Abstract
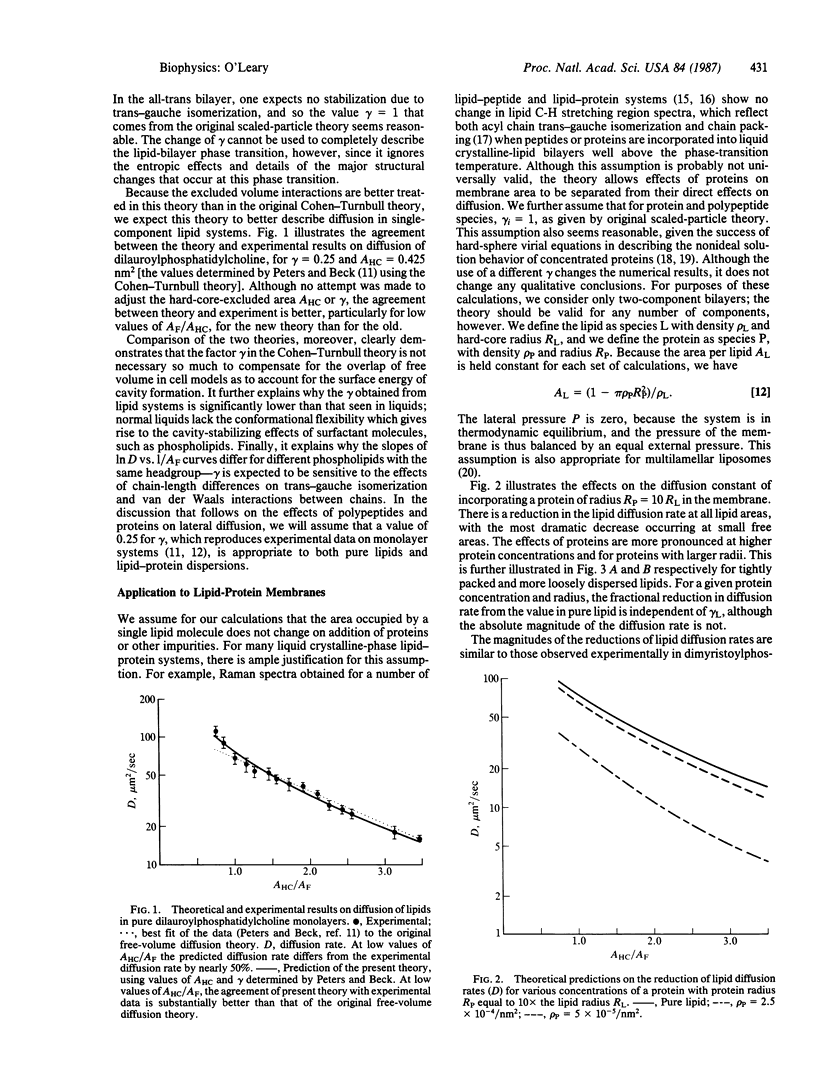
Lateral diffusion of lipids in biological membranes may be influenced by polypeptides, proteins, and other nonlipid membrane constituents. Using concepts from scaled-particle theory, we extend the free-volume model for lipid diffusion to membranes having an arbitrarily large number of components. This theory clarifies the interpretation of the free-volume theory, better reproduces the free-area dependence of lipid lateral diffusion rates, and quantitatively predicts the experimental observation that the lateral diffusion rates of membrane lipids are significantly reduced when proteins or polypeptides are incorporated in the membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eisinger J., Flores J., Petersen W. P. A milling crowd model for local and long-range obstructed lateral diffusion. Mobility of excimeric probes in the membrane of intact erythrocytes. Biophys J. 1986 May;49(5):987–1001. doi: 10.1016/S0006-3495(86)83727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Hou Y., Derzko Z., Wojcieszyn J., Organisciak D. Lipid lateral diffusion in the surface membrane of cells and in multibilayers formed from plasma membrane lipids. Biochemistry. 1981 Sep 1;20(18):5268–5275. doi: 10.1021/bi00521a027. [DOI] [PubMed] [Google Scholar]
- Lavialle F., Levin I. W. Raman spectroscopic study of the interactions of dimyristoyl- and 1-palmitoyl-2-oleoylphosphatidylcholine liposomes with myelin proteolipid apoprotein. Biochemistry. 1980 Dec 23;19(26):6044–6050. doi: 10.1021/bi00567a015. [DOI] [PubMed] [Google Scholar]
- O'Leary T. J., Ross P. D., Lieber M. R., Levin I. W. Effects of cyclosporine A on biomembranes. Vibrational spectroscopic, calorimetric and hemolysis studies. Biophys J. 1986 Apr;49(4):795–801. doi: 10.1016/S0006-3495(86)83707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Beck K. Translational diffusion in phospholipid monolayers measured by fluorescence microphotolysis. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7183–7187. doi: 10.1073/pnas.80.23.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. D., Briehl R. W., Minton A. P. Temperature dependence of nonideality in concentrated solutions of hemoglobin. Biopolymers. 1978 Sep;17(9):2285–2288. doi: 10.1002/bip.1978.360170920. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Minton A. P. Analysis of non-ideal behavior in concentrated hemoglobin solutions. J Mol Biol. 1977 May 25;112(3):437–452. doi: 10.1016/s0022-2836(77)80191-5. [DOI] [PubMed] [Google Scholar]
- Saxton M. J. Lateral diffusion in an archipelago. Effects of impermeable patches on diffusion in a cell membrane. Biophys J. 1982 Aug;39(2):165–173. doi: 10.1016/S0006-3495(82)84504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. L., Jr Phosphatidylcholine bilayers. A theoretical model which describes the main and the lower transitions. Biochim Biophys Acta. 1981 Apr 22;643(1):161–167. doi: 10.1016/0005-2736(81)90228-5. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Clegg R. M., Hallmann D. Translational diffusion of lipids in liquid crystalline phase phosphatidylcholine multibilayers. A comparison of experiment with theory. Biochemistry. 1985 Jan 29;24(3):781–786. doi: 10.1021/bi00324a037. [DOI] [PubMed] [Google Scholar]


