Abstract
Many of the pathologies linked to Helicobacter pylori are caused by the ability of the bacteria to induce chronic inflammation in the stomach of the host. One of the major transcription factors that regulate inflammation is NFκB, which is constitutively activated in many cancers including some gastric cancers. H. pylori has been shown to activate NFκB using several different bacterial components and host signaling pathways in cell-type and strain-specific ways. Our recent studies demonstrate that H. pylori utilizes its virulence factor CagA to target signaling molecule TAK1 for the activation of NFκB. In this article, we will summarize our findings together with other recent progress in the H. pylori-mediated activation of NFκB and discuss the role of CagA and TAK1 in the H. pylori-mediated activation of NFκB and gastric diseases.
Key words: Helicobacter pylori, NF-κB, CagA, TAK1, TRAF6, ubiquitination
Approximately half of the world's population is infected with H. pylori, a gram-negative bacterium which colonizes the stomach and is linked to gastritis, gastric ulcers, MALT lymphoma and gastric adenocarcinoma.1 Most infections with H. pylori are persistent, which causes a steady state of inflammation in the stomach. Chronic inflammation has been linked to many diseases, including numerous types of cancers.2 NFκB, a master regulator of pro-inflammatory cytokines and anti-apoptotic signaling molecules, is one of the most well-studied transcription factors activated by H. pylori infection. There are many pathways that lead to the activation of NFκB, and many stimuli, including lipopolysaccharide (LPS), peptidoglycan and TNFα.3 The binding of these ligands to their receptors leads to the activation of signaling pathways which converge upon the phosphorylation and activation of the IκB kinase (IKK) complex. This kinase complex in turn phosphorylates and induces the degradation of IκBα, which in unstimulated conditions binds and sequesters NFκB within the cytoplasm. NFκB, a heterodimer of p50 and RelA/p65, is released and moves into the nucleus, where it undergoes various post-translational modifications and transactivates its target genes.4 Due to the important role of NFκB in inflammation-related diseases and cancer, how H. pylori activates NFκB has been a long-standing question.
H. pylori colonize the mucosal layer of the stomach, attaching themselves to the gastric epithelium via various bacterial adhesins and epithelial receptors.5 The epithelium is therefore the first point of contact for the bacteria in the host. Immune cells attracted to the site of infection by cytokines released from the epithelial cells also respond to H. pylori.1 This further amplifies the immune response from the infection and the resulting inflammation damages the mucosal layer, which can result in ulcers, gastritis and adenocarcinoma.1 Although H. pylori effects responses from both epithelial cells and immune cells including monocytes and lymphocytes, the H. pylori-induced inflammatory response generated by gastric epithelial cells is the most studied.
Infection with cagPAI-positive H. pylori is associated with more severe outcomes in the inflammation-linked illnesses mentioned as compared to infection with cagPAI-negative H. pylori.6,7 The approximately 40 kb cytotoxin-associated gene (cag) pathogenicity island contains genes which encode a type 4 secretion system (T4SS) and a pathogenicity factor called cytotoxin-associated gene A, or CagA.8 A protein ranging in size from 120–145 kDa, CagA is injected into the host epithelial cells via the T4SS, where it acts on a number of different signaling pathways leading to inflammation and cell scattering.9
CagA-Dependent and Independent Activation of NFκB by H. pylori
A role for CagA in the activation of NFκB and the production of IL-8 has been suggested by many studies. For example, using an interleukin-8 (IL-8) promoter-reporter assay, Sharma et al. showed the requirement of CagA for NFκB activation.10 It was also shown that ectopically expressed CagA induced NFκB translocation into the nucleus, and also induced IL-8 production in gastric epithelial cells.11,12 Additionally, NFκB activation and inflammation was markedly less in the gastric antra of Mongolian gerbils infected with cagA-deficient H. pylori as compared to infection with wild-type H. pylori.13 However, the exact function of CagA in NFκB activation is still unclear. Our recent studies demonstrate a clear dependence on the presence of CagA for NFκB activation by H. pylori in gastric epithelial cells.14 We showed that wild-type H. pylori, but not the cagA-deficient isogenic mutant, was capable of inducing the degradation of IκBα and the phosphorylation of RelA, enhancing the DNA-binding of NFκB, and activating NFκB-driven expression of IL-8 and TNFα.14
Despite the essential role of the cagPAI in the activation of NFκB and the induction of IL-8 in epithelial cells, the requirement for CagA, not just the T4SS, for the inflammatory response has been a point of contention nearly since the discovery of the pathogenicity island. It has been reported that the T4SS, but not CagA, is required for H. pylori-mediated production of IL-8 since T4SS mutants failed to induce IL-8.15 However, as the T4SS translocates CagA, impaired NFκB activation by mutants in which the T4SS is defective cannot rule out the possibility that the important component is, in fact, CagA. Another concern regarding the requirement of CagA for H. pylori-mediated NFκB activation is the potential for “polar effects” from the deletion of the cagA gene.16 Nevertheless, as cagA is monocistronic and transcribed in a different direction than other genes comprising the cag pathogenicity island (cagPAI),8 it is unlikely that the H. pylori isogenic cagA mutant strains are exerting “polar effects” on other genes within the cagPAI. Concordantly, no “polar effects” have been reported for the cagA-deficient H. pylori strains in the literature.
While CagA has clearly been shown to be essential for the activation of NFκB, it must be noted that this requirement could be H. pylori strain-specific. The considerable variation in the sequences of CagA from different H. pylori strains led to the designation of “Western” and “Eastern” strains based on the sequences surrounding the phosphorylation domains in the C terminus of the gene.17 These differences correlate with the ability of the CagA protein to associate with SHP-2 and induce the “hummingbird” phenotype.17 Sequence variations within the CagA multimerization domain also influence these CagA-induced phenotypes within host cells.18 In addition to affecting the “hummingbird” phenotype, strain variations have also been linked to variations in IL-8 production. Exchanging cagA genes allows low IL-8-inducing H. pylori strains to be converted into high inducing strains and vice versa.11 We hypothesize that these effects may be caused by increased or decreased abilities of these CagA proteins to bind and activate upstream signaling components, such as TAK1, influencing the downstream activity of NFκB. However, the sequences required for CagA to induce NFκB activation remain elusive and need further investigation.
Additionally, the requirement for CagA in the activation of NFκB could also be cell-type specific. CagA seems to not be essential for the H. pylori-induced activation of NFκB in cells other than gastric epithelial cells. In macrophages, H. pylori activated NFκB via TLR2 (for induction of IL-6 and IL-1β) and TLR4 (for induction of IL-12, IL-10 and IL-8).19,20 Findings similar to those of the activation in macrophages were reported in lymphocytes by Ohmae et al. In human B lymphocytes, H. pylori activates not only the NFκB classical pathway, but also the alternative pathway, which involves the processing of p100, likely by TLR4 recognition of H. pylori LPS.21
In addition to LPS, other bacterial components are also utilized by H. pylori for the activation of NFκB.22 For example, recent papers have shown that bacterial peptidoglycan (PG) is delivered via outer membrane vesicles through the T4SS into host cells where it binds to pattern recognition receptor NOD1, which signals to activate NFκB.23–25 It appears that CagA and PG target different cellular signaling molecules for the NFκB activation. However, one remaining question is why H. pylori utilizes various components for NFκB activation. One possibility is that CagA and PG synergistically activate NFκB to obtain the maximal inflammatory response in gastric epithelial cells. Another possibility is that CagA and PG might compensate for each other. In H. pylori strains where CagA is not a strong NFκB inducer, PG might play a dominant role in initiating the inflammatory response. Finally, it is also possible that CagA and PG selectively activate specific subsets of NFκB target genes. While CagA might be mainly responsible for the expression of proinflammatory cytokines, the PG-NOD1 pathway may be more important for the expression of antimicrobial peptides,25 since the PG-NOD1-mediated inflammatory response might be due largely to the activation of AP-1-dependent expression of IL-8.26 Further experiments will be needed to differentiate among these possibilities.
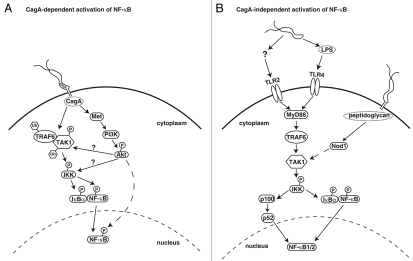
It is clear that the model system and the strain of bacteria that are used to study the H. pylori-induced activation of NFκB can affect the requirement for various components of the bacteria (Fig. 1). From the other side, the host proteins utilized by the various components of H. pylori add another layer of complexity to the H. pylori-induced activation of NFκB.
Figure 1.
H. pylori activation of NFκB via CagA-dependent and CagA-independent pathways. (A) CagA-dependent activation of NFκB. CagA is injected into host epithelial cells where it interacts with TRAF6 and TAK1 or Met to activate NFκB through TAK1 or Akt, respectively. Akt then activates IKK directly or indirectly via TAK1. Akt could also activate NFκB by indirect phosphorylation of RelA. (B) CagA-independent activation of NFκB. Host immune cells are stimulated by H. pylori products, such as LPS, via TLR pathways for the activation of NFκB. Alternatively, peptidoglycan injected into cells via the T4SS stimulates Nod1 activity leading to NFκB activation. Solid arrow: known interaction or activation; solid arrow with question mark: proposed interaction or activation; dashed arrow: activation through multiple steps.
Host Signaling Molecules Utilized by H. pylori to Activate NFκB
While great efforts have been made to identify the H. pylori components for NFκB activation and inflammation, determining the molecules within the host cells that transduce NFκB signaling also attracts much attention. Many cellular signaling molecules have been suggested to be involved in H. pylori-mediated NFκB activation in epithelial cells or in immune cells. Besides Nod1, which has been discussed above, H. pylori was shown to induce the interaction between PAK1 and NIK, a kinase which in turn activates the IKK complex for NFκB activation.27 Also, interference with MyD88, TRAF2, TRAF6 or TAK1 signaling was shown to downregulate H. pylori activation of NFκB.14,19,28 While roles for MyD88, TRAF6 and TAK1 have been validated by siRNA knockdown, some of these studies used dominant negative forms of the signaling molecules which are apt to have non-specific effects. Depletion of these signaling molecules using siRNA will be able to further confirm these findings. Furthermore, whether some of these signaling molecules are involved in the CagA-dependent or -independent H. pylori-mediated activation of NFκB is not clear.
Our recent studies give biochemical evidence to show that CagA activates NFκB by hijacking TAK1 in gastric epithelial cells. We demonstrate that H. pylori activation of NFκB requires TAK1, and that CagA increases the TAK1-dependent activation of NFκB.14 In our findings, CagA is injected into the host epithelial cells, where it binds to TAK1 and enhances its TRAF6-dependent lysine (K) 63-linked ubiquitination. Emerging evidence suggests that activation of TAK1 and IKK is regulated by TRAF6-mediated K63-linked ubiquitination of TRAF6 itself, IRAK1 and NEMO.29 Different from K48-linked polyubiquitination, which represents a signal for degradation by the proteasome, K63-linked polyubiquitination acts as scaffolding to bring signaling molecules in a pathway into contact to aid in the transference of a signal.30 K63-linked ubiquitination is important for the kinase activity of TAK1, since inhibiting its ubiquitination abolished its ability to activate NFκB. Also, we found that CagA enhances this activity as measured by TAK1 autophosphorylation and by the ability of TAK1 to activate the downstream kinase complex IKK, which is directly phosphorylated and activated by TAK1.14
Consistently, two other studies also show that TRAF6-mediated K63-linked ubiquitination is essential for the activation of TAK1 and NFκB.31,32 The ubiquitinated lysine residue(s) of TAK1 in response to H. pylori infection remains unidentified. It has been shown that lysine 34 of TAK1 is ubiquitinated by TRAF6 in response to TGFβ, and ubiquitination of this lysine correlates with the activity of TAK1.33 However, this lysine does not seem to be involved in the H. pylori-induced activation of NFκB, since mutation of lysine 34 to arginine did not affect TAK1 ubiquitination or its ability to activate NFκB, indicating that another unidentified lysine residue(s) might also undergo K63-linked ubiquitination leading to the activation of IKK and NFκB.14 In this regard, two lysine residues in TAK1 have recently been identified to be ubiquitinated by TRAF6, and the K63-linked ubiquitination of these two sites are important for the activation of TAK1 and NFκB.31,32 It would be of great interest to determine whether these two sites are involved in the H. pylori-mediated ubiquitination of TAK1.
The way in which CagA enhances the ubiquitination of TAK1 is not yet understood. CagA is associated with the membrane when it enters the host cell, and has been shown to oligomerize.34,35 It is possible that its oligomerization recruits TRAF6, an interaction which we showed in vitro, which enhances its E3 ligase activity and thus the ubiquitination of TAK1. Further supporting this, our data shows that oligomerization-deficient CagA does not activate NFκB.14 CagA may also act as a connecting protein to bring TRAF6 and TAK1 into proximity to enhance the ubiquitination of TAK1. Another possibility is that CagA may inhibit deubiquitination by A20 or CYLD, two de-ubiquitinases shown to remove ubiquitin chains from TRAF6 and TAK1,36–38 thereby enhancing the overall ubiquitination of TAK1.
How the ubiquitination of TAK1 activates the kinase is also unclear. It is expected that, like other K63-linked ubiquitinated signaling molecules, the K63-linked polyubiquitin chain functions as a scaffold to recruit other signaling molecules which are essential for the activation of TAK1. For example, MEKK3 has been shown to be recruited to the ubiquitinated TAK1, and this recruitment is required for the activation of TAK1.31 Also, TAK1 ubiquitination may aid in its activity by recruiting substrates, such as the IKK complex. The ubiquitin-binding domain of the regulatory subunit NEMO has been shown to be important in the activation of IKK,29 and it may be that it binds to the ubiquitin chains on TAK1.
While our studies clearly demonstrate the importance of TAK1 and TAK1 ubiquitination in CagA-dependent H. pylori-mediated NFκB activation, studies from others also suggest that some other molecules might be targeted by CagA and involved in the CagA-dependent NFκB activation. Suzuki et al. reported that CagA interacted with the hepatocyte growth factor receptor Met, resulting in the activation of PI3K and Akt, which led to the activation of NFκB and β-catenin.39 Since CagA binds Met, an intramembrane protein, and is also known to oligomerize,35 this binding and oligomerization may also lead to the recruitment of TRAF6. TRAF6 is recruited to the membrane by the dimerization of other membrane receptors, and it dimerizes in turn and becomes an active E3 ubiquitin ligase,30 which we found to be required for the ubiquitination of TAK1.14 Akt was also recently found to be ubiquitinated by TRAF6, which proved important for its phosphorylation and activation.40 Although a role for Akt has been suggested in the activation of NFκB by H. pylori,39,41 how Akt activates NFκB is not clear. Akt might activate IKK directly or indirectly through TAK1, or Akt might activate NFκB by inducing the phosphorylation of RelA39,41 (Fig. 1). While TAK1 is activated in vitro by binding to unanchored ubiquitin,42 Akt might function as a kinase for the in vivo phosphorylation and activation of TAK1. It is also possible that Akt may act in conjunction with TAK1 to fully activate the IKK complex via phosphorylation of IKK1/2.
Interestingly, TAK1 is involved in nearly every pathway that is activated by H. pylori, including the JNK, ERK and p38 MAPK pathways.43 We suggest that our CagA-dependent mechanism of activation for TAK1 in NFκB activation may also contribute to the downstream activations of these other pathways, furthering our understanding of the role which CagA plays in the host cells. Determining the precise way by which CagA stimulates the TRAF6-dependent ubiquitination and activation of TAK1 would clarify the H. pylori-induced inflammatory response, and would provide new information that could be used to combat development of the diseases linked to H. pylori infection.
Acknowledgements
This work was supported in part by the Indirect Cost Recovery provided by University of Illinois at Urbana-Champaign and by NIH grant DK-085158 to LF Chen. Acacia Lamb is a recipient of the CMB-TG.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/11587
References
- 1.Ibraghimov A, Pappo J. The immune response against Helicobacter pylori—a direct linkage to the development of gastroduodenal disease. Microbes Infect. 2000;2:23–25. doi: 10.1016/s1286-4579(00)01261-2. [DOI] [PubMed] [Google Scholar]
- 2.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–842. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayden MS, Ghosh S. Shared principles in NFkappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Chen LF, Greene WC. Shaping the nuclear action of NFκB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 5.Rubinsztein-Dunlop S, Guy B, Lissolo L, Fischer H. Identification of two new Helicobacter pylori surface proteins involved in attachment to epithelial cell lines. J Med Microbiol. 2005;54:427–434. doi: 10.1099/jmm.0.45921-0. [DOI] [PubMed] [Google Scholar]
- 6.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 7.Kuipers EJ, Perez-Perez GI, Meuwissen SG,, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 8.Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 10.Sharma SA, Tummuru MK, Blaser MJ, Kerr LD. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappaB in gastric epithelial cells. J Immunol. 1998;160:2401–2407. [PubMed] [Google Scholar]
- 11.Brandt S, Kwok T, Hartig R, Konig W, Backert S. NFkappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SY, Lee YC, Kim HK, Blaser MJ. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol. 2006;8:97–106. doi: 10.1111/j.1462-5822.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- 13.Shibata W, Hirata Y, Maeda S, Ogura K, Ohmae T, Yanai A, et al. CagA protein secreted by the intact type IV secretion system leads to gastric epithelial inflammation in the Mongolian gerbil model. J Pathol. 2006;210:306–314. doi: 10.1002/path.2040. [DOI] [PubMed] [Google Scholar]
- 14.Lamb A, Yang XD, Tsang YH, Li JD, Higashi H, Hatakeyama M, et al. Helicobacter pylori CagA activates NFkappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242–1249. doi: 10.1038/embor.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naumann M. Pathogenicity island-dependent effects of Helicobacter pylori on intracellular signal transduction in epithelial cells. Int J Med Microbiol. 2005;295:335–341. doi: 10.1016/j.ijmm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Fischer W, Puls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337–1348. doi: 10.1046/j.1365-2958.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 17.Higashi H, Tsutsumi R, Fujita A, Yamazaki S, Asaka M, Azuma T, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA. 2002;99:14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu HS, Saito Y, Umeda M, Murata-Kamiya N, Zhang HM, Higashi H, et al. Structural and functional diversity in the PAR1b/MARK2-binding region of Helicobacter pylori CagA. Cancer Sci. 2008;99:2004–2011. doi: 10.1111/j.1349-7006.2008.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda S, Akanuma M, Mitsuno Y, Hirata Y, Ogura K, Yoshida H, et al. Distinct mechanism of Helicobacter pylori-mediated NFkappaB activation between gastric cancer cells and monocytic cells. J Biol Chem. 2001;276:44856–44864. doi: 10.1074/jbc.M105381200. [DOI] [PubMed] [Google Scholar]
- 20.Obonyo M, Sabet M, Cole SP, Ebmeyer J, Uematsu S, Akira S, et al. Deficiencies of myeloid differentiation factor 88, Toll-like receptor 2 (TLR2), or TLR4 produce specific defects in macrophage cytokine secretion induced by Helicobacter pylori. Infect Immun. 2007;75:2408–2414. doi: 10.1128/IAI.01794-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohmae T, Hirata Y, Maeda S, Shibata W, Yanai A, Ogura K, et al. Helicobacter pylori activates NFkappaB via the alternative pathway in B lymphocytes. J Immunol. 2005;175:7162–7169. doi: 10.4049/jimmunol.175.11.7162. [DOI] [PubMed] [Google Scholar]
- 22.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 23.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 24.Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010;12:372–385. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 25.Grubman A, Kaparakis M, Viala J, Allison C, Badea L, Karrar A, et al. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 26.Allison CC, Kufer TA, Kremmer E, Kaparakis M, Ferrero RL. Helicobacter pylori Induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J Immunol. 2009;183:8099–8109. doi: 10.4049/jimmunol.0900664. [DOI] [PubMed] [Google Scholar]
- 27.Neumann M, Foryst-Ludwig A, Klar S, Schweitzer K, Naumann M. The PAK1 autoregulatory domain is required for interaction with NIK in Helicobacter pylori-induced NFkappaB activation. Biol Chem. 2006;387:79–86. doi: 10.1515/BC.2006.011. [DOI] [PubMed] [Google Scholar]
- 28.Hirata Y, Ohmae T, Shibata W, Maeda S, Ogura K, Yoshida H, et al. MyD88 and TNF receptor-associated factor 6 are critical signal transducers in Helicobacter pylori-infected human epithelial cells. J Immunol. 2006;176:3796–3803. doi: 10.4049/jimmunol.176.6.3796. [DOI] [PubMed] [Google Scholar]
- 29.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, et al. Two mechanistically and temporally distinct NFkappaB activation pathways in IL-1 signaling. Sci Signal. 2009;2:66. doi: 10.1126/scisignal.2000387. [DOI] [PubMed] [Google Scholar]
- 32.Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, et al. Lysine 63-linked polyubiquitination of transforming growth factor-[beta]-activated kinase 1 at lysine 158 is required for tumor necrosis factor [alpha] and interleukin-1[beta]-induced I[kappa]B kinase/nuclear factor-[kappa]B and c-JUN N-terminal kinase/activator protein 1 activation. J Biol Chem. 2009 doi: 10.1074/jbc.M109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, et al. The type I TGFbeta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 34.Higashi H, Yokoyama K, Fujii Y, Ren S, Yuasa H, Saadat I, et al. EPIYA motif is a membrane-targeting signal of Helicobacter pylori virulence factor CagA in mammalian cells. J Biol Chem. 2005;280:23130–23137. doi: 10.1074/jbc.M503583200. [DOI] [PubMed] [Google Scholar]
- 35.Ren S, Higashi H, Lu H, Azuma T, Hatakeyama M. Structural basis and functional consequence of Helicobacter pylori CagA multimerization in cells. J Biol Chem. 2006;281:32344–32352. doi: 10.1074/jbc.M606172200. [DOI] [PubMed] [Google Scholar]
- 36.Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NFkappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 37.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 38.Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–1138. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeshima E, Tomimori K, Kawakami H, Ishikawa C, Sawada S, Tomita M, et al. NFkappaB activation by Helicobacter pylori requires Akt-mediated phosphorylation of p65. BMC Microbiol. 2009;9:36. doi: 10.1186/1471-2180-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Xia ZP, Sun L, Chen X, Pineda G, Jiang X, Adhikari A, et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 2009;461:114–119. doi: 10.1038/nature08247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keates S, Keates AC, Warny M, Peek RM, Jr, Murray PG, Kelly CP. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J Immunol. 1999;163:5552–5559. [PubMed] [Google Scholar]