Abstract
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) damages dopaminergic neurons in the substantia nigra pars compacta (SNpc) as seen in Parkinson's disease. Here, we show that the pro-apoptotic protein Bax is highly expressed in the SNpc and that its ablation attenuates SNpc developmental neuronal apoptosis. In adult mice, there is an up-regulation of Bax in the SNpc after MPTP administration and a decrease in Bcl-2. These changes parallel MPTP-induced dopaminergic neurodegeneration. We also show that mutant mice lacking Bax are significantly more resistant to MPTP than their wild-type littermates. This study demonstrates that Bax plays a critical role in the MPTP neurotoxic process and suggests that targeting Bax may provide protective benefit in the treatment of Parkinson's disease.
Parkinson's disease (PD) is a common neurodegenerative disorder whose cardinal clinical features include tremor, slowness of movement, stiffness, and postural instability (1). These disabling symptoms are primarily due to a profound deficit in striatal dopamine content that results from the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the consequent loss of their projecting nerve fibers in the striatum (2, 3). Although several approved drugs do alleviate PD symptoms, their chronic use often is associated with debilitating side effects (4), and none seem to dampen the progression of the disease. Moreover, the development of effective neuroprotective therapies is impeded by our limited knowledge of the mechanism by which SNpc dopaminergic neurons die in PD. Thus far, however, significant insights into the pathogenesis of PD have been achieved by the use of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which replicates in humans and nonhuman primates a severe and irreversible PD-like syndrome (5). In several mammalian species, MPTP reproduces most of the biochemical and pathological hallmarks of PD, including the dramatic degeneration of dopaminergic neurons (5).
Mounting evidence indicates that highly regulated cell death-associated molecular pathways could participate in the relentless demise of neurons in degenerative diseases (6, 7), including PD (8). In keeping with this, Bax (9) has emerged as a pro-cell death driving force within the central decision point constituted by the Bcl-2 family that modulates the activation of downstream effectors of cell death such as caspases (7). It is also clear that Bax is required for the death of several types of neurons in the peripheral and central nervous systems during both normal development and pathological situations (10–18). In light of its critical role within the programmed cell death machinery and its importance in neuronal death, Bax appears as a particularly appealing target for therapeutic interventions aimed at hampering neurodegeneration. Consistent with a potential pivotal role for Bax in SNpc neuronal death, here we show that Bax is highly expressed in the SNpc and that its ablation attenuates SNpc developmental neuronal apoptosis. We demonstrate that there is a dramatic up-regulation of Bax mRNA and protein in the SNpc of adult mice after MPTP administration. These changes parallel the time course of MPTP-induced dopaminergic neurodegeneration. We also show that mutant mice lacking Bax are resistant to MPTP compared with their wild-type littermates, thus indicating that Bax is a key factor in MPTP-induced SNpc dopaminergic neurodegeneration.
Materials and Methods
Animals and Treatment.
C57/bl mice heterozygous for Bax were mated to yield F1 offspring with Bax−/−, Bax+/−, and wild-type genotypes. Tail DNA was prepared and screened for both the normal and the mutant allele by using a single PCR. The normal allele was amplified by using an exon 5 forward primer (0.64 μM: 5′-TGATCAGAACCATCATG-3′) and an intron 5 reverse primer (0.64 μM: 5′-GTTGACCAGAGTGGCGTAGG-3′), which together generated a 304-bp product. The mutant allele was amplified with a neo/pgk primer (0.16 μM: 5′-CCGCTTCCATTGCTCAGCGG-3′) and the same intron 5 reverse primer, which together generated a 507-bp product. Cycling parameters were 1 min at 94°C, 55°C, and 72°C each for a total of 30–35 cycles. The primer ratio was adjusted to allow amplification of both products simultaneously with preferential amplification of the wild-type allele to assure correct genotyping of the Bax-deficient animals. All mice used in this study were treated according to National Institutes of Health guidelines for Care and Use of Laboratory Animals and with the approval of Columbia University's Institutional Animal Care and Use Committee. Eight-week-old male mice received one i.p. injection of MPTP-HCl per day (30 mg/kg per day of free base; Research Biochemicals, Natick, MA) for 5 consecutive days and were killed at 0, 2, 4, 7, 21, and 42 days after the last injection; control mice received saline injections only. Both saline and MPTP animals then were divided into two groups. The first group was perfused and brains were used for immunohistochemistry, whereas the second group of mice were killed, and brains were quickly removed, dissected (midbrain, striatum, cerebellum, and cortex), snap-frozen on dry ice, and stored at −80°C for Western blot, immunoprecipitation, and reverse transcriptase–PCR analysis. MPTP use and safety precautions were as described (19).
Immunohistochemistry and Double Immunofluorescence.
Immunohistochemistry was performed as described by Vila et al. (20) with a polyclonal antibody to Bax (1:500; polyclonal; PharMingen). Immunostained sections then were counterstained with thionin. To examine the colocalization of Bax with tyrosine hydroxylase (TH), a double immunofluorescence technique was performed by using the same polyclonal anti-Bax antibody (1:200 dilution) and a monoclonal antibody to TH (1:200 dilution; Boheringer Mannheim). Sections were examined on green, red, and double (green + red) filters by using confocal microscopy.
Striatal Lesions with Quinolinic Acid (QA).
After Metofane inhalation, mouse pups aged postnatal day seven of either sex received an intrastriatal injection of 0.5 μl of a 480 nmol solution of QA dissolved in PBS at pH 7.4 as described (21). One day after the QA injection, animals were perfused and brains were processed for morphological analysis.
Immunoblots and Immunoprecipitation.
For Western blot analysis, total tissue proteins were isolated in 50 mM Tris⋅HCl, pH 7.0/150 mM NaCl/5 mM EDTA/1% SDS/1% Nonidet P-40/protease inhibitors (Mini mixture; Roche Diagnostics, Indianapolis, IN). Incubation with primary antibody was performed overnight at 4°C with monoclonal antibodies to Bax (1:1,500 dilution; Santa Cruz Biotechnology) or Bcl-2 (1:500 dilution, Transduction Laboratories, Lexington, KY) and, as an internal control, a monoclonal antibody to β-actin (1:5,000, Sigma). Films were quantified by using the National Institutes of Health image analysis system. For immunoprecipitation, frozen samples from saline-injected mice and MPTP-intoxicated animals (at day 4 after the last MPTP injection) were homogenized in 10 vol (wt/vol) of 10 mM Hepes (pH 7.20) containing 0.25% Nonidet P-40, 142.5 mM KCl, 5 mM MgCl2, 1 mM EGTA, and one tablet of protease inhibitor mixture. Then, 250 μg of protein was incubated (overnight, 4°C) with 3 μg of a polyclonal antibody to Bcl-2 (N-19, Santa Cruz Biotechnology) and further processed for immunoprecipitation and immunoblotting as described by Ara et al. (22). Here, blots were immunostained with either a monoclonal antibody to Bax (1:1,500 dilution; Santa Cruz Biotechnology) or a monoclonal antibody to Bcl-2 (1:1,000 dilution; Transduction Laboratories).
RNA Extraction and Reverse Transcriptase–PCR.
Total RNA was extracted from midbrain, striatal, and cerebellar samples from saline and chronic MPTP-treated animals and used for reverse transcriptase–PCR analysis as described by Vila et al. (20). The Bax primer sequences were 5′-CTGAGCTGACCTTGGAGC-3′ (forward) and 5′-GACTCCAGCCACAAAGATG-3′ (reverse). As an internal control, β-actin cDNA was coamplified by using primer sequences 5′-CTTTGATGTCACGCACGATTTC-3′ (forward) and 5′-GGGCCGCTCTA GGCACCAA-3′ (reverse). Each PCR cycle consisted of denaturation at 94°C for 5 min, annealing at 55°C for 1 min, and extension at 72°C for 1.5 min, followed by a final 10-min extension at 72°C. PCR amplification was carried out for 30 cycles for Bax and 22 cycles for β-actin by using a Perkin–Elmer GeneAmp 9700 Thermal Cycler.
Stereology and Quantification of Apoptotic Neurons.
The total number of TH-positive SNpc neurons was counted in the different groups of animals at 21 days after the last MPTP or saline injection by using the optical fractionator method as described by Liberatore et al. (23). This unbiased method of cell counting is not affected by either the volume of reference (SNpc) or the size of the counted elements (neurons). Immunostaining was performed with a polyclonal antibody to TH (1:1,000; Calbiochem), and sections were counterstained with thionin. Quantification of the number of apoptotic neurons in the SNpc of MPTP- and saline-injected mice was assessed as described (21). Morphological criteria to identify apoptotic figures included shrinkage of cellular body, chromatin condensation, and the presence of distinct, round, well-defined chromatin clumps, demonstrated by thionin staining (21).
Measurement of Striatal Dopamine, 3,4-Dihydroxyphenylacetic Acid, and Homovanillic Acid Levels.
HPLC with electrochemical detection was used to measure striatal levels of dopamine, 3,4-dihydroxyphenylacetic acid, and homovanillic acid by using a method that has been described by Przedborski et al. (24), with minor modifications of the mobile phase. At 21 days after the last MPTP injection, animals were killed and striata were dissected out and processed for HPLC measurement. The modified mobile phase consisted of 0.15 M monochloroacetic acid, pH 3.0, 200 mg/liter sodium octyl sulfate, 0.1 mM EDTA, 4% acetonitrile, and 2.5% tetrahydrofuran.
Measurement of Striatal MPP+ Levels.
HPLC with UV detection (wavelength, 295 nm) was used to measure striatal MPP+ levels as described by Przedborski et al. (24). Groups of Bax+/−, Bax−/−, and wild-type littermates were killed at 90 and 180 min after one i.p. injection of 30 mg/kg MPTP, and the striata were dissected and processed for HPLC.
Statistical Analysis.
All values are expressed as the mean ± SEM with time, treatment, or genotype as the independent factors. When ANOVA showed significant differences, pair-wise comparisons between means were tested by Newman-Keuls post hoc testing. In all analysis, the null hypothesis was rejected at the 0.05 level.
Results
High Expression of Bax in SNpc Dopaminergic Neurons.
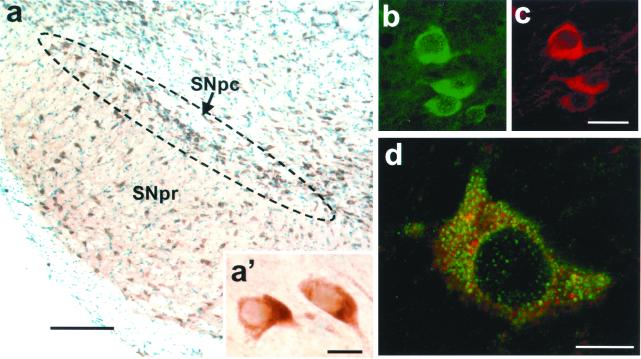
Relevant to the potential role of Bax in PD neurodegeneration, we found that virtually all neurons of the SNpc exhibit conspicuous levels of Bax protein, as evidenced by immunohistochemistry (Fig. 1a). SNpc neurons are primarily dopaminergic and secondarily GABAergic (25). Thus, to confirm that dopaminergic neurons do contain Bax protein, we performed double immunohistochemistry for TH, the rate-limiting enzyme in dopamine synthesis, and Bax. Examination by confocal microscopy demonstrated that all TH-positive neurons expressed Bax (Fig. 1 b and c) and, as expected given the ubiquitous expression of Bax in the brain, that Bax was expressed by both TH-positive and TH-negative neurons. Most Bax-positive SNpc dopaminergic neurons showed a prominent punctate immunoreactivity superimposed onto a diffuse cytoplasmic immunostaining (Fig. 1d), which is consistent with the known subcellular distribution of Bax in both mitochondria and cytosol (9, 26).
Figure 1.
Bax expression in SNpc dopaminergic neurons of adult mice. (a) Bax is highly expressed in SNpc neurons, as assessed by immunohistochemistry; sections are counterstained with thionin. (a′) High magnification of Bax-immunostained neurons in the SNpc. (b and c) Double immunofluorescence with antibodies to Bax and TH confirms that Bax (in green) is expressed in dopaminergic neurons (in red). (d) Confocal microscopy analysis of Bax-positive dopaminergic neurons (Bax + TH immunostaining) shows a robust punctate immunoreactivity superimposed onto a diffuse cytoplasmic immunostaining. [Scale bars: 200 μm (a), 10 μm (a′ and d), and 30 μm (b and c).]
Bax Modulates Developmental Cell Death in the SNpc.
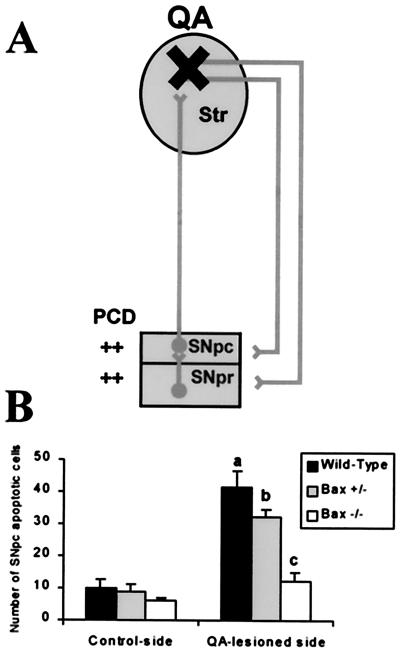
During development, neurons in the SNpc undergo an intense naturally occurring cell death process (21, 27). Dying neurons exhibit the morphological characteristics of apoptosis and their numbers are modulated by the size of striatum (21, 27), the brain structure in which SNpc neuron projections form synapses. Indeed, 24 h after unilateral destruction of the striatum with a local injection of the excitotoxin QA at postnatal day seven, wild-type pups showed four times more apoptotic neurons in the SNpc ipsilateral to the lesion compared with the contralateral side (Fig. 2). Age-matched mutant pups heterozygous (Bax+/−) or homozygous (Bax−/−) for the Bax null mutation showed a gene dosage-dependent reduction of SNpc apoptotic neurons after QA administration (Fig. 2).
Figure 2.
Bax regulates natural and QA-induced developmental neuronal death in the SNpc. (a) Schematic representation of the model of induced apoptotic cell death in the SNpc by unilateral destruction of the striatum (i.e., the target) at postnatal day seven with a local injection of QA. (b) 24 h after the lesion, wild-type mice (n = 5) exhibit a substantial number of dying neurons with a definite morphology of apoptosis in the contralateral SNpc and a dramatic increase in this number in the SNpc ipsilateral to the QA lesion. Age-matched mutant mice deficient for Bax (Bax+/− and Bax−/−, n = 4 per group) exhibit a striking lower number of SNpc apoptotic neurons after QA administration. a, P < 0.05, compared with wild-type control-side; b, P < 0.05, compared with Bax+/− control-side; c, P < 0.05, compared with wild-type and Bax+/− QA-lesioned sides but not significant when compared with Bax−/− control side; Newman-Keuls post hoc analysis.
MPTP Stimulates Bax Expression in Ventral Midbrain.
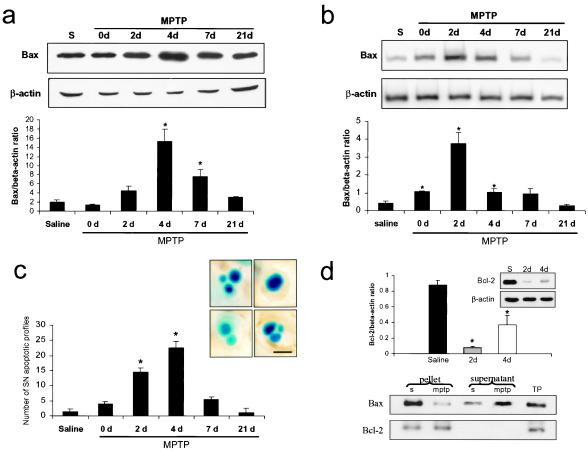
In saline-injected mice, there was a high constitutive expression of Bax protein in the ventral midbrain (Fig. 3a). After systemic MPTP administration, there was a dramatic up-regulation of Bax protein in this brain region (Fig. 3a), in agreement with a previous study (28). This change occurred in a time-dependent manner, with protein levels peaking at 4 days after the last MPTP injection (+668%), then progressively returning to control levels (Fig. 3a). This alteration was not only time-dependent but was also region-specific as MPTP-intoxicated mice showed no Bax up-regulation, at any of the time points studied, in striatum or in cerebellum, two brain regions devoid of neuronal loss after MPTP administration.
Figure 3.
Bax expression in the ventral midbrain after MPTP intoxication. (a) Bax protein levels in the ventral midbrain (n = 3 mice per group) were assessed by Western blot analysis. (b) Bax mRNA expression in the ventral midbrain was quantified by reverse transcriptase–PCR (n = 3–5 mice per group). (c) Bax protein and mRNA up-regulation coincide with the time course of apoptotic-induced cell death in the SNpc. Morphological criteria to identify apoptotic figures, as illustrated in photomicrographs, included shrinkage of cellular body, chromatin condensation, and the presence of distinct, round, well-defined chromatin clumps, demonstrated by thionin staining. (Scale bar, 5 μm.) (d) Bcl-2 protein expression (Upper) and immunoprecipitation (Lower) after MPTP intoxication. Bcl-2 protein levels are decreased in the ventral midbrain of MPTP-intoxicated mice at days 2 and 4 after the last MPTP injection (n = 3–5 mice per group). At day 4 after the last injection, ventral midbrain proteins (n = 4 mice per group) were subjected to immunoprecipitation with a polyclonal antibody to Bcl-2. The amount of Bax coimmunoprecipitated with Bcl-2 appeared less abundant in the pellets of MPTP-intoxicated mice than in those of saline-injected animals. This was associated with increased Bax immunoreactivity in the supernatant. S, saline; TP, total proteins. *, P < 0.05, compared with saline-injected animals; Newman-Keuls post hoc analysis. Error bars indicate SEM.
MPTP Increases Bax mRNA Levels in Ventral Midbrain.
Given the change in Bax protein after MPTP injections, we also investigated whether this change was associated with Bax transcriptional alterations. In saline-injected mice, there was a constitutive level of Bax transcript in the ventral midbrain (Fig. 3b). In MPTP-injected mice, there was a time-dependent increase in the level of Bax transcript, which peaked at 2 days after the last MPTP injection (+364%), then progressively returned to the level of controls by day 7 (Fig. 3b). Bax mRNA up-regulation was also region-specific as it was not detected in the striatum nor in the cerebellum of MPTP-intoxicated animals.
Time Course of MPTP-Induced Apoptotic Neuronal Death.
Quantification of apoptotic cells in the SNpc of MPTP- and saline-injected mice indicates that apoptotic neuronal death culminated between days 2 and 4 after the last MPTP injection (Fig. 3). Morphological criteria used to identify apoptotic cells were previously validated (21) and included shrinkage of cellular body, chromatin condensation, and presence of distinct, round, well-defined chromatin clumps, demonstrated by thionin staining.
MPTP Decreases Bax:Bcl-2 Heterodimerization in the Ventral Midbrain.
Several members of the Bcl-2 family, such as Bcl-2, can bind to Bax to form Bax:Bcl-2 heterodimers, hence antagonizing Bax pro-cell death properties (9). Accordingly, we determined the levels of Bcl-2 protein as well as its capacity to heterodimerize with Bax protein in ventral midbrain of MPTP-intoxicated mice, at the peak of MPTP-induced apoptotic neuronal death. In striking contrast with Bax up-regulation, Bcl-2 protein levels, as assessed by Western blot, were dramatically decreased in ventral midbrain of MPTP-intoxicated mice compared with saline-injected animals, 2 and 4 days after the last injection (Fig. 3d). Furthermore, the amount of Bax that coimmunoprecipitated with Bcl-2 at this time point, using an anti-Bcl-2 antibody, was much less in MPTP-intoxicated mice than in saline-injected animals (Fig. 3d). Consistent with this finding, the amount of Bax that escaped coimmunoprecipitation using an anti-Bcl-2 antibody was much greater in MPTP-intoxicated mice than in saline-injected animals (Fig. 3d). The ratio of these proteins indicates that most of the Bax protein could be inactivated by Bcl-2 in saline-injected mice whereas there is an excess of unopposed Bax in MPTP-injected mice.
Bax-Deficient Mice Are Resistant to MPTP Intoxication.
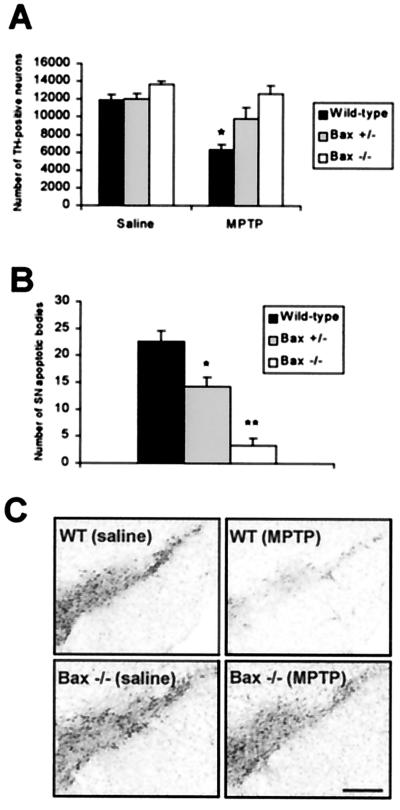
To confirm the involvement of Bax in MPTP-induced neuronal death, we compared the effects of MPTP in Bax+/− and Bax−/− mice and in their wild-type littermates. In saline-injected mice, no significant changes in stereological counts of SNpc dopaminergic neurons, defined by TH immunostaining, were detected between the different groups of mice (Fig. 4A). In wild-type mice, MPTP caused a dramatic loss of SNpc TH-positive neurons, which was accompanied by a large number of apoptotic neurons (Fig. 4 A and B). MPTP can down-regulate phenotypic markers such as TH (29), thus it is important to indicate that the TH/Nissl ratio of neuronal counts did not differ between saline- and MPTP-injected wild-type mice (saline = 1.78 ± 0.05 vs. MPTP = 1.77 ± 0.03; n = 3 per group; Student's t test), confirming that the reduction in TH-positive neurons corresponds to an actual loss of neurons. In contrast to the situation in wild-type animals, MPTP failed to affect SNpc TH-positive neuronal counts in Bax−/− and caused only a mild reduction of these numbers in Bax+/− mice (Fig. 4A). Similarly, the number of MPTP-induced SNpc apoptotic neurons was significantly smaller in Bax−/− and, to a lesser extent, in Bax+/− than in wild-type animals (Fig. 4B). Although less striking than the loss of the SNpc cell body counts, the loss in striatal dopaminergic nerve terminals after MPTP administration, as assessed by measuring the levels of dopamine and its two main metabolites 3,4-dihydroxyphenylacetic acid and homovanillic acid, was also markedly attenuated in Bax−/− and Bax+/− mice, compared with their wild-type littermates (Table 1).
Figure 4.
Bax-deficient mice are resistant to MPTP neurotoxic effect. (A) Stereological counts of TH-positive neurons in the SNpc were performed in Bax-deficient mice and their wild-type littermates at day 21 after the last injection (n = 3–5 mice per group). In wild-type mice, only 53% of the SNpc TH-positive neurons survived MPTP administration. In contrast, 81% of SNpc TH-positive neurons survived in Bax+/− mice and no loss of TH-positive cells was found in Bax−/− animals under an identical MPTP regimen. *, P < 0.05, compared with saline-injected wild-type animals; Newman-Keuls post hoc analysis. (B) At the peak of apoptotic cell death (day 4 after the last MPTP injection), Bax−/− mice (n = 3) presented 85% reduction in the number of apoptotic profiles in the SNpc compared with MPTP-intoxicated control animals (n = 4). In Bax+/− animals (n = 4), these numbers were reduced by 37%. *, P < 0.05 compared with MPTP-intoxicated control animals; **, P < 0.05 compared with MPTP-intoxicated control animals and MPTP-intoxicated Bax+/− mice; Newman-Keuls post hoc analysis). Error bars indicate SEM. (C) Photomicrographs of TH-immunostained sections with thionin counterstain, illustrating the results in A. (Scale bar, 400 μm.)
Table 1.
Striatal monoamine levels (ng/mg tissue)
| Mice | Dopamine | DOPAC | HVA |
|---|---|---|---|
| Saline | |||
| Wild type | 12.2 ± 0.2 | 2.1 ± 0.2 | 1.8 ± 0.1 |
| Bax+/− | 13.3 ± 0.3 | 2.0 ± 0.1 | 1.7 ± 0.1 |
| Bax−/− | 13.4 ± 0.4 | 2.4 ± 0.1 | 1.6 ± 0.2 |
| MPTP | |||
| Wild type | 0.5 ± 0.1 | 0.3 ± 0.05 | 0.2 ± 0.01 |
| Bax+/− | 3.1 ± 0.3* | 0.6 ± 0.06 | 0.6 ± 0.04* |
| Bax−/− | 4.1 ± 0.2** | 1.3 ± 0.2** | 1.0 ± 0.1** |
DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid. *, P < 0.05, compared to MPTP-injected wild-type mice; **, P < 0.05, compared to MPTP-injected wild-type and MPTP-injected Bax+/− mice; Newman-Keuls post hoc test. Data represent means ± SEM for 4–6 mice per group.
MPP+ Production in Bax-Deficient Mice.
The main determining factor of MPTP neurotoxic potency is its conversion in the brain to 1-methyl-4-phenylpyridinium ion (MPP+) (30). To confirm that the resistance of Bax−/− mice is due to the absence of the Bax gene and not to an alteration in the brain's production of MPP+, we measured striatal content of MPP+ at different time points after MPTP administration. At no time point does the striatal content of MPP+ differ significantly among Bax+/−, Bax−/−, and wild-type littermate mice (Table 2).
Table 2.
Striatal MPP+ levels (μg/g striatum) in Bax-deficient and wild-type mice
| Mice | 90 min | 180 min |
|---|---|---|
| Wild type | 8.5 ± 1.0 | 5.7 ± 0.9 |
| Bax+/− | 7.6 ± 1.9 | 5.1 ± 0.3 |
| Bax−/− | 10.0 ± 1.0 | 5.4 ± 0.8 |
HPLC measurements of striatal MPP+ levels in wild-type and Bax-deficient mice were determined at 90 and 180 min after a single i.p. MPTP injection (30 mg/kg). n = 4 animals per group. Values represent the mean ± SEM.
Discussion
Bax is widely expressed in the central nervous system, where it is detected primarily in neurons (9, 31). Herein, we show that almost all neurons of the SNpc, especially all dopaminergic neurons, contain abundant amounts of Bax protein (Fig. 1), likely located both at mitochondria and in cytosol (9). We also demonstrate that Bax controls the apoptotic demise of SNpc dopaminergic neurons during development, because its ablation attenuates SNpc developmental cell death in immature animals (Fig. 2). These findings confirm a key role for Bax in the fate of SNpc neurons, thus setting the stage for Bax being a potential culprit in the degeneration of SNpc dopaminergic neurons in PD.
To test the contribution of Bax in PD neurodegeneration, we used the experimental model produced by the parkinsonian neurotoxin MPTP (5). Because the mode of cell death in PD may be, at least in part, apoptotic (8), we selected a MPTP regimen that kills SNpc dopaminergic neurons by apoptosis (32). This regimen induces a time-dependent apoptotic cell death in the SNpc that is maximal between 2 and 4 days after the last dose of MPTP (Fig. 3c). Relevant to the known pro-apoptotic role of Bax, we found that the time course of SNpc apoptotic neuronal death coincides with that of increased levels of Bax mRNA and protein in ventral midbrain after MPTP administration (Fig. 3 a and b). The opposite image was found for Bcl-2 in that at 2 and 4 days post-MPTP ventral midbrain Bcl-2 protein levels were markedly reduced. These findings suggest that, during the MPTP-induced neurodegenerative process, the finely tuned balance between cell death agonists, such as Bax, and cell death antagonists, such as Bcl-2, is upset in the ventral midbrain, leading to a situation in which molecular pro-apoptotic forces dominate (33). In this context, an aspect related to Bax function is its capacity to form heterodimers with Bcl-2 and homomultimers with itself (34). In saline-injected mice, the amount of Bax can theoretically be neutralized by Bcl-2 as evidenced by the majority of Bax in heterodimers. Whereas, in MPTP-injected mice excess Bax exists free of neutralizing interaction with Bcl-2 (Fig. 3d). Taken together, our data suggest that, after MPTP administration, a cascade of deleterious events is set in motion within which Bax up-regulation and Bcl-2 down-regulation are key factors. Consistent with this scenario, the observed neuroprotective effects provided by Bcl-2 overexpression against MPTP (35, 36) may reflect its capacity to counter Bax.
Consistent with the involvement of Bax in the MPTP neurotoxic process is our demonstration that no significant loss of SNpc dopaminergic neurons was observed in Bax−/− mice and that approximately 81% of SNpc dopaminergic neurons survived in Bax+/− mice compared with their wild-type littermates after MPTP administration (Fig. 4). Similarly, there were significantly fewer apoptotic neurons in the SNpc of Bax+/− and Bax−/− after MPTP administration compared with wild-type controls (Fig. 4). The resistance of the SNpc dopaminergic neurons in Bax knockout mice was accompanied by a significant, although less prominent, sparing of striatal dopamine contents (Table 1). The latter suggests that Bax ablation protects against SNpc neuronal death, but still allows some changes in gene expression and/or alterations in dopamine synthesis. Relevant to this, is our previous demonstration that TH, the rate-limiting enzyme in dopamine synthesis, is inactivated by tyrosine nitration after MPTP administration (22).
We also found that ablation of Bax was not associated with alterations in the formation of MPTP active metabolite, MPP+ (Table 2), which is the most significant modulating factor of MPTP potency (30).
In light of the results reported above, including the resistance of Bax-deficient mice to the neurotoxic effects of MPTP, we argue that Bax is a critical effector molecule in MPTP-mediated cell death. Given the mode of action of MPTP and Bax, it is possible that the mitochondrion is key to the observed neuroprotection. Models of Bax activation indicate its oligomerization may result in a homomultimeric pore (37), a VDAC-containing pore (38), or a permeabilization of mitochondrial outer membrane (39) to release cytochrome c. Several lines of evidence indicate that translocation of mitochondrial cytochrome c to the cytosol is a critical event in the mitochondrial-dependent activation of effector caspases such as caspase-3 and ensuing cell death (40). Providing credence to this proposed sequence of events in PD is the observation that caspase-3 is indeed activated in postmortem SNpc samples from parkisonian patients (41). Once inside dopaminergic neurons, MPP+ is actively concentrated within mitochondria, where it inhibits complex I of the electron transport chain (5). This inhibition leads to a deficit in ATP formation and to an increase in reactive oxygen species production (5), which, in turn, cause an energy crisis and oxidative stress. As with other situations, mitochondrial dysfunction seen after MPTP administration ultimately can trigger large amplitude swelling often attributed to the opening of the permeability transition pore complex (PTPC). Alternatively, the opening of the PTPC can lead to several dramatic consequences, including a dissipation of the mitochondrial transmembrane potential and a release to the cytosol of proteins normally confined to the mitochondria, such as cytochrome c (42).
Collectively, our results indicate that Bax plays a pivotal role in SNpc dopaminergic neuronal death in the MPTP mouse model likely by acting in injured neurons before the onset of irreversible cell death events. Whether blocking events downstream of Bax also can protect these cells remains to be determined. Ablation of the cell executioner, caspase-3, dramatically decreases neuronal death during development (43). However, whether inhibition of caspases downstream of mitochondria will prove sufficient to interfere with adult-onset pathological stimuli or merely shift the mode of death of severely injured neurons remains uncertain. Because of the striking similarities between the MPTP model and PD, the present study raises the possibility that Bax plays a critical role in the neurodegenerative process of PD and thus that targeting Bax could open new neuroprotective avenues for this disabling neurological disease.
Acknowledgments
We thank Norma Romero and Caiping Chen for assisting in the genotyping, Eric Smith for his editorial assistance, and Theresa Swayne for her technical assistance with confocal microscopy. This study was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke Grants R29 NS37345, RO1 NS38586, and P50 NS38370, the Department of Defense (DAMD 17–99-1–9474), the Parkinson's Disease Foundation, the Lowenstein Foundation, the Smart Foundation, the Muscular Dystrophy Association, the Amyotrophic Lateral Sclerosis Association, and Project-ALS. M.V. is recipient of a fellowship from the Human Frontier Science Program Organization.
Abbreviations
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- SNpc
substantia nigra pars compacta
- PD
Parkinson's disease
- TH
tyrosine hydroxylase
- QA
quinolinic acid
- MPP+
1-methyl-4-phenylpyridinium ion
References
- 1.Fahn S, Przedborski S. In: Merritt's Neurology. Rowland L P, editor. New York: Lippincott Williams & Wilkins; 2000. pp. 679–693. [Google Scholar]
- 2.Hornykiewicz O, Kish S J. In: Parkinson's Disease. Yahr M, Bergmann K J, editors. New York: Raven; 1987. pp. 19–34. [Google Scholar]
- 3.Pakkenberg B, Moller A, Gundersen H J G, Mouritzen A, Pakkenberg H. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostic V, Przedborski S, Flaster E, Sternic N. Neurology. 1991;41:202–205. doi: 10.1212/wnl.41.2_part_1.202. [DOI] [PubMed] [Google Scholar]
- 5.Przedborski S, Jackson-Lewis V, Djaldetti R, Liberatore G, Vila M, Vukosavic S, Almer G. Restor Neurol Neurosci. 2000;16:135–142. [PubMed] [Google Scholar]
- 6.Rudin C M, Thompson C B. Annu Rev Med. 1997;48:267–281. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 7.Pettmann B, Henderson C E. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- 8.Burke R E, Kholodilov N G. Ann Neurol. 1998;44:S126–S133. doi: 10.1002/ana.410440719. [DOI] [PubMed] [Google Scholar]
- 9.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 10.Deckwerth T L, Elliott J L, Knudson C M, Johnson E M J, Snider W D, Korsmeyer S J. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 11.Shindler K S, Latham C B, Roth K A. J Neurosci. 1997;17:3112–3119. doi: 10.1523/JNEUROSCI.17-09-03112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White F A, Keller-Peck C R, Knudson C M, Korsmeyer S J, Snider W D. J Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deckwerth T L, Easton R M, Knudson C M, Korsmeyer S J, Johnson E M., Jr Exp Neurol. 1998;152:150–162. doi: 10.1006/exnr.1998.6846. [DOI] [PubMed] [Google Scholar]
- 14.Selimi F, Vogel M W, Mariani J. J Neurosci. 2000;20:5339–5345. doi: 10.1523/JNEUROSCI.20-14-05339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doughty M L, De Jager P L, Korsmeyer S J, Heintz N. J Neurosci. 2000;20:3687–3694. doi: 10.1523/JNEUROSCI.20-10-03687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong M J, Murray M R, Gosink E C, Russell H R, Srinivasan A, Kapsetaki M, Korsmeyer S J, McKinnon P J. Proc Natl Acad Sci USA. 2000;97:889–894. doi: 10.1073/pnas.97.2.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenmann S, Engel S, Gillardon F, Bahr M. Cell Death Differ. 1999;6:673–682. doi: 10.1038/sj.cdd.4400538. [DOI] [PubMed] [Google Scholar]
- 18.Jackson A C. Acta Neuropathol. 1999;98:288–294. doi: 10.1007/s004010051082. [DOI] [PubMed] [Google Scholar]
- 19.Przedborski, S., Jackson-Lewis, V., Naini, A., Jakowec, M., Petzinger, G., Miller, R. & Akram, M. (2000) J. Neurochem., in press. [DOI] [PubMed]
- 20.Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. J Neurochem. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- 21.Jackson-Lewis V, Vila M, Djaldetti R, Guegan C, Liberatore G, Liu J, O'Malley K L, Burke R E, Przedborski S. J Comp Neurol. 2000;424:476–488. doi: 10.1002/1096-9861(20000828)424:3<476::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Ara J, Przedborski S, Naini A B, Jackson-Lewis V, Trifiletti R R, Horwitz J, Ischiropoulos H. Proc Natl Acad Sci USA. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberatore G T, Jackson-Lewis V, Vukosavic S, Mandir A S, Vila M, McAuliffe W G, Dawson V L, Dawson T M, Przedborski S. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 24.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson V L, Dawson T M. Proc Natl Acad Sci USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dray A. Neuroscience. 1979;4:1407–1439. doi: 10.1016/0306-4522(79)90048-4. [DOI] [PubMed] [Google Scholar]
- 26.Hsu Y T, Wolter K G, Youle R J. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janec E, Burke R E. Mol Cell Neurosci. 1993;4:30–35. doi: 10.1006/mcne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 28.Hassouna I, Wickert H, Zimmermann M, Gillardon F. Neurosci Lett. 1996;204:85–88. doi: 10.1016/0304-3940(96)12323-5. [DOI] [PubMed] [Google Scholar]
- 29.Tatton W G, Kwan M M, Verrier M C, Seniuk N A, Theriault E. Brain Res. 1990;527:21–31. doi: 10.1016/0006-8993(90)91056-m. [DOI] [PubMed] [Google Scholar]
- 30.Giovanni A, Sieber B A, Heikkila R E, Sonsalla P K. J Pharmacol Exp Ther. 1991;257:691–697. [PubMed] [Google Scholar]
- 31.Sugimoto T, Xiao C, He Y F, Ichikawa H. Neurosci Lett. 1996;215:37–40. doi: 10.1016/s0304-3940(96)12946-3. [DOI] [PubMed] [Google Scholar]
- 32.Tatton N A, Kish S J. Neuroscience. 1997;77:1037–1048. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- 33.Vander Heiden M G, Thompson C B. Nat Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- 34.Gross A, Jockel J, Wei M C, Korsmeyer S J. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Matthews R T, Schulz J B, Klockgether T, Liao A W, Martinou J C, Penney J B, Jr, Hyman B T, Beal M F. J Neurosci. 1998;18:8145–8152. doi: 10.1523/JNEUROSCI.18-20-08145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Offen D, Beart P M, Cheung N S, Pascoe C J, Hochman A, Gorodin S, Melamed E, Bernard R, Bernard O. Proc Natl Acad Sci USA. 1998;95:5789–5794. doi: 10.1073/pnas.95.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito M, Korsmeyer S J, Schlesinger P H. Nat Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu S, Narita M, Tsujimoto Y. Nature (London) 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 39.Kluck R M, Esposti M D, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber M J, Green D R, Newmeyer D D. J Cell Biol. 1999;147:809–822. doi: 10.1083/jcb.147.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 41.Hartmann A, Hunot S, Michel P P, Muriel M P, Vyas S, Faucheux B A, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, et al. Proc Natl Acad Sci USA. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. . (First Published February 25, 2000; 10.1073/pnas.040556597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroemer G, Dallaporta B, Resche-Rigon M. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 43.Kuida K, Zheng T S, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell R A. Nature (London) 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]