Abstract
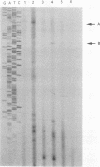
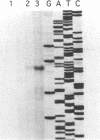
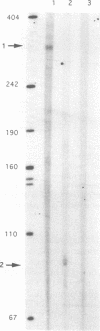
Clostridium perfringens is a source of food poisoning in humans and animals because of production of a potent enterotoxin (CPE). To study the regulation of the cpe gene in C. perfringens, we cloned and sequenced the cpe promoter regions and N-terminal domains from three strains. The cpe promoter region from one strain contained a 45-bp insertion compared with previously published sequences. This insertion was also found in two (of five) other Cpe+ strains. cpe gene expression in C. perfringens was measured by using translational fusions of each promoter type to the Escherichia coli gusA gene, which codes for beta-glucuronidase. For either promoter type, cpe-gusA expression was undetectable throughout exponential growth but increased dramatically at the beginning of the stationary phase. To measure cpe expression in Bacillus subtilis, cpe-gusA fusions were integrated into the B. subtilis chromosome. Both types of promoter exhibited moderate expression during exponential growth; cpe expression increased threefold at the beginning of the stationary phase. Transcriptional start sites were determined by primer extension and in vitro transcription assays. For C. perfringens, both types of promoter gave the same 5' end, 197 bp upstream of the translation start (50 bp downstream of the 45-bp insertion). In B. subtilis, however, the 5' end was internal to the 45-bp insertion, suggesting the use of a different promoter than that utilized by C. perfringens.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. P., Blaschek H. P. Factors involved in the electroporation-induced transformation of Clostridium perfringens. FEMS Microbiol Lett. 1990 Jul;58(2):217–220. doi: 10.1111/j.1574-6968.1990.tb13981.x. [DOI] [PubMed] [Google Scholar]
- Bannam T. L., Rood J. I. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid. 1993 May;29(3):233–235. doi: 10.1006/plas.1993.1025. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brynestad S., Iwanejko L. A., Stewart G. S., Granum P. E. A complex array of Hpr consensus DNA recognition sequences proximal to the enterotoxin gene in Clostridium perfringens type A. Microbiology. 1994 Jan;140(Pt 1):97–104. doi: 10.1099/13500872-140-1-97. [DOI] [PubMed] [Google Scholar]
- Canard B., Saint-Joanis B., Cole S. T. Genomic diversity and organization of virulence genes in the pathogenic anaerobe Clostridium perfringens. Mol Microbiol. 1992 Jun;6(11):1421–1429. doi: 10.1111/j.1365-2958.1992.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Czeczulin J. R., Hanna P. C., McClane B. A. Cloning, nucleotide sequencing, and expression of the Clostridium perfringens enterotoxin gene in Escherichia coli. Infect Immun. 1993 Aug;61(8):3429–3439. doi: 10.1128/iai.61.8.3429-3439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Ileal loop fluid accumulation and production of diarrhea in rabbits by cell-free products of Clostridium perfringens. J Bacteriol. 1969 Oct;100(1):86–94. doi: 10.1128/jb.100.1.86-94.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H., Sebald M. Sporulation and enterotoxin production by mutants of Clostridium perfringens. J Bacteriol. 1972 Apr;110(1):378–391. doi: 10.1128/jb.110.1.378-391.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alvarado J. S., Labbé R. G., Rodriguez M. A. Sporulation and enterotoxin production by Clostridium perfringens type A at 37 and 43 degrees C. Appl Environ Microbiol. 1992 Apr;58(4):1411–1414. doi: 10.1128/aem.58.4.1411-1414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier T., Cole S. T. Studies of UV-inducible promoters from Clostridium perfringens in vivo and in vitro. Mol Microbiol. 1988 Sep;2(5):607–614. doi: 10.1111/j.1365-2958.1988.tb00069.x. [DOI] [PubMed] [Google Scholar]
- Goldner S. B., Solberg M., Jones S., Post L. S. Enterotoxin synthesis by nonsporulating cultures of Clostridium perfringens. Appl Environ Microbiol. 1986 Sep;52(3):407–412. doi: 10.1128/aem.52.3.407-412.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna P. C., Wnek A. P., McClane B. A. Molecular cloning of the 3' half of the Clostridium perfringens enterotoxin gene and demonstration that this region encodes receptor-binding activity. J Bacteriol. 1989 Dec;171(12):6815–6820. doi: 10.1128/jb.171.12.6815-6820.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower K. I., Wnek A. P., McClane B. A. Evidence that alterations in small molecule permeability are involved in the Clostridium perfringens type A enterotoxin-induced inhibition of macromolecular synthesis in Vero cells. J Cell Physiol. 1989 Sep;140(3):498–504. doi: 10.1002/jcp.1041400314. [DOI] [PubMed] [Google Scholar]
- Iwanejko L. A., Routledge M. N., Stewart G. S. Cloning in Escherichia coli of the enterotoxin gene from Clostridium perfringens type A. J Gen Microbiol. 1989 Apr;135(4):903–909. doi: 10.1099/00221287-135-4-903. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R. G., Duncan C. L. Evidence for stable messenger ribonucleic acid during sporulation and enterotoxin synthesis by Clostridium perfringens type A. J Bacteriol. 1977 Feb;129(2):843–849. doi: 10.1128/jb.129.2.843-849.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R. G., Rey D. K. Raffinose increases sporulation and enterotoxin production by Clostridium perfringens type A. Appl Environ Microbiol. 1979 Jun;37(6):1196–1200. doi: 10.1128/aem.37.6.1196-1200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grice S. F., Shih C. C., Whipple F., Sonenshein A. L. Separation and analysis of the RNA polymerase binding sites of a complex Bacillus subtilis promoter. Mol Gen Genet. 1986 Aug;204(2):229–236. doi: 10.1007/BF00425503. [DOI] [PubMed] [Google Scholar]
- Mathiopoulos C., Sonenshein A. L. Identification of Bacillus subtilis genes expressed early during sporulation. Mol Microbiol. 1989 Aug;3(8):1071–1081. doi: 10.1111/j.1365-2958.1989.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Geoffroy C., Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991 Mar;59(3):1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. P., Bukusoglu G., Sonenshein A. L. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J Bacteriol. 1992 Jul;174(13):4361–4373. doi: 10.1128/jb.174.13.4361-4373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik H. S., Duncan C. L. Rapid detection and quantitation of Clostridium perfringens enterostoxin by counterimmunoelectrophoresis. Appl Environ Microbiol. 1977 Aug;34(2):125–128. doi: 10.1128/aem.34.2.125-128.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood J. I., Cole S. T. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev. 1991 Dec;55(4):621–648. doi: 10.1128/mr.55.4.621-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Slack F. J., Mueller J. P., Sonenshein A. L. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993 Aug;175(15):4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack F. J., Mueller J. P., Strauch M. A., Mathiopoulos C., Sonenshein A. L. Transcriptional regulation of a Bacillus subtilis dipeptide transport operon. Mol Microbiol. 1991 Aug;5(8):1915–1925. doi: 10.1111/j.1365-2958.1991.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Cami B., Brevet J., Cote R. Isolation and characterization of rifampin-resistant and streptolydigin-resistant mutants of Bacillus subtilis with altered sporulation properties. J Bacteriol. 1974 Oct;120(1):253–265. doi: 10.1128/jb.120.1.253-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme-Jongsten M., Wernars K., Notermans S. Cloning and sequencing of the Clostridium perfringens enterotoxin gene. Antonie Van Leeuwenhoek. 1989 Aug;56(2):181–190. doi: 10.1007/BF00399981. [DOI] [PubMed] [Google Scholar]
- Wnek A. P., McClane B. A. Preliminary evidence that Clostridium perfringens type A enterotoxin is present in a 160,000-Mr complex in mammalian membranes. Infect Immun. 1989 Feb;57(2):574–581. doi: 10.1128/iai.57.2.574-581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]