Abstract
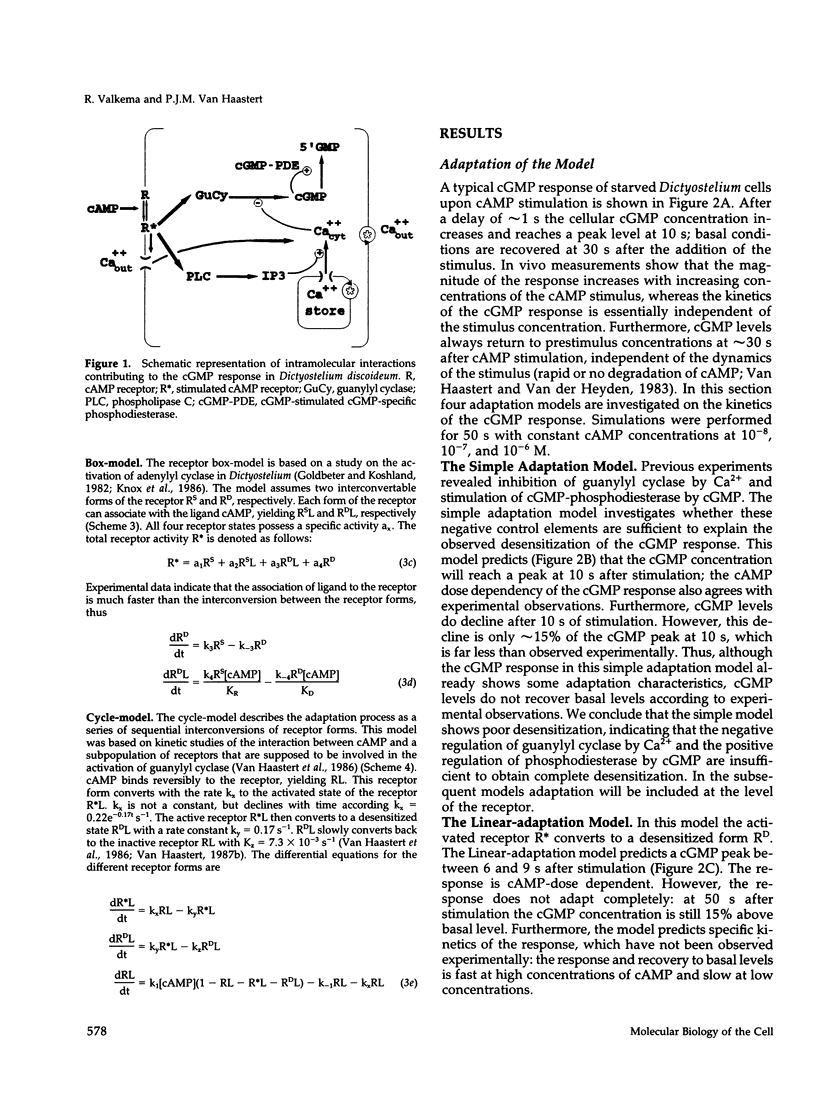
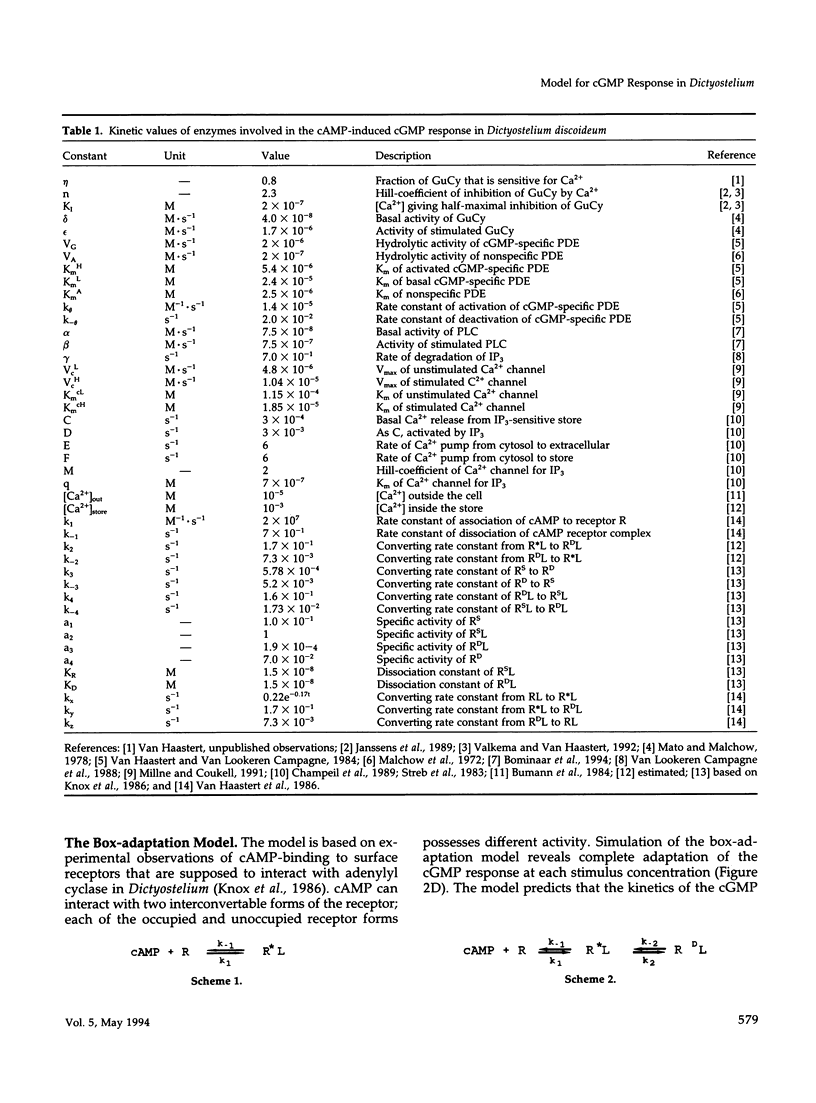
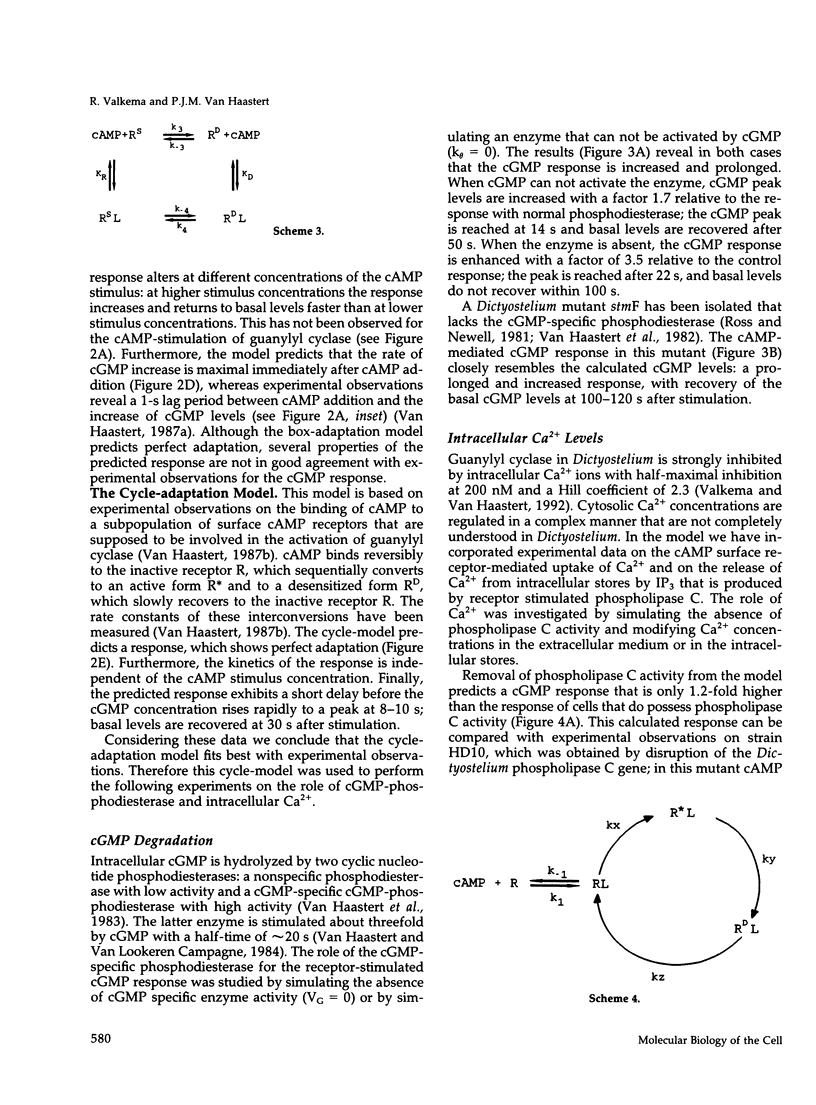
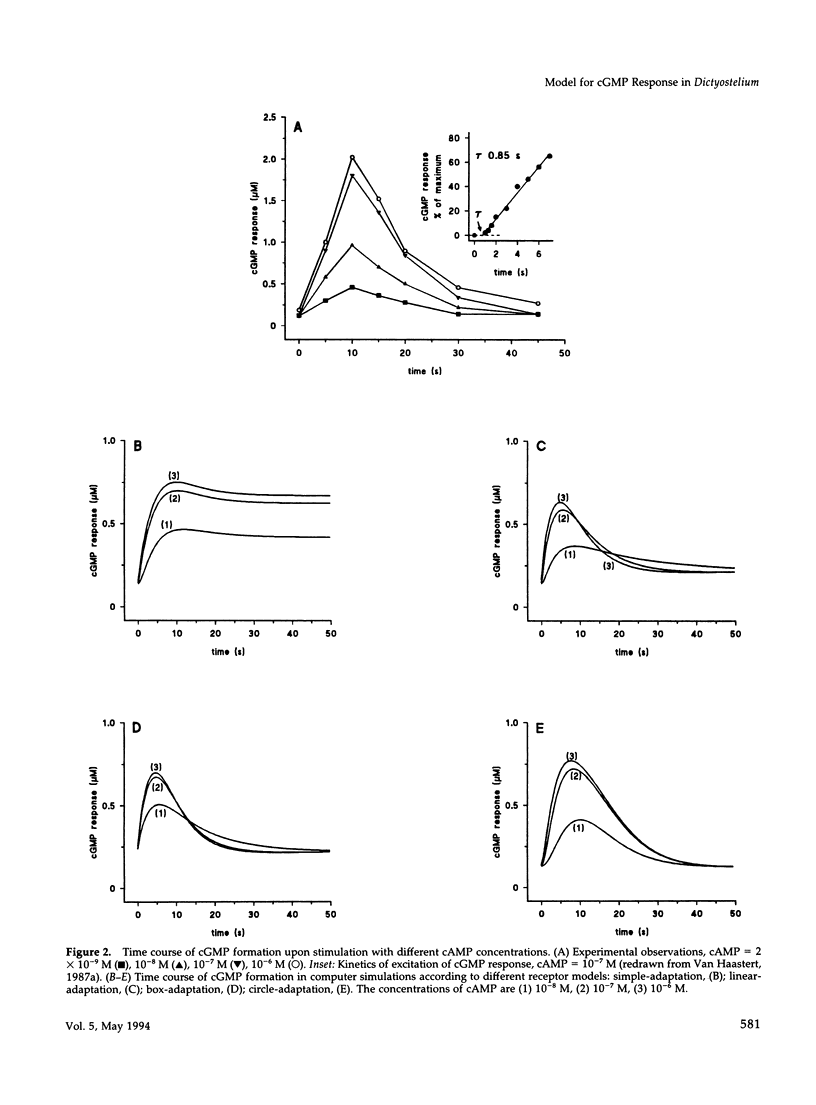
In Dictyostelium discoideum extracellular cyclic AMP (cAMP), as shown by previous studies, induces a transient accumulation of intracellular cyclic guanosine-5'-monophosphate (cGMP), which peaks at 10 s and recovers basal levels at 30 s after stimulation, even with persistent cAMP stimulation. Additional investigations have shown that the cAMP-mediated cGMP response is built up from surface cAMP receptor-mediated activation of guanylyl cyclase and hydrolysis of cGMP by phosphodiesterase. The regulation of these activities was measured in detail on a seconds time-scale, demonstrating complex adaptation of the receptor, allosteric activation of cGMP-phosphodiesterase by cGMP, and potent inhibition of guanylyl cyclase by Ca2+. In this paper we present a computer model that combines all experimental data on the cGMP response. The model is used to investigate the contribution of each structural and regulatory component in the final cGMP response. Four models for the activation and adaptation of the receptor are compared with experimental observations. Only one model describes the magnitude and kinetics of the response accurately. The effect of Ca2+ on the cGMP response is simulated by changing the Ca2+ concentrations outside the cell (Ca2+ influx) and in stores (IP3-mediated release) and changing phospholipase C activity. The simulations show that Ca2+ mainly determines the magnitude of the cGMP accumulation; simulations are in good agreement with experiments on the effect of Ca2+ in electropermeabilized cells. Finally, when cGMP-phosphodiesterase activity is deleted from the model, the simulated cGMP response is elevated and prolonged, which is in close agreement with the experimental observations in mutant stmF that lacks this enzyme activity. We conclude that the computer model provides a good description of the observed response, suggesting that the main structural and regulatory components have been identified.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Maeda Y., Iijima T. Transient increase of the intracellular Ca2+ concentration during chemotactic signal transduction in Dictyostelium discoideum cells. Differentiation. 1988 Dec;39(2):90–96. doi: 10.1111/j.1432-0436.1988.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Alcantara F., Monk M. Signal propagation during aggregation in the slime mould Dictyostelium discoideum. J Gen Microbiol. 1974 Dec;85(2):321–334. doi: 10.1099/00221287-85-2-321. [DOI] [PubMed] [Google Scholar]
- Bominaar A. A., Kesbeke F., Van Haastert P. J. Phospholipase C in Dictyostelium discoideum. Cyclic AMP surface receptor and G-protein-regulated activity in vitro. Biochem J. 1994 Jan 1;297(Pt 1):181–187. doi: 10.1042/bj2970181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumann J., Wurster B., Malchow D. Attractant-induced changes and oscillations of the extracellular Ca++ concentration in suspensions of differentiating Dictyostelium cells. J Cell Biol. 1984 Jan;98(1):173–178. doi: 10.1083/jcb.98.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champeil P., Combettes L., Berthon B., Doucet E., Orlowski S., Claret M. Fast kinetics of calcium release induced by myo-inositol trisphosphate in permeabilized rat hepatocytes. J Biol Chem. 1989 Oct 25;264(30):17665–17673. [PubMed] [Google Scholar]
- Chang Y. Y. Cyclic 3',5'-adenosine monophosphate phosphodiesterase produced by the slime mold Dictyostelium discoideum. Science. 1968 Jul 5;161(3836):57–59. doi: 10.1126/science.161.3836.57. [DOI] [PubMed] [Google Scholar]
- Drayer A. L., Van der Kaay J., Mayr G. W., Van Haastert P. J. Role of phospholipase C in Dictyostelium: formation of inositol 1,4,5-trisphosphate and normal development in cells lacking phospholipase C activity. EMBO J. 1994 Apr 1;13(7):1601–1609. doi: 10.1002/j.1460-2075.1994.tb06423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Cyclic AMP stimulates accumulation of inositol trisphosphate in Dictyostelium. J Cell Sci. 1987 Mar;87(Pt 2):221–229. doi: 10.1242/jcs.87.2.221. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Inositol 1,4,5-triphosphate induces calcium release from a non- mitochondrial pool in amoebae of Dictyostelium. Biochim Biophys Acta. 1986 Aug 1;887(3):335–340. doi: 10.1016/0167-4889(86)90163-1. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Inositol 1,4,5-trisphosphate and calcium stimulate actin polymerization in Dictyostelium discoideum. J Cell Sci. 1986 Jun;82:41–51. doi: 10.1242/jcs.82.1.41. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Hülser D., Malchow D., Wick U. Cell communication by periodic cyclic-AMP pulses. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 6;272(915):181–192. doi: 10.1098/rstb.1975.0080. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Koshland D. E., Jr Simple molecular model for sensing and adaptation based on receptor modification with application to bacterial chemotaxis. J Mol Biol. 1982 Nov 5;161(3):395–416. doi: 10.1016/0022-2836(82)90246-7. [DOI] [PubMed] [Google Scholar]
- Green A. A., Newell P. C. Evidence for the existence of two types of cAMP binding sites in aggregating cells of Dictyostelium discoideum. Cell. 1975 Oct;6(2):129–136. doi: 10.1016/0092-8674(75)90003-3. [DOI] [PubMed] [Google Scholar]
- Henderson E. J. The cyclic adenosine 3':5'-monophosphate receptor of Dictyostelium discoideum. Binding characteristics of aggregation-competent cells and variation of binding levels during the life cycle. J Biol Chem. 1975 Jun 25;250(12):4730–4736. [PubMed] [Google Scholar]
- Janssens P. M., De Jong C. C., Vink A. A., Van Haastert P. J. Regulatory properties of magnesium-dependent guanylate cyclase in Dictyostelium discoideum membranes. J Biol Chem. 1989 Mar 15;264(8):4329–4335. [PubMed] [Google Scholar]
- Knox B. E., Devreotes P. N., Goldbeter A., Segel L. A. A molecular mechanism for sensory adaptation based on ligand-induced receptor modification. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2345–2349. doi: 10.1073/pnas.83.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konijn T. M. Cyclic AMP as a first messenger. Adv Cyclic Nucleotide Res. 1972;1:17–31. [PubMed] [Google Scholar]
- Konijn T. M., Van De Meene J. G., Bonner J. T., Barkley D. S. The acrasin activity of adenosine-3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama H., Ishida S., Van Haastert P. J. Non-chemotactic Dictyostelium discoideum mutants with altered cGMP signal transduction. J Cell Biol. 1993 Dec;123(6 Pt 1):1453–1462. doi: 10.1083/jcb.123.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Newell P. C. Evidence that cyclic GMP regulates myosin interaction with the cytoskeleton during chemotaxis of Dictyostelium. J Cell Sci. 1988 May;90(Pt 1):123–129. doi: 10.1242/jcs.90.1.123. [DOI] [PubMed] [Google Scholar]
- Malchow D., Böhme R., Rahmsdorf H. J. Regulation of phosphorylation of myosin heavy chain during the chemotactic response of Dictyostelium cells. Eur J Biochem. 1981 Jun;117(1):213–218. doi: 10.1111/j.1432-1033.1981.tb06324.x. [DOI] [PubMed] [Google Scholar]
- Malchow D., Gerisch G. Short-term binding and hydrolysis of cyclic 3':5'-adenosine monophosphate by aggregating Dictyostelium cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2423–2427. doi: 10.1073/pnas.71.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow D., Nägele B., Schwarz H., Gerisch G. Membrane-bound cyclic AMP phosphodiesterase in chemotactically responding cells of Dictyostelium discoideum. Eur J Biochem. 1972 Jun 23;28(1):136–142. doi: 10.1111/j.1432-1033.1972.tb01894.x. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Konijn T. M. Chemotaxis and binding of cyclic AMP in cellular slime molds. Biochim Biophys Acta. 1975 Apr 7;385(2):173–179. doi: 10.1016/0304-4165(75)90345-1. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Krens F. A., van Haastert P. J., Konijn T. M. 3':5'-cyclic AMP-dependent 3':5'-cyclic GMP accumulation in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2348–2351. doi: 10.1073/pnas.74.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato J. M., Malchow D. Guanylate cyclase activation in response to chemotactic stimulation in Dictyostelium discoideum. FEBS Lett. 1978 Jun 1;90(1):119–122. doi: 10.1016/0014-5793(78)80311-1. [DOI] [PubMed] [Google Scholar]
- McRobbie S. J., Newell P. C. Chemoattractant-mediated changes in cytoskeletal actin of cellular slime moulds. J Cell Sci. 1984 Jun;68:139–151. doi: 10.1242/jcs.68.1.139. [DOI] [PubMed] [Google Scholar]
- Milne J. L., Coukell M. B. A Ca2+ transport system associated with the plasma membrane of Dictyostelium discoideum is activated by different chemoattractant receptors. J Cell Biol. 1991 Jan;112(1):103–110. doi: 10.1083/jcb.112.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaichi K., Cubitt A. B., Pitt G. S., Firtel R. A. Amino acid substitutions in the Dictyostelium G alpha subunit G alpha 2 produce dominant negative phenotypes and inhibit the activation of adenylyl cyclase, guanylyl cyclase, and phospholipase C. Mol Biol Cell. 1992 Jul;3(7):735–747. doi: 10.1091/mbc.3.7.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P., Hall E. M., Bonner J. T. Folic acid as second chemotactic substance in the cellular slime moulds. Nat New Biol. 1972 Jun 7;237(75):181–182. doi: 10.1038/newbio237181a0. [DOI] [PubMed] [Google Scholar]
- Pannbacker R. G., Bravard L. J. Phosphodiesterase in Dictyostelium discoideum and the chemotactic response to cyclic adenosine monophosphate. Science. 1972 Mar 3;175(4025):1014–1015. doi: 10.1126/science.175.4025.1014. [DOI] [PubMed] [Google Scholar]
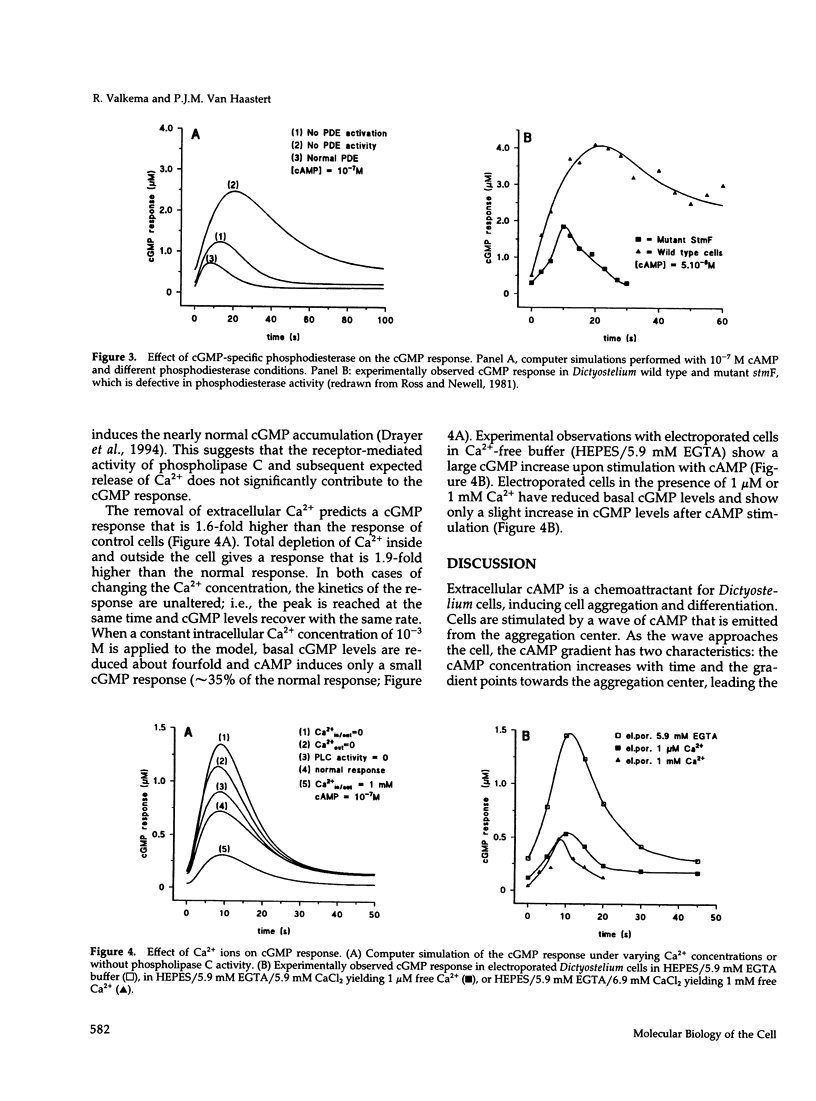
- Ross F. M., Newell P. C. Streamers: chemotactic mutants of Dictyostelium discoideum with altered cyclic GMP metabolism. J Gen Microbiol. 1981 Dec;127(2):339–350. doi: 10.1099/00221287-127-2-339. [DOI] [PubMed] [Google Scholar]
- Saran S., Nakao H., Tasaka M., Iida H., Tsuji F. I., Nanjundiah V., Takeuchi I. Intracellular free calcium level and its response to cAMP stimulation in developing Dictyostelium cells transformed with jellyfish apoaequorin cDNA. FEBS Lett. 1994 Jan 3;337(1):43–47. doi: 10.1016/0014-5793(94)80626-8. [DOI] [PubMed] [Google Scholar]
- Schaap P., Wang M. Interactions between adenosine and oscillatory cAMP signaling regulate size and pattern in Dictyostelium. Cell. 1986 Apr 11;45(1):137–144. doi: 10.1016/0092-8674(86)90545-3. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Valkema R., Van Haastert P. J. Inhibition of receptor-stimulated guanylyl cyclase by intracellular calcium ions in Dictyostelium cells. Biochem Biophys Res Commun. 1992 Jul 15;186(1):263–268. doi: 10.1016/s0006-291x(05)80802-2. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J., De Vries M. J., Penning L. C., Roovers E., Van der Kaay J., Erneux C., Van Lookeren Campagne M. M. Chemoattractant and guanosine 5'-[gamma-thio]triphosphate induce the accumulation of inositol 1,4,5-trisphosphate in Dictyostelium cells that are labelled with [3H]inositol by electroporation. Biochem J. 1989 Mar 1;258(2):577–586. doi: 10.1042/bj2580577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J. Differential effects of temperature on cAMP-induced excitation, adaptation, and deadaptation of adenylate and guanylate cyclase in Dictyostelium discoideum. J Cell Biol. 1987 Nov;105(5):2301–2306. doi: 10.1083/jcb.105.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J. Kinetics and concentration dependency of cAMP-induced desensitization of a subpopulation of surface cAMP receptors in Dictyostelium discoideum. Biochemistry. 1987 Nov 17;26(23):7518–7523. doi: 10.1021/bi00397a047. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J. Relationship between adaptation of the folic acid and the cAMP mediated cGMP response in Dictyostelium. Biochem Biophys Res Commun. 1983 Aug 30;115(1):130–136. doi: 10.1016/0006-291x(83)90979-8. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J. Sensory adaptation of Dictyostelium discoideum cells to chemotactic signals. J Cell Biol. 1983 Jun;96(6):1559–1565. doi: 10.1083/jcb.96.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J., Van Lookeren Campagne M. M. Transient kinetics of a cGMP-dependent cGMP-specific phosphodiesterase from Dictyostelium discoideum. J Cell Biol. 1984 Feb;98(2):709–716. doi: 10.1083/jcb.98.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J., Van der Heijden P. R. Excitation, adaptation, and deadaptation of the cAMP-mediated cGMP response in Dictyostelium discoideum. J Cell Biol. 1983 Feb;96(2):347–353. doi: 10.1083/jcb.96.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lookeren Campagne M. M., Erneux C., Van Eijk R., Van Haastert P. J. Two dephosphorylation pathways of inositol 1,4,5-trisphosphate in homogenates of the cellular slime mould Dictyostelium discoideum. Biochem J. 1988 Sep 1;254(2):343–350. doi: 10.1042/bj2540343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haastert P. J., de Wit R. J., Janssens P. M., Kesbeke F., DeGoede J. G-protein-mediated interconversions of cell-surface cAMP receptors and their involvement in excitation and desensitization of guanylate cyclase in Dictyostelium discoideum. J Biol Chem. 1986 May 25;261(15):6904–6911. [PubMed] [Google Scholar]
- van Haastert P. J., van Lookeren Campagne M. M., Ross F. M. Altered cGMP-phosphodiesterase activity in chemotactic mutants of Dictyostelium discoideum. FEBS Lett. 1982 Oct 18;147(2):149–152. doi: 10.1016/0014-5793(82)81029-6. [DOI] [PubMed] [Google Scholar]


