Abstract
Acyloxy nitroso compounds hydrolyze to nitroxyl (HNO), a nitrogen monoxide with distinct chemistry and biology. Ultraviolet-visible spectroscopy and mass spectrometry show hydrolysis rate depends on pH and ester group structure with the observed rate being trifluoroacetate (3) > acetate (1) > pivalate (2). Under all conditions, 3 rapidly hydrolyzes to HNO. A combination of spectroscopic, kinetic and product studies show that addition of thiols increases the decomposition rate of 1 and 2 leading to hydrolysis and HNO. Under conditions that favor thiolates, the thiolate directly reacts with the nitroso group yielding oximes without HNO formation. Biologically, 3 behaves like Angeli's salt demonstrating thiol-sensitive nitric oxide-mediated soluble guanylate cyclase-dependent vasorelaxation, suggesting HNO-mediated vasorelaxation. The slow HNO-donor 1 demonstrates weak thiol-insensitive vasorelaxation indicating HNO release kinetics determine HNO bioavailability and activity. These results show that acyloxy nitroso compounds represent new HNO donors capable of vasorelaxation depending on HNO release kinetics.
Introduction
Nitroxyl (HNO) is a nitrogen-containing compound chemically related to the well-known signaling agent nitric oxide (NO) through one-electron reduction and protonation. 1, 2 Nitroxyl demonstrates separate chemistry and biology compared to NO, which has driven studies to better understand its chemical reactions, potential endogenous formation, and the development of HNO-based therapeutics.1-10 Endogenous mechanisms of HNO formation have been proposed including direct enzymatic production from nitric oxide synthases, heme protein-mediated oxidation of various substrates and non-enzymatic reactions between thiols and S-nitrosothiols.11-15 Possible specific functions for endogenously produced HNO range from antioxidant,16 to vasorelaxant,17-25 to cytotoxic mediator.26 Some of these HNO associated activities may occur via oxidation to NO, and subsequent stimulation of NO-signaling and various 1-electron acceptors have been proposed to mediate this biochemistry including ferric heme proteins, flavins and quinones.21, 22
Irrespective of endogenous HNO formation, a parallel line of work entails the development of HNO donors as therapeutics.1-10 This area stems from previous studies demonstrating that HNO mediates the action of cyanamide,27, 28 a drug used to treat alcoholism. Exciting newer studies show the HNO donor, (sodium trioxodinitrate, Angeli's salt, AS) exerts positive inotropic effects in both normal and failing hearts, and decreases venous pressures, therapeutic properties optimal for the treatment of heart failure.5, 29 An increasing understanding of HNO reactivity reveals NO-independent mechanisms of biological action and identifies thiols as critical reaction targets. Recent studies suggest thiols represent the primary reaction target for HNO and likely mediate much of their biological activity.5 For example, nitroxyl inhibits the thiol containing enzymes aldehyde dehydrogenase (ALDH) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), showing both reversible and irreversible components of inhibition.27 The majority of the actions of HNO on cardiac tissue (increasing inotropy and calcium sensitivity) have also been attributed to HNO interaction with key thiols.29-31 Consistent with this concept, we showed HNO selectively modifies mitochondrial thiols.32
The chemical behavior of HNO (rapid dimerization and dehydration to nitrous oxide, N2O) requires the use of HNO donors in these studies.6 A current major limitation in HNO-based therapeutic development and our understanding of HNO biology is the reliance on a select few HNO-donors.2, 5, 10, 33 The most widely utilized is Angeli's salt, which also produces nitrite that possesses its own biological activity.34, 35 Development of structurally distinct HNO donors with diverse release rates would complement the majority of studies using AS, and form the basis of more efficient formulations.
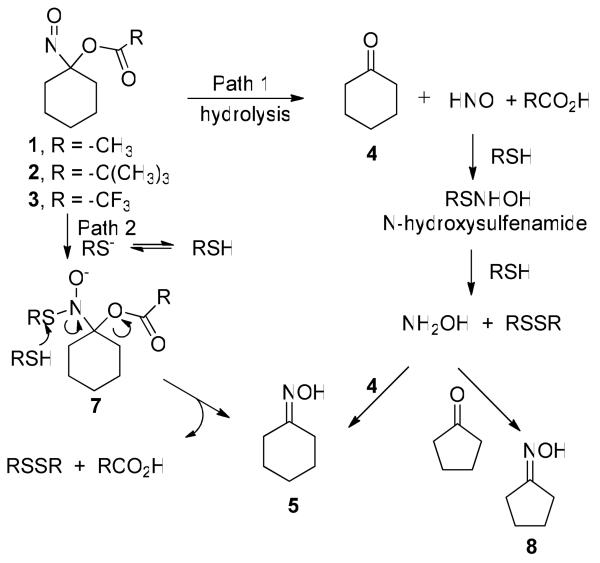
Previously, we reported acyloxy nitroso compounds as new hydrolytic HNO donors that relax pre-constricted rat aorta (Scheme 1).36 Here, we further characterize the kinetics, products, mechanism of HNO release and the vasoactive properties of three (1-3, Scheme 1) acyloxy nitroso compounds. These results provide detailed decomposition rate information and reveal the ability of acyloxy nitroso compounds to directly react with thiolate ions to form oximes and disulfides. The chemical reactivity of this new class of HNO donors provides a basis to explain the observed ability of these compounds to relax pre-constricted rat aorta.
Scheme 1.
Materials and Methods
Chemistry
General
Cyclohexanone oxime, 2,2-dimethylpropanoic acid, lead (IV) tetraacetate, [bis(trifluoroacetoxy)iodo]benzene, reduced glutathione (GSH), oxidized glutathione (GSSG), N-acetyl-DL-penicillamine (NAP), N-acetyl-L-cysteine (NAC), dithiothreitol (DTT) and thiophenol (TP) were purchased from Sigma-Aldrich Chemical Company and used as received. Analytical thin layer chromatography (TLC) was performed on silica gel plates with C-4 Spectroline 254 indicator. Visualization was accomplished with UV light and 20% phosphomolybdic acid solution in EtOH. Solvents for extraction and purification were technical grade and used as received. 1H NMR and 13C NMR spectra were recorded using a Bruker Advance 300 MHz NMR spectrometer. Chemical shifts are given in ppm (δ); multiplicities are indicated by s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and br (broadened). HT Laboratories, San Diego, CA, performed ESI analyses. Combustion elemental analysis (performed by Atlantic Microlab, Inc., Norcross, GA) provided >95% purity of any newly described compounds. UV-Vis spectrometry was performed on a Cary 100 Bio UV-Vis spectrophotometer (Varian, Walnut Creek, CA).
1-Nitrosocyclohexyl Acetate (NCA, 1).36
Synthesized as previously described to give 1 as a blue oil (4.67 g, 57%).
1-Nitrosocyclohexyl Pivalate (NCP, 2)
A solution of cyclohexanone oxime (4.88 g, 43.16 mmol) in CH2Cl2 (50 mL) was added dropwise with stirring to a solution of lead (IV) tetraacetate (19.14 g, 43.16 mmol) and 2,2-dimethylpropanoic acid (44.08 g, 431.6 mmol) in CH2Cl2 (200 mL) at 0°C. A blue color appeared with the addition of the oxime. The mixture was stirred at 0°C for one hour and at room temperature for 3 hrs. Water (50 mL) was added, the layers separated, and the CH2Cl2 layer was washed with saturated sodium bicarbonate (10 × 50 mL), dried over MgSO4, filtered, and concentrated. The crude residue was purified by passing through a short pad of silica gel (20:1 hexanes:EtOAc, Rf = 0.77) to give 2 as a blue oil (4.32 g, 47%). UV/vis (MeOH): λmax = 665 nm, ε = 20.7 M−1 cm−1; 1H NMR (benzene-d6) 1.2 (s, 9H) 1.2 – 1.8 (m, 10H); 13C NMR (benzene-d6) 21.9, 24.9, 27.2, 29.3, 39.2, 122.8, 175.5; ESI m/z 212 [100%, M – 1]; Anal. Calcd. For C11H19NO3: C, 61.95; H, 8.98, N, 6.57; Found: C, 61.71, H, 9.01, N, 6.45.
1-Nitrosocyclohexyl Trifluoroacetate (NCTFA, 3)
A solution of cyclohexanone oxime (0.57 g, 5 mmol) in CH2Cl2 (10 mL) was added dropwise with stirring to a solution of [bis(trifluoroacetoxy)iodo]benzene (2.15 g, 5 mmol) in CH2Cl2 (10 mL) at 0 °C. A blue color appeared with the addition of the oxime. After 1 hr at 0 °C, the reaction mixture was allowed to warm to room temperature and stirred for another 1 hr. After disappearance of the oxime as judged by TLC, the solvent was evaporated and the residue was purified through a short pad of silica gel (hexanes) to give 3 as blue oil (0.74g, 65% yield), and characterized as previously described.36
Gas Chromatographic Detection of Nitrous Oxide.36
Substrate (0.1 mmol) was placed in a 25 mL round bottom flask, sealed with rubber septa and flushed with argon. Solvent (2 mL, either MeOH, 1:1 MeOH:Tris buffer (50 mM, pH = 7.6), 1:1 MeOH:0.1 M NaOH, 1:1 MeOH:0.1 M KOH, or 1:1 MeOH:1 M HCl) was added via syringe. In some samples, GSH or NAP (0.2 mmol) was added to the solvent before injection. After certain times, headspace (250 μL) was injected into a 7890A Agilent Technologies gas chromatograph equipped with a thermal conductivity detector and a 6 ft. × 1/8 in. Porapack Q column. The oven operated at 40 °C for 5 minutes and then 150 °C for 4.5 minutes. The purged packed inlet with a total flow (He carrier gas) at 18 mL/min and a septum purge flow of 3 mL/min was kept constant at 140 °C and the back detector with a reference flow of 9 mL/min and a make-up flow of 6 mL/min was kept constant at 150 °C. The retention time of N2O was 2.5 min and the yields were calculated from a standard curve of N2O purchased from Matheson Tri-Gas. This experiment was repeated measuring headspace injections every 30 min for 9 hrs to evaluate kinetic measurements.
UV-Vis Assay for the decomposition of 1 and 2.36
A solution of Tris buffer (40 mM, pH = 7.0, 1.0 mL) was added to a solution of 1 (70 μmol, 12 mg) in MeOH (1 mL) at room temperature in a sealed cuvette. UV-Vis measurements were taken every 5 min for 60 min at 665 nm. In other experiments, a solution of NaOH, (70 mM, 1 mL) was used and similar measurements made. Similarly, a solution of Tris buffer (40 mM, pH = 7.0, 0.5 mL) was added to a solution of 2 (51 μmol, 10.9 mg) in MeOH (1 mL) at room temperature in a sealed cuvette. UV-Vis measurements were taken every 30 min for 4 hrs at 665 nm. In other experiments, a solution of NaOH, (0.1, 0.2, 0.5 M, 1 mL) was used and similar measurements made.
UV-Vis Assay for the reaction of 1 and 2 with thiols
A solution of GSH (35.1 μmol, 10.8 mg) in Tris buffer (40 mM, pH = 7.0, 0.5 mL) was added to a solution of 1 (35.1 μmol, 6 mg) in MeOH (0.5 mL) at room temperature in a sealed cuvette. UV-Vis measurements were taken every 5 min for 4 hrs at 665 nm. Similar experiments were performed using varying concentrations of GSH (70.2 μmol, 21.6 mg or 175 μmol, 53.7 mg), GSH (175 μmol, 53.7 mg) in NaOH (35.1 mM, 0.5 mL) or GSSG (35.1 μmol, 21.5 mg). Other experiments were done using NAC (35.1, 70.2, 175 μmol). Similarly, a solution of GSH (51 μmol, 15.6 mg) in Tris buffer (40 mM, pH = 7.0, 0.5 mL) was added to a solution of 2 (51 μmol, 10.9 mg) in MeOH (1 mL) at room temperature in a sealed cuvette. UV-Vis measurements were taken every 30 min for 4 hrs at 665 nm. Similar experiments were performed using varying concentrations of GSH (102 μmol, 31.2 mg or 260 μmol, 79.8 mg). In other experiments, a solution of NaOH (0.5 M, 1 mL) was used instead of buffer and GSSG (51 μmol, 21.5 mg) instead of GSH.
Product isolation
Following UV-Vis analysis, the MeOH was removed to leave an aqueous fraction. This fraction was extracted with CH2Cl2 (3 × 10 mL), the organic layers were combined, dried, filtered and concentrated to give a residue that was analyzed using TLC, and 1H, and 13C NMR spectroscopy. In other experiments, DTT (340 μmol, 52.4 mg) in MeOH (12 mL) was added and stirred at room temperature for 24 hrs till the disappearance of the blue color. The MeOH was removed and the residue dissolved in EtOAc and extracted with 1 M NaOH. After the organic layer was dried with MgSO4, the products of the reaction were identified by TLC and chemical shift analysis of the 1H and 13C NMR as compared to the standards: cyclohexanone, cyclohexanone oxime, GSH, GSSG, reduced and oxidized DTT.
GC-MS analysis of the reaction of 1-3 with thiols
A solution of acyloxy nitroso compound 1, 2 or 3 (0.3 mmol) in solvent (10 mL, MeOH, 1:1 MeOH:Tris buffer (50 mM, pH = 7.6), 1:1 MeOH:1 M NaOH) was stirred overnight. In some experiments GSH, NAC, TP (0.75 mmol) and/or cyclopentanone (1.5, 3 or 6 mmol) was added. The MeOH was evaporated, CH2Cl2 (2 mL) was added, the organic layer was separated and an aliquot (1 μL) was injected onto a 7890A Agilent Technologies gas chromatograph coupled to a 5957C Agilent Technologies inert XL EI/CI MSD with triple axis detector run in electron impact mode, and a 5-MS capillary column (30 m, 0.25 micron film, 0.25 mm OD) were used. The oven operated at 50 °C for 1 minute and ramped to 250 °C for 24 minutes for a total run time of 25 minutes. The purged packed inlet was kept constant at 250 °C and had a total flow (He carrier gas) of 61 mL/min with a pressure of 7.65 psi and a septum purge flow of 1 mL/min °C. Products were identified by retention time analysis compared to standard cyclohexanone, cyclohexanone oxime and cyclopentanone oxime (0.1 mg/mL) each. The amount of the cyclohexanone and cyclohexanone oxime formed were calculated using area under the peak. For the kinetic analysis of these reactions, MeOH, Tris buffer (50 mM, pH = 7.6) or 1 M NaOH (5 mL) was added to a solution of 1, 2 or 3 (0.3 mmol) and cyclopentanone oxime (internal standard, 500 μL, 0.5 mg/mL) in MeOH (4.5 mL). In other experiments NAC or TP (0.75 mmol) was added. Aliquots (1 μL) of these solutions were injected into the GC-MS spectrometer using the previous method every 30 min for 24 hrs. The amount of cyclohexanone, cyclohexanone oxime and acyloxy nitroso compound was calculated by comparing their area under the peak to the peak of the cyclopentanone oxime internal standard.
13C NMR measurements of the reaction of 1-3 with with thiols
A solution of GSH (2.5 mmol, 767.5 mg) in Tris buffer (50 mM, pH = 7.6) or 1 M NaOH (5 mL) and compound (1- 3, 1 mmol) and cyclopentanone (10 mmol, 884 μL) in MeOH (5 mL) was stirred overnight at room temperature. The MeOH was evaporated and the aqueous layer extracted with CH2Cl2 (2 mL). The organic layer was separated, dried with MgSO4 and evaporated. The products were dissolved in CDCl3 for 13C NMR measurements. The products were identified by chemical shift analysis compared to standards of cyclopentanone, cyclopentanone oxime, cyclohexanone and cyclohexanone oxime.
Pharmacology
Materials
Angeli's salt (AS) and MahmaNonoate (MNO) were purchased from Cayman Chemicals. Manganese (III) meso-(tetrakis(4-sulfonato-phenyl))porphyrinate (MnIIITSPP) was purchased from Frontier Scientific. All other reagents were purchased from Sigma Aldrich Chemical Company. Indomethacin and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxidecarboxy-PTIO (C-PTIO) were dissolved in 100% EtOH or MeOH, respectively. Acyloxy nitroso compounds (1 and 3) were solubilized in 100% dimethyl sulfoxide (DMSO). The concentration of 1 was determined spectrophotometrically (ε667 = 20.7 M−1cm−1) while the concentration of 3 was calculated by formula weight.36 All HNO-donor solutions were diluted in vessel relaxation experiments to a final solvent concentration of 0.1% (v/v). No effects of these solvents were observed on vasoactive responses at these concentrations (not shown). AS and MNO were dissolved in 0.1N NaOH and its concentration determined spectrophotometrically (ε237 = 6100 M−1 cm−1 and ε250 = 8 mM−1 cm−1 respectively).37 Krebs-Henseleit buffer (KH) was used for all studies and consisted of the following composition (final concentrations: 118.0 mM NaCl, 27.2 mM NaHCO3, 4.8 mM KCl, 1 mM KH2PO4, 1.2 mM MgSO4, 11.1 mM D-glucose, 0.03 mM EDTA) with a pH of 7.4 at 37°C and 5% CO2 perfusion. Male Sprague-Dawley rats (200-250 g) were purchased from Harlan (Indianapolis, IN, USA). Animals were housed in approved facilities, exposed to 12 hr light / dark cycles and provided food and water ad libitum. All experiments were conducted according to Institutional Animal Care and Use Committee approved protocols.13
Vessel Bioassay Studies
For all vessel experiments thoracic aorta from male Sprague-Dawley rats were used as previously described after cardiac puncture.38 Briefly, aortas were isolated and submerged in KH buffer maintained at room temperature. Vessels were cleansed of fat and connective tissue and segmented into 2-4 mm rings. Vessel rings were placed in vessels bioassay chambers (Radnoti Glass Technologies-Monrovia, CA, USA) containing 15 ml of KH buffer equilibrated at 37°C and perfused with a gas mixture comprising of 21% O2/ 5% CO2/ 74% N2, pH 7.45. A basal tension of 2 g was applied and vessel rings were allowed to equilibrate for 1 hr. Following equilibration, a hyperpolarizing concentration of KCl (70 mM) was added to the baths to check for viability and assess maximum constriction.
To assess the effects of O2 on HNO-donor mediated vasodilation, vessels were equilibrated to 21 or 1% O2 gas mixtures (containing 5% CO2 and balanced with N2). Gas was precisely delivered by mass flow controllers (Sierra Instruments, California, USA) set to 0.15 L/min as previously described.38 In all experiments, vessels were pretreated with nitric oxide synthase inhibitor L-NG-nitroarginine methyl ester (L-NAME, 100 μM) and indomethacin (5 μM) to inhibit cyclooxygenase. Vessels were pre-constricted to approximately 50-75% of maximal KCl constriction with phenylephrine (PE, 100 nM). AS, 1 or 3 dependent vasodilation was assessed by cumulative administration of increasing concentrations. Experiments were performed in the absence or presence of C-PTIO (200 μM), superoxide dismutase (SOD, 100 U/ml), GSH (0-1 mM), NAC (1 mM), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 1 μM) or MnIIITSPP (20 μM) which were added after PE and 5 mins before initiating MNO, AS, 1, or 3 concentration response. Vasodilatory effects of cumulative MNO, AS, 1, or 3 additions were determined by measuring the delta tension and expressing this as a percent relaxation with respect to the maximal PE constriction. Only one donor was used per aorta preparation with between 1-2 concentration response curves evaluated for each vessel segment; no differences in responses observed in consecutive runs (not shown) consistent with recent data showing no tolerance using AS.17 EC50s were obtained by fitting concentration-dependent dilation data non-linear curve fitting using sigmoidal curve with variable slope algorithm using GraphPad Prism software.
In a subset of experiments, after exposure to different NO/HNO-donors, the effects of sodium nitroprusside (SNP), an endothelium independent vasodilator were evaluated. In all cases SNP was able to fully dilate vessels indicating that different NO or HNO-donors did not compromise the ability of vessels to respond to vasoactive stimuli (not shown).
Statistics
Concentration-dependent vessel relaxation curves were fitted to sigmoidal dose-response algorithms by non-linear regression curve fitting and analyzed by 2-way RM-ANOVA with Bonferonni post-test to evaluate significance. When directly comparing measured EC50, unpaired t-test was used. Significance was set at P < 0.05. All data analysis was performed on GraphPad Prism 5.
Results
Synthesis
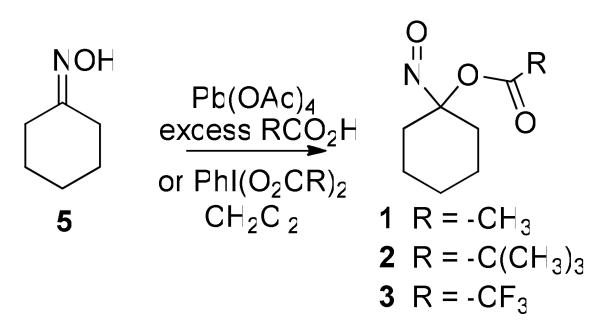
Oxidation of cyclohexanone oxime (5) with lead tetraacetate generates 1-nitrosocyclohexyl acetate (1) and a similar oxidation in the presence of excess 2,2-dimethylpropanoic acid or trifluoroacetic acid (5 to 10 equivalents) yields 1-nitrosocyclohexyl pivalate or trifluoroacetate (2-3, Scheme 2). Oxidation of cyclohexanone oxime (5) with [bis(trifluoroacetoxy)iodo]benzene provides 1-nitrosocyclohexyl trifluoroacetate (3, Scheme 2).39 1H and 13C NMR spectroscopy, mass spectrometry and elemental analysis confirm the structure of these acyloxy nitroso compounds, which exist as bright blue oils.
Scheme 2.
Acyloxy nitroso compound decomposition kinetics and products
Previous work shows that 1-nitrosocyclohexyl acetate (1) demonstrates reasonable stability at neutral pH.36 Monitoring the disappearance of the absorption at 667 nm using ultraviolet-visible (UV-Vis) spectroscopy or the peak representing the major mass fragment (M+-NO) of the acyloxy nitroso compound by gas chromatography-mass spectrometry (GC-MS) provides kinetic information regarding the decomposition of 1. Incubation of 1 in a 1:1 mixture of MeOH:Tris buffer (50 mM, pH = 7.6) at room temperature results in the exponential decrease of 1 over time with a rate constant of k = 8.6 × 10−4 min−1 and a t1/2 = 800 min (UV-Vis spectroscopy) and k = 7.8 × 10−4 min−1 and a t1/2 = 890 min (GC-MS). Compound 1 decomposes much slower in MeOH with a rate constant of k = 2.1 × 10−4 min−1 and a t1/2 = 3261 min. These kinetic results stand in contrast to the previously reported rapid decomposition of 1 in MeOH:0.1 N NaOH (t1/2 = 0.8 min). 1-Nitrosocyclohexyl pivalate (2) decomposes very slowly in a 1:1 mixture of MeOH:Tris buffer (50 mM, pH = 7.6) at room temperature as judged by UV-Vis or GC-MS experiments with a rate constant k = 2.5 × 10−4 min−1 and a t1/2 = 2268 min. UV-Vis experiments also show that 2 does not appreciably decompose over 4 hrs in a mixture of MeOH:1 eq. NaOH (Supporting Information). 1-Nitrosocyclohexyl trifluoroacetate (3) decomposes immediately upon addition of water or buffer to a MeOH solution of 3 as determined by the visual disappearance of the deep blue color.
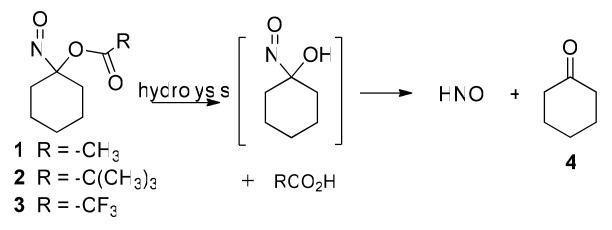
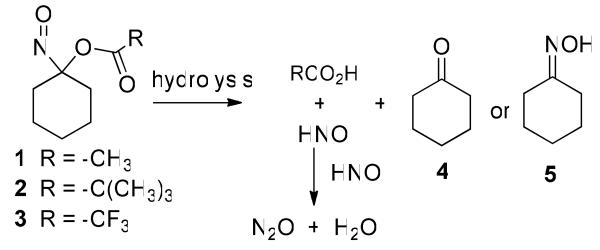
Gas chromatographic headspace analysis of N2O, the dimerization and dehydration product of HNO, provides evidence for HNO intermediacy during the decomposition of 1-3 (Scheme 3, Table 1). The results with 1 and 3 appear similar to those previously reported under neutral and basic conditions (Entries 1, 2 and 7-10).36 Under buffered conditions (Entry 1), the rate of N2O formation matches the rate of decomposition of 1 for approximately the first 3 hrs but decreases as decomposition of the acyloxy nitroso compound continues (Supporting Information). Under basic conditions, N2O formation occurs within the first hour consistent with the observed decomposition kinetics (Entry 2, Supporting Information). Acid-catalyzed hydrolysis of 1 appears slightly less efficient compared to basic hydrolysis in terms of HNO formation (Entry 1 vs. Entry 3). Addition of GSH (1 or 2 equivalents) to these incubations quenches N2O production in each case (<2%) providing further evidence for the intermediacy of HNO in these reactions. 1-Nitrosocyclohexyl pivalate (2) does not produce N2O (HNO) under neutral buffered, basic or acidic conditions (Entries 4-6) (Supporting Information).
Scheme 3.
Table 1.
Product formation from the decomposition of 1-3.
| N2O (%) | Organic | ||||
|---|---|---|---|---|---|
| Entry | Compound | Conditions | 2 hr | 24 hr | 4:5 |
| 1 | 1 | MeOH/Tris (50 mM, pH 7.6) | 12 | 39 | 84:16 |
| 2 | 1 | MeOH/0.1 NaOH | 50 | 36 | 97:3 |
| 3 | 1 | MeOH/HCl | 14 | 29 | 91:9 |
| 4 | 2 | MeOH/Tris (50 mM, pH 7.6) | 2 | 4 | 86:14 |
| 5 | 2 | MeOH/0.1 NaOH | 2 | 10 | 85:15 |
| 6 | 2 | MeOH/HCl | 2 | 8 | 94:6 |
| 7 | 3 | MeOH/Tris (50 mM, pH 7.6) | 57 | 54 | 97:3 |
| 8 | 3 | Tris (50 mM, pH 7.6) | 8 | 17 | - |
| 9 | 3 | MeOH | 1 | 10 | 100:0 |
| 10 | 3 | MeOH/0.1 NaOH | 100:0 | ||
A combination of TLC, NMR spectroscopy and GC-MS experiments reveals the formation of cyclohexanone (4) and cyclohexanone oxime (5) from the decomposition of 1-3 over time (much longer for 2) in MeOH/buffer and MeOH/basic conditions (Scheme 3, Table 1). In general, cyclohexanone (4) represents the major product from the decomposition of 1-3 with smaller amounts of cyclohexanone oxime (5) being formed (Table 1). No other cyclohexanone-derived products were identified and starting material was completely consumed during these incubations as judged by both UV-Vis spectroscopy and GC-MS.
Acyloxy nitroso compound decomposition kinetics and products in the presence of thiols
Kinetic UV-Vis spectroscopic experiments monitoring the disappearance of the absorption at 667 nm show that the addition of GSH to a solution of 1 in MeOH:TRIS buffer (40 mM, pH = 7.0) increases the rate of acyloxy nitroso compound decomposition (Supporting Information). The addition of GSH (5 equiv.) completely consumes 2, which does not appreciably decompose under neutral or basic conditions over 4 hrs (Supporting Information). Table 2 summarizes more detailed kinetic information regarding the decomposition of 1 in the presence of thiols from GC-MS experiments monitoring the disappearance of the major mass fragment (M+-NO) of the acyloxy nitroso compound. Reaction of 1 occurs much more rapidly in a MeOH:buffer mixture than in MeOH alone (Table 2, Entries 1 and 5). Addition of NAC approximately doubles the rate of decomposition in MeOH:buffer and has little effect on the rate in MeOH (Table 2, Entries 2, 3, 6 and 7). Addition of thiophenol (TP) to the reaction mixtures increases the rate of decomposition nearly 200-fold in MeOH:buffer and 30-fold in MeOH (Table 2, Entries 4 and 8). Similar to 1, treatment of 2 with TP results in its exponential decrease over time with a rate constant of k = 1.5 × 10−1 min−1 and a t1/2 = 4.6 min.
Table 2.
Kinetics of the decomposition of 1 in the presence of thiols.
| Entry | Conditions | Thiol (eq) | k (min−1) | t1/2 (min) |
|---|---|---|---|---|
| 1 | MeOH/Tris (50 mM, pH 7.6) | - | 7.8 × 10−4 | 890 |
| 2 | MeOH/Tris (50 mM, pH 7.6) | NAC (2.5) | 1.5 × 10−3 | 453 |
| 3 | MeOH/Tris (50 mM, pH 7.6) | NAC (5) | 1.6 × 10−3 | 434 |
| 4 | MeOH/Tris (50 mM, pH 7.6) | TP (2.5) | 1.5 × 10−1 | 4.6 |
| 5 | MeOH | - | 2.1 × 10−4 | 3261 |
| 6 | MeOH | NAC (2.5) | 2.1 × 10−4 | 3261 |
| 7 | MeOH | NAC (5) | 5.2 × 10−4 | 1324 |
| 8 | MeOH | TP (2.5) | 6.0 × 10−3 | 113 |
Addition of thiols to the decomposition of 1-3 under buffered conditions dramatically alters the observed reaction products by 1) abolishing N2O formation, 2) generating a disulfide product and 3) changing the ratio of cyclohexanone (4):cyclohexanone oxime (5) produced (Scheme 3). Table 3 shows the ratio of 4:5 for the decomposition of 1-3 in the presence of different thiols in a mixture of MeOH:Tris buffer (50 mM, pH 7.6). In general and in contrast to reactions in the absence of thiol (Table 1), cyclohexanone oxime (5) represents the major product from these decompositions of 1 and 2 with smaller amounts of 4 being formed (Table 3). The decomposition of 3 in the presence of GSH produces cyclohexanone (4) as the major product although the percentage of 5 formed increases compared to the non-thiol reaction (Table 3, Entry 7 and Table 1, Entry 7). No other cyclohexanone-derived products were identified and starting material was completely consumed during these incubations as judged by both UV-Vis spectroscopy and GC-MS. Kinetic GC-MS experiments reveal the formation of 4 and 5 occurs at the same rate as the disappearance of starting material (Supporting Information).
Table 3.
Product formation from the decomposition of 1-3 in the presence of thiols.
| Entry | Compound | Thiol 2.5(equiv.) |
4:5 | 4: 5 (with Cyclopentanone 10 equiv.) |
|---|---|---|---|---|
| 1 | 1 | GSH | 26:74 | 87:13 |
| 2 | 1 | TP | 7:92 | 25:75 |
| 3 | 1 | NAC | 5:95 | 95:5 |
| 4 | 2 | GSH | 5:95 | 88:12 |
| 5 | 2 | TP | 18:82 | 33:67 |
| 6 | 2 | NAC | 4:96 | 90:10 |
| 7 | 3 | GSH | 73:28 | 98:2 |
Addition of cyclopentanone to these reactions further influences the ratio of cyclohexanone (4):cyclohexanone oxime (5) produced (Table 3). Incubation of 1 or 2 with GSH or NAC under in the presence of cyclopentanone produces 4 as the major product with only small amounts of 5 (Table 3, Entries 1, 3, 4, and 6) and increases the amount of 4 formed during the decomposition of 3 in the presence of GSH (Table 3, Entry 7). Incubation of 1 or 2 with TP in the presence of cyclopentanone increases the amount of 4 formed but 5 remains the major component of the reaction mixture (Table 3, Entries 2 and 5). GC-MS and 13C NMR experiments confirm the formation of cyclopentanone oxime in these reactions (Supporting Information).
Similar experiments conducted in a 1:1 mixture of MeOH: 1.0 N NaOH reveal further changes in the ratio of cyclohexanone (4):cyclohexanone oxime (5) produced in the presence and absence of cyclopentanone (Table 4). Generally, incubation of 1 or 2 with GSH, TP or NAC under basic conditions produces cyclohexanone oxime (5) as the major product with only small amounts of cyclohexanone (4) being formed (Table 4, Entries 1-6). Exposure of 3 to these conditions (with GSH) yields mainly cyclohexanone (4) (Table 4, Entry 7). Unlike the results from reactions in buffered solution, the addition of cyclopentanone to these reactions did not significantly increase the amount of 4 produced and had little change on the overall ratio of 4:5 formed (Table 4).
Table 4.
Product formation from the decomposition of 1-3 in the presence of thiols under basic conditions.
| Entry | Compound | Thiol (2.5 equiv.) |
4:5 | 4:5 (with Cyclopentanone 10 equiv.) |
|---|---|---|---|---|
| 1 | 1 | GSH | 2:98 | 12:88 |
| 2 | 1 | TP | 21:79 | 21:79 |
| 3 | 1 | NAC | 10:90 | 10:90 |
| 4 | 2 | GSH | 9:91 | 9:91 |
| 5 | 2 | TP | 14:86 | 11:89 |
| 6 | 2 | NAC | 2:98 | 6:94 |
| 7 | 3 | GSH | 97:3 |
Reaction of HNO with acyloxy nitroso compounds
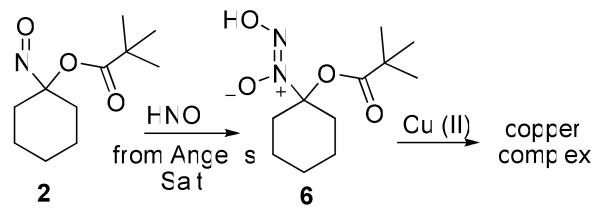
Kinetic UV-Vis spectroscopic experiments show the disappearance of the absorption at 667 nm of 2 upon the addition of AS within 20 minutes at room temperature in MeOH:buffer (Supporting Information). Control experiments show that nitrite, another product of AS decomposition, does not affect the UV-Vis absorbance of 2. Addition of copper (II) sulfate to this reaction mixture generates a species with a UV-Vis absorbance at 653 nm, similar to the spectrum of Cu(II) sulfate and cupferron (Supporting Information).
Vasodilation assays
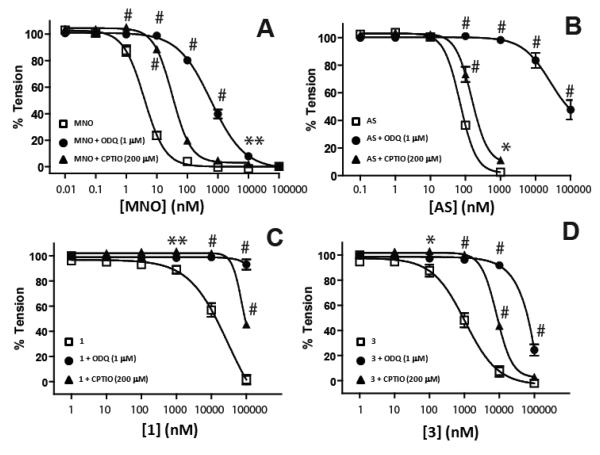
Figure 1 shows that Angeli's salt, 1, 3 and MNO stimulated vasodilation in a concentration dependent manner with the potency being MNO > AS > 3 > 1 consistent with our previous studies.36 Figure 1 shows that in each case vasodilation occurs by NO-dependent activation of soluble guanylate cyclase (sGC) as indicated by inhibition of relaxation in the presence of either the sGC inhibitor ODQ or the NOscavenger C-PTIO.
Figure 1. Role of NO in HNO-donor mediated vasodilation.
MNO (Panel A). AS (Panel B), 1 (Panel C) or 3 (Panel D) dependent vasodilation was assessed using rat thoracic aorta in the absence (□) or presence of either ODQ (1μM, ●) or CPTIO (200μM, ▲). Data show mean ± SEM (n = 3-4) by *P < 0.05, **P < 0.01, # P < 0.001 by 2-way RM-ANOVA with Bonferroni post-test for ODQ effect relative to control condition. NAC effect was not significantly different relative to control. In some cases error bars are not observed and were smaller than symbol size.
Effect of Oxygen on HNO-donor dependent dilation
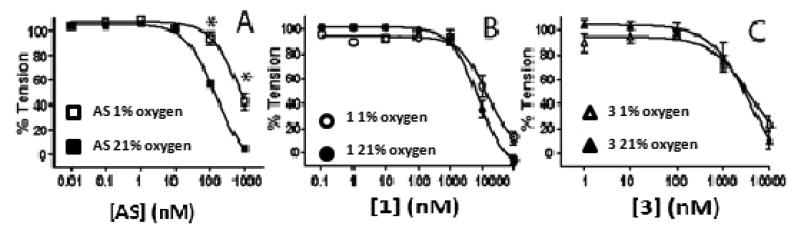
Recent studies underscore the importance of varying oxygen tensions and specifically lower oxygen tensions (that are more consistent with in vivo situations), in modulating vasodilatory mechanisms of nitrovasodilators.38 HNO represents an intriguing nitrosovasodilator due to the potential competing reaction between HNO and oxygen leading to the formation of reactive nitrogen species that could affect HNO signaling.40, 41 Figure 2 shows the HNO-donor dependent dilation assessed at 21% O2 and 1% O2 and reveals that lowering oxygen tensions inhibited AS dependent dilation but had no effect on 1 or 3 dependent-vasodilation.
Figure 2. Effect of oxygen tension on HNO-donor mediated vasodilation.
AS (Panel A), 1 (Panel B) or 3 (Panel C) mediated vasodilation was assessed at either 21% O2 (filled symbols) or 1% O2 (open symbols).). Data show mean ± SEM (n = 3). *P < 0.001, by 2-way RM-ANOVA with Bonferroni post-test relative to 21% O2 condition.
Effects of SOD on HNO-dependent relaxation at high and low oxygen tension
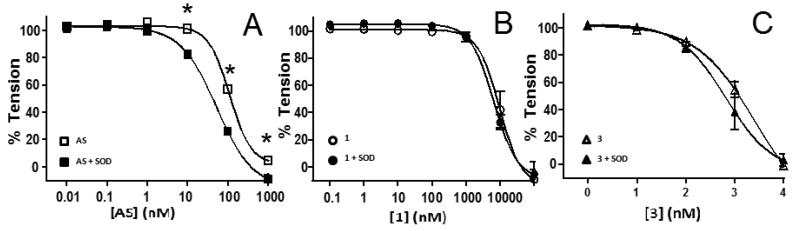
Previous studies suggest that the cupric ion (Cu2+) in SOD oxidizes HNO to NO possibly mediating NO-signaling from HNO.25 A second distinct mechanism by which SOD may potentiate HNO-dependent vasodilation is superoxide scavenging, thereby preventing rapid NO-scavenging (and peroxynitrite formation) and improving NO-dependent vasodilation.42 Figure 3 shows the effects of SOD on AS, 1 and 3 dependent-vasodilation. SOD significantly potentiated vasodilation by AS (indicated by leftward shift in concentration-dependent relaxation), but did not significantly change the vasodilation efficiency of 1 or 3.
Figure 3. Effect of SOD on HNO-donor mediated vasodilation.
AS (Panel A), 1 (Panel B) or 3 (Panel C) mediated vasodilation was assessed at 21% O2 in the presence (filled symbols) or absence of (open symbols) SOD (100U/ml). Data show mean ± SEM (n = 3-4). *P < 0.001, by 2-way RM-ANOVA with Bonferroni post-test relative to with SOD condition.
Selective effects of thiols towards HNO-donor dependent vasodilation
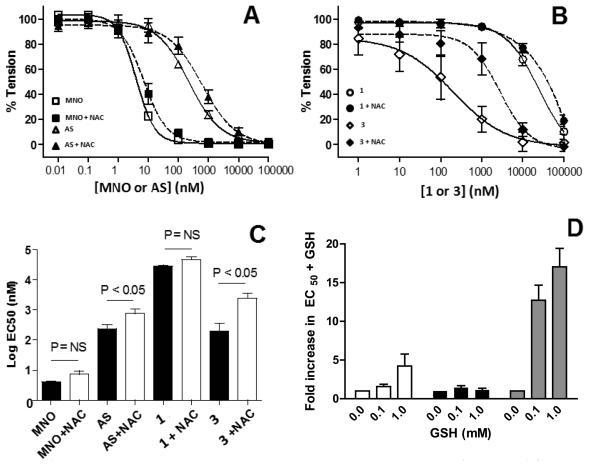
Rapid and selective reactivity with reduced thiols likely underlies many of the biological effects of HNO and thiols have been used experimentally to inhibit and implicate a role for HNO. Figure 4A and B shows the effects of NAC on MNO, AS, 1 and 3 dose-dependent vasodilation. Figure 4C shows the respective EC50 for vasodilation in the presence and absence of NAC. NAC had no significant effect on MNO nor 1 mediated vasodilation and elicited small but significant right shifts in AS and 3 mediated vasodilation as indicated by significant increases in EC50. To further probe thiol dependence of HNO-donor dependent stimulation of NO-signaling, we assessed the effects of differing doses of GSH, which were added to vessel bioassay chambers prior to HNO donors. Figure 4D shows that in a concentration dependent manner, GSH inhibited (indicated by increased EC50) vasodilation stimulated by AS and 3, but had no effect on 1 mediated dilation.
Figure 4. Effects of NAC or GSH on MNO and HNO-donor dependent dilation.
Vasodilation induced by the MNO, Angelis salt (AS), 1 or 3 was determined in the absence or presence of either N-acetylcysteine (NAC, 1mM) (Panels A,B,C) or varying concentrations of GSH (0-10mM) (Panel D). Panel C shows EC50 in the presence and absence of NAC. P-values show significance by unpaired T-test. Panel D shows fold-changes in EC50 relative to the control (i.e. absence of GSH) with an increase in the fold indicating inhibition of vasodilation. Data show mean ± SEM (n = 3-4). P-values show significance by 1-way ANOVA. Clear bars = AS, black bars = 1, grey bars = 3.
Effects of MnIIITPPS on MNO and HNO donor mediated Vasodilation
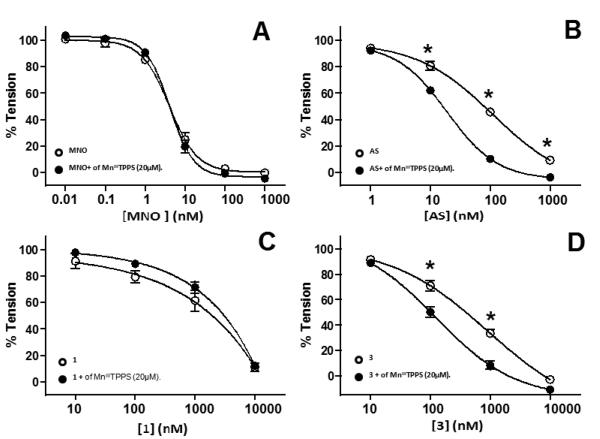
Figure 5 shows that the proposed HNO scavenger MnIIITPPS in fact potentiates vasodilation elicited by AS and 3, but had no effect on 1 nor MNO dependent effects response paralleling the distinct vasodilation mechanisms of AS and 3 versus 1 observed in Figure 4.43
Figure 5. Effects of MnIIITPPS on MNO and HNO-donor dependent vasodilation.
Vasodilation stimulated by either MNO (Panel A) AS (Panel B), 1 (Panel C) or 3 (Panel D) was assessed in the absence (○) or presence (●) of MnIIITPPS (20μM). Data show mean ± SEM (n=2-3). *P < 0.001 by 2-way RM-ANOVA with Bonferronni post-test relative to corresponding with MnIIITPPS condition.
Discussion
A more comprehensive array of HNO donor compounds is needed to provide better experimental tools to study HNO biology and aid in the development of HNOtherapeutics. We and others have recently synthesized and characterized novel HNO donor compounds that are structurally distinct from Angeli's salt and release HNO at different rates upon hydrolysis at neutral pH.36 Here, we detail the chemical decomposition of acyloxy nitroso compounds to HNO, describe their reactions with thiols and thiolate ions and compare the reactivity of 1 and 3 with AS and an NO-donor (MNO) using vasodilation as a biological end point.17-19, 21, 22, 24
Decomposition and HNO Release from Acyloxy Nitroso Compounds
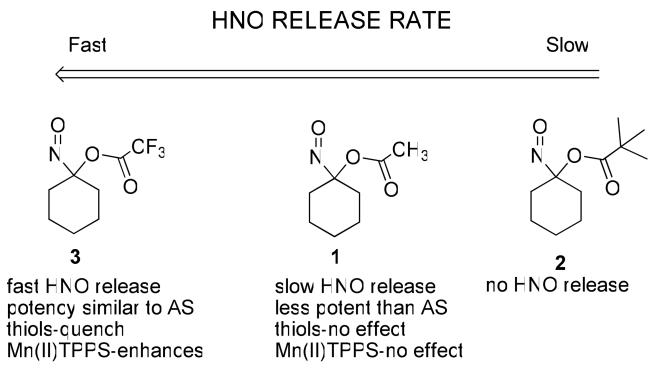
Acyloxy nitroso compounds (1-3) hydrolyze to yield an unstable α-hydroxy nitroso intermediate that collapses to cyclohexanone and HNO (Scheme 1, Scheme 5, Path 1). This mechanism predicts the rate and amount of HNO formation should directly relate to the ease of ester hydrolysis for 1-3 (with the prediction of 3 > 1 > 2). Kinetic decomposition studies show the water-sensitive trifluoroacetate group facilitates hydrolysis and 3 rapidly decomposes upon mixing with any aqueous solvent.44 The t-butyl ester resists hydrolysis and 2 decomposes very slowly in either neutral (t1/2 = 2268 min) or basic conditions. 1-Nitrosocyclohexyl acetate (1) rapidly hydrolyzes under basic aqueous conditions favoring hydrolysis (t1/2 = 0.8 min) but predictably hydrolyzes slowly in neutral buffered conditions (t1/2 = 800-890 min).36 Regardless of the rate, cyclohexanone represents the major organic product for each of these reactions indicating a hydrolytic pathway (Table 1). These results generally segregate 1-3 into three groups regarding kinetic decomposition (and presumably HNO release) at neutral pH with 1 being a slow HNO 2 not being an HNO donor (or extremely slow HNO donor) and 3 being a rapid donor, HNO donor similar to Angeli's salt (t1/2 = 2.3 min).45
Scheme 5.
Gas chromatographic headspace identification of N2O from the aqueous-based decomposition of 1-3 parallels the kinetic studies. 1-Nitrosocyclohexyl trifluoroacetate (3), which decomposes fastest, yields the largest amount of N2O (57%) within 2 h in a mixture of MeOH:buffer compared to 1 (12%) and 2 (2%, Table 1). Incubation of 1 under basic conditions facilitates hydrolysis of the acetate group and subsequent N2O formation but has little effect on N2O release from 2 that contains the hydrolysis resistant t-butyl ester (Table 1). Identification of N2O, the dimerization/dehydration product of HNO,6 provides evidence for HNO intermediacy, and N2O quenching by glutathione, which rapidly reacts with HNO,46 supports this assertion. Earlier work also shows potassium ferricyanide reduces N2O formation from the decomposition of 1 and 3, presumably through the iron (III)-mediated oxidation of HNO to NO.36 The decomposition of 3 in the presence of methemoglobin yields iron (II) nitrosyl hemoglobin, a characteristic reaction of HNO, which supports HNO release from 3.36
While these gas chromatographic results strongly imply HNO release from 1 and 3, the less than expected quantitative amounts of N2O clearly reveals limitations to this indirect method of HNO detection. This assay relies on HNO dimerization making the rate and amount of N2O formation dependent on the square of HNO concentration. As the amount of donor and HNO decrease with time, the rate of dimerization decreases, which may explain the decrease in rate of N2O formation compared to rate of donor decomposition observed with 1 (Supporting Information). Also, a very slow HNO donor, such as 2, would only produce a low steady-state HNO concentration (and low N2O formation). The reaction of HNO with other species including residual oxygen, solvent components or other organic by-products would also reduce N2O formation. Indeed, Angeli's salt-generated HNO directly reacts with the nitroso group of the acyloxy nitroso compound (2), similar to C-nitroso compounds, to give a proposed “cupferron-like” derivative (6, Scheme 4).47 Addition of copper (II) to this complex generates a species with absorption properties similar to a cupferron-copper complex. The observation of small amounts of cyclohexanone oxime (5) in the neutral decomposition of 1-3 clearly indicates the occurrence of non-HNO producing reactions (Table 1). Such reactions may contribute to decreased N2O yields and clearly show the ability of HNO to react with other organic molecules including the parent donor. The gas chromatographic identification of N2O provides a reasonable marker for HNO formation but further evidence should be gathered for new HNO donors and improvements in HNO detection remains a priority for advancing HNO chemistry and biology.
Scheme 4.
Reactions of Acyloxy Nitroso Compounds withThiols
The addition of GSH to the decomposition of 1-3 under neutral or basic conditions quenches N2O formation, presumably through thiol trapping of the nascent HNO. While the decomposition of 1-3 in the absence of thiol gives cyclohexanone (Table 1), the decomposition of 1 and 2 under neutral and basic conditions in the presence of GSH, NAC or TP forms disulfide and cyclohexanone oxime (5) as the major organic products (Tables 3 and 4). Decomposition of 3 in the presence of GSH predominately gives cyclohexanone under both neutral and basic conditions (Table 3, Entry 7 and Table 4, Entry 7). Scheme 5 (Path 1) shows a reasonable pathway for the formation of these products via HNO. Hydrolysis of the acyloxy nitroso compound produces HNO, the corresponding carboxylic acid and cyclohexanone (4). In the presence of GSH or NAC, the thiol reacts with HNO to generate the proposed N-hydroxysulfenamide intermediate and further thiol reduction gives disulfide and hydroxylamine (Scheme 5). Hydroxylamine condenses with cyclohexanone to form cyclohexanone oxime (Scheme 5, Path 1).
However, the addition of NAC or GSH (thiol pKa's = 9.5 and 8.7, respectively)48 under buffered conditions to 1 or 2 increases the rate of acyloxy nitroso compound decomposition as judged by UV-Vis spectroscopy (Table 2 and Supporting Information). Addition of thiophenol (pKa = 6.6)49 to 1 or 2 under buffered conditions further increases their rate of decomposition (Table 2). The rapid reaction of 2 under these conditions, which does not produce N2O over the same time period in the absence of thiol, suggests a non-hydrolytic (and non-HNO) mediated decomposition pathway. Based on the pKa's of the thiols examined and the pH of the incubation buffer, these results strongly suggest the direct reaction of thiolates with the acyloxy nitroso compounds (Scheme 5, Path 2). Thiolate addition to the nitroso group would disrupt the n to p* transition resulting in decreased absorbance and generate an organic N-hydroxysulfenamide intermediate (7, Scheme 5, Path 2). Attack of a second thiol (or thiolate) on 7 would generate disulfide and cyclohexanone oxime (5) without the intermediacy of HNO (Scheme 5, Path 2). The increased rate of reaction with TP compared to GSH or NAC provides evidence for thiolate ion involvement. Overall, these results show that in the presence of thiols, acyloxy nitroso compounds can decompose through two different paths, only one of which involves HNO, to form identical products (disulfides and oximes).
Experiments using cyclopentanone as a trap for hydroxylamine provide a method for separating the two decomposition paths of acyloxy nitroso compounds in the presence of thiols. Only Path 1 (Scheme 5) includes HNO and hydroxylamine and the addition of a second ketone (cyclopentanone) could trap any hydroxylamine to generate cyclopentanone oxime (8) and preserve any originally-generated cyclohexanone (4, Scheme 5). The addition of excess cyclopentanone to the decomposition of 1 under neutral buffered conditions in the presence of GSH reverses the ratio of 4:5 from 26:74 to 87:13, a similar ratio to decomposition in the absence of thiol (Tables 1 and 3). Similar experiments using NAC also reverse the ratio of 4:5 formed from 1 and the addition of cyclopentanone to the decomposition of 2 in the presence of these thiols yields a similar reversal in the ratio of 4:5 (Table 3). GC-MS experiments show the formation of 8 during these reactions and provide strong evidence that 1 and 2 primarily decompose through a path that involves free hydroxylamine (Scheme 5, Path 1). However, the decomposition of 1 and 2 in the presence of excess cyclopentanone and TP predominantly forms cyclohexanone oxime (5, Table 3), which suggests these reactions do not generate hydroxylamine and proceed through the thiolate of thiophenol via Path 2. Decomposition of 1 or 2 in the presence of GSH, NAC or TP under basic conditions (that ensure thiolate formation) yields cyclohexanone oxime (5) as the major product regardless of the presence of cyclopentanone providing strong evidence that acyloxy nitroso compounds directly react with thiolate ions through Path 2 (Table 4). Regardless of conditions (pH, presence/absence of thiol or cyclopentanone), 3 primarily forms cyclohexanone (4) suggesting a primarily hydrolytic decomposition (Path 1, Scheme 5).
Based on these results, the reactivity and HNO-donating ability appears to depend on the structure of the acyloxy nitroso compound, the presence of thiols, the pKa of these thiols and the pH of the reaction medium. Compound 3, which contains the hydrolytically-sensitive trifluoroacetate group, rapidly hydrolyzes (generating HNO) regardless of the reaction conditions or presence of thiols (Path 1, Scheme 5). Compounds 1 and 2 hydrolyze (at different rates) to HNO in the absence of thiols or under conditions that favor the thiol (rather than thiolate) ionization state (Path 1, Scheme 5). However, these compounds appear to directly react through thiolate addition to the nitroso group (Path 2, Scheme 5) under conditions that favor the thiolate ionization state. These findings indicate acyloxy nitroso compounds may demonstrate different reactivity (including their ability to release HNO) depending on their structure and the presence of thiols/thiolates. This differing reactivity with thiols compared to thiolates could have important implications in targeting specific highly reactive low pKa thiol residues in various proteins.
Vasorelaxation Properties and Structure-Activity Relationships
Angeli's salt (AS) elicits vasodilation of rabbit thoracic aorta and bovine intrapulmonary arteries through soluble guanylate cyclase (sGC) activation.50 The sGC inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and NO-scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (C-PTIO) block this relaxation providing evidence for NO-mediated sGC activation suggesting some oxidative metabolism of HNO to NO.18, 51 While early work indicates that NO is the only nitrogen monoxide capable of sGC activation,52-54 recent reports provide evidence for HNO-mediated sGC activation,55 a finding that has also been challenged.56 Our previous studies show that acyloxy nitroso compounds 1 and 3 relax pre-constricted rat aorta similar to other HNO donors including AS.36 Compared to AS (EC50 = 0.082 μM), 1 is much less potent (EC50 = 10 μM) and 3 slightly (~10-fold) less potent (EC50 = 0.88 μM) indicating some differences in the biological actions of these HNO donors.50
The current vasorelaxation studies show differences between the acyloxy nitroso compounds 1 and 3 compared to AS and each other. The very poor water solubility and extremely slow decomposition rate of 2 prevented the reliable assay of its activity. Both ODQ and C-PTIO inhibit vasodilation induced by 1 and 3 revealing this activity likely occurs through NO-mediated activation of sGC, similar to AS (Figure 1). Experiments with bovine lung sGC indicate that 3 acts as a competent sGC activator presumably through HNO formation,55 but the C-PTIO inhibition observed in these studies suggests NO-mediated activity. Overall, the acyloxy nitroso compounds 1 and 3 appear to elicit vasodilation through NO mediated activation of sGC in this system.
The most surprising and interesting results were the similarity in vasodilatory mechanisms between AS and 3 compared to 1 (Figures 4 and 5). Reduced thiols (NAC and GSH, (Figure 4)) and the putative HNO trapping porphyrin (MnIIITPPS) differentially altered the observed biological response of AS and 3 compared to 1 (Figures 4 and 5). Multiple studies show that HNO rapidly reacts with thiols with a measured rate constant for reaction with GSH of ~106M−1s−1.8 Addition of thiols also experimentally discerns HNO and NO-dependent pathways as thiols selectively scavenge HNO with little effect on NO (confirmed by the lack of any effect of NAC on MNO-dependent vasodilation). Consistent with this concept and supporting previous work 17, 24, NAC and GSH inhibited AS-mediated vasodilation (Fig 4). Surprisingly, these thiols failed to modulate dilation by 1 suggesting a distinct vasodilatory mechanism for this HNO donor compared to AS. Moreover, GSH did inhibit dilation mediated by 3, a compound structurally similar to 1 but which releases HNO at a faster rate. Further evidence illustrating a distinct mechanism of action of 1 was provided by the lack of effect of the addition of MnIIITPPS. This water-soluble porphyrin reacts with HNO via a reductive nitrosylation reaction yielding the corresponding MnII-NO complex.43 Figure 5 shows that MnIIITPPS potentiates vasodilatation in the presence of AS and 3 suggesting reductive nitrosylation yields bioactive NO. However, MnIIITPPS had no effect on 1 similar to the reactivity profile observed with GSH. MnIIITPPS did not affect MNO-dependent responses suggesting direct reactions with NO do not play a role. Together these data highlight the similarities in the biological activity of AS and the trifluoroacetate (3) and illustrate the differences compared to the structurally similar but slower HNO releasing acetate (1).
The chemical findings appear to provide some insight to the biological action of the acyloxy nitroso compounds (1 and 3) relative to AS. Variation of the ester group structure changes the HNO release profile and separates 1-3 into 3 groups of HNO donors (Scheme 6). Compound 3 generates HNO rapidly with a release profile most similar to Angeli's salt, the most commonly used HNO donor. Even in the presence of thiols, 3 rapidly hydrolyzes and the lack of cyclohexanone oxime formation suggests some interaction of the thiol with the trifluroacetate group (perhaps during hydrolysis). Compound 1 slowly hydrolyzes under neutral conditions making it a slow HNO donor, while under these conditions 2 does not hydrolyze to a significant extent in the same time frame. Both 1 and 2, preferentially react with thiolates at the nitroso group under appropriate conditions. These chemical properties identify 3 as an HNO donor similar to Angeli's salt, a compound that releases HNO rapidly upon introduction to water. Compound 1 releases HNO much more slowly (and 2 even slower) and both possess potential reactivity with thiolates providing other mechanisms for biological activity. Given this chemistry, AS and 3 would be expected to demonstrate similar biological properties attributed to the rapid release of HNO (they exhibit similar potencies). Compound 1 releases HNO much more slowly, reacts with thiolates and induces vasorelaxation. Confirmed HNO traps (thiols and metal porphyrins) affect AS and 3 in a similar fashion with thiols quenching activity and the manganese porphyrin enhancing activity presumably through thiol reduction of HNO and metal heme-mediated oxidation of HNO to NO, respectively (Scheme 6). Neither of these agents alters the activity of 1, which strongly suggests that under these conditions 1 does not generate HNO or very little HNO and acts through a separate mechanism.
Scheme 6.
The rate of HNO release appears as the major chemical difference between AS and the acyloxy nitroso compound (3) compared to the structurally similar 1. Similar to NO donors, differences in the rate of HNO release could greatly alter both the observed chemical and biological behavior of HNO donors. Compounds that release HNO quickly (AS and 3) will generate a relatively greater concentration of HNO at certain times compared to a slow HNO donor at the same concentration. The dimerization pathway will also be more prevalent for a rapid HNO donor. Given the high reactivity of HNO, larger concentrations of HNO from rapid HNO donors may alter biological targets (protein thiols, heme or metal groups) in a different fashion than a slow HNO donor. For example, a rapid HNO donor generating higher concentrations of HNO will modify more thiols/targets in an unselective manner in a given protein as compared to a slow HNO donor that would likely modify the most reactive thiol/target and perhaps lead to a specific effect. In this work, the observed activity of AS and 3 appear to be the result of rapid HNO release and that of 1 from a slow HNO donor that may be eliciting the observed effects through different mechanisms. Also, the level of oxygen or the presence of SOD had little effect on the activity of 1 and 3 but SOD enhanced the activity of Angeli's salt and decreased oxygen tension decreased AS ability to elicit vasorelaxation (Figures 2 and 3). Given the differences in the rate of HNO release, the differences between AS and 1 may be expected but the differences between AS and 3 with regards to oxygen tension and SOD remain unclear if both are acting as rapid HNO donors. Possible explanations include that oxygen and/or SOD react differently with the donor molecule (AS or 3) or that the different mechanisms (protonation vs. hydrolysis) results in the difference. These results clearly show that the overall chemistry of the donor molecule (including HNO, NO or other donors) must be accounted for when evaluating the observed biological actions for a given compound.
Conclusions
Nitroxyl (HNO) has emerged as a unique nitrogen monoxide demonstrating different chemistry and biology from the better-known redox related compound, nitric oxide. Acyloxy nitroso compounds release HNO through hydrolysis and the rate of hydrolysis depends on pH and the structure of the ester portion of the molecule with the observed rate being trifluoroacetate (3) > acetate (1) > pivalate (2). Under all conditions, 3 rapidly hydrolyzes to HNO making this compound most similar to Angeli's salt. Thiol addition to the decomposition of the acetate (2) and pivalate (3) increases the rate of reaction and alters product distribution. Conditions that favor thiols lead to hydrolysis and HNO, while conditions that favor thiolates generate oximes without HNO formation. Biologically, the trifluoroacetate (3) behaves similarly to Angeli's salt demonstrating thiol-sensitive nitric oxide-mediated soluble guanylate cyclase-dependent vasorelaxation, which strongly suggests HNO-mediated vasorelaxation. In contrast, the slow HNO-donor acetate (1) demonstrates weak thiol-insensitive vasorelaxation indicating that the rate of HNO release from these structurally similar compounds determines HNO bioavailability and activity. In general, these results show that 1) acyloxy nitroso compounds act as HNO donors upon hydrolysis, 2) the rate of hydrolysis clearly dictates the observed biological response and 3) the overall chemistry of the HNO donor (regardless of structural similarity) must be considered in evaluation of the biological properties.
Supplementary Material
Acknowledgements
RPP acknowledges funds from the National Institutes of Health (HL092624) and a Cardiovascular Pathophysiology Training Fellowship to TSI. SBK acknowledges support from the National Institutes of Health (HL62198) and the Egyptian Ministry of Higher Education through a Joint Supervision Channel Program (MES).
Abbreviations
- ALDH
aldehyde dehydrogenase
- AS
Angeli's salt
- C-PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxidecarboxy-PTIO
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HNO
nitroxyl
- KH
Krebs-Henseleit buffer
- L-NAME
L-NG-nitroarginine methyl ester
- MNO
MahmaNonoate
- MnIIITSPP
Manganese (III) meso-(tetrakis(4-sulfonato-phenyl)) porphyrinate
- PE
phenylephrine
- NAC
N-acetyl-L-cysteine
- NO
nitric oxide
- NAP
Nacetyl-DL-penicillamine
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- sGC
soluable guanylate cyclase
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- TP
thiophenol
Footnotes
Supporting Information Availability
1H and 13C NMR data for compound 2, along with UV-vis and GC-MS kinetic decomposition data for compounds 1-3 is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.DuMond JF, King SB. The Chemistry of Nitroxyl (HNO) Releasing Compounds. Antioxidants & Redox Signaling. 2010 doi: 10.1089/ars.2010.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miranda KM. The chemistry of nitroxyl (HNO) and implications in biology. Coordination Chemistry Reviews. 2005;249(3-4):433–455. [Google Scholar]
- 3.Fukuto JM, Bianco CL, Chavez TA. Nitroxyl (HNO) signaling. Free Radical Biology and Medicine. 2009;47(9):1318–1324. doi: 10.1016/j.freeradbiomed.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Irvine JC, Ritchie RH, Favaloro JL, Andrews KL, Widdop RE, Kemp-Harper BK. Nitroxyl (HNO): the Cinderella of the nitric oxide story. Trends in Pharmacological Sciences. 2008;29(12):601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Paolocci N, Jackson MI, Lopez BE, Miranda K, Tocchetti CG, Wink DA, Hobbs AJ, Fukuto JM. The pharmacology of nitroxyl (HNO) and its therapeutic potential: Not just the Janus face of NO. Pharmacology & Therapeutics. 2007;113(2):442–458. doi: 10.1016/j.pharmthera.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner FT, Hughes MN. The Aqueous Solution Chemistry of Nitrogen in Low Positive Oxidation States. Comments in Inorganic Chemistry. 1988;7:215–234. [Google Scholar]
- 7.Bartberger MD, Fukuto JM, Houk KN. On the acidity and reactivity of HNO in aqueous solution and biological systems. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(5):2194–2198. doi: 10.1073/pnas.041481598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King SB, Nagasawa HT. Chemical approaches toward generation of nitroxyl. Nitric Oxide, Pt C. 1999:211–220. doi: 10.1016/s0076-6879(99)01084-8. [DOI] [PubMed] [Google Scholar]
- 10.Miranda KM, Nagasawa HT, Toscano JP. Donors of HNO. Current Topics in Medicinal Chemistry. 2005;5(7):647–664. doi: 10.2174/1568026054679290. [DOI] [PubMed] [Google Scholar]
- 11.Wong PSY, Hyun J, Fukuto JM, Shirota FN, DeMaster EG, Shoeman DW, Nagasawa HT. Reaction between S-nitrosothiols and thiols: Generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37(16):5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 12.Spencer NY, Patel NK, Keszler A, Hogg N. Oxidation and nitrosylation of oxyhemoglobin by S-nitrosoglutathione via nitroxyl anion. Free Radical Biology and Medicine. 2003;35(11):1515–1526. doi: 10.1016/j.freeradbiomed.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt HHHW, Hofmann H, Schindler U, Shutenko ZS, Cunningham DD, Feelisch M. No .NO from NO synthase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnelle DR, Stamler JS. NO+, NO., and NO− Donation by S-Nitrosothiols - Implications for Regulation of Physiological Functions by S-Nitrosylation and Acceleration of Disulfide Formation. Archives of Biochemistry and Biophysics. 1995;318(2):279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 15.Wei CC, Wang ZQ, Hemann C, Hille R, Stuehr DJ. A tetrahydrobiopterin radical forms and then becomes reduced during N-omega-hydroxyarginine oxidation by nitric-oxide synthase. J Biol Chem. 2003;278(47):46668–46673. doi: 10.1074/jbc.M307682200. [DOI] [PubMed] [Google Scholar]
- 16.Lopez BE, Shinyashiki M, Han TH, Fukuto JM. Antioxidant actions of nitroxyl (HNO) Free Radical Biology and Medicine. 2007;42(4):482–491. doi: 10.1016/j.freeradbiomed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Irvine JC, Favaloro JL, Widdop RE, Kemp-Harper BK. Nitroxyl anion donor, Angeli's salt, does not develop tolerance in rat isolated aortae. Hypertension. 2007;49(4):885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- 18.Favaloro JL, Kemp-Harper BK. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc Res. 2007;73(3):587–596. doi: 10.1016/j.cardiores.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Irvine JC, Favaloro JL, Kemp-Harper BK. NO- activates soluble guanylate cyclase and K-v channels to vasodilate resistance arteries. Hypertension. 2003;41(6):1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- 20.Crawford JH, White CR, Patel RP. Vasoactivity of S-nitrosohemoglobin: role of oxygen, heme, and NO oxidation states. Blood. 2003;101(11):4408–4415. doi: 10.1182/blood-2002-12-3825. [DOI] [PubMed] [Google Scholar]
- 21.Nelli S, McIntosh L, Martin W. Role of copper ions and cytochrome P450 in the vasodilator actions of the nitroxyl anion generator, Angeli's salt, on rat aorta. European Journal of Pharmacology. 2001;412(3):281–289. doi: 10.1016/s0014-2999(00)00845-1. [DOI] [PubMed] [Google Scholar]
- 22.Buyukafsar K, Nelli S, Martin W. Formation of nitric oxide from nitroxyl anion: role of quinones and ferricytochrome c. British Journal of Pharmacology. 2001;132(1):165–172. doi: 10.1038/sj.bjp.0703812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis A, Li CG, Rand MJ. Differential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. British Journal of Pharmacology. 2000;129(2):315–322. doi: 10.1038/sj.bjp.0703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pino RZ, Feelisch M. Bioassay Discrimination between Nitric-Oxide (NO. and Nitroxyl (NO−) Using L-Cysteine. Biochemical and Biophysical Research Communications. 1994;201(1):54–62. doi: 10.1006/bbrc.1994.1668. [DOI] [PubMed] [Google Scholar]
- 25.Fukuto JM, Hobbs AJ, Ignarro LJ. Conversion of Nitroxyl (HNO) to Nitric-Oxide (NO) in Biological-Systems - the Role of Physiological Oxidants and Relevance to the Biological-Activity of HNO. Biochemical and Biophysical Research Communications. 1993;196(2):707–713. doi: 10.1006/bbrc.1993.2307. [DOI] [PubMed] [Google Scholar]
- 26.Wink DA, Feelisch M, Fukuto J, Chistodoulou D, Jourd'heuil D, Grisham MB, Vodovotz Y, Cook JA, Krishna M, DeGraff WG, Kim S, Gamson J, Mitchell JB. The cytotoxicity of nitroxyl: Possible implications for the pathophysiological role of NO. Archives of Biochemistry and Biophysics. 1998;351(1):66–74. doi: 10.1006/abbi.1997.0565. [DOI] [PubMed] [Google Scholar]
- 27.DeMaster EG, Redfern B, Nagasawa HT. Mechanisms of inhibition of aldehyde dehydrogenase by nitroxyl, the active metabolite of the alcohol deterrent agent cyanamide. Biochemical Pharmacology. 1998;55(12):2007–2015. doi: 10.1016/s0006-2952(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 28.Nagasawa HT, Demaster EG, Redfern B, Shirota FN, Goon JW. Evidence for Nitroxyl in the Catalase-Mediated Bioactivation of the Alcohol Deterrent Agent Cyanamide. Journal of Medicinal Chemistry. 1990;33(12):3120–3122. doi: 10.1021/jm00174a001. [DOI] [PubMed] [Google Scholar]
- 29.Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O'Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng HP, Kass DA, Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circulation Research. 2007;100(1):96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai TY, Tian Y, Tocchetti CG, Katori T, Murphy AM, Kass DA, Paolocci N, Gao WD. Nitroxyl increases force development in rat cardiac muscle. Journal of Physiology-London. 2007;580(3):951–960. doi: 10.1113/jphysiol.2007.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(18):10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiva S, Crawford JH, Ramachandran A, Ceaser EK, Hillson T, Brookes PS, Patel RP, Darley-Usmar VM. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem J. 2004;379(Pt 2):359–366. doi: 10.1042/BJ20031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda KM, Katori T, de Holding CLT, Thomas L, Ridnour LA, MeLendon WJ, Cologna SM, Dutton AS, Champion HC, Mancardi D, Tocchetti CG, Saavedra JE, Keefer LK, Houk KN, Fukuto JM, Kass DA, Paolocci N, Wink DA. Comparison of the NO and HNO donating properties of diazeniumdiolates: Primary amine adducts release HNO in vivo. Journal of Medicinal Chemistry. 2005;48(26):8220–8228. doi: 10.1021/jm050151i. [DOI] [PubMed] [Google Scholar]
- 34.Gladwin MT, Raat NJH, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291(5):H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 35.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nature Chemical Biology. 2005;1(6):308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 36.Sha X, Isbell TS, Patel RP, Day CS, King SB. Hydrolysis of acyloxy nitroso compounds yields nitroxyl (HNO) Journal of the American Chemical Society. 2006;128(30):9687–9692. doi: 10.1021/ja062365a. [DOI] [PubMed] [Google Scholar]
- 37.He XJ, Azarov I, Jeffers A, Presley T, Richardson J, King SB, Gladwin MT, Kim-Shapiro DB. The potential of Angeli's salt to decrease nitric oxide scavenging by plasma hemoglobin. Free Radical Biology and Medicine. 2008;44(7):1420–1432. doi: 10.1016/j.freeradbiomed.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isbell DC, Voros S, Yang Z, DiMaria JM, Berr SS, French BA, Epstein FH, Bishop SP, Wang H, Roy RJ, Kemp BA, Matsubara H, Carey RM, Kramer CM. Interaction between bradykinin subtype 2 and angiotensin II type 2 receptors during post-MI left ventricular remodeling. Am J Physiol Heart Circ Physiol. 2007;293(6):H3372–3378. doi: 10.1152/ajpheart.00997.2007. [DOI] [PubMed] [Google Scholar]
- 39.Calvet G, Dussaussois M, Blanchard N, Kouklovsky C. Lewis acid-promoted hetero Diels-Alder cycloaddition of α-acetoxynitroso dienophiles. Organic Letters. 2004;6(14):2449–2451. doi: 10.1021/ol0491336. [DOI] [PubMed] [Google Scholar]
- 40.Espey MG, Miranda KM, Thomas DD, Wink DA. Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radic Biol Med. 2002;33(6):827–834. doi: 10.1016/s0891-5849(02)00978-4. [DOI] [PubMed] [Google Scholar]
- 41.Kirsch M, de Groot H. Formation of peroxynitrite from reaction of nitroxyl anion with molecular oxygen. J Biol Chem. 2002;277(16):13379–13388. doi: 10.1074/jbc.M108079200. [DOI] [PubMed] [Google Scholar]
- 42.White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, Gianturco SH, Gore J, Freeman BA, et al. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci U S A. 1994;91(3):1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marti MA, Bari SE, Estrin DA, Doctorovich F. Discrimination of nitroxyl and nitric oxide by water-soluble Mn(III) porphyrins. Journal of the American Chemical Society. 2005;127(13):4680–4684. doi: 10.1021/ja044632n. [DOI] [PubMed] [Google Scholar]
- 44.Bender ML. Mechanisms of Catalysis of Nucleophilic Reactions of Carboxylic Acid Derivatives. Chemical Reviews. 1960;60(1):53–113. [Google Scholar]
- 45.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of NO with Nucleophiles as Agents for the Controlled Biological Release of Nitric-Oxide - Vasorelaxant Effects. Journal of Medicinal Chemistry. 1991;34(11):3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 46.Doyle MP, Mahapatro SN, Broene RD, Guy JK. Oxidation and Reduction of Hemoproteins by Trioxodinitrate(II) - the Role of Nitrosyl Hydride and Nitrite. Journal of the American Chemical Society. 1988;110(2):593–599. [Google Scholar]
- 47.Shoeman DW, Nagasawa HT. The reaction of nitroxyl (HNO) with nitrosobenzene gives cupferron (N-nitrosophenylhydroxylamine) Nitric Oxide-Biology and Chemistry. 1998;2(1):66–72. doi: 10.1006/niox.1998.0166. [DOI] [PubMed] [Google Scholar]
- 48.Podhradsky D, Drobnica L, Kristian P. Reactions of Cysteine, Its Derivatives, Glutathione, Coenzyme-a, and Dihydrolipoic Acid with Isothiocyanates. Experientia. 1979;35(2):154–155. doi: 10.1007/BF01920581. [DOI] [PubMed] [Google Scholar]
- 49.Pankratov N, Shalabai AV. Quantum-Chemical Evaluation of the Protolytic Properties of Thiophenols. Journal of Structural Chemistry. 2004;45(5):756–761. [Google Scholar]
- 50.Fukuto JM, Chiang K, Hszieh R, Wong P, Chaudhuri G. The Pharmacological Activity of Nitroxyl - a Potent Vasodilator with Activity Similar to Nitric-Oxide and or Endothelium-Derived Relaxing Factor. Journal of Pharmacology and Experimental Therapeutics. 1992;263(2):546–551. [PubMed] [Google Scholar]
- 51.Favaloro JL, Kemp-Harper BK. Redox variants of NO (NO{middle dot} and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am J Physiol Heart Circ Physiol. 2009;296(5):H1274–1280. doi: 10.1152/ajpheart.00008.2009. [DOI] [PubMed] [Google Scholar]
- 52.Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends in Pharmacological Sciences. 1997;18(12):484–491. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- 53.Griffiths C, Wykes V, Bellamy TC, Garthwaite J. A new and simple method for delivering clamped nitric oxide concentrations in the physiological range: Application to activation of guanylyl cyclase-coupled nitric oxide receptors. Molecular Pharmacology. 2003;64(6):1349–1356. doi: 10.1124/mol.64.6.1349. [DOI] [PubMed] [Google Scholar]
- 54.Poulos TL. Soluble guanylate cyclase. Current Opinion in Structural Biology. 2006;16(6):736–743. doi: 10.1016/j.sbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Miller TW, Cherney MM, Lee AJ, Francoleon NE, Farmer PJ, King SB, Hobbs AJ, Miranda KM, Burstyn JN, Fukuto JM. The Effects of Nitroxyl (HNO) on Soluble Guanylate Cyclase Activity Interactions at Ferrous Hemes and Cysteine Thiols. Journal of Biological Chemistry. 2009;284(33):21788–21796. doi: 10.1074/jbc.M109.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeller A, Wenzl MV, Beretta M, Stessel H, Russwurm M, Koesling D, Schmidt K, Mayer B. Mechanisms Underlying Activation of Soluble Guanylate Cyclase by the Nitroxyl Donor Angeli's Salt. Molecular Pharmacology. 2009;76(5):1115–1122. doi: 10.1124/mol.109.059915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.