Abstract
The diphtheria toxin T-domain translocates the catalytic C-domain across the endosomal membrane in response to acidification. To elucidate the role of histidine protonation in modulating pH-dependent membrane action of the T-domain, we have used site-directed mutagenesis coupled with spectroscopic and physiological assays. Replacement of H257 with an arginine (but not with a glutamine) resulted in dramatic unfolding of the protein at neutral pH, accompanied by a substantial loss of helical structure and greatly increased exposure of the buried residues W206 and W281. This unfolding and spectral shift could be reversed by the interaction of the H257R mutant with model lipid membranes. Remarkably, this greatly unfolded mutant exhibited WT-like activity in channel formation, N-terminus translocation and cytotoxicity assays. Moreover, membrane permeabilization caused by H257R mutant occurs already at pH 6, where wild type protein is inactive. We conclude that protonation of H257 acts as a major component of the pH-dependent conformational switch, resulting in destabilization of the folded structure in solution and thereby promoting the initial membrane interactions necessary for translocation.
Keywords: membrane protein folding, lipid bilayer insertion, histidine protonation, tryptophan fluorescence, conformational switch
Diphtheria toxin enters the cell via the endosomal pathway, which is shared by many other toxins, including botulinum, tetanus, and anthrax 1; 2; 3; 4; 5. A key step in cell infection is the translocation of the catalytic unit from the endosome into the cytosol, accomplished by the translocation domain (T-domain, Fig. 1A). Diphtheria toxin T-domain is a small, 178-residue protein fragment that forms a separate, tightly folded domain at neutral pH but changes its conformation under acidic conditions inside the endosome. It then inserts into the lipid bilayer and translocates its own N-terminus and the attached catalytic domain across the membrane 6; 7. The crystallographic structure of the soluble toxin is known 8, and numerous studies have addressed the interactions of the T-domain with lipid bilayers 9; 10; 11; 12; 13; 14; 15; 16; 17; 18; 19; 20. But a detailed picture of the molecular mechanism of its action is yet to emerge. Recently we have suggested that the acid-induced membrane action of the T-domain is modulated by the following two titration transitions: (a) formation of the membrane-competent state in bulk solution and (b) formation of the insertion-competent state on the membrane interface 21. We have designed a systematic mutagenesis strategy in order to identify the key residues, protonation of which is responsible for triggering these transitions. Here we report that a single amino acid replacement (H257R) triggers substantial conformational change in the T-domain at neutral pH, without compromising its physiological activity, thus providing an opportunity for dissecting its complex insertion pathway in subsequent structural and thermodynamic studies.
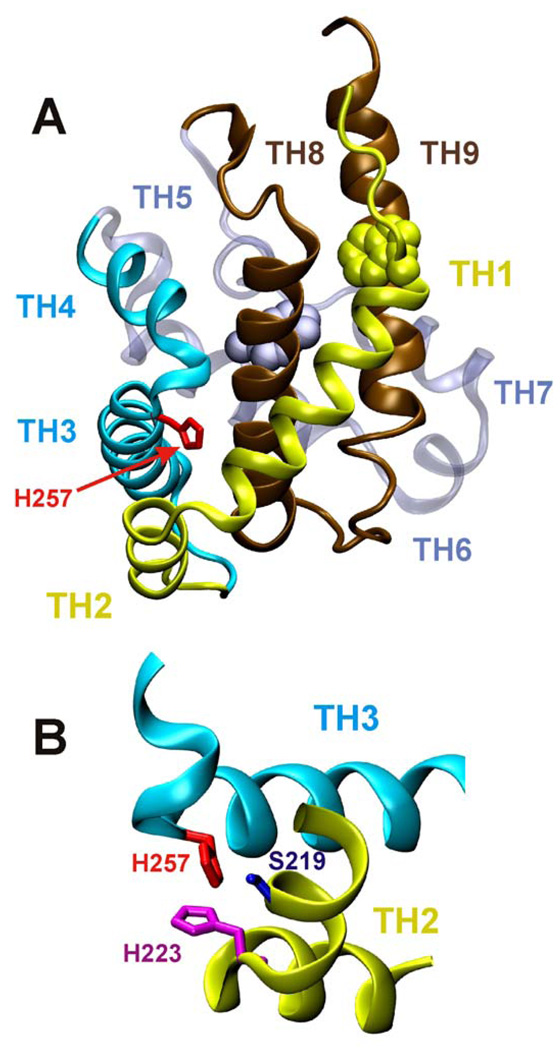
Figure 1.
Crystallographic structure of the WT T-domain of diphtheria toxin (A) represented as backbone ribbon. Histidine 257 (red) is positioned between helices TH1-2 (yellow) and TH3-4 (blue). Other regions of protein are: the consensus membrane insertion domain TH8-9 in brown, helices Th6-7 in grey. Two tryptophan residues are shown as CPK models: W206 in yellow and W281 in grey. Lower panel (B) represents another view of the region surrounding H257 (only neighboring structural elements are shown for clarity).
Two groups of residues are expected to undergo protonation in the range of pH relevant to physiological changes inside the endosome: acidic residues (E and D), which will lose negative charge upon acidification, and histidines, which will gain a positive charge. Histidine protonation has been implicated in biological activity of other toxins, including anthrax 22 and aerolysin 23. It has been suggested that the protonation of the six native histidines of the T-domain makes a favorable thermodynamic contribution to the formation of the interfacial intermediate state of the T-domain 16 and is implicated in the modulation of insertion by anionic lipids 21. The role of histidines in the action of T-domain has been addressed by Perier et al. 19, who studied the membrane interactions of a series of mutants with H-to-F replacements. The authors concluded that key histidines undergo a concerted protonation, and they suggest that the pair His223–His257 and His251 are important mostly for unfolding, while His322–His323 and His251 are important mostly for membrane binding. This study, however, did not address the physiological activity of the mutants, nor did it attempt to modulate the effect of histidine protonation by introducing charge mutation. Moreover, replacement with phenylalanines results in the potential introduction of strong, non-native hydrophobic interactions with the lipid bilayer. According to the Wimley-White experimental hydrophobicity scales, H-to-F mutation provides up to an exuberant 2.5 kcal/mole in additional favorable free energy for membrane interaction per single replaced residue 24; 25. It is not difficult to imagine how these additional hydrophobic interactions would compensate for the lost electrostatic interactions of histidines with the charged membrane interfaces, thus complicating the interpretation of the results. To resolve these shortcomings and ambiguities, we have designed an alternative mutagenesis strategy, which is based on the comparison of the biophysical and physiological properties of the T-domain WT with those of (a) mutants with neutral, but not hydrophobic residues (H-to-Q replacement) and (b) those with pH-independent positive charge (H-to-R replacements).
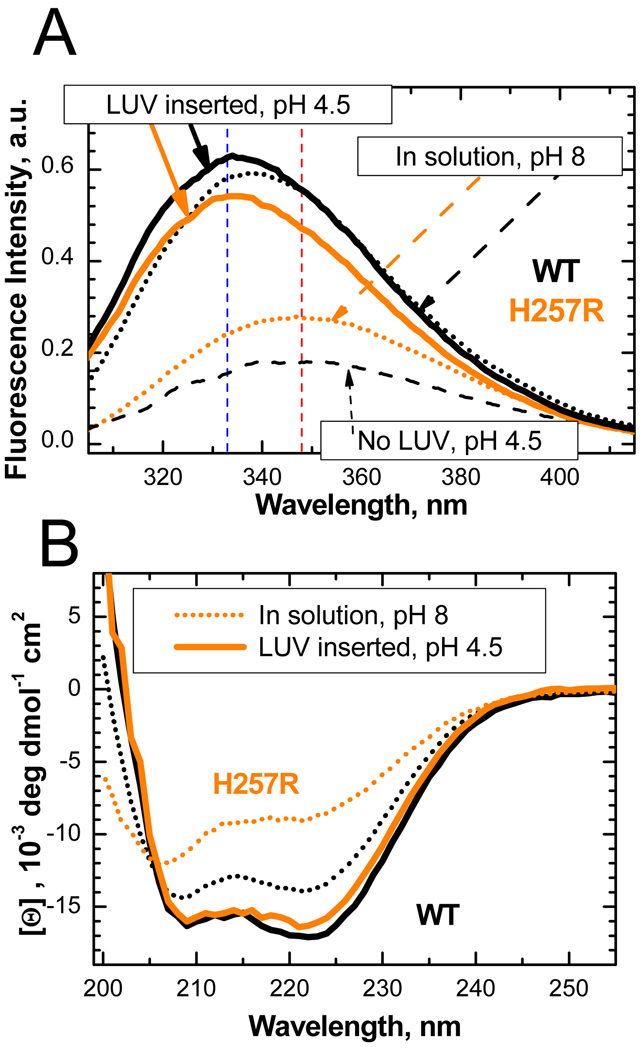
We have systematically replaced (one by one and in groups) all six native histidines of the T-domain with either glutamine or arginine residues and evaluated their folding in solution by means of circular dichroism (CD). Some replacements caused pronounced misfolding, while others had moderate effect on changes of secondary structure (see Fig. S1). The most intriguing result was obtained with substitutions of H257: a replacement with the neutral glutamine caused little effect, while replacement with the charged arginine caused substantial unfolding. This unfolding is also revealed by the red shift of intrinsic fluorescence originating from W206 and W281 and the decrease of their combined quantum yield (Fig. 2A, orange dotted line). Such spectral changes are consistent with an increased exposure of the fluorophores to an aqueous environment, and are similar to those observed for WT T-domain during the formation of the membrane-competent state at acidic pH (dashed black line). The latter state, however, is prone to significant self-aggregation17; 26, which makes it difficult to study and is likely the reason why the unfolded state is not observed at low pH by CD spectroscopy 17; 26. Remarkably, membrane insertion of the H257R mutant leads to a significant intensity increase and blue spectral shift (solid orange line), to the point that the position of the fluorescence spectrum is identical to that of WT (solid black line), and their quantum yields are close. Similarly, the ellipticity of the mutant is also strongly increased upon membrane insertion and becomes almost identical to that of the WT protein (Fig. 2B). Thus, both CD and fluorescence data indicate that H257R, while largely unfolded in solution, gains its native structure when inserted into the membranes. In contrast, the H257Q mutant behaves essentially as WT does in equilibrium CD and fluorescence experiments (not shown).
Figure 2.
Comparison of intrinsic fluorescence (A) and secondary structure (B) of T-domain WT (black) and H257R mutant (orange). (A) Intrinsic fluorescence spectra of WT (black) and unfolded H257R mutant (orange) in solution at neutral pH (dotted lines) and in membrane-inserted conformation at acidic pH (solid lines). Fluorescence of H257R in solution is red-shifted (maximum indicated by a vertical red dashed line) and has low intensity, both of which indicate increased exposure of W206 and W281 to aqueous environment (this spectrum is also similar to that of the membrane-competent form of WT at acidic pH, shown by dashed line). Membrane insertion results in increase of fluorescence, and its blue shift corresponds to that of WT (vertical blue dashed line indicates maxima of both membrane-inserted proteins), suggesting similar environments of the tryptophan residues in membrane complexes of WT and H257R. (B) CD spectrum of H257R mutant at neutral pH (orange dotted line) exhibits reduced ellipticity compared to that of WT (black dotted line), indicating significant loss of helical secondary structure. Membrane insertion at acidic conditions leads to recovery of native-like secondary structure (solid lines). Experimental details: WT and mutant proteins were expressed in E. coli and purified as described in 21. Fluorescence measurements were performed using a SPEX Fluorolog FL3-22 steady-state fluorescence spectrometer (Jobin Yvon, Edison, NJ) as described previously 21 using excitation wavelength of 292 nm. CD measurements were collected in cuvettes of 1 mm path using Jasco-720 spectropolarimeter (Japan Spectroscopic Company, Tokyo) as described previously 26. Samples contained 1 µM (fluorescence) or 2 µM (CD) of T-domain and, when present, 25% POPC/75% POPG LUV containing 1 mM of total lipid.
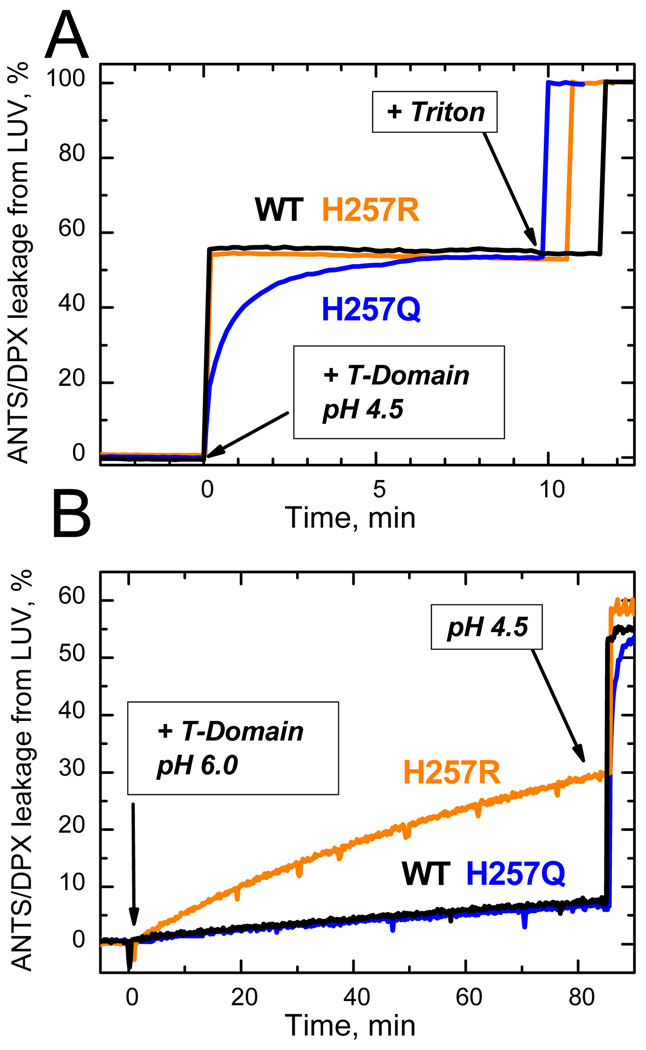
To further evaluate the membrane action of T-domain mutants, we have performed three independent measurements of activity: vesicle permeabilization, channel formation and translocation in planar bilayers, and cytotoxicity. First, we determined vesicle content leakage by measuring fluorescence associated with the release of the fluorophore/quencher pair ANTS/DPX preloaded into the vesicles upon T-domain insertion. Whereas both mutants exhibit similar final levels of permeabilization at pH 4.5, the kinetics of release caused by H257Q mutant is much slower than that of H257R or WT (Fig. 3A). This indicates that removing the positive charge on His-257 significantly affects pH-triggered conformational switching in the T-domain but doesn’t eliminate it, suggesting that such switching is redundant (i.e., can be triggered by multiple residues). Consistent with this mechanism, introducing a pH-independent positive charge at this position is expected to result in an increased activity at neutral pH, which is indeed observed for the H257R mutant (Fig. 3B).
Figure 3.
Leakage of lipid vesicle contents resulting from membrane insertion of the T-domain WT and H257R and H257Q mutants, monitored by increase of fluorescence from ANTS/DPX-loaded LUV. Membrane interactions were initiated at zero time by adding stocks of T-domain to vesicular samples at pH 4.5 and 6.0. Both mutants demonstrated a similar level of membrane-disrupting activity at pH 4.5, although the kinetics of membrane action was slower in H257Q (A). Correspondingly, H257R showed substantially greater membrane disruption at pH 6.0 than did WT or H257Q (B). The traces for WT measured at pH 6.0 and 4.5 coincide with those for the respectively neutral and charged replacements of H257, indicating that the protonation of this residue in WT is responsible for triggering its pH-dependent membrane action. Samples contained 0.2 µM T-domain and 25% POPC/75% POPG LUV containing 0.2 mM of total lipid. Other experimental details are described in 17.
We have complemented vesicle permeabilization assays with more specific studies of physiological function, such as measurements of the voltage-dependent conductivity caused by the T-domain in planar lipid bilayers (Fig. 4A, B) and measurements of cytotoxicity performed with the entire three-domain diphtheria toxin (Fig. 4C). We measured the current response under voltage-clamp conditions in planar bilayers between the two chambers: the cis-chamber with a buffer of pH 5.3 into which the proteins were injected from a stock solution and the trans-chamber with a buffer of pH 7.2. Such measurements have been used extensively in studies of T-domain to identify the open-channel state topology, investigate N-terminus translocation, and establish that an active channel is formed by monomeric T-domain 6; 12; 27. Our results indicate that, similarly to WT, both mutants are capable of making channels in planar bilayers that would open at positive voltages and close at negative ones (Fig. 4A, B). This channel-closing is a hallmark of the N-terminus translocation across the bilayer 6 and is caused by interaction of the N-terminal poly-His tag (which was kept uncleaved for these measurements) with the channel on the trans-side of the planar bilayer. Because the activity observed in such experiments varies from membrane to membrane preparation, after the mutant-induced activity leveled off, we added WT protein to each of the membranes used to measure activity of mutants, to serve as a standard. The activity (and thus the number of active channels) is somewhat lower for the mutants than it is for WT, which could be explained in numerous ways, such as by lower membrane partitioning, formation of inactive channels, or partial misfolding. Given these limitations and the overall nature of any experiment with planar bilayers, our results are consistent with the WT–like activity of both mutants in terms of channel properties and their ability to translocate their N-termini.
Figure 4.
Functional activity of H257R and H257Q mutants studied using electrophysiological (top panels) and cytotoxicity assays (bottom panel). The results indicate near-WT activity in both H257Q and H257R mutants. Panels A and B show a record of the transmembrane current (upper trace) at ±30 mV voltage (lower trace) vs time. For each record, mutant T-domain was added to the cis compartment at the start of the record (first arrow). The current increased as channels opened at +30 mV. At −30 mV, the current rapidly decayed toward zero, and quickly recovered upon the return to +30 mV, as expected for T-domain with an amino-terminal histidine-tag 6. After the number of channels inserted into the membrane stabilized, an equal concentration of wild-type T-domain was added to the cis compartment (second arrow), causing an increase in the current as wild-type channels opened. A. Mutant H257R was added to a concentration of 0.8 nM, resulting in the opening of about 300 channels. After 0.8 nM wild-type T-domain was added, the total number of channels increased to about 1000. B. 1.0 nM of mutant H257Q generated about 300 channels. After the addition of 1.0 nM wild-type T-domain, there were about 2400 channels in total. Methods: Asolectin planar bilayers were formed and voltage-clamp recording was performed as previously described 32. The voltage is defined as the potential of the cis compartment, relative to that of the opposite trans compartment. The aqueous solutions contained 1 M KCl, 2 mM CaCl2, 1 mM EDTA, and either 30 mM MES, pH 5.3 (cis) or 20 mM HEPES, pH 7.2 (trans).
(C) Cytotoxicity assays were performed using the protein synthesis inhibition assay 29. Briefly, CHO-K1 cells (10,000 cells/well) were intoxicated by diphtheria toxin constructs for 24 hrs at 37° C after which incorporation of L-[4, 5-3H] leucine was measured. Both H257Q and H257R mutants exhibit activity indistinguishable from that of the WT toxin.
The ultimate test of physiological activity is the cytotoxicity assay, which utilizes the entire full-sequence diphtheria toxin (normally these studies are performed with a weakened strain carrying a E148S mutation in the catalytic domain to reduce the toxic potency 28). Cell death is determined by monitoring the inhibition of protein synthesis 29. We have introduced arginine and glutamine replacements for histidine 257 in the context of the entire toxin and evaluated the resulting cytotoxic activity. Our data indicate that both H257R and H257Q mutants retain the wild-type activity (Fig. 4C).
The crystallographic structure of the folded T-domain consists of nine helices of various lengths (TH1-9; Fig. 1A), eight of which completely surround the most hydrophobic one, TH8. Acidification of the endosomal environment triggers a massive conformational refolding/insertion transition, ultimately allowing helices TH8-9 to adopt a transmembrane topology and the N-terminus to be translocated across the lipid bilayer. Histidine 257 is a relatively buried residue, located between helices TH3 and TH4, with a side chain contributing to the interface with the other two N-terminal helices, TH1 and TH2 (Fig. 1B). This histidine can potentially interact with either H223 of TH2 or S219 of TH1 (insert). Protonation of H257 is expected to destabilize interactions between N-terminal helices, which will then destabilize the hydrophobic core of the protein. Introduction of a positively charged side chain at this position in the H257R mutant results in substantial unfolding, which manifests itself in the loss of secondary structure and exposure of the buried residues W206 and W281 into an aqueous environment (Fig. 2). Our estimates, based on fitting with reference spectra using CONTIN/LL procedure of Sreerama and Woody 30, suggest that this mutant has only ~30–50% (depending on the reference set) of helical structure, compared to ~80% in WT. This conformational change shifts the pH range for membrane action of the T-domain toward neutral pH values (Fig. 3B). On the other hand, total elimination of the charge in the H257Q mutant substantially slows down membrane permeabilization at acidic pH (Fig. 3A).
As pointed out by Yang and Honig in their theoretical study 31 the pH-dependent unfolding in proteins appears to be due to individual groups located on protein interfaces which have anomalous pKa’s. In our future studies we intend to determine if this holds true for diphtheria toxin T-domain by performing theoretical and experimental exploration of thermodynamic stability of the protein and its mutants. The evidence presented here indicates that protonation of H257 acts as a pH-sensitive switch that triggers conformational change, resulting in membrane insertion. H257 is not the only residue involved in conformational switching; this appears to have multiple controls. Replacement of this histidine with arginine allows one to selectively turn on a part of the conformational machinery at higher pH, while retaining the overall physiological function. This opens up unique possibilities, such as using the H257R mutant for probing structural and thermodynamic properties of the T-domain at intermediate stages along the refolding/insertion pathway.
Supplementary Material
Acknowledgement
We are grateful to Dr. S.H. White for his valuable comments and to Mr. M.A. Myers for his editorial assistance.
ABBREVIATIONS
- T-domain
diphtheria toxin T-domain
- WT
wild type (T-domain)
- LUV
large unilamellar vesicles
- POPC
palmitoyloleoylphosphatidylcholine
- POPG
palmitoyloleoylphosphatidylglycerol
- ANTS
8-aminonaphthalene-1,3,6 trisulfonic acid
- DPX
p-xylene-bis-pyridinium bromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This research was supported by NIH grants GM-069783 (A.S.L), GM-29210 (A.F.) and AI-022021 (R.J.C).
REFERENCE LIST
- 1.Hoch DH, Romero-Mira M, Ehrlich BE, Finkelstein A, DasGupta BR, Simpson LL. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: Relevance to translocation of proteins. Proc.Natl.Acad.Sci.USA. 1985;82:1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K. Botulinum toxin as a biological weapon: Medical and public health management. JAMA. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 3.Neale EA. Moving across membranes. Nature Struct.Biol. 2003;10:2–3. doi: 10.1038/nsb0103-2. [DOI] [PubMed] [Google Scholar]
- 4.Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nature Struct.Biol. 2003;10:13–18. doi: 10.1038/nsb879. [DOI] [PubMed] [Google Scholar]
- 5.Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45–70. doi: 10.1146/annurev.cellbio.19.111301.140655. [DOI] [PubMed] [Google Scholar]
- 6.Senzel L, Huynh PD, Jakes KS, Collier RJ, Finkelstein A. The diphtheria toxin channel-forming T domain translocates its own NH2-terminal region across planar bilayers. J.Gen.Physiol. 1998;112:317–324. doi: 10.1085/jgp.112.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh KJ, Senzel L, Collier RJ, Finkelstein A. Translocation of the catalytic domain of diphtheria toxin across planar phospholipid bilayers by its own T domain. Proc.Natl.Acad.Sci.USA. 1999;96:8467–8470. doi: 10.1073/pnas.96.15.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett MJ, Eisenberg D. Refined structure of monomeric diphtheria toxin at 2.3 Å resolution. Protein Sci. 1994;3:1464–1475. doi: 10.1002/pro.5560030912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh KJ, Zhan H, Cui C, Hideg K, Collier RJ, Hubbell WL. Organization of diphtheria toxin T domain in bilayers: A site-directed spin labeling study. Science. 1996;273:810–812. doi: 10.1126/science.273.5276.810. [DOI] [PubMed] [Google Scholar]
- 10.Oh KJ, Zhan H, Cui C, Altenbach C, Hubbell WL, Collier RJ. Conformation of the diphtheria toxin T domain in membranes: A site-directed spin-labeling study of the TH8 helix and TL5 loop. Biochemistry. 1999;38:10336–10343. doi: 10.1021/bi990520a. [DOI] [PubMed] [Google Scholar]
- 11.Kachel K, Ren JH, Collier RJ, London E. Identifying transmembrane states and defining the membrane insertion boundaries of hydrophobic helices in membrane-inserted diphtheria toxin T domain. J.Biol.Chem. 1998;273:22950–22956. doi: 10.1074/jbc.273.36.22950. [DOI] [PubMed] [Google Scholar]
- 12.Senzel L, Gordon M, Blaustein RO, Oh KJ, Collier RJ, Finkelstein A. Topography of diphtheria toxin's T domain in the open channel state. J.Gen.Physiol. 2000;115:421–434. doi: 10.1085/jgp.115.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao G, London E. Behavior of diphtheria toxin T domain containing substitutions that block normal membrane insertion at Pro345 and Leu307: Control of deep membrane insertion and coupling between deep insertion of hydrophobic subdomains. Biochemistry. 2005;44:4488–4498. doi: 10.1021/bi047705o. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Malenbaum SE, Kachel K, Zhan HJ, Collier RJ, London E. Identification of shallow and deep membrane-penetrating forms of diphtheria toxin T domain that are regulated by protein concentration and bilayer width. J.Biol.Chem. 1997;272:25091–25098. doi: 10.1074/jbc.272.40.25091. [DOI] [PubMed] [Google Scholar]
- 15.Chenal A, Savarin P, Nizard P, Guillain F, Gillet D, Forge V. Membrane protein insertion regulated by bringing electrostatic and hydrophobic interactions into play. A case study with the translocation domain of the diphtheria toxin. J.Biol.Chem. 2002;277:43425–43432. doi: 10.1074/jbc.M204148200. [DOI] [PubMed] [Google Scholar]
- 16.Ladokhin AS, Legmann R, Collier RJ, White SH. Reversible refolding of the diphtheria toxin T-domain on lipid membranes. Biochemistry. 2004;43:7451–7458. doi: 10.1021/bi036157w. [DOI] [PubMed] [Google Scholar]
- 17.Palchevskyy SS, Posokhov YO, Olivier B, Popot JL, Pucci B, Ladokhin AS. Chaperoning of Insertion of Membrane Proteins into Lipid Bilayers by Hemifluorinated Surfactants: Application to Diphtheria Toxin. Biochemistry. 2006;45:2629–2635. doi: 10.1021/bi052257l. [DOI] [PubMed] [Google Scholar]
- 18.Montagner C, Perier A, Pichard S, Vernier G, Menez A, Gillet D, Forge V, Chenal A. Behavior of the N-Terminal Helices of the Diphtheria Toxin T Domain during the Successive Steps of Membrane Interaction. Biochemistry. 2007;46:1878–1887. doi: 10.1021/bi602381z. [DOI] [PubMed] [Google Scholar]
- 19.Perier A, Chassaing A, Raffestin S, Pichard S, Masella M, Menez A, Forge V, Chenal A, Gillet D. Concerted protonation of key histidines triggers membrane interaction of the diphtheria toxin T domain. J Biol Chem. 2007;282:24239–24245. doi: 10.1074/jbc.M703392200. [DOI] [PubMed] [Google Scholar]
- 20.Posokhov YO, Rodnin MV, Das SK, Pucci B, Ladokhin AS. FCS study of the thermodynamics of membrane protein insertion into the lipid bilayer chaperoned by fluorinated surfactants. Biophys J. 2008;95:L54–L56. doi: 10.1529/biophysj.108.141002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyrychenko A, Posokhov YO, Rodnin MV, Ladokhin AS. Kinetic intermediate reveals staggered pH-dependent transitions along the membrane insertion pathway of the diphtheria toxin T-domain. Biochemistry. 2009;48:7584–7594. doi: 10.1021/bi9009264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wimalasena DS, Cramer JC, Janowiak BE, Juris SJ, Melnyk RA, Anderson DE, Kirk KL, Collier RJ, Bann JG. Effect of 2-fluorohistidine labeling of the anthrax protective antigen on stability, pore formation, and translocation. Biochemistry. 2007;46:14928–14936. doi: 10.1021/bi701763z. [DOI] [PubMed] [Google Scholar]
- 23.Buckley JT, Wilmsen HU, Lesieur C, Schulze A, Pattus F, Parker MW, van der Goot FG. Protonation of histidine-132 promotes oligomerization of the channel-forming toxin aerolysin. Biochemistry. 1995;34:16450–16455. doi: 10.1021/bi00050a028. [DOI] [PubMed] [Google Scholar]
- 24.Wimley WC, White SH. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nature Struct.Biol. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 25.White SH, Wimley WC. Hydrophobic interactions of peptides with membrane interfaces. Biochim.Biophys.Acta. 1998;1376:339–352. doi: 10.1016/s0304-4157(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 26.Rodnin MV, Posokhov YO, Contino-Pepin C, Brettmann J, Kyrychenko A, Palchevskyy SS, Pucci B, Ladokhin AS. Interactions of fluorinated surfactants with diphtheria toxin T-domain: testing new media for studies of membrane proteins. Biophys J. 2008;94:4348–4357. doi: 10.1529/biophysj.107.126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon M, Finkelstein A. The number of subunits comprising the channel formed by the T domain of diphtheria toxin. J Gen Physiol. 2001;118:471–480. doi: 10.1085/jgp.118.5.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbieri JT, Collier RJ. Expression of a mutant, full-length form of diphtheria toxin in Escherichia coli. Infect Immun. 1987;55:1647–1651. doi: 10.1128/iai.55.7.1647-1651.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanke SR, Milne JC, Benson EL, Collier RJ. Fused polycationic peptide mediates delivery of diphtheria toxin A chain to the cytosol in the presence of anthrax protective antigen. Proc Natl Acad Sci U S A. 1996;93:8437–8442. doi: 10.1073/pnas.93.16.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sreerama N, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 31.Yang A-S, Honig B. On the pH dependence of protein stability. J.Mol.Biol. 1993;231:459–474. doi: 10.1006/jmbi.1993.1294. [DOI] [PubMed] [Google Scholar]
- 32.Kienker PK, Jakes KS, Finkelstein A. Protein translocation across planar bilayers by the colicin Ia channel-forming domain: where will it end? J Gen Physiol. 2000;116:587–597. doi: 10.1085/jgp.116.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.