Abstract
Background:
Ovarian transposition is the anatomical relocation of the ovaries from the pelvis to the abdomen. Transposition is beneficial in women who are to undergo pelvic radiation, because it allows maintenance of ovarian function and preservation of assisted reproductive capacity.
Methods:
The da Vinci surgical system (Intuitive Surgical™, Mountainview, CA, USA) was used to perform an endoscopic ovarian transposition. The ovaries were mobilized on their respective infundibulopelvic ligaments and sutured to the ipsilateral pericolic gutters.
Results:
A series of laboratory sessions using the da Vinci system was completed at our institution's training facility. Surgical experience included cadaveric pelvic dissection and abdominopelvic procedures on anesthetized porcine models. Additional didactic and laboratory training, including a certification examination, was obtained from Intuitive Surgical, Inc. The first clinical case of robotically assisted endoscopic ovarian transposition was performed.
Conclusions:
Robotically assisted endoscopy was successfully used for ovarian transposition.
Keywords: Laparoscopy, da Vinci robotic system, Ovarian transposition
INTRODUCTION
Anatomical relocation of the ovaries from the pelvis to the abdomen allows preservation of ovarian function in the majority of women so treated. This is especially important in premenopausal women who are desirous of continued ovarian function to prevent premature menopause and maintain assisted reproductive capacity. Endoscopic pelvic surgery often results in less morbidity and reduced postoperative recovery time compared with laparotomy. Conventional laparoscopy, however, is limited by its monocular visibility and mobility constraints, such that endoscopic suturing is often cumbersome and time consuming. Robotically assisted endoscopy is potentially a more efficient method of dissection and suturing.
Ovarian transposition was performed on a 32-year-old, gravida 3, para 3-0-0-3 female, who had undergone a radical hysterectomy and bilateral pelvic lymphadenectomy for a Stage I-B1 cervical squamous cell carcinoma 4 weeks earlier. Ovarian transposition was not performed at the time of the radical hysterectomy in keeping with the patient's wishes that this be done only if postoperative radiotherapy was inevitable (ie, multiple lymph node metastases). Intraoperative frozen section analysis of enlarged lymph nodes showed no evidence of metastatic disease, and all lymph nodes were negative on final histopathologic analysis. The primary tumor, however, showed extensive lymphovascular space invasion, which is an independent risk factor for tumor recurrence.1 Recurrence risk may be reduced by pelvic radiotherapy, with permanent ovarian failure as a consequence.
MATERIALS AND METHODS
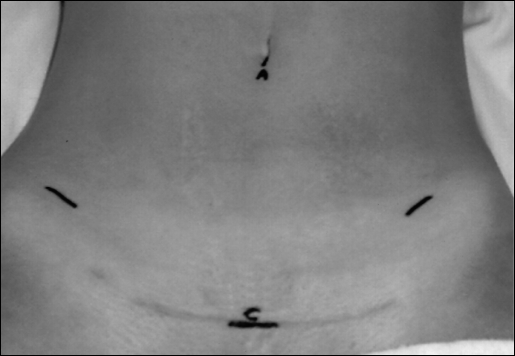
After an informed discussion, the patient elected to undergo ovarian transposition prior to adjunctive treatment. She was taken to the operative theater, administered general anesthesia, and placed in the dorsal lithotomy position. Laparoscopic insertion sites were marked on the abdomen (Figure 1). A subumbilical incision was made for initial insufflation using the Veress needle, and laparoscopic trocar and camera placement. A 10-mm suprapubic incision was initially used as an accessory instrument port. Incisions in the right and left lower quadrants were used as instrument ports. These 10-mm lower quadrant port sites were placed a hand's width apart (approximately 12 cm) at roughly 90-degree angles from the suprapubic port. Standard laparoscopic instrumentation was used to survey the anatomy and initiate adhesiolysis.
Figure 1.
Laparoscopic insertion sites placed in the subumbilical region and beneath outline of swimsuit to minimize visibility. The port placed at the umbilicus (A) was used as an accessory port. The suprapubic port (C) was used for the da Vinci camera port.
A robotically assisted endoscopy was then performed. The da Vinci surgical cart was moved into place below the pelvis, with the surgical arms reaching over the patient's abdomen (Figure 2). The da Vinci camera was introduced through the suprapubic port, such that the camera was now located low in the pelvis with visibility directed toward the abdomen. The robotic arms were secured to instruments entering through the right and left lower abdomen. The umbilical incision was now used as an accessory hand-held instrument port.
Figure 2.
Intraoperative photograph of the da Vinci system with surgeon's console (arrow), vision cart (double arrow) shown in the background, and surgical cart with robotic arms visible in the foreground.
At this time, the surgeon (KLM) was seated at the surgeon's console, as the surgical assistant (JSW) remained at the patient's side in a sterile fashion. Adhesiolysis, retroperitoneal dissection, and isolation of the ureters were completed with Potts scissors, spatula cautery, and blunt dissection directed from the surgeon's console. The ovaries and infundibulopelvic ligaments were isolated, and the retroperitoneal dissections continued above the pelvic brim until the ovaries could be easily mobilized on their intact blood supply. The right ovary was relocated to the abdomen and placed in the right pericolic gutter, well beyond the anticipated radiation field (Figure 3). The ovarian ligament remnant was secured to the psoas muscle and overlying peritoneum with 3 interrupted 3-0 silk suture ligatures. A needle driver and microforceps were used for suturing, and knots were secured via the instrument tie technique. Similarly, the left ovary was mobilized to the left pericolic gutter and fixed to the left psoas muscle and overlying peritoneum (Figure 4). Three small vascular clips were placed on the ovarian ligament remnant bilaterally to enable radiographic localization.
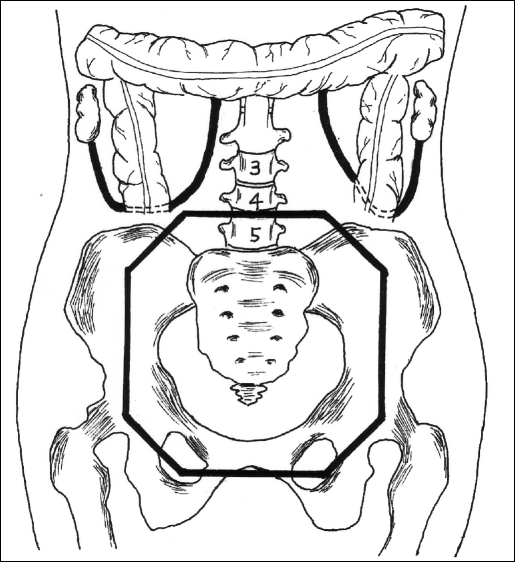
Figure 3.
Schematic of ovaries transposed to the ipsilateral paracolic gutters where they are secured beyond the radiation treatment field (heavy octagonal line).
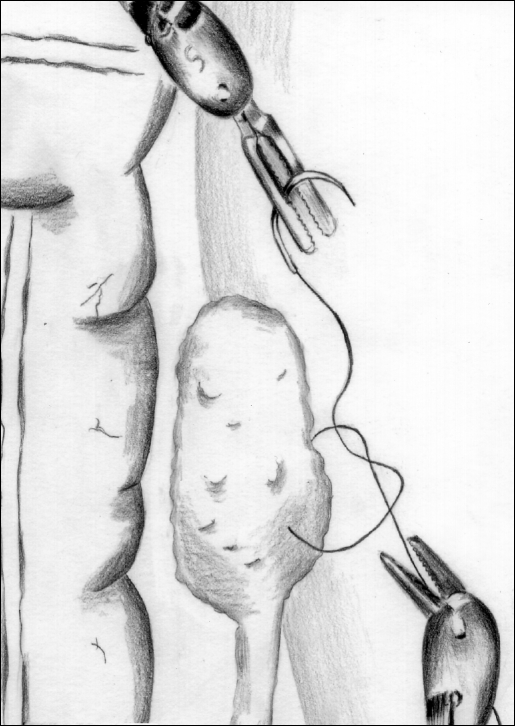
Figure 4.
Suturing the left ovary to the pericolic peritoneum is facilitated by enhanced articulation of the robotic instruments.
The vascular pedicles and ovarian tissue were inspected and noted to be without tension, torsion, or compromise. Small bleeders were rendered hemostatic with a unipolar cautery hook. The tissue was irrigated, and under direct visualization the instruments were removed. The surgical cart was retracted away from the patient, and the fascial and skin incisions were closed.
RESULTS
Our preclinical experience with the da Vinci surgical system includes didactic and laboratory training certification sessions as directed by Intuitive Surgical, Inc. Additionally, cadaveric pelvic dissection and a series of dissections with anesthetized porcine models have been completed. Procedures performed include retroperitoneal dissection with ureterolysis(3), bilateral salpingoophorectomy(2), ovarian mobilization on intact gonadal vessels(2), hysterectomy(2), bilateral pelvic lymphadenectomy(2), adhesiolysis(1), and acquisition of hemostasis via repair of controlled venous and arterial lacerations(2).
The first clinical case of ovarian transposition with robotically assisted endoscopic techniques was successfully performed. No immediate or late complications occurred. Approximately 20 minutes were required to bring the surgical cart into the operative field, ensure proper positioning, and secure the camera and instrument arms. Technically, the most difficult segment of the procedure was adhesiolysis in dissecting the ovaries free from the pelvic sidewalls (∼108 min). Times utilized for mobilization of the infundibulopelvic ligaments and suturing the ovaries into place were 24 minutes for the right side and 18 minutes for the left side.
This patient was admitted for overnight observation and discharged home in stable condition on the morning of postoperative day 1. She subsequently completed 5000 cGy of external beam radiotherapy, with ovarian shielding. Therapy included daily 180 cGy fractions with the 4-field technique to encompass the pelvic region (Figure 3). Postirradiation levels of 4.9 mIU/mLl FSH and 2.5 mIU/mL LH confirmed preservation of ovarian function. At a recent clinical follow-up, the patient was asymptomatic and without evidence of disease.
DISCUSSION
The da Vinci endoscopic instrument system is a novel technology. It consists of 3 main components: (1) the surgeon's console, (2) the surgical cart, and (3) the vision cart. The surgeon's console is located remotely from the patient and does not require sterile preparation. It is composed of a computer, video monitor, and instrument controls. The surgeon sits at the console, placing thumb and forefinger in the hand-controlled manipulators, and views the operative field through the video monitor.
Coordinated hand and foot movements control the camera and instruments within the surgical field. Movements of the surgeon's wrist and fingers are translated into exacting complementary movements by the surgical instruments. The surgical cart is mobile and can be locked into position adjacent to the surgical table, partially within the sterile field. This contains the instrument arms, camera arm, and setup joints for optimal positioning of the camera and instruments. The vision cart allows all members of the surgical team to visualize the procedure and confirm proper equipment functioning via a monitoring and feedback system.2
Robotically assisted technology has diverse clinical applications. It has been successfully used for minimally invasive cardiac, gastrointestinal, and pelvic surgical procedures.3–9 Standard endoscopy is limited in part by monocular vision, restricted instrument articulation and reduced tactile sensation. Computer-enhanced robotic technology enables binocular 3-dimensional visibility and vastly improved instrument articulation. The computer also eliminates unintentional hand tremors. Accordingly, complex surgical tasks may potentially be performed with greater efficiency. The current technology, however, provides inadequate tactile feedback. Traumatic tissue injury and suture breakage may initially be problematic owing to lack of tension sensitivity in the surgeon's master controls.2 Positioning of the surgical cart is important for proper instrument alignment and to avoid interference with the anesthesia. Mechanical limitations and steric hindrance of the robotic arms are avoided by appropriate port placement and adequate spacing. Additionally, changing the position of the patient or surgical table is not feasible once the apparatus is engaged. Careful positioning avoids the inevitable time consumption of disengaging and re-engaging the system if any significant position change is required.
As with any new technique, an inevitable learning curve exists. Our basic work during laboratory sessions provided a fundamental familiarity with the robotic system, enabling us to move forward with this, our first clinical case. The setup and operative times for this ovarian transposition surgery were longer than if performed via laparotomy. It is our observation that short suture segments (6-8 cm) enhance the efficiency of surgical knot tying. Economy of time is maximized if suture and needle are introduced and subsequently withdrawn through the hand-held accessory port, and sutures are cut by the surgical assistant. This precludes the need for instrument change because the suture can be passed from the assistant's instrument to the surgeon's instruments under direct visualization.
The first robotically assisted endoscopic ovarian transposition was successfully performed. The learning curve with novel technology is steep, and the scope of clinical utility for robotic-assisted endoscopy is yet to be defined. Undoubtedly, further experience will significantly reduce the time required for robotically assisted endoscopic procedures. Additional laboratory and clinical investigations are ongoing at our institution.
Acknowledgments:
The authors wish to express sincere gratitude to Rachel Molpus for her assistance with the medical illustration. Disclosure: The authors have no financial interest in any commercial device, equipment, instrument, or drug that is mentioned in this article. The authors have no conflicts of interest.
Contributor Information
Kelly L. Molpus, Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Omaha, Nebraska, USA..
June S. Wedergren, Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Omaha, Nebraska, USA..
Mark A. Carlson, Department of Surgery, University of Nebraska Medical Center, Omaha, Nebraska, USA..
References:
- 1. Buckley SL, Tritz DM, Van Le L, et al. Lymph node metastases and prognosis in patients with stage IA2 cervical cancer. Gynecol-Oncol. 1996;63(1):4–9 [DOI] [PubMed] [Google Scholar]
- 2.The Insite™ Vision System User Manual. Moutain View, Calif: Intuitive Surgical Inc; 1999 [Google Scholar]
- 3. Loulmet D, Carpentier A, d'Attellis N, et al. Endoscopic Coronary Artery Bypass Grafting with the Aid of Robotic Assisted Instruments. J Thoracic Cardiovasc. Surg 1999;118(1):4–10 [DOI] [PubMed] [Google Scholar]
- 4. Carpentier A, Loulmet D, Aupecle B, Berrebi A, Relland J. Computer-assisted cardiac surgery. Lancet. 1999;353(9150):379–380 [DOI] [PubMed] [Google Scholar]
- 5. Dogan S, Aybek T, Andressen E, et al. Totally endoscopic coronary artery bypass grafting on cardiopulmonary bypass with robotically enhanced telemanipulation: report of forty-five cases. J Thorac Cardiovasc Surg. 2000;123(6):1125–1131 [DOI] [PubMed] [Google Scholar]
- 6. Falk V, Autschbach R, Krakor R, et al. Computer-enhanced mitral valve surgery: Toward a total endoscopic procedure. Eur J Cardiothorac Surg. 1999;15(3):260–264 [DOI] [PubMed] [Google Scholar]
- 7. Cadiere GB, Himpens J, Vertruyen M, Favretti F. The world's first obesity surgery performed by a surgeon at a distance. Obes Surg. 1999;9(2):206–209 [DOI] [PubMed] [Google Scholar]
- 8. Cadiere GB, Himpens J, Vertruyen M, Bruyns J, Fourtanier G. Gundoplicature selon Nissen réalisée à distance du patient par robotique. Ann Chir. 1999;53(2):137–141 [PubMed] [Google Scholar]
- 9. Degueldre M, Vandromme J, Huong PT, Cadiere GB. Robotically assisted laparoscopic microsurgical tubal reanastomosis: a feasibility study. Fertil Steril. 2000;74:1020–1022 [DOI] [PubMed] [Google Scholar]