Abstract
Proton magnetic resonance spectroscopy (1H MRS) is a non-invasive imaging technique that permits measurement of particular compounds or metabolites within the tissue of interest. In the brain, 1H MRS provides a snapshot of the neurochemical environment within a defined volume of interest. A search of the literature demonstrates the widespread utility of this technique for characterizing tumors, tracking the progress of neurodegenerative disease, and for understanding the neurobiological basis of psychiatric disorders. As of relatively recently, 1H MRS has found its way into substance abuse research, and it is beginning to become recognized as a valuable complement in the brain imaging toolbox that also contains positron emission tomography (PET), single photon emission computed tomography (SPECT), and functional magnetic resonance imaging (fMRI). Drug abuse studies employing 1H MRS have identified a number biochemical changes in the brain. The most consistent alterations across drug class were reductions in N-acetylaspartate and elevations in myo-inositol, while changes in choline, creatine, and amino acid transmitters also were abundant. Together, the studies discussed herein provide evidence that drugs of abuse may have a profound impact on neuronal health, energy metabolism and maintenance, inflammatory processes, cell membrane turnover, and neurotransmission, and these biochemical changes may underlie the neuropathology within brain tissue that subsequently gives rise to the cognitive and behavioral impairments associated with drug addiction.
Keywords: proton magnetic resonance spectroscopy, human, brain imaging, drug abuse
Introduction
Proton magnetic resonance spectroscopy (1H MRS) is a non-invasive neuroimaging technique that has become a useful tool for a number of applications in drug abuse research. As reviewed herein, 1H MRS is gaining popularity in studies aimed at elucidating the cerebral mechanisms underlying drug-induced neuronal injury and the subsequent behavioral and cognitive changes that can contribute to addiction. Beyond simply determining the cerebral consequences of drug abuse, however, 1H MRS has the potential for tracking disease and/or treatment progression. For instance, studies examining the effect of short-term abstinence on the brain of alcoholics demonstrated metabolic changes that were indicative of neuronal and glial regeneration1–5, while Streeter et al.6 attempted to correlate changes in neurotransmission to efficacy of candidate treatments for cocaine dependence. Recently, Meyerhoff and Durazzo7 proposed the idea of correlating levels and patterns of metabolites not only with cognition and behavior, but with genetic information in order to understand better alcohol use disorder. Taken together, 1H MRS is emerging as an informative technique that will become an invaluable instrument for understanding the etiology of drug abuse, as well as for monitoring the long-term recovery from this disease.
I. MEASURABLE COMPOUNDS
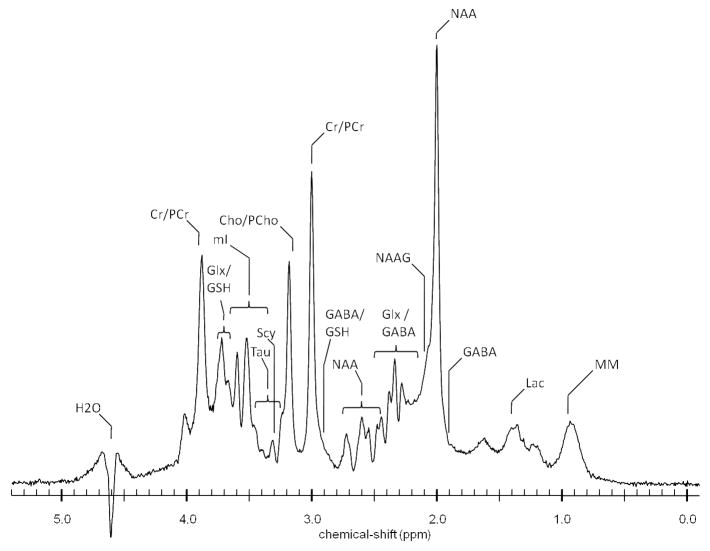
In 1H MRS, the visible spectrum depends on the energy absorbed by specific organic molecules, which is determined by the number of hydrogen atoms in the compound as well as in its environment (for a brief overview of the basic principles of MRS, please see8). In the brain, current detection limits permit the quantification of a number of chemical peaks in the spectrum once the overwhelming water and lipid signals are suppressed (Figure 1). Most commonly reported include peaks for the metabolites N-acetylaspartate (NAA), creatine (Cr), choline (Cho), and myo-inositol (mI). Also visible are the metabolically available cellular pools of the amino acids γ-aminobutyric acid (GABA), glutamate (Glu), and glutamine (Gln). To some extent, the visible proton spectrum has begun to transform the way in which drug abuse researchers think about the mechanistic landscape underlying addiction. Neurotransmitters that have dominated the literature such as acetylcholine, norepinephrine, dopamine, and serotonin, are of insufficient concentrations to be visible with the current technology. Similarly, a number of second messengers and ribonucleic acids also are outside of range. However, a good deal of information has been provided by measuring those MR-visible metabolites.
Figure 1.
Proton spectrum acquired at 4 tesla from a 16cc voxel within the parieto-occipital cortex of a 26-year old healthy male volunteer. Cho/PCho: choline/phosphocholine, Cr/PCr: creatine/phosphocreatine, GABA: γ-aminobutyric acid, Glx: glutamate/glutamine, GSH: glutathione, H2O: water, Lac: lactate, mI: myo-inositol, MM: macromolecules, NAA: N-acetylaspartate, NAAG: N-acetylaspartylglutamate, Scy: scyllo-inositol, Tau: taurine. [Courtesy of J. Eric Jensen, Ph.D., Brain Imaging Center, McLean Hospital/Harvard Medical School].
N-acetylaspartate
The most prominent signal in the water-suppressed proton spectrum arises from NAA (for a comprehensive review on NAA, see 9). Although approximately 15–25% of this signal is due to the contribution of N-acetylaspartylglutamate (NAAG),10 the spectral peak (at 2.02 ppm) primarily is attributable to the acetyl groups of NAA. Brain concentrations of NAA are rather high11 and relatively homogeneous,12 although there is significantly more NAA in grey matter than in white matter.13 Moreover, Nadler and Cooper14 demonstrated that NAA is localized almost exclusively to neuronal tissue in comparison to glia, thus rendering NAA a neuronal marker. Reductions in NAA often have been interpreted as being representative of neuronal damage and/or loss (e.g.,15–18), or markers for reduction in synaptic density, as well as dysregulated neuronal function and/or neurotransmission (see discussion19). Alternatively, as discussed thoroughly in Stork and Renshaw,20 NAA may serve as a measure of mitochondrial function due to its role in mitochondrial energy production. Taken together, these studies suggest that NAA sub serves several putative functions within the brain.
Creatine
Similar to NAA, Cr is distributed evenly throughout the brain, with higher concentrations in grey matter.12 The Cr peaks (at 3.03 and 3.94 ppm) are more accurately referred to as total Cr (tCr), since Cr and phosphocreatine (PCr) are indistinguishable from one another. Together the Cr/PCr system has been believed to provide a stable source of energy that typically has been used as an internal reference to normalize 1H MRS data (e.g., 21) However, concentrations of Cr and PCr have been shown to be unstable not only across disease states,22–23 but it can vary across regions of healthy brain and with age.24
Choline
While Cho is the precursor of acetylcholine within cholinergic neurons, it is the Cho-containing compounds glycerophosphocholine and phosphocholine that contribute to the Cho peak (at 3.2 ppm). Since both are involved in the synthesis and degradation of cellular membranes,25 they are found in higher concentrations within myelin, and therefore within white matter.12 The distribution of Cho throughout the brain also is regionally-dependent;12 as a result, the Cho resonance has the potential to vary quite a bit across disease states, particularly when membrane turnover has been implicated in the etiology.25–26
myo-inositol
The exact significance of the mI peak (at 3.56 ppm) is unclear, as there is still much speculation about the precise function of mI within the brain. It has been suggested that mI is an osmoregulator27 or that it contributes to glucose storage.28 Other studies have shown that mI is an integral component of the calcium-mobilizing phosphatidylinositol (PI) second messenger system,29 and abnormalities in this system may contribute to the pathophysiology of several psychiatric illnesses.30 Most commonly though, mI is used as a marker for glial content.31
Glutamate, Glutamine, and GABA
Glu, Gln, and GABA not only maintain an optimal balance between excitation and inhibition within the brain, but they work together to regulate neuronal energy metabolism (see32). Although the Glu, Gln, and GABA signals are derived from the large metabolically active cellular pools, they are notoriously difficult to measure with 1H MRS. A number of methods have been developed to circumvent the complicated spectral patterns that arise from their overlapping resonances,33 but typically the peak (at 2.4 ppm) which is attributed primarily to resonances arising from both Glu and Gln, is considered “Glx”. Similarly, the GABA peak (at 3.03 ppm) comprises Cr and macromolecules34 as well as homocarnosine.35 Despite a preponderance of evidence supporting critical roles for altered Glu and/or GABA neurotransmission underlying drug addiction (see reviews36–37), the majority of spectroscopic studies have restricted their investigations to metabolites that are comparatively less technically demanding to measure.
II. USING 1H MRS TO UNDERSTAND ILLICIT DRUG USE
PSYCHOSTIMULANTS
Amphetamine congeners
Amphetamine (AMPH), methamphetamine (METH), and its derivative 3,4-methylenedioxymethamphetamine (MDMA or Ecstasy), are highly abused CNS stimulants that have been shown to produce long-lasting neurotoxic effects. A wealth of data exists demonstrating that administration of AMPH is toxic to dopaminergic neurons, MDMA is toxic primarily to serotonergic neurons, and METH damages both dopaminergic and serotonergic nerve terminals while also altering the glutamatergic system (e.g., see reviews38–39; 40). This stimulant-induced toxicity is believed to be mediated by oxidative stress and activation of apoptotic pathways, and subsequently, may contribute to the observed cognitive, neurological, and psychiatric disturbances that persist following prolonged use.41–42
Amphetamine
To date, the 1H MRS studies examining the neurochemical effects of AMPH have not been from a drug abuse perspective, but rather, AMPH has been administered as a lithium-sensitive model of mania.43–44 Specifically, dextroamphetamine was administered to healthy volunteers in order to address the hypothesis that lithium-induced depletion of inositol underlies the anti-manic effects of treatment.45–46 While the results from a preliminary study indicated that dextroamphetamine increased levels of mI (as a ratio of PCr-Cr) in the temporal lobe,44 a follow-up study using a sequence optimized for mI47 failed to demonstrate any change in mI within the dorsal medial prefrontal cortex.43 The authors concluded that when all things were considered dextroamphetamine had no effect on mI, and thus, changes in mI cannot be relied upon solely to understand the etiology of mania. However, in the context of drug abuse, these data suggest that although AMPH is toxic to dopaminergic neurons,39 AMPH-induced reductions in dopamine synthesis, dopamine metabolites, and dopamine uptake48–51 may not be manifested as glial disturbances or inflammatory processes. Of course it should be noted that the clinical MRS studies mentioned here examined an acute dose of dextroamphetamine, while the other studies cited employed a chronic regimen of AMPH administration.
Methamphetamine
METH has been shown to have long-lasting neurotoxic effects in both preclinical and clinical studies. Human neuroimaging studies of METH abusers in particular have demonstrated profound drug-induced changes not only in the dopaminergic52–53 and serotonergic54 neurotransmitter systems, but also in cerebral glucose metabolism,55–57 and the structural integrity of the brain.58–61 Ernst et al.62 was the first group to report alterations in metabolite concentrations in METH users relative to healthy controls. In the basal ganglia, they found that NAA and Cr both were reduced,62 while concentrations of Cho and mI were elevated in frontal gray matter.62 Subsequent studies corroborated their findings by reporting both reductions in NAA/Cr63–65 and elevations in Cho/NAA64–65 within frontal gray matter, frontal white matter elevations in mI,66 and reduced Cr + PCr/Cho in the basal ganglia.67 Although together these studies imply that METH exposure leads to subsequent neuronal injury, presently the literature contains only a few studies that provide evidence supporting this hypothesis.
Ernst et al.62 also demonstrated an inverse relationship between the concentration of NAA in frontal white matter and cumulative lifetime METH exposure, while more recently Sung et al.66 showed that NAA was reduced in METH users who had consumed a ‘large’ cumulative dose relative to those who had consumed a ‘small’ dose.66 The reduction in NAA also was shown to be correlated with reduced levels of attentional control as measured by interference on the Stroop test.65 Finally, decreased levels of Cr + PCr/Cho were correlated not only with longer duration of METH use, but also with severity of residual psychiatric symptoms resulting from METH use.67 Together, these findings suggest a tangible functional relationship between METH-induced neurochemical alterations and cognitive impairment and/or psychiatric disturbances.
In addition to the effect duration of use may have on metabolite levels and subsequent neuronal injury, varying durations of abstinence from METH use may impact reported values of metabolites. For example, although reductions in NAA have not been consistent across studies, differences in the reported periods of abstinence among participants may explain the apparent discrepancies. When abstinence ranged from 0 to approximately four months NAA levels were reduced compared to controls,62–63,65 while NAA was not significantly decreased compared to controls when the duration of abstinence was approximately two years.66 Moreover, NAA was shown to be positively correlated with duration of abstinence, suggesting not only that metabolite levels may recover, but that the neurotoxicity produced by METH may be reversed over time.66 While Nordahl et al.64 contradicted this hypothesis by showing no differences in NAA/Cr between those individuals who had been abstinent for 1–5 years (long) vs. those who had been abstinent for six months or less (short), they reported higher ratios of Cho/NAA in the brains of ‘short’ relative to the ‘long’ abstinence participants. These results imply that Cho normalizes as abstinence increases, and they underscore further the hypothesis that the brain may recover from METH-induced insult over time. However, functional studies demonstrating a reversal in altered metabolite levels concomitant with improvements in cognitive processes and/or psychiatric symptoms have yet to be undertaken.
Recently, Ernst and Chang40 extended their findings beyond NAA, Cr, Cho, and mI, to include spectroscopic measures of METH-induced adaptations in glutamatergic neurotransmission. Preclinical studies have shown that in addition to serotonin and dopamine, glutamate levels are altered by METH,68 which may contribute to the mechanisms underlying METH’s neurotoxicity.69 Glx was shown to be low in frontal gray matter, but not in frontal white matter or basal ganglia. Levels of Glx were lowest at the beginning of abstinence (≤ 1 month), and while correlational analysis suggested a normalization of Glx over time with progressive abstinence, a down-regulation of glutamate and/or glutamine during early METH abstinence may have potentially significant implications with respect to METH craving. Participants in the Ernst and Chang40 study who reported experiencing symptoms of craving had lower Glx in the frontal cortex relative to those who did not; this finding was in agreement not only with a preclinical study that demonstrated reduced levels of basal glutamate during cocaine-seeking,70 but also with clinical studies that have demonstrated a decrease in drug-taking and cue-reactivity when cocaine-dependent participants were administered medication to increase concentrations of basal glutamate in the brain.71–72 Although these findings have not been replicated in METH users, Ernst and Chang’s report40 not only provided novel evidence of glutamatergic dysfunction associated with METH use in humans, but it has provided an impetus for improving the methods to permit the measurement of glutamate and glutamine separately for use in drug abuse research.
3,4-methylenedioxymethamphetamine
Despite discrepancies between the animal and human literature, the preponderance of evidence supporting the toxic potential of MDMA suggests that there are residual alterations in serotonergic neurotransmission among human MDMA users.73 The ramifications of this putative neurotoxicity include the emergence of neurological, psychiatric, and somatic disturbances that have been associated with serotonergic imbalance. Accordingly, case reports suggest that MDMA use may lead to the appearance of a host of problems such as psychosis as well as anxiety, panic, and depressive disorders (for example, see74–77). Similarly, more in-depth reports describe MDMA-induced sleep disturbances and cognitive deficits (for references, see78).
In 1H MRS studies it has been hypothesized that similar to METH, regular users of MDMA exhibit neuronal loss or dysfunction and/or glial activation. Indeed, levels of NAA (expressed as ratios of both Cr and Cho) have been demonstrated to be reduced in frontal gray matter79–80 and approaching a significant reduction in the left hippocampus,81 while mI was elevated in parietal white matter.82 Moreover, study participants who had lifetime histories of heavy use (i.e., taking upwards of 700+ tablets of ecstasy) exhibited deficits in delayed verbal recall that were strongly associated with the prefrontal reductions in NAA.79
The majority of studies, however, have not supported these hypothesized alterations in NAA and/or mI. In fact, there is little consistency among reports of 1H MRS data (reviewed83). NAA was found to decrease within brain regions that mediate verbal memory in association with lifetime cannabis use in MDMA polydrug abusers,84 while it was unchanged in single voxels placed within frontal gray or parietal white matter82,85–87, neocortex,81 hippocampus,89 or occipital regions.82,85–88 Similarly, mI was unaffected in many of the same regions.80,84,86–88 Although these studies suggest that MDMA does not induce lasting neuronal injury, it should be noted that they evaluated participants who reported polydrug abuse. In fact, it is difficult to find pure MDMA abusers within the population, as most also co-abuse cannabis, alcohol, or other stimulants.90 Additionally, participants reported variable estimates of lifetime MDMA exposure. However, data obtained in non-human primates demonstrated reductions in hypothalamic NAA after exposure to a recreational dose of MDMA.91 Furthermore, MDMA use in general has been associated with impaired delayed memory function even in the absence of changes in levels of NAA and mI (e.g.,92), together suggesting that the sensitivity of current methods may not permit the measurement of long-term neuroadaptations that occur as a result of repeated exposure to MDMA.
Cocaine
Cocaine, like the other psychostimulants, has cognitive, neurological, and psychiatric consequences associated with prolonged use. These effects may be due in part to cocaine’s vasoconstrictive effects which are believed to underlie cocaine-related strokes, intracranial hemorrhage, and persistent perfusion deficits (for more details, please see93), but they also may be attributed to its ability to increase intracellular calcium,94 thereby facilitating seizure activity95 and/or cell death.96 A number of studies have shown that biochemical mechanisms within the brain (i.e., alterations in brain metabolites) also are associated with cocaine use and may contribute to the etiology of cocaine-induced neuronal dysfunction.
Among the changes in brain metabolites believed to result from prolonged cocaine use, alterations in NAA have been the most commonly reported. Retrospective studies that examined the effects of cocaine have demonstrated decreased thalamic NAA in current cocaine users19 as well as decreased levels of NAA in mid-frontal gray matter regions among abstinent cocaine-dependent individuals97 relative to healthy normal controls. However, neither Chang et al.98 nor Ke et al.99 observed any alterations in NAA when they examined mid-occipital gray or temporoparietal white matter of abstinent individuals or the left prefrontal lobe of current users, respectively. The predominating dogma regarding the significance of NAA suggests that while levels of NAA may be dynamic and reflective of ongoing processes within neurons (reductions in NAA observed in neurological disease states or brain injury have been shown to be reversible100–101), the decrease in NAA associated with chronic cocaine use may result from loss or damage to neurons, a reduction of synaptic density, or even a cocaine-induced depletion of brain monoamines (see discussion19). Indeed, a number of imaging studies have shown reductions in brain volume or tissue density consistent with cocaine-induced injury 102–104.
In contrast, opposite findings have been reported after a single acute administration of cocaine. A preliminary report described a significant increase in thalamic NAA (as well as a non-significant increase in Cho) following administration of cocaine to cocaine-dependent participants.105 Similarly, in men who were only occasional users, an intravenous infusion of cocaine resulted in a dose-dependent increase in NAA (as well as in Cho) within the left basal ganglia.106 While these results are consistent with an increase in cocaine-induced phospholipid turnover, Christensen et al.106 hypothesized that cocaine’s ability to inhibit Na+/K+-ATPase107 may have led to augmented intracellular water content and cellular swelling,108–109 and this subsequent osmotic stress may have affected the transverse relaxation times (T2*) of the NAA and Cho peaks. Although the significance of these collective results still is unclear, NAA may be regulated differentially by acute cocaine vs. prolonged exposure to cocaine over time.
Several other brain metabolites also are believed to be modified following chronic cocaine abuse such as Cr, mI, and GABA. Levels of Cr and mI were shown to be elevated in the temporoparietal white matter of abstinent users, and were correlated with the frequency and duration of use, respectively.98 A follow-up study examining frontal white matter regions in a younger, less cocaine-experienced cohort found similar results, albeit to a lesser extent.97 Elevations in mI typically are hypothesized to represent increased glial hypertrophy and/or proliferation, and may suggest a reactive process in response to the chronic insult incurred in the brain by cocaine. Glial hypertrophy subsequently may have led to the increase in Cr since glial cells contain more Cr than neuronal cells,31 and Cr levels typically are assumed to remain stable (but see110). In order to complicate things further, Chang et al.97 also reported on sex-related differences in metabolite levels among cocaine users. While males in that study exhibited a reduction in NAA (gray matter) in addition to elevated Cr (white matter) and mI (both white and gray matter), the only abnormality observed in females was elevated mI in frontal white matter. If these differences in metabolite levels truly indicate differences in cocaine-induced brain injury, then not only are they in agreement with previous work showing that women experience fewer cerebral perfusion deficits,111 but they also provide further support for examining the role of gonadal hormones as potential therapeutics.
Two recent studies have shown that cocaine-dependent individuals have lower prefrontal99 and occipital112 GABA levels than healthy controls. In the prefrontal cortex, there was a 30% difference in GABA between groups,99 while there was a 23% difference in GABA in the occipital cortex.112 The finding within the prefrontal cortex in particular is significant because the profound impairments in inhibitory control, executive functioning, and decision-making displayed by cocaine-dependent individuals have been localized repeatedly to prefrontal cortical regions.113 Interestingly, the GABA system has been a promising target for therapeutics aimed at treating cocaine dependence (see review114). However it is unclear at this time if increasing levels of GABA, particularly in the prefrontal cortex, is sufficient to have clinical significance with respect to treatment for addiction (e.g.,6).
OPIATES
Opiates such as morphine and heroin are powerful analgesics with high abuse liability. Synthetic opioid compounds such as methadone also possess high abuse potential, although methadone’s utility extends beyond pain relief to include playing an integral role in the process of opiate detoxification. In addition to the risk of respiratory depression, viral infection, and/or liver damage associated with intravenous administration, current and former opiate abusers tend to display persistent neurocognitive deficits that may result from opiate-induced brain injury (see review115). Like many other drugs of abuse, the rewarding effects of opiates are mediated primarily by the mesocorticolimbic dopamine system.116 It is prefrontal regions, however, that have been implicated in a number of neuropsychological studies demonstrating profound impairments in executive functioning,115 as well as in brain imaging studies demonstrating reduced activity117–119 and cerebral blood flow120–123 in opiate-dependent individuals. Moreover, both T2-weighted MRI124 and voxel-based morphometry125 in opiate-dependent participants have revealed prefrontal white matter hyperintensities and reduced gray matter density, respectively, indicative of neuropathology.
To date, comparatively fewer studies have employed proton MRS to study opiate-induced alterations in neurochemistry. The most commonly reported alteration in metabolite concentration is a non-specific reduction in NAA. For example, NAA was reduced to a similar extent in both the dorsal anterior cingulate119 and frontal gray matter126 of opiate-dependent participants maintained on opioid replacement therapy. Case reports also demonstrated cerebellar white matter reductions in NAA among individuals who suffered from heroin-induced leukoencephalopathy, a toxic spongiform encephalopathy resulting from inhaling heroin vapors.127–128 That the opiate-induced alteration in NAA is similar to that observed among cocaine97 and methamphetamine 62 abusers suggests that changes in NAA may be non-specific. Indeed, it has been hypothesized that reductions in NAA are indicative of the cerebral hypoxic-ischemic events associated with drug abuse in a more general sense.126
Other notable changes in brain chemistry include an increase in lactate in those patients who suffered from leukoencephalopathy.127–128 Since elevated lactate levels typically are observed as a result of mitochondrial dysfunction (e.g.,129), this finding was interpreted to be indicative of abnormal cellular energy metabolism resulting from the neuropathology associated with this particular condition; this change was not seen in opiate-dependent individuals maintained on stable methadone or buprenorphine.119 In addition to elevations in lactate, opiate dependence also has been shown to be associated with a decrease in Glx within the dorsal anterior cingulate.119 Although it is unknown what the subsequent reduction in neuronal excitability contributes to the processes that accompany and/or underlie opiate addiction, the reduction in Glx may represent abnormalities in reward-based learning and decision making, or in the modulation of dopaminergic neurotransmission.119 Interestingly, despite little clinical spectroscopic evidence supporting the involvement of lactate and Glx in opiate dependence, a recent study in the preclinical literature described similar changes in both the thalamus and somatosensory cortex of rats treated chronically with morphine.130 Together, these data invite further investigation regarding the role of energy metabolism and neuronal excitability in opiate dependence.
CANNABIS
Of all the illicit drugs of abuse, cannabis causes the most controversy. While reports indicate that cannabis-based drugs provide relief to those who suffer from chronic pain or disease-induced spasticity, there is a vast literature demonstrating impairment in cognitive function, increased incidence of psychotic behavior, and not surprisingly, risk of abuse and/or dependence (see review131). Moreover, although cannabis is believed by many to be harmless, both preclinical132–133 and clinical134 findings indicate that chronic cannabis use is neurotoxic and has harmful effects on the integrity of brain tissue.
In as much as chronic cannabis consumption surely has an effect on neurochemistry, there only are a couple of studies describing such investigations. Chang et al.135 demonstrated that in the basal ganglia of individuals who had smoked marijuana almost daily for at least one year, levels of NAA, Cho, and Glu were reduced. Glu also was reduced in the thalamus, while Cr was elevated in this region.135 In occasional or recreational users, NAA was reduced in the dorsolateral prefrontal cortex, but no metabolite changes were found in the anterior cingulate, striatum, thalamus, or hippocampus.136 The implication of both studies was that the reduction in NAA could be interpreted as a marker for neuronal dysfunction, although the specific functional significance of such impairment is unclear. Neuropsychological testing returned equivocal results as Chang et al.135 did not find any deficits in their participants. In contrast, the younger cohort examined by Hermann et al.136 exhibited cannabis-related deficits on tests assessing attention as well as short-term memory. While there was no correlation between those neuropsychological results and the dorsolateral prefrontal NAA decrease,136 it is intriguing not only that similar findings have been reported in studies investigating the neurochemical basis of schizophrenia,137–138 but cannabis use is believed to be a risk factor for schizophrenia in genetically predisposed individuals.139 Finally, while these results all together highlight the difficulty in drawing inferences about brain function from observed changes in brain metabolites, they provide support for the argument that cannabis indeed may have neurotoxic effects within the brain.
III.USING 1H MRS TO UNDERSTAND LICIT DRUG USE
ALCOHOL
The neurotoxicity of chronic alcohol consumption has been revealed by imaging and histopatholgical studies showing significant atrophy in the brains of alcoholics (see reviews140–141). Regions of the brain that appear to be the most sensitive to the effects of chronic alcohol include the neocortex, limbic system, and cerebellum. Subsequently, alcoholics exhibit a host of cognitive and behavioral abnormalities including deficits in executive functioning, learning and memory, as well as problems with emotion and personality142.
In order to understand better the neurobiological substrates of the profound brain damage common in alcoholism, a number of 1H MRS studies have been performed (reviewed recently7). Reduced levels of NAA2–5 143–146,148 and Cho2–3,5, 143–144,148–150 were observed as putative evidence of general neuronal dysfunction in the gray and white matter brain regions of treatment-seeking alcohol-dependent volunteers. Also, levels of mI were elevated, particularly during short-term abstinence from alcohol.147 This finding suggests a temporary increase in glial activation or an attempt to regulate cell volume in a state of alcohol-induced osmotic stress.147 Although metabolite reductions did not correlate necessarily with specific brain atrophy, recovery of NAA was correlated with a gain in global brain volume.1 The recovery of NAA and/or Cho observed in abstinence1–5,151 is consistent with a study that found no differences in metabolites between healthy individuals and those who had been alcohol-abstinent for two years.152 Moreover, the reversal of those metabolite abnormalities sometimes was correlated with cognitive improvements.1–2,5 Together, these findings suggest that similar to the alcohol-induced structural and functional deficits that are at least partially reversible with abstinence (see references147), the metabolite abnormalities associated with those impairments also may recover in time.
Metabolite alterations also were observed in studies examining cohorts of active drinkers relative to light drinkers or treatment-seeking abstinent alcoholics. Compared to light drinkers, active heavy drinkers exhibited lower levels of frontal white mater NAA, as well as higher levels of Cho, Cr, and mI in parietal gray matter.153 While active heavy drinkers also exhibited elevated levels of Cho, Cr, and mI across a number of gray and white matter regions compared to abstinent alcoholics,154 NAA was higher in the current drinkers.154 These slightly different patterns of alcohol-induced effects on metabolites in the actively drinking cohort could reflect any number of factors that may modulate neurochemistry differentially (e.g., age, gender, co-morbid psychiatric illness, lifetime exposure to alcohol, withdrawal symptoms, etc.).
Aside from these metabolic alterations, changes in glutamatergic and/or GABAergic neurotransmission have been implicated in the etiology of alcohol abuse (see reviews36,155). Consistent with chronic alcohol-induced GABAA receptor abnormalities and the subsequent glutamatergic hyperactivity observed during withdrawal,156–157 Glx and Glu/Cr were increased in the basal ganglia150 and the anterior cingulate149 of detoxified alcoholics, respectively, while GABA was reduced by approximately 30% in the absence of any other metabolic changes within the occipital cortex.158 Interestingly, Mason et al.159 found no differences in occipital GABA levels when comparing alcohol-dependent and healthy volunteers. However, their data revealed that during early abstinence the alcohol-dependent patients who were smokers had significantly lower levels of GABA relative to the non-smokers.159 While a thorough examination of these data in the context of the literature regarding GABAergic mechanisms mediating alcohol dependence and withdrawal is beyond the scope of this review, a brief discussion of the effect smoking has on the neurochemical findings in alcohol-dependent individuals is warranted and can be found in the next section.
NICOTINE
Nicotine is the component of tobacco that gives rise to the addictive properties of cigarette smoking. Nicotine is an agonist at nicotinic acetylcholine receptors, and like most other drugs of abuse, exerts its reinforcing effects ultimately by increasing dopaminergic neurotransmission within the mesolimbic reward circuitry.160 A great deal of the nicotine literature has focused on understanding how nicotine and/or smoking may enhance neurotransmission within cortico-basal ganglia-thalamic circuits161 to have subsequent effects on learning, memory, and attention (see reviews162, 163), as well as reward processing and dependence.164–165 Brain imaging studies in particular have demonstrated that acute and chronic exposure to nicotine and/or cigarette smoking results in decreased global brain activity but focal activations within prefrontal regions, thalamus, and the visual system.166 Moreover, nicotine also has been associated with morphological abnormalities in frontal subregions and cerebellum.164–165
Preclinical studies have implicated GABA and glutamate in the neurobiological effects of nicotine,167 and the clinical literature employing 1H MRS supports this claim. Nicotine-dependent women exhibited lower baseline GABA compared to nicotine-dependent men within the occipital cortex,168 and comparisons of nicotine-dependent women to nonsmoking women revealed reductions during the follicular phase of the menstrual cycle that could not be attributed to differences in smoking habits. Although subsequent investigations have not followed up on those preliminary smoking-related sex differences in GABA, Gallinat and Schubert169 demonstrated that hippocampal Glu was unchanged between smokers, former smokers, and individuals who had never smoked. In contrast, examination of other metabolites revealed that hippocampal (but not ACC) NAA levels were reduced when smokers were compared to nonsmokers.170 This finding is consistent with a body of preclinical work suggesting that nicotine has neurotoxic effects on hippocampal neurons (e.g.,171–173). To the extent that NAA is a marker for synaptic density and/or neuronal viability,19 the null result in the ACC does not support previous work demonstrating reduced grey matter volume and grey matter density within that brain region of smokers compared to nonsmokers.164–165 However, involvement of NAA has been corroborated by a report showing that chronic cigarette smoking was correlated with lower midbrain NAA.143 Moreover, that study also demonstrated reductions in Cho within the midbrain and cerebellar vermis,143 together confirming that nicotine and/or smoking has an adverse effect on neuronal function.
As previously discussed, alcohol has a deleterious effect on the brain similar to that of smoking. Interestingly, upwards of 80% of alcohol-dependent individuals also smoke regularly.174–175 It has been argued that consumption of alcohol facilitates the consumption of nicotine, and subsequently, the co-dependent population may represent a sub-population having unique needs with respect to smoking and/or drinking cessation.176 However, while chronic cigarette smoking was shown to be detrimental to gray matter tissue volume and perfusion in alcohol-dependent individuals,163,177 most of the studies demonstrating the effect of alcohol on brain metabolites failed to control for the effects of concurrent nicotine dependence.1–2,4–5,144–151 Therefore, the extent to which those reported alcoholism-induced metabolite alterations reflected the effects of the combination is unknown.
Studies in which smokers were separated from nonsmokers to determine how smoking affected brain metabolites and neurocognitive functioning in alcohol dependence found that during the first week of abstinence smokers had lower frontal, midbrain, and medial temporal lobe NAA,143,178 as well as lower Cho within midbrain143 and medial temporal lobe.178 After a month of sobriety, smokers exhibited less metabolite recovery while their nonsmoking counterparts exhibited increases in NAA and Cho, as well as more improved cognitive performance.3,178 Together, these findings indicate that smoking not only compounds the brain damage resulting from alcohol dependence, but it also influences the brain’s recovery from the chronic alcoholic insult. Although it is not known how the long-term trajectory of recovery beyond one month is affected by smoking, these preliminary data support the campaign to encourage treatment for both dependencies simultaneously.
TOLUENE
Toluene (methyl benzene) is an organic solvent that is the main component of many commercial and household products such as paint, thinner, glue, and lighter fluid. Because it is legal and readily accessible, it is one of the most commonly abused substances among adolescents.179 The adolescent brain is particularly vulnerable to toluene-induced toxicity not only because toluene’s highly lipophilic nature leads to its accumulation in the lipid-rich white matter regions of the brain,180–181 but also because the proportion of white matter is increased during this time of neuronal development consequent to an increase in myelination.182 Accordingly, much of the damage observed in the brains of individuals who were exposed to toluene chronically was localized to white matter regions (in addition to periventricular and subcortical regions)183 and correlated significantly with neurological,184 psychological,185 and cognitive deficits.186
Although the brain abnormalities as well as subsequent encephalopathy and neuropsychological deficits associated with toluene abuse in humans have been acknowledged in a number of publications,187–190 there is no consensus regarding the mechanism underlying this damage. However, a number of animal studies have demonstrated changes in neurochemistry by measuring levels of acetylcholine,191 dopamine,192–193 GABA,194 and glutamate,195 following administration of toluene. Similarly, the few studies employing MRS in humans have used this technology as a way to probe the neurochemical processes underlying toluene exposure in an effort to provide mechanistic substantiation of toluene-induced brain damage. For example, NAA was reduced within white matter in two individuals (ages 19 and 20) who had abused organic solvents for 6–7 years and who also experienced symptoms of encephalopathy such as unsteady gait, tremor, diplopia, dysarthria, and impaired IQ relative to healthy controls.196 Likewise, young adults (ages 15–23) who had abused paint thinner for 2–3 years had lower levels of NAA as well as higher levels of mI in cerebral and cerebellar white matter, but not in the thalamus, relative to their age-matched controls.197 Moreover, these neurochemical changes were correlated with the self-reported duration of use.197
A reduction in NAA typically has been interpreted to represent a reduction in neuronal number or loss of neuro-axonal integrity in general,9 but these results were interpreted to be indicative primarily of axonopathy. Because NAA levels were spared within the thalamus, an area with higher neuronal density compared to the white matter and cerebellum, the authors concluded that the etiology of toluene encephalopathy did not involve the targeting of neurons per se.197 This conclusion is supported by other studies that failed to identify neuronal loss or morphological abnormalities,183,198 but demonstrated degeneration of axons,199 in post-mortem tissue. Additionally, the elevated levels of mI observed by Aydin et al.197 may reflect toluene-induced gliosis and astrocytic activation rather than neuronal death following chronic exposure to toluene. Indeed, glial cell marker proteins have been shown to be increased, particularly in cerebellum, of rats exposed to toluene in a chronic dosing paradigm.200–202 Taken together, these data suggest that axonopathy and gliosis seem to underlie the encephalopathy observed following chronic toluene exposure.
It has been suggested that in addition to gliosis, the demyelination observed in post-mortem tissue183,199 also may underlie the toluene-induced white matter lesions observed with MRI.184 This hypothesis has been supported by findings in the basal ganglia showing that levels of Cho/NAA and Cho/Cr + PCr (but not NAA/Cr + PCr or mI/Cr + PCr) were elevated among abstinent toluene abusers relative to controls.203 An increase in the Cho peak as measured with MRS is indicative of an increase in membrane phospholipids which may be released during membrane decomposition, thereby representing active demyelinating processes.25 However, five of the 12 participants in this study also were taking neuroleptic medication to manage their psychiatric symptoms, and Cho has been shown previously to be sensitive to this type of medication.204–206 Moreover, alterations in white matter Cho did not reach statistical significance in the results reported by Aydin et al.197, together suggesting that demyelination may occur in toluene abuse, but most likely it is not the primary mechanism of neurotoxicity.
IV.SYNTHESIS OF METABOLIC CHANGES IN DRUG ABUSE AND FUTURE DIRECTIONS
Of all the changes in metabolites (Table 1) and amino acids (Table 2) that have been measured to date, there is considerable overlap across drug classes (Table 3). Reductions in NAA and elevations in mI were observed almost universally, thus indicating that drugs of abuse in general have a profound impact on neuronal health, energy metabolism, and inflammatory processes. The next most common metabolite changes involved alterations in Cho and Cr, suggesting that methamphetamine, cocaine, cannabis, and alcohol influence cell membrane turnover as well as energy maintenance. Methamphetamine, opiates, cannabis, and alcohol were found to alter Glx to some extent, while GABA was reduced by cocaine, alcohol, and nicotine, together suggesting that drugs of abuse impact neurotransmission. While not all drugs of abuse were associated with changes in all the metabolites represented, it should be noted that not every study measured every visible metabolite. In fact, quantifying metabolites with strongly coupled spins such as mI, Glx, and GABA require specific advanced techniques as well as stronger magnetic field strength.47,207 However, as 1H MRS becomes more widely recognized as a means to evaluate the substrates of drug action, the technology has the potential to evolve into a more automated and user-friendly procedure.
Table 1.
Summary of reported metabolite changes with drugs of abuse
| NAA | Cho | Cr | mI | |
|---|---|---|---|---|
| Amphetaminea | -------------------- | -------------------- | -------------------- | Increase (TL) None (PFC) |
| Methamphetamineb | Decrease (BG, FGM) | Increase (FGM) | Decrease (BG) | Increase (FGM, FWM) |
| MDMAc | Decrease (FGM, HP) None (FGM, PWM, NC, HP, OCC) |
-------------------- | -------------------- | Increase (PWM) None (FGM, PWM, OCC) |
| Cocained | Decrease (FGM, TH) Increase (BG, TH) None (OCC, PWM) |
Increase (BG) | Increase (PWM) | Increase (FGM, PWM) |
| Opiatese | Decrease (ACC, FGM, CBL) | -------------------- | -------------------- | -------------------- |
| Cannabisf | Decrease (BG, PFC) | Decrease (BG) | Increase (TH) | |
| Alcoholg | Decrease (CBL, FGM, FWM, TH, TL) | Decrease (PGM, TH, CBL) Increase (PGM, TL) |
Increase (PGM) | Increase (ACC, PGM, TH) |
| Nicotineh | Decrease (HP) None (ACC) |
-------------------- | -------------------- | -------------------- |
| Toluenei | Decrease (CBL, CWM) None (BG, TH) |
Increase (BG) | -------------------- | Increase (CBL, CWM) None (BG, TH) |
ACC= anterior cingulate, BG= basal ganglia, CBL= cerebellar white matter, CWM= cerebral white matter, FGM= frontal gray matter, FWM= frontal white matter, HP= hippocampus, NC= neocortex, OCC=occipital cortex, PFC= prefrontal cortex, PGM= parietal gray matter, PWM= parietal white matter, TH= thalamus, TL= temporal lobe
Ernst et al. 2000; Nordahl et al. 2002, 2005; Salo et al. 2007; Sung et al. 2007; Sekine et al. 2002
Chang et al. 1999; Cowan et al. 2007; Daumann et al. 2004; de Win et al. 2007, 2008; Obergriesser et al. 2001; Reneman et al. 2001, 2002
Table 2.
Summary of reported amino acid changes with drugs of abuse
| Glx | GABA | |
|---|---|---|
| Methamphetamine | FGM: Decrease (Ernst and Chang, 2008) | -------------------- |
| Cocaine | --------------------- | PFC: Decrease (Ke et al. 2004) OCC: Decrease (Hetherington et al. 2000) |
| Opiates | ACC: Decrease (Yücel et al. 2007) | --------------------- |
| Cannabis | BG, TH: Decrease (Chang et al. 2006) | --------------------- |
| Alcohol | ACC: Increase (Lee et al. 2007) BG: Increase (Miese et al. 2006) |
OCC: Decrease (Behar et al. 1999); No change (Mason et al. 2006) |
| Nicotine | HP: No change (Gallinat and Schubert, 2007) | OCC: Decrease (Epperson et al. 2005) |
ACC= anterior cingulate, BG= basal ganglia, FGM= frontal gray matter, HP= hippocampus, OCC=occipital cortex, PFC= prefrontal cortex, TH= thalamus
Table 3.
Simplified summary of overlapping metabolite findings across drug classes
| Metabolite | Decrease | Increase |
|---|---|---|
| NAA | Methamphetamine, MDMA, Cocaine, Opiates, Cannabis, Alcohol, Nicotine, Toluene | Cocaine (acute administration) |
| Cho | Cannabis, Alcohol | Methamphetamine, Cocaine, Alcohol |
| Cr | Methamphetamine | Cocaine, Cannabis, Alcohol |
| mI | ------ | Amphetamine, Methamphetamine, MDMA, Cocaine, Alcohol, Toluene |
| Glx | Methamphetamine, Opiates, Cannabis | Alcohol |
| GABA | Cocaine, Alcohol, Nicotine | ------ |
These 1H MRS data add a new dimension to the existing wealth of knowledge regarding the detrimental effects of drugs of abuse on the brain. Specifically though, what have we learned about addiction from measuring brain metabolites? Studies investigating the etiology of other brain diseases have begun using 1H MRS to probe functional relationships between metabolite alterations and other measures of pathology. For example, levels of NAA have been shown to correlate with hippocampal volume, memory, and intelligence in patients with medial temporal lobe epilepsy (e.g.,208). Similarly, drug abuse research has begun to benefit from examining the relationships between metabolites and drug-induced impairments in neurocognitive function. Correlation analyses have revealed associations between reduced NAA in frontal regions and attentional control as well as impaired verbal memory among participants who had histories of abusing methamphetamine65 and MDMA,79 respectively. These results are in agreement with other research that has shown a correlation between levels of frontal NAA and measures of selective attention209 and memory.210 Moreover, they agree with findings obtained in alcoholics showing that various measures of learning and memory improved as levels of NAA (and Cho) recovered during abstinence.3 In general, a great deal of research has shown that deficits in attention211 and memory212 may contribute to processes that underlie drug-seeking behavior, thereby necessitating an understanding of the neurochemical mechanisms mediating those processes and subsequent behavioral output.
Although other metabolite-behavior relationships have been demonstrated (e.g., Ernst and Chang40 showed that reduced frontal Glx correlated with ratings of craving while Sekine et al.67 correlated reduced Cr in the basal ganglia with the severity of residual psychiatric symptoms after use), all together, the information gleaned from 1H MRS studies at this point mostly seem to corroborate decades of previous research. Moreover, several studies have demonstrated that metabolite reductions recover during abstinence from alcohol1–5,151 or methamphetamine,64,66 suggesting that perhaps the long-term neuroadaptations that maintain drug-seeking and drug-taking behaviors cannot be explained by the particular changes in neurochemistry that are measurable with 1H MRS. Meyerhoff and Durazzo7 suggested recently that in addition to relating metabolite levels to cognition, genetic polymorphism data might provide an additional piece of information that could help elucidate not only the neurobiological mechanisms underlying substance abuse disorder, but also to identify risk factors and disease severity.
Indeed, the strength of this technology in drug abuse research may lie in its utility as a diagnostic tool to predict treatment matching, to monitor the progress of treatment, or to prevent relapse. Although currently 1H MRS is used routinely to monitor the course of treatment and determine therapeutic strategies in a number of other brain illnesses such as tumors, multiple sclerosis, and Alzheimer’s disease,213 the potential of these applications has not been explored thoroughly in the context of drug abuse. A recent study by Streeter et al.6 demonstrated the use of 1H MRS as a tool for assessing treatment efficacy. The study aimed to correlate changes in the level of prefrontal GABA with reduced cocaine consumption in cocaine-dependent volunteers after eight weeks of treatment with modest doses of the candidate therapeutics pramipexole or venlafaxine. Previous studies had shown that GABA was reduced in the left prefrontal lobe of cocaine-dependent volunteers,99 and that GABA-enhancing drugs reduced cocaine self-administration in laboratory animals214–215 and humans.216–219 Although both pharmacologic treatments increased levels of GABA significantly relative to placebo, cocaine use was not reduced in either treatment group.6 However, the maximum increase in GABA was still approximately 19% lower than control levels,99 suggesting that dose of drug may have been partly to blame. Regardless, this study is a prime example demonstrating the usefulness of 1H MRS within a treatment paradigm.
Although this review focused on metabolite changes that occur following exposure to drugs of abuse in the adult brain, drug abuse may begin in childhood and/or adolescence, and exposure may occur even in utero. 1H MRS has the potential to be an invaluable method for studying these vulnerable populations. The lack of ionizing radiation makes 1H MRS suited for undertaking longitudinal studies, particularly during the more active periods of brain development. In fact, 1H MRS already has been used to begin understanding the biochemical maturation of the brain not only in terms of specific metabolites, but also in terms of water content, relaxation properties, and myelination.220–221 In drug abuse, 1H MRS was used in a small study that employed children to examine the neurotoxic effects of prenatal exposure to methamphetamine on the developing brain.222 Findings showed that despite the absence of structural abnormalities, exposed children (ages 3–16) had relatively normal levels of NAA but elevated levels of Cr in the striatum relative to age-matched controls.222 These results were in contrast to the reduction of Cr (and NAA) observed in this region of adults who have abused methamphetamine.62,67 They suggest that although methamphetamine-induced biochemical alterations occur in both children and adults, prenatal exposure to methamphetamine can disrupt energy metabolism differentially in children, which may have clinical implications with respect to cognitive function in these individuals as their development progresses.222 Moreover, these results underscore the importance of utilizing 1H MRS technology to study the effects of drugs of abuse in the developing brain.
Finally, 1H MRS may prove to be a useful modality for studying the etiology of addiction in general. A great deal of research has suggested that chronic drug abuse shares neurobiological underpinnings with other addictive disorders such as bulimia nervosa, pathological gambling, and sexual addiction.223 Although to date the findings have been restricted to the effects of those reinforcers on the monoamine and opioid receptor system and their downstream signaling cascades, it is likely that other addictive disorders would yield to study with 1H MRS just as well. In fact, it would be interesting to determine if pathological gambling, eating, or sexual appetite reduced NAA to a similar extent as drugs of abuse. While it is unlikely that these non-chemical reinforcers would reduce neuronal health and/or viability on their own (independent of a history of head trauma or some type of brain injury), a reduction in NAA would give pause with respect to its hypothesized function as an outcome measure indicating neuronal viability. Such results may hint at metabolite differences as predisposing factors, although the recovery of NAA during abstinence from drugs of abuse argues against that idea. However, 1H MRS studies investigating the effects of natural reinforcers on brain chemistry would help elucidate some of the neurobiological mechanisms contributing to reward and reinforcement in general.
Other spectroscopy methods
Without going into much detail, it should be noted that in addition to 1H, 31P and 13C MRS also hold a great deal of promise for in vivo drug abuse research. Phosphorous spectra contain information regarding phospholipid metabolism, tissue bioenergetics, and pH. Specifically, the major peaks in the spectra correspond to phosphomonoesters (PME) and phosphodiesters (PDE), both of which contribute to phospholipid metabolism.224–225 Major peaks also correspond to high-energy phosphates phosphocreatine (PCr), inorganic phosphate (Pi), and α-, β-, and γ-nucleoside triphosphate (NTP). Using 31P MRS, several studies have reported abnormalities in phospholipid and bioenergetic metabolism in the brains of cocaine-dependent polydrug abusers226–227 and heroin-dependent individuals early in their methadone maintenance therapy.228–229 These results are consistent with those obtained using 1H MRS, altogether suggesting drug-induced changes in cerebral bioenergetic states that may shift with increased abstinence and/or treatment, as well as putative membrane changes that may be associated with neuronal viability.19,82,119,126
13C MRS has been developed relatively recently as a non-invasive measure of specific metabolic fluxes within the human brain. This technique exploits the rapid synthesis of glutamate, glutamine, and GABA by monitoring the incorporation of 13C atoms of labeled precursors (e.g., [1–13C]-glucose) into the intermediate metabolites of the tricarboxylic acid (TCA) and glutamate-glutamine cycles.230 Subsequently, 13C MRS provides information regarding the basic mechanisms governing glial-neuronal interactions, particularly with respect to glutamatergic function.231 Previous 13C MRS studies have demonstrated an inextricable link between glutamate neurotransmission and glucose consumption (which undoubtedly has been advantageous for interpreting the brain activation observed using functional imaging modalities),232–233 but the potential for this technique to be used drug abuse research is untouched. In fact, the use of 13C MRS in human psychiatric research in general is still in its infancy, most likely owing in part to the trade-off between long infusion times of substrate or reduced sensitivity when it is administered orally.234 However, the need to understand better how abnormalities in glia and/or amino acid neurotransmission contribute to the etiology of substance abuse (as well as a multitude of other psychiatric illnesses), will drive the technology to evolve.
Limitations
When considering the 1H MRS findings as a whole, there are several methodological and technical and limitations to take into account. First, many of the drug abuse studies summarized here relied on self-report of retrospective drug use. Although self-report is a critical aspect of many drug abuse studies, underreporting drug use is common, it varies across participant populations and with specific drug of abuse,235 and it can become challenging when the purity of the drug consumed is in question (e.g., only 63% of ecstasy pills contain actual MDMA).236 Moreover, MDMA abusers in particular are notorious for being polydrug abusers,90 further complicating interpretation of any metabolite alterations in this group of individuals. The retrospective study design is problematic in that one cannot draw inferences about a causative link between drug intake and putative neurobiological consequences. However, given the ethical issues surrounding administering potentially toxic drugs of abuse to human volunteers (e.g., cocaine’s effects extend beyond brain injury and include cardiovascular damage),237 most studies to date have used this design which, while somewhat dissatisfying, is at least consistent across studies.
Although 1H MRS has proven to be an amazing research tool, there are limitations to this technology with respect to the acquisition, quantification, and interpretation of the spectra. A number of these practical issues have been described at length elsewhere (please see238–239), but a few shortcomings stand out. For example, 1H MRS does not have the spatial or temporal resolution of some other imaging techniques. The user must consider employing a single-voxel design versus chemical-shift imaging of a slab across the brain (potentially upwards of 40–50 voxels). A single-voxel design permits control of localization, improved shimming and water suppression, and requires shorter measurement times,240–241 but these advantages are predicated on having chosen the correct voxel for the measurement of interest. Chemical-shift imaging, on the other hand, can cover a larger region of interest, but as a result it precludes precise localization, requires a longer acquisition time, and increases field inhomogeneity.240–241 Importantly, neither design is capable of providing information about a specific compartment where metabolite changes are taking place. For instance, while the overall concentration of glutamate in the brain is approximately 12 mM, concentrations vary between gray matter and white matter due to different rates of synthesis and oxidation across tissue.28 Moreover, 1H MRS does not permit differentiation between the cytosolic vs. vesicular pools within those gross compartments.242 Therefore, it is difficult to pinpoint the origin of any metabolite changes, which ultimately does limit interpretation of spectral changes.
Similarly, regardless of the fact that the chemical-shift approach allows the investigator to obtain multiple spectra simultaneously while the single-voxel approach results in one spectrum, the spectra themselves are only “snapshots” of the neurochemical environment during the acquisition. Although temporal resolution is determined in part by the strength of the magnetic field, even at high-fields (≥ 3 T) data is acquired on the order of minutes.207 This timeframe is acceptable for studies that employ retrospective or longitudinal designs, but using 1H MRS for human behavioral pharmacology during acute drug administration (e.g., 243) may benefit from faster acquisition. Taken together, although 1H MRS provides a non-invasive window into neurochemical changes associated with substance abuse, it still can be considered a crude measurement.
Conclusions
Neuroimaging techniques such as 1H MRS are invaluable tools for understanding the brain. The studies presented here suggest that reduced NAA and elevated mI may be neurochemical hallmarks of drug abuse-induced injury within the brain. Whether these changes are a result of the effects of the chemicals on the brain specifically or whether they reflect the neurobiology driving the addictive process in general (i.e., impairments in motivation-reward, affect regulation, and behavioral inhibition as reviewed223), is unknown. In addition to gaining insight regarding the neurochemical outcomes of drug abuse, 1H MRS also may provide an opportunity to match individuals with the most suitable treatment, monitor treatment efficacy, and predict and/or prevent relapse. Consequently, 1H MRS potentially could have a profound impact on future drug abuse research.
Acknowledgments
This work was supported by the National Institute on Drug Abuse grants K01 DA023659 (SCL) and K24 DA151116 (PFR).
References
- 1.Bartsch AJ, Homola G, Biller A, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- 2.Bendszus M, Weijers HG, Wiesbeck G, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. AJNR Am J Neuroradiol. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- 3.Durazzo TC, Gazdzinski S, Rothlind JC, et al. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alcohol Clin Exp Res. 2006;30:539–551. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 4.Ende G, Welzel H, Walter S, et al. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Parks MH, Dawant BM, Riddle WR, et al. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin Exp Res. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- 6.Streeter CC, Hennen J, Ke Y, et al. Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafazine treatment. Psychopharmacology. 2005;182:516–526. doi: 10.1007/s00213-005-0121-5. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhoff DJ, Durazzo TC. Proton magnetic resonance spectroscopy in alcohol use disorders: a potential new endophenotype. Alcohol Clin Exp Res. 2008;32:1146–1158. doi: 10.1111/j.1530-0277.2008.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore CM, Frederick BB, Renshaw PF. Brain biochemistry using magnetic resonance spectroscopy: relevance to psychiatric illness in the elderly. J Geriatr Psychiatry Neurol. 1999;12:107–117. doi: 10.1177/089198879901200304. [DOI] [PubMed] [Google Scholar]
- 9.Moffett JR, Ross B, Arun P, et al. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pouwels PJ, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed. 1997;10:73–78. doi: 10.1002/(sici)1099-1492(199704)10:2<73::aid-nbm448>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Tallan HH, Moore S, Stein WH. N-Acetyl-L-aspartic acid in brain. J Biol Chem. 1956;219:257–264. [PubMed] [Google Scholar]
- 12.Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- 13.Inglese M, Rusinek H, George IC, et al. Global average gray and white matter N-acetylaspartate concentration in the human brain. Neuroimage. 2008;41:270–276. doi: 10.1016/j.neuroimage.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadler JV, Cooper JR. N-acetyl-aspartic acid content of human neural tumours and bovine peripheral nervous tissues. J Neurochem. 1972;19:313–319. doi: 10.1111/j.1471-4159.1972.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 15.Brooks WM, Friedman SD, Gasparovic C. Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil. 2001;16:149–164. doi: 10.1097/00001199-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Chen JG, Charles HC, Barboriak DP, et al. Magnetic resonance spectroscopy in Alzheimer’s disease: focus on N-acetylaspartate. Acta Neurol Scand Suppl. 2000;176:20–26. doi: 10.1034/j.1600-0404.2000.00303.x. [DOI] [PubMed] [Google Scholar]
- 17.Demougeot C, Marie C, Giroud M, et al. N-acetylaspartate: a literature review of animal research on brain ischemia. J Neurochem. 2004;90:776–783. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- 18.Simone IL, Tortorella C, Federico F. The contribution of (1)H-magnetic resonance spectroscopy in defining the pathophysiology of multiple sclerosis. Ital J Neurol Sci. 1999;20:S241–S245. doi: 10.1007/s100729970004. [DOI] [PubMed] [Google Scholar]
- 19.Li SJ, Wang Y, Pankiewicz J, et al. Neurochemical adaptation to cocaine abuse: reduction of N-acetyl aspartate in thalamus of human cocaine abusers. Biol Psychiatry. 1999;45:1481–1487. doi: 10.1016/s0006-3223(98)00230-3. [DOI] [PubMed] [Google Scholar]
- 20.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 21.Bonavita S, Di Salle F, Tedeschi G. Proton MRS in neurological disorders. Eur J Radiol. 1999;30:125–131. doi: 10.1016/s0720-048x(99)00051-0. [DOI] [PubMed] [Google Scholar]
- 22.Frey BN, Stanley JA, Nery FG, et al. Abnormal cellular energy and phopholipid metabolism in the left dorsolateral prefrontal cortex of medication-free individuals with bipolar disorder: an in vivo 1H MRS study. Bipolar Disord. 2007;9(Suppl 1):119–127. doi: 10.1111/j.1399-5618.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- 23.Yerli H, Ağildere AM, Ozen O, et al. Evaluation of cerebral glioma grade by using normal side creatine as an internal reference in multi-voxel 1H-MR spectroscopy. Diagn Interv Radiol. 2007;13:3–9. [PubMed] [Google Scholar]
- 24.King KG, Glodzik L, Liu S, et al. Anteroposterior hippocampal metabolic heterogeneity: three-dimensional multivoxel proton 1H MR spectroscopic imaging-initial findings. Radiology. 2008;249:242–250. doi: 10.1148/radiol.2491071500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller BL. A review of chemical issues in 1H NRM spectroscopy: N-acetyl-L aspartate, creatine and choline. NMR Biomed. 1991;4:47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Toledo E, Kelley RE, Minager A. Role of magnetic resonance spectroscopy in diagnosis and management of multiple sclerosis. Neurol Res. 2006;28:280–283. doi: 10.1179/016164106X98161. [DOI] [PubMed] [Google Scholar]
- 27.Nakanishi T, Turner RJ, Burg MB. Osmoregulatory changes in myo-inositol transport by renal cells. Proc Natl Acad Sci USA. 1989;86:6002–6006. doi: 10.1073/pnas.86.15.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross BD. Biochemical considerations in 1H spectroscopy. Glutamate and glutamine; myo-inositol and related metabolites. NMR Biomed. 1991;4:59–63. doi: 10.1002/nbm.1940040205. [DOI] [PubMed] [Google Scholar]
- 29.Berridge MJ, Irvine RF. Inositol phosphates and cell signaling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle to psychiatric disorders–focus on magnetic resonance spectroscopy (MRS) studies. Hum Psychopharmacol. 2005a;20:309–326. doi: 10.1002/hup.693. [DOI] [PubMed] [Google Scholar]
- 31.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 32.Behar KL, Rothman DL. In vivo nuclear magnetic resonance studies of glutamate-gamma-aminobutyric acid-glutamine cycling in rodent and human cortex: the central role of glutamine. J Nutr. 2001;131:2498S–2504S. doi: 10.1093/jn/131.9.2498S. [DOI] [PubMed] [Google Scholar]
- 33.Mullins PG, Chen H, Xu J, et al. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn Reson Med. 2008;60:964–969. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- 34.Behar KL, Rothman DL, Spencer DD, et al. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- 35.Kish SJ, Perry TL, Hansen S. Regional distribution of homocarnosine, homocarnosine-carnosine synthetase and homocarnosinase in human brain. J Neurochem. 1979;32:1629–1636. doi: 10.1111/j.1471-4159.1979.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 36.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torregrossa MM, Kalivas PW. Microdialysis and the neurochemistry of addiction. Pharmacol Biochem Behav. 2008;90:261–272. doi: 10.1016/j.pbb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;18:E337–E347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricaurte GA, McCann UD. Neurotoxic amphetamine analogues: effects in monkeys and implications for humans. Ann N Y Acad Sci. 1992;648:371–382. doi: 10.1111/j.1749-6632.1992.tb24586.x. [DOI] [PubMed] [Google Scholar]
- 40.Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol. 2008;3:165–172. doi: 10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cadet JL, I, Krasnova N, Jayanthi S, et al. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- 42.Lundqvist T. Cognitive consequences of cannabis use: Comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 43.McGrath BM, McKay R, Dave S, et al. Acute dextro-amphetamine administration does not alter brain myo-inositol levels in humans and animals: MRS investigations at 3 and 18.8 T. Neurosci Res. 2008;61:351–359. doi: 10.1016/j.neures.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Silverstone PH, O’Donnell T, Ulrich M, et al. Dextro amphetamine increases phosphoinositol cycle activity in volunteers: an MRS study. Hum Psychopharmacol. 2002;17:425–429. doi: 10.1002/hup.434. [DOI] [PubMed] [Google Scholar]
- 45.Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidyl-inositol responses in brain and salivary glands. Biochem J. 1982;206:587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 47.Kim H, Thompson RB, Hanstock CC, et al. Variability of metabolite yield using STEAM or PRESS sequences in vivo at 3.0 T, illustrated with myo-inositol. Magn Reson Med. 2005b;53:760–769. doi: 10.1002/mrm.20434. [DOI] [PubMed] [Google Scholar]
- 48.Melega WP, Quintana J, Raleigh MJ, et al. 6-[18F]fluoro-L-DOPA-PET studies show partial reversibility of long-term effects of chronic amphetamine in monkeys. Synapse. 1996;22:63–69. doi: 10.1002/(SICI)1098-2396(199601)22:1<63::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 49.Ridley RM, Baker HF, Owen F, et al. Behavioural and biochemical effects of chronic amphetamine treatment in the vervet monkey. Psychopharmacology. 1982;78:245–251. doi: 10.1007/BF00428159. [DOI] [PubMed] [Google Scholar]
- 50.Steranka LR, Sanders-Bush E. Long-term effects of continuous exposure to amphetamine on brain dopamine concentration and synaptosomal uptake in mice. Eur J Pharmacol. 1980;65:439–443. doi: 10.1016/0014-2999(80)90351-9. [DOI] [PubMed] [Google Scholar]
- 51.Wagner GC, Ricaurte GA, Johanson CE, et al. Amphetamine induces depletion of dopamine and loss of dopamine uptake sites in caudate. Neurology. 1980;30:547–550. doi: 10.1212/wnl.30.5.547. [DOI] [PubMed] [Google Scholar]
- 52.McCann UD, Wong DF, Yokoi F, et al. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volkow ND, Chang L, Wang GJ, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001a;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 54.Sekine Y, Ouchi Y, Takei N, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- 55.London ED, Simon SL, Berman SM, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 56.Volkow ND, Chang L, Wang GJ, et al. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001b;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- 57.Wang GJ, Volkow ND, Chang L, et al. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- 58.Chang L, Cloak C, Patterson K, et al. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung A, I, Lyoo K, Kim SJ, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- 60.Kim SJ, I, Lyoo K, Hwang J, et al. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- 61.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ernst T, Chang L, Leonido-Yee M, et al. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 63.Nordahl TE, Salo R, Possin K, et al. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry Res. 2002;116:43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 64.Nordahl TE, Salo R, Natsuaki Y, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- 65.Salo R, Nordahl TE, Natsuaki Y, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 66.Sung YH, Cho SC, Hwang J, et al. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend. 2007;88:28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 67.Sekine Y, Minabe Y, Kawai M, et al. Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms. A proton MRS study. Neuropsychopharmacology. 2002;27:453–461. doi: 10.1016/S0893-133X(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 68.Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit Rev Neurobiol. 2005;17:87–118. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 70.Baker DA, McFarland K, Lake RW, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 71.LaRowe SD, Myrick H, Hedden S, et al. Is cocaine desire reduced by N– acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 72.Mardikian PN, LaRowe SD, Hedden S, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of methylenedioxyamphetamines (MDMA; ecstasy) in humans: how strong is the evidence for persistent brain damage? Addiction. 2006a;101:348–361. doi: 10.1111/j.1360-0443.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 74.Creighton FJ, Black DL, Hyde CE. ‘Ecstasy’ psychosis and flashbacks. Br J Psychiatry. 1991;159:713–715. doi: 10.1192/bjp.159.5.713. [DOI] [PubMed] [Google Scholar]
- 75.Fetter JC. Mirtazepine for MDMA-induced depression. Am J Addict. 2005;14:300–301. doi: 10.1080/10550490590966137. [DOI] [PubMed] [Google Scholar]
- 76.Pallanti S, Mazzi D. MDMA (Ecstasy) precipitation of panic disorder. Biol Psychiatry. 1992;32:91–95. doi: 10.1016/0006-3223(92)90145-p. [DOI] [PubMed] [Google Scholar]
- 77.Soar K, Parrott AC, Fox HC. Persistent neuropsychological problems after 7 years of abstinence from recreational Ecstasty (MDMA): a case study. Psychol Rep. 2004;95:192–196. doi: 10.2466/pr0.95.1.192-196. [DOI] [PubMed] [Google Scholar]
- 78.McCann UD, Peterson SC, Ricaurte GA. The effect of catecholamine depletion by alpha-methyl-para-tyrosine on measures of cognitive performance and sleep in abstinent MDMA users. Neuropsychopharmacology. 2007;32:1695–1706. doi: 10.1038/sj.npp.1301302. [DOI] [PubMed] [Google Scholar]
- 79.Reneman L, Majoie CB, Schmand B, et al. Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol Psychiatry. 2001;50:550–554. doi: 10.1016/s0006-3223(01)01177-5. [DOI] [PubMed] [Google Scholar]
- 80.Reneman L, Majoie CB, Flick H, et al. Reduced N-acetylaspartate levels in the frontal cortex of 3,4-methylenedioxymethamphetamine (Ecstasy) users: preliminary results. AJNR Am J Neuroradiol. 2002;23:231–237. [PMC free article] [PubMed] [Google Scholar]
- 81.Daumann J, Fischermann T, Pilatus U, et al. Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neurosci Lett. 2004;362:113–116. doi: 10.1016/j.neulet.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Chang L, Ernst T, Grob CS, et al. Cerebral (1)H MRS alterations in recreational 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users. J Magn Reson Imaging. 1999a;10:521–526. doi: 10.1002/(sici)1522-2586(199910)10:4<521::aid-jmri4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 83.Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology. 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- 84.Cowan RL, Joers JM, Dietrich MS. N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: A 3 T magnetic resonance spectroscopy study. Pharmacol Biochem Behav. 2009;92:105–110. doi: 10.1016/j.pbb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Win MM, Reneman L, Jager G, et al. A prospective cohort study on sustained effects of low-dose ecstasy use on the brain in new ecstasy users. Neuropsychopharmacology. 2007;32:458, 470. doi: 10.1038/sj.npp.1301225. [DOI] [PubMed] [Google Scholar]
- 86.de Win MM, Jager G, Booij J, et al. Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain. 2008a;131(Pt 11):2936–2945. doi: 10.1093/brain/awn255. [DOI] [PubMed] [Google Scholar]
- 87.de Win MM, Jager G, Booij J, et al. Neurotoxic effects of ecstasy on the thalamus. Br J Psychiatry. 2008b;193:289–296. doi: 10.1192/bjp.bp.106.035089. [DOI] [PubMed] [Google Scholar]
- 88.Cowan RL, Bolo NR, Dietrich M, et al. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Obergriesser T, Ende G, Braus DF, et al. Hippocampal 1H-MRSI in ecstasy users. Eur Arch Psychiatry Clin Neurosci. 2001;251:114–116. doi: 10.1007/s004060170044. [DOI] [PubMed] [Google Scholar]
- 90.Gouzoulis-Mayfrank E, Daumann J. The confounding problem of polydrug use in recreational ecstasty/MDMA users: a brief overview. J Psychopharmacol. 2006b;20:188–193. doi: 10.1177/0269881106059939. [DOI] [PubMed] [Google Scholar]
- 91.Meyer JS, Brevard ME, Piper BJ, et al. Neural effects of MDMA as determined by functional magnetic resonance imaging and magnetic resonance spectroscopy in awake marmoset monkeys. Ann N Y Acad Sci. 2006;1074:365–376. doi: 10.1196/annals.1369.036. [DOI] [PubMed] [Google Scholar]
- 92.McCardle K, Luebbers S, Carter JD, et al. Chronic MDMA (ecstasy) use, cognition and mood. Psychopharmacology. 2004;173:434–439. doi: 10.1007/s00213-004-1791-0. [DOI] [PubMed] [Google Scholar]
- 93.Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA. 1998;279:376–380. [PubMed] [Google Scholar]
- 94.Du C, Yu M, Volkow ND, et al. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26:11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meldrum BS. Cell damage in epilepsy and the role of calcium in cytotoxicity. Adv Neurol. 1986;44:849–855. [PubMed] [Google Scholar]
- 96.Schanne FA, Kane AB, Young EE, et al. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 97.Chang L, Ernst T, Strickland T, et al. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry. 1999b;156:716–722. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- 98.Chang L, Mehringer CM, Ernst T, et al. Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biol Psychiatry. 1997;42:1105–1114. doi: 10.1016/s0006-3223(97)00135-2. [DOI] [PubMed] [Google Scholar]
- 99.Ke Y, Streeter CC, Nassar LE, et al. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 100.Cendes F, Andermann F, Dubeau F, et al. Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy. Evidence from proton MR spectroscopic imaging. Neurology. 1997;49:1525–153. doi: 10.1212/wnl.49.6.1525. [DOI] [PubMed] [Google Scholar]
- 101.De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34:721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- 102.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matochik JA, London ED, Eldreth DA, et al. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 104.Sim ME, I, Lyoo K, Streeter CC, et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- 105.Li SJ, Wang Y, Risinger R, et al. Acute cocaine administration induces NAA increase detected by 1H MRS. Proceedings of the International Society of Magnetic Resonance in Medicine, USA. 1998;6:1714. [Google Scholar]
- 106.Christensen JD, Kaufman MJ, Frederick B, et al. Proton magnetic resonance spectroscopy of human basal ganglia: response to cocaine administration. Biol Psychiatry. 2000;48:685–692. doi: 10.1016/s0006-3223(00)00897-0. [DOI] [PubMed] [Google Scholar]
- 107.Lien R, Mishra OP, Graham E, et al. Alteration of brain cell membrane function following cocaine exposure in the fetal guinea pig. Brain Res. 1994;637:249–254. doi: 10.1016/0006-8993(94)91240-8. [DOI] [PubMed] [Google Scholar]
- 108.Hertz L, Code WE, Sykova E. Ions, water and energy in brain cells: A synopsis of interrelations. Can J Physiol Pharmacol. 1992;70:S100–S106. doi: 10.1139/y92-250. [DOI] [PubMed] [Google Scholar]
- 109.Olson J, Sankar R, Holtzman D, et al. Energy-dependent volume regulation in primary cultured cerebral astrocytes. J Cell Physiol. 1986;128:209–215. doi: 10.1002/jcp.1041280211. [DOI] [PubMed] [Google Scholar]
- 110.Li BS, Wang H, Gonen O. Metabolite ratios to assumed stable creatine levels may confound the quantification of proton brain MR spectroscopy. Magn Reson Imaging. 2003;21:923–928. doi: 10.1016/s0730-725x(03)00181-4. [DOI] [PubMed] [Google Scholar]
- 111.Kaufman MJ, Levin JM, Maas LC, et al. Cocaine-induced cerebral vasoconstriction differs as a function of sex and menstrual cycle phase. Biol Psychiatry. 2001;49:774–781. doi: 10.1016/s0006-3223(00)01091-x. [DOI] [PubMed] [Google Scholar]
- 112.Hetherington HP, Pan JW, Telang F, et al. Reduced brain GABA levels in cocaine abusers. Proceedings of the International Society for Magnetic Resonance in Medicine, USA. 2000;8:523. [Google Scholar]
- 113.Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102:33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- 114.Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 115.Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate use. Neuropsychol Rev. 2007;17:299–315. doi: 10.1007/s11065-007-9041-y. [DOI] [PubMed] [Google Scholar]
- 116.Wise RA, Bozarth MA. Action of drugs of abuse on brain reward systems: An update with specific attention to opiates. Pharmacol Biochem Behav. 1982;17:239–243. doi: 10.1016/0091-3057(82)90076-4. [DOI] [PubMed] [Google Scholar]
- 117.Ersche KD, Fletcher PC, Lewis SJG, et al. Abnormal frontal activations related to decision making in current and former amphetamine and opiate dependent individuals. Psychopharmacology. 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Forman SD, Dougherty GG, Casey BJ, et al. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 119.Yücel M, Lubman DI, Harrison BJ, et al. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12:611, 691–702. doi: 10.1038/sj.mp.4001955. [DOI] [PubMed] [Google Scholar]
- 120.Danos P, Kasper S, Grünwald F, et al. Pathological regional cerebral blood flow in opiate-dependent patients during withdrawal: a HMPAO-SPECT study. Neuropsychobiology. 1998;37:194–199. doi: 10.1159/000026502. [DOI] [PubMed] [Google Scholar]
- 121.Galynker II, Watras-Ganz S, Miner C, et al. Cerebral metabolism in opiate-dependent subjects: effects of methadone maintenance. Mt Sinai J Med. 2000;67:381–387. [PubMed] [Google Scholar]
- 122.Krystal JH, Woods SW, Kosten TR, et al. Opiate dependence and withdrawal: preliminary assessment using single photon emission computerized tomography (SPECT) Am J Drug Alcohol Abuse. 1995;21:47–63. doi: 10.3109/00952999509095229. [DOI] [PubMed] [Google Scholar]
- 123.Rose JS, Branchey M, Buydens-Branchey L, et al. Cerebral perfusion deficits in early and late opiate withdrawal: a technetium-99m-HMPAO SPECT study. Psychiatry Res. 1996;67:39–47. doi: 10.1016/0925-4927(96)02663-7. [DOI] [PubMed] [Google Scholar]
- 124.Lyoo IK, Streeter CC, Ahn KH, et al. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res. 2004;131:2229–2237. doi: 10.1016/j.pscychresns.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 125.Lyoo IK, Pollack MH, Silveri MM, et al. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology. 2006;184:139–144. doi: 10.1007/s00213-005-0198-x. [DOI] [PubMed] [Google Scholar]
- 126.Haselhorst R, Dürsteler-MacFarland KM, Scheffler K, et al. Frontocortical N-acetylaspartate reduction associated with long-term i.v. heroin use. Neurology. 2002;58:305–307. doi: 10.1212/wnl.58.2.305. [DOI] [PubMed] [Google Scholar]
- 127.Kriegstein AR, Shungu DC, Millar WS, et al. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (“chasing the dragon”) Neurology. 1999;53:1765–1773. doi: 10.1212/wnl.53.8.1765. [DOI] [PubMed] [Google Scholar]
- 128.Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol. 2008;63:146–152. doi: 10.1016/j.crad.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 129.Mathews PM, Andermann F, Silver K, et al. Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology. 1993;43:2484–2490. doi: 10.1212/wnl.43.12.2484. [DOI] [PubMed] [Google Scholar]
- 130.Xiang Y, Gao H, Zhu H, et al. Neurochemical changes in brain induced by chronic morphine treatment: NMR studies in thalamus and somatosensory cortex of rats. Neurochem Res. 2006;31:1255–1261. doi: 10.1007/s11064-006-9158-z. [DOI] [PubMed] [Google Scholar]
- 131.Murray RM, Morrison PD, Henquet C, et al. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- 132.Chan GC, Hinds TR, Impey S, et al. Hippocampal neurotoxicity of Δ9– tetrahydrocannabinol. J Neurosci. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Landfield PW, Cadwallader LB, Vinsant S. Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Res. 1988;443:47–62. doi: 10.1016/0006-8993(88)91597-1. [DOI] [PubMed] [Google Scholar]
- 134.Yücel M, Solowij N, Respondek C, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008a;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 135.Chang L, Cloak C, Yakupov R, et al. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hermann D, Sartorius A, Welzel H, et al. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61:1281–1289. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 137.Bertolino A, Sciota D, Brudaglio F, et al. Working memory deficits and levels of N-acetylaspartate in patients with schizophreniform disorder. Am J Psychiatry. 2003;160:483–489. doi: 10.1176/appi.ajp.160.3.483. [DOI] [PubMed] [Google Scholar]
- 138.Molina V, Sánchez J, Reig S, et al. N-acetyl-aspartate levels in the dorsolateral prefrontal cortex in the early years of schizophrenia are inversely related to disease duration. Schizophr Res. 2005;73:209–219. doi: 10.1016/j.schres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 139.Henquet C, Di Forti M, Morrison P, et al. Gene-environment interplay between cannabis and psychosis. Schizophr Bull. 2008;34:1111–1121. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol. 1999;58:381–387. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- 141.Sullivan EV. Human brain vulnerability to alcoholism: evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio (NIAAA Research Monograph No. 34) National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 2000. pp. 473–508. [Google Scholar]
- 142.Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Durazzo TC, Gazdzinski S, Banys P, et al. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- 144.Jagannathan NR, Desai NG, Raghunathan P. Brain metabolite changes in alcoholism: an in vivo proton magnetic resonance spectroscopy (MRS) study. Magn Reson Imaging. 1996;14:553–557. doi: 10.1016/0730-725x(96)00048-3. [DOI] [PubMed] [Google Scholar]
- 145.Schweinsburg BC, Taylor MJ, Alhassoon OM, et al. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin Exp Res. 2001;25:924–934. [PubMed] [Google Scholar]
- 146.Schweinsburg BC, Alahassoon OM, Taylor MJ, et al. Effects of alcoholism and gender on brain metabolism. Am J Psychiatry. 2003;160:1180–1183. doi: 10.1176/appi.ajp.160.6.1180. [DOI] [PubMed] [Google Scholar]
- 147.Schweinsburg BC, Taylor MJ, Videen JS, et al. Elevated myo-inositol in gray matter of recently detoxified but not long-term abstinent alcoholics: a preliminary MR spectroscopy study. Alcohol Clin Exp Res. 2000;24:699–705. [PubMed] [Google Scholar]
- 148.Seitz D, Widmann U, Seeger U, et al. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcohol Clin Exp Res. 1999;23:158–163. [PubMed] [Google Scholar]
- 149.Lee E, Jang DP, Kim JJ, et al. Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport. 2007;18:1511–1514. doi: 10.1097/WNR.0b013e3282ef7625. [DOI] [PubMed] [Google Scholar]
- 150.Miese F, Kircheis G, Wittsack HJ, et al. 1H-MR spectroscopy, magnetization transfer, and diffusion-weighted imaging in alcoholic and nonalcoholic patients with cirrhosis with hepatic encephalopathy. AJNR Am J Neuroradiol. 2006;27:1019–1026. [PMC free article] [PubMed] [Google Scholar]
- 151.Martin PR, Gibbs SJ, Nimmerrichter AA, et al. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:1078–1082. doi: 10.1111/j.1530-0277.1995.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 152.O’Neill J, V, Cardenas A, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:1673–1682. [PubMed] [Google Scholar]
- 153.Meyerhoff DJ, Blumenfeld R, Truran D, et al. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gazdzinski S, Durazzo TC, Weiner MW, et al. Are treated alcoholics representative of the entire population with alcohol use disorders? A magnetic resonance study of brain injury. Alcohol. 2008a;42:67–76. doi: 10.1016/j.alcohol.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vengeliene V, Bilbao A, Molander A, et al. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hoffman PL, Tabakoff B. Alcohol dependence: a commentary on mechanisms. Alcohol Alcohol. 1996;31:333–340. doi: 10.1093/oxfordjournals.alcalc.a008159. [DOI] [PubMed] [Google Scholar]
- 157.Tsai GE, Ragan P, Chang R, et al. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry. 1998;155:726–732. doi: 10.1176/ajp.155.6.726. [DOI] [PubMed] [Google Scholar]
- 158.Behar KL, Rothman DL, Petersen KF, et al. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- 159.Mason GF, I, Petrakis L, de Graaf RA, et al. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 160.Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- 161.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamo-cortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- 162.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 163.Gazdzinski S, Durazzo TC, Studholme C, et al. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- 164.Brody AL, Mandelkern MA, Jarvik ME, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- 165.Gallinat J, Meisenzahl E, Jacobsen LK, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- 166.Brody AL. Functional brain imaging of tobacco use and dependence. J Psychiatr Res. 2006;40:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Markou A. Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Epperson CN, O’Malley S, Czarkowski KA, et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40:64–67. doi: 10.1055/s-2007-970144. [DOI] [PubMed] [Google Scholar]
- 170.Gallinat J, Lang UE, Jacobsen LK, et al. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. J Clin Psychopharmacol. 2007;27:80–84. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- 171.Barros DM, Galhardi FG, Ribas Ferreira JL, et al. The benefits and drawbacks of nicotine exposure in the cortex and hippocampus of old rats. Neurotoxicity. 2007;28:562–568. doi: 10.1016/j.neuro.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 172.Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Trauth JA, Seidler FJ, McCook EC, et al. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- 174.Burling TA, Ziff DC. Tobacco smoking: a comparison between alcohol and drug abuse inpatients. Addict Behav. 1988;13:185–190. doi: 10.1016/0306-4603(88)90010-x. [DOI] [PubMed] [Google Scholar]
- 175.DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- 176.Littleton J, Barron S, Prendergast M, et al. Smoking kills (alcoholics)! Shouldn’t we do something about it? Alcohol Alcohol. 2007;42:167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- 177.Gazdzinski S, Durazzo TC, Jahng GH, et al. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: a preliminary magnetic resonance study. Alcohol Clin Exp Res. 2006;30:947–958. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gazdzinski S, Durazzo TC, Yeh PH, et al. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008b;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yücel M, Takagi M, Walterfang M, et al. Toluene misuse and long-term harms: a systematic review of the neuropsychological and neuroimaging literature. Neurosci Biobehav Rev. 2008b;32:910–926. doi: 10.1016/j.neubiorev.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 180.Gerasimov MR. Brain uptake and biodistribution of [11C]toluene in nonhuman primates and mice. Methods Enzymol. 2004;385:334–349. doi: 10.1016/S0076-6879(04)85018-3. [DOI] [PubMed] [Google Scholar]
- 181.Gospe SM, Jr, Calaban MJ. Central nervous system distribution of inhaled toluene. Fundam Appl Toxicol. 1988;11:540–545. doi: 10.1016/0272-0590(88)90118-2. [DOI] [PubMed] [Google Scholar]
- 182.Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42:335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 183.Rosenberg NL, Kleinschmidt-DeMasters BK, Davis KA, et al. Toluene abuse causes diffuse central nervous system white matter changes. Ann Neurol. 1988;23:611–614. doi: 10.1002/ana.410230614. [DOI] [PubMed] [Google Scholar]
- 184.Aydin K, Sencer S, Demir T, et al. Cranial MR findings in chronic toluene abuse by inhalation. AJNR Am J Neuroradiol. 2002;23:1173–1179. [PMC free article] [PubMed] [Google Scholar]
- 185.Filley CM, Heaton RK, Rosenberg NL. White matter dementia in chronic toluene abuse. Neurology. 1990;40:532–534. doi: 10.1212/wnl.40.3_part_1.532. [DOI] [PubMed] [Google Scholar]
- 186.Yamanouchi N, Okada S, Kodama K, et al. Effects of MRI abnormalities on WAIS-R performance in solvent abusers. Acta Neurol Scand. 1997;96:34–39. doi: 10.1111/j.1600-0404.1997.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 187.Ikeda M, Tsukagoshi H. Encephalopathy due to toluene sniffing. Report of a case with magnetic resonance imaging. Eur Neurol. 1990;30:347–349. doi: 10.1159/000117371. [DOI] [PubMed] [Google Scholar]
- 188.Lubman DI, Yücel M, Lawrence AJ. Inhalant abuse among adolescents: neurobiological considerations. Br J Pharmacol. 2008;154:316–326. doi: 10.1038/bjp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rosenberg NL, Grigsby J, Dreisbach J, et al. Neuropsychologic impairment and MRI abnormalities associated with chronic solvent abuse. J Toxicol Clin Toxicol. 2002;41:209–210. doi: 10.1081/clt-120002883. [DOI] [PubMed] [Google Scholar]
- 190.Zur J, Yule W. Chronic solvent abuse. 1. Cognitive sequelae. Child Care Health Dev. 1990;16:1–20. doi: 10.1111/j.1365-2214.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 191.Stengard K. Effects of toluene inhalation on extracellular striatal acetylcholine release studied with microdialysis. Pharmacol Toxicol. 1994;75:115–118. doi: 10.1111/j.1600-0773.1994.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 192.Gerasimov MR, Schiffer WK, Marstellar D, et al. Toluene inhalation produces regionally specific changes in extracellular dopamine. Drug Alcohol Depend. 2002;65:243–51. doi: 10.1016/s0376-8716(01)00166-1. [DOI] [PubMed] [Google Scholar]
- 193.Riegel AC, Zapata A, Shippenberg TS, et al. The abused inhalant toluene increases dopamine release in the nucleus accumbens by directly stimulating ventral tegmental area neurons. Neuropsychopharmacology. 2007;32:1558–1569. doi: 10.1038/sj.npp.1301273. [DOI] [PubMed] [Google Scholar]
- 194.Stengard K, Tham R, O’Connor WT, et al. Acute toluene exposure increases extracellular GABA in the cerebellum of rat: A microdialysis study. Pharmacol Toxicol. 1993;73:315–318. doi: 10.1111/j.1600-0773.1993.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 195.Win-Shwe TT, Mitsushima D, Nakajima D, et al. Toluene induces rapid and reversible rise of hippocampal glutamate and taurine neurotransmitter levels in mice. Toxicol Lett. 2007;168:75–82. doi: 10.1016/j.toxlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 196.Noda S, Yamanouchi N, Okada S, et al. Proton MR spectroscopy in solvent abusers. Ann N Y Acad Sci. 1996;801:441–444. doi: 10.1111/j.1749-6632.1996.tb17466.x. [DOI] [PubMed] [Google Scholar]
- 197.Aydin K, Sencer S, Ogel K, et al. Single-voxel proton MR spectroscopy in toluene abuse. Magn Reson Imaging. 2003;21:777–785. doi: 10.1016/s0730-725x(03)00175-9. [DOI] [PubMed] [Google Scholar]
- 198.Ladefoged O, Strange P, Møller A, et al. Irreversible effects in rats of toluene (inhalation) exposure for six months. Pharmacol Toxicol. 1991;68:384–390. doi: 10.1111/j.1600-0773.1991.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 199.Escobar A, Aruffo C. Chronic thinner intoxication: clinicopathologic report of a human case. J Neurol Neurosurg Psychiatry. 1980;43:986–994. doi: 10.1136/jnnp.43.11.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Baydas G, Reiter RJ, Nedzvetskii VS, et al. Melatonin protects the central nervous system of rats against toluene-containing thinner intoxication by reducing reactive gliosis. Toxicol Lett. 2003;137:169–174. doi: 10.1016/s0378-4274(02)00400-9. [DOI] [PubMed] [Google Scholar]
- 201.Huang J, Asaeda N, Takeuchi Y, et al. Dose dependent effects of chronic exposure to toluene on neuronal and glial cell marker proteins in the central nervous system of rats. Br J Ind Med. 1992;49:282–286. doi: 10.1136/oem.49.4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gotohda T, Tokunaga I, Kubo S, et al. Effect of toluene inhalation on astrocytes and neurotrophic factor in rat brain. Forensic Sci Int. 2000;113:233–238. doi: 10.1016/s0379-0738(00)00215-2. [DOI] [PubMed] [Google Scholar]
- 203.Takebayashi K, Sekine Y, Takei N, et al. Metabolite alterations in basal ganglia associated with psychiatric symptoms of abstinent toluene users: a proton MRS study. Neuropsychopharmacology. 2004;29:1019–1026. doi: 10.1038/sj.npp.1300426. [DOI] [PubMed] [Google Scholar]
- 204.Ertugrul A, Uluğ B. The effect of clozapine on neuroimaging findings in schizophrenia. Psychiatr Danub. 2007;19:367–369. [PubMed] [Google Scholar]
- 205.Pedata F, Sorbi S, Pepeu G. Choline high-affinity uptake and metabolism and choline acetyltransferase activity in the striatum of rats chronically treated with neurolpetics. J Neurochem. 2006;35:606–611. doi: 10.1111/j.1471-4159.1980.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 206.Bustillo JR, Rowland LM, Lauriello J, et al. High choline concentrations in the caudate nucleus in antipsychotic-naïve patients with schizophrenia. Am J Psychiatry. 2002;159:130–133. doi: 10.1176/appi.ajp.159.1.130. [DOI] [PubMed] [Google Scholar]
- 207.Di Costanzo A, Trojsi F, Tosetti M, et al. High-field proton MRS of human brain. Eur J Radiol. 2003;48:146–153. doi: 10.1016/j.ejrad.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 208.Sawrie SM, Martin RC, Knowlton R, et al. Relationships among hippocampal volumetry, proton magnetic resonance spectroscopy, and verbal memory in temporal lobe epilepsy. Epilepsia. 2001;42:1403–1407. doi: 10.1046/j.1528-1157.2001.018301.x. [DOI] [PubMed] [Google Scholar]
- 209.Grachev ID, Kumar R, Ramachandran TS, et al. Cognitive interference is associated with neuronal marker N-acetyl aspartate in the anterior cingulate cortex: an in vivo (1)H-MRS study of the Stroop Color-Word task. Mol Psychiatry. 2001;6:529–539. doi: 10.1038/sj.mp.4000940. [DOI] [PubMed] [Google Scholar]
- 210.Ohrmann P, Siegmund A, Suslow T, et al. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naïve and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. J Psychiatry Res. 2007;41:625–634. doi: 10.1016/j.jpsychires.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 211.Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 212.Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 213.Lin A, Ross BD, Harris K, et al. Efficacy of proton magnetic resonance spectroscopy in neurological diagnosis and neurotherapeutic decision making. NeuroRx. 2005;2:197–214. doi: 10.1602/neurorx.2.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kushner SA, Dewey SL, Kornetsky C. The irreversible gamma-aminobutyric acid (GABA) transaminase inhibitor gamma-vinyl-GABA blocks cocaine self-administration in rats. J Pharmacol Exp Ther. 1999;290:797–802. [PubMed] [Google Scholar]
- 215.Stromberg M, Mackler S, Volpecelli J, et al. The effect of gamma-vinyl-GABA on the consumption of concurrently available oral cocaine and ethanol in the rat. Pharmacol Biochem Behav. 2001;68:291–299. doi: 10.1016/s0091-3057(00)00456-1. [DOI] [PubMed] [Google Scholar]
- 216.Brebner K, Childress A, Roberts D. A potential role for GABA(B) agonists in the treatment of pyschostimulant addiction. Alcohol Alcohol. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- 217.Gonzalez G, Sevarino K, Sofuoglu M, et al. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction. 2003;98:1625–1632. doi: 10.1046/j.1360-0443.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- 218.Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 219.Winhusen TM, Somoza EC, Harrer JM, et al. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005;100(Suppl 1):68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- 220.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 221.Pouwels PJ, Brockmann K, Kruse B, et al. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res. 1999;46:474–485. doi: 10.1203/00006450-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 222.Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- 223.Goodman A. Neurobiology of addiction. An integrative review. Biochem Pharmacol. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 224.Murphy EJ, Rajagopalan B, Brindle KM, et al. Phospholipid bilayer contribution to 31P NMR spectra in vivo. Magn Reson Med. 1989;12:282–289. doi: 10.1002/mrm.1910120218. [DOI] [PubMed] [Google Scholar]
- 225.Pettegrew J, Kopp S, Minshew N, et al. 31P Nuclear magnetic resonance studies of phosphoglyceride metabolism in developing and degenerating brain: preliminary observations. J Neuropath Exp Neurol. 1987;46:419–430. doi: 10.1097/00005072-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 226.Christensen JD, Kaufman MJ, Levin JM, et al. Abnormal cerebral metabolism in polydrug abusers during early withdrawal: a 31P MR spectroscopy study. Magn Reson Med. 1996;35:658–663. doi: 10.1002/mrm.1910350506. [DOI] [PubMed] [Google Scholar]
- 227.MacKay S, Meyerhoff DJ, Dillon WP, et al. Alteration of brain phospholipid metabolites in cocaine-dependent polysubstance abusers. Biol Psychiatry. 1993;34:261–264. doi: 10.1016/0006-3223(93)90080-w. [DOI] [PubMed] [Google Scholar]
- 228.Kaufman MJ, Pollack MH, Villafuerte RA, et al. Cerebral phosphorus metabolite abnormalities in opiate-dependent polydrug abusers in methadone maintenance. Psychiatry Res. 1999;90:143–152. doi: 10.1016/s0925-4927(99)00017-7. [DOI] [PubMed] [Google Scholar]
- 229.Silveri MM, Pollack MH, Diaz CI, et al. Cerebral phosphorus metabolite and transverse relaxation time abnormalities in heroin-dependent subjects at onset of methadone maintenance treatment. Psychiatry Res. 2004;131:217–226. doi: 10.1016/j.pscychresns.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 230.de Graaf RA, Mason GF, Patel AB, et al. In vivo 1H-[13C]-NMR spectroscopy of cerebral metabolism. NMR Biomed. 2003;16:339–357. doi: 10.1002/nbm.847. [DOI] [PubMed] [Google Scholar]
- 231.Magistretti PJ, Pellerin L, Rothman DL, et al. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 232.Mason GF, Gruetter R, Rothman DL, et al. Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J Cereb Blood Flow Metab. 1995;15:12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- 233.Sibson NR, Dhankhar A, Mason GF, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mason GF, Krystal JH. MR spectroscopy: its potential role for drug development for the treatment of psychiatric diseases. NMR Biomed. 2006;19:690–701. doi: 10.1002/nbm.1080. [DOI] [PubMed] [Google Scholar]
- 235.Neale J, Robertson M. Comparisons of self-report data and oral fluid testing in detecting drug use amongst new treatment clients. Drug Alcohol Depend. 2003;71:57–64. doi: 10.1016/s0376-8716(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 236.Baggot M, Heifets B, Jones RT, et al. Chemical analysis of ecstasy pills. JAMA. 2000;284:2190. doi: 10.1001/jama.284.17.2190. [DOI] [PubMed] [Google Scholar]
- 237.Nahas GG. The experimental use of cocaine in human subjects. Bull Narc. 1990;42:57–62. [PubMed] [Google Scholar]
- 238.Capizzano AA, Jorge RE, Acion LC, et al. In vivo proton magnetic resonance spectroscopy in patients with mood disorders: a technically oriented review. J Magn Reson Imaging. 2007;26:1378–1389. doi: 10.1002/jmri.21144. [DOI] [PubMed] [Google Scholar]
- 239.Frahm J, Michaelis T, Klaus-Dietmar M, et al. Localized NMR spectroscopy In Vivo. NMR Biomed. 1989;2:188–195. doi: 10.1002/nbm.1940020504. [DOI] [PubMed] [Google Scholar]
- 240.De Stefano N, Filippi M, Miller D, et al. Guidelines for using proton MR spectroscopy in multicenter clinical MS studies. Neurology. 2007;69:1942–1952. doi: 10.1212/01.wnl.0000291557.62706.d3. [DOI] [PubMed] [Google Scholar]
- 241.Hsu YY, Chang C, Chang CN, et al. Proton MR spectroscopy in patients with complex partial seizures: single-voxel spectroscopy versus chemical-shift imaging. AJNR Am J Neuroradiol. 1999;20:643–651. [PMC free article] [PubMed] [Google Scholar]
- 242.Valette J, Chaumeil M, Guillermier M, et al. Diffusion-weighted NMR spectroscopy allows probing of 13C labeling of glutamate inside distinct metabolic compartments in the brain. Magn Reson Med. 2008;60:306–311. doi: 10.1002/mrm.21661. [DOI] [PubMed] [Google Scholar]
- 243.Licata SC, Jensen JE, Penetar DM, et al. A therapeutic dose of zolpidem reduces thalamic GABA in healthy volunteers: a proton MRS study at 4 Tesla. Psychopharmacology. 2009;203:819–829. doi: 10.1007/s00213-008-1431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]