S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992 Nov;119(4):905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Bellot F., Honegger A. M., Ullrich A., Schlessinger J., Zilberstein A. Tyrosine kinase activity is essential for the association of phospholipase C-gamma with the epidermal growth factor receptor. Mol Cell Biol. 1990 Feb;10(2):435–441. doi: 10.1128/mcb.10.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel R. R., Brodeur S. R., Shalloway D., Laudano A. P. Selective binding of activated pp60c-src by an immobilized synthetic phosphopeptide modeled on the carboxyl terminus of pp60c-src. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10696–10700. doi: 10.1073/pnas.88.23.10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Parsons J. T. Focal adhesion kinase: an integrin-linked protein tyrosine kinase. Trends Cell Biol. 1993 Aug;3(8):258–262. doi: 10.1016/0962-8924(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Shalloway D. The cell cycle and c-Src. Curr Opin Genet Dev. 1993 Feb;3(1):26–34. doi: 10.1016/s0959-437x(05)80337-5. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992 Dec 11;71(6):891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. Identification of a novel tyrosine kinase as a major substrate. J Biol Chem. 1992 Sep 25;267(27):19031–19034. [PubMed] [Google Scholar]
- Zhu G., Decker S. J., Saltiel A. R. Direct analysis of the binding of Src-homology 2 domains of phospholipase C to the activated epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9559–9563. doi: 10.1073/pnas.89.20.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]