Abstract
The human adenovirus E4orf4 protein is toxic in both human tumor cells and Saccharomyces cerevisiae. Previous studies indicated that most of this toxicity is dependent on an interaction of E4orf4 protein with the B55 class of regulatory subunits of protein phosphatase 2A (PP2A) and in yeast with the B55 homolog Cdc55. We have found previously that E4orf4 inhibits PP2A activity against at least some substrates. In an attempt to understand the mechanism of this inhibition, we used a genetic approach to identify residues in the seven-bladed β-propeller proteins B55α and Cdc55 required for E4orf4 binding. In both cases, amino-terminal polypeptides composed only of blade 1 and at least part of blade 2 were found to bind E4orf4 and overexpression blocked E4orf4 toxicity in yeast. Furthermore, certain amino acid substitutions in blades 1 and 2 within full-length B55α and Cdc55 resulted in loss of E4orf4 binding. Recent mutational analysis has suggested that segments of blades 1 and 2 present on the top face of B55α form part of the “substrate-binding groove.” Additionally, these segments are in close proximity to the catalytic C subunit of the PP2A holoenzyme. Thus, our results are consistent with the hypothesis that E4orf4 binding could affect the access of substrates, resulting in the failure to dephosphorylate some PP2A substrates.
Previous studies by our group and others showed that the 114-residue product of early region 4 of human adenoviruses, termed E4orf4, induces cell death in both human tumor cells and Saccharomyces cerevisiae (18-20, 22-27, 31, 38, 41-43). Cell death is p53 independent and resembles apoptosis in some human cancer cell lines (19, 20, 24, 36, 41); however, with human H1299 lung carcinoma cells, death is caspase independent and appears to occur by mitotic catastrophe following mitotic arrest (20, 22, 23), which we believe may induce a variety of death processes, depending on the physiological state or type of cell. Induction of toxicity by E4orf4 in yeast is also associated with mitotic arrest (18, 38), and we have shown recently that this effect may result from premature activation of the APCCdc20 anaphase-promoting complex and inefficient progression through mitosis (31).
Early studies showed that E4orf4 associates with protein phosphatase 2A (PP2A) holoenzymes through interactions with the B55α regulatory subunit (17). E4orf4 also interacts with Cdc55, the yeast homolog of B55α (18, 31, 38). E4orf4 contains an arginine-rich sequence that can act as a potent nuclear and nucleolar targeting sequence (30) but which also is critical for binding to B55α (25, 42) and Cdc55 (31, 38). This interaction is required for E4orf4-mediated cell killing. In S. cerevisiae, deletion of CDC55 eliminates much of the E4orf4-induced loss of cell viability (18, 38). In human tumor cells, E4orf4 mutants that fail to bind B55α or Cdc55 (termed class I by our group) are defective in induction of cell death. Class II mutant E4orf4 proteins coimmunoprecipitate with B55α and Cdc55 but are defective for killing (25, 31, 38, 42). These results may indicate that association with B55α and Cdc55 is required but not sufficient to promote cell death. Alternatively, although class II mutants associate, they may fail to elicit the appropriate biological effect on PP2A due to imperfect binding. Recently we have found that binding of E4orf4 to B55α inhibits PP2A activity when measured in vitro against phosphoprotein substrates and that low levels of okadaic acid or expression of the PP2A peptide inhibitor I1PP2A actually enhance E4orf4-induced cell killing (22). Our current model is that E4orf4 binding may block the access of at least some substrates to B55α/Cdc55-containing PP2A holoenzymes, thus inducing cell death through the failure to dephosphorylate key targets, including those that regulate proper mitotic progression.
PP2A is the most abundant serine/threonine phosphatase, exhibiting pleiotropic activities affecting metabolism, RNA splicing, translation, morphogenesis, development, and cell cycle progression (9, 13, 21, 32, 47). The PP2A holoenzyme exists in multiple forms as a heterotrimer composed of a catalytic C subunit, an A subunit that functions as a scaffold for holoenzyme formation, and a B regulatory subunit (28). In mammalian cells, the A and C subunits, which make up the core enzyme, are ubiquitous, and unique genes encode two forms of each. The 65-kDa A subunit is composed of 15 imperfect HEAT repeats that mediate interactions with the C and B subunits (10, 12, 39, 40, 52). To date, more than 20 mammalian B subunits have been identified, composing three classes designated B (B55 or PR55), B′ (B56 or PR61), and B" (PR48/PR72/PR130), as well as striatin and SG2NA (B‴ or PR93/PR110). Intercellular localization is controlled by B subunits, and each determines a unique range of substrate specificities for the PP2A holoenzyme (4, 6, 16). Although little sequence homology exists between classes, the members of each class are highly related. B55 proteins all have molecular masses of about 55 kDa and include four members, B55α, B55β, B55γ, and B55δ. B55α and B55δ are ubiquitous, whereas the others are expressed more selectively in certain cell types (29, 34, 49, 55). Although an earlier report suggested that E4orf4 may interact with members of the B′ class (43), our recent studies showed that E4orf4 binding could only be detected with the four members of the B55 class of PP2A regulatory subunits (22). Figure 1A shows the amino acid sequence of human B55α, which contains seven WD40 repeats and, like Bγ (50), all B55 members thus are seven-bladed β-propeller proteins (33, 45). Each propeller blade is composed of four anti-parallel β strands (a, b, c, and d) where the semiconserved WD (FQ, WK, WH, CD, WD, FD, and FQ in B55α) of the WD40 motif is located at the end of β strand c.
FIG. 1.
Sequence alignment of B55α, Cdc55, and Met30-37. (A) The amino acid sequences of PP2A B55α, and Cdc55 were aligned in relation to the crystal structure of B55α (53). Highlighted residues correspond to amino acid similarities or identities between Β55α and Cdc55. The positions (or proposed positions in the case of Cdc55) of β strands within blades 1 to 7 (e.g., β1a, etc.), the β-hairpin loops (b1 to b4), and the β-hairpin arm are indicated above the sequences. (B) The sequence of Met30-37 is shown aligned with those within blades 1 and 2 of Β55α and Cdc55 with homologous residues or regions indicated.
PP2A of S. cerevisiae greatly resembles the mammalian enzyme with respect to organization, subunit amino acid sequence, substrate specificity, and sensitivity to inhibitors (13). The catalytic C subunit is encoded by two highly homologous genes, PPH21 and PPH22 (37, 46). TPD3 encodes the A subunit, which has a structure similar to that of mammalian A subunits (51). Only two B-type regulatory subunits exist that are encoded by CDC55 and RTS1, which are highly related to the mammalian B/B55 and B′/B56 families, respectively (11, 44). As noted, we and others have shown that the toxicity of E4orf4 depends on interactions only with Cdc55 (1, 18, 31, 38). The interaction of E4orf4 with Cdc55 is necessary and sufficient to cause the coimmunoprecipitation of the PP2A A and C subunits, as is the case with B55α, indicating that E4orf4 can interact with PP2A holoenzyme complexes. Figure 1A shows the sequence of Cdc55 aligned with that of B55α and demonstrates considerable similarity or identity in critical parts of these proteins.
The work in this report was completed prior to the knowledge of detailed structural information; however, the publication of the crystal structure of the PP2A holoenzyme containing B55α (53) has now provided a much more meaningful interpretation of our results. Because of the high degree of homology, here we refer to regions in Cdc55 as “proposed” blades or β strands based on the known structure of B55α (53).
We show here that the interaction of E4orf4 with both Cdc55 and B55α involves residues within blades 1 and 2, a finding that is consistent with the idea that E4orf4 binding may block the dephosphorylation of at least some PP2A substrates.
MATERIALS AND METHODS
Cell line.
Human H1299 (p53−/−) cells (ATCC CRL-5803) were cultured in Falcon tissue culture dishes in α-minimal essential medium (Bio-Whittaker) supplemented with 10% fetal bovine serum (Bio-Whittaker), 100 U of penicillin and streptomycin per ml, and 0.292 mg of l-glutamine per ml.
Yeast strains.
For some studies, yeast strains included the cdc55::kanMX4 mutant, its isogenic wild-type strain SEY6210 (MATα leu2-3,112 ura3-52 his3-Δ200 lys2-801 trp1-Δ901 suc2-Δ9) (2), strain W303-1A (MATa ade2-1 can1-100 trp1-1 ura3-1 his3-11,15 leu2-3,112), strain L40 [MATα his3-Δ200 trp1-Δ901 leu2-3,112 ade2 LYS2::(4lexAop-HIS3) URA3::(8lexAop-LacZ) GAL4], strain Y34 (MATα ade2-1 ura3-1 his3-11 leu2-3,112 SWE1:MYC12) (54), and the cdc55::kanMX2 mutant strain in a BY4741 background (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0). Yeast culture media were prepared and yeast genetic manipulations were performed using standard techniques.
DNA plasmids.
The construction of DNA plasmids pcDNA3-HA-E4orf4 and pcDNA3-FLAG-PP2AB55α for mammalian cell expression has been described previously (25). Throughout, we have used cDNAs expressing rat B55α, which is identical in amino acid sequence to human B55α, except for the conservative change of valine at residue 310 in place of isoleucine. A similar approach was used to generate pcDNA3-HA-PP2AB55α. To make pcDNA3-GFP-PP2AB55α, the enhanced green fluorescent protein (GFP) sequence was excised from pEGFP-C1 (Clontech) using PCR with Vent DNA polymerase with the introduction of flanking HindIII (5′) and BamHI (3′) sites, and the digested PCR product was cloned into pcDNA3 (Invitrogen), producing pcDNA3-GFP. Then full-length PP2A B55α was subcloned into this vector with BamHI and SmaI restriction digestion. B55α point mutant alanine substitutions were generated by PCR using PfuTurbo DNA polymerase (Stratagene) with plasmid pcDNA-HA-PP2AB55α or pcDNA-GFP-PP2AB55α as the template and primers introducing the appropriate mutations. The identities of all mutants were confirmed by DNA sequencing.
Yeast and bacterial expression plasmids were constructed as follows. pEG202-CDC55, which expresses Cdc55 of S. cerevisiae, was generated in two steps. First, the carboxy-terminal EcoRI/XhoI fragment of the CDC55 gene from vector pcDNA3-FLAG-CDC55 was inserted into the corresponding sites of pEG202. The remaining part of the CDC55 gene was amplified by PCR and subsequently subcloned into pEG202-CDC55. Cdc55 was expressed as a LexA-Cdc55 fusion protein under the control of the ADH promoter. Plasmid pJG4-5-E4orf4, which expresses a hemagglutinin (HA)-E4orf4 fusion product, was generated by ligating the NcoI (blunted with Klenow)-and-XhoI E4orf4 fragment from pcDNA3-HA-E4orf4 into the EcoRI (blunted with Klenow) and XhoI sites of vector pJG4-5. E4orf4 was expressed under the control of the GAL1 promoter as a fusion product with the B42 transcriptional activation domain. pYES2-HA-E4orf4 was constructed by subcloning the KpnI/XbaI HA-E4orf4 fragment from plasmid pcDNA3-HA-E4orf4 into the corresponding sites of pYES2. HA-E4orf4 expression was controlled by the GAL1 promoter. pTRG-MET30-37, which expresses a 37-residue fragment of yeast MET30 (residues 344 to 380 containing predicted propeller blade 2), was generated in two steps. First, two oligonucleotides were designed to encode a 37-residue fragment of yeast MET30 that was isolated through a yeast multicopy suppressor screen. These oligonucleotides, containing EcoRI and BamHI sites at the ends, were annealed and then subcloned into the pcDNA3-FLAG vector. The KpnI (blunted with T4 DNA polymerase)-and-XhoI FLAG-MET30-37 fragment was subcloned into the BamHI (blunted with Klenow) and XhoI sites on pTRG. All pTRG-derived plasmids express an RNA polymerase α (RNAP-α) fusion protein. pTRG-B55α(1-124), which encodes residues 1 to 124 of B55α, was constructed by subcloning the KpnI (blunted with T4 DNA polymerase)-and-XhoI fragment from the pcDNA3-FLAG-PP2AB55α vector into the BamHI (blunted with Klenow) and XhoI sites of the pTRG vector. The resulting vector expresses the amino-terminal 124 amino acids of B55α containing the first two WD40 repeats fused to RNAP-α. pTRG-B55α, which expresses full-length rat PP2A B55α, was generated by inserting the BstEII/XhoI fragment from the pcDNA3-FLAG-PP2AB55α vector into pTRG-B55 (1-124) to express full-length Bα fused to RNAP-α.
Random mutagenesis of yeast CDC55 by error-prone PCR.
Random mutagenesis of the S. cerevisiae CDC55 gene was carried out by error-prone PCR. Briefly, CDC55 was cloned into the pEG202 vector and expressed as a LexA-Cdc55 fusion product. This plasmid was used as a PCR template. Two PCR primers were used to amplify CDC55 from plasmid pEG202-CDC55. The primer sequences were CCAATTGTCGTAGATCTTCG (forward) and GTCTAACTCCTTCCTTTTCG (reverse), and recombinant Taq DNA polymerase was used (Pharmacia). To increase the mutation frequency, different concentrations of MgCl2 and MnCl2 were employed in the PCR buffer. Eight PCRs were prepared with MgCl2 and MnCl2 concentrations, respectively, of 1.5 mM and 2.0 mM, 1.5 mM and 1.25 mM, 1.5 mM and 2.5 mM, 1.5 mM and 3.75 mM, 1.5 mM and 5 mM, 2.75 mM and 2.5 mM, 3.8 mM and 3.75 mM, and 5 mM and 5 mM. To each reaction mixture, 10 U of Taq DNA polymerase, 4 ng of plasmid template, and a 100 pM concentration of each primer were added. After denaturing at 94°C for 2 min, 30 cycles of 94°C for 1 min, 45°C for 1.3 min, and 72°C for 2 min were run, ending with 72°C for 10 min. The eight PCR products were then pooled and purified (Qiagen PCR purification kit). The Cdc55 coding sequences in PCR products were flanked with 87 bp at the 5′ end and 85 bp at the 3′ end of the plasmid DNA sequence. The flanking sequences in the PCR product were designed for in vivo recombination.
Coimmunoprecipitation assay.
Yeast strain BY4741 cdc55::kanMX2 was cotransformed (5) with either the empty pYES2 vector or pYES2 expressing HA-E4orf4, together with one of the following amino-terminally FLAG-tagged Cdc55 point mutant forms subcloned into the p425GAL1 DNA vector (ATCC), created by site-directed mutagenesis: Cdc55 (T42P), Cdc55 (S191A), Cdc55 (M250T), and Cdc55 (S191A/M250T). Cells were grown overnight in 2% glucose-containing synthetic complete medium, transferred to 2% raffinose medium for 2 h, and finally resuspended in 2% galactose-2% raffinose medium for 6 h to induce both Cdc55 point mutations and E4orf4 expression. Cells were lysed with yeast lysis buffer, whole-cell extracts were prepared, and coimmunoprecipitation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blotting were performed as previously described (31).
In mammalian cells, extracts prepared in buffer (50 mM Tris-HCl [pH 7.5], 250 mM NaCl, 5 mM EDTA, 0.1% Triton X-100, 0.5% NP-40, 1 μg/ml aprotinin, 1 μg/ml phenylmethylsulfonyl fluoride) were clarified by centrifugation at 13,000 × g for 10 min, and then immunoprecipitations were carried out as described previously (25). Mouse anti-HA monoclonal antibody 16B12 or 12CA5 (BabCo) was used to recognize the HA tag.
Screening for Cdc55 mutants defective for E4orf4 binding and toxicity.
cdc55 yeast cells were first transformed with pJG4-5-E4orf4 plasmid DNA expressing E4orf4 driven by the GAL1 promoter and grown in a large volume and then transformed with Cdc55-specific PCR products prepared as described above and EcoRI/BamHI-linearized plasmid pEG202. In vivo recombination was achieved by growing yeast on selection medium containing 2% glucose. Colonies were replicated on plates with selection medium supplemented with 2% raffinose and 2% galactose. As E4orf4 expression is induced by galactose, colonies that grew must express Cdc55 mutant products that are defective for reconstituting E4orf4-induced loss of cell viability in cdc55 mutant cells. Such mutant Cdc55 products could either contain point mutations or represent truncated forms of Cdc55. To eliminate the latter, the colonies were replicated on plates with medium containing 300 nM rapamycin. Therefore, colonies growing on rapamycin plates must express truncated or otherwise functionally defective forms of Cdc55. The colonies that did not grow were selected, and plasmid DNAs were prepared. CDC55 mutations were identified by DNA sequencing.
Multicopy suppressor screening of yeast genomic DNA for E4orf4-induced cell toxicity.
To screen for sequences that suppress E4orf4-induced cell toxicity, a yeast genomic DNA library was cloned into pJG4-5 expression vectors and the DNA was used to transform yeast strain W303-1A. E4orf4-induced toxicity was enhanced by coexpression of wild-type CDC55. The plasmids, expressing Cdc55 as a LexA fusion product under the control of the ADH promoter, and E4orf4 driven by the GAL1 promoter, were first transformed into yeast strain W303-1A. Transformed yeast cells were allowed to grow in medium supplemented with 2% glucose in a large volume and further transformed with the pJG4-5-based yeast genomic library expressed as fusion products with the B42 transcriptional activation domain fusion protein. Such cells were allowed to grow on selection medium containing 2% raffinose and 2% galactose. Colonies that grew must express suppressors of E4orf4-induced cell toxicity. Plasmids were rescued from these resistant colonies and then sequenced.
Competitive binding between E4orf4 and fragments of Cdc55 propeller blades.
Locations of WD40 repeats were predicted on SwissProt, and individual propeller blades within Cdc55 were determined. The following structural mutant forms of Cdc55 were constructed with an amino-terminal FLAG tag in the p425GAL1 DNA vector: blade 1, blade 1-2, blade 3-5, blade 1-5, blade 1Δ, blade 2Δ, and blade 3-5Δ. These constructs were cotransformed into the Y34 yeast strain with either the empty pYES2 vector or pYES2 with HA-E4orf4 subcloned. Culturing of these cells and cell growth assays were performed as previously described (31).
Bacterial two-hybrid assay.
The bacterial two-hybrid interaction assay was principally carried out according to the manufacturer's protocol (Stratagene); however, some modifications were added to the protocol. Briefly, we first transformed the bacterial reporter strain with bait vector pBT and target vector pTRG and selected for transformants on plates containing tetracycline, chloramphenicol, and kanamycin. The colonies were then streaked onto plates containing different concentrations of ampicillin to demonstrate protein-protein interactions.
RESULTS
Generation of S. cerevisiae Cdc55 mutants defective for E4orf4 binding and toxicity.
As little was known about the detailed structure of either B55α or Cdc55 at the time we commenced, we took advantage of yeast genetics to identify residues important in the binding of E4orf4 to Cdc55. E4orf4-induced loss of viability is low in cdc55 mutant yeast strains; however, reconstitution with wild-type CDC55 restores full E4orf4 toxicity (38). Thus, for the initial studies, the yeast CDC55 gene was subjected to random mutagenesis by error-prone PCR-based techniques. PCR products were then cloned into a yeast two-hybrid bait vector, pEG202, that expresses a fusion product containing the LexA DNA-binding domain driven by the ADH promoter. E4orf4 coding sequences were inserted into a prey vector, pJG4-5, to express E4orf4 fused to the B42 transcriptional activation domain driven by the GAL1 promoter. The E4orf4 expression vector was first transformed into cdc55 mutant yeast (derived from an SEY6210 background), and then the randomly mutagenized Cdc55-expressing vector DNAs were introduced. Transformants were selected by growth on glucose selection medium and then replicated on plates containing raffinose and galactose to induce E4orf4 expression. Colonies that grew on these plates represented either Cdc55 point mutants that were resistant to E4orf4 toxicity or Cdc55 mutants (truncated forms or point mutants) that were functionally inactive, including those that failed to form functional holoenzymes. To eliminate the latter potentially extensive category of mutants, colonies were replicated on plates containing 300 nM rapamycin, as the toxicity of this drug requires a functional Cdc55 subunit. Therefore, any CDC55 mutant that reconstitutes rapamycin toxicity must be generally functional. Thus, although some mutants of potential interest may be lost, this approach provides a meaningful start to our genetic analysis of E4orf4 binding. A total of 12 such colonies were isolated, and the plasmid DNAs expressing these CDC55 mutants were rescued. To verify function, these mutants were transformed into cdc55 mutant yeast and tested for resistance to E4orf4 toxicity. Figure 2A (plates at the left) shows that although cdc55 mutant yeast reconstituted with wild-type CDC55 lost viability following the expression of E4orf4, all of the mutants were resistant to E4orf4 toxicity, as expected; however, when tested on plates containing 300 nM rapamycin, only two mutants (G9 and A12) reconstituted rapamycin toxicity (Fig. 2A, plates at the right). To determine if the products of mutant CDC55 genes bind to E4orf4, they were introduced into yeast strain L40 for two-hybrid interaction analysis. Figure 2B shows that all of the mutants interacted with E4orf4, except for mutants G9 and G8. Mutant G8 was nonfunctional and thus probably not informative; however, mutant G9, which did not bind to E4orf4 but complemented rapamycin toxicity, also did not support E4orf4-induced loss of cell viability, suggesting that the amino acid alterations in mutant G9, while affecting E4orf4 binding and toxicity, did not affect its role in rapamycin toxicity. Table 1 summarizes the mutations present in these CDC55 mutants following full sequence analysis, as well as their phenotypes. Two of these mutants appeared to be quite informative. First, mutant C10 contains a stop signal following residue 93 of Cdc55, thus explaining its failure to reconstitute sensitivity to rapamycin and E4orf4; however, the fact that it was able to bind to E4orf4 suggested that at least some interaction with E4orf4 can occur with a region composed only of the amino-terminal 93 residues of Cdc55 that included proposed β7d, all of blade 1, and β2a of blade 2. This mutant protein was designated Cdc55 (1-93) for further studies described below. Second, mutant G9 was found to contain two missense mutations and two silent mutations. The two missense mutations affected amino acids 42 (T42P; proposed β1b, see Fig. 1A) and 403 (S403R; within the proposed loop between β6c and β6d, see Fig. 1A). Although the importance of the S403R mutation remains unresolved, it seemed likely that the T42P alteration may play some role in loss of E4orf4 binding considering the results obtained with Cdc55 (1-93) just described.
FIG. 2.
CDC55 mutagenesis and analysis. (A) Identification of mutants. cDNAs expressing CDC55 mutants isolated from primary screening were retransformed into cdc55 mutant yeast along with a cDNA expressing HA-E4orf4. Transformants were first selected on the plates containing selection medium supplemented with 2% glucose, and growing colonies were streaked onto the plates with medium supplemented with 2% raffinose and 2% galactose to assess E4orf4 killing (left) or onto plates also containing 300 nM rapamycin to assess rapamycin sensitivity (right). The mutants are described in detail in Table 1. (B) Interaction of E4orf4 with yeast CDC55 mutants using the yeast two-hybrid assay. Yeast strain L40 were cotransformed with cDNAs expressing the indicated LexA-Cdc55 fusion products and with vector DNA expressing E4orf4 fused to a transcriptional activation domain. Transformed yeast was streaked onto nonselective plates (+histidine) and selection plates (−histidine) and allowed to grow at 30°C for 3 days. The mutants are described in detail in Table 1. V, parental vector pEG202; WT, wild-type CDC55.
TABLE 1.
Cdc55 mutants and their phenotypesa
| Mutant | Binding to E4orf4 | Complementation of E4orf4 killing | Complementation of rapamycin killing | Mutations |
|---|---|---|---|---|
| A3 | Yes | No | No | A112G (T36A), T307C (F101L), A703C (T233P), T909 (Del.), A1196T (N397I) |
| A8 | Yes | No | No | G49A (G15S), A428G (D141G), T785C (L260S), T1450G, C1452T (S479L), 1558G (Ins.) |
| A12 | Yes | No | Yes | C80T (A25V), A321G, A617G (D204G), A646G (I214V), A914G (N303S), A1133G (Y376C), G1552A (A516T) |
| B12 | Yes | No | No | T312C, A412T (K136Stop), 850T (Ins.), A902G, A904G, A1197G, C1445 (Del.) |
| C10 | Yes | No | No | T65C (I20T), T209C (F68S), A228G, C286T (R94Stop), A434C, G454T, T551C, G573A, T581C, A684T, A936G, A989T, A1532G, A1540G |
| D6 | Yes | No | No | T210 (Del.), T626G, A648G, A823C, C824G, A829 (Del.), T1238C, A1558G |
| D9 | Yes | No | No | T158A (L51H), A1159G (N385D), A1223G (N406S) |
| F3 | Yes | No | No | T67A, T474 (Del.), T1225C, A1562G |
| G4 | Yes | No | No | A191G (Y62C), A251G (E82G), A322G (N106D), G397T (G131W) |
| G8 | No | No | No | A429G (D141), 610G (Ins.), T787C |
| G9 | No | No | Yes | A130C (T42P), T429C, A798G, A1213C (S403R) |
| G12 | Yes | No | No | T308A (F101Y), A359G (K118R), C579T (S191L), T756C (M250T) |
Listed are the Cdc55 mutants isolated and characterized as described in Fig. 2 to 4 in terms of binding to E4orf4, complementation of E4orf4 killing, and reconstitution of sensitivity to rapamycin in cdc55 mutant strains. Each was fully sequenced, and the changes from the wild-type sequence are indicated in the rightmost column, which lists the nucleotides and amino acids affected (e.g., T36A) and, when appropriate, the type of change, such as a deletion (Del.) or insertion (Ins.).
To examine this possibility more directly, a series of cDNA constructs was made that expressed wild-type FLAG-Cdc55 and, by site-directed mutagenesis, FLAG-Cdc55 (T42P), FLAG-Cdc55 (S191A), FLAG-Cdc55 (M250T), and FLAG-Cdc55 (S191A/M250T) that harbored alterations in proposed β1b, β3a, β4b, and β3a/β4b, respectively. Yeast strain BY4741 cdc55 cells were transformed with these cDNAs along with a cDNA expressing HA-E4orf4, and cell extracts were immunoprecipitated with anti-FLAG antibodies and then subjected to Western blotting using anti-HA antibodies to detect the coimmunoprecipitation of E4orf4 with the various Cdc55 proteins. Reciprocal coimmunoprecipitation was also done. Figure 3 shows that all of the Cdc55 mutant species interacted efficiently with E4orf4, except Cdc55 (T42P). These results indicated that the T42P mutation alone was sufficient to prevent binding of E4orf4, thus implicating β1b of Cdc55 in this interaction.
FIG. 3.
Analysis of E4orf4 binding by Cdc55 point mutants. BY4741 cdc55 mutant yeast was cotransformed with DNA vectors expressing FLAG-tagged Cdc55 proteins containing amino acid substitutions generated by site-directed mutagenesis and HA-E4orf4. Cell extracts were immunoprecipitated (IP) using either anti-FLAG (upper panels) or anti-HA (lower panels) antibodies and examined by Western blotting to examine E4orf4 binding. The mutants are indicated.
Effect of Cdc55 fragments on E4orf4 toxicity.
We next determined if expression of Cdc55 (1-93) could block E4orf4-induced toxicity in wild-type yeast. Wild-type yeast cells (SEY6210) were cotransformed with cDNAs expressing mutant C10/Cdc55 (1-93) and E4orf4, and Fig. 4 shows that expression of this portion of Cdc55 blocked E4orf4-induced loss of viability (plate at the left) but had no effect on rapamycin toxicity when these cells were plated in the presence of this drug (plate at the right). Figure 4 also shows that another mutant, D9 with point mutations in proposed β1b and β6c, that bound to E4orf4 but failed to reconstitute rapamycin sensitivity (Table 1) also blocked E4orf4 toxicity in wild-type yeast and had little effect on rapamycin toxicity in these cells. Previous studies with human tumor cells indicated that E4orf4 cell killing is highly dose dependent (22). Thus, it is likely that binding of E4orf4 to Cdc55 (1-93) or nonfunctional D9 mutant Cdc55 protein was sufficient to reduce interactions with wild-type Cdc55 to lessen toxicity.
FIG. 4.
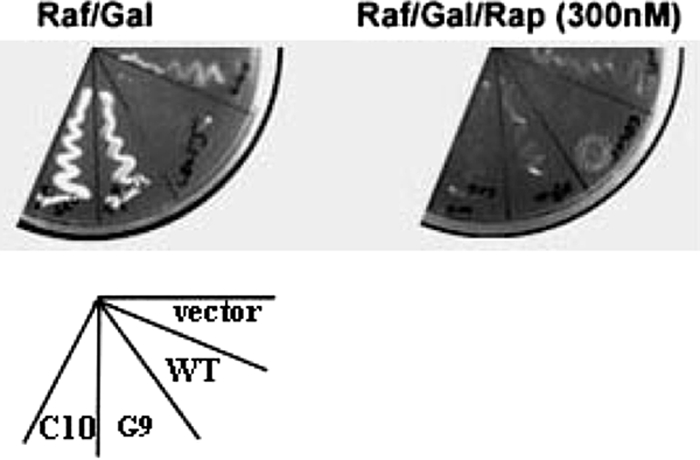
Suppression of E4orf4 killing of wild-type yeast by CDC55 mutants. Yeast strain SEY6210 cells were transformed with vector DNAs expressing Cdc55 mutant protein C10 or D9 and E4orf4 fused to a transcriptional activation domain driven by the GAL1 promoter. Transformed yeast cells were selected on plates with medium supplemented with 2% glucose. Colonies were then streaked onto plates with medium supplemented with 2% raffinose (Raf) and 2% galactose (Gal) to activate E4orf4 expression in the absence (left) or presence (right) of 300 nM rapamycin (Rap).
Multicopy suppressor screens of yeast.
Following our finding that expression of Cdc55 (1-93) reduced E4orf4 toxicity in yeast, we undertook multicopy suppressor screens to identify other yeast polypeptides that might exhibit similar properties. Identification of such species might provide further insights into E4orf4-binding sequences. We have found previously that, for reasons that remain to be determined, overexpression of Cdc55 in wild-type yeast strains enhances E4orf4 toxicity (D. E. Roopchand and P. E. Branton, unpublished results). Thus, a yeast DNA library driven by the GAL1 promoter was introduced into wild-type strain W303-1A yeast that had been pretransformed with plasmid DNAs expressing Cdc55 driven by the ADH promoter and E4orf4 driven by the GAL1 promoter. From about 2 million transformed colonies, 4 E4orf4-resistant colonies were identified. Following rescue and reanalysis, only one plasmid DNA was confirmed to block E4orf4 toxicity by overexpression in wild-type yeast (Fig. 5A). Sequence analysis showed that this DNA encoded a 37-amino-acid fragment of yeast Met30. Met30 is an F-box protein that also possesses seven WD40 repeats and is involved in sulfur metabolism, protein ubiquitination, and perhaps other functions (7, 8, 14, 15, 35). Figure 1B shows that some amino acid homology was apparent between this fragment, which maps within the Met30 WD40 repeat, specifically, predicted β2b-β2c (3) and the predicted sequence of blade 1, and perhaps even more with that of blade 2 of Cdc55 and B55α. Thus, it was possible that, like Cdc55 (1-93), this Met30 fragment might block E4orf4 toxicity by binding E4orf4 to sequester it and thus diminish its interaction with Cdc55.
FIG. 5.

Analysis of E4orf4 binding to the Met30 fragment and an amino-terminal B55α polypeptide. (A) Expression of the yeast Met30 fragment blocks killing by E4orf4. Yeast strain W303-1A previously transformed with pYES2-HA-E4orf4 and pEG202-CDC55 was transformed by vector DNA expressing the 37-residue Met30 fragment. Transformed yeast cells were streaked onto glucose plates or raffinose (Raf) and galactose (Gal) plates and allowed to grow at 30°C for 5 days. Sectors are indicated. (B) Bacterial two-hybrid analysis of E4orf4 interactions with fragments of Met30 and B55α and full-length B55α. Bacterial two-hybrid reporter strain XL1-Blue MRF′KAN was cotransformed with DNAs expressing a pTRG RNAP-α fusion product with the Met30 fragment, B55α (1-124), or full-length B55α, with E4orf4 fused to lambda-cl DNA-binding protein. Transformed cells were first grown on non-ampicillin-containing plates and then streaked onto plates supplemented with 125 μg/ml ampicillin. Cells were allowed to grow at 37°C for 24 h. LGF2, pBT-LGF2 interaction control plasmid encoding the dimerization domain (40 amino acids) of the Gal4 transcriptional activator protein; Gal11, pTRG-GAL11 interaction control plasmid encoding a domain (90 amino acids) of the mutant form of the Gal11 protein; Orf4, pBT-E4orf4; Met30, pTRG-MET30-37 expressing the yeast MET30 fragment; B55 (1-124), pTRG-B55α (1-124) expressing amino acids 1 to 124 of mammalian PP2A B55α; B55, pTRG-B55α expressing full-length mammalian PP2A B55α. No vector, contains just the reporter strain without plasmid transformation.
E4orf4 binds to the Met30 fragment and an amino-terminal fragment of B55α.
To confirm that this Met30 fragment binds to E4orf4, a bacterial two-hybrid assay was employed. In addition, to determine if a similar region of B55α could, like Cdc55 (1-93), also interact with E4orf4, cDNAs were prepared that encoded full-length B55α or an amino-terminal fragment containing residues 1 to 124 (β7d, all of blade 1, and β2a-c up to the hairpin arm) of B55α. Figure 5B shows by two-hybrid analysis that all three of these species interacted with E4orf4, suggesting that E4orf4 interacts with comparable regions in both Cdc55 and mammalian B55α. In an attempt to demonstrate that these fragments of Met30 and B55α interact with E4orf4 in human cells, additional immunoprecipitation and Western blotting were conducted with human H1299 cells. Although significant levels of both of the fragment species could be detected in these cells, we were not able to demonstrate reproducibly the coimmunoprecipitation of E4orf4 (data not shown). It is possible that the interaction with E4orf4 may be either transient or at too low an affinity to be preserved under the immunoprecipitation conditions employed.
Analysis of E4orf4 binding to various portions of Cdc55.
Thus far, the data suggested that E4orf4 binding appeared to involve residues present in proposed blade 1 and possibly blade 2 of Cdc55, as well as B55α. These findings were somewhat surprising, as it was not expected that fragments of such propeller proteins would fold properly into a conformation resembling that present in the full-length protein. Nevertheless, cDNAs were generated that expressed a range of FLAG-tagged versions of Cdc55 that contained various combinations of proposed Cdc55 blades. These were tested for binding to HA-E4orf4 in coimmunoprecipitation assays conducted with yeast cell extracts; however, no further insights into E4orf4 binding were obtained (data not shown). We also examined the ability of these Cdc55 polypeptides to protect against E4orf4 toxicity following overexpression in wild-type yeast. Figure 6 shows that only expression of the blade 1-2 fragment relieved E4orf4-induced loss of viability. An additional observation unrelated to E4orf4 killing was the fact that overexpression of full-length Cdc55 alone caused a significant suppression of cell growth. This finding has also been reported by others (48), and at present, this phenomenon is not understood.
FIG. 6.
Inhibition of E4orf4 toxicity by overexpression of various portions of Cdc55. Constructs expressing various portions of Cdc55 or the Cdc55 (T42P) mutant protein, as indicated, were prepared and cotransformed into wild-type yeast cells with either a vector (V) control or plasmid DNA containing HA-E4orf4. Yeast was serially diluted and spotted onto solid medium containing glucose (left) or medium containing raffinose and galactose (right) to induce E4orf4 expression.
Identification of residues in B55α required for E4orf4 binding.
As blades 1 and 2 of B55α appear able to interact with E4orf4 and in Cdc55 an amino acid substitution in proposed β1b of Cdc55 eliminated E4orf4 binding, a series of point mutations in conserved residues in the sequence encoding B55α was generated by site-directed mutagenesis. Because of the sequence similarity to the Met30 polypeptide (see Fig. 1B) found to bind E4orf4, alterations in conserved amino acids present in β1b and β2b and following loops of B55α were included. Figure 7A shows that most of the mutant B55α proteins interacted with E4orf4 as efficiently as wild-type B55α, including T45A (β1b), T114A (β2b), D47A (β1b), D116A (loop 2b-c), K48A (loop 1b-c), and K117A (loop 2b-c); however, L43A (β1b) and L112A (β2b) both interacted at reduced levels with E4orf4, and L43A/L112A (β1b/β2b) was almost totally defective for binding (Fig. 7A and B). These results suggested clearly that β1b and β2b play a role in E4orf4 binding. Thus, it seems possible that E4orf4 interacts with both B55α and Cdc55 using similar regions of blades 1 and 2.
FIG. 7.
Analysis of E4orf4 binding to B55α proteins containing amino acid substitutions. (A) H1299 cells cotransfected with plasmid vectors expressing FLAG-E4orf4 and HA-tagged B55α wild-type (WT) or mutant proteins. Equal amounts of cell extracts were immunoprecipitated (IP) by anti-HA antibody, followed by Western blotting using anti-FLAG antibody (top panels). Whole-cell extracts were immunoblotted using anti-FLAG (middle panels) or anti-HA (bottom panels) antibodies to reveal the expression of E4orf4 and B55α, respectively. (B) A study similar to that in panel A, except that GFP-tagged rather than HA-tagged B55α constructs were used and FLAG-E4orf4 was immunoprecipitated by anti-FLAG antibody, followed by Western blotting using anti-GFP antibody (top panel). Whole-cell extracts were immunoblotted using anti-GFP (middle panel) or anti-FLAG (bottom panel) antibodies to reveal the expression of B55α and E4orf4, respectively. Mutants are indicated.
DISCUSSION
The present results indicated that while interactions with other regions may occur, with both B55α and Cdc55, polypeptides containing blade 1 and at least a portion of blade 2 appeared to be sufficient to bind to the adenovirus E4orf4 protein and that alteration of individual residues present in these regions can limit such binding. The significance of these results became apparent upon examination of the recent crystal structure of the PP2A holoenzyme containing B55α (53) (Fig. 8). This structure revealed that the top face of the B55α seven-bladed β propeller possesses an acidic substrate-binding groove, whereas part of the bottom face interacts with the A subunit, predominantly through a β-hairpin arm formed by extended β strands β2c and β2d. The C subunit of the holoenzyme forms minimal interactions with B55α that are mediated via Leu87 (located on loop 1d-2a, which connects the first two blades). Shi and colleagues generated three amino acid substitutions at residues present in the proposed top face acidic substrate-binding groove of B55α that prevented the dephosphorylation (and thus binding) of the PP2A substrate Tau (53). These included E27R (loop preceding β1a), K48E (loop 1b-c), and D197K (loop 3b-c). We also examined the effects of altering Lys48; however, we generated a more conservative substitution (to alanine) and found it had no effect on E4orf4 binding. Figure 8 shows that Leu43 and Leu112, found to reduce E4orf4 binding when converted to alanine residues, were present in β1b and β2b in the interior of the B55α molecule. Thus, the loss of E4orf4 binding by this conservative substitution was perhaps surprising; however, this alteration could have produced effects on the spacing of binding sites present at the carboxy-terminal end of these β strands and following loops, which are present in the predicted substrate-binding groove. In Cdc55, we identified a T42P mutation, present probably in β1b, that totally eliminated E4orf4 binding. Alteration of the comparable Thr45 residue in B55α to alanine had no obvious effect on E4orf4 binding; however, the T42P mutation in Cdc55 might have introduced a much more dramatic change in conformation, as proline cannot accommodate the mandatory H bonds within the β sheet. It should be remembered, however, that the G9 mutant containing this and another substitution was able to complement cdc55 mutant yeast for sensitivity to rapamycin, suggesting that while affecting E4orf4 binding, it was otherwise functional.
FIG. 8.
Structure of the PP2A holoenzyme containing B55α. (A) Ribbon diagram of the holoenzyme, where the B55α subunit is colored from blue to red. The acidic groove on the top face of B55α is also shown. (B) Close-up view of the B55α subunit highlighting several residues which have been subjected to mutational studies.
We conducted a number of studies in which we attempted to examine E4orf4 binding to various portions of B55α and Cdc55 (data not shown). Apart from the amino-terminal regions, no significant binding was detected. As was the case with the Met30 fragment, it is possible that these amino-terminal polypeptides existed in a rather loose conformation that permitted E4orf4 binding. Indeed, the interaction appeared to be sufficient to relieve E4orf4-induced toxicity upon the overexpression of these polypeptides in wild-type yeast. Although not yet demonstrated in yeast, in human tumor cells, E4orf4 toxicity is dose dependent (22). Thus, the release from toxicity in these experiments seemed likely to occur due to the binding of E4orf4 to these fragments, thus reducing the levels available to bind to full-length Cdc55.
E4orf4 was also found to bind to a 37-amino-acid fragment of Met30 corresponding to predicted blade 2 of this seven WD40 repeat protein. Interestingly, the highest degree of homology between this region of Met30 and blade 1 of Cdc55 and B55α lies within the b β strand and the following loop (see Fig. 1B), again suggesting that these sequences may be of particular importance in binding. We considered that Met30 could represent another potential target for E4orf4; however, we now believe that such is not the case, as in further experiments we were unable to demonstrate that E4orf4 and full-length yeast Met30 proteins interact (D. E. Roopchand, M. Z. Mui, and P. E. Branton, unpublished results).
In summary our data suggest that E4orf4 appears to interact with residues in blades 1 and 2 of both B55α and Cdc55. Residues from these blades and from work with Tau residues in blade 3 contribute to a region of B55α located within the predicted acidic substrate-binding groove near the region of interaction with the catalytic C subunit loop (loop 1d-2a). Although the structure of Cdc55 has not yet been determined, the high degree of similarity or identity shared with B55α, especially in these regions (Fig. 1), supports the possibility that E4orf4 may bind very similarly to both proteins. Of further interest, an extended basic region in Tau has been implicated in binding to B55α (53). Previous genetic analysis showed that the region of E4orf4 involved in binding to both B55α and Cdc55 is a very basic arginine-rich stretch (25, 27, 30, 31), suggesting that this region may interact with at least a portion of the acidic substrate-binding groove.
Our previous studies suggested that E4orf4 binding reduced the activity of B55α-containing holoenzymes and that at high levels of E4orf4, toxicity may result from the failure to dephosphorylate key substrates involved in mitotic and cell cycle progression (22). We also proposed that this inhibition may result from the failure of such holoenzymes to interact with at least some of their normal substrates, and the present studies are certainly consistent with this hypothesis. We have no direct evidence that E4orf4 binds to the substrate-binding groove, and in fact, several other possibilities exist in which, for example, E4orf4 binding to another part of the B subunit might alter the conformation of the substrate-binding groove or the interaction with the catalytic C subunit to reduce phosphatase activity. Nevertheless, with the assistance of the detailed structure of B55α, it should now be possible to generate much more meaningful mutants that will allow us to map the E4orf4 binding site in greater detail and to assess the binding of various B55α substrates in the presence or absence of E4orf4. Such information might also suggest that E4orf4 could be used as a more general tool to identify B55-specific PP2A substrates.
ADDENDUM IN PROOF
A previous study generated a fairly extensive set of Cdc55 mutants and tested them in yeast for holoenzyme formation and E4orf4 protein binding (R. Koren, L. Rainis, and T. Kleinberger, J. Biol. Chem. 279:48598-48606, 2004). Although many of these contained multiple amino acid changes and thus were difficult to interpret, additional insights into E4orf4 binding may result from further analysis of some of the residues identified as affecting interactions with E4orf4 protein.
Acknowledgments
This work was supported by grants to P.E.B. from the Canadian Cancer Society through the National Cancer Institute of Canada, from the Canadian Institutes of Health Research (CIHR), from the Fonds de la recherché en santé du Québec (FRSQ), and from GeminX Biotechnologies Inc. (Montréal). M.Z.M. received support from a McGill University Faculty of Medicine internal studentship and a studentship from CIHR. D.E.R. and P.B. received studentships and fellowships from the FRSQ.
Footnotes
Published ahead of print on 3 November 2010.
REFERENCES
- 1.Afifi, R., R. Sharf, R. Shtrichman, and T. Kleinberger. 2001. Selection of apoptosis-deficient adenovirus E4orf4 mutants in Saccharomyces cerevisiae. J. Virol. 75:4444-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, J. L., and H. Bussey. 1993. The yeast KRE9 gene encodes an O glycoprotein involved in cell surface beta-glucan assembly. Mol. Cell. Biol. 13:6346-6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunson, L. E., C. Dixon, A. LeFebvre, L. Sun, and N. Mathias. 2005. Identification of residues in the WD-40 repeat motif of the F-box protein Met30p required for interaction with its substrate Met4p. Mol. Genet. Genomics 273:361-370. [DOI] [PubMed] [Google Scholar]
- 4.Cegielska, A., S. Shaffer, R. Derua, J. Goris, and D. M. Virshup. 1994. Different oligomeric forms of protein phosphatase 2A activate and inhibit simian virus 40 DNA replication. Mol. Cell. Biol. 14:4616-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D. C., B. C. Yang, and T. T. Kuo. 1992. One-step transformation of yeast in stationary phase. Curr. Genet. 21:83-84. [DOI] [PubMed] [Google Scholar]
- 6.Ferrigno, P., T. A. Langan, and P. Cohen. 1993. Protein phosphatase 2A1 is the major enzyme in vertebrate cell extracts that dephosphorylates several physiological substrates for cyclin-dependent protein kinases. Mol. Biol. Cell 4:669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles, S. Veronneau, S. Dow, A. Lucau-Danila, K. Anderson, B. Andre, A. P. Arkin, A. Astromoff, M. El-Bakkoury, R. Bangham, R. Benito, S. Brachat, S. Campanaro, M. Curtiss, K. Davis, A. Deutschbauer, K. D. Entian, P. Flaherty, F. Foury, D. J. Garfinkel, M. Gerstein, D. Gotte, U. Guldener, J. H. Hegemann, S. Hempel, Z. Herman, D. F. Jaramillo, D. E. Kelly, S. L. Kelly, P. Kotter, D. LaBonte, D. C. Lamb, N. Lan, H. Liang, H. Liao, L. Liu, C. Luo, M. Lussier, R. Mao, P. Menard, S. L. Ooi, J. L. Revuelta, C. J. Roberts, M. Rose, P. Ross-Macdonald, B. Scherens, G. Schimmack, B. Shafer, D. D. Shoemaker, S. Sookhai-Mahadeo, R. K. Storms, J. N. Strathern, G. Valle, M. Voet, G. Volckaert, C. Y. Wang, T. R. Ward, J. Wilhelmy, E. A. Winzeler, Y. Yang, G. Yen, E. Youngman, K. Yu, H. Bussey, J. D. Boeke, M. Snyder, P. Philippsen, R. W. Davis, and M. Johnston. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387-391. [DOI] [PubMed] [Google Scholar]
- 8.Goh, P. Y., and U. Surana. 1999. Cdc4, a protein required for the onset of S phase, serves an essential function during G(2)/M transition in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:5512-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg, Y. 1999. Protein phosphatase 2A: who shall regulate the regulator? Biochem. Pharmacol. 57:321-328. [DOI] [PubMed] [Google Scholar]
- 10.Groves, M. R., N. Hanlon, P. Turowski, B. A. Hemmings, and D. Barford. 1999. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell 96:99-110. [DOI] [PubMed] [Google Scholar]
- 11.Healy, A. M., S. Zolnierowicz, A. E. Stapleton, M. Goebl, A. A. DePaoli-Roach, and J. R. Pringle. 1991. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol. 11:5767-5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemmings, B. A., C. Adams-Pearson, F. Maurer, P. Muller, J. Goris, W. Merlevede, J. Hofsteenge, and S. R. Stone. 1990. Alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry 29:3166-3173. [DOI] [PubMed] [Google Scholar]
- 13.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353:417-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser, P., K. Flick, C. Wittenberg, and S. I. Reed. 2000. Regulation of transcription by ubiquitination without proteolysis: Cdc34/SCF(Met30)-mediated inactivation of the transcription factor Met4. Cell 102:303-314. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser, P., R. A. Sia, E. G. Bardes, D. J. Lew, and S. I. Reed. 1998. Cdc34 and the F-box protein Met30 are required for degradation of the Cdk-inhibitory kinase Swe1. Genes Dev. 12:2587-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamibayashi, C., R. Estes, R. L. Lickteig, S. I. Yang, C. Craft, and M. C. Mumby. 1994. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem. 269:20139-20148. [PubMed] [Google Scholar]
- 17.Kleinberger, T., and T. Shenk. 1993. Adenovirus E4orf4 protein binds to protein phosphatase 2A, and the complex down regulates E1A-enhanced junB transcription. J. Virol. 67:7556-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornitzer, D., R. Sharf, and T. Kleinberger. 2001. Adenovirus E4orf4 protein induces PP2A-dependent growth arrest in Saccharomyces cerevisiae and interacts with the anaphase-promoting complex/cyclosome. J. Cell Biol. 154:331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavoie, J. N., C. Champagne, M. C. Gingras, and A. Robert. 2000. Adenovirus E4 open reading frame 4-induced apoptosis involves dysregulation of Src family kinases. J. Cell Biol. 150:1037-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavoie, J. N., M. Nguyen, R. C. Marcellus, P. E. Branton, and G. C. Shore. 1998. E4orf4, a novel adenovirus death factor that induces p53-independent apoptosis by a pathway that is not inhibited by zVAD-fmk. J. Cell Biol. 140:637-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechward, K., S. Zolnierowicz, and B. A. Hemmings. 1999. Eukaryotic translation termination factor 1 associates with protein phosphatase 2A and targets it to ribosomes. Biochemistry (Mosc.) 64:1373-1381. [PubMed] [Google Scholar]
- 22.Li, S., C. Brignole, R. Marcellus, S. Thirlwell, O. Binda, M. J. McQuoid, D. Ashby, H. Chan, Z. Zhang, M. J. Miron, D. C. Pallas, and P. E. Branton. 2009. The adenovirus E4orf4 protein induces G2/M arrest and cell death by blocking protein phosphatase 2A activity regulated by the B55 subunit. J. Virol. 83:8340-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, S., A. Szymborski, M. J. Miron, R. Marcellus, O. Binda, J. N. Lavoie, and P. E. Branton. 2009. The adenovirus E4orf4 protein induces growth arrest and mitotic catastrophe in H1299 human lung carcinoma cells. Oncogene 28:390-400. [DOI] [PubMed] [Google Scholar]
- 24.Livne, A., R. Shtrichman, and T. Kleinberger. 2001. Caspase activation by adenovirus e4orf4 protein is cell line specific and is mediated by the death receptor pathway. J. Virol. 75:789-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcellus, R. C., H. Chan, D. Paquette, S. Thirlwell, D. Boivin, and P. E. Branton. 2000. Induction of p53-independent apoptosis by the adenovirus E4orf4 protein requires binding to the Balpha subunit of protein phosphatase 2A. J. Virol. 74:7869-7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcellus, R. C., J. N. Lavoie, D. Boivin, G. C. Shore, G. Ketner, and P. E. Branton. 1998. The early region 4 orf4 protein of human adenovirus type 5 induces p53-independent cell death by apoptosis. J. Virol. 72:7144-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcellus, R. C., J. G. Teodoro, T. Wu, D. E. Brough, G. Ketner, G. C. Shore, and P. E. Branton. 1996. Adenovirus type 5 early region 4 is responsible for E1A-induced p53-independent apoptosis. J. Virol. 70:6207-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin, M., R. Kettmann, and F. Dequiedt. 2010. Recent insights into protein phosphatase 2A structure and regulation: the reasons why PP2A is no longer considered as a lazy passive housekeeping enzyme. Biotechnol. Agron. Soc. Environ. 14:243-252. [Google Scholar]
- 29.Mayer, R. E., P. Hendrix, P. Cron, R. Matthies, S. R. Stone, J. Goris, W. Merlevede, J. Hofsteenge, and B. A. Hemmings. 1991. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry 30:3589-3597. [DOI] [PubMed] [Google Scholar]
- 30.Miron, M. J., I. E. Gallouzi, J. N. Lavoie, and P. E. Branton. 2004. Nuclear localization of the adenovirus E4orf4 protein is mediated through an arginine-rich motif and correlates with cell death. Oncogene 23:7458-7468. [DOI] [PubMed] [Google Scholar]
- 31.Mui, M. Z., D. E. Roopchand, M. S. Gentry, R. L. Hallberg, J. Vogel, and P. E. Branton. 2010. Adenovirus protein E4orf4 induces premature APCCdc20 activation in Saccharomyces cerevisiae by a protein phosphatase 2A-dependent mechanism. J. Virol. 84:4798-4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mumby, M. C., and G. Walter. 1993. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol. Rev. 73:673-699. [DOI] [PubMed] [Google Scholar]
- 33.Neer, E. J., C. J. Schmidt, R. Nambudripad, and T. F. Smith. 1994. The ancient regulatory-protein family of WD-repeat proteins. Nature 371:297-300. [DOI] [PubMed] [Google Scholar]
- 34.Pallas, D. C., W. Weller, S. Jaspers, T. B. Miller, W. S. Lane, and T. M. Roberts. 1992. The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J. Virol. 66:886-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patton, E. E., C. Peyraud, A. Rouillon, Y. Surdin-Kerjan, M. Tyers, and D. Thomas. 2000. SCF(Met30)-mediated control of the transcriptional activator Met4 is required for the G(1)-S transition. EMBO J. 19:1613-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert, A., M. J. Miron, C. Champagne, M. C. Gingras, P. E. Branton, and J. N. Lavoie. 2002. Distinct cell death pathways triggered by the adenovirus early region 4 ORF 4 protein. J. Cell Biol. 158:519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronne, H., M. Carlberg, G. Z. Hu, and J. O. Nehlin. 1991. Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol. Cell. Biol. 11:4876-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roopchand, D. E., J. M. Lee, S. Shahinian, D. Paquette, H. Bussey, and P. E. Branton. 2001. Toxicity of human adenovirus E4orf4 protein in Saccharomyces cerevisiae results from interactions with the Cdc55 regulatory B subunit of PP2A. Oncogene 20:5279-5290. [DOI] [PubMed] [Google Scholar]
- 39.Ruediger, R., M. Hentz, J. Fait, M. Mumby, and G. Walter. 1994. Molecular model of the A subunit of protein phosphatase 2A: interaction with other subunits and tumor antigens. J. Virol. 68:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruediger, R., D. Roeckel, J. Fait, A. Bergqvist, G. Magnusson, and G. Walter. 1992. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol. Cell. Biol. 12:4872-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shtrichman, R., and T. Kleinberger. 1998. Adenovirus type 5 E4 open reading frame 4 protein induces apoptosis in transformed cells. J. Virol. 72:2975-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shtrichman, R., R. Sharf, H. Barr, T. Dobner, and T. Kleinberger. 1999. Induction of apoptosis by adenovirus E4orf4 protein is specific to transformed cells and requires an interaction with protein phosphatase 2A. Proc. Natl. Acad. Sci. U. S. A. 96:10080-10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shtrichman, R., R. Sharf, and T. Kleinberger. 2000. Adenovirus E4orf4 protein interacts with both Balpha and B′ subunits of protein phosphatase 2A, but E4orf4-induced apoptosis is mediated only by the interaction with Balpha. Oncogene 19:3757-3765. [DOI] [PubMed] [Google Scholar]
- 44.Shu, Y., H. Yang, E. Hallberg, and R. Hallberg. 1997. Molecular genetic analysis of Rts1p, a B′ regulatory subunit of Saccharomyces cerevisiae protein phosphatase 2A. Mol. Cell. Biol. 17:3242-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, T. F., C. Gaitatzes, K. Saxena, and E. J. Neer. 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24:181-185. [DOI] [PubMed] [Google Scholar]
- 46.Sneddon, A. A., P. T. Cohen, and M. J. Stark. 1990. Saccharomyces cerevisiae protein phosphatase 2A performs an essential cellular function and is encoded by two genes. EMBO J. 9:4339-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stark, M. J. 1996. Yeast protein serine/threonine phosphatases: multiple roles and diverse regulation. Yeast 12:1647-1675. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson, L. F., B. K. Kennedy, and E. Harlow. 2001. A large-scale overexpression screen in Saccharomyces cerevisiae identifies previously uncharacterized cell cycle genes. Proc. Natl. Acad. Sci. U. S. A. 98:3946-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strack, S., D. Chang, J. A. Zaucha, R. J. Colbran, and B. E. Wadzinski. 1999. Cloning and characterization of B delta, a novel regulatory subunit of protein phosphatase 2A. FEBS Lett. 460:462-466. [DOI] [PubMed] [Google Scholar]
- 50.Strack, S., R. Ruediger, G. Walter, R. K. Dagda, C. A. Barwacz, and J. T. Cribbs. 2002. Protein phosphatase 2A holoenzyme assembly: identification of contacts between B-family regulatory and scaffolding A subunits. J. Biol. Chem. 277:20750-20755. [DOI] [PubMed] [Google Scholar]
- 51.van Zyl, W., W. Huang, A. A. Sneddon, M. Stark, S. Camier, M. Werner, C. Marck, A. Sentenac, and J. R. Broach. 1992. Inactivation of the protein phosphatase 2A regulatory subunit A results in morphological and transcriptional defects in Saccharomyces cerevisiae. Mol. Cell. Biol. 12:4946-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter, G., F. Ferre, O. Espiritu, and A. Carbone-Wiley. 1989. Molecular cloning and sequence of cDNA encoding polyoma medium tumor antigen-associated 61-kDa protein. Proc. Natl. Acad. Sci. U. S. A. 86:8669-8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, Y., Y. Chen, P. Zhang, P. D. Jeffrey, and Y. Shi. 2008. Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated Tau dephosphorylation. Mol. Cell 31:873-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, H., W. Jiang, M. Gentry, and R. L. Hallberg. 2000. Loss of a protein phosphatase 2A regulatory subunit (Cdc55p) elicits improper regulation of Swe1p degradation. Mol. Cell. Biol. 20:8143-8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zolnierowicz, S., C. Csortos, J. Bondor, A. Verin, M. C. Mumby, and A. A. DePaoli-Roach. 1994. Diversity in the regulatory B-subunits of protein phosphatase 2A: identification of a novel isoform highly expressed in brain. Biochemistry 33:11858-11867. [DOI] [PubMed] [Google Scholar]








