Abstract
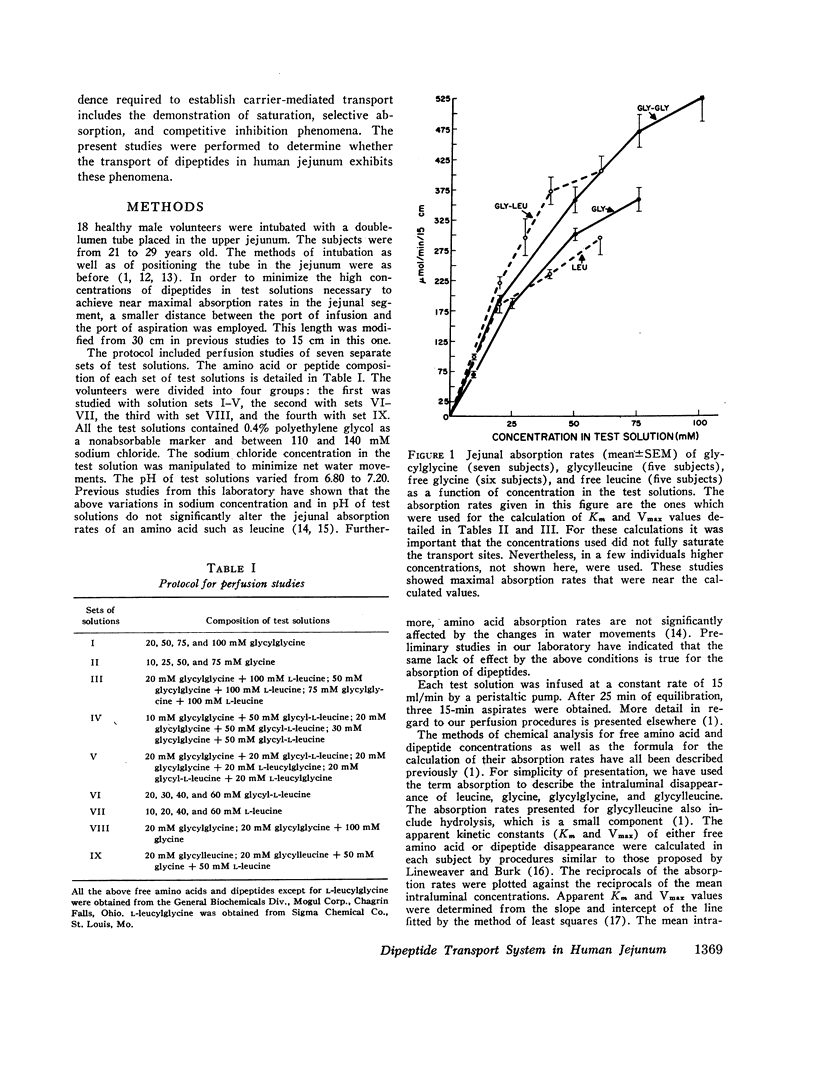
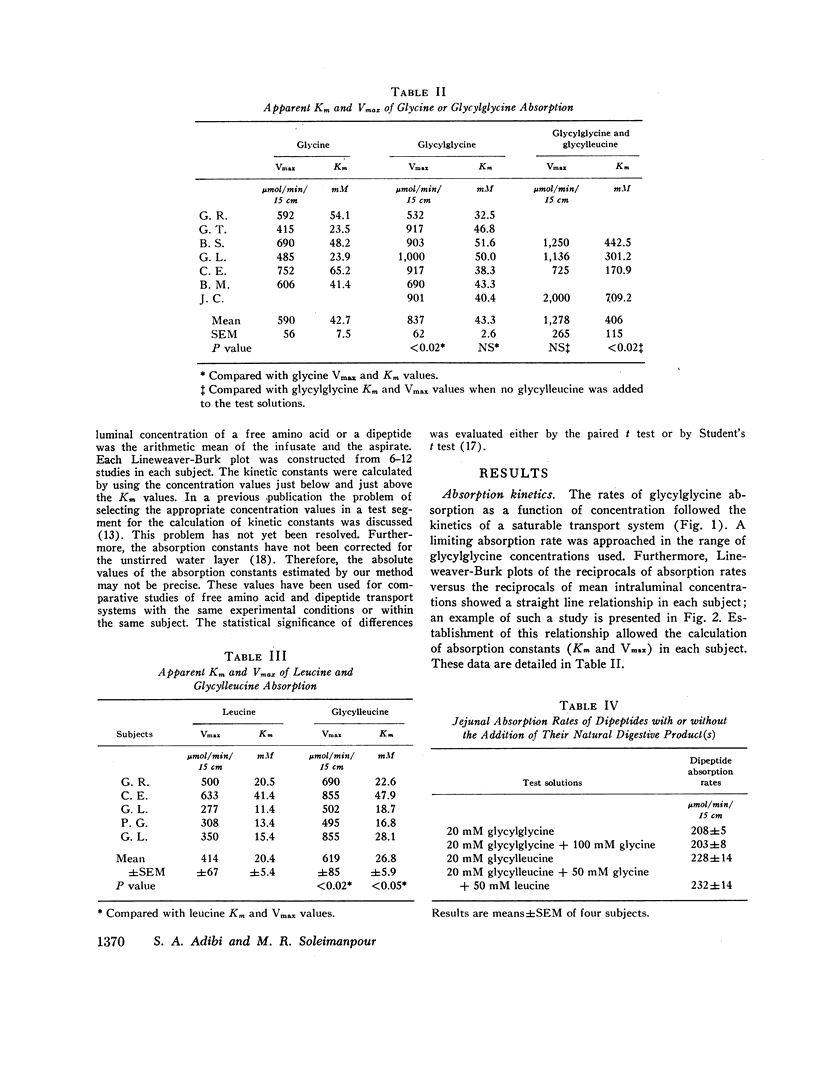
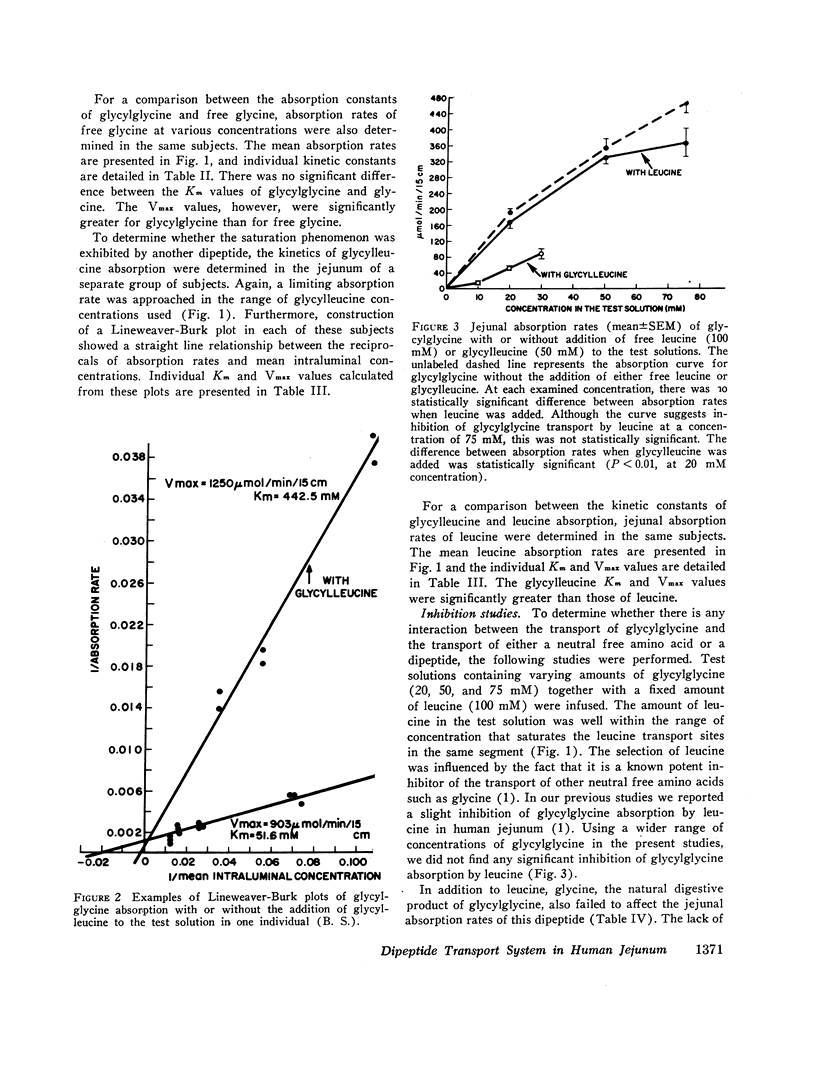
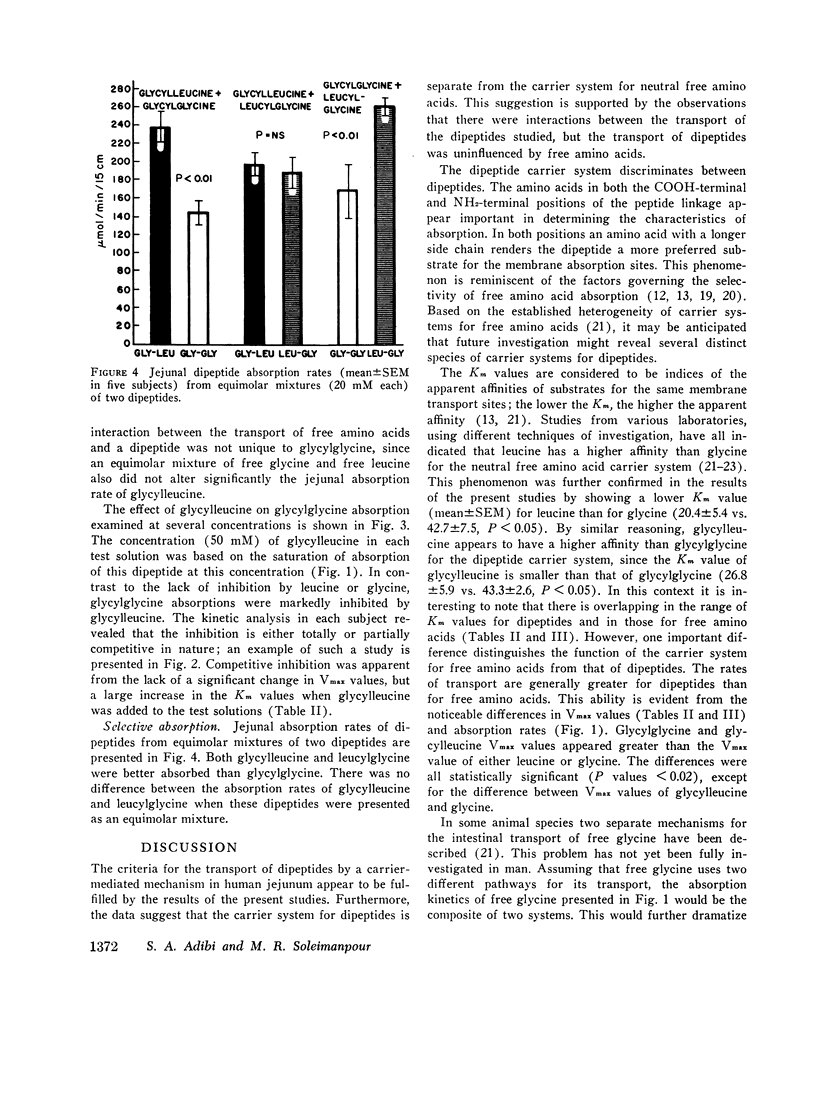
The present studies were performed to determine whether dipeptide absorption in human jejunum exhibits the characteristics of carrier-mediated transport. 15-cm jejunal segments from human volunteers were perfused with test solutions containing varying amounts of either glycylglycine, glycylleucine, glycine, leucine, glycylglycine with leucine or glycine, glycylglycine with glycylleucine, or glycylleucine with an equimolar mixture of free glycine and leucine. Jejunal absorption rates of both glycylglycine and glycylleucine followed the kinetics of a saturable process. The Km value in millimoles/liter of glycylglycine was significantly greater than the Km value of glycylleucine (43.3±2.6 vs. 26.8±5.9, P < 0.05); and the Km value of glycine was also significantly greater than the Km value of leucine (42.7±7.5 vs. 20.4±5.4, P < 0.05). While overlapping occurred among the Km values of free amino acids and dipeptides, the transport kinetics of dipeptides were characterized by higher Vmax values (in micromoles per minute per 15 centimeters) than those of free amino acids. For example, the Vmax values for glycylglycine and glycine were 837±62 and 590±56, respectively (P < 0.02). While jejunal absorption rates of glycylglycine were not significantly affected by free leucine or free glycine, they were competitively inhibited by glycylleucine. The jejunal absorption rate of glycylleucine was not significantly altered by an equimolar mixture of free glycine and leucine. The selective absorption of dipeptides was investigated by infusing three equimolar mixtures, each containing two different dipeptides. Among the three dipeptides examined, glycylglycine was the least absorbed. There was no significant difference between the absorption of glycylleucine and leucylglycine.
The above studies suggest that absorption of both glycylglycine and glycylleucine is mediated by a carrier which is not shared with free neutral amino acids; and that both COOH- and NH2-terminal amino acids appear to be influential in imposing the affinity of a dipeptide for the absorption sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison J. M., Burston D., Matthews D. M. Evidence for active transport of the dipeptide glycylsarcosine by hamster jejunum in vitro. Clin Sci. 1972 Dec;43(6):907–911. doi: 10.1042/cs0430907. [DOI] [PubMed] [Google Scholar]
- Adibi S. A., Gray S. J. Intestinal absorption of essential amino acids in man. Gastroenterology. 1967 May;52(5):837–845. [PubMed] [Google Scholar]
- Adibi S. A., Gray S. J., Menden E. The kinetics of amino acid absorption and alteration of plasma composition of free amino acids after intestinal perfusion of amino acid mixtures. Am J Clin Nutr. 1967 Jan;20(1):24–33. doi: 10.1093/ajcn/20.1.24. [DOI] [PubMed] [Google Scholar]
- Adibi S. A. Leucine absorption rate and net movements of sodium and water in human jejunum. J Appl Physiol. 1970 Jun;28(6):753–757. doi: 10.1152/jappl.1970.28.6.753. [DOI] [PubMed] [Google Scholar]
- Adibi S. A., Mercer D. W. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest. 1973 Jul;52(7):1586–1594. doi: 10.1172/JCI107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi S. A., Ruiz C., Glaser P., Fogel M. R. Effect of intraluminal pH on absorption rates of leucine, water, and electrolyes in human jejunum. Gastroenterology. 1972 Oct;63(4):611–618. [PubMed] [Google Scholar]
- Adibi S. A. The influence of molecular structure of neutral amino acids on their absorption kinetics in the jejunum and ileum of human intestine in vivo. Gastroenterology. 1969 May;56(5):903–913. [PubMed] [Google Scholar]
- Asatoor A. M., Bandoh J. K., Lant A. F., Milne M. D., Navab F. Intestinal absorption of carnosine and its constituent amino acids in man. Gut. 1970 Mar;11(3):250–254. doi: 10.1136/gut.11.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatoor A. M., Cheng B., Edwards K. D., Lant A. F., Matthews D. M., Milne M. D., Navab F., Richards A. J. Intestinal absorption of two dipeptides in Hartnup disease. Gut. 1970 May;11(5):380–387. doi: 10.1136/gut.11.5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatoor A. M., Harrison B. D., Milne M. D., Prosser D. I. Intestinal absorption of an arginine-containing peptide in cystinuria. Gut. 1972 Feb;13(2):95–98. doi: 10.1136/gut.13.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D., WOOLEY S. O. GLYCYLGLYCINE UPTAKE IN STREPTOCOCCI AND A POSSIBLE ROLE OF PEPTIDES IN AMINO ACID TRANSPORT. Arch Biochem Biophys. 1964 Apr;105:51–57. doi: 10.1016/0003-9861(64)90234-6. [DOI] [PubMed] [Google Scholar]
- Cook G. C. Comparison of intestinal absorption rates of glycine and glycylglycine in man and the effect of glucose in the perfusing fluid. Clin Sci. 1972 Sep;43(3):443–453. doi: 10.1042/cs0430443. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Sallee V. L., Wilson F. A. Unstirred water layers and absorption across the intestinal mucosa. Gastroenterology. 1971 Dec;61(6):932–934. [PubMed] [Google Scholar]
- FINCH L. R., HIRD F. J. The uptake of amino acids by isolated segments of rat intestine. II. A survey of affinity for uptake from rates of uptake and competition for uptake. Biochim Biophys Acta. 1960 Sep 23;43:278–287. doi: 10.1016/0006-3002(60)90438-8. [DOI] [PubMed] [Google Scholar]
- Fleshler B., Butt J. H., Wismar J. D. Absorption of glycine and L-alanine by the human jejunum. J Clin Invest. 1966 Sep;45(9):1433–1441. doi: 10.1172/JCI105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizer W. D., Laster L. Peptide hydrolase activities of the mucosa of human small intestine. J Clin Invest. 1969 Jan;48(1):210–228. doi: 10.1172/JCI105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier M. D., Holdsworth C. D., McColl I., Perrett D. Dipeptide absorption in man. Gut. 1972 Dec;13(12):965–969. doi: 10.1136/gut.13.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier M. D., Holdsworth C. D., Perrett D., Thirumalai C. Intestinal depeptide transport in normal and cystinuric subjects. Clin Sci. 1972 Nov;43(5):659–668. doi: 10.1042/cs0430659. [DOI] [PubMed] [Google Scholar]
- Lindberg T. Intestinal dipeptidases. Dipeptidase activity in the mucosa of the gastrointestinal tract of the adult human. Acta Physiol Scand. 1966 Apr;66(4):437–443. doi: 10.1111/j.1748-1716.1966.tb03221.x. [DOI] [PubMed] [Google Scholar]
- Lis M. T., Crampton R. F., Matthews D. M. Rates of absorption of a dipeptide and the equivalent free amino acid in various mammalian species. Biochim Biophys Acta. 1971 Apr 13;233(2):453–455. doi: 10.1016/0005-2736(71)90342-7. [DOI] [PubMed] [Google Scholar]
- MATTHEWS D. M., LASTER L. KINETICS OF INTESTINAL ACTIVE TRANSPORT OF FIVE NEUTRAL AMINO ACIDS. Am J Physiol. 1965 Apr;208:593–600. doi: 10.1152/ajplegacy.1965.208.4.593. [DOI] [PubMed] [Google Scholar]
- NEWEY H., SMYTH D. H. Cellular mechanisms in intestinal transfer of amino acids. J Physiol. 1962 Dec;164:527–551. doi: 10.1113/jphysiol.1962.sp007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab F., Asatoor A. M. Studies on intestinal absorption of amino acids and a dipeptide in a case of Hartnup disease. Gut. 1970 May;11(5):373–379. doi: 10.1136/gut.11.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W. Oligopeptide transport in Escherichia coli. Specificity with respect to side chain and distinction from dipeptide transport. J Biol Chem. 1968 Jun 25;243(12):3395–3403. [PubMed] [Google Scholar]
- Rubino A., Field M., Shwachman H. Intestinal transport of amino acid residues of dipeptides. I. Influx of the glycine residue of glycyl-L-proline across mucosal border. J Biol Chem. 1971 Jun 10;246(11):3542–3548. [PubMed] [Google Scholar]
- Silk D. B., Perrett D., Clark M. L. Intestinal transport of two dipeptides containing the same two neutral amino acids in man. Clin Sci Mol Med. 1973 Sep;45(3):291–299. doi: 10.1042/cs0450291. [DOI] [PubMed] [Google Scholar]
- Tarlow M. J., Seakins J. W., Lloyd J. K., Matthews D. M., Cheng B., Thomas A. J. Absorption of amino acids and peptides in a child with a variant of Hartnup disease and coexistent coeliac disease. Arch Dis Child. 1972 Oct;47(255):798–803. doi: 10.1136/adc.47.255.798. [DOI] [PMC free article] [PubMed] [Google Scholar]


