Abstract
Lower respiratory tract and systemic cell-mediated immunity have been studied in rabbits after infection with Listeria monocytogenes or Diplococcus pneumoniae. Respiratory tract cell-mediated immunity was evaluated by direct and indirect assays of migration inhibitory factor (MIF) production. Systemic delayed hypersensitivity was determined by means of intradermal testing with appropriate antigens.
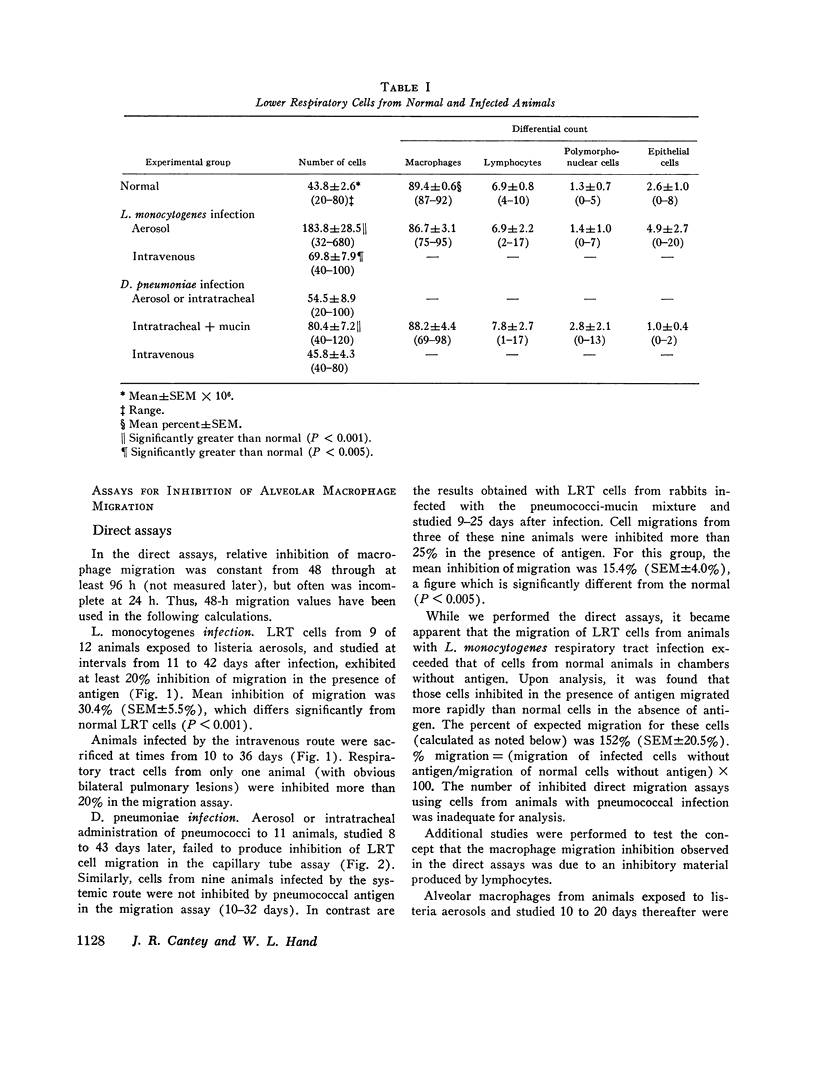
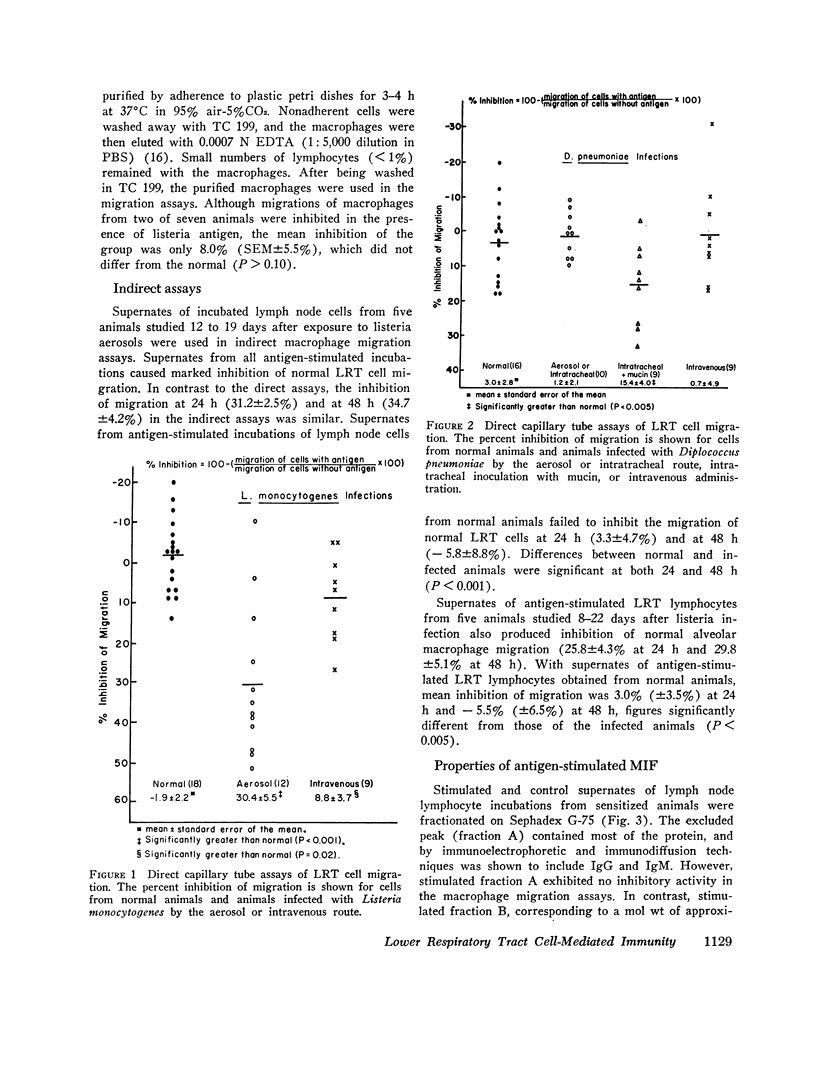
Aerosol exposure to listeria was followed by markedly increased numbers of free lower respiratory tract cells. These cells manifested antigen-stimulated inhibition of migration (mean inhibition of migration = 30.4%). Pneumococcal pneumonia was associated with similar but less dramatic changes. Intravenous administration of organisms was uncommonly followed by inhibition of lower respiratory tract cells in direct migration assays.
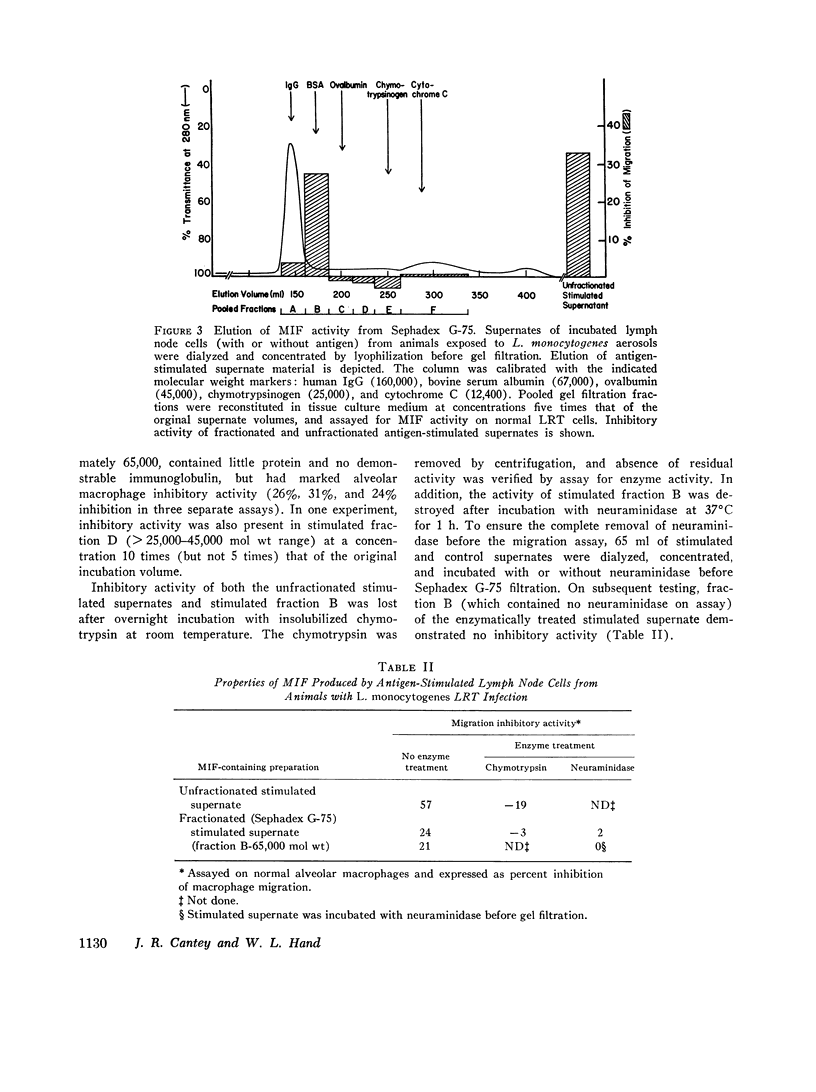
Fractionated MIF, as well as crude supernates of antigen-stimulated lower respiratory tract and lymph node lymphocytes from animals exposed to listeria aerosols, caused inhibition of normal alveolar macrophage migration. MIF, produced by lymph node lymphocytes, has a molecular weight of approximately 65,000 and is inactivated by chymotrypsin or neuraminidase.
Delayed dermal hypersensitivity to listeria antigen was observed in 54 of 55 animals exposed to listeria aerosols and in all 9 animals infected by the intravenous route. Delayed dermal reactions to pneumococcal sonicate antigen (but not capsular polysaccharide) followed D. pneumoniae respiratory tract infection in 19 of 28 animals, and was elicited in 5 of 6 animals after intravenous infection.
Both local (macrophage migration inhibition) and systemic delayed hypersensitivity followed bacterial infection of the lower respiratory tract. MIF activity was shown to be one mechanism for inhibition of alveolar macrophage migration.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartfeld H., Atoynatan T. N-acetylcysteine inactivates migration inhibitory factor and delayed hypersensitivity reactions. Nat New Biol. 1971 Jun 2;231(22):157–159. doi: 10.1038/newbio231157a0. [DOI] [PubMed] [Google Scholar]
- Bennett B., Bloom B. R. Reactions in vivo and in vitro produced by a soluble substance associated with delayed-type hypersensitivity. Proc Natl Acad Sci U S A. 1968 Mar;59(3):756–762. doi: 10.1073/pnas.59.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Lymphocyte mediators and cellular hypersensitivity. N Engl J Med. 1973 Jan 18;288(3):143–149. doi: 10.1056/NEJM197301182880311. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Page D. A., Matthew M., Wolstencroft R. A. Role of lymphocyte activation products (LAP) in cell-mediated immunity. I. Preparation and partial purification of guinea-pig LAP. Clin Exp Immunol. 1972 Jan;10(1):25–47. [PMC free article] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Galindo B., Myrvik Q. N. Migratory response of granulomatous alveolar cells from BCG-sensitized rabbits. J Immunol. 1970 Jul;105(1):227–237. [PubMed] [Google Scholar]
- Godal T., Rees R. J., Lamvik J. O. Lymphocyte-mediated modification of blood-derived macrophage function in vitro; inhibition of growth of intracellular mycobacteria with lymphokines. Clin Exp Immunol. 1971 Apr;8(4):625–637. [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., Cantey J. R. Antibacterial mechanisms of the lower respiratory tract. I. Immunoglobulin synthesis and secretion. J Clin Invest. 1974 Feb;53(2):354–362. doi: 10.1172/JCI107567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise E. R., Han S., Weiser R. S. In vitro studies on the mechanism of macrophage migration inhibition in tuberculin sensitivity. J Immunol. 1968 Nov;101(5):1004–1015. [PubMed] [Google Scholar]
- Henney C. S., Waldman R. H. Cell-mediated immunity shown by lymphocytes from the respiratory tract. Science. 1970 Aug 14;169(3946):696–697. doi: 10.1126/science.169.3946.696. [DOI] [PubMed] [Google Scholar]
- Jurgensen P. F., Olsen G. N., Johnson J. E., 3rd, Swenson E. W., Ayoub E. M., Henney C. S., Waldman R. H. Immune response of the human respiratory tract. II. Cell-mediated immunity in the lower respiratory tract to tuberculin and mumps and influenza viruses. J Infect Dis. 1973 Dec;128(6):730–735. doi: 10.1093/infdis/128.6.730. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect Immun. 1971 Oct;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu R. W., Eddleston A. L., Hadden J. W., Good R. A. Mechanism of action of migration inhibitory factor (MIF). I. Evidence for a receptor for MIF present on the peritoneal macrophage but not on the alveolar macrophage. J Exp Med. 1972 Sep 1;136(3):589–603. doi: 10.1084/jem.136.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney J. J., Waksman B. H. Activation of normal rabbit macrophage monolayers by supernatants of antigen-stimulated lymphocytes. J Immunol. 1970 Nov;105(5):1138–1145. [PubMed] [Google Scholar]
- Musher D. M., Ratzan K. R., Weinstein L. The effect of Listeria monocytogenes on resistance to pneumococcal infection. Proc Soc Exp Biol Med. 1970 Nov;135(2):557–560. [PubMed] [Google Scholar]
- Nash D. R., Holle B. Local and systemic cellular immune responses in guinea-pigs given antigen parenterally or directly into the lower respiratory tract. Clin Exp Immunol. 1973 Apr;13(4):573–583. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp J. R., Johnson J. E., 3rd, Spencer J. C. Amplification of migratory inhibition factor production during the first 48 hours of exposure to antigen. Infect Immun. 1973 Nov;8(5):781–786. doi: 10.1128/iai.8.5.781-786.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold H. G., David J. R. Further studies on migration inhibitory factor (MIF): evidence for its glycoprotein nature. J Immunol. 1971 Oct;107(4):1090–1098. [PubMed] [Google Scholar]
- Remold H. G., Katz A. B., Haber E., David J. R. Studies on migration inhibitory factor (MIF): recovery of MIF activity after purification by gel filtration and disc electrophoresis. Cell Immunol. 1970 May;1(1):133–145. doi: 10.1016/0008-8749(70)90066-3. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Meyers O. L., David J. R. An in vitro assay for cellular hypersensitivity in man. J Immunol. 1970 Jan;104(1):95–102. [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Detection of delayed hypersensitivity in mice injected with ribonucleic acid-protein fractions of Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):384–389. doi: 10.1128/iai.6.3.384-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J. C., Waldman R. H., Johnson J. E., 3rd Local and systemic cell-mediated immunity after immunization of guinea pigs with live or killed m. tuberculosis by various routes. J Immunol. 1974 Apr;112(4):1322–1328. [PubMed] [Google Scholar]
- Spitler L., Huber H., Fudenberg H. H. Inhibition of capillary migration by antigen-antibody complexes. J Immunol. 1969 Feb;102(2):404–411. [PubMed] [Google Scholar]
- Thompson H. C., Snyder I. S. Protection against pneumococcal infection by a ribosomal preparation. Infect Immun. 1971 Jan;3(1):16–23. doi: 10.1128/iai.3.1.16-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor D. E., Jureziz R. E., Veach S. R., Miller E., Dray S. Cell migration inhibition factor released by antigen from human peripheral lymphocytes. Nature. 1968 Aug 17;219(5155):755–757. doi: 10.1038/219755a0. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- WOOD W. B., Jr Studies on the cellular immunology of acute bacterial infections. Harvey Lect. 1951;Series 47:72–98. [PubMed] [Google Scholar]
- Waldman R. H., Spencer C. S., Johnson J. E., 3rd Respiratory and systemic cellular and humoral immune responses to influenza virus vaccine administered parenterally or by nose drops. Cell Immunol. 1972 Feb;3(2):294–300. doi: 10.1016/0008-8749(72)90168-2. [DOI] [PubMed] [Google Scholar]
- Warr G. A., Martin R. R. In vitro migration of human alveolar macrophages: effects of cigarette smoking. Infect Immun. 1973 Aug;8(2):222–227. doi: 10.1128/iai.8.2.222-227.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherbee R. E. Induction of systemic delayed hypersensitivity during experimental viral infection of the respiratory tract with a myxovirus or paramyxovirus. J Immunol. 1973 Jul;111(1):157–163. [PubMed] [Google Scholar]
- Yamamoto K., Anacker R. L. Macrophage Migration Inhibition Studies with Cells from Mice Vaccinated with Cell Walls of Mycobacterium bovis BCG: Characterization of the Experimental System. Infect Immun. 1970 Jun;1(6):587–594. doi: 10.1128/iai.1.6.587-594.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


