Abstract
Lactococcus lactis is used extensively for the production of various cheeses. At every stage of cheese fabrication, L. lactis has to face several stress-generating conditions that result from its own modification of the environment as well as externally imposed conditions. We present here the first in situ global gene expression profile of L. lactis in cheeses made from milk concentrated by ultrafiltration (UF-cheeses), a key economical cheese model. The transcriptomic response of L. lactis was analyzed directly in a cheese matrix, starting from as early as 2 h and continuing for 7 days. The growth of L. lactis stopped after 24 h, but metabolic activity was maintained for 7 days. Conservation of its viability relied on an efficient proteolytic activity measured by an increasing, quantified number of free amino acids in the absence of cell lysis. Extensive downregulation of genes under CodY repression was found at day 7. L. lactis developed multiple strategies of adaptation to stressful modifications of the cheese matrix. In particular, expression of genes involved in acidic- and oxidative-stress responses was induced. L. lactis underwent unexpected carbon limitation characterized by an upregulation of genes involved in carbon starvation, principally due to the release of the CcpA control. We report for the first time that in spite of only moderately stressful conditions, lactococci phage is repressed under UF-cheese conditions.
Lactic acid bacteria, particularly Lactococcus lactis, have a long history of use in milk fermentation, from small-scale traditional operations to well-controlled industrial applications. Recent developments of molecular tools have unraveled the genetics, physiology, and metabolism of this economically very important microorganism. However, interpretation has always been limited by the lack of knowledge regarding in situ bacterial physiology. Technological properties (e.g., acidification, proteolytic or lipolytic activity, and bacteriocin production) can easily be shown and quantified in vitro but have hardly ever been verified in a complex solid matrix, due to local intrinsic factors. There have been several successful attempts to measure the DNA and rRNA extracted directly from a solid food (or environmental) matrix in order to estimate either the predominant species and/or the overall level of metabolic activity of the species (11, 33). In a few cases, the expression of genes of technological interest from extracted mRNAs has been checked (42). The global gene or protein expression of L. lactis has been characterized in milk (15, 34) but not directly in cheese, the corresponding solid dairy matrix.
Over the last few years, functional-genomics approaches, including transcriptomics, have been increasingly used to obtain global gene expression profiles, thereby providing a comprehensive view of microorganism physiology. So far, such global approaches in food microbiology and in situ have been poorly documented. Recently, Bachmann et al. presented the genetic responses of L. lactis in mixed cultures in mini-Gouda-type cheeses (1). By use of a recombinant in vivo expression technology (R-IVET) assay (2), they targeted expressed genes in semihard cheeses and compared them to those in a laboratory medium (M17). Under these conditions, they observed several gene inductions during cheese fermentation; in particular, they observed that genes involved in nitrogen metabolism were overexpressed, indicating amino acid starvation.
In this study, the physiological behavior of L. lactis was analyzed directly in the dairy food matrix, enabling the dynamics of the in situ response to be established. The transcriptome of L. lactis was analyzed in a model cheese matrix at different stages during the fermentation and ripening processes, i.e., in the mid-exponential phase and in the early and late stationary growth phases.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
Lactococcus lactis subsp. lactis biovar diacetylactis LD61 (Soredab, La Boissière Ecole, France) was grown in cheeses made with ultrafiltrated (UF) retentate (UF-cheeses) as described by Ulve et al. (38). Briefly, milk was microfiltrated to remove contaminating bacteria and then ultrafiltrated to obtain a retentate (corresponding to milk concentrated 5.5-fold) as described by Hannon et al. (19). The UF retentate had the same composition as that used by Ulve et al. (38), with a final fat concentration of 5.23%, a pH of 6.54, and the following specifications: dry matter, 258.5 g/kg; fat, 52.3 g/kg; lactose, 40.9 g/kg; total nitrogen, 140.15 g/kg; noncaseinic nitrogen, 26.9 g/kg; nonprotein nitrogen, 1.61 g/kg; and salt, 6.2 g/kg.
L. lactis LD61 was grown for 15 h in M17 broth supplemented with 5 g/liter of lactose. UF retentate was heated to 25°C and inoculated with L. lactis at a concentration equivalent to 2 × 106 CFU/ml before 0.3 μl/ml of rennet (Maxiren 180; DSM Food Specialties, Delft, Netherlands) was added. UF-cheeses (30 g) were incubated for 8 h at 30°C and then transferred at 12°C in an incubation chamber. Cheese samples were taken for cell counting and chemical analysis immediately following inoculation and after 2 h, 8 h, 24 h, and 7 days of growth of L. lactis in the cheese. The numbers of CFU were determined after the separation of cells from the cheese as described by Ulve et al. (38), by plating the cells on M17 agar supplemented with 5 g/liter of lactose.
Chemical analysis of UF-cheeses.
The methods for determining sugar, organic acid, and free-amino acid concentrations were adapted from Michalski et al. (28). Each sample of cheese (20 g) was diluted four times in distilled water, homogenized using a Waring blender for three 20-s mixing sequences at high speed, and finally incubated for 1 h at 40°C. Then two different treatments were completed, depending on the analysis. For the determination of sugar and organic-acid contents, samples were centrifuged for 30 min at 4°C (3,000 × g). The supernatants were then filtrated on Whatman grade 40 paper and diluted with the same volume of H2SO4 at 0.02 N. After a 30-min centrifugation (10,000 × g; 4°C), the protein pellet was discarded and the clear solution was collected for determination of the sugar and organic-acid concentrations by high-performance liquid chromatography (HPLC) by using an Aminex A-6 ion exchange column (Dionex, Sunnyvale, CA) at 55°C with 0.01 N H2SO4 as the eluent at a flow rate of 0.4 ml/min. Both UV (210 nm) and refractometric detectors were used.
The free-amino acid content was determined after deproteinization of the sample by sulfosalicylic acid (Merck-Eurolab, Grosseron S.A., Saint Herblain, France) according to the method of Mondino et al. (29). Aqueous cheese extracts (1 ml) were treated with 50 mg of sulfosalicylic acid, shaken for 15 s, and incubated for 1 h at 4°C. The mixtures were centrifuged at 5,000 × g for 15 min at 4°C. The supernatants were filtered through a 0.45-μm-pore-size membrane (Sartorius, Palaiseau, France), and the filtrate was diluted six times with a 0.2-mol/liter lithium citrate buffer (pH 2.2) before being injected. Amino acid analysis by ion exchange chromatography was carried out using a Pharmacia LKB Alpha Plus amino acid analyzer (Amersham Pharmacia Biotech Europe).
RNA extraction.
Cells were extracted from cheese samples (10 g) after 2 h, 8 h, 24 h, and 7 days of growth in cheese and were lysed according to the protocol published by Ulve et al. (38). Cell pellets were resuspended in a solution composed of Tris-EDTA (20 mmol/liter of Tris-HCl and 2 mmol/liter of EDTA [pH 8]) buffer and RLT buffer (from the Qiagen RNeasy minikit; Qiagen, Courtaboeuf, France) at a ratio of 30/70 (vol/vol). During RNA isolation, degradation is inhibited by the immediate suspension of the cells in RLT buffer, which is a stabilizing solution, and by always keeping the temperature lower than 4°C during all the different steps of the protocol. RNA was isolated according to the instructions in the RNeasy minikit. After the addition of 200 μl of chloroform to the RNA extracts and centrifugation (20 min at 12,000 × g; 4°C), the aqueous phase was purified using an RNeasy kit (Qiagen, Courtaboeuf, France) according to the manufacturer's instructions. Then DNase treatments of the RNA samples were performed using a DNA-free kit (Ambion, Austin, TX) according to the manufacturer's instructions. Quantification of the RNA and its contamination by proteins were assessed spectrophotometrically using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Rockland, DE), and the quality of the preparation was evaluated using an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA). The absence of contamination of the RNA samples by genomic DNA was confirmed by quantitative PCR.
Transcriptomic analyses and statistical treatments.
Membrane spotting and analytical support were provided by the Genopole biochips platform (Toulouse, France). Gene expression was measured by using nylon arrays displaying 1,948 open reading frames (ORFs) of L. lactis IL1403 of the 2,310 ORFs identified in the genome by Bolotin et al. (4). The PCR set was provided by Eurogentec and was spotted in duplicates on arrays. Since the PCR set was designed for the L. lactis strain IL1403, we verified, by comparative genomic hybridization (CGH) experiments, that the genomic content of the strain used in this work (L. lactis LD61) was very similar to the IL1403 strain (P. Tan-a-ram, T. Cardoso, M. Daveran-Mingot, S. Kanchanatawee, P. Loubière, L. Girbal, and M. Cocaign-Bousquet, submitted for publication). A constant amount (10 μg) of RNA extracted from cheese samples (see above) was retrotranscribed. Synthesis of radiolabeled cDNA, nylon array hybridizations, and washings were performed as previously described in Redon et al. (35). Membranes were exposed to a phosphorimager screen for 3 days and scanned with a phosphorfluoroimager (Storm 860; Molecular Dynamics, MD). For each sampling time (2 h, 8 h, 24 h, and 7 days), at least three independent analyses were performed, corresponding to different UF-cheese fabrications.
Hybridization signals were quantified and statistically treated as previously described (10, 34, 35). Briefly, the local background was removed, and signals were normalized by the mean intensity of the corresponding membrane. Expression ratios were calculated using the 2-h sample as a reference. Only expression ratios higher than 1.2 or lower than 0.8 were considered. Student t tests were performed, and the statistical significances of the expression ratios were evaluated using the Student t test (P < 0.05). These selected genes corresponded to a false discovery rate (FDR) lower than 10%.
A functional analysis was performed according to the gene categorization established by Bolotin et al. (4). In order to determine expression changes at the levels of the functional categories and subcategories (global tendencies), the levels of enrichment of over- or underexpressed genes in the different groups were calculated with the Wilcoxon test as previously described (10). All the genes were used in this test without any selection, and a P value lower than 0.05 was considered to be significant. Table 1 shows the expression profiles of the genes discussed in this paper.
TABLE 1.
Genes differentially expressed over time during growth of L. lactis LD61 in UF-cheese identified by the Student and FDR tests
| Functional category and family | Gene | Intensity ratio or pW (increased [up] or decreased [down] expression)a |
||
|---|---|---|---|---|
| 8 h | 1 day | 7 days | ||
| Amino acid biosynthesis | ||||
| Aromatic amino acid family | 0.0221 (up) | |||
| trpG | 2.7 | |||
| tyrA | 0.7 | 1.3 | ||
| Aspartate family | asnB | 0.5 | ||
| hom | 0.6 | |||
| lysA | 0.7 | 0.6 | ||
| metB2 | 0.5 | 4.9 | ||
| thrA | 0.6 | |||
| Branched-chain family | ||||
| ilvD | 0.6 | |||
| Glutamate family | 0.0008 (down) | |||
| gltA | 0.4 | |||
| Serine family | 0.0201 (down) | |||
| cysK | 3.0 | |||
| glyA | 1.7 | |||
| Cellular processes | ||||
| Chaperones | 0.0396 (up) | 0.0206 (up) | ||
| dnaK | 1.8 | 1.6 | 3.8 | |
| groES | 1.6 | 1.6 | ||
| Biosynthesis of cofactors, prosthetic groups, and carriers | ||||
| Thioredoxin, glutaredoxin, and glutathione | 0.0398 (up) | |||
| gpo | 1.7 | |||
| gshR | 1.6 | |||
| trxB2 | 1.5 | |||
| Cell envelope | 0.0030 (down) | 0.0403 (down) | ||
| Murein sacculus and peptidoglycan | murC | 1.6 | ||
| Fatty acid and phospholipid metabolism | 0.0070 (up) | |||
| Fatty acid and phospholipid metabolism | 0.0070 (up) | |||
| fabI | 1.5 | 1.6 | ||
| fadA | 1.2 | 1.2 | ||
| lplL | 2.5 | 3.1 | ||
| Central intermediary metabolism | 0.0172 (up) | |||
| Degradation of polysaccharides | 0.0477 (up) | |||
| chiA | 1.8 | 1.4 | ||
| yucG | 1.8 | 2.1 | ||
| Other | 0.0355 (down) | |||
| glgA | 0.6 | |||
| glgC | 1.4 | 1.5 | 0.6 | |
| glgD | 0.5 | |||
| Energy metabolism | 6.8E−5 (up) | 5.7E−5 (up) | 0.0020 (up) | |
| Aerobic | noxB | 1.7 | ||
| noxE | 2.2 | 3.2 | ||
| Amino acids and amines | arcB | 2.4 | ||
| arcC1 | 1.4 | |||
| ATP-PMF conversion | 0.0012 (up) | |||
| atpD | 1.6 | 1.2 | ||
| atpF | 1.6 | |||
| atpG | 1.7 | |||
| Electron transport | qor | 1.6 | ||
| Fermentation | 0.0187 (up) | 0.0221 (up) | 0.0385 (up) | |
| adhA | 1.7 | 2.4 | 2.1 | |
| aldC | 1.6 | |||
| butA | 2.9 | 2.3 | ||
| butB | 2.2 | 3.8 | 3.0 | |
| pfl | 3.0 | 2.1 | ||
| pflA | 1.6 | |||
| Glycolysis | 0.0213 (up) | 0.0038 (up) | 0.0016 (up) | |
| enoA | 1.5 | 1.8 | ||
| gapA | 3.2 | 3.6 | ||
| pgk | 1.8 | 1.6 | ||
| Pyruvate dehydrogenase | 0.0007 (up) | 0.0010 (up) | 0.0007 (up) | |
| pdhA | 2.4 | 2.3 | 2.7 | |
| pdhB | 1.7 | 1.9 | 2.4 | |
| pdhC | 2.6 | 2.6 | 2.9 | |
| pdhD | 1.8 | 2.1 | 2.3 | |
| Sugars | 2.1E−5 (up) | 2.1E−7 (up) | 0.0002 (up) | |
| bglA | 1.8 | |||
| galK | 2.0 | 3.6 | 1.6 | |
| galM | 4.6 | 3.5 | 2.4 | |
| galT | 1.9 | 3.6 | ||
| lacC | 2.6 | 2.2 | ||
| lacZ | 2.8 | |||
| scrK | 2.5 | 1.4 | ||
| xylA | 1.8 | |||
| yrcA | 2.6 | |||
| uxuB | 1.6 | |||
| TCA cycle | 0.0024 (down) | 0.0039 (down) | ||
| citC | 1.5 | 0.5 | 0.6 | |
| citD | 1.9 | 0.3 | 0.6 | |
| citE | 2.2 | |||
| citF | 2.5 | |||
| Other categories | 0.0014 (down) | 0.0043 (down) | 0.0001 (down) | |
| Adaptations and atypical conditions | 0.0308 (up) | |||
| clpE | 1.6 | 2.7 | ||
| clpP | 1.6 | |||
| dinF | 1.6 | |||
| grpE | 1.7 | 2.6 | ||
| hrcA | 1.4 | 1.4 | ||
| Phage-related functions and prophages | 0.0123 (down) | 0.0288 (down) | 0.0002 (down) | |
| pi103 | 0.5 | 0.7 | ||
| pi107 | 0.8 | 0.5 | ||
| pi111 | 1.4 | |||
| pi124 | 1.5 | |||
| pi125 | 1.3 | |||
| pi127 | 0.6 | |||
| pi128 | 0.7 | |||
| pi139 | 0.5 | 0.4 | 0.4 | |
| pi143 | 1.6 | |||
| pi145 | 0.3 | 0.4 | ||
| pi147 | 0.5 | |||
| pi202 | 1.4 | |||
| pi203 | 1.5 | 1.5 | ||
| pi217 | 0.5 | |||
| pi226 | 0.6 | 0.5 | 0.6 | |
| pi229 | 1.4 | |||
| pi233 | 1.4 | |||
| pi239 | 1.3 | 1.4 | 1.6 | |
| pi251 | 0.7 | |||
| pi316 | 2.0 | |||
| pi317 | 0.7 | 0.7 | ||
| pi322 | 1.3 | |||
| pi326 | 1.5 | |||
| pi327 | 1.4 | |||
| pi330 | 0.5 | |||
| pi345 | 0.6 | 0.5 | ||
| pi349 | 0.7 | 0.6 | ||
| pi350 | 1.9 | |||
| pi358 | 0.6 | |||
| pip | 0.6 | |||
| ps101 | 0.7 | 0.6 | ||
| ps104 | 1.4 | |||
| ps106 | 1.3 | 1.5 | ||
| ps116 | 0.3 | 0.2 | ||
| ps117 | 0.6 | |||
| ps119 | 0.6 | |||
| ps122 | 0.6 | 0.5 | ||
| ps123 | 0.6 | 0.4 | ||
| ps201 | 0.7 | 0.8 | ||
| ps203 | 0.6 | |||
| ps301 | 0.5 | 0.5 | ||
| ps302 | 0.5 | 0.5 | ||
| ps303 | 0.3 | 0.2 | ||
| ps304 | 0.5 | |||
| ps305 | 0.6 | 0.5 | ||
| ps306 | 1.5 | |||
| ps316 | 0.7 | |||
| Purines, pyrimidines, nucleosides, and nucleotides | 0.0153 (up) | 5.5E−9 (up) | ||
| Purine ribonucleotide biosynthesis | 3.5075E−5 (up) | 1.4022E−8 (up) | ||
| purC | 2.1 | 2.7 | ||
| purD | 1.8 | 3.7 | ||
| purK | 2.0 | |||
| purM | 2.7 | |||
| purN | 3.5 | |||
| Replication | ||||
| DNA replication, restriction, modification, recombination, and repair | dnaE | 1.3 | ||
| Translation | 0.0253 (down) | 1.9659E−7 (down) | 0.0026 (down) | |
| Degradation of proteins, peptides, and glycopeptides | pepC | 0.4 | ||
| pepN | 0.5 | |||
| pepO | 0.4 | 0.3 | ||
| pepP | 2.0 | |||
| pepXP | 0.6 | 0.5 | ||
| Ribosomal proteins: synthesis and modification | 2.4E−6 (down) | 0.0410 (down) | ||
| rplB | 0.2 | |||
| rplM | 0.6 | |||
| rplQ | 0.4 | 0.4 | ||
| rplU | 0.5 | |||
| rplV | 0.4 | 0.5 | ||
| rpmGB | 0.4 | |||
| rpmJ | 0.5 | 0.4 | ||
| rpsA | 0.6 | |||
| rpsB | 0.5 | |||
| rpsC | 0.5 | 0.5 | ||
| rpsJ | 0.5 | |||
| rpsK | 0.6 | 0.7 | ||
| rpsL | 0.9 | |||
| rpsN | 0.6 | 0.5 | ||
| rpsN2 | 0.5 | 0.5 | ||
| Transcription | ||||
| RNA synthesis and modification and DNA transcription | rpoD | 1.5 | 2.1 | |
| Transport and binding proteins | 0.0012 (down) | 0.0058 (down) | ||
| Amino acids, peptides, and amines | 0.0120 (down) | 0.0011 (down) | ||
| arcD1 | 0.7 | 0.7 | 0.6 | |
| arcD2 | 0.5 | |||
| ctrA | 0.3 | 0.2 | ||
| lysQ | 1.5 | 1.4 | ||
| oppA | 0.2 | 0.1 | ||
| oppB | 0.2 | 0.1 | ||
| oppC | 0.2 | 0.1 | ||
| oppD | 0.3 | 0.1 | ||
| oppF | 0.2 | 0.1 | ||
| optC | 0.6 | |||
| optD | 0.4 | 0.5 | ||
| optS | 0.2 | 0.2 | ||
| yagE | 0.7 | 0.7 | 0.7 | |
| ydgB | 0.5 | |||
| ydgC | 0.6 | |||
| yjgC | 1.9 | |||
| ylcA | 1.5 | 2.0 | ||
| yshA | 4.1 | |||
| Carbohydrates, organic alcohols, and acids | 0.0104 (up) | 0.0291 (up) | 0.0263 (up) | |
| msmK | 3.4 | 4.0 | ||
| rbsB | 2.7 | 1.7 | ||
| yngE | 1.7 | |||
| yngF | 1.4 | 2.0 | ||
| ypcG | 2.1 | 4.3 | 1.9 | |
| ypcH | 4.3 | 2.1 | ||
| ypdA | 3.1 | |||
| PTS system | 0.0052 (up) | 0.0055 (up) | 5.7E−5 (up) | |
| celB | 1.6 | 3.1 | 2.2 | |
| ptbA | 1.6 | 1.7 | ||
| ptcA | 1.8 | 2.5 | 1.7 | |
| ptcB | 2.9 | 2.3 | ||
| ptcC | 2.5 | 2.3 | ||
| ptND | 1.6 | 2.0 | ||
| Unknown | 1.8E−71 (down) | 1.2E−56 (down) | 4.2E−70 (down) | |
| yahB | 1.4 | |||
| yiaD | 1.5 | |||
| yjaB | 2.0 | 1.8 | ||
| yobA | 1.6 | |||
| ytgH | 2.6 | |||
The p-Wilcoxon values (pW) that allow calculation of gene enrichment in the different subcategories were calculated with all the genes of the functional subcategories and are given in the table only if significant (lower than the cutoff value of 0.05). PMF, proton motive force; TCA, tricarboxylic acid; PTS, phosphotransferase.
RT-PCR.
In order to confirm and complete the results from the transcriptomic analyses, quantitative reverse transcription (RT)-PCR experiments were carried out. cDNA was synthesized using a high-capacity cDNA archive kit (Applied Biosystems, Warrington, United Kingdom) as recommended by the manufacturer. Quantitative RT-PCR was performed using an Opticon 2 real-time PCR detector (Bio-Rad, Hercules, CA). The mixture contained power SYBR green PCR master mix (1×) (Applied Biosystems, Warrington, UK), each primer (0.5 μM) (sequences are shown in Table 2), and a cDNA template. Thermal cycling consisted of 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. Quantitative RT-PCR for all experimental time points was performed in triplicate (three independent biological replicates). Standard curves were generated to calculate the copy number of each gene in each sample. The gyrB gene was used as an internal standard for normalization, as previously described for milk (5). The stability of mRNA expression of gyrB in L. lactis was verified by using the geNorm VBA applet for Microsoft Excel (40). Gene expression was thus expressed relative to gyrB expression in each cDNA preparation and was calculated according to the following formula: number of copies of each gene/number of copies of gyrB. Statistical analysis was performed using analysis of variance (ANOVA); P values lower than 0.05 identified genes showing significant changes in expression over time. For direct comparisons with transcriptomic data, the results of quantitative reverse transcription-PCR were expressed as differences (n-fold) in transcript concentrations from those of the reference point. Ratios of gene expression levels analyzed by quantitative reverse transcription-PCR agreed with the differences observed by the transcriptomic approach (Table 2).
TABLE 2.
Comparison of microarray and RT-qPCR dataa
| Gene | Locus tag | Primer sequences |
Ratio of expression at indicated time as determined by: |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Transcriptome analysis |
RT-qPCR |
||||||||
| Forward | Reverse | 8 h | 1 day | 7 days | 8 h | 1 day | 7 days | ||
| bcaT | L0086 | GTTTGCTTTCACCATTGTTTAACT | ATTAAAAGCCTATCGAACAAAGGAT | 1.1 | 0.8 | 0.4 | 4.2 | 1.2 | 4.6E−02 |
| codY | L0243 | GTCCATTAGCAATCTTCCCAGA | CCACATGATAAATGTTCCCAAAC | 1.1 | 1.1 | 1.0 | 1.5 | 1.8 | 1.5 |
| serA | L0084 | CCACGATTATGAATTTTCCAAAG | CTACAATCCCTTTACTGGCACAC | 1.4 | 0.9 | 0.7 | 3.6 | 0.6 | 0.3 |
| cysK | L0089 | GAGGTTCAGTTAAAGACCGGATT | CTGTGTTCCCAGAGGTAGGTTC | 1.2 | 1.0 | 3.0 | 16.6 | 14.9 | 20.1 |
| gltD | L114827 | AGTCATTGGTGGTGGAGATACAG | CAGTGGGTAAACTTGGTGTGATT | 1.2 | 0.3 | 0.3 | 2.5 | 0.1 | 0.1 |
| lacC | L0032 | CAAAGATTGCTTCTAGTTCTTCTCG | GTGAAGATTTCTATGAGCGTTTGAT | 2.6 | 2.2 | 1.5 | 2.7 | 1.1 | 3.3 |
| gapA | L0004 | GTCTTGTTTTGCCTGAACTTTCT | TCGTCAACTGTAACGTGTTTTTC | 1.4 | 3.2 | 3.6 | 2.4 | 26.0 | 96.3 |
| gapB | L0005 | AACTCAAGGTCGTTTTGATGGTA | GTTAGCTGGGTTAGATTCAGCAG | 1.1 | 1.6 | 1.8 | 2.1 | 3.2 | 3.8 |
| pdhB | L0034 | CAAGCAAAATATGGAGAAGAACG | ATAATTGGGTGGAAACCTTGTGT | 1.7 | 1.9 | 2.4 | 1.3 | 7.3 | 3.6 |
| aldB | L0321 | TTTCACAAAATCAACCCGAATAC | GGAGATGATAACCAGCAACACTC | 1.4 | 1.2 | 1.1 | 4.7 | 2.7 | 2.6 |
| butA | L118271 | GGTAAAGATGATGAATGGGGAAT | GGCCAGCTAAGAATGAAACAAC | 1.5 | 2.9 | 2.3 | 5.0 | 3.8 | 11.3 |
| noxE | L196579 | ATTTCCTGCAATTATTTCACTCTTG | AATCGGCCTAGAAGTTTCATTTAGT | 0.9 | 2.2 | 3.2 | 1.0 | 9.4 | 7.6 |
| murF | L141766 | ATATAACGCGACGAATGCTATGT | CGATTTCTGGTCAGCTCTACTTC | 0.8 | 0.8 | 0.9 | 1.8 | 1.4 | 1.3 |
| dnaK | L178206 | TGGTGTCTTCACAAAATTGATTG | TGAATATCTACAGCGGGTTGATT | 1.8 | 1.6 | 3.8 | 3.6 | 2.3 | 33.6 |
| chiA | L9964 | GACTTGGTCAGTAAATTGGGATG | GGTTGATTGTTAAAGAGCATTGG | 1.2 | 1.8 | 1.4 | 2.7 | 4.9 | 37.9 |
| pepN | L102360 | TTTCCTCGATATTGACCGTAAAA | AAGTGTAAACCTTTGGCATGAAA | 1.3 | 0.9 | 0.5 | 1.8 | 1.1 | 0.5 |
| gyrB | L0283 | GGATGGAATTGCTGTTGAAGTAG | CTAAAACCTTGCTCATGTGTTCC | ||||||
| pi139 | L69762 | GGTTTGCATCTAAAACAAGAACG | TTGGACATTATCAATCACAGCAG | ||||||
| pi302 | L14521 | AATTCATCAAATTTCGGTTTAAGA | AAAAAGTAAGCTAGCATGAATGTG | ||||||
RT-qPCR, quantitative reverse transcription-PCR.
RESULTS AND DISCUSSION
Growth of L. lactis in UF-cheeses and transcriptome profiling.
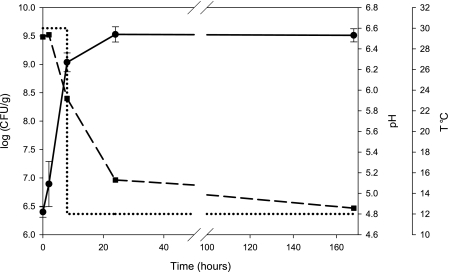
The growth of L. lactis subsp. lactis biovar diacetylactis strain LD61 in UF-cheeses was followed for 7 days (see Materials and Methods). The kinetics of growth, medium acidification, and temperature shift are reported in Fig. 1. The temperature was decreased from 30°C to 12°C after 8 h of culture (just after cheese sampling). UF-cheeses solidified upon rennet action between 2 h and 8 h of culture. Growth of L. lactis LD61 started immediately after inoculation without a noticeable lag phase. A growth deceleration phase was observed between 8 h and 24 h of culture, concomitant with the temperature decrease. The maximal lactococcal population (∼5 × 109 CFU/g) was reached after 24 h and, interestingly, remained stable between 24 h and 7 days (168 h) of culture. This strain of L. lactis has been previously shown to be able to lyse (30). However, the absence of cell lysis during the ripening of UF-cheeses has been observed previously with another L. lactis strain (19). As expected, L. lactis grown in UF-cheese demonstrated a classical homolactic metabolism, with lactate as the main fermentation product and a carbon conversion yield from lactose to lactate of over 0.80 (Table 3). While the L. lactis population remained constant after 24 h, in the stationary phase the consumption of lactose and production of lactate went on, leading to a slow postacidification rate. Between 24 h and 7 days, the pH decreased from 5.1 to 4.9.
FIG. 1.
Fermentation profiles of L. lactis strain LD61 under UF-cheese-making conditions. The kinetics of L. lactis growth (•), pH decrease (▪), and temperature shift (dotted line) are shown.
TABLE 3.
Lactose and citrate consumption and lactate production during the 7 days of culture
| Time of sampling (h) | Lactose (mmol/kg) | Lactate (mmol/kg) | Citrate (mmol/kg) | Carbon conversion yield (Ylactate/lactose [molC/molC])a | pH |
|---|---|---|---|---|---|
| 2 | 126 ± 24 | 2.7 ± 0.1 | 12.0 ± 0.4 | ND | 6.5 |
| 8 | 113 ± 10.8 | 49.1 ± 2.7 | 3.3 ± 0.6 | 0.82 | 5.9 |
| 24 | 86.8 ± 1.2 | 147.1 ± 17.1 | 0 | 0.81 | 5.1 |
| 168 | 57.9 ± 5.8 | 311.1 ± 98.1 | 0 | 0.86 | 4.8 |
Y, yield; molC, moles of carbon; ND, not determined.
The in situ L. lactis response during the cheese-making process was analyzed by a whole-genome transcriptomic approach. The transcriptome was analyzed in dynamics at four sampling times (i.e., at 2 h, 8 h, 24 h, and 7 days). The numbers of detected genes decreased slightly during the cheese-making process but remained high over time, representing nearly 70% of the genome (Table 4). This confirmed that good-quality results were obtained from RNA extracted in situ in the cheese matrix, even in the late stationary phase. In accordance with this finding, the total RNA extracted from L. lactis grown in UF-cheese was not degraded, even after 7 days, as confirmed by Agilent electrophoresis profiles (38). Compared to that at the 2-h time point sampling, the numbers of genes that were differentially expressed increased from 8 h to 24 h but stayed stable between 24 h and 7 days (∼460 genes) (Table 4). Around 530 genes were differentially expressed between 2 h and 24 h, but only ∼200 genes were differentially expressed between 24 h and 7 days, indicating that major transcriptional changes occurred for up to 24 h of the growth phase. Key elements of the adaptation of L. lactis to modified growth conditions (temperature and pH variations, evolution of substrate availability, and matrix composition) occurred before 24 h. Note that these various metabolic adaptations were always well distributed between over- and underexpression, revealing balanced metabolic activity of L. lactis LD61 in this particular but probably usual environment (Table 4). Most of the differentially expressed genes are given in Table 1.
TABLE 4.
Transcriptomic data for L. lactis LD61 in UF-cheese
| Description | Value at indicated sampling time |
|||
|---|---|---|---|---|
| 2 h | 8 h | 1 day | 7 days | |
| No. of genes detecteda | 1,677 | 1,513 | 1,392 | 1,375 |
| % of genes expressed | 82 | 74 | 68 | 67 |
| No. of genes with modified expression | 160 | 433 | 432 | |
| No. of overexpressed genes | 69 | 171 | 185 | |
| No. of underexpressed genes | 91 | 262 | 247 | |
Genes with spot intensities above the mean intensity of “empty” spots plus two standard deviations.
The metabolic activity of L. lactis slowed down but was maintained for 7 days.
L. lactis remained viable and metabolically active until day 7 in UF-cheese, as strongly indicated by slow rates of lactose consumption and acidification (Table 3) but also by the absence of mRNA degradation (see above). Consistent with this active metabolic state, punctual inductions of genes involved in important metabolic functions were observed exclusively in the late stationary phase. For instance, dnaE, essential for replication, was overexpressed at day 7, as was rpoD (ratio, 2.1), which codes for the RNA polymerase subunit, and murC, which is involved in cell wall biosynthesis (Table 1).
Although we confirmed that some genes maintained their metabolic statuses, the transcriptomic profile illustrated a general slowdown of L. lactis activity after 24 h, corresponding to growth arrest. Genes encoding the translation machinery, particularly ribosomal proteins (rplB, rplM, rplQ, rplU, and rplV; rpmGB and rpmJ; and rpsA, rpsB, rpsC, rpsJ, rpsK, rpsL, rpsN, and rpsN2), were underexpressed. This negative control was massive and was observed at the level of the whole subcategory (with significant p-Wilcoxon values of 2.5 × 10−6 and 4.1 × 10−2 at 24 h and 7 days, respectively [Table 1]). This underexpression of ribosomal protein is a distinctive signature of a decrease in the growth rate of L. lactis (10) and is in agreement with the reduced metabolic activity and growth arrest of L. lactis after 24 h. The growth arrest was also accompanied by the induction of a large number of genes involved in the general stress response. Several genes encoding chaperones and proteases (clpE and clpP, groES, and the hrcA-grpE-dnaK regulon) were overexpressed, as previously observed in milk (34), as were the general-stress-inducible gene ytgH, a homologue of the gls24 gene in Enterococcus faecalis (14), and the DNA damage-inducible gene dinF.
Induction of these stress-related genes clearly demonstrated that L. lactis had to adapt to substantial modifications of its environment during the UF-cheese-making process, which together contributed to L. lactis growth arrest, even though cells remained active and viable at day 7. In fact, UF-cheese is a complex matrix in which several parameters can influence L. lactis growth and metabolism and provoke nutritional limitations (e.g., free-amino acid limitation and carbon starvation) and/or various physicochemical stresses (pH, oxygen, or temperature). In order to identify the major environmental change(s) sensed by L. lactis during the UF-cheese-making process, transcriptomic profiles were unraveled and the signatures of various metabolic modifications and individual stress responses were searched out.
An efficient proteolytic system to cope with the small free-amino acid concentrations of UF-cheeses.
Milk contains small concentrations of free amino acids and peptides and high levels of proteins. In UF-cheese, milk proteins (casein and whey proteins), but not free amino acids, are concentrated by ultrafiltration to avoid the draining step. The initial levels of free amino acids were low in the UF-cheese (Table 5). In particular, the level of arginine, which can be used as an energy source for cell growth and maintenance and which was totally consumed by L. lactis LD61 at 24 h, was low in the UF-cheese (as in other cheeses). Apart from arginine, most amino acids were produced by proteolysis and accumulated progressively during fermentation, as illustrated by the 4-fold increase in the total free-amino acid content of the UF-cheese at day 7 compared to the initial composition (Table 5). Alanine, lysine, proline, valine, leucine, and particularly glutamate, with a concentration of 2,529 nmol/ml, were the preponderant amino acids quantified in the UF-cheese at day 7. A large accumulation of glutamate in the UF-cheese starting at 24 h is an interesting cheese property, as glutamate can be further metabolized by secondary flora, promoting the production of flavor compounds (3). Proline and lysine were the two main amino acids quantified during casein proteolysis in the UF-cheese at 8 h, in agreement with previous studies on caseinolysis (24).
TABLE 5.
Concentrations of free amino acids in UF-cheese over timea
| Amino acid | Initial concn in UF retentate (nmol/ml) | Concn (nmol/ml) in UF-cheese at indicated sampling time |
|||
|---|---|---|---|---|---|
| 2 h | 8 h | 1 day | 7 days | ||
| Thr | 9 | 10 | X | 26 | 578 |
| Ser | 13 | 17 | X | 20 | 292 |
| Gly | 81 | 78 | 18 | 69 | 272 |
| Met | ND | ND | ND | ND | 13 |
| Val | 40 | 39 | 52 | 439 | 1,338 |
| Ile | ND | ND | ND | 19 | 174 |
| Leu | ND | ND | ND | 125 | 699 |
| Tyr | ND | ND | 29 | 103 | 189 |
| Phe | ND | ND | 26 | 50 | 275 |
| Lys | 33 | 37 | 279 | 590 | 832 |
| His | ND | ND | 63 | 117 | 158 |
| Arg | 10 | 21 | 24 | ND | ND |
| Pro | ND | ND | 303 | 796 | 973 |
| Ala | 49 | 33 | 44 | 151 | 871 |
| Asp | 15 | 15 | 121 | 162 | 213 |
| Asn | ND | ND | ND | ND | 302 |
| Glu | 232 | 240 | 267 | 1,167 | 2,529 |
| Gln | ND | ND | ND | ND | 386 |
| Cit | ND | ND | 29 | 136 | 224 |
| Orn | ND | ND | ND | 31 | 162 |
| Total | 4,051 | 4,009 | 5,398 | 8,674 | 16,562 |
Cys and Trp were not detected at any sampling time. X, missing value; ND, not detected.
The large amounts of accumulated free amino acids confirmed the efficiency of the proteolytic system in L. lactis LD61 in UF-cheese. The first step in casein utilization by L. lactis is performed by a cell envelope proteinase, PrtP, that is regulated at the transcription level (27). In L. lactis LD61, the gene encoding PrtP is on a plasmid and was not spotted on the nylon membrane used for this experiment (a design based on the plasmid-free strain IL1403). prtP expression was thus determined by quantitative RT-PCR and showed a 10-fold increase in induction between 2 h and day 7 (data not shown). Concomitantly with this efficient proteolysis, nitrogen metabolism was globally downregulated. First, the expression of genes involved in oligopeptide transport (oppA, oppB, oppC, oppD, and oppF and optC, optD, and optS) decreased substantially after 24 h. Furthermore, only one peptidase was overexpressed at 24 h (pepP), while most of the others were underexpressed at 24 h (pepXP and pepO) and at 7 days (pepXP, pepO, pepN, and pepC). Second, the expression of most of the genes involved in amino acid transport decreased. Genes involved in arginine transport (arcD1 and arcD2), the genes yagE, ydgB, and ydgC, which encode amino acid permeases, and ctrA, which was recently demonstrated to be involved in branched-chain amino acid transport (9), were downexpressed. Only four genes involved in amino acid transport (lysQ, yjgC, ylcA, and yshA) were overexpressed at day 7. Lastly, genes involved in amino acid biosynthesis displayed more-balanced expression changes, with punctual regulation both up and down, except for the genes of the glutamate family, which were substantially underexpressed at day 7 (p-Wilcoxon value, 8.3 × 10−4 [Table 1]). Generally, these expression modifications were in agreement with the relative level of free amino acids accumulated in the UF-cheese. Genes involved in the biosynthesis of cysteine (cysK), methionine (metB2), tyrosine (tyrA), tryptophane (trpG), and glycine (glyA) were overexpressed at 7 days. These amino acids accumulated in smaller amounts in the UF-cheese (Table 5). Conversely, genes involved in the biosynthesis of the predominant accumulated amino acids were repressed (ilvD, lysA, and the genes of the glutamate family).
This global negative regulation of nitrogen metabolism was likely mediated by the pleiotropic transcriptional repressor CodY (16, 17). Numerous genes known to be under CodY control (hom; ilvD; gltA; thrA; pepC; pepN and pepO; ctrA; asnB; glgA, glgC, and glgD; oppA, oppB, oppC, oppD, and oppF; and optD and optS) were indeed downregulated at 7 days. However, upregulation at 7 days of prtP, another target of CodY, suggested that its expression was governed by an additional regulator(s). This positive regulation while general downregulation of peptidases was observed could confer bitterness to the final UF-cheese. Repression of the CodY regulon at day 7 was in accordance with the accumulation in UF-cheeses of large amounts of amino acids, including isoleucine, leucine, and valine, which act as corepressors of CodY. Conversely to our results, Bachmann et al. observed a global induction of CodY-responsive genes as a result of amino acid starvation in Gouda-type cheeses involving the overexpression of various genes of amino acid and peptide transport and metabolism (1). Thus, in the UF-cheese, L. lactis LD61 did not undergo any nitrogen limitation due to the efficient proteolytic system of L. lactis LD61.
Multiple strategies of adaptation to several stresses encountered by L. lactis.
Growth deceleration occurred after 8 h, while the pH was still high (5.9), significantly higher than the 4.7 limit for growth of this L. lactis strain in milk (34). Thus, the decrease in pH and the corresponding lactic acid inhibition could not entirely account for the growth arrest. We previously mentioned that proteolysis was efficiently active all through the 7 days of culture. Apart from its role in nitrogen supply, the release of amino acids via proteolysis generated ammonium that contributed to alkylination of the media and counteracted acidification. In agreement with such moderate acidic stress, only a mitigated transcriptomic response was observed for the three main mechanisms shown to confer acid resistance, i.e., the proton-translocating F0F1-ATPase involved in the expulsion of protons from the cell cytoplasm, the arginine deiminase, and the citrate decarboxylation pathways, both involved in pH homeostasis and energy production (8, 13, 39). First, only 3 of the 7 genes encoding the different subunits of the F1F0-ATPase complex were overexpressed in the UF-cheese (atpG and atpD at 8 h and atpD and atpF at day 7). Second, genes involved in citrate metabolism, citC, citD, citE, and citF, were only transiently overexpressed at 8 h in accordance with the consumption of citrate (Table 3). However, at 8 h, the pH of the medium was still high (5.9) and corresponded to conditions of low stress, indicating that this mechanism was not related to acidic/lactic stress but more likely to energy metabolism. Finally, genes involved in the deiminase pathway (ADI), such as the ADI pathway activator ahrC (23) and genes arcB and arcC1, were punctually overexpressed in the dynamic, while both major genes involved in the arginine/ornithine antiport, arcD1 and arcD2, were underexpressed (Table 1). Accordingly, in the UF-cheese, citrulline appeared at 8 h (i.e., at a nonstressing pH), whereas ornithine was produced later, at 24 h, at pH 5.1 (Table 3). This early citrulline production at 8 h was consistent with ADI pathway activity at optimal pH conditions (39) and thus confirmed the lack of severe acidic stress in the UF-cheese. The ADI pathway was, in comparison, strongly induced in milk culture of the same L. lactis strain (34), but the rate of acidification was significantly higher. In the UF-cheese, the pH reached 4.8 at day 7, while in milk, the pH decreased to 4.9 after 11 h of culture. The smaller decrease in pH in UF-cheese than in milk, due in part to the high buffering capacity of this matrix, resulted in a lower level of acidic/lactic stress.
In addition to the specific response of L. lactis to media acidification, we noticed the induction of several genes known to be part of the oxidative-stress response. Similar to the L. lactis response to aeration, overexpression of several genes shown to be induced in aerated conditions (32) was also observed in the cheese matrix at 7 days. Three genes coding for a UspA-like protein (yahB, yjaB, and yobA), a quinone reductase (qor), genes involved in fatty acid metabolism (fabI, fadA, and lplL), and two transporters (yshA and yxbD) showed increased expression in the UF-cheese after 24 h. noxE, the gene coding for the major cytoplasmic NADH oxidase, was overexpressed, with a ratio of 3.2 at 7 days, as were noxB and yiaD, coding respectively for NADH dehydrogenase and NADH reductase (Table 1). Last but not least, genes involved in the biosynthesis of cysteine and methionine (cysK and metB2), trxB2, which codes for a thioredoxin reductase, and genes involved in glutathione metabolism (gpo and gshR) were overexpressed in the stationary phase (Table 1). An oxidative-stress response can be linked to the presence of oxygen but also to oxygen-independent stresses such as the stringent response, the acid stress response, and growth rate modifications (10, 12). In controlled anaerobic conditions in milk, Raynaud et al. (34) already observed a partial response to oxygen, including the expression of trxB2; gpo; butA and butB; qor; and pdhB and pdhD (34). In UF-cheese, oxygen was present in the environment, probably at nonlimiting levels, given the small size of the cheeses (30 g). However, we cannot exclude that this oxidative-stress response may also be part of a more global cross-protection response related to other stresses encountered in UF-cheese. Notably, no cold shock proteins were found to be induced after the temperature shift to 12°C, but most of them are induced at lower temperatures and only transiently (31). Their likely weak and transient induction could thus have been missed in our experiment.
Lastly, a massive overexpression of genes involved in central carbon metabolism was observed in the UF-cheese. Genes involved in the utilization of lactose/galactose via the Leloir pathway (lacZ and galM, galK, and galT) or via the tagatose pathway (lacC) were overexpressed at 24 h and/or at 7 days. In addition, several genes involved in the utilization of carbon sources other than the lactose available in the UF-cheese were also overexpressed. Genes encoding transport proteins for various carbon sources, such as cellobiose, saccharose, mannose, ribose, β-glucosides, and other undefined carbon substrates (yngE and yngF; ypcG and ypcH; msmK; ypdA; rbsB; celB; ptbA; ptcA, ptcB, and ptcC; and ptn D), as well as genes specifically involved in these different sugar metabolisms (scrK, xylA, bglA, yrcA, and uxuB) or the degradation of polysaccharides (chiA and yucG) were upregulated. Downstream in the metabolic pathway, genes involved in glycolysis, gapA, pgk, and enoA, coding respectively for glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, and enolase, were overexpressed at 24 h and 7 days. Finally, the expression of several genes involved in alternative pathways of pyruvate conversion was also increased after 24 h, even if L. lactis metabolism remained homolactic throughout the culture. Genes coding for the subunits of the pyruvate dehydrogenase (pdhA, pdhB, pdhC, and pdhD), the alcohol dehydrogenase (adhA), and the pyruvate formate lyase (pfl) were induced at 24 h and day 7 (Table 1). Likewise, genes involved in the production of diacetyl and 2,3-butanediol (aldC, butA, and butB) were also overexpressed following the exponential phase (Table 1).
Such massive overexpression of the central carbon metabolism is the main characteristic of carbon starvation (36), due mainly to the release of the CcpA control (41). Many genes repressed by CcpA were induced in the UF-cheese after 24 h, including genes of the gal operon (galK, galM, and galT), pyruvate metabolism (pdhB, pdhC, and pdhD; butA and butB; and adhA), and cellobiose transport (ptcA, ptcB, and ptcC). Similarly, genes related to nitrogen metabolism (cysK and glyA) and purine metabolism (purC, purD, purK, purM, and purN), known to be repressed by CcpA, were induced (Table 1). In accordance with carbon catabolic repression being relieved, no activation of the las operon was observed. As shown in Table 3, lactose was not exhausted during the 7 days of culture; thus, the induction of the carbon starvation response could be related to a decrease in lactose flux consumption provoked by the temperature decrease. This lactose flux consumption decrease was effectively visualized by the reduction of L. lactis activity from 8 h onwards. Such apparent carbon limitation in spite of lactose being left in the UF-cheese underlines local limitations in the cheese matrix (lactose diffusion gradient), since this phenomenon was not observed in noncoagulating milk by Raynaud et al. (34).
In conclusion, L. lactis encountered several stresses in UF-cheese, including acidic and oxidative stresses, as well as a drop in temperature and an unexpected carbon limitation. The L. lactis strain LD61 induced several specific responses to counteract these stressful or nonoptimal conditions. Nevertheless, stresses encountered by L. lactis were progressive, allowing L. lactis to adapt by multiple strategies, which in turn may account for the maintenance of cell viability through 7 days. In other words, the multiple stresses encountered by L. lactis LD61 in UF-cheese could be considered moderately stressful for this strain.
Expression of phage-related genes was repressed.
The autolysis of lactic acid bacteria is of special importance for optimum cheese maturation by the release of intracellular enzymes (peptidases, esterases, and enzymes of amino acid catabolism) (26). Despite their commercial success, UF-cheeses have been reported repeatedly to be far slower in ripening than traditional cheeses (22). Several hypotheses have been proposed to explain this disadvantage, such as the high whey protein concentration or the large buffering capacity, which could delay the rate of autolysis (18, 37). Even though several solutions to the question of what influences the rate of proteolysis in UF-cheeses have been proposed, the genetic components controlling autolysis in this matrix have been poorly studied. Lysis in L. lactis involves bacterial peptidoglycan hydrolases, such as AcmA, AcmB, AcmC, AcmD, and YjgB (6, 20, 21), capable of hydrolyzing bonds in their own protective cell wall peptidoglycan, or prophage induction under environmental-stress-releasing endolysins (25, 26).
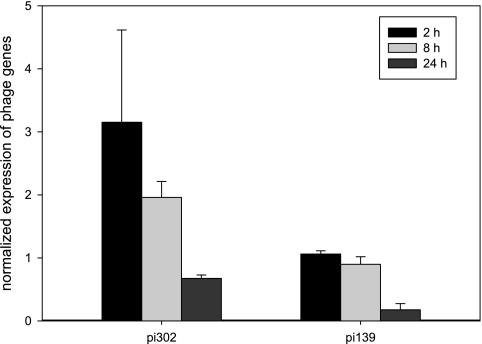
Previously, a drastic reduction of autolysis was observed in L. lactis subsp. cremoris strain AM2 when it was cured from its prophage (25). However, this result was not confirmed in UF-cheeses (18). Both strains, with or without prophages, showed a delayed autolysis, while in traditional cheeses, the AM2 strain was highly autolytic (7). In our study with UF-cheese, no autolysin genes were induced, and the whole category of phage-related genes was underexpressed (significant p-Wilcoxon value of 2 × 10−4 [Table 1]). Quantitative RT-PCR was carried out to confirm this result (Fig. 2). Both of the phage-related genes pi139 and pi302 were effectively repressed over time. To our knowledge, this is the first time that lactococci phage repression under UF-cheese conditions has been clearly reported. A high level of stress is considered to be a cause of prophage induction (10, 34, 36, 40). However, we showed that in UF-cheese, the stress conditions were progressive and moderate, which may have prevented prophage induction.
FIG. 2.
Gene expression profiles of some phage-related genes evaluated by quantitative RT-PCR during the process of making UF-cheese with L. lactis LD61.
Concluding remarks.
In this study, the global gene expression of L. lactis in UF-cheese was determined from the early exponential to the late stationary phase, allowing the dynamics of L. lactis adaptation to this complex matrix to be unraveled. The major constraint related to the low levels of free amino acids in the cheese matrix was bypassed by an efficient proteolytic system. Nevertheless, during the UF-cheese-making process, L. lactis had to cope with several stresses, including a temperature decrease to 12°C, acidic and oxidative stresses, and carbon limitation, although lactose was left in the cheese matrix. L. lactis counteracted these progressive stresses by multiple efficient strategies, remaining metabolically active and viable for 7 days. The lack of severe stresses in UF-cheese may, in part, account for the noninduction of prophages under these conditions and thus to delayed autolysis. This transcriptomic analysis of L. lactis was performed in a controlled and flexible cheese matrix but can easily be adapted to other cheese compositions and cheese-making processes. This work thus opens new perspectives for research for a better understanding of the physiology of L. lactis in situ.
Acknowledgments
Marina Cretenet is the recipient of a CIFRE Ph.D. fellowship from the Centre National Interprofessionnel de l'Economie Laitière. This research was supported by a grant from the Agence Nationale de la Recherche (GenoFerment Project).
Footnotes
Published ahead of print on 12 November 2010.
REFERENCES
- 1.Bachmann, H., L. de Wilt, M. Kleerebezem, and J. E. T. van Hylckama Vlieg. 2010. Time-resolved genetic responses of Lactococcus lactis to a dairy environment. Environ. Microbiol. 12:1260-1270. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, H., M. Kleerebezem, and J. E. T. van Hylckama Vlieg. 2008. High-throughput identification and validation of in situ-expressed genes of Lactococcus lactis. Appl. Environ. Microbiol. 74:4727-4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beresford, T. P., N. A. Fitzsimons, N. L. Brennan, and T. M. Cogan. 2001. Recent advances in cheese microbiology. Int. Dairy J. 11:259-274. [Google Scholar]
- 4.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. [sic] lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borezée-Durant, E., A. Hiron, J. C. Piard, and V. Juillard. 2009. Dual role of the oligopeptide permease Opp3 during growth of Staphylococcus aureus in milk. Appl. Environ. Microbiol. 75:3355-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buist, G., J. Kok, K. J. Leenhouts, M. Dabrowska, G. Venema, and A. J. Haandrikman. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 177:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapot-Chartier, M., C. Deniel, M. Rousseau, L. Vassal, and J. Gripon. 1994. Autolysis of two strains of Lactococcus lactis during cheese ripening. Int. Dairy J. 4:251-269. [Google Scholar]
- 8.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 9.den Hengst, C. D., M. Groeneveld, O. P. Kuipers, and J. Kok. 2006. Identification and functional characterization of the Lactococcus lactis CodY-regulated branched-chain amino acid permease BcaP (CtrA). J. Bacteriol. 188:3280-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dressaire, C., E. Redon, H. Milhem, P. Besse, P. Loubiere, and M. Cocaign-Bousquet. 2008. Growth rate regulated genes and their wide involvement in the Lactococcus lactis stress responses. BMC Genomics 9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ercolini, D. 2004. PCR-DGGE fingerprinting: novel strategies for detection of microbes in food. J. Microbiol. Methods 56:297-314. [DOI] [PubMed] [Google Scholar]
- 12.Frees, D., F. K. Vogensen, and H. Ingmer. 2003. Identification of proteins induced at low pH in Lactococcus lactis. Int. J. Food Microbiol. 87:293-300. [DOI] [PubMed] [Google Scholar]
- 13.García-Quintáns, N., C. Magni, D. de Mendoza, and P. Lopez. 1998. The citrate transport system of Lactococcus lactis subsp. lactis biovar diacetylactis is induced by acid stress. Appl. Environ. Microbiol. 64:850-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giard, J. C., N. Verneuil, Y. Aufray, and A. Hartke. 2002. Characterization of genes homologous to the general stress-inducible gene gls24 in Enterococcus faecalis and Lactococcus lactis. FEMS Microbiol. Lett. 206:235-239. [DOI] [PubMed] [Google Scholar]
- 15.Gitton, C., M. Meyrand, J. H. Wang, C. Caron, A. Trubuil, A. Guillot, and M. Y. Mistou. 2005. Proteomic signature of Lactococcus lactis NCDO763 cultivated in milk. Appl. Environ. Microbiol. 71:7152-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guédon, E., P. Serror, S. D. Ehrlich, P. Renault, and C. Delorme. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol. Microbiol. 40:1227-1239. [DOI] [PubMed] [Google Scholar]
- 17.Guedon, E., B. Sperandio, N. Pons, S. D. Ehrlich, and P. Renault. 2005. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in firmicutes. Microbiology 151:3895-3909. [DOI] [PubMed] [Google Scholar]
- 18.Hannon, J. A., S. M. Deutsch, M. N. Madec, J. Y. Gassi, M. P. Chapot-Chartier, and S. Lortal. 2006. Lysis of starters in UF cheeses: behaviour of mesophilic lactococci and thermophilic lactobacilli. Int. Dairy J. 16:324-334. [Google Scholar]
- 19.Hannon, J. A., C. Lopez, M. N. Madec, and S. Lortal. 2006. Altering renneting pH changes microstructure, cell distribution, and lysis of Lactococcus lactis AM2 in cheese made from ultrafiltered milk. J. Dairy Sci. 89:812-823. [DOI] [PubMed] [Google Scholar]
- 20.Huard, C., G. Miranda, Y. Redko, F. Wessner, S. J. Foster, and M. P. Chapot-Chartier. 2004. Analysis of the peptidoglycan hydrolase complement of Lactococcus lactis: identification of a third N-acetylglucosaminidase, AcmC. Appl. Environ. Microbiol. 70:3493-3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huard, C., G. Miranda, F. O. Wessner, A. Bolotin, J. Hansen, S. J. Foster, and M. P. Chapot-Chartier. 2003. Characterization of AcmB, an N-acetylglucosaminidase autolysin from Lactococcus lactis. Microbiology 149:695-705. [DOI] [PubMed] [Google Scholar]
- 22.Koning, P., R. Boer, P. Both, and P. Nooy. 1981. Comparison of proteolysis in a low-fat semi-hard type of cheese manufactured by standard and by ultrafiltration techniques. Neth. Milk Dairy J. 35:35-46. [Google Scholar]
- 23.Larsen, R., G. Buist, O. P. Kuipers, and J. Kok. 2004. ArgR and AhrC are both required for regulation of arginine metabolism in Lactococcus lactis. J. Bacteriol. 186:1147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence, R. C., L. K. Creamer, and J. Gilles. 1987. Texture development during cheese ripening. J. Dairy Sci. 70:1748-1760. [Google Scholar]
- 25.Lepeuple, A. S., L. Vassal, B. Cesselin, A. Acroix-Buchet, J. C. Gripon, and M. P. Chapot-Chartier. 1998. Involvement of a prophage in the lysis of Lactococcus lactis subsp. cremoris AM2 during cheese ripening. Int. Dairy J. 8:667-674. [Google Scholar]
- 26.Lortal, S., and M. P. Chapot-Chartier. 2005. Role, mechanisms and control of lactic acid bacteria lysis in cheese. Int. Dairy J. 15:857-871. [Google Scholar]
- 27.Marugg, J., R. Kranenburg, P. Laverman, G. Rutten, and W. Vos. 1996. Identical transcriptional control of the divergently transcribed prtP and prtM genes that are required for proteinase production in Lactococcus lactis SK11. J. Bacteriol. 178:1525-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalski, M.-C., B. Camier, V. Briard, N. Leconte, J.-Y. Gassi, H. Goudédranche, F. Michel, and J. Fauquant. 2004. The size of native milk fat globules affects physico-chemical and functional properties of Emmental cheese. Lait 84:343-358. [Google Scholar]
- 29.Mondino, A., G. Bongiovanni, S. Fumero, and L. Rossi. 1972. An improved method of plasma deproteination with sulphosalicylic acid for determining amino acids and related compounds. J. Chromatogr. 74:255-263. [DOI] [PubMed] [Google Scholar]
- 30.Nouaille, S., S. Even, C. Charlier, Y. Le Loir, M. Cocaign-Bousquet, and P. Loubiere. 2009. Transcriptomic response of Lactococcus lactis in mixed culture with Staphylococcus aureus. Appl. Environ. Microbiol. 75:4473-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panoff, J. M., S. Legrand, B. Thammavongs, and P. Boutibonnes. 1994. The cold shock response in Lactococcus lactis subsp. lactis. Curr. Microbiol. 29:213-216. [Google Scholar]
- 32.Pedersen, M. B., C. Garrigues, K. Tuphile, C. Brun, K. Vido, M. Bennedsen, H. Mollgaard, P. Gaudu, and A. Gruss. 2008. Impact of aeration and heme-activated respiration on Lactococcus lactis gene expression: identification of a heme-responsive operon. J. Bacteriol. 190:4903-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randazzo, C. L., S. Torriani, A. D. L. Akkermans, W. M. de Vos, and E. E. Vaughan. 2002. Diversity, dynamics, and activity of bacterial communities during production of an artisanal Sicilian cheese as evaluated by 16S rRNA analysis. Appl. Environ. Microbiol. 68:1882-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raynaud, S., R. Perrin, M. Cocaign-Bousquet, and P. Loubiere. 2005. Metabolic and transcriptomic adaptation of Lactococcus lactis subsp. lactis biovar diacetylactis in response to autoacidification and temperature downshift in skim milk. Appl. Environ. Microbiol. 71:8016-8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redon, E., P. Loubiere, and M. Cocaign-Bousquet. 2005. Role of mRNA stability during genome-wide adaptation of Lactococcus lactis to carbon starvation. J. Biol. Chem. 280:36380-36385. [DOI] [PubMed] [Google Scholar]
- 36.Redon, E., P. Loubiere, and M. Cocaign-Bousquet. 2005. Transcriptome analysis of the progressive adaptation of Lactococcus lactis to carbon starvation. J. Bacteriol. 187:3589-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saboya, L. V., H. Goudedranche, J. L. Maubois, A. L. S. Lerayer, and S. Lortal. 2001. Impact of broken cells of lactococci or propionibacteria on the ripening of Saint-Paulin UF-cheeses: extent of proteolysis and GC-MS profiles. Lait 81:699-713. [Google Scholar]
- 38.Ulve, V. M., C. Monnet, F. Valence, J. Fauquant, H. Falentin, and S. Lortal. 2008. RNA extraction from cheese for analysis of in situ gene expression of Lactococcus lactis. J. Appl. Microbiol. 105:1327-1333. [DOI] [PubMed] [Google Scholar]
- 39.van de Guchte, M., P. Serror, C. Chervaux, T. Smokvina, S. Ehrlich, and E. Maguin. 2002. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82:187-216. [PubMed] [Google Scholar]
- 40.Vandesompele, J., K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe, and F. Speleman. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zomer, A. L., G. Buist, R. Larsen, J. Kok, and O. P. Kuipers. 2007. Time-resolved determination of the CcpA regulon of Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 189:1366-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zúñiga, M., M. D. Miralles, and G. Perez-Martinez. 2002. The product of arcR, the sixth gene of the arc operon of Lactobacillus sakei, is essential for expression of the arginine deiminase pathway. Appl. Environ. Microbiol. 68:6051-6058. [DOI] [PMC free article] [PubMed] [Google Scholar]