SUMMARY
To test whether severe ascorbic acid deficiency in macrophages affects progression of early atherosclerosis, we used fetal liver cell transplantation to generate atherosclerosis-prone apolipoprotein E-deficient (apoE−/−) mice that selectively lacked the ascorbate transporter (SVCT2) in hematopoietic cells, including macrophages. After 13-weeks of chow diet, apoE−/− mice lacking the SVCT2 in macrophages had surprisingly less aortic atherosclerosis, decreased lesion macrophage numbers, and increased macrophage apoptosis compared to control-transplanted mice. Serum lipid levels were similar in both groups. Peritoneal macrophages lacking the SVCT2 had undetectable ascorbate, increased susceptibility to H2O2-induced mitochondrial dysfunction and apoptosis, decreased expression of genes for COX2, IL1β, IL6, and decreased lipopolysaccharide-stimulated NF-κB and anti-apoptotic gene expression. These changes were associated with decreased expression of both the receptor for advanced glycation end products (RAGE) and HIF-1α, either or both of which could have been proximal causes of decreased macrophage activation and apoptosis in ascorbate-deficient macrophages.
Keywords: Antioxidants, atherosclerosis, macrophages, ascorbic acid, apolipoprotein E deficiency
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease characterized by infiltration of peripheral blood monocytes into the arterial intima, where they differentiate into macrophages [1]. These macrophages engulf modified lipoproteins such as oxidized low density lipoproteins (oxLDL) and become the foam cells that form the bulk of early atherosclerotic lesions. When activated by oxidized lipid, macrophages further intensify inflammatory responses and lesion progression by producing cytokines, oxygen radicals, proteases, and factors of the complement cascade [2]. The oxidative stress generated by macrophages in turn plays a key role in perpetuating the atherosclerotic process. It is plausible that antioxidants will decrease oxidative stress and thus might ameliorate atherosclerosis. However, clinical trials of supplements with antioxidants such as vitamin C (ascorbic acid) or vitamin E (α-tocopherol) have not shown benefits [3–5]. Demonstrating the converse, that low levels of the antioxidant vitamin worsen atherosclerosis, has been shown for vitamin E in mice [6, 7], but this is more difficult to prove for ascorbic acid, since most mammals can synthesize it from glucose.
It was reported many years ago that guinea pigs with early scurvy developed lesions in the proximal aorta that resembled those seen in atherosclerosis [8], and that these resolved with vitamin C replacement [9]. Guinea pigs, like humans, have lost the enzyme catalyzing the last step in ascorbate synthesis, gulonolactone oxidase (gulo). More recent studies by Maeda and colleagues showed that vitamin C deficiency in gulo−/− mice caused disruption of the endothelium and the elastic lamina in the aortic sinus, but did not induce lesions characteristic of human atherosclerosis [10]. A subsequent study from the same group involved gulo−/− mice that were also prone to atherosclerosis due to deletion of apolipoprotein E (apoE). Results of that study showed that low vitamin C intake and low systemic ascorbate levels (25–30% of normal) decreased lesion collagen content by 30%, but failed to modify progression of early aortic sinus lesions [11]. We recently reported that both combined vitamin C and vitamin E deficiency as well as a more severe but still partial systemic vitamin C deficiency alone did significantly increase atherosclerosis progression in apoE−/− mice [12].
Since the macrophage is the major cell type in these early lesions, it seems the best cell in which to establish the molecular mechanism by which cellular ascorbate deficiency affects atherosclerosis progression. Freshly prepared human monocytes have ascorbate concentrations of about 3 mM [13], or some 60-fold those in plasma. This steep gradient, which is likely generated by a high affinity energy- and sodium-dependent ascorbate transporter (slc23a2, SVCT2) [14], also persists in thjoglycollate-elicited mouse peritoneal macrophages. These cells also have initial intracellular ascorbate concentrations of about 3 mM, and retain ascorbate at concentrations of 2 mM after 3 days of culture in ascorbate-free media [15]. If this is also true for macrophages in aortic atherosclerosis, it could explain why severe systemic ascorbate deficiency was required to increase atherosclerosis in apoE−/−/gulo−/− mice. The SVCT2 gene has been knocked out in the mouse, and the resulting pups, even though capable of ascorbate synthesis, have very low levels of ascorbate in brain, adrenal, muscle, and other organs [16]. Unfortunately, they also die shortly after birth with respiratory failure and intra-parenchymal brain hemorrhage. To circumvent this obstacle and to obtain mice with very low levels of macrophage ascorbate, we used the approach of fetal liver cell (FLC) transplantation [17]. This allowed us to generate atherosclerosis-prone apoE−/− mice that lack SVCT2 in hematopoietic cells (SVCT2−/−), including monocytes destined to become macrophages. As expected, aortic sinus lesion macrophages lacked SVCT2 and thioglycollate-elicited peritoneal macrophages lacked both SVCT2 and ascorbate. Contrary to expectations, however, apoE−/− mice lacking the SVCT2 in macrophages and other hematopoietic cells had decreased atherosclerotic lesion size and fewer lesion macrophages compared to transplanted apoE−/− mice with normal macrophage SVCT2 expression. Failure of lesions to form and progress in mice lacking macrophage SVCT2 was due to increased macrophage apoptosis and decreased inflammatory activation, effects correlated with deactivation of HIF-1α and the RAGE/NF-κB pathway.
Materials and methods
SVCT2−/− FLC isolation and transplantation
SVCT2+/− mice were a generous gift from Dr. Robert Nussbaum (NIH, NHGRI) [16]. The mice, originally on the 129/SvEvTac background, were backcrossed over 9 generations to apoE−/− mice on the C57BL/6 background to generate SVCT2+/−/apoE−/− mice. To produce SVCT2−/−/apoE−/− embryos, female and male SVCT2+/−/apoE−/− mice were mated and pregnancy was determined by the presence of a vaginal plug. On day 14–16 of gestation, the pregnant mice were killed by cervical dislocation under isoflurane anesthesia and embryos were dissected free. The tails of embryos were taken for genotyping. Primers for the SVCT2 were CAT CTG TGC GTG CAT AGT AGC, CAC CGT GGC CCT CAT TG, TCT GAG CCC AGA AAG CGA AG, and GAT GGACGG CAT ACA AGT TC. Genotyping for apoE gene was carried out with primers designed by the Jackson laboratory (Bar Harbor, ME). Fetal gender was determined as described previously and cells from males were used for transplanting [17]. Isolated fetal liver cells were cryopreserved using Synth-a-freeze cryopreservation media (Catalog # R-005-50, Invitrogen, Carlsbad, CA). Eight week old male SVCT2+/+/apoE−/− recipient mice on C57BL/6 background (Jackson Laboratory, Bar Harbor, ME) were lethally irradiated (9 Gy) from a cesium gamma source. Four hours later, 5×106 FLCs were injected into the retro-orbital vein. After transplantation, the mice were placed on a rodent chow diet (catalog no. 5010; PMI Feeds Inc) for 13 weeks. These studies were approved by the Vanderbilt University Animal Care and Use Committee.
Serum Lipids and Lipoprotein Distribution Analysis
Serum total cholesterol and triglyceride levels were determined on samples obtained from mice fasted for 4 hours. Fast performance liquid chromatography was performed on an HPLC system model 600 (Waters Corporation, Milford, MA) using a Superose 6 column (Pharmacia, Piscataway, NJ).
Analysis of Aortic Lesions
Mice were killed under general anesthesia and 30 ml of saline was flushed through the left ventricle. The heart was removed, embedded in Tissue Tek OCT solution (100498-158, VWR Scientific, Suwanee, GA), and snap-frozen in liquid nitrogen. The entire aortas were dissected, pinned out “en face”, stained by Sudan IV, and lesion size analysis was carried out as described [18]. Cryosections of the proximal aorta of 10 μm thickness were prepared as described [18], starting at the aortic sinus and continuing distally according to the method of Paigen, et al. [19], adapted for computer analysis [20]. Aortic sections were stained with Sudan IV and counterstained with hematoxylin. Images of 15 sections of each aorta were captured on an Axioskop microscope (Carl Zeiss Inc., Thornwood, NY) and analyzed with an imaging program (KS 300, release 2.0, Kontron Electronik GmbH, Eching, Germany).
To detect macrophages, 5 μm serial cryosections of the proximal aorta were fixed in cold acetone and incubated with a rat antibody against mouse macrophage-derived foam cells, MOMA-2 (Accurate Chemical, Westbury, NY) as described [18]. Some sections were double stained using rat antibody MOMA-2 and rabbit antibodies to RAGE (Abcam Inc., Cambridge, MA) or SVCT2 (Santa Cruz Biotechnology, Santa Cruz, CA), then treated with secondary goat antibodies to rat and rabbit IgG labeled with Alexa Flour 488 and Alexa Flour 594 (both from Invitrogen, Carlsbad, CA). Photomicroscopy was assessed by an Olympus AX70 microscope with the DP72 camera.
To assess apoptosis in aortic lesions, serial 5-micron cryosections of the proximal aorta were fixed in 4% paraformaldehyde in PBS, treated with 3% citric acid and apoptotic cells were detected by a terminal deoxynucleotidyl transferase-mediated dUTP nick-end-labeling (TUNEL) technique using the In Situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN) as described [21]. TUNEL-positive (TUNEL+) cells were counted in 4 different sections from each aorta. The same approach was used to stain TUNEL+ peritoneal macrophages.
Assay of ascorbate in tissues and of ascorbate and GSH in peritoneal macrophages
Peripheral blood monocytes were isolated using density-gradient centrifugation of plasma with Histopaque-1077, collecting macrophages at the plasma-Hisopaque interface according to the manufacturer’s instructions (Sigma/Aldrich Chemical Co., St. Louis, MO). Thioglycollate-elicited peritoneal macrophages were isolated by peritoneal lavage and placed in culture as previously described [21] from mice reconstituted with either SVCT2+/+/apoE−/− or SVCT2−/−/apoE−/− FLCs. Ascorbate contents of peritoneal macrophages seeded in 6-well plates were determined by a microtiter plate assay [22]. Cells were covered with 100 μl of 25% metaphosphoric acid to lyse and extract, then 400μl water was added. Of the extract, 20 μl was placed in triplicate in a black opaque 96-well plate and 200 μl assay buffer (1M sodium acetate, pH 5.5, 1 mM Tempol, and 1mM o-phenylenediamine) was added. After 30 min in the dark, fluorescence was read at 345 nm excitation and 425 nm emission and compared to a standard curve. For assay of glutathione 1μl of the extract was assayed in similar plates with an assay buffer of 0.1M sodium phosphate buffer with 1mM o-phthaladehyde. After 30 min fluorescence was measured as for ascorbate and compared to a standard curve.
Assay of ascorbate transport in isolated peritoneal macrophages
This was performed in 96-well plates using 3 μM [1-14C]ascorbic acid (0.05 μCi, Perkin Elmer Life and Analytical Sciences, Inc., Boston, MA). At the times indicated, uptake was stopped and extracellular radioactivity removed by 3 rinses with room temperature phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate dibasic, 2 mM potassium phosphate, monobasic, pH 7.4). To extract radioactivity 40 μl of 0.1M NaOH was added with mixing and 20 μl of the extract was transferred to a 96-well plate for counting (Optiplate, Perkin Elmer, Waltham, MA,). Scintillation fluid (Microscint 20, Perkin Elmer, Waltham, MA) in a volume of 200 μl was added, the plate was shaken 10min in a plate shaker, and radioactivity was assayed (TopCount, Perkin Elmer, Waltham, MA).
Mitochondrial labeling and TUNEL staining of macrophages
Thioglycollate-elicited peritoneal macrophages were isolated from mice that had been reconstituted with either SVCT2+/+/apoE−/− or SVCT2−/−/apoE−/− FLCs. Two days later, macrophages were pre-incubated with serum-free Dulbecco’s modified essential media for 2 hours and treated with a fresh media containing different amounts of H2O2 (Sigma) for 16 hours. For labeling mitochondria, cells were incubated with 200 nM of the cell-permeable dye MitoTracker Red CMXRos (Invitrogen, Carlsbad, CA) for 20 min at 37 °C. The cells were then fixed with 4% paraformaldehyde-PBS and assessed an Olympus AX70 microscope with the DP72 camera. For TUNEL staining, apoptotic cells were detected using the In Situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN) as described previously [21]. TUNEL-positive (TUNEL+) cells were counted in 4–5 representative fields (containing ~ 1000 cells) of cultured macrophages.
Analysis of macrophage phagocytosis
Phagocytosis was assessed using FluoSpheres (carboxylate-modified microspheres, 2 μm, red fluorescence, Invitrogen # F8826). These microspheres were pre-coated in 1% solution of serum bovine albumin at pH 9.0. Peritoneal macrophages were serum-starved for 18 hours in RPMI medium and then treated with FluoSpheres for 1 hour at 37°C. Cells were vigorously washed 5 times in ice-cold PBS and fixed in 2% paraformaldehyde (Sigma-Aldrich Chemical Co., St. Louis, MO). Phagocytosis was assessed either directly with an Olympus AX70 microscope with a DP72 camera or by flow cytometry using a BD LSRFortessa cell analyzer (Becton, Dickinson and Company).
RNA Isolation and real-time PCR
Total RNA was isolated and relative quantification of the target mRNA was performed as described [21]. The gene expression assays (Applied Biosystems, Foster City, CA) were normalized with 18S ribosomal RNA or β-actin as an endogenous control.
Western blotting
Cells were lysed on ice with a lysis buffer (Cell Signaling Technology, Danvers, MA) containing protease (Sigma-Aldrich Chemical Co., St. Louis, MO) and phosphatase (Pierce Chemical Co., Rockford, IL) inhibitor cocktails. Protein concentrations were determined with the DC Protein assay kit (Bio-Rad Laboratories, Hercules, CA). Lysates (20 or 100 μg/lane) were resolved by NuPAGE Bis-Tris elecrophoresis (Invitrogen, Carlsbad, CA) and transferred onto nitrocellulose membranes (BioRad Laboratories, Hercules, CA). Blots were probed with a goat antibody to SVCT2, a rabbit antibody to β–actin (Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit antibody to HIF-1α (Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit anti- RAGE (Abcam, Cambridge, MA). Donkey anti goat and anti rabbit infrared labeled secondary antibodies were visualized and quantified using the Odyssey detection system (Li-Cor Biosciences, Lincoln, NE). Protein levels were quantified by densitometry with normalization for β-actin in the same lane.
Immunocytochemistry and double staining were performed using monoclonal rat antibody MOMA-2 (AbD Serotec, Raleigh, NC), rabbit antibodies to RAGE (Abcam, Waltham, MA) and SVCT2 (catalog #9926, Santa Cruz Biotechnology, Santa Cruz, CA) as described [23].
Statistical Analysis
Data are expressed as mean ± standard errors. Statistical differences in mean serum lipids and aortic lesion areas between the groups were determined by non-paired t-testing or by the Mann-Whitney test for non-parametric data.
RESULTS
Characterization of SVCT2+/+/apoE−/− mice expressing SVCT2−/− hematopoietic cells
Since SVCT2−/− pups die immediately after birth [16], we used the FLC transplantation approach [17]to generate apoE −/− chimeric mice with SVCT2−/− hematopoietic cells. Eight week old male apoE−/− mice were lethally irradiated and transplanted with male SVCT2+/+/apoE−/− (n = 18) or SVCT2−/−/apoE−/− (n = 19) FLCs. The recipient mice were fed a chow diet for 13 weeks. Survival of the two groups was similar during this period and the SVCT2−/− recipients did not show signs of scurvy (lethargy, poor grooming, and hunched posture). Further, body weight was not different between the two groups (Table 1). Since weight loss is an early sign of scurvy in mice [10], these results support the clinical observations that the SVCT2−/− FLC recipients did not have scurvy. As expected, plasma cholesterol was markedly elevated and triglycerides were modestly elevated, but both were similar in the two groups (Table 1). Whereas brain cortex ascorbate contents were similar in each group, spleen ascorbate contents were decreased to 30% of controls in spleens of SVCT2−/− FLC recipients (Table 1). Since spleen has a much higher content of hematopoietic cells than brain, these results suggest that (a) spleen was reconstituted with FLC-derived cells and (b) that SVCT2 is needed in to maintain normal ascorbate levels in the spleen.
Table 1.
Body weights, lipid levels, and ascorbate contents1
| SVCT2+/+ FLC recipients | SVCT2−/− FLC recipients | |
|---|---|---|
| Weight (grams) | 26 ± 0.5 | 25 ± 0.6 |
| Cholesterol (mg/dl) | 496 ± 30 | 486 ± 28 |
| Triglycerides (mg/dl) | 213 ± 21 | 188 ± 13 |
| Serum ascorbate (μM) | 75 ± 23 | 37 ± 4* |
| Brain ascorbate (μmol/g wet wt) | 3.8 ± 0.6 | 4.0 ± 0.9 |
| Spleen ascorbate (μmol/g wet wt) | 4.5 ± 1.3 | 1.5 ± 1.6* |
Results are shown from at least 13 mice for each gender and treatment group.
An asterisk indicates p < 0.05 compared to SVCT2+/+ FLC recipients.
Transplantation of FLCs lacking the SVCT2 suppresses early atherosclerosis
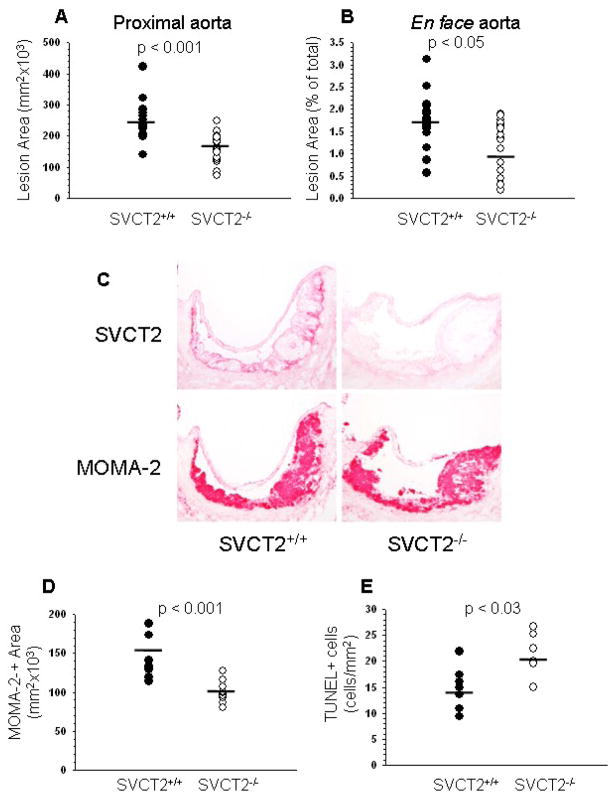
Quantitative analysis of cross sections of the aortic sinus showed that apoE−/− mice reconstituted with SVCT2−/−/apoE−/− FLCs had significantly smaller (42%) lesions compared to mice transplanted with SVCT2+/+/apoE−/− FLCs (145876 ± 13314 μm2vs. 251957 ± 13804 μm2; p < 0.001; Fig. 1A). Similarly, en face analysis of distal atherosclerotic lesions in pinned out aortas stained with Sudan IV revealed that apoE−/− mice reconstituted with SVCT2−/− FLCs had a 25% decrease in distal atherosclerosis compared to control apoE−/− mice transplanted with SVCT2+/+ FLCs (1.25 ± 0.13% vs. 1.67 ± 0.14%, respectively; p < 0.036; Fig. 1B).
Figure 1.
Atherosclerosis, SVCT2 expression and apoptosis in mouse atherosclerotic lesions. (A, B) Image analysis data obtained from serial sections of aortic sinus (A) and en face aorta (B) of mice reconstituted with apoE−/− FLCs either expressing the SVCT2 (SVCT2+/+, n = 18) or lacking it (SVCT2−/−, n = 19). (C) Immunostaining of sequential random aortic sinus sections for the SVCT2 (top panels) and macrophages (MOMA-2, bottom panels) in apoE−/− mice transplanted with SVCT2+/+ or SVCT2−/− FLCs. (D) Image analysis of MOMA-2 immunostaining from mice transplanted with SVCT2+/+ FLCs or mice transplanted with SVCT2−/− FLCs. (E) Image analysis of TUNEL-positive cells in proximal aortic lesions from mice transplanted with SVCT2+/+ FLCs or from mice transplanted with SVCT2−/− FLCs. In A, B, D and E, bars indicate mean values.
Deficiency of the SVCT2 in hematopoietic cells decreases macrophage number and increases macrophage apoptosis in aortic lesions
Mice that received SVCT2+/+ FLCs expressed SVCT2 in aortic sinus lesions, with most intense staining of cells at the base of the lesion (Fig. 1C, top-left panel). On the other hand, there was no SVCT2 staining in aortic sinus lesions of mice that received SVCT2−/− FLCs (Fig. 1C, top-right panel). Immunostaining of representative lesions with the macrophage-specific marker MOMA-2 showed that these cells made up essentially all of the lesions from both groups (Fig. 1C, bottom panels). Quantification of macrophage immunostaining in serial sections of aortic sinus showed that recipients of SVCT2−/− FLCs had a 28% decrease in macrophage-derived foam cells in atherosclerotic lesions compared to recipients of SVCT2+/+ FLCs (100098 ± 39884 μm2 vs. 138713 ± 7769 μm2; p < 0.001; Fig. 1D).
To assess apoptosis as a possible cause of this decrease in macrophage number, serial sections from aortic sinus were stained with the TUNEL technique. Mice that received SVCT2−/− FLCs had a 51% increase in the percentage of TUNEL-positive cells per atherosclerotic lesion area compared to mice that received SVCT2+/+ FLCs (Fig. 1E). These results suggest that decreased lesion size in mice that received SVCT2−/− FLCs may be due to accelerated apoptosis of ascorbate-deficient macrophages.
SVCT2 expression and function in macrophages
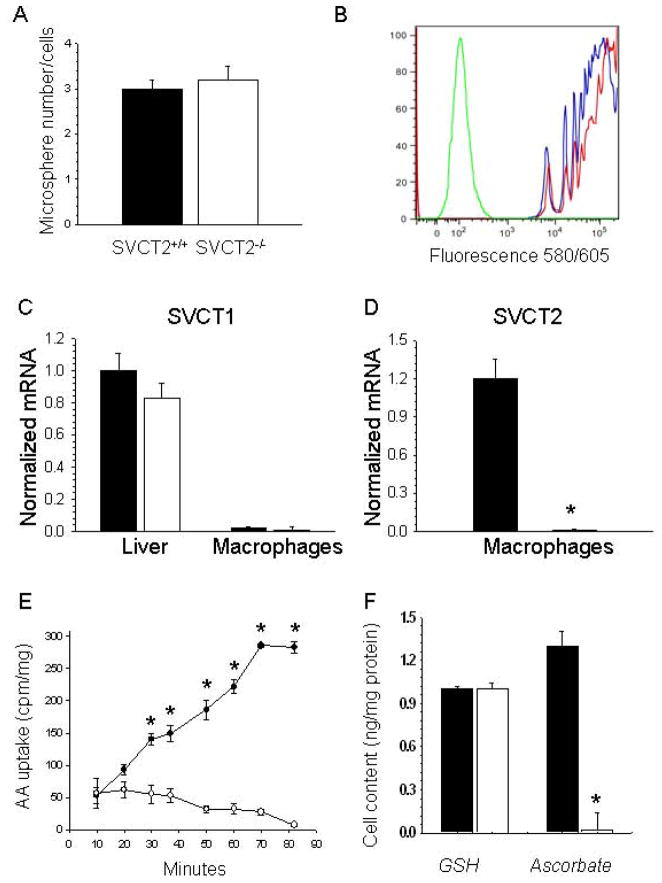
To examine more closely the role of the SVCT2 in macrophage ascorbate accumulation as it relates to apoptosis, we prepared thioglycollate-stimulated peritoneal macrophages from both groups of mice. There were no statistical significant differences between yields of thioglycollate-elicitedperitoneal macrophages isolated from recipient mice reconstituted with SVCT2+/+ (n = 16) or SVCT2−/− (n = 12) FLCs (23.3 ± 2.1 cells ×106/ml vs. 24.7 ± 3.2 cells ×106/ml; p = 0.71, respectively). Similarly, there were no differences between numbers of Histopaque-islolated blood mononuclear cells from mice transplanted with SVCT2+/+ (n = 4) or SVCT2−/− (n = 3) FLCs (6.2 ± 1.1 vs. 6.3 ± 0.4 cells ×106/ml, respectively; p = 0.92). Macrophages prepared from SVCT2−/− mice also had no difference in phagocytosis of fluorescent beads compared to macrophages from SVCT2+/+ mice, either in the number of particles engulfed per macrophage (Fig. 2A, p = 0.73) or by flow cytometric analysis of macrophage bead content (Fig. 2B). Together, these results suggest that peritoneal macrophages from mice lacking the SVCT2 did not have impaired chemotaxis or capacity for phagocytosis.
Figure 2.
Macrophage phagocytosis and ascorbate transporter expression and function. Liver tissue and peritoneal macrophages were isolated from apoE−/− mice reconstituted either with SVCT2+/+ (■) or SVCT2−/− (□) FLCs. Phagocytosis of fluorescent microspheres was allowed to continue for 1 hour at 37°C, followed either by counting of macrophage bead content (A) or by flow cytometry (B). For (B), the green line represents control non-phagocytosing macrophages, the red line macrophages from mice transplanted with SVCT2−/− FLCs, and the blue line macrophages from mice that received SVCT2+/+ FLCs. Gene expression levels of SVCT1 (C) and SVCT2 (D) were measured in liver and macrophages by real-time PCR. Graphs show results from 3 mice per group, with an asterisk indicating p < 0.05 compared to the control group. Radiolabeled ascorbate uptake over 90 min was measured in peritoneal macrophages in 4 experiments, with an asterisk indicating p < 0.05 compared to time zero (E). Macrophage ascorbate and GSH were measured in macrophages from each group that were cultured for 2 days after harvesting (F). Results are shown from 4 experiments, with an asterisk indicating p < 0.05 compared to control ascorbate.
We next examined expression of SVCT1 ascorbate transporter isoform. This transporter is expressed most strongly in epithelial cells of the intestine and kidney as well as in hepatocytes [14]; for this study it was necessary to confirm that it is not present in macrophages. As shown in Fig. 2C, whereas SVCT1 mRNA was abundant in liver of both groups of recipient mice, it was absent in peritoneal macrophages. As expected, SVCT2 mRNA was highly expressed in macrophages from mice that received SVCT2+/+ FLCs, but was undetectable in macrophages from mice that received SVCT2−/− FLCs (Fig. 2D). These data indicate that (a) macrophages do not express SVCT1 and (b) that macrophages from SVCT2−/− recipient mice also lack SVCT2 expression.
Absence of the SVCT2 in macrophages from mice receiving SVCT2−/− FLCs was confirmed by the finding that these cells failed to transport ascorbate over 90 min of incubation, compared with a progressive increase in cellular accumulation of radiolabeled ascorbate in macrophages from mice that received SVCT2+/+ FLCs (Fig. 2E). Despite 4 days of culture in the absence of added ascorbate, macrophages from mice that received SVCT2+/+ FLCs had intracellular ascorbate concentrations that were about twice those of GSH on a molar basis (Fig. 2F). On the other hand, macrophages from mice that received SVCT2−/− FLCs contained no detectable ascorbate (Fig. 2F). Intracellular GSH concentrations were similar in both groups, indicating that oxidative stress due to ascorbate deficiency was not severe enough to alter GSH concentrations in thioglycollate-activated macrophages.
SVCT2/ascorbate deficiency increases sensitivity of macrophages to H2O2-induced mitochondrial dysfunction and apoptosis
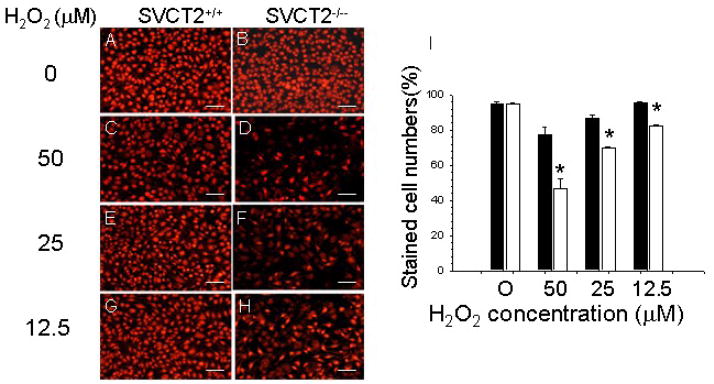
Next we examined in vitro the impact of SVCT2 deficiency on macrophage mitochondrial function as a precursor to apoptosis. To generate cells for these studies, we lethally irradiated and transplanted male C57BL6 mice with FLCs from male apoE−/−/SVCT2+/+ (n = 13) or apoE−/−/SVCT2−/− (n = 10) mice. Ten weeks post-transplantation, thioglycollate-elicited peritoneal macrophages were isolated and their response to oxidative stress was investigated. First, we examined whether oxidative stress due to hydrogen peroxide (H2O2) differentially affected mitochondrial function in the two groups. In these experiments, apoE−/−/SVCT2+/+ and apoE−/−/SVCT2−/− macrophages were pre-incubated with serum-free media for 2 hours, and then treated with fresh culture media containing different amounts of H2O2 for 18 hours and stained with MitoTracker red. This probe is selectively retained by mitochondria, where it is oxidized to the corresponding fluorescent form. Untreated control macrophages of both groups were stained brightly with this dye (Fig. 3A–B) and there was no difference between the two groups when untreated cells were quantified as the percent stained of total cells (Fig. 3I). Oxidative stress due to H2O2 decreased fluorescent staining in both types of cells (Fig. 3C–H), but there were significantly fewer (<50%) stained cells in SVCT2−/−/apoE−/− macrophages than in SVCT2+/+/apoE−/− macrophages (Fig. 3I).
Figure 3.
SVCT2 deficiency impairs mitochondrial function in response to H2O2. (A–H) Staining by MitoTracker red of mitochondria in SVCT2+/+ and SVCT2−/− macrophages treated with the indicated amounts of H2O2. The scale bars represent 50 μm. (I) Mitotracker red staining of SVCT2+/+ (■) and SVCT2−/− (□) macrophages from 3 mice in each group, with an asterisk indicating p < 0.05 compared to SVCT2+/+ macrophages at each H2O2 concentration.
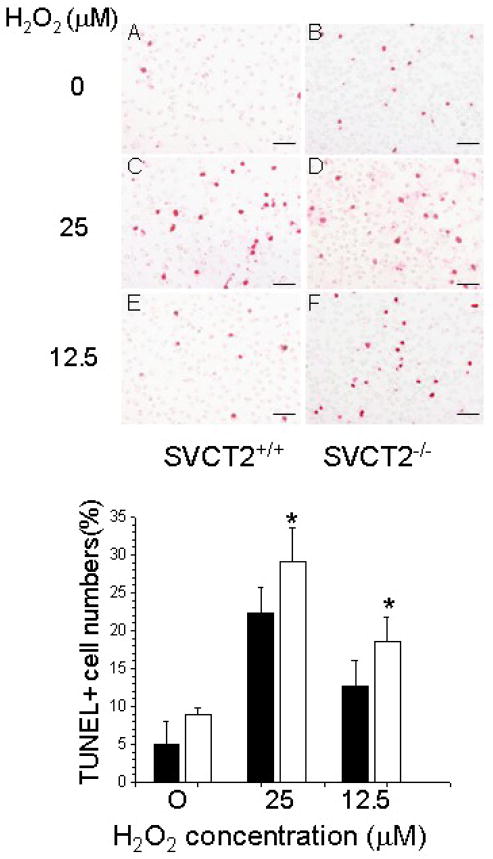
To assess whether increased oxidative stress due to H2O2 also increased apoptosis, macrophages from both groups were pre-incubated with serum-free media for 1 h and treated in the same media with increasing amounts of H2O2 for 16 hours. Whereas H2O2 treatment increased the number of TUNEL+ cells in both groups (Fig. 4, top panel), the effect was more pronounced in SVCT2−/−/apoE−/− macrophages than in control SVCT2+/+/apoE−/− macrophages at each H2O2 concentration (Fig. 4, bottom panel). Together these data show that SVCT2/ascorbate deficiency in thioglycollate-elicited macrophages (a) impairs mitochondrial function in response to H2O2, (b) increases basal apoptosis, and (c) increases apoptosis in response to H2O2.
Figure 4.
SVCT2 deficiency increases TUNEL+ cell numbers in response to H2O2. Top panel: TUNEL staining of SVCT2+/+ and SVCT2−/− macrophages and treated with increasing concentrations of H2O2 (A–F). The scale bars represent 50 μm. Bottom Panel: Percent of TUNEL+ macrophages from 3 mice for SVCT2+/+ (■) and SVCT2−/− (□) macrophages. An asterisk indicates p < 0.05 compared to SVCT2+/+ macrophages.
SVCT2/ascorbate deficiency suppresses the RAGE/NF-κB pathway in macrophages
The finding that severe macrophage ascorbate deficiency increased macrophage apoptosis prompted a search for the molecular mechanism underlying the effect. Recent studies have demonstrated that oxidative stress and hyperglycemia cause formation of advanced glycation end products (AGEs) that form by non-enzymatic glycation of oxidized proteins, lipids and nucleic acids [24, 25]. AGEs are ligands for the receptor for advanced glycation end-products (RAGE), which is abundantly expressed in vascular macrophages [26] and initiates cellular signals that activate NF-κB [27]. To test whether the RAGE/NF-κB pathway is affected by SVCT2/ascorbate deficiency, we examined RAGE gene and protein expression levels in thioglycollate-elicited macrophages. As shown in Fig. 5A, RAGE mRNA was abundantly expressed in SVCT2+/+/apoE−/− macrophages, but very little expression was seen in SVCT2−/−/apoE−/− macrophages. Further, RAGE protein expression in SVCT2−/−/apoE−/− macrophages was decreased to nearly half that seen in control SVCT2+/+/apoE−/− macrophages (Fig. 5B). As expected, MOMA-2-positive cells of atherosclerotic lesions abundantly expressed RAGE in both groups (Fig. 5C). Although RAGE staining appears more evident in the lesion areas of mice reconstituted with SVCT2+/+/apoE−/− FLCs compared to mice with SVCT2−/−/apoE−/− FLCs (Fig. 5C, bottom panels), this approach is not quantitative.
Figure 5.
Lack of SVCT2 suppresses RAGE gene and protein expression in peritoneal macrophages and aortic sinus lesions. RAGE mRNA (A) and protein (B) levels were measured in untreated SVCT2+/+ (■) and SVCT2−/− (□) macrophages from 3 mice in each group. An asterisk indicates p < 0.05 compared to control macrophages. (C) Representative immunostaining for RAGE (top panels) and macrophages (bottom panels) of cross sections of aortic sinus of mice reconstituted with SVCT2+/+/apoE−/− (left-hand panels) and SVCT2−/−/apoE−/− (right-hand panels) FLCs.
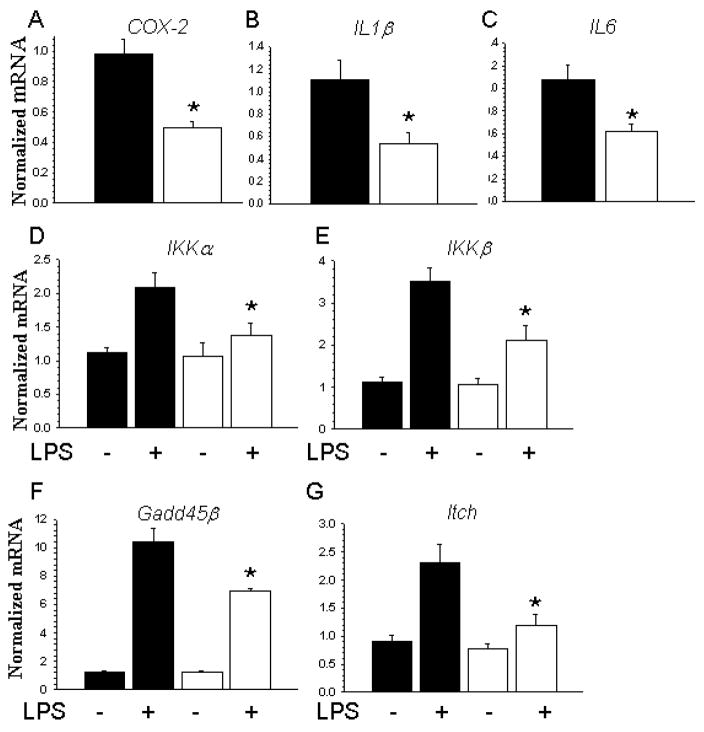
To determine the effect of in vivo deficiency of SVCT2 and ascorbate on the activation state of peritoneal macrophages, we measured inflammatory and anti-apoptotic gene expression by real time PCR. As shown in Fig. 6A–C, peritoneal macrophages from mice that received SVCT2−/−/apoE−/− FLCs had baseline 50% decreases in mRNA expression of COX-2, IL1β and IL6 compared to control macrophages from mice that received SVCT2+/+/apoE−/− FLCs. There were no differences in the expression levels of TNFα and Akt1 genes between cells (data not shown). Baseline expression of both NF-κB-related genes (IKKα and IKKβ, Fig. 6D, E) and anti-apoptotic genes (Gadd45 and Itch, Fig. 6F, G) was similar between macrophage types. However, when macrophages were treated with lipopolysaccharide (LPS, 50 ng/ml) for 5 hours, the expected increase in expression of these genes was blunted in macrophages obtained from mice that received SVCT2−/−/apoE−/− FLCs.
Figure 6.
SVCT2 deficiency in macrophages suppresses NF-κB-related and anti-apoptotic genes. (A–C) Basal expression of mRNA for inflammation-related genes (COX-2, IL1β and IL6) in untreated SVCT2+/+ (■) and SVCT2−/− (□) macrophages from 3 mice in each group. Data are shown as mean ± SEM, with an asterisk indicating p < 0.05 compared to the control group. (D–G) SVCT2+/+ (■) and SVCT2−/− (□) macrophages from 3 mice in each group were treated with 50 nM LPS for 5 hours, followed by measurement of mRNA for NF-κB-related (IKKα, IKKβ) and anti-apoptotic (Gadd45β and Itch) genes. An asterisk indicates p < 0.05 compared to control LPS treatment for the respective group.
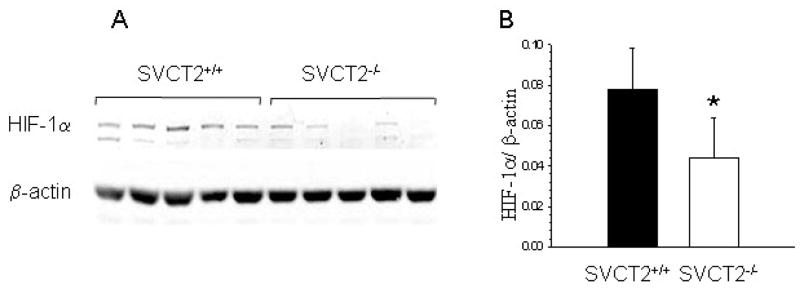
Because ascorbate has been implicated in the post-translational control of HIF-1α, we compared expression of this transcription factor in peritoneal macrophages prepared from the two groups. As shown in Fig. 7, HIF-1α protein expression was decreased in macrophages lacking the SVCT2, compared to macrophages that received the SVCT2 in FLCs. Together, these results suggest that severe ascorbate-deficiency decreased inflammatory activation associated with decreased activity of the RAGE/NF-κB pathway, as well as increased apoptosis due to decreased function of anti-apoptotic genes.
Figure 7.
Macrophage SVCT2 deficiency inhibits expression of HIF-1α. HIF-1α protein expression is shown for macrophages either lacking or expressing the SVCT2 from 5 mice (A). In (B) the results are shown relative to actin expression, with an asterisk indicating p < 0.05 compared to control macrophages.
DISCUSSION
Selective loss of the SVCT2 in hematopoietic cells due to transplant of SVCT2−/− FLCs caused severe ascorbate deficiency in peritoneal macrophages and spleen in atherosclerosis-prone mice. Since the only known function of the SVCT2 is to transport ascorbate into cells [14], the effects observed in this study can be attributed to cellular ascorbate deficiency. Macrophage ascorbate deficiency, which did not cause scurvy or affect serum lipid levels in apoE−/− mice, suppressed formation of early atherosclerotic lesions. This was largely explained by finding fewer macrophage foam cells in the lesions of mice reconstituted with SVCT2−/−/apoE−/− FLCs. Apoptosis was increased both in the lesions and in peritoneal macrophages derived from mice that received SVCT2−/− FLCs. This was likely the main cause of decreased macrophage number in the artery wall, since peritoneal macrophage recruitment and function did not differ between the two groups. In contrast to neutrophils that die soon after activation, macrophages are relatively long-lived cells [28] that typically die by apoptosis rather than necrosis [29]. In the complex lesions of advanced atherosclerosis, macrophage apoptosis contributes to necrotic core formation and plaque expansion [30, 31], probably because of defective clearance of apoptotic macrophages [29]. In earlier stages of atherosclerosis (i.e., fatty streak stage), decreased macrophage apoptosis appears to promote progression to more advanced lesions [32, 33], whereas increased apoptosis inhibits lesion progression [21, 34, 35]. This apparent paradox has been attributed to efficient clearance of apoptotic cells by phagocytes in early atherosclerotic lesions [29, 33]. These considerations support the contention that the increases in apoptosis in SVCT2/ascorbate-deficient macrophages contributed significantly to the decreases in early lesion size observed in the present study.
Ascorbate is an excellent antioxidant, so it is possible that lack of the antioxidant effects of ascorbate is the cause of increased apoptosis in lesion macrophages. Although the lack of change in intracellular GSH in SVCT2−/− peritoneal macrophages suggest that they were not under severe oxidative stress, they did show both increased susceptibility to mitochondrial dysfunction leading to apoptosis when challenged with H2O2. Intracellular ascorbate has been reported to protect macrophages from apoptosis induced by a variety of oxidative stresses [36–39], including oxidized LDL [40, 41]. However, it should be noted that an anti-apoptotic effect of ascorbate supplementation has not been uniformly observed in either endothelial cells [42] or macrophages [43]in vitro. These discordant results in cell culture studies likely relate to such factors as different intracellular ascorbate concentrations, use of different cell types, or to different oxidative stressors. Nonetheless, the present results show that lack of appreciable intracellular ascorbate increases macrophage apoptosis in atherosclerotic lesions in vivo, as well as ex vivo in H2O2-treated peritoneal macrophages. The level of intracellular ascorbate required to prevent macrophage apoptosis in atherosclerotic lesions cannot be assessed from our studies, but is likely to be low, since a severe but still incomplete ascorbate deficiency actually increased atherosclerosis in apoE−/− mice [12].
Ascorbate is also involved in regulation of intracellular iron, so it is possible that its deficiency in macrophages could have predisposed to apoptosis due to changes in iron distribution. For example, macrophages are known to sequester intracellular iron in lysosomes, a process that requires ascorbate for reduction of Fe3+ to Fe2+ by a cytochrome b561 with homology to DcytB [44]. It is possible that lack of ascorbate in macrophages and associated failure to utilize the cytochrome b561 trans-membrane oxidoreductase in lysosomes may prevent lysosomal Fe2+ uptake, thereby increasing intracellular iron. Further, we have recently shown in endothelial cells that intracellular ascorbate modestly decreases Fe2+ that can be chelated by PhenGreen SK [45], so that decreased cell ascorbate would increase the labile iron pool. Both of these effects would increase mitochondrial iron uptake, where it could contribute both to increased generation of radical species and to increased mitochondrial-dependent apoptosis.
Ascorbate also serves as a cofactor to various mono- and dioxygenase enzymes that are required for the synthesis of dopamine and carnitine, amidation of neuropeptides, and hydroxylation of proline and lysine residues in certain proteins, including procollagen and HIF-1α [46, 47]. Of these functions of ascorbate, effects on carnitine and HIF-1α could have contributed to the observed effects. Since carnitine synthesized in mitochondria is essential for fatty acid oxidation [48], a local deficiency of carnitine may cause mitochondrial dysfunction during oxidative stress in SVCT2−/−/ascorbate-deficient macrophages, ultimately leading to apoptosis. Also, ascorbate deficiency could increase cellular HIF-1α, which is required for myeloid-dependent inflammation [49]. Ascorbate serves to maintain the non-heme iron in the HIF-1α prolyl hydroxylase in a reduced and active form [50]. This enzyme hydroxylates key prolines and lysines on HIF-1α, which then targets it for proteosomal destruction [51]. Thus, ascorbate serves, at least acutely, to decrease HIF-1α levels, an effect that has been well documented when HIF-1α is increased by hypoxia and heavy metals in several cell types, including cancer cells [52], endothelial cells [53] and peritoneal neutrophils [54]. In the latter, hypoxia-stimulated HIF-1α enhances neutrophil survival by inhibiting apoptosis [55]. Ascorbate deficiency increases HIF-1α protein expression and inhibits neutrophil apoptosis, even under normoxic conditions [54]. However, rather than an increase, we found a modest decrease in HIF-1α protein expression in macrophages lacking the SVCT2 and thus ascorbate. How long term in vivo ascorbate depletion might decrease peritoneal macrophage HIF-1α expression remains to be determined. However, it is unlikely to be due to decreased RAGE expression, since inhibition of hypoxia-induced HIF-1α hypoxic neurons decreases RAGE expression, an effect likely mediated by a consensus sequence for HIF-1α on the RAGE promoter [56]. Rather, at least part of the effect of ascorbate deficiency to decrease RAGE and downstream effectors in macrophages could be due to decreased HIF-1α.
It is plausible that suppression of the RAGE/NF-κB pathway contributed to our finding of increased apoptosis in SVCT2−/− macrophages. We found decreased macrophage cytokine and RAGE gene expression, as well as decreased LPS-stimulated NF-kB and anti-apoptotic gene expression in SVCT2−/− macrophages. All of the observed changes could relate to decreases in RAGE. RAGE is a pattern recognition cell-surface receptor that binds diverse ligands, including AGEs, members of the S100/calgranulin family, high mobility group box-1 (HMGB1, amphoterin), and the amyloid β-peptide [57]. Ligand binding and activation of RAGE amplifies immune and inflammatory responses [57]. Its decrease in SVCT2−/− macrophages may well have accounted for the observed decreases in basal mRNA expression of COX-2 and the macrophage cytokines IL1β and IL6, as well as decreased LPS-stimulated activation of the NF-κB pathway, all of which may have contributed to decreased atherosclerosis in mice receiving SVCT2−/− macrophages.
The role of RAGE in atherosclerosis progression has been confirmed by the demonstration that atherosclerosis-prone mice lacking RAGE had decreased lesion size and progression [58, 59]. Aortic endothelial cells express RAGE, and systemic or endothelial cell-specific knockout of RAGE significantly suppressed atherosclerosis in apoE−/− mice [58]. A primary decrease in RAGE also suppressed levels of its inflammatory ligands S100/calgranulins and HMGB1 in that study. A more recent study confirmed a decrease in diet-induced atherosclerosis in LDLR−/− mice lacking RAGE [59]. It also showed that RAGE-deficient mice had decreased macrophage content in atherosclerotic plaques, as well as decreased oxidative stress in the vessel wall. Whether the decrease in macrophage number due to absence of RAGE in those studies was due to apoptosis was not investigated. That apoptosis was involved is suggested by the results of our study and by the previous finding that targeted knockdown of RAGE in tumor cells led to decreased cell survival due to apoptosis [60]. On the other hand, activation of RAGE in mouse peritoneal macrophages by treatment with HMGB1 also increases apoptosis [61] and AGE’s acting through RAGE generate cellular oxidative stress leading to apoptosis [62, 63]. A resolution to these apparently contradictory findings is to invoke the concept of hormesis and suggest that a basal constitutive function of RAGE is required to limit apoptosis, but that enhanced RAGE expression and function upon binding of inflammatory ligands promotes apoptosis. That the RAGE/NF-κB pathway functions in this manner is supported by the finding that constitutive activation of the NF-κB pathway is essential for macrophage survival [64].
In conclusion, our data show that severe macrophage ascorbate depletion due to lack of the SVCT2 decreases atherosclerotic lesion size in apoE−/− mice and that this is associated with decreases in macrophage numbers and increased macrophage apoptosis. A plausible cause of increased lesion macrophage apoptosis is a decrease in the RAGE/NF-κB pathway, which in turn could have been due, at least in part, to decreased HIF-1α. The mechanisms by which ascorbate deficiency decreases RAGE and HIF-1α remain to be determined.
Acknowledgments
This work was supported by NIH grant DK050435 to JMM, HL065709 and HL057986 to SF, HL065405 and HL086988 to MFL, and by center/core grants DK59637, GM15431, and DK020593.
Abbreviations
- AGE
advanced glycation end products
- apoE
apolipoprotein E
- FLC
fetal liver cells
- HIF-1α
hypoxia-inducible factor-1α
- HMGB1
high mobility group box-1
- RAGE
receptor for advanced glycation end products
- SVCT2
sodium-dependent vitamin C transporter 2
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S35–S40. doi: 10.1038/sj.ijo.0802498. [DOI] [PubMed] [Google Scholar]
- 3.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–8. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 5.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terasawa Y, Ladha Z, Leonard SW, Morrow JD, Newland D, Sanan D, et al. Increased atherosclerosis in hyperlipidemic mice deficient in α–tocopherol transfer protein and vitamin E. Proc Natl Acad Sci U S A. 2000;97:13830–4. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarna C, Wu BJ, Choy K, Mori T, Croft K, Cynshi O, et al. Protective effect of vitamin E supplements on experimental atherosclerosis is modest and depends on preexisting vitamin E deficiency. Free Radic Biol Med. 2006;41:722–30. doi: 10.1016/j.freeradbiomed.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Willis GC. An experimental study of the intimal ground substance in atherosclerosis. Can Med Assoc J. 1953;69:17–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Willis GC. The reversibility of atherosclerosis. Can Med Assoc J. 1957;77:106–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA. 2000;97:841–6. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakata Y, Maeda N. Vulnerable atherosclerotic plaque morphology in apolipoprotein E-deficient mice unable to make ascorbic acid. Circulation. 2002;105:1485–90. doi: 10.1161/01.cir.0000012142.69612.25. [DOI] [PubMed] [Google Scholar]
- 12.Babaev VR, Li L, Shah S, Fazio S, Linton MF, May JM. Combined Vitamin C and Vitamin E Deficiency Worsens Early Atherosclerosis in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.209502. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem. 1990;265:2584–7. [PubMed] [Google Scholar]
- 14.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen X-Z, Wang YX, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–5. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 15.May JM, Li L, Qu ZC, Huang J. Ascorbate uptake and antioxidant function in peritoneal macrophages. Arch Biochem Biophys. 2005;440:165–72. doi: 10.1016/j.abb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Sotiriou S, Gispert S, Cheng J, Wang YH, Chen A, Hoogstraten-Miller S, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nature Med. 2002;8:514–7. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- 17.Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo. J Clin Invest. 1999;103:1697–705. doi: 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babaev VR, Patel MB, Semenkovich CF, Fazio S, Linton MF. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J Biol Chem. 2000;275:26293–9. doi: 10.1074/jbc.M002423200. [DOI] [PubMed] [Google Scholar]
- 19.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–40. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 20.Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, et al. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci U S A. 1997;94:4647–52. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babaev VR, Chew JD, Ding L, Davis S, Breyer MD, Breyer RM, et al. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell Metab. 2008;8:492–501. doi: 10.1016/j.cmet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vislisel JM, Schafer FQ, Buettner GR. A simple and sensitive assay for ascorbate using a plate reader. Anal Biochem. 2007;365:31–9. doi: 10.1016/j.ab.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–91. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–97. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price CL, Knight SC. Advanced glycation: a novel outlook on atherosclerosis. Curr Pharm Des. 2007;13:3681–7. doi: 10.2174/138161207783018608. [DOI] [PubMed] [Google Scholar]
- 26.Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2275–81. doi: 10.1161/ATVBAHA.108.175992. [DOI] [PubMed] [Google Scholar]
- 27.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–97. [PubMed] [Google Scholar]
- 28.Bainton DF. The cells of inflammation: a general view. In: Weissman G, editor. Handbook of Inflammation. Amsterdam: Elsevier/North-Holland Biomedical Press; 1980. pp. 1–25. [Google Scholar]
- 29.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–64. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 30.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–66. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–8. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Thewke DP, Su YR, Linton MF, Fazio S, Sinensky MS. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol. 2005;25:174–9. doi: 10.1161/01.ATV.0000148548.47755.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 34.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, et al. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–13. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Liu B, Wang P, Dong X, Fernandez-Hernando C, Li Z, et al. Phospholipase C beta3 deficiency leads to macrophage hypersensitivity to apoptotic induction and reduction of atherosclerosis in mice. J Clin Invest. 2008;118:195–204. doi: 10.1172/JCI33139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoshiba K, Tamaoki J, Nagai A. Acute cigarette smoke exposure induces apoptosis of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1392–L1401. doi: 10.1152/ajplung.2001.281.6.L1392. [DOI] [PubMed] [Google Scholar]
- 37.Vandana S, Ram S, Ilavazhagan M, Kumar GD, Banerjee PK. Comparative cytoprotective activity of vitamin C, E and beta-carotene against chromium induced oxidative stress in murine macrophages. Biomed Pharmacother. 2006;60:71–6. doi: 10.1016/j.biopha.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Miike T, Shirahase H, Jino H, Kunishiro K, Kanda M, Kurahashi K. Effects of an anti-oxidative ACAT inhibitor on apoptosis/necrosis and cholesterol accumulation under oxidative stress in THP-1 cell-derived foam cells. Life Sci. 2008;82:79–84. doi: 10.1016/j.lfs.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Ichiki A, Miyazaki T, Nodera M, Suzuki H, Yanagisawa H. Ascorbate inhibits apoptosis of Kupffer cells during warm ischemia/reperfusion injury. Hepatogastroenterology. 2008;55:338–44. [PubMed] [Google Scholar]
- 40.Asmis R, Wintergerst ES. Dehydroascorbic acid prevents apoptosis induced by oxidized low-density lipoprotein in human monocyte-derived macrophages. Eur J Biochem. 1998;255:147–55. doi: 10.1046/j.1432-1327.1998.2550147.x. [DOI] [PubMed] [Google Scholar]
- 41.Chi X, May JM. Oxidized lipoprotein induces the macrophage ascorbate transporter (SVCT2): protection by intracellular ascorbate against oxidant stress and apoptosis. Arch Biochem Biophys. 2009;485:174–82. doi: 10.1016/j.abb.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vissers MC, Lee WG, Hampton MB. Regulation of apoptosis by vitamin C. Specific protection of the apoptotic machinery against exposure to chlorinated oxidants. J Biol Chem. 2001;276:46835–40. doi: 10.1074/jbc.M107664200. [DOI] [PubMed] [Google Scholar]
- 43.Harris LK, Mann GE, Ruiz E, Mushtaq S, Leake DS. Ascorbate does not protect macrophages against apoptosis induced by oxidised low density lipoprotein. Arch Biochem Biophys. 2006;455:68–76. doi: 10.1016/j.abb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Zhang DL, Su D, Berczi A, Vargas A, Asard H. An ascorbate-reducible cytochrome b561 is localized in macrophage lysosomes. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbagen.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 45.May JM, Qu ZC. Chelation of intracellular iron enhances endothelial barrier function: A role for vitamin C? Arch Biochem Biophys. 2010 doi: 10.1016/j.abb.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chatterjee IB, Majumder AK, Nandi BK, Subramanian N. Synthesis and some major functions of vitamin C in animals. Ann N Y Acad Sci. 1975;258:24–47. doi: 10.1111/j.1749-6632.1975.tb29266.x. [DOI] [PubMed] [Google Scholar]
- 47.Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta Gen Subj. 2002;1569:1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 48.Rebouche CJ. Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr. 1991;54:1147S–52S. doi: 10.1093/ajcn/54.6.1147s. [DOI] [PubMed] [Google Scholar]
- 49.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirsilä M, Koivunen P, Gunzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–80. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- 51.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 52.Salnikow K, Kasprzak KS. Ascorbate depletion: a critical step in nickel carcinogenesis? Environ Health Perspect. 2005;113:577–84. doi: 10.1289/ehp.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vissers MC, Gunningham SP, Morrison MJ, Dachs GU, Currie MJ. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic Biol Med. 2007;42:765–72. doi: 10.1016/j.freeradbiomed.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 54.Vissers MC, Wilkie RP. Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1{alpha} J Leukoc Biol. 2007;81:1236–44. doi: 10.1189/jlb.0806541. [DOI] [PubMed] [Google Scholar]
- 55.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-kappaB activity. J Exp Med. 2005;201:105–15. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pichiule P, Chavez JC, Schmidt AM, Vannucci SJ. Hypoxia-inducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia/ischemia. J Biol Chem. 2007;282:36330–40. doi: 10.1074/jbc.M706407200. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–55. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–94. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun L, Ishida T, Yasuda T, Kojima Y, Honjo T, Yamamoto Y, et al. RAGE mediates oxidized LDL-induced pro-inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovasc Res. 2009;82:371–81. doi: 10.1093/cvr/cvp036. [DOI] [PubMed] [Google Scholar]
- 60.Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ. 2010;17:666–76. doi: 10.1038/cdd.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu XM, Yao YM, Liang HP, Liu F, Dong N, Yu Y, et al. Effect of high mobility group box-1 protein on apoptosis of peritoneal macrophages. Arch Biochem Biophys. 2009;492:54–61. doi: 10.1016/j.abb.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 62.Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–52. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J, Song M, Yu S, Gao P, Yu Y, Wang H, et al. Advanced glycation endproducts alter functions and promote apoptosis in endothelial progenitor cells through receptor for advanced glycation endproducts mediate overpression of cell oxidant stress. Mol Cell Biochem. 2010;335:137–46. doi: 10.1007/s11010-009-0250-y. [DOI] [PubMed] [Google Scholar]
- 64.Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-kappaB activation to maintain A1 expression and mitochondrial homeostasis. Mol Cell Biol. 2000;20:8855–65. doi: 10.1128/mcb.20.23.8855-8865.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]